Abstract
Attention deficit hyperactivity disorder (ADHD) is strongly affected by sex, but sex chromosomes’ effect on brain attention networks and cognition are difficult to examine in humans. This is due to significant etiologic heterogeneity among diagnosed individuals. In contrast, individuals with Turner syndrome (TS), who have substantially increased risk for ADHD symptoms, share a common genetic risk factor related to the absence of the X-chromosome, thus serving as a more homogeneous genetic model. Resting-state functional MRI was employed to examine differences in attention networks between girls with TS (n = 40) and age- sex- and Tanner-matched controls (n = 33). We compared groups on resting-state functional connectivity measures from data-driven independent components analysis (ICA) and hypothesis-based seed analysis. Using ICA, reduced connectivity was observed in both frontoparietal and dorsal attention networks. Similarly, using seeds in the bilateral intraparietal sulcus (IPS), reduced connectivity was observed between IPS and frontal and cerebellar regions. Finally, we observed a brain-behavior correlation between IPS-cerebellar connectivity and cognitive attention measures. These findings indicate that X-monosomy contributes affects to attention networks and cognitive dysfunction that might increase risk for ADHD. Our findings not only have clinical relevance for girls with TS, but might also serve as a biological marker in future research examining the effects of the intervention that targets attention skills.
Keywords: attention deficit hyperactivity disorder, attention networks, executive function, resting-state imaging, Turner syndrome
Introduction
Attention deficit hyperactivity disorder (ADHD) is the most common human neurodevelopmental disorder, affecting up to 10% of children in the United States of America. ADHD symptoms are behavioral manifestations of neural dysfunction. Accordingly, significant efforts have been made toward finding brain correlates of the disorder’s core symptoms (Bush 2011). One approach to investigating neural circuits underlying ADHD symptoms is resting-state functional MRI (rsfMRI), a neuroimaging method that measures low-frequency correlations between blood-oxygen-level dependent (BOLD) time points across brain regions (Tian et al. 2007; Dosenbach et al. 2008).
In general, findings from seed-based analyses of rsfMRI data from individuals with ADHD (mostly males) show reduced strength of anticorrelated (negative) activity between default mode network and dorsal anterior cingulate cortex relative to controls. However, these results are not consistent across studies. Given varied analytic methods including independent components analysis (ICA), small-world network analysis, graph theory and seed-based approaches as well as varied age groups, male to female ratios, ADHD symptomatology and relatively small cohorts, direct comparison of findings across studies is difficult (Uddin et al. 2010). Beyond these limitations, an inherent and significant limitation to rsfMRI imaging studies in ADHD is the heterogeneous nature of the disorder, which affects our ability to detect consistent differences in brain structure or function in individuals with ADHD relative to controls. Addressing this issue, Uddin et al. suggests that clinical pediatric resting-state studies should be comprised of relatively homogenous clinical and control groups to allow for meaningful interpretations.
Turner syndrome (TS), a relatively common genetic condition in females caused by the absence of most or all of 1 X-chromosome (Stochholm et al. 2006), is associated with significantly increased risk for ADHD in the context of normal overall intellectual function (Russell et al. 2006). We have recently shown that ADHD-associated behavioral and cognitive problems are prevalent in TS (up to 50% of girls with TS) and comparable in severity to those found in children with idiopathic ADHD (Green et al. 2015). This finding is aligned with the strong effect of sex on ADHD manifestations and the effects of genes of the X chromosomes (MAOA (Bonvicini et al. 2016), HTR2C (Li et al. 2006; Xu et al. 2009), and STS (Trent et al. 2012, 2013)) on attention and hyperactivity. Thus, the study of brain correlates in girls with TS caused by X-monosomy not only provides a human model that is relatively homogenous from the standpoint of a shared etiologic risk factor, but also enables a unique look into the effect of X-chromosome absence on attention networks and ADHD-related symptoms.
To the best of our knowledge, only one study to date has examined resting-state functional connectivity (RSFC) in girls with TS. This study used a graph theory-based approach to measure whole brain functional connectivity strength in 22 girls with TS and 17 age-matched controls. Girls with TS were shown to have reduced functional connectivity in the cuneus, right cerebellum, bilateral intraparietal sulcus (IPS), and bilateral angular gyrus (Xie et al. 2015). Limited by methodology and relatively small sample size, this comparison did not provide information about specific attention networks such as the dorsal attention network (DAN) and frontoparietal network (Dosenbach et al. 2008; Petersen and Posner 2012) nor did it account for potential confounding factors such as pubertal development.
Here, for the first time, we examine how TS affects RSFC in large-scale attentional networks relative to age- and sex-matched typically developing controls. Group differences in RSFC were examined using both model-free ICA and model-driven seed-based analysis. For ICA-based analysis, group differences in connectivity within large-scale brain attention networks (the DAN and the frontoparietal network) were assessed. For seed-based analysis, voxel-wise whole brain connectivity for a set of a priori selected seed-regions within these same networks based on known anatomical differences in the IPS (Molko et al. 2003; Bray et al. 2011) was examined at the group-level. Altered RSFC in girls with TS compared with controls was hypothesized to be associated with cognitive functioning differences related to attention.
Materials and Methods
Participants
Participants with TS were recruited through the National Turner Syndrome Society and the Turner Syndrome Foundation, a local network of physicians, and advertisement on the Stanford University School of Medicine website. Control participants were recruited through local print media and parent networks. Exclusion criteria for both groups included premature birth (gestational age < 34 weeks), low-birth weight (<2000 g), and known diagnosis of a major psychiatric condition (i.e., psychotic or mood disorder) or current neurological disorder, including seizures. All participants in the TS group had X-monosomy, confirmed by karyotype reports supplied by families. Girls with TS exhibiting mosaic or uncommon structural karyotypes were excluded. The local Institutional Review Board of the Stanford University School of Medicine approved this study and informed written consent was obtained from a legal guardian for all participants. Written assent was obtained from participants over 7 years of age.
For this study, 86 participants (47 TS and 39 controls) were scanned. Thirteen participants (7 TS, 6 controls) were excluded based on 2 data-usability criteria: 1) ≥5 min of artifact-free rsfMRI data (Power et al. 2015), after scrubbing 2) a mean frame-wise displacement (FD) of ≤0.2 mm. The final analysis included 73 participants (40 TS, 33 controls) ages 4.7–16.2 years, with groups matched for sex, age, Tanner stage (Table 1 and see Supplementary Table S1), number of scrubbed frames, mean FD after scrubbing, and duration of clean (after scrubbing) resting-state data (see Supplementary materials).
Table 1.
Demographics
| Controls | TS | P-value | |
|---|---|---|---|
| n | 33 | 40 | |
| Age range (years) | 5.3–14.2 | 4.7–16.2 | |
| At risk for attention problems/hyperactivitya | 2 (6%) | 15 (37.5%) | |
| Mean (SD) | Mean (SD) | ||
| Age | 10.5 (2.2) | 11.2 (2.8) | NS |
| Attention problems | 45.6 (7.4) | 52.6 (9.4) | <0.001 |
| Hyperactivity | 45.1 (9.9) | 54.5 (13.0) | <0.001 |
| FSIQ | 116.1 (11.8) | 96.7 (13.8) | <0.001 |
| VIQ | 118.6 (14.4) | 107.1 (15.7) | <0.01 |
| PIQ | 115.3 (11.3) | 96.2 (15.4) | <0.001 |
| Working memory | 107.0 (10.9) | 92.8 (12.7) | <0.001 |
| Processing speed | 103.9 (15.9) | 87.1 (13.8) | <0.001 |
| NEPSY attention and EF domain | |||
| Auditory attention | 10.9 (2.3) | 8.7 (3.8) | <0.01 |
| Response set | 11.3 (2.0) | 9.2 (3.3) | <0.01 |
| Inhibition | |||
| Naming | 10.3 (3.8) | 7.7 (3.3) | <0.01 |
| Inhibition (contrast) | 11.2 (3.3) | 9.0 (3.0) | <0.01 |
| Switching (contrast) | 10.7 (3.2) | 9.4 (3.3) | =0.08 |
| NEPSY visuo-spatial domain | |||
| Arrows | 10.7 (2.7) | 6.5 (3.9) | <0.001 |
| Picture Puzzles | 11.4 (3.5) | 6.7 (3.5) | <0.001 |
Note: FSIQ, full scale intelligence quotient; PIQ, performance intelligence quotient; VIQ, verbal intelligence quotient. NEPSY—NEuroPSYchological assessment.
aBASC-2, Behavior Assessment System for Children, Second Edition. Participants were considered be at risk for Attention problems/Hyperactivity if T score were equal or above 60.
Cognitive Assessment
Participants were administered cognitive assessments appropriate for their age (Wechsler 2002, 2003). Attention, executive function, and visual–spatial abilities were assessed using the developmental NEPSY-2 (Korkman 2004) (see Supplementary materials for full description).
MRI Data Acquisition
Imaging data were acquired on a 3-Tesla GE MR750 scanner (GE Healthcare) with an 8-channel head coil, including high-resolution T1-weighted structural images (sagittal slices, repetition time 8.2 ms; echo time 3.2 ms; flip angle 12°; field of view 240 × 192 mm; matrix 256 × 256; 176 slices; voxel size = 1.0 × 1.0 × 1.0 mm). A gradient echo imaging pulse sequence was used to acquire 6 min 06 s of rsfMRI data (T2-weighted images) with the following parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 80°, FOV = 22 cm × 22 cm, 30 slices, matrix = 64 × 64, voxel size = 3.4 × 3.4 × 5 mm. Participants were instructed to relax and remain still in the scanner and to close their eyes without falling asleep.
Data Preprocessing
Standard rsfMRI preprocessing was performed using the Configurable Pipeline for the Analysis of Connectomes (C-PAC version 0.3.4; cp-indi.github.io/docs/user/rnotes.html). Preprocessing included discarding the first 10 volumes (or TRs) of data for signal stabilization, slice timing correction, motion correction (FSL MCFLIRT), skull stripping (FSL BET), grand mean scaling, spatial smoothing (FWHM 4 mm), and a temporal band-pass filter (0.01 Hz < f < 0.1 Hz).
Additionally, nuisance signal correction was implemented by regressing out 1) linear and quadratic trends; 2) mean time-series from white matter (WM) and cerebrospinal fluid; 3) 24 motion parameters obtained by motion correction (the 6 motion parameters of the current volume and the preceding volume, plus each of these values squared); and 4) signals extracted using the CompCor algorithm (Behzadi et al. 2007). To ensure that group differences in RSFC were not influenced by spurious motion-related noise, scrubbing (“censuring”) was performed (Power et al. 2012, 2015). FD and DVARS (D referring to temporal derivative of time course, VARS referring to root mean square variance over voxels) were used in union to censure data points. In addition, to accommodate temporal smoothing of BOLD data, we also marked 1 frame back and 2 frames forward from any marked frames where FD/DVARS threshold (determined using fsl_motion_outliers command) was crossed.
ICA-Based within-Network Connectivity Analysis
To test for alterations in the large-scale attention networks, we performed data-driven ICA. We examined differences in within-network RSFC in the DAN and frontoparietal network with the hypothesis that relative hypoconnectivity would be observed in TS for each network. Specifically, we used group-based ICA and dual regression methodology (Filippini et al. 2009). This analysis involves 3 main steps. First, data-driven spatial maps are created by running group-ICA (Filippini et al. 2009) on temporally concatenated data from equal numbers of participants from both groups. Second, the group-ICA components are then dual-regressed into the subject space (Filippini et al. 2009). Third, we examine within-network connectivity differences in 2 large neural networks (DAN and frontoparietal network) associated with attention. The group-level analysis was performed by contrasting subject-specific spatial maps for the 2 large-scale networks while controlling for age and verbal intelligence quotient (IQ). To determine significant group differences, FSL’s randomize permutation tool was used. This uses a threshold-free cluster enhancement procedure at a family-wise error (P < 0.05) with 10 000 iterations (Winkler et al. 2014).
Seed-Based Connectivity Analysis
To test for alterations in IPS connectivity, we compared whole brain connectivity to bilateral IPS between participants with TS and controls. Extracted time-series from 2 seed locations were modeled with GLM analysis using the fMRI Expert Analysis Tool (Beckmann et al. 2003). The seeds were located on bilateral left and right IPS (left x = −32, y = −63, z = 46 and right x = 30, y = −45, z = 43), which independently and consistently have been shown to have reduced volume in TS relative to controls (Molko et al. 2003; Bray et al. 2011). For the group-level comparisons, age and verbal IQ were used as covariates of no interest. Group-level maps were cluster corrected using a standard value of Z = 2.3 and FWE P < 0.05.
Brain-Behavior Correlations Exploratory Analysis
Pearson correlations were computed to explore associations between rsfMRI results and scores representing the following neurocognitive domains: Attention (NEPSY Auditory Attention, Response Set); Executive function (NEPSY Naming, Inhibition, Switching); Visuo-spatial (NEPSY Arrows and Picture Puzzles), and behavior (BASC-2 Attention Problems and Hyperactivity). These cognitive-behavioral data were analyzed for associations with ICA-based within-network connectivity (mean Z’s extracted from the 5 clusters of significant between-group effects, Fig. 1) and IPS-whole brain connectivity (mean Z’s extracted from the 6 clusters of significant between-group effects). Results (P-values) were adjusted for multiple comparisons within each neurocognitive domain using Bonferroni correction. Between-group correlation differences were then examined using regression with neurocognitive score as the outcome variable y, group and connectivity values in brain region as predictors. Interaction terms for group and brain region were also included in the model.
Figure 1.
Data-driven independent component analysis. Between-group comparisons revealed several clusters of reduced connectivity in the TS group in the frontoparietal and DAN. (A) frontoparietal network (yellow), clusters of reduced activity (red). (B) DAN (green), clusters of reduced activity (red). Table: summary of cluster location and size.
Results
The groups did not significantly differ in age, sex, or Tanner pubertal stage (all P > 0.05). However, girls with TS had significantly lower IQ scores compared to controls. As expected (Hong et al. 2009), verbal IQ scores showed the smallest gap (11.5 points on average) between groups. Girls with TS also had significantly higher scores on the BASC-2 attention problems and hyperactivity subscales, and lower scores on the NEPSY attention, executive function, and visuo-spatial domains compared to controls (Table 1). Tanner pubertal stage, growth and estrogen hormone status are reported in Supplementary Table S1.
TS is Associated with Reduced Intrinsic Connectivity in Large-Scale Attention Networks
Between-group comparisons revealed several clusters of reduced connectivity in the DAN and frontoparietal networks in the TS group. Results are summarized in Figure 1. Full description of all other connectivity networks can be found in Supplementary Table S2.
TS is Associated with Reduced Left and Right IPS Connectivity to Frontal Regions
Between-group comparisons showed hypoconnectivity in TS of the left IPS with extensive bilateral frontal regions including dorsal lateral prefrontal cortex, paracingulate, anterior cingulate gyrus, paracingulate gyrus (2 clusters: 388 voxels, P < 0.0001, Z = 4.1; 179 voxels, P < 0.001, z = 3.73) and cerebellar regions (2 clusters: 375 voxels, P < 0.0001, Z = 3.97; 233, P < 0.0001, z = 3.83) (Fig. 2). Hypoconnectivity of the right IPS with a cluster at the junction of superior frontal sulcus and precentral sulcus (97 voxels, P < 0.05, Z = 3.83) and hyperconnectivity with extensive regions in bilateral occipital cortices and the precuneus in the parietal lobe (1118 voxels, P < 0.0001, Z = 4.18) were also observed in TS relative to controls (Fig. 2).
Figure 2.
IPS seed-based analysis. (A) Between-group comparisons revealed several clusters of reduced connectivity in the TS group between the left IPS and extensive bilateral frontal regions including dorsal lateral prefrontal cortex, paracingulate, anterior cingulate gyrus, paracingulate gyrus, and cerebellar regions. (B) Hypoconnectivity of the right IPS with a cluster at the junction of the superior frontal sulcus and precentral sulcus and hyperconnectivity with extensive regions in bilateral occipital cortices and the precuneus in the parietal lobe was also observed in TS relative to controls. Color bars represent standard cluster-corrected z-stats (using threshold of z = 2.3) at FWE P < 0.05.
Correlations with Cognitive Measures of Attention, Executive Function, and Visuo-spatial Abilities
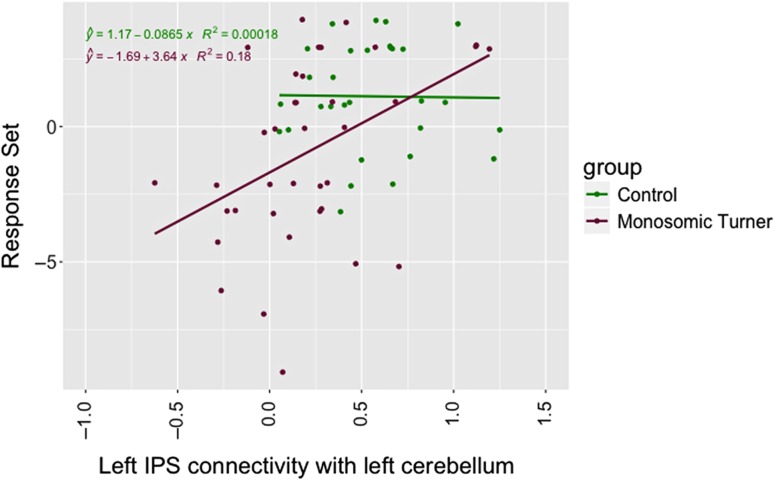
After correcting for multiple comparisons, connectivity estimates between the left IPS seed and the left cerebellum cluster were correlated with the Response Set subscale (correlation estimates = 0.427, P = 0.03, Bonferroni corrected) in TS while no significant correlations were found in the control group (P = NS). Connectivity estimates between the left IPS and the left cerebellum cluster were lower in TS (mean 144 ± 0.21) compared with controls (mean 0.345 ± 0.21, t = −2.8, P = 0.007). A regression model found a significant difference in brain-behavior correlation (group × connectivity estimates interaction term) between the groups (F = 6.7, df = 3,63, P < 0.001, R2 = 0.2; group × connectivity estimates interaction term, t = 2.1 and P = 0.037) (Fig. 3). Also, connectivity estimates between the left IPS and the right occipital and cerebellum cluster were positively correlated with the Response Set subscale in TS (correlation estimates = 0.428, P = 0.03, Bonferroni corrected) while no significant correlations were found in the control group (P = NS). Connectivity estimates between the left IPS and the right occipital and cerebellum cluster were lower in TS (mean 0.211 ± 0.38) compared with controls (mean 0.546 ± 0.30, t = −2.6, P = 0.0125). A regression model did not find a significant between-group difference in brain-behavior correlations, though the P-value for group × connectivity interaction term approached significance (F = 3.7, df = 3,63, P < 0.001, R2 = 0.2; group × connectivity estimates interaction term, t = 1.9 and P = 0.059). No correlation between seed-based connectivity estimate measures and cognitive abilities were found for other neurocognitive domains, nor for ADHD behavioral measures (attention problems and hyperactivity).
Figure 3.
Relationship between left IPS and the left cerebellum cluster connectivity estimate values and Response Set inhibition scores in TS and control groups. A regression line estimating the overall trend of the data was added for illustration purposes.
No correlation between ICA connectivity estimate (DAN and frontoparietal networks) measures and cognitive abilities were found for any neurocognitive domains, nor for ADHD behavioral measures (attention problems and hyperactivity).
Discussion
The DAN is central to orienting attention, while the frontoparietal network is essential to executive control. While measures of resting-state connectivity of these networks help to demonstrate their distinct yet anatomically overlapping spontaneous neural activity in healthy controls (Fox et al. 2006; Petersen and Posner 2012), such networks have not yet been examined in TS, a condition where affected individuals are at particularly high risk for attentional dysfunction and ADHD symptoms. Using ICA analysis, we identified a specific pattern of hypoconnectivity in both dorsal and frontoparietal attention systems in girls with TS compared to controls. Using a seed-based approach, we identified a pattern of bilateral IPS hypoconnectivity with frontal regions, left IPS hypoconnectivity with cerebellar regions, and right IPS hyperconnectivity with parieto-occipital regions. Positive brain-behavior correlations were specifically identified for the Response Inhibition subtest in the TS group, suggesting downstream effects of X-chromosome monosomy on these attention networks.
Hypoconnectivity in DAN and Frontoparietal Control Network in TS Identified with a Data-Driven Approach
The functional complexity and anatomical overlap of the DAN and frontoparietal control network make analyses of these 2 systems challenging, particularly in neurobiologically heterogeneous clinical populations such as children with ADHD. Here, we describe resting-state dysregulation in girls with TS, a relatively homogenous population with respect to sharing an identifiable genetic risk factor. For the first time, we describe hypoconnectivity in the prefrontal cortex region of the frontoparietal attention network. We also provide independent support for resting-state dysregulation within parietal-occipital regions of the DAN (Xie et al. 2015). Our findings of disrupted connectivity in both attention networks in TS are in line with lower scores on cognitive tasks that measure attention in individuals with TS compared with controls. Specifically, lower Response Set scores in the TS group might indicate poor ability to shift attention and maintain a new and complex set of instructions. Overall, these cognitive results replicate findings from an independent sample of girls with TS using the NEPSY (Green et al. 2015).
The DAN and frontoparietal network were hypothesized to differentiate between TS and controls. Our hypothesis was based on our previous findings of deficits in executive functions and elevated levels of inattention and hyperactivity symptoms in girls with TS (Lepage et al. 2011; Green et al. 2015). These symptoms appeared relatively independent of other cognitive features such as visuo-spatial and sensorimotor weaknesses, typically associated with TS (Hong et al. 2009). However, our ICA findings (see Supplementary Table S2) show that other networks, specifically the sensorimotor network, also robustly differentiate between TS and controls. Overall, we find hypoconnectivity in attention networks as well as in other networks in TS. These neuronal findings aligned with cognitive and behavioral findings of a TS effect on executive function as well as an effect on visuo-spatial and sensory motor abilities.
Connectivity Pattern for Bilateral IPS in TS Identified with a Seed-Based Approach
The IPS is central to both the DAN and frontoparietal control network (Petersen and Posner 2012). Within the DAN, we found hypoconnectivity of the right IPS with a cluster at the junction of the superior frontal sulcus and precentral sulcus—a region that corresponds to the frontal eye field. Additionally, within the frontoparietal control network, we found hypoconnectivity of the left IPS with clusters at the bilateral dorsal lateral prefrontal cortex, paracingulate, anterior cingulate gyrus, and paracingulate gyrus. The anatomical location of these clusters overlap with resting-state findings from non-syndromic ADHD populations (Fair et al. 2010; Liston et al. 2011).
Our results are consistent with those from an independent study (Xie et al. 2015) that identified bilateral IPS hypoconnectivity in girls with TS. These results are also in line with task-based functional imaging studies showing reduced activation in frontal and parietal regions in girls with TS (Haberecht et al. 2001; Tamm et al. 2003; Hart et al. 2006) during working memory and response inhibition. Specifically, during a working memory task, Bray et al. found reduced connectivity of the right IPS with the left IPS and left middle frontal gyri (Bray et al. 2013). The results presented here, indicating an effect of TS on the frontoparietal control network, are also supported by structural neuroimaging studies showing differences in the morphology of the parietal lobes (Brown et al. 2004; Raznahan et al. 2010; Green et al. 2014), and WM pathways linking frontal and parietal regions in TS (Holzapfel et al. 2006; Yamagata et al. 2012). In contrast to the observation of overall hypoconnectivity, hyperconnectivity was observed for right IPS with extensive occipital regions in the TS group. The occipital lobe has been identified by our group and others as structurally affected by X-monosomy (Cutter et al. 2006; Hong et al. 2014). It is possible that the observed resting hyperconnectivity might reflect a compensatory mechanism within the dorsal visual stream.
Hypoconnectivity in the TS group was identified for the left IPS with cerebellar regions. As identified in healthy controls, the IPS has strong RSFC with cerebellar (Crus I and II) and prefrontal regions (O’Reilly et al. 2010). In TS, Xie et al. (2015) found hypoconnectivity in the right cerebellum. Structural imaging studies have observed increased gray matter volume of the cerebellum in girls with TS compared to controls (Cutter et al. 2006; Hong et al. 2014). Here, we observed that the scores in the Response Set subtest, measuring attention and inhibition, were lower in girls with TS compared to controls. The performance on the Response Set task positively correlated with the connectivity of the left IPS and cerebellar regions. This correlation, detected only in the TS group, points to aberrant functional connectivity between the IPS and cerebellar regions as a potential neural correlate of aberrant attentional function in this condition.
Limitations
Although our results are based on the largest rsfMRI TS sample to date, some limitations should be noted. First, several factors are known to confound connectivity results, namely, age, sex, pubertal stage, and cognitive functioning. Our groups were matched for age, sex, and Tanner stage but not IQ. Further, a relatively wide age range could impact ICA-based connectivity results by combining data across neurodevelopmental stages. We addressed these limitations by including cognitive function (VIQ) and age as covariates of no interest in our analyses. Finally, connectivity estimates are known to be affected by head movements during data collection, especially in children (Power et al. 2012). We employed state-of-the-art motion correction approaches to reduce the effect of head movement in estimating connectivity.
Conclusions
In this study, we measured connectivity of the DAN and frontoparietal network in a population of girls with TS. Our findings suggest an effect of TS on resting-state connectivity (both in ICA and seed-based analyses) and attention abilities. We also found between-group differences in how IPS and cerebellar connectivity were related to specific attention measures. These findings suggest that absence of X-chromosome and related haploinsufficency of X-linked genes (as occur in typically developing males with XY genotype) contribute to attention network dysfunction and increased risk for ADHD. The brain-behavior correlations found in this study might serve as a biological marker in future research examining the effects of an intervention that targets attention skills.
Supplementary Material
Notes
The authors would like to sincerely thank all the girls and families who kindly volunteered to participate. The authors would like to thank the Turner Syndrome Society and the Turner Syndrome Foundation that made this work possible. Conflict of Interest: None declared.
Funding
The National Institute of Mental Health (NIMH) (MH099630), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (HD049653), and the Sharon Levine Foundation to A.L.R. D.S.H. was supported by funding from the National Institute of Mental Health (NIMH) (MH092170). The funding sources mentioned above had no role in the study design or collection, analysis and interpretation of the data. Dr. Reiss is an unpaid medical advisor for the Turner Syndrome Society and Turner Syndrome Foundation.
References
- Beckmann CF, Jenkinson M, Smith SM. 2003. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 20:1052–1063. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvicini C, Faraone SV, Scassellati C. 2016. Attention-deficit hyperactivity disorder in adults: a systematic review and meta-analysis of genetic, pharmacogenetic and biochemical studies. Mol Psychiatry. 21:872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, Hirt M, Jo B, Hall SS, Lightbody AA, Walter E, Chen K, Patnaik S, Reiss AL. 2011. Aberrant frontal lobe maturation in adolescents with fragile X syndrome is related to delayed cognitive maturation. Biol Psychiatry. 70:852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, Hoeft F, Hong DS, Reiss AL. 2013. Aberrant functional network recruitment of posterior parietal cortex in Turner syndrome. Hum Brain Mapp. 34:3117–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WE, Kesler SR, Eliez S, Warsofsky IS, Haberecht M, Reiss AL. 2004. A volumetric study of parietal lobe subregions in Turner syndrome. Dev Med Child Neurol. 46:607–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G. 2011. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 69:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter WJ, Daly EM, Robertson DM, Chitnis XA, van Amelsvoort TA, Simmons A, Ng VW, Williams BS, Shaw P, Conway GS, et al. 2006. Influence of X chromosome and hormones on human brain development: a magnetic resonance imaging and proton magnetic resonance spectroscopy study of Turner syndrome. Biol Psychiatry. 59:273–283. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. 2008. A dual-networks architecture of top-down control. Trends Cogn Sci. 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TGC, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT. 2010. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 68:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. 2009. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Nat Acad Sci USA. 106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. 2006. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Nat Acad Sci USA. 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Bade Shrestha S, Chromik LC, Rutledge K, Pennington BF, Hong DS, Reiss AL. 2015. Elucidating X chromosome influences on attention deficit hyperactivity disorder and executive function. J Psychiatr Res. 68:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Chromik LC, Mazaika PK, Fierro K, Raman MM, Lazzeroni LC, Hong DS, Reiss AL. 2014. Aberrant parietal cortex developmental trajectories in girls with Turner syndrome and related visual-spatial cognitive development: a preliminary study. Am J Med Genet B Neuropsychiatr Genet. 165B:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberecht MF, Menon V, Warsofsky IS, White CD, Dyer-Friedman J, Glover GH, Neely EK, Reiss AL. 2001. Functional neuroanatomy of visuo-spatial working memory in Turner syndrome. Hum Brain Mapp. 14:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SJ, Davenport ML, Hooper SR, Belger A. 2006. Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain. 129:1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel M, Barnea-Goraly N, Eckert MA, Kesler SR, Reiss AL. 2006. Selective alterations of white matter associated with visuospatial and sensorimotor dysfunction in turner syndrome. J Neurosci. 26:7007–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D, Scaletta Kent J, Kesler S. 2009. Cognitive profile of Turner syndrome. Dev Dis Res Rev. 15:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DS, Hoeft F, Marzelli MJ, Lepage JF, Roeltgen D, Ross J, Reiss AL. 2014. Influence of the X-chromosome on neuroanatomy: evidence from Turner and Klinefelter syndromes. J Neurosci. 34:3509–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M. 2004. NEPSY: A tool for comprehensive assessment of neurocognitive disorders in children In: Hersen M, Goldstein G, Beers SR, editors. The handbook of psychological assessment volume 1: intellectual and neuropsychological assessment. New York: John Wiley & Sons; p. 157–176. [Google Scholar]
- Lepage JF, Dunkin B, Hong DS, Reiss AL. 2011. Contribution of executive functions to visuospatial difficulties in prepubertal girls with Turner syndrome. Dev Neuropsychol. 36:988–1002. [DOI] [PubMed] [Google Scholar]
- Li J, Wang Y, Zhou R, Zhang H, Yang L, Wang B, Faraone SV. 2006. Association between polymorphisms in serotonin 2C receptor gene and attention-deficit/hyperactivity disorder in Han Chinese subjects. Neurosci Lett. 407:107–111. [DOI] [PubMed] [Google Scholar]
- Liston C, Malter Cohen M, Teslovich T, Levenson D, Casey BJ. 2011. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biol Psychiatry. 69:1168–1177. [DOI] [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, Le Bihan D, Cohen L, Dehaene S. 2003. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 40:847–858. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. 2010. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 20:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. 2012. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 35:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE. 2015. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 105:536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Cutter W, Lalonde F, Robertson D, Daly E, Conway GS, Skuse DH, Ross J, Lerch JP, Giedd JN, et al. 2010. Cortical anatomy in human X monosomy. Neuroimage. 49:2915–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell HF, Wallis D, Mazzocco MM, Moshang T, Zackai E, Zinn AR, Ross JL, Muenke M. 2006. Increased prevalence of ADHD in Turner syndrome with no evidence of imprinting effects. J Pediatr Psychol. 31:945–955. [DOI] [PubMed] [Google Scholar]
- Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. 2006. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. 91:3897–3902. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. 2003. Abnormal prefrontal cortex function during response inhibition in turner syndrome: functional magnetic resonance imaging evidence. Biol Psychiatry. 53:107–111. [DOI] [PubMed] [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, et al. 2007. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 400:39–43. [DOI] [PubMed] [Google Scholar]
- Trent S, Dean R, Veit B, Cassano T, Bedse G, Ojarikre OA, Humby T, Davies W. 2013. Biological mechanisms associated with increased perseveration and hyperactivity in a genetic mouse model of neurodevelopmental disorder. Psychoneuroendocrinology. 38:1370–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent S, Dennehy A, Richardson H, Ojarikre OA, Burgoyne PS, Humby T, Davies W. 2012. Steroid sulfatase-deficient mice exhibit endophenotypes relevant to attention deficit hyperactivity disorder. Psychoneuroendocrinology. 37:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. 2010. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci. 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 2002. Wechsler Preschool and Primary Scale of Intelligence, 3rd. ed. (WPPSI-III). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D. 2003. Wechsler intelligence scale for children, 4th ed. (WISC-IV): technical and interactive manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. 2014. Permutation inference for the general linear model. Neuroimage. 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Yang J, Zhang Z, Zhao C, Bi Y, Zhao Q, Pan H, Gong G. 2015. The effects of the X chromosome on intrinsic functional connectivity in the human brain: evidence from Turner syndrome patients. Cereb Cortex. 27:474–484. [DOI] [PubMed] [Google Scholar]
- Xu X, Brookes K, Sun B, Ilott N, Asherson P. 2009. Investigation of the serotonin 2C receptor gene in attention deficit hyperactivity disorder in UK samples. BMC Res Notes. 2:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata B, Barnea-Goraly N, Marzelli MJ, Park Y, Hong DS, Mimura M, Reiss AL. 2012. White matter aberrations in prepubertal estrogen-naive girls with monosomic Turner syndrome. Cereb Cortex. 22:2761–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.