Abstract
A working memory of obstacles is essential for navigating complex, cluttered terrain. In quadrupeds, it has been proposed that parietal cortical areas related to movement planning and working memory may be important for guiding the hindlegs over an obstacle previously cleared by the forelegs. To test this hypothesis, parietal areas 5 and 7 were reversibly deactivated in walking cats. The working memory of an obstacle was assessed in both a visually dependent and tactilely dependent paradigm. Reversible bilateral deactivation of area 5, but not area 7, altered hindleg stepping in a manner indicating that the animals did not recall the obstacle over which their forelegs had stepped. Similar deficits were observed when area 5 deactivation was restricted to the delay during which obstacle memory must be maintained. Furthermore, partial memory recovery observed when area 5 function was deactivated and restored within this maintenance period suggests that the deactivation may suppress, but not eliminate, the working memory of an obstacle. As area 5 deactivations incurred similar memory deficits in both visual and tactile obstacle working memory paradigms, parietal area 5 is critical for maintaining the working memory of an obstacle acquired via vision or touch that is used to modify stepping for avoidance.
Keywords: cat, cortical cooling, locomotion, obstacle avoidance, working memory
Introduction
Neural mechanisms for walking must maintain equilibrium of the moving animal while adapting gait for the environment and the current goals of the animal (Forssberg et al. 1980; Takakusaki 2013; Drew and Marigold 2015). While spinal locomotor networks can generate rhythmic activity in motor neurons for basic gait on a level surface (Grillner 2011; Takakusaki 2013), uneven or cluttered terrain engages supraspinal structures related to motor planning and working memory (Drew et al. 2008; Drew and Marigold 2015). In particular, a working memory of environmental obstacles is essential for navigation in walking mammals. As humans, this memory system affords us the ability to walk through a complex or cluttered setting without looking directly at our feet as we step around or over obstacles (Patla and Vickers 1997; Mohagheghi et al. 2004). In quadrupeds, this memory system is especially important for guiding hindleg stepping. As an obstacle previously cleared by the forelegs is no longer directly visible to the animal once it has passed under the body, a working memory of the obstacle is used to modify subsequent hindleg steps (Wilkinson and Sherk 2005; McVea and Pearson 2006, 2007a; McVea et al. 2009; Whishaw et al. 2009).
Furthermore, such step modulations can also occur without any visual input of an obstacle. A sudden stumble over an unexpected obstacle evokes the stumbling corrective reaction (Forssberg 1979), resulting in limb hyperflexion to lift the leg over an impeding obstacle. Recently, such tactilely acquired working memory of an obstacle was demonstrated to be able to persist for many minutes, and could be used to coordinate appropriate hindleg stepping if forward locomotion was delayed after foreleg clearance (Wong et al. 2016). Moreover, cooling-induced deactivation of parietal area 5 resulted in altered hindleg stepping indicative of a forgotten obstacle. As inactivation of area 5 via lesions results in similar deficits in the working memory of an obstacle acquired visually (McVea et al. 2009), area 5 appears to be important for the working memory of an obstacle, regardless of the sensory modality through which obstacle information is obtained.
Working memory involves the acquisition and maintenance of relevant sensory stimuli used to guide future behaviours (Jonides et al. 2008). Previous electrophysiological recordings in walking cats revealed a population of cells in area 5 that discharge strongly as an obstacle passes beneath the body (Lajoie et al. 2010). If forward progress of the cat is delayed, this increased neural activity is sustained as long as the cat remains straddling the obstacle between its fore- and hindlegs. Such sustained activity during delayed response tasks is regarded as representing the maintenance of relevant sensory stimuli in memory (Fuster and Alexander 1971; Eriksson et al. 2015). Thus area 5 is hypothesized to contribute specifically to maintaining the working memory of an obstacle, although its causal role in working memory maintenance remains to be demonstrated. Furthermore, area 5 contributions to working memory acquisition have yet to be evaluated. While aforementioned studies employing lesions to elucidate area 5 function can only demonstrate overall contributions to working memory-guided obstacle locomotion, transient, temporally controlled cortical deactivations achieved with cooling can be used to dissociate the role of area 5 in working memory acquisition versus maintenance.
In the present study, the working memory of an obstacle previously cleared by the forelegs was examined in walking cats. To assess the role of area 5 in the acquisition and maintenance of the working memory of an obstacle, cooling loops were placed bilaterally over parietal area 5 in 3 cats. Additionally, control cooling loops were placed bilaterally over an adjacent region of parietal area 7 to ensure that any observed changes in gait following area 5 deactivation were due to the specific cooling of area 5 and not a result of cooling in general. Obstacle working memory was assessed while individually deactivating area 5 or 7. By varying the duration that hindleg obstacle clearance was delayed, parietal cortex contributions to obstacle working memory were assessed in a delay-dependent manner. Additionally, both bilateral and unilateral parietal cortex deactivations were performed in the same animals to assess possible laterality of the working memory system. Finally, by varying the onset and offset of cooling, parietal areas were deactivated throughout memory testing or during specific phases to assess their contributions to obstacle working memory acquisition versus maintenance. Parietal cortex contributions were assessed in both a visually dependent obstacle working memory test adapted from McVea et al. (2009) and a tactile (visually independent) test designed to evoke the stumbling corrective reaction (Wong et al. 2016). Altogether, these experiments revealed the critical role of parietal area 5 in maintaining the working memory of an obstacle obtained with or without vision, used to ensure proper obstacle negotiation.
Materials and Methods
Overview
Parietal cortex contributions to obstacle working memory were examined in 3 adult (>6M) female domestic cats obtained from a commercial breeding facility (Liberty Labs, NY). All animals were housed in an enriched colony environment. Food intake was regulated during testing days such that moist food was provided during each testing session. Additionally, animals were offered dry food for 1 h at the end of each day. Water was provided ad libitum. Each animal received bilateral cryoloops over parietal areas 5 and 7. Each area was bilaterally cooled during both visually dependent and tactilely dependent obstacle working memory testing paradigms. When behavioral testing was completed, cryoloops were exposed on the surface of the brain and a thermal imaging camera was used to visualize the extent of cortical deactivation. Animals were then perfused and the brains were fixed and removed from the cranium. Brains were then frozen, coronally sectioned, and processed for Nissl, cytochrome oxidase, and SMI-32. Reconstructions of deactivation loci were compared with areal boundaries revealed with SMI-32 to confirm accurate cryoloop placement. All animals were previously examined in a study of memory-guided stumbling correction (Wong et al. 2016). All trials included in the present study are distinct from trials examined in the previous study. All procedures were conducted in compliance with the National Research Council’s “Guide for the Care and Use of Laboratory Animals” (eighth edition; 2011) and the Canadian Council on Animal Care’s “Guide to the Care and Use of Experimental Animals” (1993), and were approved by the University of Western Ontario Animal Use Subcommittee of the University Council on Animal Care.
Apparatus
The same apparatus described in Wong et al. (2016), Wong and Lomber (2017) was used to assess visual and tactile obstacle working memory in the present study. Each cat was trained to walk along an 8-foot long runway. Halfway along the apparatus, an 8.7 cm high obstacle could be raised onto or lowered from the surface using a lever mounted underneath the runway. An ethernet camera mounted on a tripod recorded all trials at 54 frames per second using Contemplas (Kempten, GER) motion detection software.
Obstacle Memory Testing
To examine visually dependent obstacle working memory, each animal was trained to walk along a runway towards an obstacle (Fig. 1A). Food was placed on an elevated platform on the far side of the obstacle to encourage the animal to step over the obstacle with their forelegs only. Animals were allowed to eat from the platform for delays ranging from less than a second to 2 min. Delays were varied in order to assess any possible delay-dependent effects of obstacle working memory. During this delay, the obstacle was covertly lowered, becoming flush with the walkway, to prevent further visual or tactile inputs. The food was then moved forward to encourage the animal to resume walking and hindlegs steps were observed. Such trials comprised the visual obstacle present condition. Additionally, an equivalent number of trials where the animal approached the food platform on the far side of the lowered obstacle comprised the visual obstacle absent condition. In these control trials, forward progress was similarly delayed for up to 2 min as the animal ate. Stepping in these visual obstacle absent trials was examined to ensure that animals did not develop a learned obstacle avoidance strategy of sustained overstepping regardless of whether the obstacle was present or absent. In both visual obstacle present and obstacle absent conditions, there was never any tactile contact between the cat and the obstacle. The tactile-dependent obstacle working memory paradigm was identical to previously described procedures (Wong et al. 2016). Briefly, each animal approached the food platform in the absence of any obstacle (Fig. 1B). As the animal ate, the obstacle was covertly raised onto the walkway directly below the food dish to prevent any visual input of the obstacle. The food was moved to encourage the animal to continue walking forwards, causing the front legs to contact the obstacle before stepping over it. The animal’s interest in food was sufficient in maintaining the gaze forwards, preventing any visual input of the obstacle during the trial. As the animal continued to eat, the obstacle was covertly removed from the walkway, before forward locomotion was again resumed. Such trials comprised the tactile obstacle present condition. Additionally, trials where the obstacle was raised onto then immediately removed from the walkway during the initial approach comprised the tactile obstacle absent condition. In these control trials, removal of the obstacle precluded any contact. Stepping in these tactile obstacle absent trials was examined to ensure that animals did not develop a learned avoidance response of chronic overstepping.
Figure 1.
Visually dependent and tactilely dependent testing paradigms used to assess obstacle working memory. (A) Schematic depicting the visual obstacle working memory test where each animal would see and step over an 8.7 cm high obstacle with their forelegs to reach food placed on an elevated platform. As the animal ate, the obstacle was covertly lowered becoming flush with the walkway. Following a variable delay period, the food was moved forwards to encourage the animal to resume walking. Hindleg stepping was measured to assess working memory of the obstacle. Horizontal blue and red bars (i–iv) represent variations in cooling onset (blue) and offset (red) used to examine overall parietal cortex contributions to the working memory task as a whole (i), or to distinct phases of working memory acquisition (ii) and working memory maintenance (iii–iv). (B) Schematic depicting the tactile obstacle working memory test where each animal would approach the food platform in the absence of the obstacle. As the animal ate, it could not see that the obstacle was covertly raised beneath the food platform. By moving the food forward, the forelegs would contact the obstacle before stepping over it. During the subsequent delay period, the obstacle was lowered becoming flush with the walkway. As in the visual obstacle working memory paradigm, hindleg stepping when walking resumed was examined to assess obstacle working memory. Horizontal blue and red bars (i–iii) represent variations in cooling onset (blue) and offset (red) used to examine overall parietal cortex contributions to the working memory task as a whole (i), or to distinct phases of working memory acquisition (ii) and working memory maintenance (iii).
Surgical Procedures
Cryoloops (Lomber et al. 1999) were implanted bilaterally over areas 5 and 7 (Fig. 2) according to previously reported surgical procedures (Lomber et al. 1999, 2010; Lomber and Payne 2000a, 2000b; Lomber and Malhotra 2008).Cooling loops were shaped from 23-guage stainless steel hypodermic tubing to conform to each area examined. For surgical implantation, craniotomies exposed parietal areas 5 and 7 in each hemisphere. Individual cryoloops were positioned with the loop in direct contact with the cortical surface for each area. The base of each loop was secured to the skull with dental acrylic anchored to stainless steel screws, before closing the craniotomies with additional dental acrylic.
Figure 2.
Cortical areas deactivated in parietal cortex shown on the right hemisphere of a cat brain. (A) Lateral view of the right cat cerebrum showing parietal areas 5 and 7 examined in the current study. D—dorsal, A—anterior. (B) Cooling loops in contact with areas 5 and 7 of the right hemisphere photographed at the time of implantation. Adapted with permission from Wong et al. (2016).
Working Memory Testing and Reversible Cooling Deactivation
Following surgical implantation and approximately 2 weeks of recovery, obstacle working memory was examined using both visual and tactile obstacle memory paradigms. Each testing day began with trials conducted in the absence of any cooling (warm condition). A second block of trials then began with a maintenance phase cooling trial, where cryoloops in contact with the parietal areas were cooled to 3.0 ± 1.0 °C to completely deactivate all cortical layers (Lomber and Payne 2000a). In these trials where parietal cortex deactivation was restricted to working memory maintenance, cooling was initiated immediately following foreleg obstacle clearance (Fig. 1A-iii,B-iii). Subsequent memory delays lasted around 60 s or longer to allow cortical temperatures to reach 3.0 ± 1.0 °C for complete cortical deactivation. Maintenance phase cooled trials were then followed by more trials where parietal areas remained deactivated throughout the entire obstacle working memory test (Fig. 1A-i,B-i). This cooling block ended with a final acquisition phase cooled trial, where cooling was stopped immediately after foreleg clearance of the obstacle (Fig. 1A-ii,B-ii). Once cooling was terminated, memory delays exceeded 60 s to permit restoration of cortical temperature and full functional restoration before walking resumed. A final “warm” block of trials re-established baseline stepping. Cortical temperatures were monitored closely throughout testing to confirm the duration and depth of deactivation. Each testing block consisted of trials where the obstacle was present interspersed with trials where the obstacle was absent for both visual and tactile variations in order to prevent habituation to the obstacle and development of a learned avoidance response. Either bilateral or unilateral deactivations were performed on a given testing day.
Data Analysis
Videos were analyzed using custom written scripts in Matlab (MathWorks, Natick, MA). Steps were tracked by an investigator who was blind with respect to which experimental condition each trial belonged to during video analyses. Step height was measured at the peak of each step as the vertical height of the toe above the walking surface when the toe reached the highest point in the step. Additionally, step clearance was measured as the step height directly above the lowered obstacle. The horizontal distance between the toe and obstacle at the peak of each step was also measured. Trials of the same experimental condition from the 3 animals were combined for subsequent statistical testing due to similarities in peak step height, step clearance, and the step peak to obstacle distance between all 3 animals.
To assess working memory-guided obstacle locomotion in visual and tactile obstacle paradigms, step height for each leg was compared between obstacle present and obstacle absent trials, in accordance with previous studies examining obstacle working memory in quadrupedal animals (McVea and Pearson 2006, 2007b; McVea et al. 2009; Whishaw et al. 2009; Setogawa et al. 2014; Wong et al. 2016). This was done for the following reasons: within the warm condition, elevated step height in obstacle present trials in comparison to obstacle absent trials would indicate that the animal accurately remembered the presence of the obstacle, demonstrating intact obstacle working memory. Observing relatively lower step height in obstacle absent trials would also ensure that the animals did not overlearn an avoidance response, and that elevated stepping in obstacle present trials was truly indicative of working memory. Additionally, step height comparisons between obstacle present and obstacle trials when cryoloops were cooled could be used to ensure that parietal cortex deactivations did not induce any motor deficits; any observed attenuations of step height thus reflect deficits in working memory. Thus a 1-way multivariate ANOVA was used to compare peak step height for each leg for each trial type (visual obstacle present, visual obstacle absent, tactile obstacle present, tactile obstacle absent). A Bonferroni correction was applied to account for multiple comparisons and statistical significance was accepted at P < 0.0125. For each of the 4 steps, paired t-tests were conducted to compare step clearances as well as the step peak to obstacle distance between visual and tactile obstacle present trials. Bonferroni corrections were applied to account for multiple comparisons and statistical significance was accepted at P < 0.0125.
To assess parietal cortex contributions to obstacle working memory, a 1-way multivariate ANOVA was conducted to assess the effect of cooling condition (warm, area 5 bilaterally cooled, or area 7 bilaterally cooled) on peak step height for all 4 legs in obstacle present and obstacle absent trials. Statistical significance was accepted at P < 0.00625 to account for multiple comparisons. Additionally, step clearances and step peak to obstacle distances were compared between the 3 cooling conditions with 1-way multivariate ANOVAs, with significance accepted at P < 0.0125. Similar analyses were conducted when cooling was temporally restricted to either working memory acquisition or working memory maintenance phases.
To examine the effects of unilateral deactivation, a 2-way multivariate ANOVA was conducted to examine the effects of deactivation locus (left area 5 or right area 5) and leading leg (ipsilateral or contralateral to the site of deactivation) on peak step height. Due to significant interaction effects, unilaterally cooled trials were examined separately according to which hemisphere was cooled, and whether the hindleg ipsilateral or contralateral to the site of cooling was the first to step. For each unilateral cooling condition, peak step heights, step clearances, and the distances between the step peak and obstacle were compared to stepping in warm trials and trials where area 5 was bilaterally deactivated.
Terminal Procedures
Following all behavioral testing, each cat was anesthetized with sodium pentobarbital (25–30 mg/kg, i.v.) and a craniotomy was made to expose the implanted cooling loops on the surface of the brain. Each cryoloop was individually cooled to the same temperature used during behavioral testing (3.0 ± 1.0 °C) and photographed with a thermal imaging camera to capture the extent of deactivation. After each area was photographed, anesthesia was deepened with sodium pentobarbital (40 mg/kg, i.m.) and the animal was transcardially perfused. The brain was removed, frozen and cut in 60 μm coronal sections and collected serially. Sections from the first of 5 series, separated by 300 μm intervals, were processed with Nissl stain. Series 2 was processed with cytochrome oxidase (Payne and Lomber 1996). Nissl and cytochrome oxidase stained sections were examined to ensure that repeated deactivations did not alter the cortical structure of parietal areas cooled over the testing period. Series 3 was processed with the monoclonal antibody SMI-32 (Covance, Emeryville, CA) for areal border delineation (van der Gucht et al. 2001; Mellott et al. 2010; Wong et al. 2014). Series 4 and 5 were retained as spares to process with any of the above methods as need. Reacted sections were mounted onto gelatinized slides, cleared and coverslipped.
Cooling Deactivation Assessment
Alignment of deactivation sites with area 5 or 7 was confirmed in each animal by comparing thermal photographs with Nissl and SMI-32 processed tissue. Area 5 and area 7 borders delineated in SMI-32 stained sections confirmed that deactivation loci were contained within each area of interest, with minor spread into flanking cortices.
Results
Visual or Tactile Information About an Obstacle can be used for Working Memory-Guided Obstacle Locomotion
Working memory-guided obstacle locomotion was assessed in cats (n = 3) using both a visually dependent and tactile-dependent obstacle working memory paradigm. The height of each step was compared between obstacle present and obstacle absent conditions for both visual and tactile paradigms. A 1-way multivariate analysis revealed a significant effect of the trial type (visual obstacle present, visual obstacle absent, tactile obstacle present, tactile obstacle absent) on peak step height (F(12, 617) = 165.0, P < 0.0001). Step height of all 4 legs was significantly affected by trial type (leading foreleg F(3, 236) = 1148.6, P < 0.0001; trailing foreleg F(3, 236) = 1383.4, P < 0.0001; leading hindleg F(3, 236) = 670.5, P < 0.0001; trailing hindleg F(3, 236) = 268.8, P < 0.0001). For all 4 legs, post hoc Tukey tests indicated that step height was significantly higher in obstacle present trials for both visual and tactile paradigms (Fig. 3A–D). Furthermore, for tactile obstacle present trials, foreleg stepping was significantly higher than stepping in visual obstacle present trials (P < 0.0001 for all comparisons), with mean peak step heights of 13.2 ± 1.6 cm and 12.5 ± 1.5 cm for leading and trailing foreleg steps, respectively, in tactile obstacle present trials, and step heights of 11.9 ± 1.2 cm and 11.6 ± 1.0 cm for leading and trailing foreleg steps, respectively, in visual obstacle present trials (Fig. 3A,B). The opposite pattern was observed for the leading hindleg step. In tactile obstacle present trials, peak step height for the leading hindleg steps was 9.6 ± 1.5 cm, which was significantly lower than in visual obstacle present trials where mean step height was 10.5 ± 1.7 cm (P = 0.0014; Fig. 3C). For the trailing hindleg, peak step height did not differ between the visual and tactile paradigms, with mean heights of 7.6 ± 1.4 cm and 7.4 ± 1.7, respectively (Fig. 3D).
Figure 3.
Visual or tactile information about an obstacle can be used for working memory-guided obstacle locomotion. (A–D) Mean peak step height ± SD in obstacle present and obstacle absent trials for both visual and tactile obstacle working memory paradigms for leading foreleg (A), trailing foreleg (B), leading hindleg (C), and trailing hindleg steps (D). For both visual and tactile obstacle present trials, stepping of all 4 legs was significantly elevated over stepping in obstacle absent trials, demonstrating the ability to use visual or tactile information about an obstacle to modify stepping. In obstacle present trials, foreleg stepping was significantly higher in tactile obstacle working memory trials. Conversely, leading hindleg steps were significantly higher in visual obstacle working memory trials, while trailing hindleg steps were similar between the 2 paradigms. (E) Mean step clearance ± SD for all 4 legs in visual (V) and tactile (T) obstacle present trials. Step clearance only differed between visual and tactile trials for the trailing foreleg, where clearance was significantly higher in the tactile paradigm. (F) Mean step peak to obstacle distance ± SD for all 4 legs in visual (V) and tactile (T) obstacle present trials. Both leading and trailing forelegs tended to peak later after passing over the obstacle in tactile trials. Conversely, leading hindleg steps tended to peak sooner before passing over the obstacle in visual trials. Step trajectories were similar between visual and tactile paradigms for the trailing hindleg. *P < 0.0125, **P < 0.001, ***P < 0.0001.
Additionally, step clearance was measured as the difference between obstacle height and step height directly above the lowered obstacle (Fig. 3E). While step clearance was generally similar between visual and tactile obstacle memory paradigms, mean clearance of the trailing foreleg step was significantly higher in tactile trials at 3.0 ± 2.0 cm, in comparison to mean step clearance in visual trials at 2.5 ± 1.0 cm (P = 0.0062). Furthermore, the horizontal distance between step peak and the obstacle was measured to assess step trajectory (Fig. 3F). Foreleg steps tended to peak further after passing over the obstacle in tactile trials than in visual trials (leading: P = 0.0008; trailing: P = 0.0014). In contrast, leading hindleg steps tended to peak before passing over the obstacle in visual trials with a mean step peak to obstacle distance of −0.9 ± 2.2 cm, while leading hindleg steps tended to peak just after the obstacle in tactile trials with a mean distance of 0.1 ± 1.8 cm (P = 0.004). Step trajectories were similar between visual and tactile trials for the trailing hindleg. Overall, despite the differences between stepping in visual and tactile obstacle memory paradigms, significantly elevated stepping in obstacle present versus obstacle absent trials indicates the ability to use visual or tactile information about an obstacle to modulate stepping for memory-guided obstacle avoidance.
Visual Obstacle Working Memory Relies on Parietal Area 5
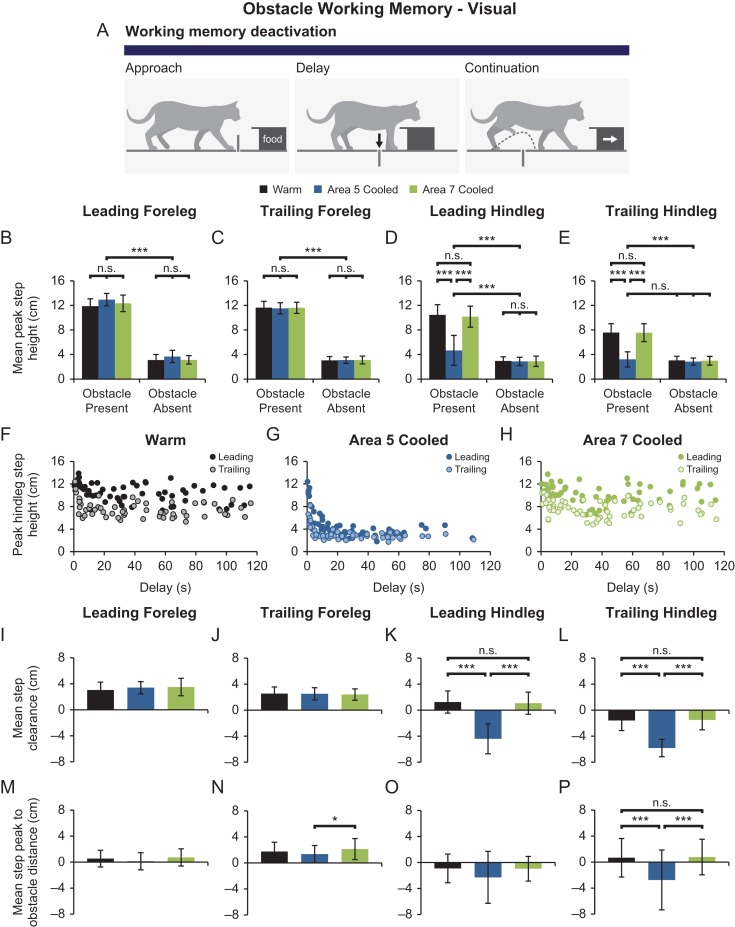
To assess parietal cortex contributions to visual obstacle memory, cryoloops implanted over areas 5 or 7 were then bilaterally cooled prior to obstacle approach, and sustained throughout the delay and continuation phases for the visual obstacle memory paradigm (Fig. 4A). Such deactivation of neither area 5 nor area 7 affected foreleg stepping in obstacle present or obstacle absent trials (Fig. 4B,C). Leading and trailing foreleg steps remained significantly higher in obstacle present trials than in obstacle absent trials whether visual obstacle memory was assessed with parietal areas warm, when area 5 was deactivated, or when area 7 was deactivated (P < 0.0001 for all comparisons). However, in comparison to both warm and area 7 cooled conditions, deactivation of area 5 resulted in significantly lower hindleg stepping, with mean peak step heights of 4.7 ± 2.4 cm and 3.2 ± 1.2 cm for leading and trailing hindleg steps, respectively (P < 0.0001 for all comparisons; Fig. 4D,E). In comparison to obstacle absent trials for any condition, leading hindleg steps remained significantly higher in obstacle present trials when area 5 was deactivated (P < 0.0001 for all comparisons; Fig. 4D). In contrast, area 5 deactivation reduced trailing hindleg step height such that it no longer differed from stepping in obstacle absent trials (Fig. 4E). Differences in leading and trailing hindleg stepping are further visualized by examining scatter plots of peak step height over time (Fig. 4F–H). While leading steps are higher than trailing steps in warm and area 7 cooled conditions (Fig. 4F,H), step height remains relatively stable across delays of up to 2 min. When area 5 was deactivated during trials where animals were permitted to walk continuously over the obstacle (such that delays were only a maximum of a few seconds), hindleg steps were similar to obstacle height, indicating intact memory-guided obstacle locomotion. However, as demonstrated by the exponential decay of step height over time (Fig. 4G), step height was rapidly attenuated with increasing delays when area 5 was deactivated.
Figure 4.
Bilateral deactivation of parietal area 5, but not area 7, results in working memory deficits in the visually dependent obstacle memory test. (A) Schematic of the visual obstacle working memory paradigm with the blue horizontal bar indicating the use of cooling throughout the entire test. (B–E) Mean peak step height ± SD in visual obstacle present and obstacle absent trials for leading foreleg (B), trailing foreleg (C), leading hindleg (D), and trailing hindleg steps (E) in warm, area 5 cooled, and area 7 cooled conditions. (F–H) Scatter plots of peak step height versus delay for leading and trailing hindlegs in warm (F), area 5 cooled (G), and area 7 cooled trials (H). While foreleg steps were unaffected by cooling, deactivation of area 5 resulted in attenuated hindleg step height. (I–L) Mean step clearance ± SD for all 4 legs in each of the 3 cooling conditions. Area 5 deactivation similarly resulted in reduced hindleg step clearance in comparison to warm and area 7 cooled conditions. (M–P) Mean step peak to obstacle distance ± SD for all 4 legs in the 3 cooling conditions. Leading step trajectories did not differ between cooling conditions. However, in comparison to area 5 cooled trials, trailing forelegs peaked further from the obstacle after passing over it in area 7 cooled trials. Additionally, while trailing hindleg steps peaked just after passing over the obstacle in warm and area 7 cooled trials, area 5 cooling resulted in steps peaking well before passing over the obstacle. *P < 0.0125, ***P < 0.0001, n.s.—not significant.
Furthermore, step clearance was similarly affected by parietal cortex deactivation. In comparison to step clearances in the warm condition, neither leading nor trailing foreleg step clearances were affected by bilateral area 5 or area 7 cooling (Fig. 4I,J). However, both leading and trailing hindleg step clearances were significantly reduced to −4.4 ± 2.3 cm (P < 0.0001) and −5.8 ± 1.3 cm (P < 0.0001), respectively, when area 5 was cooled (Fig. 4K,L). When area 7 was deactivated, hindleg step clearances did not differ from the step clearances in the warm condition. Additionally, in comparison to the warm condition, foreleg step trajectories were not affected by area 5 or area 7 cooling (Fig. 4M,N). However, the mean step peak to obstacle distance was significantly lower when area 5 was cooled in comparison to the area 7 cool condition, with mean distances of 1.4 ± 1.3 cm and 2.1 ± 1.6 cm, respectively (P = 0.0108; Fig. 4N). While leading hindleg step trajectories did not differ with parietal cortex deactivation (Fig. 4O), trailing hindleg steps peaked well before passing the obstacle with a mean step peak to obstacle distance of −2.7 ± 4.6 cm (Fig. 4P). In comparison, trailing hindleg steps typically peaked after passing the obstacle in both warm and area 7 cooled conditions, with mean distances of 0.7 ± 3.0 cm (P < 0.0001) and 0.8 cm ± 2.7 cm (P < 0.0001), respectively. Overall, these alterations in hindleg stepping indicate significant obstacle memory deficits with bilateral parietal area 5, but not area 7, deactivation (Fig. 10, row 1).
Figure 10.
Summary diagram illustrating the dissociation of parietal cortex contributions to obstacle memory. Deactivation of area 5, but not area 7, resulted in altered hindleg step height and trajectories demonstrative of impaired obstacle memory. ↓↓—complete deficit; ↓—incomplete deficit.
Area 5 in 1 Hemisphere may Affect Obstacle Locomotion of Both Hindlegs
In order to evaluate a possible lateralization of area 5 contributions to obstacle memory, area 5 was deactivated unilaterally. Leading and trailing hindleg steps appeared to be differentially affected by unilateral cooling depending on whether the leading hindleg was ipsilateral or contralateral the site of area 5 deactivation. Thus a 2-way multivariate ANOVA was conducted to examine the effects of deactivation locus (left area 5 or right area 5) and leading hindleg (ipsilateral or contralateral to the site of deactivation) on peak step height. This revealed a significant interaction between deactivation locus and leading leg (F(2, 224) = 8.1, P = 0.0004). Further analyses of unilateral area 5 contributions to obstacle memory compared hindleg stepping between warm and bilateral area 5 cooled conditions for both left and right area 5 cooled trials according to the identity of the leading hindlimb (Fig. 5). The results of left area 5 deactivations are detailed here; however, note that hindleg stepping is similarly affected by right area 5 deactivations (Fig. 5, right).
Figure 5.
Obstacle working memory deficits following unilateral area 5 deactivation were dependent on which hindleg led. (A,B) Mean peak step height ± SD for leading and trailing hindleg steps in warm obstacle present trials, trials where area 5 was bilaterally cooled, or trials where left area 5 was cooled and the ipsilateral (A) or contralateral (B) hindleg led. (C,D) Mean peak step height ± SD for leading and trailing hindleg steps when right area 5 was cooled and the ipsilateral (C) or contralateral (D) hindleg led compared to warm and bilateral area 5 cooled conditions. (E,F) Mean step clearance ± SD for leading and trailing hindleg steps when left area 5 was cooled and the ipsilateral (E) or contralateral (F) hindleg led compared to warm and bilateral cooled conditions. (G,H) Mean step clearance ± SD for leading and trailing hindleg steps when right area 5 was cooled and the ipsilateral (G) or contralateral (H) hindleg led compared to warm and bilateral cooled conditions. Regardless of whether area 5 was cooled in the left or right hemisphere, when the ipsilateral hindleg led, step height and clearance was significantly attenuated in the contralateral hindleg only. However, stepping of both legs was affected when the contralateral hindleg led. (I–L) Mean step peak to obstacle distance ± SD for leading and trailing hindleg steps. In comparison to bilateral area 5 deactivation, unilateral cooling did not affect trailing hindleg trajectory relative to the warm condition. *P < 0.0125, **P < 0.001, ***P < 0.0001, n.s.—not significant.
For trials where left area 5 was cooled and the ipsilateral (left) hindleg led, a 1-way multivariate analysis revealed a significant effect of the cooling condition (warm, bilateral area 5 deactivation, or left area 5 deactivation) on peak step height (F(4, 366) = 112.4, P < 0.0001; Fig. 5A). Both leading (F(2, 164) = 138.6, P < 0.0001) and trailing (F(2, 164) = 132.9, P < 0.0001) hindleg step height were significantly affected by the cooling condition. Post hoc Tukey tests revealed that while bilateral area 5 deactivation resulted in significantly reduced leading and trialing hindleg step height in comparison to the warm condition (P < 0.0001), step height was only significantly reduced in the contralateral (right) trailing hindleg when left area 5 was cooled (P < 0.0001). Notably, trailing step height was not reduced to the same extent as in the bilateral deactivation condition, as trailing step height was significantly higher when left area 5 was cooled and the left hindleg led (P < 0.0001). Peak step height of the ipsilateral (left) leading hindleg did not differ from the warm condition. In contrast, when left area 5 was cooled and the contralateral (right) hindleg led, both leading and trailing hindleg step heights were significantly reduced in comparison to the warm condition (Fig. 5B). Peak step height of the contralateral (right) leading hindleg was significantly reduced to 5.4 ± 2.6 cm (P < 0.0001 in comparison to warm), such that it did not differ from leading hindleg step height when area 5 was bilaterally deactivated. However, despite a significant reduction in step height to a mean of 6.5 ± 2.6 cm (P = 0.0076 in comparison to the warm condition), ipsilateral (left) trailing hindleg steps remained significantly higher than trailing hindleg steps when area 5 was bilaterally deactivated (P < 0.0001).
Accordingly, changes in step clearance paralleled changes in peak step height with unilateral area 5 deactivations (Fig. 5E–H). Overall, in comparison to the warm condition, step clearance was only significantly reduced in trailing hindleg steps if the ipsilateral hindleg led (P < 0.0001 for both comparisons; Fig. 5E,G); ipsilateral leading step clearances were unaffected. However, step clearance was significantly reduced in both hindlegs if the hindlimb contralateral to the site of deactivation led (P < 0.0001 in comparison to the warm condition for both comparisons; Fig. 5F,H). For example, when left area 5 was cooled, step clearance of the contralateral (right) leading hindleg was reduced to −3.6 ± 2.6 cm such that it did not differ from clearance of the leading hindleg in bilaterally cooled trials (Fig. 5F). In contrast, clearance of the ipsilateral (right) trailing hindleg was significantly higher than in bilaterally cooled trials (P < 0.0001). Furthermore, when left area 5 was cooled and the ipsilateral hindleg led, leading hindleg steps peaked significantly closer to the obstacle than when area 5 was bilaterally cooled (P = 0.0152, Fig. 5I). When the contralateral hindleg led and left area 5 was cooled, both leading and trailing steps peaked after passing the obstacle unlike bilaterally cooled trials (P < 0.0001 for both comparisons; Fig. 5J).
Altogether, pronounced memory deficits restricted to the contralateral trailing leg when the ipsilateral hindlimb led suggest that area 5 in 1 hemisphere may be essential for guiding the contralateral leg over a remembered obstacle (Fig. 10, row 2). However, similar memory deficits in both leading and trailing hindleg steps when the contralateral leg led suggest that leading hindlimb steps can influence trailing hindlimb steps (Fig. 10, row 3). Ultimately, memory-guided obstacle avoidance likely involves bilateral area 5 contributions.
Area 5 is Necessary for Memory Maintenance, but Insufficient for Working Memory Acquisition
To further specify cortical contributions to obstacle memory, parietal areas were bilaterally deactivated during different phases of the visual obstacle memory test (Figs 6–8). First, we examined the effect of deactivating area 5 during the initial approach towards the obstacle, encompassing the memory acquisition phase (Fig. 6A). A 1-way multivariate ANOVA revealed a significant effect of the cooling condition on peak step height (F(10, 856) = 116.9, P < 0.0001). With mean peak step heights of 7.7 ± 2.7 cm and 5.2 cm for leading and trailing hindlegs, respectively, steps following bilateral area 5 deactivation during memory acquisition remained significantly lower than steps in time-matched warm trials (P < 0.0001 for both comparisons). However, both leading and trailing steps remained significantly higher than stepping in all obstacle absent trials (P < 0.0001 for all comparisons). Consequently, leading and trailing step clearances were also significantly lower in comparison to time-matched warm trials (P < 0.0001 for both comparisons, Fig. 6D,E). In contrast, area 7 deactivation restricted to obstacle memory acquisition did not affect step height or step clearance in comparison to the warm condition. Furthermore, step trajectories were similar between cooling conditions in obstacle present trials, as the mean step peak to obstacle distance did not differ significantly between warm or acquisition cooled trials or area 5 or area 7 (Fig. 6F,G). Thus in comparison to the marked changes in hindleg stepping with bilateral area 5 deactivation throughout the entire visual obstacle memory paradigm, acquisition phase deactivation of area 5 resulted in partial or incomplete deficits in obstacle memory (Fig. 10, row 4).
Figure 6.
Bilateral cortical cooling was restricted to the approach phase to assess cortical contributions to the acquisition of visual obstacle memory. (A) Schematic of the visual obstacle memory paradigm with the blue and red horizontal bar depicting the restriction of cooling (blue) to the approach phase of the task. (B,C) Mean peak step heights ± SD of leading (B) and trailing (C) hindlegs for each cooling condition for trials where the obstacle was present or absent. While area 5 deactivation during memory acquisition attenuated hindleg step height relative to warm and area 7 cooled conditions, steps remained significantly higher than stepping in obstacle absent trials. (D,E) Mean step clearance ± SD of leading (D) and trailing (E) hindlegs for each cooling condition. Acquisition phase cooling of area 5 significantly reduced hindleg step clearances in comparison to warm and area 7 cooled conditions. (F,G) Mean step peak to obstacle distance ± SD for leading (F) and (G) trailing hindlegs did not differ between conditions. ***P < 0.0001, n.s.—not significant.
Figure 8.
Bilateral cortical deactivation and reactivation during the memory maintenance phase of the visual obstacle memory paradigm. (A) Schematic of the visual obstacle memory paradigm with the red and blue horizontal bar depicting the onset and offset of cooling (blue) during the delay phase of the task. (B,C) Mean peak step heights ± SD of leading (B) and trailing (C) hindlegs for each cooling condition for trials where the obstacle was present or absent. While area 5 deactivation and reactivation during memory maintenance attenuated hindleg step height relative to warm and area 7 cooled conditions, steps remained significantly higher than stepping in obstacle absent trials. (D,E) Mean step clearance ± SD of leading (D) and trailing (E) hindlegs for each cooling condition. Area 5 deactivation and reactivation during memory maintenance resulted in significantly reduced hindleg step clearances in comparison to warm and area 7 cooled conditions. (F–G) Mean step peak to obstacle distance ± SD for leading (F) and (G) trailing hindlegs did not differ between conditions. ***P < 0.0001, n.s.—not significant.
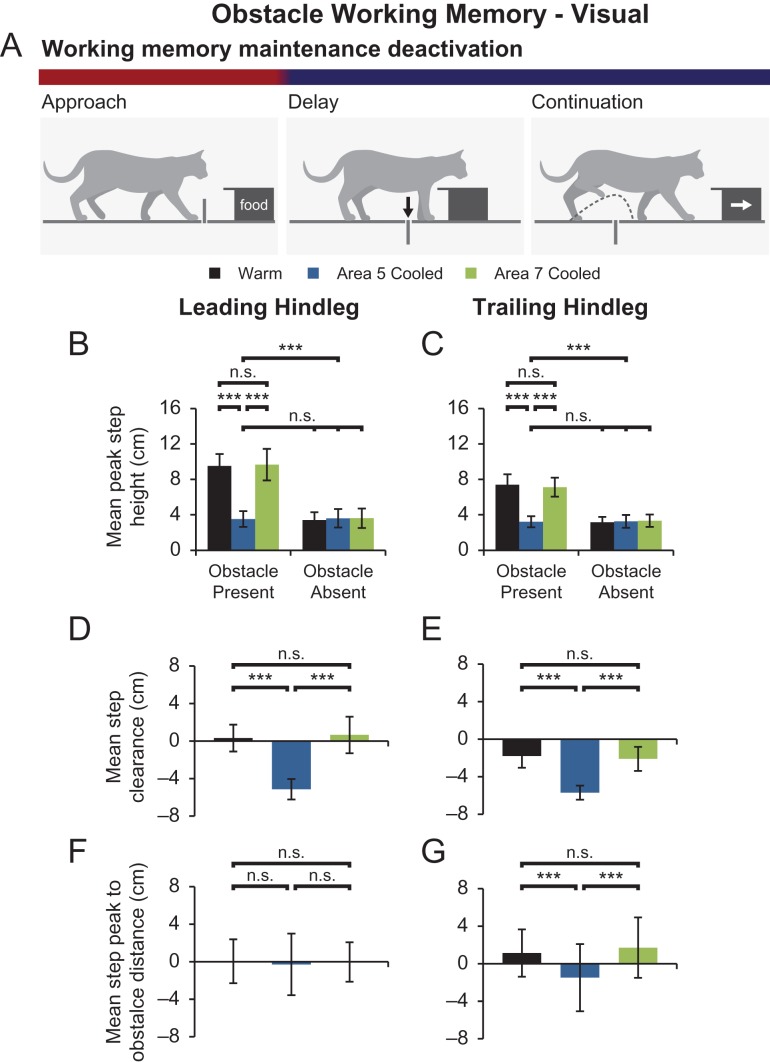
Next, the effect of deactivating area 5 during the delay was examined to assess parietal cortex contributions to obstacle memory maintenance (Fig. 7A). In these trials, maintenance phase deactivation of area 5 resulted in hindleg step heights similar to those observed in obstacle absent trials (Fig. 7B,C). In comparison to both time-matched warm trials and trials where area 7 was deactivated during memory maintenance, leading and trailing steps were significantly lower, with mean step heights of 3.5 ± 0.9 cm and 3.6 cm ± 1.0 cm, respectively (P < 0.0001 for all comparisons). Mean step clearances were consequently reduced to −5.1 ± 1.1 cm and −5.7 ± 0.7 cm for leading and trailing hindlegs, respectively, which were both significantly lower than step clearances in warm and area 7 cooled trials (P < 0.0001 for all comparisons; Fig. 7D,E). While the distance between the leading step peak and obstacle did not differ significantly between cooling conditions, trailing steps peaked before the obstacle in area 5 maintenance cooled trials, which differed significantly from both warm and area 7 maintenance cooled trials (P < 0.0001 for both comparisons; Fig. 7F,G). Overall, these changes in hindleg stepping were similar to those observed when area 5 was cooled throughout the entire visual obstacle memory task. Thus deactivation of area 5, but not 7, during memory maintenance was sufficient in reproducing memory deficits observed when area 5 was deactivated throughout the entire memory test (Fig. 10, row 5).
Figure 7.
Bilateral cortical cooling was initiated during the delay phase to assess cortical contributions to the maintenance of visual obstacle memory. (A) Schematic of the visual obstacle memory paradigm with the red and blue horizontal bar depicting the initiation of cooling (blue) during the delay phase of the task. (B,C) Mean peak step heights ± SD of leading (B) and trailing (C) hindlegs for each cooling condition for trials where the obstacle was present or absent. (D,E) Mean step clearance ± SD of leading (D) and trailing (E) hindlegs for each cooling condition. Maintenance phase cooling of area 5 resulted in significantly reduced hindleg step heights and clearances. (F,G) Mean step peak to obstacle distance ± SD for leading (F) and (G) trailing hindlegs for each condition. While leading hindleg step trajectories did not differ between conditions, area 5 deactivation resulted in trailing hindleg steps peaking sooner before passing over the obstacle. ***P < 0.0001, n.s.—not significant.
Finally, parietal areas were deactivated and reactivated within the memory maintenance phase (Fig. 8A). Memory delays ranged from 140 s to around 240 s to permit complete cortical deactivation and subsequent restoration of neural activity before walking resumed. Additional warm trials were conducted to ensure comparisons between trials with similar memory delays. Area 5 deactivation and reactivation during memory maintenance resulted in mean step heights of 6.9 ± 1.7 cm and 5.0 ± 1.1 cm for leading and trailing legs, respectively, which was significantly lower than stepping in both time-matched warm trials (leading: 9.1 ± 1.7 cm, P < 0.0001; trailing: 6.9 ± 1.6 cm, P < 0.0001; Fig. 8B,C), and trials where area 7 was deactivated and reactivated during memory maintenance (leading: 9.0 ± 1.3 cm, P < 0.0001; trailing: 6.2 ± 1.4 cm, P < 0.0001). Consequently, such area 5 deactivation and reactivation resulted in leading and trailing step clearances of −2.1 ± 1.8 cm and −4.0 ± 1.1 cm, respectively, which were both significantly lower in comparison to the warm condition (P < 0.0001 for both comparisons; Fig. 8D,E). While leading hindleg step clearances similarly differed between area 5 and area 7 cooled trials (Fig. 8D), trailing hindleg step clearances were not differentially affected by area 5 or area 7 deactivation and reactivation during memory maintenance (Fig. 8E). Furthermore, the distance between the step peak and obstacle did not differ significantly between any of the 3 cooling conditions (Fig. 8F,G). Overall, area 5 deactivation and reactivation during memory maintenance resulted in partial or incomplete memory deficits, similar to those observed when area 5 was deactivated during obstacle memory acquisition (Fig. 10, row 6).
Area 5 Contributes Similarly to Tactile Obstacle Working Memory
To compare parietal cortex involvement between visual and tactile obstacle memory, areas 5 or 7 were bilaterally deactivated throughout the entire tactile obstacle memory paradigm, or specifically during memory acquisition or memory maintenance (Fig. 9A). While deactivation of neither area 5 nor area 7 throughout the entire tactile obstacle memory paradigm affected stepping of the forelegs, both leading and trailing hindleg step height was significantly reduced to 4.7 ± 2.4 cm and 3.2 ± 1.2 cm, respectively, for obstacle present trials in comparison to both warm and area 7 cooled conditions (P < 0.0001 for all comparisons; Fig. 9B,C). Moreover, area 5 deactivation resulted in hindleg step heights in obstacle present trials that did not differ significantly from obstacle absent trials. Such memory deficits were similar to those observed in visual obstacle memory trials (Fig. 9D,E). For leading hindleg steps, area 5 deactivation resulted in a 55.4% reduction from step height measured in visual trials performed warm, and a 63.1% reduction from step height measured in tactile trials performed warm (Fig. 9D). In contrast, area 7 deactivation resulted in 2.8% and 2.7% reduction from step height in warm visual and warm tactile trials, respectively. Similarly, trailing hindleg step height was reduced by 57.7% and 62.2% when area 5 was cooled during visual and tactile trials, respectively (Fig. 9E). In contrast, area 7 deactivation during visual and tactile trials only reduced trailing hindleg step height by 0.2% and 4.2%, respectively. Thus in both visual and tactile obstacle memory paradigms, deactivation of area 5, but not 7, resulted in profound obstacle memory deficits (Fig. 10, rows 1 and 7).
Figure 9.
Bilateral deactivation of parietal area 5 during the tactile obstacle memory paradigm results in deficits similar to those observed in the visual obstacle memory paradigm. (A) Schematic of the tactile obstacle memory paradigm with blue and red horizontal bars depicting the duration of cooling extending throughout the entire task (i), or restriction of cooling (blue) to the approach phase (ii) or delay and continuation phases of the task (iii). (B,C) Mean peak step height ± SD of leading (B) and trailing (C) hindlegs for obstacle present and obstacle trials performed with parietal areas warm (no cooling), or when area 5 or area 7 was bilaterally deactivated. Deactivation of area 5, but not 7, resulted in substantial reductions from leading (D) and trailing (E) hindleg step height observed in warm obstacle present trials for both visual and tactile paradigms. (F–I) Area 5 deactivation restricted to the memory acquisition phase of the tactile obstacle memory test attenuated hindleg stepping in obstacle present trials. The percent reduction in step height from warm trials was greater in tactile trials for both hindlegs. However, deficits were not as pronounced as in trials where area 5 was cooled throughout the entire task. (J–M) Area 5 deactivation restricted to the memory maintenance phase of the tactile paradigm attenuated hindleg stepping in obstacle present trials to a similar extent in visual and tactile paradigms. Deficits were similar to those observed when area 5 was cooled throughout the entire task. ***P < 0.0001, n.s.—not significant.
When area 5 deactivation was restricted to the memory acquisition phase of the tactile paradigm, leading and trailing hindleg step heights were significantly reduced to 5.5 ± 2.2 cm and 4.5 ± 1.7 cm for obstacle present trials, in comparison to warm time-matched trials (leading: 9.1 ± 2.3 cm, P < 0.0001, Fig. 8F; trailing: 7.3 ± 1.9 cm, P < 0.0001, Fig. 9F,G). However, these steps remained significantly higher than steps in obstacle absent trials (P < 0.0001 for all comparisons), suggesting only partial or incomplete deficits in the memory of obstacle height. Overall, such changes in hindleg step height represented a 40.0% and 38.6% reduction from leading and trailing step height, respectively, in time-matched tactile warm trials (Fig. 9H,I). In contrast, area 5 deactivation during visual memory acquisition resulted in a 19.0 % and 30.0 % reduction from leading and trailing step height, respectively, in time-matched visual trials. Area 7 deactivation during memory acquisition did not substantially reduce step height, with only a 3.7% and 2.6% reduction in leading step height in visual and tactile trials, respectively, and a 3.1% and 1.5% reduction in trailing step height in visual and tactile trials, respectively (also see Fig. 10 rows 4 and 8).
When area 5 was deactivated during the tactile memory maintenance phase, both leading and trailing hindleg steps were significantly reduced to peak heights of 3.5 ± 0.8 cm and 3.0 ± 0.6 cm, respectively, relative to both warm and area 7 maintenance cooled trials (P < 0.0001 for all comparisons; Fig. 9J,K). As in trials where area 5 was cooled throughout the entire task, tactile maintenance cooling of area 5 resulted in attenuated leading and trailing step heights such that they did not differ from stepping in obstacle absent trials. Such memory deficits were similar to those observed in the visual obstacle memory paradigm (Fig. 9L,M; compare Fig. 10 rows 5 and 9). Maintenance phase deactivation of area 5 resulted in a 62.9% and 62.1% reduction from leading hindleg step height in time-matched warm visual and tactile trials, respectively. Trailing hindleg steps were reduced by 56.7% and 58.1% when area 5 deactivation was restricted to visual and tactile obstacle memory maintenance, respectively. In contrast, maintenance phase deactivation of area 7 resulted in leading and trailing hindleg step heights that were actually 1.7% and 5.3% higher than time-matched warm trials. Trailing hindleg steps were reduced by a mere 3.8% and 2.0% when area 7 was deactivated during visual and tactile obstacle memory maintenance, respectively.
Overall, deactivation of area 5, but not 7, resulted in profound obstacle memory deficits for both visual and tactile paradigms (Fig. 10). While memory deficits were similar when area 5 was cooled throughout obstacle memory paradigms or restricted to memory maintenance, step heights were reduced to a lesser extent when area 5 deactivation was restricted to obstacle memory acquisition, especially in the visual paradigm.
Discussion
Working Memory-Guided Obstacle Avoidance can Rely on Vision or Somatosensation
In comparison to stepping in obstacle absent trials, elevated stepping of all 4 legs in visual and tactile obstacle present trials demonstrates the ability to use visual or tactile information about an obstacle to modify leg movements for avoidance. Moreover, elevated hindleg stepping following delays tested up to 2 min illustrates the ability to retain information about an obstacle in memory. In both visual and tactile obstacle present trials, foreleg steps exceeded the height of the obstacle by around 3 cm, ensuring an adequate margin of safety between the foot and obstacle (Patla et al. 1991). Furthermore, in tactile trials, contact with the obstacle produced higher foreleg steps relative to foreleg stepping modified by visual input of the obstacle. Additionally, trailing foreleg step clearance was also higher in tactile obstacle present trials, and both forelegs steps peaked later after passing over the obstacle following tactile input. These differences in foreleg stepping can be attributed to the reflexive activation of knee flexors and ankle extensors upon foreleg contact, which withdraw the leg from the obstacle (Andersson et al. 1978). This rapid compensatory response mediated by spinal locomotor mechanisms ensures that the legs are lifted well above the obstacle for avoidance (Forssberg 1979). In contrast, visual inputs acquired at least 2 steps before the obstacle can adjust stepping in a feedforward manner (Drew et al. 1996; Patla and Vickers 1997; Mohagheghi et al. 2004). Resulting steps are therefore not as excessively elevated as in tactile trials, demonstrating more efficient obstacle locomotion without incurring extraneous energy costs (Patla et al. 1991).
Foreleg steps were also notably higher than hindleg steps for both visual and tactile obstacle present trials. While foreleg stepping is modified directly by visual inputs or fast reflexive pathways initiated by tactile inputs, hindleg stepping following a delay is modified by memory dependent processes. The resulting attenuation of hindleg step height is similar to the target undershooting bias associated with memory-guided reaching (Westwood et al. 2003). Such target undershooting is thought to reflect uncertainty about target location, and ensures that reaches do not collide with the target incurring time-consuming reversals in movement direction. A similar uncertainty about obstacle size and location likely exists when hindleg clearance is delayed and obstacle information must be retained in working memory. While undershooting leg height would likely result in the foot colliding with the target, less energy is required relative to overshooting obstacle height. As the obstacle used for the present study is relatively benign, attenuated hindleg stepping likely reflects a strategy invoked with uncertainty about obstacle height that opts to minimize energy expenditure given the low risk of serious danger.
In visual obstacle present trials, leading hindleg steps were higher and peaked sooner before passing over the obstacle. In tactile obstacle present trials, having the hindleg peak closer to the actual location of the obstacle may indicate a more accurate representation of obstacle location retained in working memory. However, if steps do not reach their maximal point until after passing the obstacle, the leg may not be elevated sufficiently for clearance by the time it actually reaches the obstacle. Thus having the foot peak sooner in a step may reflect a cautious strategy to maximize the opportunity for successful avoidance. Additionally, with relatively higher leading hindleg step heights and clearances in visual obstacle present trials, obstacle avoidance would be more successful in visual than tactile trials. This may be attributed to a more accurate representation of obstacle height and location acquired visually during the initial approach.
In contrast to differences in leading hindleg steps, trailing hindleg steps did not differ in terms of peak height, clearance, or step peak to obstacle distance between visual and tactile trials. Trailing hindleg steps were also notably lower than leading hindleg steps. With mean step clearances falling below the height of the obstacle, working memory-guided modifications to trailing hindleg steps would have been insufficient for successful obstacle avoidance in either paradigm. In humans, a similar pattern of increased failures in trailing versus leading limb obstacle crossings have been demonstrated in tests of working memory-guided obstacle locomotion (Heijnen et al. 2014). Thus insufficient trailing limb clearance may reflect common limitations of obstacle working memory mechanisms in bipedal and quadrupedal animals, regardless of whether obstacle information is acquired visually or tactilely. Importantly, despite these insufficiencies in step modulation for obstacle clearance, both leading and trailing hindleg steps were significantly elevated in obstacle present trials. Thus despite differences in how obstacle information is acquired, the resulting working memory-guided step modulations may be executed by similar mechanisms, which appear to include parietal area 5.
Area 5 Contributes to Memory-Guided Obstacle Locomotion Regardless of Input Sensory Modality
Within the same animal, deactivating identical sites within area 5 produced similar working memory deficits in visual and tactile paradigms. Furthermore, as temporally restricted deactivations resulted in similar patterns of working memory impairment in both visual and tactile paradigms, area 5 may store information about an obstacle regardless of input sensory modality. While area 5 has been previously examined in studies of visuomotor processing, area 5 in the cat has been traditionally regarded as a higher order somatosensory area (Avendaño et al. 1988). In addition to receiving visual (Squatrito et al. 1981; Avendaño et al. 1988) and corollary motor inputs (Ghosh 1997), area 5 is primed to receive tactile information about an obstacle. Direct projections from primary sensory cortex (Jones and Powell 1970) enable area 5 to respond to cutaneous inputs (Sakata et al. 1973; Scannell et al. 1995), such as the collision of the forelegs with an obstacle. Thus it is perhaps unsurprising that the present study demonstrates the role of area 5 in tasks dependent on visual or tactile information about the environment. However, it remains to be determined if previously described memory delay-related activity recorded in area 5 during a similar visual obstacle memory task (Lajoie et al. 2010) is similarly present in our tactile variation. Specifically, elucidating whether it is identical or distinct neural populations that are active during visual and tactile variations will provide insights into the nature of the neural signals observed in area 5. For example, if distinct subpopulations of area 5 neurons are recruited for visual and tactile obstacle working memory maintenance, then area 5 may indeed store visual or tactile information about the obstacle, respectively, during the working memory delay. Conversely, if the same group of neurons are recruited regardless of sensory input modality, area 5 may be more closely related to retaining the impending motor intention for elevated hindleg stepping. Alternatively, delay-related neural activity in area 5 may not be purely sensory or purely motor in nature. As the resiliency of visual or tactile obstacle working memory is improved if the forelegs have cleared the obstacle (McVea and Pearson 2007a; Wong et al. 2016), efference motor commands and proprioceptive information about foreleg movements may also contribute to the neural signal observed in area 5 (Lajoie et al. 2012). These diverse inputs to area 5 from visual, somatosensory, and motor cortices could be integrated to form a representation of the body in relation to near objects, or body schema (Graziano and Botvinick 2002; Ivanenko et al. 2011), used to guide locomotor movements. Delay-related neural activity may represent such higher order awareness of the obstacle beneath the body that could be used to modulate hindleg stepping when walking resumes.
Utility of Transient, Reversible Deactivations
While studies employing lesions have been essential in elucidating the functional role of particular brain regions, such permanent damage precludes the ability to assess the contributions of an area to distinct stages of working memory. In the present study, cooling permitted temporal control to cortical deactivations, allowing discrete parietal areas to be switched “on” or “off” during different phases of working memory testing. Restricting area 5 deactivation to the working memory maintenance phase was sufficient in reproducing memory deficits observed when area 5 was cooled throughout visual or tactile tests. Previous electrophysiological recordings in walking cats reported a subset of area 5 cells that exhibit sustained activation when an obstacle passes under the body and remains straddled between the fore- and hindlimbs if forward locomotion is paused (Lajoie et al. 2010)—equivalent to the working memory maintenance phase of the present study. Thus working memory deficits resulting from maintenance phase deactivation are likely due to the silencing of such cells, implicating their direct involvement in maintaining the working memory of an obstacle when locomotion is delayed.
Additionally, area 5 deactivation to the working memory acquisition phase resulted in partial working memory deficits. It must be noted, however, that the temporal resolution of cooling-induced deactivations is admittedly not as precise as optogenetically induced inhibition (compare with Kopec et al. 2015). While cooling offers greater temporal control to cortical deactivations in comparison to those achieved pharmacologically (compare with Winters and Bussey 2005), a span of about 6–16 s typically separates cooling onset or offset from the silencing or restoration of neural activity, respectively (Lomber et al. 1999). In comparison, optogenetic approaches ensures inhibition onset and offset within 60 ms of laser stimulation (Kopec et al. 2015). This temporal limitation of cooling-induced deactivation reflects the thermodynamic properties of cortical tissue. As such, the delays used to examine parietal cortex contributions to obstacle working memory maintenance and acquisition were sufficiently long enough to permit deactivation or reactivation of parietal areas following cooling onset or offset, respectively. However, despite these efforts to separate working memory acquisition from maintenance, it is possible that despite terminating cooling immediately following foreleg clearance over the obstacle, neurons remained inactive into the early stages of the working memory maintenance phase. As such, we must acknowledge that the observed partial memory deficits may result from area 5 deactivation during working memory acquisition and early working memory maintenance. Future work employing more temporally precise deactivation techniques will provide further insight into the role of area 5 in obstacle working memory acquisition in the walking cat.
Redundant Working Memory Systems Involve Multiple Brain Regions
Obstacle working memory recovery (albeit partial) following restoration of area 5 function during memory maintenance suggests that maintenance phase deactivation of area 5 may suppress, but not completely eliminate the working memory of the obstacle. Such recovery may be possible if information about the obstacle is relayed to area 5 continuously or repetitively during working memory maintenance. This would implicate another region or structure in the working memory circuitry, although the identity of such an area or areas, and its connectivity to area 5 remains elusive. Given the incomplete memory recovery, it is possible that reverberating activity (Hebb 1949; Sejnowski 1999) between area 5 and another area is responsible for maintaining obstacle working memory. This configuration suggests that deactivating area 5 during early working memory maintenance reduces the overall activity of this reverberating circuitry. Thus despite restoring area 5 function later in the maintenance phase, the memory of the obstacle may be incomplete or less robust, resulting in only partial memory recovery. Similarly, partial working memory deficits were observed following acquisition phase area 5 deactivation. Such deficits may arise if cortical cooling interferes with the relay of information about the obstacle to area 5, again implicating another area or region in the working memory system.
The involvement of other brain regions in working memory-related processes would establish functional redundancies that provide safeguarding mechanisms preventing data loss (Li et al. 2016; Yu 2016). As no brain structure appears to be unique or specific to working memory (Eriksson et al. 2015), memory-related processing is likely distributed across and involves multiple brain areas (Fuster and Bressler 2012). In addition to parietal area 5, working memory-guided obstacle locomotion may also recruit prefrontal (Fuster and Alexander 1971; Goldman-Rakic 1995), premotor (Simon et al. 2002; Lorey et al. 2011), and motor cortical areas (Tomasino and Gremese 2016). Furthermore, the possibility of subcortical contributions to working memory-guided obstacle locomotion cannot be overlooked. Transient optogenetic inactivation of both cortical and subcortical brain regions in the rat revealed contributions of a frontal cortical region and the superior colliculus in both the acquisition and maintenance of working memory for orienting (Kopec et al. 2015). Thus while our discrete deactivations of a single area of parietal cortex resulted in behaviorally relevant memory impairments, these results likely demonstrate the role of a single player within a network of multiple areas and regions that mediate working memory-guided obstacle avoidance. Further electrophysiological and anatomical work will aid in identifying other players in the obstacle working memory circuitry.
Conclusion
These results demonstrate parietal cortex contributions to a working memory system required for hindleg obstacle avoidance in quadrupeds. While area 7 has little or no contribution to obstacle memory, altered hindleg stepping following deactivation of area 5 demonstrates the critical role of area 5 in maintaining the working memory of an obstacle acquired visually or tactilely (Fig. 10). Furthermore, incomplete working memory deficits or partial working memory recovery following restoration of area 5 function during visual working memory maintenance suggests that maintenance phase deactivations may suppress but not eliminate obstacle working memory. Furthermore, partial working memory deficits following acquisition phase deactivation of area 5 suggest that area 5 is necessary but insufficient for acquiring the working memory of an obstacle. As this simple behavioral task involves mechanisms related to locomotion, motor planning, working memory, and spatial representation of the environment, the observed memory deficits strongly implicate area 5 in all of these processes.
Supplementary Material
Notes
We thank Drs. B.D. Corneil, M.A. Goodale, D.F. Sherry, and P. Gribble for helpful discussions and comments on the project and manuscript. We thank Gary Wong, Haley Campbell, and Amy Cardinal for helping train and test the animals. We also thank Pam Nixon for assistance with the surgical implantations and care of the animals. Conflict of Interest: None declared.
Funding
We gratefully acknowledge the support of the Canadian Institutes of Health Research, Natural Science and Engineering Research Council of Canada (NSERC), and the Canada Foundation for Innovation. C.W. was supported by an Alexander Graham Bell Canada Graduate Scholarship from NSERC.
References
- Andersson O, Forssberg H, Grillner S, Lindquist M. 1978. Phasic gain control of the transmission in cutaneous reflex pathways to motoneurones during “fictive” locomotion. Brain Res. 149:503–507. [DOI] [PubMed] [Google Scholar]
- Avendaño C, Rausell E, Perez-Aguilar D, Isorna S. 1988. Organization of the association cortical afferent connections of area 5: a retrograde tracer study in the cat. J Comp Neurol. 278:1–33. [DOI] [PubMed] [Google Scholar]
- Drew T, Andujar JE, Lajoie K, Yakovenko S. 2008. Cortical mechanisms involved in visuomotor coordination during precision walking. Brain Res Rev. 57:199–211. [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Kably B, Lavoie S. 1996. Role of the motor cortex in the control of visually triggered gait modifications. Can J Physiol Pharmacol. 74:426–442. [PubMed] [Google Scholar]
- Drew T, Marigold DS. 2015. Taking the next step: cortical contributions to the control of locomotion. Curr Opin Neurobiol. 33:25–33. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Vogel EK, Lansner A, Bergström F, Nyberg L. 2015. Neurocognitive architecture of working memory. Neuron. 88:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H. 1979. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J Neurophysiol. 42:936–953. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J. 1980. The locomotion of the low spinal cat. I. Coordination within a hindlimb. Acta Physiol Scand. 108:269–281. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. 1971. Neuron activity related to short-term memory. Science. 173:652–654. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bressler SL. 2012. Cognit activation: a mechanism enabling temporal integration in working memory. Trends Cogn Sci. 16:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. 1997. Cytoarchitecture of sensorimotor areas in the cat cerebral cortex. J Comp Neurol. 388:354–370. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. 1995. Cellular basis of working memory. Neuron. 14:477–485. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Botvinick MM. 2002. How the brain represents the body: insights from neurophysiology and psychology In: Prinz W, Hommel B, editors. Common mechanisms in perception and action: attention and performance. Oxford: Oxford University press; p. 136–157. [Google Scholar]
- Grillner S. 2011. Control of locomotion in bipeds, tetrapods, and fish, comprehensive physiology. Hoboken, NJ, USA: John Wiley & Sons, Inc. [Google Scholar]
- Hebb DO. 1949. Organization of behavior. New York: John Wiley and Sons. [Google Scholar]
- Heijnen MJH, Romine NL, Stumpf DM, Rietdyk S. 2014. Memory-guided obstacle crossing: more failures were observed for the trail limb versus lead limb. Exp Brain Res. 232:2131–2142. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Dominici N, Daprati E, Nico D, Cappellini G, Lacquaniti F. 2011. Locomotor body scheme. Hum Mov Sci. 30:341–351. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TPS. 1970. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 93:793–820. [DOI] [PubMed] [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. 2008. The mind and brain of short-term memory. Annu Rev Psychol. 59:193–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec CD, Erlich JC, Brunton BW, Deisseroth K, Brody CD. 2015. Cortical and subcortical contributions to short-term memory for orienting movements. Neuron. 88:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie K, Andujar J-E, Pearson KG, Drew T. 2010. Neurons in area 5 of the posterior parietal cortex in the cat contribute to interlimb coordination during visually guided locomotion: a role in working memory. J Neurophysiol. 103:2234–2254. [DOI] [PubMed] [Google Scholar]
- Lajoie K, Bloomfield LW, Nelson FJ, Suh JJ, Marigold DS. 2012. The contribution of vision, proprioception, and efference copy in storing a neural representation for guiding trail leg trajectory over an obstacle. J Neurophysiol. 107:2283–2293. [DOI] [PubMed] [Google Scholar]
- Li N, Daie K, Svoboda K, Druckmann S. 2016. Robust neuronal dynamics in premotor cortex during motor planning. Nature. 532:459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S. 2008. Double dissociation of “what” and “where” processing in auditory cortex. Nat Neurosci. 11:609–616. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Meredith MA, Kral A. 2010. Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci. 13:1421–1427. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR. 2000. a. Translaminar differentiation of visually guided behaviors revealed by restricted cerebral cooling deactivation. Cereb Cortex. 10:1066–1077. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR. 2000. b. Contributions of cat posterior parietal cortex to visuospatial discrimination. Vis Neurosci. 17:701–709. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Horel JA. 1999. The cryoloop: an adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neural function. J Neurosci Methods. 86:179–194. [DOI] [PubMed] [Google Scholar]
- Lorey B, Pilgramm S, Bischoff M, Stark R, Vaitl D, Kindermann S, Munzert J, Zentgraf K. 2011. Activation of the parieto-premotor network is associated with vivid motor imagery—a parametric FMRI study. PLoS ONE. 6:e20368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVea DA, Pearson KG. 2006. Long-lasting memories of obstacles guide leg movements in the walking cat. J Neurosci. 26:1175–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVea DA, Pearson KG. 2007. a. Stepping of the forelegs over obstacles establishes long-lasting memories in cats. Curr Biol. 17:R621–R623. [DOI] [PubMed] [Google Scholar]
- McVea DA, Pearson KG. 2007. b. Contextual learning and obstacle memory in the walking cat. Integr Comp Biol. 47:457–464. [DOI] [PubMed] [Google Scholar]
- McVea DA, Taylor AJ, Pearson KG. 2009. Long-lasting working memories of obstacles established by foreleg stepping in walking cats require area 5 of the posterior parietal cortex. J Neurosci. 29:9396–9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott JG, Van der Gucht E, Lee CC, Carrasco A, Winer JA, Lomber SG. 2010. Areas of cat auditory cortex as defined by neurofilament proteins expressing SMI-32. Hear Res. 267:119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohagheghi AA, Moraes R, Patla AE. 2004. The effects of distant and on-line visual information on the control of approach phase and step over an obstacle during locomotion. Exp Brain Res. 155:459–468. [DOI] [PubMed] [Google Scholar]
- Patla AE, Prentice SD, Robinson C, Neufeld J. 1991. Visual control of locomotion: strategies for changing direction and for going over obstacles. J Exp Psychol Hum Percept Perform. 17:603–634. [DOI] [PubMed] [Google Scholar]
- Patla AE, Vickers JN. 1997. Where and when do we look as we approach and step over an obstacle in the travel path? Neuroreport. 8:3661–3665. [DOI] [PubMed] [Google Scholar]
- Payne BR, Lomber SG. 1996. Age dependent modification of cytochrome oxidase activity in the cat dorsal lateral geniculate nucleus following removal of primary visual cortex. Vis Neurosci. 13:805–816. [DOI] [PubMed] [Google Scholar]
- Sakata H, Takaoka Y, Kawarasaki A, Shibutani H. 1973. Somatosensory properties of neurons in the superior parietal cortex (area 5) of the rhesus monkey. Brain Res. 64:85–102. [DOI] [PubMed] [Google Scholar]
- Scannell JW, Blakemore C, Young MP. 1995. Analysis of connectivity in the cat cerebral cortex. J Neurosci. 15:1463–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejnowski TJ. 1999. The book of Hebb. Neuron. 24:773–776. [DOI] [PubMed] [Google Scholar]
- Setogawa S, Yamaura H, Arasaki T, Endo S, Yanagihara D. 2014. Deficits in memory-guided limb movements impair obstacle avoidance locomotion in Alzheimer’s disease mouse model. Sci Rep. 4:7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SR, Meunier M, Piettre L, Berardi AM, Segebarth CM, Boussaoud D. 2002. Spatial attention and memory versus motor preparation: premotor cortex involvement as revealed by fMRI. J Neurophysiol. 88:2047–2057. [DOI] [PubMed] [Google Scholar]
- Squatrito S, Galletti C, Battaglini PP, Sanseverino ER. 1981. An autoradiographic study of bilateral cortical projections from cat area 19 and lateral suprasylvian visual area. Arch Ital Biol. 119:21–42. [PubMed] [Google Scholar]
- Takakusaki K. 2013. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord. 28:1483–1491. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Gremese M. 2016. The cognitive side of M1. Front Hum Neurosci. 10:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gucht E, Vandesande F, Arckens L. 2001. Neurofilament protein: a selective marker for the architectonic parcellation of the visual cortex in adult cat brain. J Comp Neurol. 441:345–368. [DOI] [PubMed] [Google Scholar]
- Westwood DA, Heath M, Roy EA. 2003. No evidence for accurate visuomotor memory: systematic and variable error in memory-guided reaching. J Mot Behav. 35:127–133. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Sacrey LAR, Gorny B. 2009. Hind limb stepping over obstacles in the horse guided by place-object memory. Behav Brain Res. 198:372–379. [DOI] [PubMed] [Google Scholar]
- Wilkinson EJ, Sherk HA. 2005. The use of visual information for planning accurate steps in a cluttered environment. Behav Brain Res. 164:270–274. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. 2005. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 25:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Chabot N, Kok MA, Lomber SG. 2014. Modified areal cartography in auditory cortex following early- and late-onset deafness. Cereb cortex. 24:1778–1792. [DOI] [PubMed] [Google Scholar]
- Wong C, Wong G, Pearson KG, Lomber SG. 2016. Memory-guided stumbling correction in the hindlimb of quadrupeds relies on parietal area 5. Cereb Cortex. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Wong C, Lomber SG. 2017. Reversible cooling-induced deactivations to study cortical contributions to obstacle memory in the walking cat. J Vis Exp. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BM. 2016. Neuroscience: Fault tolerance in the brain. Nature. 532:449–450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.