Abstract
Neural stem cells in the postnatal telencephalic ventricular-subventricular zone (V-SVZ) generate new interneurons, which migrate tangentially through the rostral migratory stream (RMS) into the olfactory bulb (OB). The Sp8 and Sp9 transcription factors are expressed in neuroblasts, as well as in the immature and mature interneurons in the V-SVZ–RMS–OB system. Here we show that Sp8 and Sp9 coordinately regulate OB interneuron development: although Sp9 null mutants show no major OB interneuron defect, conditional deletion of both Sp8 and Sp9 resulted in a much more severe reduction of OB interneuron number than that observed in the Sp8 conditional mutant mice, due to defects in neuronal differentiation, tangential and radial migration, and increased cell death in the V-SVZ–RMS–OB system. RNA-Seq and RNA in situ hybridization reveal that, in Sp8/Sp9 double mutant mice, but not in Sp8 or Sp9 single mutant mice, newly born neuroblasts in the V-SVZ–RMS–OB system fail to express Prokr2 and Tshz1 expression, genes with known roles in promoting OB interneuron differentiation and migration, and that are involved in human Kallmann syndrome.
Keywords: interneuron, Kallmann syndrome, migration, olfactory bulb, Prokr2, Sp8, Sp9, Tshz1
Introduction
The mammalian olfactory bulb (OB) is composed of 2 main types of neurons: projection neurons and interneurons. While transmission of olfactory information to the pyriform and entorhinal cortex is mediated by the glutamatergic projection neurons (mitral/tufted cells), the vast majority of neurons in the OB are the GABAergic interneurons, including the granular, external plexiform and periglomerular subtypes (Shepherd et al. 2007; Nagayama et al. 2014; Lim and Alvarez-Buylla 2016). During embryonic development (~E12–E14), OB interneurons begin to be generated from neural stem/progenitor cells located primarily in the dorsal lateral ganglionic eminence (dLGE) and along the rostral migratory stream (RMS) (Wichterle et al. 2001; Stenman et al. 2003; Tucker et al. 2006; Long et al. 2007). OB interneurons continue to be generated in the postnatal and adult brain. Neural stem cells in the ventricular-subventricular zone (V-SVZ) in different regions of the V-SVZ, facing the cortex, striatum and septum, as well as in the RMS, produce different types of OB interneurons (Vergano-Vera et al. 2006; Kohwi et al. 2007; Merkle et al. 2007, 2014; Young et al. 2007; Alonso et al. 2008; Bartolini et al. 2013).
The process of OB interneuron generation in the adult brain includes several steps: (1) their initial genesis from GFAP-expressing astroglial primary neural stem cells (known as B1 cells); (2) the amplification of the lineage through the generation of intermediate neural progenitors (C cells) (Doetsch et al. 1999, 2002; Mirzadeh et al. 2008); (3) the initial differentiation as C cells give rise to the migrating neuroblasts (A cells) (Doetsch et al. 1997; Ponti et al. 2013); (4) tangential migration of neuroblasts aligning into chains along the V-SVZ and RMS into the core of the OB (the end of the RMS) (Lois and Alvarez-Buylla 1994; Lois et al. 1996); (5) radial migration of the young neurons as individual cells from the core of the OB into specific layers; and (6) final maturation and differentiation into functional interneurons (Hack et al. 2002; Carleton et al. 2003; Saghatelyan et al. 2004). These processes are normally then followed by a wave of programmed cell death that occurs after the new neurons have fully formed dendrites and synaptic spines (Petreanu and Alvarez-Buylla 2002).
The transcription factor Sp8, initially expressed in the embryonic dLGE, continues to be expressed in the postnatal and adult V-SVZ–RMS–OB. Its expression persists from the dividing neuroblasts to the vast majority of mature interneurons. (Waclaw et al. 2006; Liu et al. 2009; Wei et al. 2011; Kosaka and Kosaka 2012). Dlx5/6-Cre-IRES-EGFP (Dlx5/6-CIE) transgenic mice express both Cre recombinase and GFP under the control of the Dlx5/6 enhancer element id6/id5 in nearly all telencephalic GABAergic neurons (Zerucha et al. 2000; Stenman et al. 2003). In Dlx5/6-CIE; Sp8F/F mice (referred to herein as Sp8-CKO mice), a large number of glutamate decarboxylase 1-expressing (GAD1+) and calbindin 2 (calretinin)-expressing (CR+) interneurons in the OB granule cell layer (GCL) and glomerular layer, and about 80% parvalbumin expressing (PV+) interneurons in the OB external plexiform layer (EPL) are lost (Waclaw et al. 2006; Li et al. 2011).
Recently, we have showed that Sp9, a Sp family member which closely resembles Sp8 (Kawakami et al. 2004; Zhao and Meng 2005), is widely expressed in the embryonic ganglionic eminences and that Sp9+ cells give rise to the majority of OB interneurons (Zhang et al. 2016). Given the high homology of Sp8 and Sp9, we asked whether Sp9 regulates OB interneuron development, especially in the context of a possible interplay with Sp8.
Here, we show that, like Sp8, Sp9 is also expressed in the adult V-SVZ–RMS–OB system. Most Sp9+ cells are neuroblasts, but a few correspond to intermediate progenitors. Although germline knockout of Sp9 (Sp9-KO) did not lead to obvious defects in OB interneurons, conditional ablation of both Sp8 and Sp9 with Dlx5/6-CIE (Sp8/Sp9-DCKO) led to a much enhanced loss of OB interneurons than that observed in the Sp8-CKO mice. Close inspection of the development of OB interneurons in the double mutants revealed blockage of neuronal differentiation in embryonic and postnatal neural progenitors, defect in tangential and radial migration of neuroblasts, and increased cell death in the V-SVZ–RMS–OB system. RNA sequencing (RNA-Seq) and in situ hybridization showed that Sp8 and Sp9 coordinately induce prokineticin receptor 2 (Prokr2) and transcription factor teashirt zinc finger family member 1 (Tshz1) expression, genes essential for OB interneuron differentiation, migration and survival. These 2 genes are also known to involved in human Kallmann syndrome, which is characterized by congenital hypogonadotropic hypogonadism (due to gonadotropin-releasing hormone [GnRH] deficiency) and anosmia/hyposmia (due to defects in OB development) (Ng et al. 2005; Dode et al. 2006; Matsumoto et al. 2006; Pitteloud et al. 2007; Prosser et al. 2007; Sarfati et al. 2010; Martin et al. 2011; Ragancokova et al. 2014). Thus, our results demonstrate that Sp8 and Sp9 have crucial roles in regulating OB interneuron development.
Materials and Methods
Mice
Sp9 LacZ/+ (Zhang et al. 2016), Sp9 floxed (Zhang et al. 2016), Sp8 floxed (Bell et al. 2003), hGFAP-Cre (Zhuo et al. 2001), Dlx5/6-CIE (Stenman et al. 2003), and Rosa-YFP (Srinivas et al. 2001) mice were previously described. These mice were maintained in a mixed genetic background of C57BL/6J, 129S6, and CD1. The day of vaginal plug detection was calculated as embryonic day 0.5, and the day of birth was considered as postnatal day 0. All animal experiments described in this study were approved in accordance with institutional guidelines.
Tissue Preparation
Postnatal mice were deeply anesthetized and perfused intracardially with 4% PFA in 1× phosphate buffered saline (PBS, pH 7.4); embryonic brains were immersion fixed in 4% PFA. All brains were fixed overnight in 4% PFA, cryoprotected in 30% sucrose for at least 24 h, frozen in the embedding medium and cryosectioned.
BrdU Labeling
Single intraperitoneal injections of BrdU (100 mg/kg) were given to control and hGFAP-Cre; Sp8F/F; Sp9F/F mice at P60. Mice were sacrificed 1 h after BrdU injection.
Viral Infection
P0 mouse pups were deeply anesthetized on ice. 0.05 μl Ad-Cre (1 × 1010 cfu/ml) was injected to the dorsolateral V-SVZ (Merkle et al. 2007) using a microinjector on a stereotaxic injection station. After injection, pups were returned to their mother. Mice were sacrificed at P7 and P21.
Histochemistry
Procedures for 5-bromo-4-chloro-3-indoyl-d-galactopyranoside (X-gal) staining was performed as described previously (Wang et al. 2011). Briefly, Sp9Lacz/+ mice were perfused with 4% PFA. The 30 μm sections were washed twice (5 min each) in X-gal wash (0.1 M sodium phosphate buffer, pH 7.4, 2 mM MgCl2, 5 mM EGTA, 0.01% sodium deoxycholate, 0.02% Nonidet P-40) and stained at 37°C for 1–5 h in X-gal staining solution (X-gal wash plus 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 1 mg/ml X-gal).
Immunohistochemistry
Immunohistochemistry was performed on 12 μm cryostat sections on slides, or 30–50 μm free floating sections (Ma et al. 2013; Zhang et al. 2016). For Sp9 immunohistochemistry, sections were boiled in 10 mM sodium citrate briefly for antigen retrieval. BrdU labeled sections were incubated in 2 N HCl for 1 h at room temperature, and then rinsed in 0.1 M borate buffer twice. The following primary antibodies were used: goat anti-Sp8 (1:1000, Santa Cruz, sc-104 661), rabbit anti-Sp9 (1:500) (Zhang et al. 2016), rabbit anti-CR (1:2000, Swant, 7699/3H), mouse anti-CR (1:1000, Swant, 6B3), rabbit anti-PV (1:3000, Swant,PV25), mouse anti-PV (1/1000, Sigma, P3088), rabbit anti-CB (1:10 000, Swant,CB38), mouse anti-CB (1:10 000, Swant, 300), mouse anti-TH (tyrosine hydroxylase, 1:400, Millipore, MAB318), chicken anti-GFP (1:3000, Aves Labs, GFP-1020), goat anti-DCX (1:1000, Santa Cruz, sc-8066), mouse anti-PSA-NCAM (1:1000, Millipore, MAB5324), mouse anti-NeuN (1:500, Millipore, MAB-377), rat anti-BrdU (1:50, Accurate Chemical, OBT0030s), rabbit anti-Ascl1 (1:2000, Cosmo Bio, SK-T01-003), rabbit anti-Gsx2 (1:2000, Millipore, ABN162), and rabbit anti-cleaved Caspase-3 (1:500, Cell Signaling, 9661L).
In Situ RNA Hybridization
All in situ RNA hybridization experiments were performed using digoxigenin riboprobes on 20 μm cryostat sections (Long et al. 2009; Zhang et al. 2016). Riboprobes were made from cDNAs amplified by PCR using the following primers:
Gad1 Forward: ATGGCATCTTCCACTCCTTCG
Gad1 Reverse: TTACAGATCCTGACCCAACCTCTC
Tshz1 Forward: GAGAAGGTCACGGGCAAGGTCAGC
Tshz1 Reverse: GAGGCGAGGACACAGCATCTGCCA
Prokr2 Forward: ATGGGACCCCAGAACAGA
Prokr2 Reverse: ATGGGACCCCAGAACAGA
Reln Forward: CACCTACTACGTACCGGGACAGGA
Reln Reverse: AGGCTGTCCTGTTTCCACTGGAA
Meis1 Forward: CAACACACACTTTACACACGCACG
Meis1 Reverse: TCAGGGTTATAAGGTGTCCCTTGG
Vax1 Forward: ATGTTCGGGAAACCAGACAAAATG
Vax1 Reverse: TCAGTCCAGCGCTTTTTTCTCG
Pbx3 Forward: GGATGGACGATCAATCCAGGATG
Pbx3 Reverse: GAGTTTGGCGTGGTGGGTGAG
TH Forward: GGAGGCTGTGGTATTCGAGG
TH Reverse: ACCAGTACACCGTGGAGAGT
Microscopy
Bright field images (in situ hybridization results) and some fluorescent images (Figs 3A–F′, 4A–L, 5A,B, and 8G,G′) were imaged with Olympus BX 51 microscope using a 4× or 10× objective. Other fluorescent images were taken with Olympus FV1000 confocal microscope system using 10×, 20×, 40×, or 60× objectives. Z-stack confocal images were reconstructed using the FV10-ASW software. All images were merged, cropped and optimized in Photoshop CS5 without distortion of the original information.
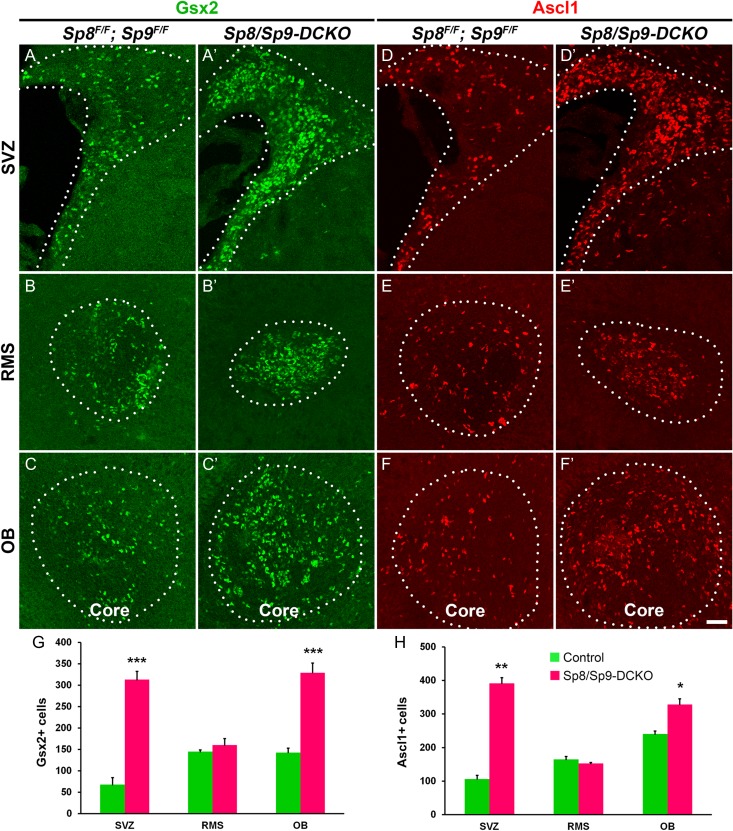
Figure 3.
Neural stem/progenitor cells accumulate in the V-SVZ and OB of postnatal Sp8/Sp9-DCKO mice. (A–F′) Representative images of Gsx2+ and Ascl1+ cells in the V-SVZ–RMS–OB of the control and Sp8/Sp9-DCKO mice at P10. (G, H) The numbers of Gsx2+ and Ascl1+ cells in the V-SVZ and OB were significantly increased in Sp8/Sp9-DCKO mice compared with controls. (Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001, n = 3, mean ± SEM). Scale bar: 50 μm.
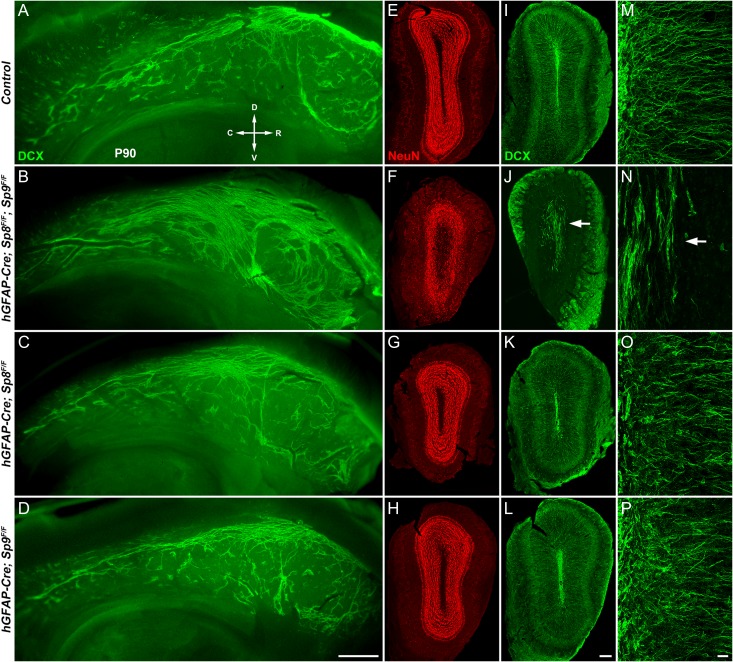
Figure 4.
Tangential and radial migration defects of neuroblasts in adult hGFAP-Cre; Sp8F/F; Sp9F/F mice. (A–D) Whole mount of the lateral wall of the lateral ventricle stained with antibody against DCX showed a marked increase in neuroblast chains in double mutants at P90. (E–L) P90 OB coronal sections stained for NeuN and DCX. Note that hGFAP-Cre; Sp8F/F; Sp9F/F double mutant OBs were smaller than control, hGFAP-Cre; Sp9F/F and hGFAP-Cre; Sp8F/F OBs. (M–P) High magnification images of DCX+ cells in OBs. Arrows indicate defects in radial migration of neuroblasts. R, rostral; C, caudal; D, dorsal; V, ventral. Scale bars: 500 μm in (D) for (A–D); 200 μm in (L) for (E–L); 20 μm in (P) for (M–P).
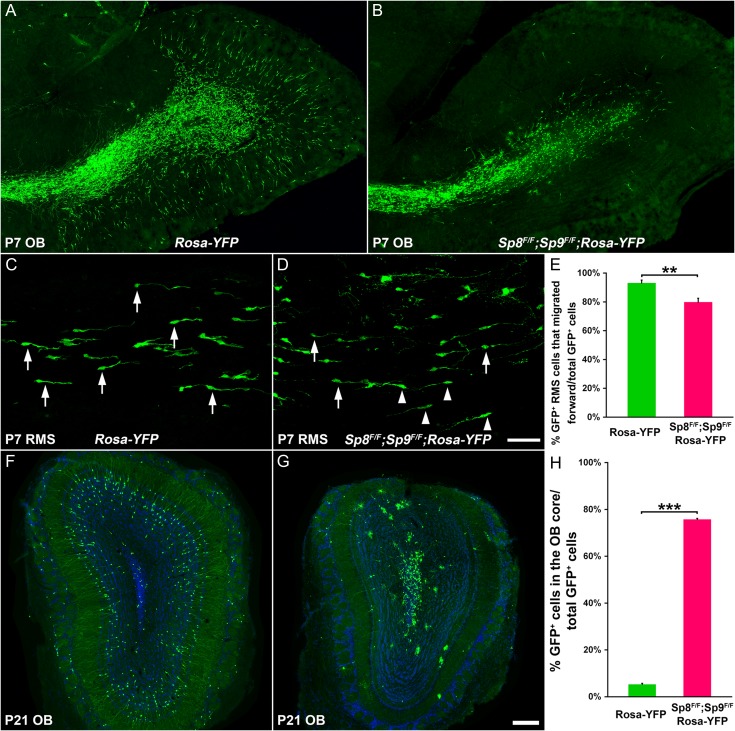
Figure 5.
Sp8 and Sp9 are required cell autonomously for neuroblast tangential and radial Migration. (A, B) Sp8 and Sp9 were deleted by injecting Ad-Cre into the P0 dorsolateral V-SVZ. GFP+ cells in the P7 OB of Rosa-YFP and Sp8F/F; Sp9F/F; Rosa-YFP mice were shown. (C, D) GFP+ migratory neuroblasts in the P7 RMS. Arrows indicate cells oriented in the direction of the OB, whereas arrowheads indicate cells oriented in the reverse direction. (E) Quantification of the percentage of GFP+ cells oriented in the forward direction. (Student’s t test, **P < 0.01, n = 4, mean ± SEM). (F, G) GFP+ cells in the P21 OB of Rosa-YFP and Sp8F/F; Sp9F/F; Rosa-YFP mice are shown. (H) Quantification of the percentage of GFP+ cells that were in the OB core. (Student’s t test, **P < 0.001, n = 3, mean ± SEM). Scale bars: 200 μm in (G) for (A, B, F, G); 50 μm in (D) for (C, D).
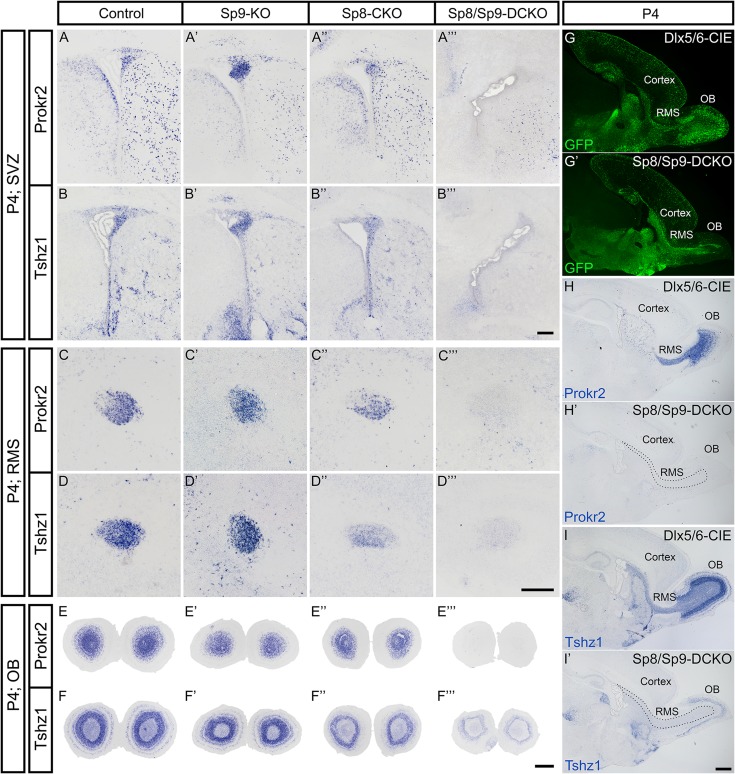
Figure 8.
Prokr2 and Tshz1 expression in the postnatal V-SVZ, RMS, and OB are dependent on Sp8 and Sp9. (A–I’) Prokr2 and Tshz1 expression in the V-SVZ, RMS, and OB were observed in coronal (A–F″′) and sagittal (G–I′) sections of control, Sp9-KO, Sp8-CKO mice at P4, whereas their expression were lost from these structures in Sp8/Sp9-DCKO mice. Note remaining Tshz1 expression in mitral/tufted cells in the Sp8/Sp9-DCKO OB (F″′, I′). Scale bars: 200 μm in B″′ for A–B″′; 200 μm in D″′ for C–D″′; 500 μm in F″′ for E–F″′; 500 μm in I′ for G–I′.
RNA-Seq
RNA-Seq analysis was performed as previously described (Zhang et al. 2016). The OBs from P11 Sp8/Sp9-DCKO mice and littermate controls (Sp8/Sp9 floxed mice without Dlx5/6-CIE) (n = 2 mice, each group), and OBs from P30 hGFAP-Cre; Sp8F/F; Sp9F/F mice and littermate controls were dissected (n = 4 mice, each group). In brief, total RNA was extracted using RNeasy Mini Kit (QIAGEN) according to the manufacturer’s protocol, quantified using NanoDrop ND-2000, and checked for RNA integrity by an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA).
RNA-seq libraries were prepared according to the Illumina TruSeq protocol, quantified with Qubit 2.0 Fluorometer (Invitrogen), examined for dot distribution with Agilent 2100, and subjected to sequencing using Illumina Hiseq 2500. Acquired data were processed to obtain raw reads, which were then extracted for genome mapping with TopHat (version 2.0.9). Levels of gene expression were reported in FPKM (Trapnell et al. 2012). A gene was considered to be expressed if it had an FPKM > 1. For a gene to be called as differentially expressed, it required a P < 0.05. Data from this experiment has been deposited in the GEO database (GSE87417).
Quantification
Sp9 expression and its colocalization with DCX in the adult OB, RMS and V-SVZ (29 000 μm2 area from the lateral V-SVZ) was quantified in 2–3 randomly chosen 30 μm sections from each mouse, and 3 adult mice were used.
Sp9 expression in the OB was quantified in 3 randomly chosen 12 μm sections for each mice (3 adult mice were used). We counted cells within a 400 000 μm2 in the GCL, EPL, or glomerular layer per section. Quantification of interneuron subtypes in the OB was performed in the same way using P30 mice.
Quantification of BrdU+, Gsx2+, and Ascl1+ cells in the dorsal–lateral V-SVZ (360 000 μm2 per section) were performed in 30 μm coronal sections, and 3 sections from each mouse brain were analyzed. BrdU+, BrdU+/DCX+, Gsx2+, and Ascl1+ cells per section in the RMS and OB core were quantified, and 3 sections from each brain were analyzed. Three mice were analyzed for each group.
For quantification of Ad-Cre labeled GFP+ cells in the RMS and OB, about 5–9 30 μm coronal or sagittal sections were counted. Overall, 4–6 brains per group were analyzed.
The numbers of cleaved Caspase-3+ cells were assessed in the V-SVZ, RMS, and OB (excluding the glomerular layer) of Sp8/Sp9-DCKO and control mice at P4, P7, and P11, and those of hGFAP-Cre; Sp8F/F; Sp9F/F mice and littermate controls at P7 and P16. Of all, 9–12 40 μm sections of V-SVZ, RMS, or OB were quantified, 3 brains per group. We also counted cleaved Caspase-3+ cells in the V-SVZ and RMS.
Statistics
Statistical significance was assessed using unpaired Student’s t test. All quantification results were presented as the mean ± SEM (standard error of mean). P values less than 0.05 were considered significant.
Results
Sp9 is Widely Expressed in the Adult V-SVZ–RMS–OB System
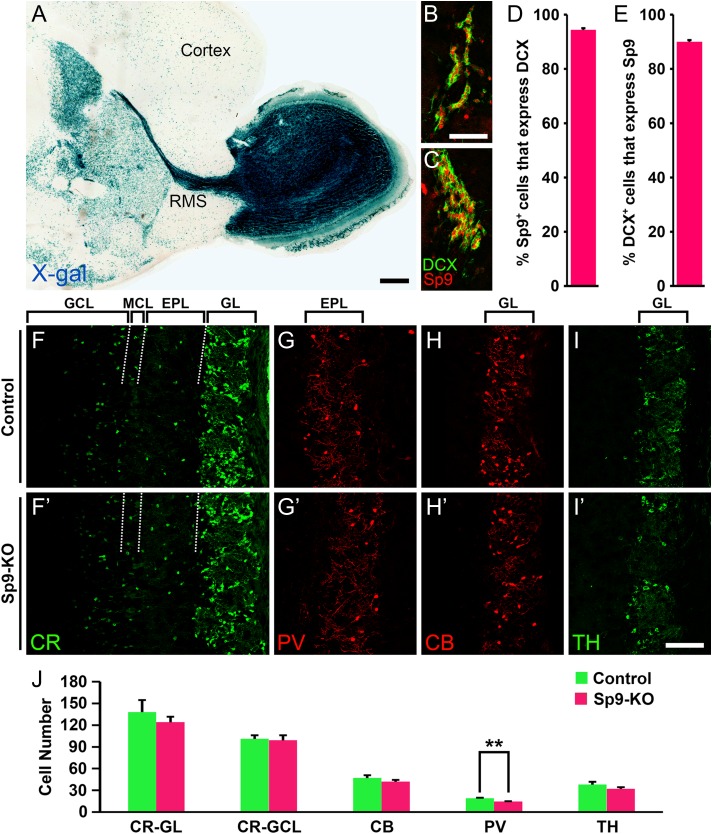
We took advantage of a Sp9LacZ null allele that expresses β-galactosidase (Zhang et al. 2016) to analyze the expression of Sp9 in the V-SVZ–RMS–OB system. X-gal staining demonstrated that Sp9 is strongly expressed in the V-SVZ–RMS–OB in adulthood (Fig. 1A). Consistent with our previous report (Zhang et al. 2016), Sp9 expression in the striatum and cortex was also observed (Fig. 1A). In the adult V-SVZ, 94.4% Sp9+ cells expressed doublecortin (DCX), a marker of newly born neurons (neuroblasts), and about 90% DCX+ cells expressed Sp9 (Fig. 1B–E). A small fraction of Sp9+ and Sp9+/DCX+ cells also expressed Ascl1 (Zhang et al. 2016), consistent with the previous report that expression of this proneural transcription factor was retained in a small population of DCX+ cells (Ponti et al. 2013).
Figure 1.
Sp9 is expressed in the DCX+ neuroblasts in the adult V-SVZ. (A) An X-gal stained P30 Sp9LacZ/+ mouse brain sagittal section showing strong Sp9 expression in the V-SVZ–RMS–OB system. (B–E) The vast majority of DCX+ V-SVZ cells expressed Sp9 and vice versa. DCX+/Sp9+ cells in the lateral (B) and dorsal lateral (C) V-SVZ are shown. (F–J) The numbers of CR+, CB+, and TH+ cells were unaffected in the Sp9-KO OB at P21, except for PV+ cells in the EPL, which showed a significant decrease. (Student’s t test, **P < 0.01, n = 3, mean ± SEM). EPL, external plexiform layer; GCL, granule cell layer; GL, glomerular layer; MCL, mitral cell layer. Scale bars: 500 μm in A; 50 μm in B for B, C; 100 μm in I′ for F–I′.
Immunostaining showed that Sp8 and Sp9 are widely expressed in the adult OB; expression in the deep GCL was stronger for Sp9 than Sp8, whereas in the superficial GCL the reverse was observed with stronger Sp8 and weaker Sp9 expression (Supplementary Fig. S1A–C). Most Sp9+ cells also expressed Sp8 and vice versa (Supplementary Fig. S1D–F″, K). About 50% CR+ cells in the GCL and 88% CR+ cells in the glomerular layer expressed Sp9 (Supplementary Fig. S1G, L). PV+ cells in the EPL expressed Sp8 (Li et al. 2011), but not Sp9 (Supplementary Fig. S1J). We also found that Sp9 was expressed by less than 10% calbindin 1 (CB)+ and TH+ cells in the glomerular layer (Supplementary Fig. S1H, I, K).
No Major OB Neurogenesis Defects are Observed in Sp9-KO Mice
There was no significant difference in the sizes of OB between Sp9-KO mice and control littermates at P20 (Zhang et al. 2016), and the laminar organization of the OB was comparable (Fig. 1F–I′). We counted numbers of interneurons (CR+, PV+, CB+, and TH+ subtypes) in the Sp9-KO OB and control OB at P21, as most Sp9-KO mice die before or around P21 (Zhang et al. 2016). The numbers of CR+, CB+, and TH+ neurons in the OB of Sp9-KO appeared normal. However the number of PV+ cells in the EPL was significantly reduced (Fig. 1J). Thus, compared with the large number of OB interneuron loss in Sp8-cKO mice (Fig. 2G–H″) (Waclaw et al. 2006; Li et al. 2011), there was only a minor OB interneuron defect in Sp9-KO mice. Interestingly, we found that Sp8 expression was upregulated in the deep GCL of the Sp9-KO OB (Supplementary Fig. S1M, N), whereas Sp9 expression was not upregulated in the Sp8-CKO OB (data not shown).
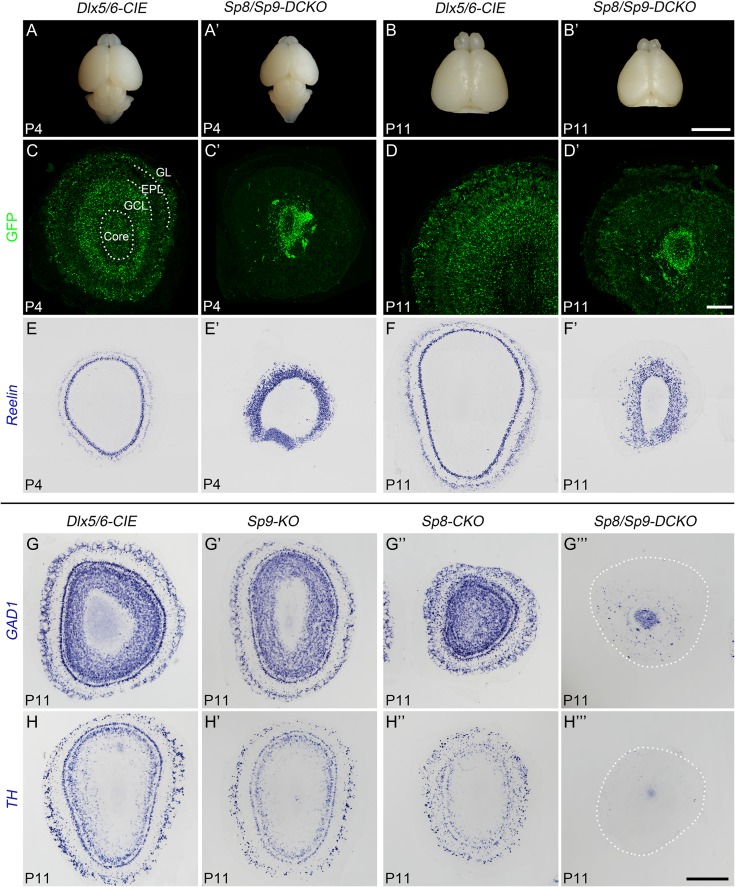
Figure 2.
Severely reduced OB interneurons in Sp8/Sp9-DCKO mice. (A–B′) The OBs and telencephalons from Sp8/Sp9-DCKO mice were smaller than Dlx5/6-CIE controls at P4 and P11. (C–F′) GFP immunostaining and Reln in situ RNA hybridization on P4 and P11 OB sections. Note that most GFP+ cells were restricted to the core of the OB in Sp8/Sp9-DCKO mice. (G–H″′) GAD1 and TH in situ RNA hybridization on P11 OB sections of control, Sp9-KO, Sp8-CKO and Sp8/Sp9-DCKO mice. TH mRNA was not detected in Sp8/Sp9-DCKO OBs. EPL, external plexiform layer; GCL, granule cell layer; GL, glomerular layer. Scale bars: 5 mm in B′ for A–B′; 200 μm in D′ for C–D′; 500 μm in H″′ for E–H″′.
Severe OB Interneuron Defects in Sp8/Sp9-DCKO Mice
The similar expression patterns of Sp8 and Sp9 in the V-SVZ–RMS–OB, and upregulation of Sp8 expression in the Sp9-KO OB suggest that they may play redundant roles in regulating OB interneuron development. Therefore, we analyzed neurogenesis in OBs of Sp8/Sp9-DCKO mice, in which Sp8 and Sp9 were deleted in all neuroblasts destined for the OB from embryonic to postnatal stage. Sp8/Sp9-DCKO mice developed weakness from birth and died between P5 and P12. Compared with Dlx5/6-CIE control OBs, Sp8/Sp9-DCKO OBs were severely reduced in size at P4 and P11 (Fig. 2A–B′). Accumulation of GFP+ cells (Dlx5/6-CIE-expressing cells) in the central core of OB was observed in the mutants, compared with the normal laminar distribution of GFP+ cells in the core to GCL, EPL and glomerular layer (Fig. 2C–D′). RNA in situ hybridization of GAD1 confirmed that most GABAergic interneurons were restricted to the core of the OB in the Sp8/Sp9-DCKO mice at P11, with very few cells in the GCL and/or glomerular layer (Fig. 2G″′). Moreover, GAD1+ cells in the Sp8/Sp9-DCKO OBs were dramatically reduced compared with control and Sp9-KO OBs (Fig. 2G,G′,G″′), and this reduction was also more pronounced than in Sp8-CKO OBs (Fig. 2G″). Notably, while TH mRNA expression was observed in OB superficial granule cells and periglomerular cells in controls, Sp9-KOs and Sp8-CKOs, we did not observe TH mRNA expression in Sp8/Sp9-DCKOs (Fig. 2H–H″′), further suggesting that nearly all mature OB interneurons are lost in the absence of Sp8/9 function. Taken together, Sp8 and Sp9 have redundant functions for OB interneuron development.
To analyze OB projection neurons, we performed in situ hybridization for reelin (Reln), which is mainly expressed by OB mitral/tufted cells (Hack et al. 2002). We found that Reln+ cells were severely disorganized in the Sp8/Sp9-DCKO OB, as there was no clear distinction between the mitral cell layer, EPL and glomerular layer (Fig. 2E–F′). The density of Reln+ cells was increased (Fig. 2E–F′), but the total number was unchanged at P11 indicating the increase of cell density was mostly due to the decrease in the OB volume. The numbers of Reln+ cells per OB sections in the P11 Sp8/Sp9-DCKO mice (1133 ± 27 cells) and controls (1097 ± 53 cells) were similar (Student’s t test, P = 0.58, n = 3), suggesting that the generation of OB projection neurons was not affected in Sp8/Sp9-DCKO.
Sp8 and Sp9 Promote Neuronal Differentiation in the V-SVZ–RMS–OB
To investigate whether Sp8 and Sp9 are required for the transition from proliferating neural progenitors to neurons, we measured the numbers of Gsx2+ and Ascl1+ neural stem/progenitor cells in the V-SVZ–RMS–OB system of Sp8/Sp9-DCKO mice and control mice at P10. Compared with controls, there was a significant increase of Gsx2+ and Ascl1+ cells in the V-SVZ and OB of double mutants (Fig. 3A–H).
To further investigate the function of Sp8 and Sp9 in regulating OB interneuron development, we conditionally deleted Sp8 and Sp9 in V-SVZ neural stem cells using the hGFAP-Cre mouse (Zhuo et al. 2001). This transgenic line exhibits excision of floxed alleles in cortical radial glial cells starting around E12, and in the vast majority of neural stem cells in the postnatal and adult V-SVZ (Malatesta et al. 2003; Lim et al. 2009). Moreover, hGFAP-Cre is not active in the ventral LGE until later stages of development (Malatesta et al. 2003). Therefore, by the time Sp8 and Sp9 are inactivated in hGFAP-Cre; Sp8F/F; Sp9F/F mice, the striatum is well developed and a substantial number of OB interneurons have been generated. In contrast to Sp8/Sp9-DCKO mice, which died between P5 and P12, the growth and survival of hGFAP-Cre; Sp8F/F; Sp9F/F mice were indistinguishable from littermate control mice (i.e., Sp8F/F; Sp9F/F).
To measure the number of cells in S-phase of the cell cycle, we injected BrdU into adult (P60) hGFAP-Cre; Sp8F/F; Sp9F/F mice, and quantified the numbers of proliferating cells in the V-SVZ–RMS–OB 1 h after BrdU injection. Compared with controls, we observed more BrdU+ cells in the mutant V-SVZ and RMS (Supplementary Fig. S2A–B′, K). Notably, cycling cells in the core of the OB appeared to be more prominent. Very few BrdU+ and BrdU+/DCX+ cells were observed in the control OB in adult mice (Supplementary Fig. S2C, J). In contrast, the OBs of hGFAP-Cre; Sp8F/F; Sp9F/F mice showed an approximately 16-fold and 60-fold increase in the number of BrdU+ and BrdU+/DCX+ cells, respectively (Supplementary Fig. S2C′, J′, K, L). Consistent with this observation, an increase of Gsx2+ and Ascl1+ progenitor cells was also observed in the V-SVZ–RMS–OB of mutant mice (Supplementary Fig. S2D–I’). Again, whereas Gsx2+ and Ascl1+ cells were rare in the adult control OBs (Supplementary Fig. S2F, I), Gsx2+ and Ascl1+ cells were greatly increased in the OB core of hGFAP-Cre; Sp8F/F; Sp9F/F mice (Supplementary Fig. S2F′, I′, M, N). Homeodomain transcription factor Gsx2 and proneural transcription factor Ascl1 have been shown to promote neural progenitor proliferation in the embryonic and adult brain (Bertrand et al. 2002; Castro et al. 2011; Pei et al. 2011; Lopez-Juarez et al. 2013; Andersen et al. 2014; Imayoshi and Kageyama 2014), whereas Sp8 and Sp9 are mainly expressed by neuroblasts and mature OB interneurons (Fig. 1A–E) (Waclaw et al. 2006; Liu et al. 2009). Therefore, the increased numbers of Gsx2+, Asc1+, and BrdU+ cells in the V-SVZ–RMS–OB in Sp8/Sp9 double mutants suggest that the process for the neural progenitors to generate immature neurons, at least partly, was blocked.
Neuroblasts Migration Defects in the hGFAP-Cre; Sp8F/F; Sp9F/F Mice
Although neuronal differentiation was inhibited, substantial neuroblasts were still produced in the V-SVZ–RMS–OB of Sp8/Sp9 double mutant mice (e.g., BrdU+ and BrdU+/DCX+ cells were seen in the mutant OBs, Supplementary Fig. 2J, J′). We then examined neuroblast migration in the lateral wall of the lateral ventricle using whole-mount staining (Doetsch and Alvarez-Buylla 1996; Mirzadeh et al. 2010). DCX+ neuroblasts are organized in chains in the SVZ of adult (P90) hGFAP-Cre; Sp8F/F; Sp9F/F mice similar to those observed in controls (Fig. 4A,B). Strikingly, however, there was an obvious increase in these neuroblast chains in mutant mice (Fig. 4A,B). Furthermore, these chains appeared disoriented in hGFAP-Cre; Sp8F/F; Sp9F/F mice compared with controls, as many were oriented perpendicular to the RMS in the anterior wall (Fig. 4B), indicative of a disturbance in tangential migration (n = 4 mice per group).
The sizes of OBs were reduced in adult hGFAP-Cre; Sp8F/F; Sp9F/F mice and the numbers of neurons within OB were significantly decreased compared with littermate controls (Fig. 4E–H). The laminar structures (i.e., GCL, EPL, and glomerular layers) of the OB were largely preserved in the mutants, as shown by NeuN (Rbfox3, a mature neuron marker), but not as clearly defined as those in the control mice (Fig. 4E,F). DCX+ neuroblasts were observed in the hGFAP-Cre; Sp8F/F; Sp9F/F OB, but based on their orientations, most of them were not able to switch from tangential to radial migration (Fig. 4J,N). As a result the DCX+ mutant cells were largely restricted to the OB core with very few present within the GCL or more superficial layers (Fig. 4I–P). Note that although hGFAP-Cre; Sp8F/F; Sp9F/F OBs have many more Gsx2+, Asc1+, BrdU+, BrdU+/DCX+ cells, the number of neuroblasts was severely reduced compared with controls (Fig. 4M–P, Supplementary Fig. S2), further suggesting an inhibition of neuronal differentiation. This in conjunction with defects in neuroblast tangential and radial migration, shows that Sp8/9 are required at multiple steps in the generation and migration of OB interneurons.
We did not observe defects of neuroblast tangential migration in the lateral wall of the lateral ventricle in Sp8 or Sp9 single mutants (Fig. 4C,D). There were also no visible defects of neuroblast radial migration in single mutant OBs (Fig. 4K,L,O,P). These results strongly suggest that Sp8 and Sp9 together are required for the tangential and radial migration of young neurons from the SVZ to different layers of the OB.
Sp8/9-deficent Neuroblasts Have a Cell-Autonomous Defect in Tangential and Radial Migration in the V-SVZ–RMS–OB System
To directly assess neuroblast tangential and radial migration, we injected Cre recombinase-expressing adenovirus (Ad-Cre) into the dorsolateral SVZ of P0 Rosa-YFP control and Sp8F/F; Sp9F/F; Rosa-YFP mice. Direction of migration was determined based on the orientation of the leading process (Sawamoto et al. 2006). In control experiments, by 1 week after Ad-Cre injection, GFP+ neuroblasts reached the OB and some of them had switched from tangential to radial migration (Fig. 5A). This is consistent with previous observations (Petreanu and Alvarez-Buylla 2002; Sawamoto et al. 2006). On the other hand, while in the mutants (Sp8F/F; Sp9F/F; Rosa-YFP mice), some GFP+ neuroblasts also reached the OB, most of them failed to switch from tangential to radial migration (Fig. 5B). In the RMS of control mice, 93% GFP+ cells were oriented in the forward direction (the direction of the OB) (Fig. 5C,E), whereas, this number was 79% in the RMS of Sp8F/F; Sp9F/F; Rosa-YFP mice (Fig. 5D,E). Therefore, loss of Sp8 and Sp9 function resulted in disorientation of tangentially migrating neuroblasts.
Three weeks after Ad-Cre injection, we counted the number of GFP+ cells in the OB. In the control mice, most GFP+ in the OB dispersed in the GCL and glomerular layers (Fig. 5F,H). Some GFP+ cells in the GCL showed branched dendrites and dendritic spines, typical features of mature OB granular cells (Fig. 5F) (Petreanu and Alvarez-Buylla 2002). In contrast, 76% of Sp8/9-deficient GFP+ cells that reached the OB remained in the core; only a small fraction of GFP+ cells were observed in the GCL and glomerular layer (Fig. 5G,H). Taken together, these results suggest that Sp8 and Sp9 are essential for both the tangential and radial migration of neuroblasts in the V-SVZ–RMS–OB system in a cell-autonomous manner.
Apoptotic Cell Death is Increased in the V-SVZ–RMS–OB of Postnatal Sp8/Sp9-DCKO Mice
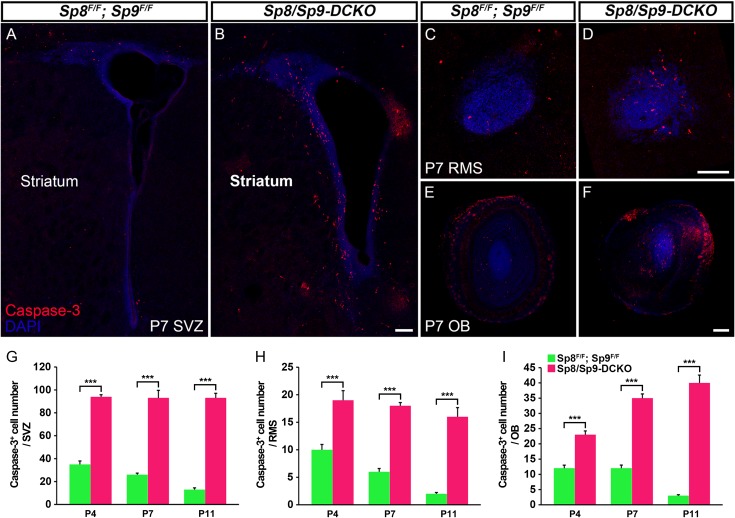
We performed cleaved Caspase-3 immunostaining to examine whether cell death contributed to the reduction of immature and mature interneurons in the OB of Sp8/Sp9-DCKO mice. At all postnatal stages analyzed (P4, P7, and P11), we found increased number of Caspase-3+ cells in the V-SVZ, RMS and OB of Sp8/Sp9-DCKO mice compared with littermate controls (Sp8/Sp9 floxed mice without Dlx5/6-CIE) (Fig. 6A–I). The increase in Caspase-3+ cells in the mutant OB was most evident in core (Fig. 6F), where most GFP+ and GAD1+ cells were located (Fig. 2C′,D′,G″′). There were also more Caspase-3+ cells in the V-SVZ, RMS and OB of hGFAP-Cre; Sp8F/F; Sp9F/F mice compared with controls at P7 and P16 (Supplementary Fig. S3A–I). Increased apoptosis in the adult V-SVZ, RMS, and OB of hGFAP-Cre; Sp8F/F; Sp9F/F mice continued to be observed, but was less conspicuous than that in the younger mice (Supplementary Fig. S3G–I). Thus, Sp8 and Sp9 are required to promote cell survival in the V-SVZ–RMS–OB system. Consistent with our previous finding that Sp9 promotes the survival of postmitotic striatopallidal projection neurons (Zhang et al. 2016), increased apoptotic cell death was also found in the Sp8/Sp9 double mutant striatum (Fig. 6A,B; Supplementary Fig. S3A, B).
Figure 6.
Apoptotic cells are significantly increased in the V-SVZ, RMS and OB in Sp8/Sp9-DCKO mice. (A–F) Cleaved Caspase-3+ cells in the V-SVZ–RMS–OB of controls and double mutants at P7. (G–I) Numbers of Caspase-3+ cells in the mutant V-SVZ, RMS and OB were significantly higher than those in controls at P4, P7, and P11. (Student’s t-test, ***P < 0.001, n = 3, mean ± SEM). Scale bars: 100 μm in B for A, B; 100 μm in D for C, D; 200 μm in F for E, F.
RNA-Seq Analysis Reveals Key Molecular Defects in the OBs of Sp8/Sp9-DCKO
To characterize the molecular changes in the OBs of Sp8/Sp9-DCKO mice, we performed RNA-Seq analysis. We compared gene expression profiles from P11 OB of Sp8/Sp9-DCKO mice to those of littermate controls (n = 2 biological replicas, GEO accession number: GSE87417). Gene expression levels were reported in FPKM (fragments per kilobase of exon per million fragments mapped) (Trapnell et al. 2012). For example, the expression levels (FPKM) of Sp8 and Sp9 genes in P11 control OB were 30.3 and 24.9, respectively. Whereas, in Sp8/Sp9-DCKO OBs at P11, Sp8 and Sp9 gene expression levels were reduced to 1.8 and 0.1 (Supplementary Table 1) demonstrating that the mutation effectively reduced OB expression of Sp8 and Sp9.
To study the impact of Sp8/Sp9 double deletion on different cell types in the OB, such as neural stem/progenitors in the OB core, mitral/tufted cells, astrocytes, and interneurons, we explored expression levels for genes that are enriched in these populations (Supplementary Table S1, S2). We found that RNA levels of OB interneuron-enriched genes were either significantly reduced (fold change, 0.15–0.3, mutants vs. controls), such as Dlx1, Dlx2, Dlx5, Dlx6, Etv1 (Er81), Gad1, Gad2, Meis2, Neto1, Pbx1, Pbx3, Rbfox3, and Tshz1 (Supplementary Table S1), or nearly undetectable, such as Meis1, Prokr2, and Vax1 (0.6, 0.5, and 0.27, FPKM, respectively) (Supplementary Table S1).
In contrast, transcription factors that are expressed in neural stem/progenitors for OB interneurons, such as Gsx1, Gsx2, Nr2e1 (Tlx), and Ascl1, were slightly increased (Supplementary Table S2). Increased expressions of Gsx2 and Ascl1 revealed by RNA-seq was consistent with our immunostaining results (Fig. 3C,C′,F–H), which supported our proposal that in the Sp8/Sp9-DCKO neural stem/progenitors have reduced neurogenesis. The expression of mitral/tufted cell markers [Eomes (Tbr2), Reln, Slc176a (Vglut2), Slc17a7 (Vglut1), Tbr1 and Tbx21], and the astrocyte markers [Aldoc, Aldh1l1, Aqp4, Fabp5, Fabp7 (Blbp), GFAP, Gja1 (Cx43), S100b, Slc1a3 (Glast1), and Vim (Vimentin)] were all significantly increased, levels ranging from a 1.5 to 4-fold change (Supplementary Table S2). We posit that this is largely due to the loss of interneurons, which changed the relative ratio of OB cell types. These RNA-Seq results further confirmed that decreased interneuron genesis was the most salient feature in the OB of Sp8/Sp9-DCKO mice.
Sp8/Sp9-DCKO dLGE Fail to Express Tshz1 and Prokr2
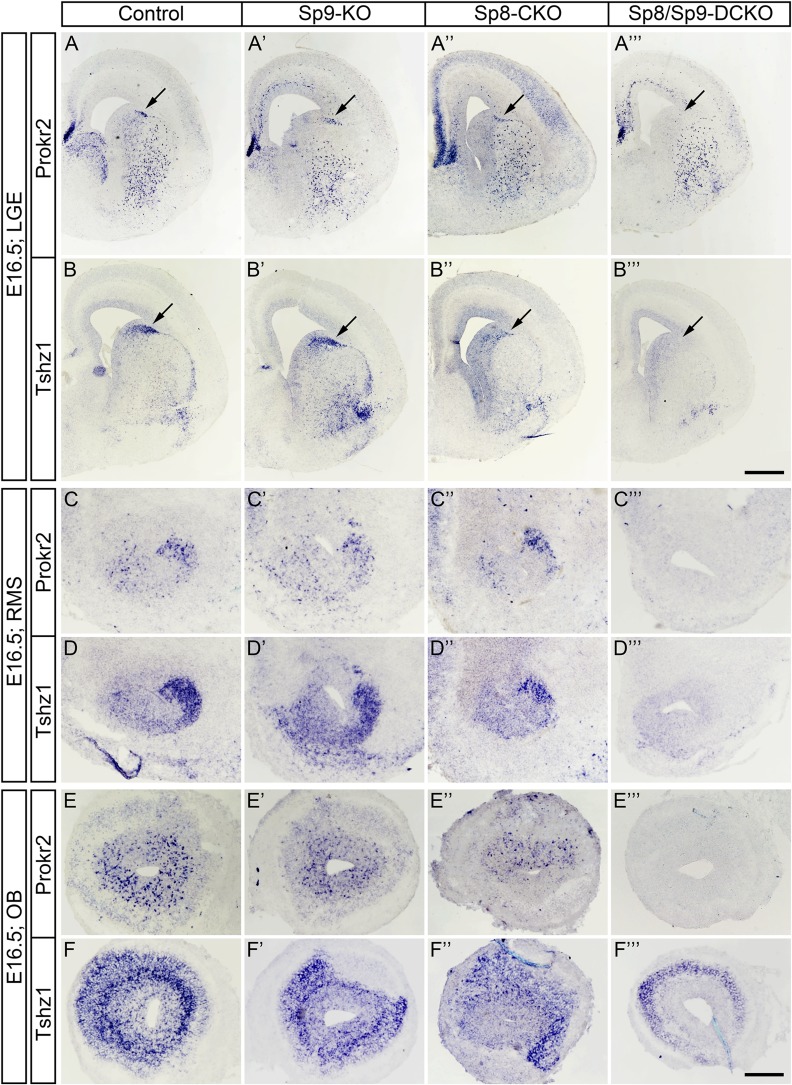
Prokineticin 2 (PROK2) and PROKR2 signaling is required for the neuronal differentiation, neuronal migration from the SVZ through the RMS to their final layers in the OB and cell survival; defects in this signaling result in Kallmann syndrome in humans (Ng et al. 2005; Dode et al. 2006; Matsumoto et al. 2006; Pitteloud et al. 2007; Prosser et al. 2007; Sarfati et al. 2010; Martin et al. 2011; Ragancokova et al. 2014). A recent study has shown that Tshz1 is required for neuronal differentiation, and is essential for neuroblast radial migration in the OB in part through directly promoting Prokr2 expression (Ragancokova et al. 2014). Our RNA-Seq analysis revealed that Prokr2 expression was lost and Tshz1 expression was significantly downregulated in the Sp8/Sp9-DCKO mouse OB (Supplementary Table S1). Moreover, removal of Sp8 and Sp9 in the V-SVZ–RMS–OB of Sp8/Sp9-DCKO and hGFAP-Cre; Sp8F/F; Sp9F/F mice led to phenotypes similar to those observed in Prok2, Prokr2, and Tshz1 constitutive or conditional mutants: reduction in OB size, loss of normal OB architecture, blockage of neuronal differentiation, defect in the tangential and radial migration of young interneurons, and increased cell death in the V-SVZ–RMS–OB (Ng et al. 2005; Matsumoto et al. 2006; Prosser et al. 2007; Ragancokova et al. 2014). Therefore, we next examined the expression of Prokr2 and Tshz1 in the V-SVZ, RMS, and OB of Sp9-KO, Sp8-CKO, and Sp8/Sp9-DCKO mice.
To study the roles of Sp8 and Sp9 in regulating Prokr2 and Tshz1 expression, we performed in situ RNA hybridization in control, Sp9-KO, Sp8-CKO, and Sp8/Sp9-DCKO mice. During embryonic development, the dLGE and RMS are the main source of OB interneurons (Stenman et al. 2003; Waclaw et al. 2006; Long et al. 2007). Consistent with this, we observed that Prokr2 and Tshz1 were expressed in the dLGE but not in the ventral LGE of control mice at E16.5 (Fig. 7A,B). There were no visible differences in the expression of Tshz1 and Prokr2 in the Sp9-KO dLGE compared with controls (Fig. 7A′,B′), while their expression levels in the dLGE of the Sp8-CKO were reduced (Fig. 7A″,B″). Interestingly, complete loss of Tshz1 and Prokr2 expression was observed in the dLGE of Sp8/Sp9-DCKO mice (Fig. 7A″′,B″′). Similarly, the expression of Prokr2 and Tshz1 in the RMS and OB, was greatly reduced or undetectable in the double mutants (Fig. 7C–F″′). A recent study shows that Tshz1 is also expressed in mitral/tufted cells (Kawasawa et al. 2016). Thus, the Tshz1 expression remaining in the Sp8/Sp9-DCKO OB (Fig. 7F″′) likely represents mitral/tufted cells, as marked by Reln in the double mutants (Fig. 2E–F′).
Figure 7.
Sp8/Sp9-DCKO mice fail to express Prokr2 and Tshz1 in the embryonic dLGE, RMS and OB. (A–F″′) Expression of Prokr2 and Tshz1 were absent in the E16.5 dLGE (arrows), RMS and OB of Sp8/Sp9-DCKO. There were no visible differences in the expression of Tshz1 and Prokr2 in the Sp9-KO compared with controls while in Sp8-CKO was slightly reduced (A″, B″). Note that Tshz1 expression in mitral/tufted cells was unaffected (F″′). Scale bars: 500 μm in B″′ for A–B″′; 200 μm in F″′ for C–F″′.
Tshz1 and Prokr2 Expression in the Postnatal V-SVZ–RMS–OB Depends on Sp8 and Sp9
In the postnatal brain, OB interneurons are derived from neural stem/progenitor cells in the lateral, dorsal (cortical), and medial (septal) V-SVZ (Vergano-Vera et al. 2006; Kohwi et al. 2007; Merkle et al. 2007, 2014; Young et al. 2007; Alonso et al. 2008). Accordingly, we observed Prokr2 and Tshz1 expression in the lateral, dorsal, and medial V-SVZ, as well as in the RMS and OB in coronal and sagittal sections of control mice at P4 (Fig. 8A–I). This is consistent with previous observations that most neuroblasts in the V-SVZ–RMS–OB system express Prokr2 and Tshz1 (Ng et al. 2005; Ragancokova et al. 2014). The expressions of Prokr2 and Tshz1 in the V-SVZ–RMS–OB in Sp9-KO and control mice were comparable, while the Sp8-CKO showed reduced expression of Prokr2 and Tshz1 (Fig. 8A–F″). Again, the expression of Prokr2 and Tshz1 in Sp8/Sp9-DCKO mice was undetectable, except for the presumed Tshz1 expression in mitral/tufted cells (Fig. 8A–I′). Interestingly, while we observed only subtle defects in Sp9-KO OB interneurons, there were more Prokr2+ and Tshz1+ cells in the Sp9-KO SVZ (Fig. 8A′,B′). This probably is due to atrophy of the striatum as most striatopallidal neurons are lost in Sp9-KO mice (Zhang et al. 2016).
To confirm our RNA-Seq results from P11 OBs (Supplementary Table S1), we have also examined Tshz1 and Prokr2 expression in the V-SVZ, RMS and OB in these mice at P11, and found the expression patterns nearly identical to those observed in P4 mice (Supplementary Fig. S4A–F″′).
Finally, we examined Tshz1 and Prokr2 expression in control and hGFAP-Cre; Sp8F/F; Sp9F/F mice at P21. Tshz1 and Prokr2 expression in the mutant RMS was barely detectable compared with controls which displayed strong staining (Supplementary Fig. S5D–G′). Of note, while Tshz1 expression was retained in a subpopulation of interneurons and mitral/tufted cells in hGFAP-Cre; Sp8F/F; Sp9F/F mice, its expression in the OB core was significantly downregulated (Supplementary Fig. S5A–B′), suggesting loss of Tshz1 expression in newly born neuroblasts, consistent with its expression pattern in the RMS (Supplementary Fig. S5F′). Prokr2 is a G protein-coupled receptor of chemokine Prok2, and its signaling promotes the neuroblast migration. Therefore, Prokr2 expression is downregulated in mature OB interneurons when these cells stop migrating (Supplementary Fig. S5C). Indeed, although a subpopulation of DCX+ neuroblasts within the core and NeuN+ interneurons in each layer were present in the OB (Fig. 4E–P), we observed very few Prokr2+ cells in the P21 OB of hGFAP-Cre; Sp8F/F; Sp9F/F mice (Supplementary Fig. S5C′).
We also performed RNA-Seq to compare gene expression profiles from P30 OB of hGFAP-Cre; Sp8F/F; Sp9F/F mice to those of littermate controls (n = 4 biological replicas, GEO accession number: GSE87417). Consistent with the RNA-Seq data from P11 Sp8/Sp9-DCKO mouse OB, interneuron specific gene expression levels showed an 2–3-fold decrease in the hGFAP-Cre; Sp8F/F; Sp9F/F mouse OB compared with controls (Supplementary Table S3, S4). We found that the expression level of DCX gene in control OB was 81.7 (FPKM), whereas it was reduced to 24.7 in the mutant OB (fold change: mutants vs. control, 0.30) (Supplementary Table S3). The expression level of Prokr2 gene in control OBs was 11.1, however, in P30 hGFAP-Cre; Sp8F/F; Sp9F/F mouse OB, Prokr2 gene expression level was reduced to 0.5 (fold change: 0.04) (Supplementary Table S3). This result further suggests that Sp8/9 promote Prokr2 expression in the OB.
Taken together, our RNA-Seq and in situ hybridization analysis showed that the expressions of Tshz1 and Prokr2 in the embryonic dLGE and in the postnatal V-SVZ–RMS–OB critically depend on Sp8 and Sp9 function (Supplementary Fig. S6).
Discussion
Our results indicate that the transcription programs regulated by Sp8 and Sp9 are essential for OB interneuron differentiation, tangential and radial migration, and cell survival (Supplementary Fig. S6). This regulation is mainly due to promoting Prokr2 and Tshz1 expression by Sp8 and Sp9, as in Sp8/Sp9-DCKO, neuroblasts in the V-SVZ–RMS–OB system fail to express Prokr2 and Tshz1. Furthermore, Sp8/Sp9-DCKO mice largely phenocopy Prokr2 and Tshz1 mutants in the V-SVZ–RMS–OB system and vice versa. This study may provide novel insights into the mechanisms underlying Kallmann syndrome.
Sp8 and Sp9 are Important for Lineage Progression From Neural Progenitors to OB interneurons
OB neurogenesis in rodents is a continuous process of neuron production from neural stem/progenitor cells throughout embryonic development and adulthood. A previous study has shown that miR-124 regulates the temporal progression of neurogenesis in the adult V-SVZ (Cheng et al. 2009). Here we propose that Sp8 and Sp9 also regulate the lineage progression of neural stem/progenitor cells in the V-SVZ and RMS (Supplementary Fig. S6). This is based on several observations:
Sp8 and Sp9 are expressed in a small fraction of Ascl1+ progenitors (Waclaw et al. 2006; Zhang et al. 2016), and are continuously expressed by dividing neuroblasts, postmitotic neuroblasts, immature and mature OB interneurons, indicating that they may control some or all steps in this neurogenic process.
Conditional inactivation of Sp8 and Sp9 results in accumulation of Gsx2+ and Ascl1+ progenitors and dividing DCX+ neuroblasts in the V-SVZ and OB, and a severe reduction in OB interneurons. Especially, although many more Gsx2+, Ascl1+, BrdU+, and BrdU+/DCX+ cells were observed in the adult hGFAP-Cre; Sp8F/F; Sp9F/F mouse OBs, there were a ~3-fold decrease of DCX expression compared with controls (Supplementary Fig. S2, Supplementary Table S3), strongly suggesting blockage of neuronal differentiation in these progenitors.
Sp8 expression in neuroblasts in the dLGE and the postnatal V-SVZ, RMS, and OB depends on the Dlx1 and Dlx2 transcription factors. Dlx1 and Dlx2 are key regulators of interneuron genesis. They are expressed in neural progenitors as well as in migratory neuroblasts in the V-SVZ–RMS–OB system (Porteus et al. 1994; Anderson et al. 1997; Doetsch et al. 2002; Lim et al. 2006, 2009; Long et al. 2007, 2009). In Dlx1 and Dlx2 double mutants, Sp8 expression is completely lost in these neurogenic regions (Long et al. 2007, 2009).
The homeodomain transcription factor Gsx2 is at the top hierarchy of LGE identity, and induces Ascl1 and Dlx2 expression in the LGE (Corbin et al. 2000; Toresson et al. 2000; Yun et al. 2001; Waclaw et al. 2009; Wang et al. 2013). In the adult V-SVZ, Gsx2 is expressed in a subset of neural stem/progenitor cells and promotes the activation and lineage progression of neural stem cells (Lopez-Juarez et al. 2013). Sp8 expression in the dLGE, adult V-SVZ and OB depends on Gsx2 (Waclaw et al. 2006; Lopez-Juarez et al. 2013) and Gsx2+ neural progenitor differentiation is blocked in the V-SVZ–RMS–OB system of Sp8/Sp9-DCKO mice.
The downstream targets of Sp8 and Sp9 are Prokr2 and Tshz1, as newly born neuroblasts fail to express Prokr2 and Tshz1 in the absence of Sp8/9 function. Note that Prokr2 and Tshz1 are required to promote neuronal differentiation and migration in the V-SVZ–RMS–OB (Ng et al. 2005; Matsumoto et al. 2006; Prosser et al. 2007; Ragancokova et al. 2014).
Cell Death in the V-SVZ–RMS–OB in the Absence of Sp8 and Sp9
Increased apoptotic cell death also contributed to the defects in the Sp8/Sp9-DCKO in the V-SVZ, RMS, and OB. Sp8 and Sp9 may be directly required for the survival of these cells. However, we cannot exclude that some of the effect on cell survival may be indirect or cell nonautonomous. The Sp8-CKO mice also showed increased apoptosis within the dLGE at late embryonic stages as well as in the postnatal RMS and OB (Waclaw et al. 2006). We have found that Sp9 promotes the survival of postmitotic striatopallidal projection neurons (Zhang et al. 2016).
Redundant Functions of Sp8 and Sp9 in Regulating OB Interneuron Development
During development, Sp8 is expressed in the cortical VZ and regulates cortical patterning (Sahara et al. 2007; Zembrzycki et al. 2007; Borello et al. 2014), whereas Sp9 is required for making most Drd2 striatopallidal medium-sized spiny neurons (Zhang et al. 2016). Sp8 also plays a role in establishing the pMN/p3 domain boundary in the spinal cord (Li et al. 2014). Therefore, Sp8 and Sp9 each has prominent unique functions that are related at least in part due to differences in their expression patterns. For the development of OB interneurons, Sp8’s function (Waclaw et al. 2006; Li et al. 2011) appears more prominent than Sp9, as we did not observe major OB interneuron defect in Sp9 single mutants (Fig. 1F–J), and upregulation of Sp8 expression is likely to compensate for the loss of Sp9 in the OB of Sp9-KO mice (Supplementary Fig. S1M, N).
In this study, we found redundant role for Sp8 and Sp9 in promoting Prokr2 and Tshz1 expression. This was evident in virtually all neuroblasts in the embryonic dLGE and in the postnatal V-SVZ, RMS, and OB, suggesting that rules governing OB interneuron genesis from embryonic to adult stages are maintained. While the defects of tangential and radial migration of neuroblasts in the Sp8 and Sp9 single mutant OB were not apparent (mainly because Prokr2 and Tshz1 expressions were largely unaffected), we demonstrate that Sp8 and Sp9 coordinately promote the tangential and radial migration of neuroblasts in the V-SVZ–RMS–OB system in a cell-autonomous manner. A similar function has been proposed for Prok2-Prokr2 signaling (Ng et al. 2005; Matsumoto et al. 2006; Prosser et al. 2007) and/or Tshz1 (Ragancokova et al. 2014). Sp8 and Sp9 may also coordinately promote Meis1, Vax1, and Pbx3 expression in the V-SVZ–RMS–OB (Supplementary Fig. S4G–L′, Supplementary Table S1), 3 additional transcription factors important for OB development (Soria et al. 2004). Within the zinc-finger domain (DNA binding domain), only one amino acid residue differs between Sp8 and Sp9 (Kawakami et al. 2004; Zhao and Meng 2005). Therefore, Sp8 and Sp9 are likely to directly bind to the same set of target genes (e.g., promoters and enhancers). The redundancy of Sp8 and Sp9 may ensure normal OB interneuron development. Clearly the deletion of these 2 transcription factors has a broad impact on OB development and particularly on the generation and migration of interneurons, a process that is continuous throughout life in rodents.
A recent study suggests that Tshz1 directly binds to a regulatory element of Prokr2 to promote its expression in neuroblasts that are migrating radially within the OB (Ragancokova et al. 2014). However, it is worth noting that Prokr2 expression in neuroblasts in the V-SVZ and RMS does not depend on Tshz1 (Ragancokova et al. 2014). In addition, Tshz1 expression in mature OB interneurons does not correlate with Prokr2 expression (Ragancokova et al. 2014) (Supplementary Fig. S5B′, C′, this study).
Sp8/Sp9 and Kallmann Syndrome
Kallmann syndrome has 2 main clinical manifestations: congenital hypogonadotropic hypogonadism and anosmia/hyposmia. Both PROK2 and PROKR2 mutations are implicated in Kallmann syndrome (Dode et al. 2006; Sarfati et al. 2010; Martin et al. 2011), and both Prok2 and Prokr2 mutant mice recapitulate the human Kallmann syndrome phenotype, including hypogonadotropic hypogonadism caused by GnRH deficiency (Ng et al. 2005; Matsumoto et al. 2006; Pitteloud et al. 2007; Prosser et al. 2007). Sp8 and Sp9 are widely expressed in the human dLGE and infant V-SVZ, RMS, and OB (Ma et al. 2013; Wang et al. 2014) (data not shown). Herein, we found that Sp8 and Sp9 coordinately promote the expression of Prokr2 and Tshz1 in the migratory neuroblasts, whereas Prokr2 and Tshz1 expression in Sp8 and Sp9 single mutants were largely unaffected. These results suggest that Sp8 and Sp9 directly regulate OB interneuron development through inducing the expression of molecules involved in human Kallmann syndrome. Future studies are needed to determine whether loss of Sp8 and Sp9 also contribute to other aspects of Kallmann syndrome, such as the generation and migration of GnRH neurons.
Author Contributions
J.L. and C.W. performed all experiments and analysis. Z.Z., Y.W., L.A., Q.L., Z.X., S.W., W.L., T.G., G.L., G.T., Y.Y., H.D. and Z.F. helped conduct experiments and analyze the data. M.H., B.C., K.C. A.A.-B. and J.L.R. helped guide the project and analyzed some results. Z.Y. designed the experiments, analyzed the results and wrote the article.
Supplementary Material
Notes
The GEO accession number for the RNA-Seq data reported in this article is GSE87417. Conflict of Interest: J.L.R. and A.A.B. are cofounders, stockholders, and currently serve on the scientific board of Neurona, a company studying the potential therapeutic use of interneuron transplantation.
Funding
Research grants to Z.Y. from National Natural Science Foundation of China (31425011, 31630032, 31421091, and 31429002), research grants to B.C. from NIH (NS089777), K.C. from NIH (MH090740 and NS044080), and J.L. Rubenstein from NIH (MH049428).
References
- Alonso M, Ortega-Perez I, Grubb MS, Bourgeois JP, Charneau P, Lledo PM. 2008. Turning astrocytes from the rostral migratory stream into neurons: a role for the olfactory sensory organ. J Neurosci. 28:11089–11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J, Urban N, Achimastou A, Ito A, Simic M, Ullom K, Martynoga B, Lebel M, Goritz C, Frisen J, et al. 2014. A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron. 83:1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. 1997. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 19:27–37. [DOI] [PubMed] [Google Scholar]
- Bartolini G, Ciceri G, Marin O. 2013. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron. 79:849–864. [DOI] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Waclaw RR, Campbell K, Potter SS, Scott WJ. 2003. Sp8 is crucial for limb outgrowth and neuropore closure. Proc Natl Acad Sci USA. 100:12195–12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. 2002. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 3:517–530. [DOI] [PubMed] [Google Scholar]
- Borello U, Madhavan M, Vilinsky I, Faedo A, Pierani A, Rubenstein J, Campbell K. 2014. Sp8 and COUP-TF1 reciprocally regulate patterning and Fgf signaling in cortical progenitors. Cereb Cortex. 24:1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. 2003. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 6:507–518. [DOI] [PubMed] [Google Scholar]
- Castro DS, Martynoga B, Parras C, Ramesh V, Pacary E, Johnston C, Drechsel D, Lebel-Potter M, Garcia LG, Hunt C, et al. 2011. A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev. 25:930–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LC, Pastrana E, Tavazoie M, Doetsch F. 2009. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 12:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. 2000. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development. 127:5007–5020. [DOI] [PubMed] [Google Scholar]
- Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, et al. 2006. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. 1996. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 93:14895–14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. 1999. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 97:703–716. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. 1997. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 17:5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. 2002. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 36:1021–1034. [DOI] [PubMed] [Google Scholar]
- Hack I, Bancila M, Loulier K, Carroll P, Cremer H. 2002. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat Neurosci. 5:939–945. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Kageyama R. 2014. bHLH factors in self-renewal, multipotency, and fate choice of neural progenitor cells. Neuron. 82:9–23. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Esteban CR, Matsui T, Rodriguez-Leon J, Kato S, Izpisua Belmonte JC. 2004. Sp8 and Sp9, two closely related buttonhead-like transcription factors, regulate Fgf8 expression and limb outgrowth in vertebrate embryos. Development. 131:4763–4774. [DOI] [PubMed] [Google Scholar]
- Kawasawa YI, Salzberg AC, Li M, Sestan N, Greer CA, Imamura F. 2016. RNA-seq analysis of developing olfactory bulb projection neurons. Mol Cell Neurosci. 74:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Petryniak MA, Long JE, Ekker M, Obata K, Yanagawa Y, Rubenstein JL, Alvarez-Buylla A. 2007. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 27:6878–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K. 2012. Further characterization of the juxtaglomerular neurons in the mouse main olfactory bulb by transcription factors, Sp8 and Tbx21. Neurosci Res. 73:24–31. [DOI] [PubMed] [Google Scholar]
- Li X, Liu Z, Qiu M, Yang Z. 2014. Sp8 plays a supplementary role to Pax6 in establishing the pMN/p3 domain boundary in the spinal cord. Development. 141:2875–2884. [DOI] [PubMed] [Google Scholar]
- Li X, Sun C, Lin C, Ma T, Madhavan MC, Campbell K, Yang Z. 2011. The transcription factor Sp8 is required for the production of parvalbumin-expressing interneurons in the olfactory bulb. J Neurosci. 31:8450–8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. 2016. The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb Perspect Biol. 8: pii: a018820. doi: 10.1101/cshperspect.a018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Huang YC, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, Ernst P, Alvarez-Buylla A. 2009. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 458:529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Suarez-Farinas M, Naef F, Hacker CR, Menn B, Takebayashi H, Magnasco M, Patil N, Alvarez-Buylla A. 2006. In vivo transcriptional profile analysis reveals RNA splicing and chromatin remodeling as prominent processes for adult neurogenesis. Mol Cell Neurosci. 31:131–148. [DOI] [PubMed] [Google Scholar]
- Liu F, You Y, Li X, Ma T, Nie Y, Wei B, Li T, Lin H, Yang Z. 2009. Brain injury does not alter the intrinsic differentiation potential of adult neuroblasts. J Neurosci. 29:5075–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. 1994. Long-distance neuronal migration in the adult mammalian brain. Science. 264:1145–1148. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. 1996. Chain migration of neuronal precursors. Science. 271:978–981. [DOI] [PubMed] [Google Scholar]
- Long JE, Garel S, Alvarez-Dolado M, Yoshikawa K, Osumi N, Alvarez-Buylla A, Rubenstein JL. 2007. Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation. J Neurosci. 27:3230–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Swan C, Liang WS, Cobos I, Potter GB, Rubenstein JL. 2009. Dlx1&2 and Mash1 transcription factors control striatal patterning and differentiation through parallel and overlapping pathways. J Comp Neurol. 512:556–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Juarez A, Howard J, Ullom K, Howard L, Grande A, Pardo A, Waclaw R, Sun YY, Yang D, Kuan CY, et al. 2013. Gsx2 controls region-specific activation of neural stem cells and injury-induced neurogenesis in the adult subventricular zone. Genes Dev. 27:1272–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Wang C, Wang L, Zhou X, Tian M, Zhang Q, Zhang Y, Li J, Liu Z, Cai Y, et al. 2013. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 16:1588–1597. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. 2003. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 37:751–764. [DOI] [PubMed] [Google Scholar]
- Martin C, Balasubramanian R, Dwyer AA, Au MG, Sidis Y, Kaiser UB, Seminara SB, Pitteloud N, Zhou QY, Crowley WF Jr. 2011. The role of the prokineticin 2 pathway in human reproduction: evidence from the study of human and murine gene mutations. Endocr Rev. 32:225–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, et al. 2006. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A. 103:4140–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez-Buylla A. 2014. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat Neurosci. 17:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. 2007. Mosaic organization of neural stem cells in the adult brain. Science. 317:381–384. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Doetsch F, Sawamoto K, Wichterle H, Alvarez-Buylla A. 2010. The subventricular zone en-face: wholemount staining and ependymal flow. J Vis Exp. 39: pii: 1938. doi: 10.3791/1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. 2008. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 3:265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama S, Homma R, Imamura F. 2014. Neuronal organization of olfactory bulb circuits. Front Neural Circuits. 8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. 2005. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 308:1923–1927. [DOI] [PubMed] [Google Scholar]
- Pei Z, Wang B, Chen G, Nagao M, Nakafuku M, Campbell K. 2011. Homeobox genes Gsx1 and Gsx2 differentially regulate telencephalic progenitor maturation. Proc Natl Acad Sci U S A. 108:1675–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. 2002. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 22:6106–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, et al. 2007. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A. 104:17447–17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G, Obernier K, Guinto C, Jose L, Bonfanti L, Alvarez-Buylla A. 2013. Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc Natl Acad Sci U S A. 110:E1045–E1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteus MH, Bulfone A, Liu JK, Puelles L, Lo LC, Rubenstein JL. 1994. DLX-2, MASH-1, and MAP-2 expression and bromodeoxyuridine incorporation define molecularly distinct cell populations in the embryonic mouse forebrain. J Neurosci. 14:6370–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, Bradley A, Caldwell MA. 2007. Olfactory bulb hypoplasia in Prokr2 null mice stems from defective neuronal progenitor migration and differentiation. Eur J Neurosci. 26:3339–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragancokova D, Rocca E, Oonk AM, Schulz H, Rohde E, Bednarsch J, Feenstra I, Pennings RJ, Wende H, Garratt AN. 2014. TSHZ1-dependent gene regulation is essential for olfactory bulb development and olfaction. J Clin Invest. 124:1214–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelyan A, de Chevigny A, Schachner M, Lledo PM. 2004. Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat Neurosci. 7:347–356. [DOI] [PubMed] [Google Scholar]
- Sahara S, Kawakami Y, Izpisua Belmonte JC, O’Leary DD. 2007. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfati J, Dode C, Young J. 2010. Kallmann syndrome caused by mutations in the PROK2 and PROKR2 genes: pathophysiology and genotype-phenotype correlations. Front Horm Res. 39:121–132. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, et al. 2006. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 311:629–632. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Willhite D, Migliore M, Greer CA. 2007. The olfactory granule cell: from classical enigma to central role in olfactory processing. Brain Res Rev. 55:373–382. [DOI] [PubMed] [Google Scholar]
- Soria JM, Taglialatela P, Gil-Perotin S, Galli R, Gritti A, Verdugo JM, Bertuzzi S. 2004. Defective postnatal neurogenesis and disorganization of the rostral migratory stream in absence of the Vax1 homeobox gene. J Neurosci. 24:11171–11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. 2003. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 23:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K. 2000. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 127:4361–4371. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker ES, Polleux F, LaMantia AS. 2006. Position and time specify the migration of a pioneering population of olfactory bulb interneurons. Dev Biol. 297:387–401. [DOI] [PubMed] [Google Scholar]
- Vergano-Vera E, Yusta-Boyo MJ, de Castro F, Bernad A, de Pablo F, Vicario-Abejon C. 2006. Generation of GABAergic and dopaminergic interneurons from endogenous embryonic olfactory bulb precursor cells. Development. 133:4367–4379. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ 2nd, Bell SM, Erdelyi F, Szabo G, Potter SS, Campbell K. 2006. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 49:503–516. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Wang B, Pei Z, Ehrman LA, Campbell K. 2009. Distinct temporal requirements for the homeobox gene Gsx2 in specifying striatal and olfactory bulb neuronal fates. Neuron. 63:451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Long JE, Flandin P, Pla R, Waclaw RR, Campbell K, Rubenstein JL. 2013. Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants. J Comp Neurol. 521:1561–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Lufkin T, Rubenstein JL. 2011. Dlx6 regulates molecular properties of the striatum and central nucleus of the amygdala. J Comp Neurol. 519:2320–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, You Y, Qi D, Zhou X, Wang L, Wei S, Zhang Z, Huang W, Liu Z, Liu F, et al. 2014. Human and monkey striatal interneurons are derived from the medial ganglionic eminence but not from the adult subventricular zone. J Neurosci. 34:10906–10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Nie Y, Li X, Wang C, Ma T, Huang Z, Tian M, Sun C, Cai Y, You Y, et al. 2011. Emx1-expressing neural stem cells in the subventricular zone give rise to new interneurons in the ischemic injured striatum. Eur J Neurosci. 33:819–830. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. 2001. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 128:3759–3771. [DOI] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. 2007. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 27:8286–8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. 2001. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 128:193–205. [DOI] [PubMed] [Google Scholar]
- Zembrzycki A, Griesel G, Stoykova A, Mansouri A. 2007. Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Dev. 2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerucha T, Stuhmer T, Hatch G, Park BK, Long Q, Yu G, Gambarotta A, Schultz JR, Rubenstein JL, Ekker M. 2000. A highly conserved enhancer in the Dlx5/Dlx6 intergenic region is the site of cross-regulatory interactions between Dlx genes in the embryonic forebrain. J Neurosci. 20:709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang Y, Wang C, Xu Z, Liang Q, An L, Li J, Liu Z, You Y, He M, et al. 2016. The zinc finger transcription factor Sp9 is required for the development of striatopallidal projection neurons. Cell Rep. 16:1431–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Meng A. 2005. Sp1-like transcription factors are regulators of embryonic development in vertebrates. Dev Growth Differ. 47:201–211. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. 2001. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 31:85–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.