Abstract
Two studies were conducted to evaluate the effects of dietary supplementation of guanidinoacetic acid (GAA) on growth performance, carcass characteristics, and meat quality in pigs from wean to finish (Exp 1) and finishing pigs fed GAA at different time periods before slaughter (Exp 2). In Exp 1, a total of 360 weaned pigs (Duroc × [Landrace × Yorkshire]) with an average initial BW of 7.17 ± 0.03 kg were randomly distributed into 3 dietary treatments consisting of 10 replicates per treatment and 12 pigs (6 barrows and 6 gilts) per replicate. Dietary treatments were a control (CON; basal diet), a basal diet + 0.08% GAA (0.08% GAA); and a basal diet + 0.12% GAA (0.12% GAA). The duration of the experiment was 150 d. At the end of the experiment, 20 pigs (10 barrows and 10 gilts) from each treatment were slaughtered for measuring carcass characteristics and meat quality. In Exp 2, 1,440 finishing pigs [(Duroc × (Landrace × Yorkshire)), 56.15 ± 0.10 kg BW)] were randomly allocated to 4 treatments with 18 replicates (20 pigs per replicate). Dietary treatments were a control diet (CON; basal), a basal diet + 0.12% GAA fed 60 d before slaughter (T1), a basal diet + 0.12% GAA fed 40 d before slaughter (T2), and a basal diet + 0.12% GAA fed 25 d before slaughter (T3). Body weight was measured at the start (120 d of age) and at the end (180 d of age) of the experiment. At the end of the study, 144 pigs (72 barrows and 72 gilts) from 4 dietary treatments (36 pigs per treatment) were slaughtered for the determination of carcass and meat quality parameters. In Exp 1, 0.12 % GAA increased (P < 0.05) ADG and G:F during starter, grower, finisher, and the overall growth period (30 to 180 d of age). Pigs fed 0.12 % GAA had improved (P < 0.05) lean meat yield in comparison with CON. There was no interaction effect among GAA supplementation and sex of the pigs. Meat quality was not affected by GAA supplementation in pigs. In Exp 2, the final BW, ADG, and lean yield of the pigs fed T1 were higher (P < 0.05) than CON and those fed T3. The carcass back-fat thickness of T1 was lower (P < 0.05) than CON. In conclusion, 0.12% GAA improved the growth performance and lean meat yield in pigs from wean to finish. Finishing pigs fed diets supplemented with 0.12% GAA 60 d before slaughter improved ADG, feed efficiency, and lean meat yield and reduced back-fat thickness compared with those fed GAA unsupplemented diets.
Keywords: guanidinoacetic acid, lean meat, performance, pig
INTRODUCTION
Guanidinoacetic acid (GAA), an amino acid derivative from arginine and glycine, is a natural precursor for the synthesis of creatine (CRE) in animal tissues (Lemme et al., 2007; Michiels et al., 2012). In animals and humans, GAA is produced in the kidney and then transported to the liver via blood circulation for the synthesis of CRE (Liu et al., 2015). CRE is located in the skeletal muscle in the form of CRE phosphate (Janicki and Buzala, 2013), and it plays an important role in energy metabolism via CRE and phosphocreatine system (Brosnan et al., 2009). The CRE and phosphocreatine system is predominant only in muscle cells, where there is an increased energy demand. The CRE and phosphocreatine system works as a backup to the ADP/ATP system to store and mobilize energy during need on short period of time (Wyss and Kaddurah-Daouk, 2000). Approximately, 1.7% of CRE and phosphocreatine are irreversibly converted to creatinine and excreted in urine (Wyss and Kaddurah-Daouk, 2000); hence, there is a constant need of CRE in animals (Michiels et al., 2012). Furthermore, about 67% daily CRE need can be met by de novo synthesis from glycine and arginine (Brosnan et al., 2009), and the rest 33% of CRE need to be supplied via the feed only, especially for animals of modern genetics. In general, CRE is not present in plant-based feed ingredients, but it is present only in animal by-products such as fish meal, poultry by-product meals, and meat and bone meal (Janicki and Buzala, 2013). There exist some restrictions for using animal by-products as feed ingredients in animal diets (Michiels et al., 2012). Therefore, dietary supplementation of CRE is advantageous for fast-growing animals like swine and poultry.
In pigs, CRE has been used in the form of CRE monohydrate to enhance growth performance and carcass quality (review by Janicki and Buzala, 2013). However, feeding CRE in pure form as a feed additive in animals has some disadvantages such as instability and higher price in comparison to GAA, which is more stable and less expensive (Baker, 2009). Therefore, GAA could be a suitable feed additive in swine and poultry. Previous studies demonstrated that GAA supplementation improved weight gain and feed efficiency compared with the negative control in broilers (Lemme et al., 2007; Ringel et al., 2008; Michiels et al., 2012). Some studies reported that CRE improved growth performance in growing-finishing pigs (Berg and Allee, 2001; Young et al., 2005; 2007). In addition, few studies indicated that GAA supplementation in finishing pigs improved meat quality (Wang et al., 2012; Liu et al., 2015).
There is dearth of information on GAA supplementation in pig diets and its effect on growth performance, carcass, and meat quality. Therefore, studies were conducted to investigate the effects of dietary supplementation of GAA on growth performance, carcass characteristics, and meat quality of pigs (starter to finisher; Exp 1) and to evaluate the GAA supplementation at different time points before slaughter in finishing pigs on growth performance, carcass characteristics, and meat quality (Exp 2). The overall hypothesis was that dietary supplementation of GAA would improve growth performance, carcass, and meat quality in pigs.
MATERIALS AND METHODS
Experiments 1 and 2 were conducted in collaboration with Institute of Agricultural Science of Southern Vietnam (IASVN), Vietnam at research facility of IASVN and at a commercial farm in southern Vietnam, respectively. All animal care procedures were approved by the Animal Care and Use Committee of Institute of Animal Science, Vietnam.
Experiment 1
A total of 360 weaned pigs (Duroc × [Landrace × Yorkshire]) with an initial BW of 7.17 ± 0.03 kg were randomly distributed to 5 dietary treatments consisting of 10 replicates per treatment and 12 pigs (6 barrows and 6 gilts) per replicate. The three dietary treatments were a control (CON; basal diet), a basal diet + 0.08% GAA (0.08% GAA), and a basal diet + 0.12% GAA (0.12% GAA). GAA was incorporated into the diet by adding the commercial feed additive (CreAMINO, >96% GAA; Evonik Industries AG, Hanau-Wolfgang, Germany). Feed and water were provided ad libitum throughout the study. The composition of the experimental diets including nutrients is presented in Table 1. The basal diets were formulated according to NRC (2012) recommendations and are maintained isonitrogenous and isoenergetic. A 4-phase feeding program was implemented which includes prestarter phase (30 to 60 d; 7 to 18 kg BW), starter phase (60 to 90 d; 18 to 35 kg BW), grower phase (90 to 120 d; 35 to 55 kg BW), and finisher phase (120 to 180 d; 55 to 100 kg BW). The total duration of the experiment was 150 d. In each phase, initial and final BW and feed intake were measured to calculate ADG, ADFI, and feed efficiency (G:F).
Table 1.
Ingredient and nutrient composition of basal diet
| Ingredients, % | Prestarter | Starter | Grower | Finisher1 |
|---|---|---|---|---|
| Corn | 50 | 58.0 | 60.0 | 48.0 |
| Rice bran | – | 10.0 | 12.0 | 32.1 |
| Soybean meal (47.5% CP) |
15.0 | 13.5 | 19.5 | 12.0 |
| Fermented soybean meal | 14.2 | 10.4 | – | – |
| Whey powder | 12.8 | – | – | – |
| Soybean oil | 3.30 | 3.40 | 4.60 | 4.10 |
| Premix2,3 | 0.25 | 0.25 | 0.25 | 0.25 |
| Salt | 0.32 | 0.46 | 0.46 | 0.43 |
| Limestone | 0.20 | 0.78 | 0.49 | 0.59 |
| Dicalcium phosphate | 3.30 | 2.40 | 2.10 | 1.80 |
| L-Lys | 0.28 | 0.30 | 0.31 | 0.37 |
| DL-Met | 0.31 | 0.30 | 0.12 | 0.12 |
| L-Thr | 0.16 | 0.18 | 0.15 | 0.20 |
| L-Trp | 0.02 | 0.03 | 0.01 | 0.03 |
| Calculated nutrient | ||||
| ME, Kcal/kg | 3360 | 3295 | 3295 | 3200 |
| CP, % | 21.0 (20.3)a | 18.0 (18.5) | 16.0 (15.4) | 14.0 (13.3) (14.2) |
| Crude fat, % | 6.62 | 7.27 | 6.15 | 6.7 |
| Crude fiber, % | 2.25 | 3.23 | 3.93 | 4.39 |
| Salt, % | 0.5 | 0.5 | 0.5 | 0.5 |
| Ca, % | 1.0 | 0.9 | 0.7 | 0.7 |
| Total, % | 0.8 | 0.67 | 0.55 | 0.55 |
| Available P, % | 0.6 | 0.44 | 0.35 | 0.35 |
| AA total basis, % | ||||
| Lys | 1.34 (1.15)a | 1.05 (1.16) | 0.91 (0.98) | 0.79 (0.93) (0.81) |
| Met | 0.48 (0.33) | 0.37 (0.51) | 0.36 (0.31) | 0.29 (0.34) (0.31) |
| Met + Cys | 0.83 (0.85) | 0.67 (0.81) | 0.59 (0.58) | 0.53 (0.59) (0.55) |
| Thr | 0.90 (0.83) | 0.73 (0.82) | 0.63 (0.65) | 0.57 (0.57) (0.56) |
| Trp | 0.30 | 0.22 | 0.18 | 0.17 (0.18) (0.19) |
| Val | 0.91 (0.92) | 0.81 (0.88) | 0.72 (0.72) | 0.64 (0.65) (0.67) |
| Arg | 1.19 (1.26) | 1.05 (1.20) | 0.93 (1.00) | 0.71 (0.91) (0.74) |
| Ile | 0.83 (0.81) | 0.72 (0.71) | 0.64 (0.61) | 0.54 (0.51) (0.55) |
| AA SID basis, % | ||||
| Lys | 1.18 | 0.92 | 0.80 | 0.69 |
| Met | 0.39 | 0.31 | 0.27 | 0.24 |
| Met + Cys | 0.71 | 0.57 | 0.50 | 0.43 |
| Thr | 0.74 | 0.60 | 0.52 | 0.46 |
| Trp | 0.26 | 0.18 | 0.16 | 0.14 |
| Val | 0.80 | 0.63 | 0.54 | 0.47 |
| Arg | 1.06 | 0.93 | 0.83 | 0.63 |
| Ile | 0.73 | 0.63 | 0.56 | 0.47 |
aFigures in parentheses are analyzed values.
1Basal diet for Exp 1 and Exp 2.
2For prestarter and, starter diets: the vitamin-micromineral premix contained the following (per kg of the premix): vitamin A, 5,000 IU; vitamin D3, 800 IU; vitamin E, 30,000 mg; vitamin K 3,200 mg; riboflavin 4,000 mg; vitamin B3, 20,000 mg; vitamin B6, 2,000 mg; vitamin B12, 16,000 mcg; pantothenic acid, 14,000 mg; Cu, 6,600 mg; Fe, 15,000 mg; manganese, 6,000; selenium, 36 mg; zinc, 18,000 mg.
3For grower and finisher diets: the vitamin-micromineral premix contained the following (per kg of the premix): vitamin A, 4,000 IU; vitamin D3, 800 IU; vitamin E, 20,000 mg; vitamin K 3,200 mg; riboflavin 4,000 mg; vitamin B3, 20,000 mg; vitamin B6, 2,000 mg; vitamin B12, 16,000 mcg; pantothenic acid, 14,000 mg; Cu, 6,600 mg; Fe, 15,000 mg; manganese, 4,800; selenium, 36 mg; zinc, 12,000 mg.
Carcass Characteristics and Meat Quality
At the end of the feeding trial, 20 pigs (10 barrows and 10 gilts) per each treatment (2 pigs per pen), representing the mean BW of the pen, were slaughtered for determining carcass and meat quality. Carcass parameters mainly include slaughter weight, carcass weight, lean meat content, lean meat percentage, and back-fat thickness on animal (before slaughter) and on carcass. Back-fat thickness measurement of live pigs was done at 9 to 11th rib by ultrasound instrument (Aloka SSD-500, ALOKA CO., LTD, Germany).
The pH value of the semimembranosus and longissmus lumborum muscle was measured using a pH meter (Minolta Chromameter, Japan) at different time points (15, 30, 60, and 90 min after slaughter). Percentage of drip loss was determined according to Honikel (1998). Briefly, meat samples were weighed, placed in a mesh bag, and then transferred to nylon bag, and stored at chilling temperature for 24 h. Percentage of drip loss was calculated as follows: 100 × (initial weight − final weight of meat sample)/initial weight. Cooking loss was determined according to USDA ARS (2014). Briefly, the raw blade meat weights were recorded and placed on a rack in a roasting pan. Distilled water (100 mL) was added to the roasting pan, which was tightly covered and placed in the center of a preheated oven at 163 °C. Initial cooking time estimates were 45 min for blade meat. The internal temperature was determined with an electronic digital thermometer. Meat samples were allowed to cool for 5 min and then reweighed.
Experiment 2
A total of 1,440 pigs (Duroc × [Landrace × Yorkshire]) (56.15 ± 0.10 kg BW) were randomly allocated to 4 dietary treatments with 18 replicates (20 pigs per replicate). Corn-soybean meal-based basal diet (Table 1) was formulated for the finisher phase and fed through the 60-d experimental period. Dietary treatments included were a control diet (CON), a basal diet + 0.12% GAA fed 60 d before slaughter (T1), a basal diet + 0.12% GAA fed 40 d before slaughter (T2), and a basal diet + 0.12% GAA fed 25 d before slaughter (T3). GAA was incorporated into the diet by adding the commercially available feed additive (CreAMINO, >96% GAA; Evonik Industries AG, Hanau-Wolfgang, Germany). Experimental diets did not contain antimicrobial growth promoters. All pigs were provided ad libitum feed and water. The BW of individual pigs and pen feed disappearance were recorded at the start (120 d of age) and end (180 d) of the experiment to calculate ADG, ADFI, and G:F. The basal diet for finishing pigs was formulated according to NRC (2012) recommendations and all the diets were isonitrogenous and isocaloric. At the end of the feeding trial, 36 pigs (18 barrows and 18 gilts) per each treatment were slaughtered for the determination of carcass and meat quality. Determination procedure was followed as per Exp 1.
Analytical Procedures
Diet samples were analyzed for DM (AOAC, 2000; method 930.15), CP (AOAC, 2000; method 968.06), and amino acids (AOAC, 2000; method 994.12). Estimation of GAA and CRE in the diet samples was done according to the procedure described by Michiels et al. (2012).
Statistical Analysis
In Exp 1, data on growth performance (n = 10 per treatment; pen as the experimental unit) were analyzed as completely randomized design using the general linear model (GLM) with the fixed effect of treatment using SAS 9.4. For carcass characteristics and meat quality (n = 20; carcass as the experimental unit), data were analyzed as a completely randomized design, the model included sex and diets as main effects as well as their interaction. If this analysis indicated significant (P < 0.05) differences among treatments, the treatment means were compared using Tukey’s test. Orthogonal polynomial contrasts were used to determine linear and quadratic effects of increasing levels of GAA (0%, 0.08%, and 0.12%) in the pig diets.
In Exp 2, data of growth performance (n = 18 per treatment; pen as the experimental unit) were analyzed as completely randomized design using the GLM with the fixed effect of treatment using SAS 9.4. For carcass and meat quality (n = 36 per treatment; carcass as the experimental unit), data were analyzed as a completely randomized design, the model included sex and diets as main effects as well as their interaction, and the experimental unit was a carcass. If this analysis indicated significant (P < 0.05) differences among treatments, the treatment means were compared using Tukey’s test.
RESULTS
Analyzed Nutrient Composition and GAA Content in the Experimental Diets
The analyzed CP and AA contents of experimental diets are presented in Table 1. Most of the analyzed AA content of starter, grower, and finisher diets were met or higher than the calculated values. The actual GAA content of the experimental diets is presented in Table 2.
Table 2.
Analyzed guanidinoacetic acid (GAA) contents (mg/kg, as is) of experimental diets
| Experimental diets | Prestarter | Starter | Grower | Finisher (Exp 1) |
Finisher (Exp 2) |
|---|---|---|---|---|---|
| CON1 | <1 | <1 | <1 | <1 | < 20 |
| 0.08% GAA2 | 944 | 764 | 698 | 846 | - |
| 0.12% GAA3 | 1,252 | 1,141 | 928 | 990 | 1,186 |
1CON- control
20.08% GAA = basal + 0.8 kg GAA/MT
30.12% GAA =basal + 1.2 kg GAA /MT
Experiment 1
Growth performance data are presented in Table 3. During starter, grower, finisher, and the over-all growth period, pigs fed 0.12% GAA had higher (P < 0.05) daily gain and feed efficiency compared with other dietary treatments. In addition, the overall ADG and G:F improved (linear; P < 0.05) due to increased supplementation of GAA (0%, 0.08%, and 0.12%) in pig diets (Table 3). Data on carcass characteristics and meat quality are presented in Table 4. There were no sex and diet interactions detected in carcass and meat quality parameters. Pigs fed increasing GAA levels (0%, 0.08%, and 0.12%) increased (linear; P < 0.05) lean meat weight, lean meat percentage, and reduced (linear; P < 0.05) back-fat thickness on carcass. In addition, back-fat thickness measured on animal decreased (P < 0.001) due to increasing GAA levels. Cooking loss tended to decrease (linear; P = 0.051) as the levels of GAA supplementation increased in pigs. However, other meat quality parameters such as post-mortem pH, and drip loss were not affected by dietary treatments. Slaughter weight, carcass weight, carcass percentage, and dripping loss were not affected by sex. Carcass from gilts had higher lean meat weight and percentage compared with barrows, whereas the back-fat thickness measured on animal and carcass was lower in barrows than gilts. The pH of longissimus muscle was lower in gilts than barrows; however, cooking loss was lower in meat from barrows than from gilts.
Table 3.
Effect of guanidinoacetic acid (GAA) supplementation on growth performance of pigs from wean to finish (Exp 1)1
| Dietary treatment | P value5 | |||||
|---|---|---|---|---|---|---|
| Item | CON1 | 0.08% GAA2 | 0.12% GAA3 | SEM 4 | Linear | Quadratic |
| Pre-starter | ||||||
| BW at 60 d, kg | 17.78b | 18.10ab | 18.28a | 0.10 | 0.002 | 0.650 |
| ADG, g | 354b | 364ab | 370a | 3.29 | 0.002 | 0.617 |
| ADFI, g | 590 | 597 | 599 | 3.62 | 0.091 | 0.497 |
| G:F | 0.60 | 0.61 | 0.62 | 0.01 | 0.042 | 0.980 |
| Starter | ||||||
| BW at 90 d, kg | 31.61b | 32.43b | 33.66a | 0.28 | <0.001 | 0.501 |
| ADG, g | 461b | 478b | 513a | 7.75 | <0.001 | 0.338 |
| ADFI, g | 941 | 950 | 952 | 11.69 | 0.532 | 0.771 |
| G:F | 0.489b | 0.503b | 0.538a | 0.004 | <0.001 | 0.046 |
| Grower | ||||||
| BW at 120 d, kg | 52.31c | 53.41b | 55.40a | 0.29 | <0.001 | 0.245 |
| ADG, g | 690b | 699ab | 725a | 7.77 | 0.004 | 0.404 |
| ADFI, g | 2012 | 2023 | 2002 | 21.84 | 0.761 | 0.546 |
| G:F | 0.344b | 0.344b | 0.361a | 0.003 | <0.001 | 0.005 |
| Finisher | ||||||
| BW at 180 d, kg | 95.3c | 97.19b | 101.0a | 0.35 | <0.001 | 0.047 |
| ADG, g | 717c | 730b | 759a | 3.70 | <0.001 | 0.093 |
| ADFI, g | 2376 | 2390 | 2378 | 7.18 | 0.866 | 0.161 |
| G:F | 0.300b | 0.305b | 0.319a | 0.002 | <0.001 | 0.085 |
| Overall (30 to 180 d) | ||||||
| ADG, g | 588c | 600b | 625a | 2.31 | <0.001 | 0.037 |
| ADFI, g | 1659 | 1670 | 1662 | 5.29 | 0.731 | 0.147 |
| G:F | 0.353c | 0.361b | 0.374a | 0.001 | <0.001 | 0.160 |
a,b,cWithin a row, means without a common superscript differ (P < 0.05).
1CON- control (basal) diet.
20.08% GAA= basal diet + 0.8 kg GAA/MT.
30.12% GAA= basal diet + 1.2 kg GAA /MT.
4SEM = standard error of mean.
5Considered significant when P < 0.05. Orthogonal polynomial contrasts were used to determine linear and quadratic effects of increasing levels of GAA (0%, 0.08%, and 0.12%) in the pig diets.
Table 4.
Effect of guanidinoacetic acid (GAA) supplementation on carcass characteristics and meat quality of finishing pigs (Exp 1)
| Item | Dietary treatment | Sex | P value5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contrast 6 | ||||||||||||
| CON1 | 0.08% GAA2 | 0.12% GAA3 | SEM 4 | Barrow | Gilt | SEM | Diet | Sex | Diet × Sex | Linear | Quadratic | |
| Slaughter weight, kg | 96.10 | 95.95 | 96.15 | 0.40 | 96.17 | 95.97 | 0.33 | 0.935 | 0.666 | 1.000 | 0.930 | 0.722 |
| Carcass weight, kg | 71.63 | 72.24 | 72.48 | 0.44 | 72.29 | 71.94 | 0.36 | 0.380 | 0.501 | 0.997 | 0.179 | 0.736 |
| Carcass, % | 74.54 | 75.30 | 75.39 | 0.37 | 75.17 | 74.97 | 0.30 | 0.207 | 0.637 | 0.995 | 0.106 | 0.461 |
| Lean meat, kg | 38.30b | 38.90b | 39.83a | 0.25 | 38.51b | 39.51a | 0.21 | <0.001 | <0.001 | 0.994 | <0.001 | 0.595 |
| Lean meat, % | 53.47b | 53.85b | 54.97a | 0.22 | 53.27b | 54.92a | 0.18 | <0.001 | <0.001 | 0.994 | <0.001 | 0.183 |
| Back-fat thickness measured on carcass, mm | 15.95a | 15.45ab | 14.77b | 0.20 | 15.89a | 14.89b | 0.16 | <0.001 | <0.001 | 1.000 | 0.001 | 0.717 |
| Back-fat thickness measured on animal7, mm | 11.93a | 11.53ab | 11.16b | 0.20 | 12.04a | 11.05b | 0.16 | 0.033 | <0.001 | 1.000 | 0.009 | 0.952 |
| Meat quality parameters | ||||||||||||
| pH of the meat after slaughter8 | ||||||||||||
| 15 min | 6.86 | 6.84 | 6.89 | 0.04 | 6.96a | 6.76b | 0.03 | 0.660 | <0.001 | 1.000 | 0.588 | 0.466 |
| 30 min | 6.63 | 6.70 | 6.63 | 0.03 | 6.75a | 6.55b | 0.03 | 0.262 | <0.001 | 1.000 | 1.000 | 0.103 |
| 60 min | 6.31 | 6.32 | 6.33 | 0.04 | 6.42a | 6.22b | 0.03 | 0.932 | <0.001 | 1.000 | 0.709 | 1.000 |
| 90 min | 5.93 | 5.97 | 5.90 | 0.04 | 6.03a | 5.84b | 0.03 | 0.443 | <0.001 | 0.999 | 0.605 | 0.245 |
| Cooking loss, % | 44.06 | 43.05 | 42.93 | 0.40 | 42.78a | 43.92b | 0.33 | 0.101 | 0.017 | 0.992 | 0.051 | 0.371 |
| Drip loss, % | 9.01 | 8.89 | 8.84 | 0.56 | 8.79 | 9.03 | 0.46 | 0.978 | 0.708 | 0.999 | 0.837 | 0.966 |
a,b,c Within a row, means without a common superscript differ (P< 0.05)
1CON- control
20.08% GAA = basal diet + 0.8 kg GAA/MT
30.12% GAA= basal diet + 1.2 kg GAA/MT
4SEM = standard error of mean
5Considered significant when P < 0.05
6Orthogonal polynomial contrasts were used to determine linear and quadratic effects of increasing levels of GAA (0, 0.08, and 0.12%) in the pig diets.
7Back-fat thickness of live pigs was done between 9 to 11th rib by ultrasound instrument (Aloka SSD-500, ALOKA Co., Ltd, Germany).
8pH measured on the semimembranosus and longissmus muscle using a pH meter (Minolta Chromameter, Japan).
Experiment 2
Data on growth performance of pigs (120 to 180 d of age) are presented in Table 5. The final BW of the pigs fed 0.12% GAA during 60 d before slaughter (T1) was higher (P < 0.05) than CON and those fed GAA during 25 d before slaughter (T4). Similarly, the ADG for T1 was higher (P < 0.05) than CON and T4. The ADFI was similar (P > 0.05) among the dietary treatments. Feed efficiency of T1 was better (P < 0.05) than CON and T3.
Table 5.
Effect of guanidinoacetic acid (GAA) supplementation on growth performance of finishing pigs (120 to180 d of age) (Exp 2)
| Item | CON1 | T12 | T23 | T34 | SEM5 | P value6 |
|---|---|---|---|---|---|---|
| BW at 120 d of age, kg | 56.18 | 56.13 | 56.13 | 56.14 | 0.10 | 0.242 |
| BW at 180 d of age, kg | 96.67b | 98.40a | 97.58ab | 97.10b | 0.31 | 0.001 |
| ADG, g/pig/day | 675b | 704a | 691ab | 682b | 5.22 | 0.001 |
| ADFI, g/pig/day | 2415 | 2417 | 2410 | 2418 | 4.51 | 0.579 |
| G:F | 0.26b | 0.29a | 0.27ab | 0.27b | 0.03 | 0.003 |
a,b,cWithin a row, means without a common superscript differ (P < 0.05).
1CON = control (basal) diet.
2T1 = basal diet + 1.2 kg GAA/MT fed 60 d before slaughter.
3T2 = basal diet + 1.2 kg GAA/MT fed 40 d before slaughter.
4T3 = basal diet + 1.2 kg GAA/MT fed 25 d before slaughter.
5SEM = standard error of mean.
6Considered significant when P < 0.05.
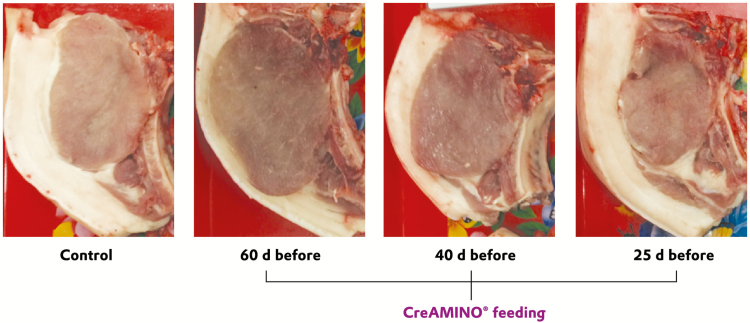
Data on carcass characteristics and meat quality of finishing pigs (Table 6) revealed that there were no interactions between diet and sex on any of the response criteria except for lean meat % (P = 0.021). Carcass characteristics and meat quality parameters were not affected by sex. Pigs fed GAA 60 d before slaughter (T1) had higher lean meat weight (kg) and lean meat percentage compared with control pigs (CON) and T3. T1 pigs had lower (P < 0.05) back-fat thickness measured on carcass than CON (Figure 1). Dietary treatments did not affect the meat quality parameters (pH, cooking loss %, and drip loss %; Table 6).
Table 6.
Effect of guanidinoacetic acid (GAA) supplementation on carcass characteristics and meat quality of finishing pigs (at 180 d of age)
| Item | Diets | Sex | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON1 | T12 | T23 | T34 | SEM5 | Barrow | Gilt | SEM | Diets | Sex | Diet × Sex | |
| Slaughter weight, kg | 96.10 | 96.17 | 95.99 | 94.04 | 0.29 | 96.00 | 96.15 | 0.21 | 0.977 | 0.622 | 0.858 |
| Carcass weight, kg | 71.66 | 72.34 | 72.11 | 72.05 | 0.32 | 72.14 | 71.94 | 0.23 | 0.514 | 0.541 | 0.858 |
| Carcass % | 74.56 | 75.24 | 75.14 | 75.01 | 0.38 | 75.16 | 74.84 | 0.27 | 0.598 | 0.407 | 0.887 |
| Lean meat weight, kg | 38.57b | 39.78a | 39.22ab | 38.88b | 0.19 | 39.00 | 39.24 | 0.14 | <0.001 | 0.212 | 0.270 |
| Lean meat content, % | 53.83b | 55.01a | 54.41ab | 53.96b | 0.22 | 54.06 | 54.55 | 0.15 | <0.001 | 0.308 | 0.027 |
| Back-fat thickness measured on carcass, mm7 | 15.94a | 14.95b | 15.36ab | 15.39ab | 0.16 | 15.37 | 15.45 | 0.11 | <0.001 | 0.579 | 0.637 |
| Back-fat thickness measured on animal, mm | 11.76 | 11.30 | 11.49 | 11.51 | 0.14 | 11.47 | 11.57 | 0.10 | 0.156 | 0.478 | 0.511 |
| Meat quality parameters | |||||||||||
| Meat pH after slaughter8 | |||||||||||
| 15 min | 6.87 | 6.88 | 6.91 | 6.90 | 0.03 | 6.90 | 6.89 | 0.02 | 0.732 | 0.621 | 0.808 |
| 30 min | 6.66 | 6.63 | 6.62 | 6.65 | 0.03 | 6.65 | 6.63 | 0.02 | 0.741 | 0.395 | 0.267 |
| 60 min | 6.32 | 6.29 | 6.31 | 6.32 | 0.03 | 6.31 | 6.30 | 0.02 | 0.850 | 0.729 | 0.723 |
| 90 min | 5.93 | 5.92 | 5.92 | 5.94 | 0.03 | 5.95 | 5.91 | 0.02 | 0.973 | 0.139 | 0.759 |
| Cooking loss, % | 13.92 | 13.30 | 13.55 | 13.74 | 0.38 | 13.81 | 13.42 | 0.26 | 0.691 | 0.302 | 0.084 |
| Drip loss, % | 7.60 | 6.85 | 7.13 | 7.31 | 0.22 | 7.42 | 7.03 | 0.16 | 0.123 | 0.081 | 0.982 |
a,b,cWithin a row, means without a common superscript differ (P< 0.05).
1CON= control (basal) diet.
2T1 = basal diet + 1.2 kg GAA/MT fed 60 d before slaughter.
3T2 = basal diet + 1.2 kg GAA/MT fed 40 d before slaughter.
4T3 = basal diet + 1.2 kg GAA/MT fed 25 d before slaughter.
5SEM = standard error of mean.
6Considered significant when P < 0.05.
7Back-fat thickness of live pigs was done between 9th and 11th rib by ultrasound instrument (Aloka SSD-500, ALOKA Co., Ltd, Germany).
8pH measured on the semimembranosus and longissmus muscle using a pH meter (Minolta Chromameter, Japan).
Figure 1.
Effects of feeding guanidinoacetic acid (GAA) at different time periods before slaughter on back-fat thickness* on carcasses of finishing pigs. *Back-fat thickness was measured on carcass of pigs using ultrasound instrument (Aloka SSD-500, ALOKA Co., Ltd. Germany). Control = basal diet. 60 d before = basal diet + 1.2 kg GAA/MT fed 60 d before slaughter. 40 d before = basal diet + 1.2 kg GAA/MT fed 40 d before slaughter. 25 d before = basal diet + 1.2 kg GAA/MT fed 25 d before slaughter.
DISCUSSION
Growth Performance
Our hypothesis was that GAA supplementation in swine diets would improve growth performance, carcass characteristics, and meat quality. In this study, pigs fed 0.12% GAA supplementation had improved overall growth performance compared with control. To the best of our knowledge, there exists paucity of information on GAA supplementation and its effect on performance of pigs in complete growth phase. In animal body, GAA is the only immediate precursor for CRE, which is not present in plant-based feed ingredients, but it is present only in animal by-products such as fishmeal or meat-bone meal (Wyss and Kaddurah-Daouk, 2000). It was assumed that majority of CRE is present in skeletal muscle (Balsom et al., 1994; Wyss and Kaddurah-Daouk, 2000); furthermore, phosphocreatine is the major form (around 2/3) of muscle CRE and acts as a primary source of energy for muscle fibers. In pigs, phosphocreatine resources in fast glycolytic muscles are higher than slow glycolytic muscles (review by Janicki and Buzala, 2013). In the current study, improved BW gain in pigs fed 0.12% GAA could be due to the formation of CRE, which further might have increased muscle protein and water retention in the skeletal muscles (Lemme et al., 2007; Michiels et al., 2012). Recently, Tossenberger et al. (2016) demonstrated that broiler chickens fed increasing levels of GAA had increased CRE concentration in breast muscle, which indicates that dietary GAA has been utilized for the synthesis of CRE in skeletal muscle. Previous studies in broilers and turkeys are in agreement with our results that GAA supplementation improved growth performance (Lemme et al., 2007, 2010; Michiels et al., 2012; Dilger et al., 2013; Heger et al., 2014). In relation to CRE monohydrate feeding, finishing pigs showed improved growth performance compared with control diet (Berg and Allee, 2001; Maddock et al., 2002; Young et al., 2007).
Carcass Characteristics and Meat Quality
Carcass and meat quality are the most important determinants in pork processing industry. In the present study, 0.12% GAA supplementation improved the lean meat yield in finishing pigs. There exists paucity of literature in pigs to support this finding. However, there were studies demonstrating that finishing pigs fed CRE monohydrate had improved growth and carcass characteristics (Berg and Allee, 2001; Young et al., 2007). In a review, Janicki and Buzala (2013) demonstrated that pigs fed CRE had increased body growth due to enhanced retention of muscle proteins and water in the skeletal muscle.
In this study, meat quality parameters such as drip loss and cooking loss were not affected due to GAA supplementation in pigs, which is in agreement with a previous study in broilers (Ringel et al., 2008). However, some studies indicated that supplementation of GAA to growing-finishing pigs increased pH, and decreased drip loss and cooking loss (Wang et al., 2012; Liu et al., 2015). Wang et al. (2012) demonstrated that pigs fed GAA at 0.08%, 0.12%, or 0.20% for 54-d linearly increased postmortem pH and quadratically declined drip loss. However, Janicki and Buzala (2013) indicated in the review that the rate and extent of drop in pH during conversion of muscle to meat did not influence the meat quality characteristics. After slaughter, the muscle pH progressively drops from 7.4 to about 5.6 to 5.7 within 6 to 8 h. In our study, the average meat pH falls under this range. Further work need to be conducted to validate the effects of GAA on meat quality in growing-finishing pigs.
Carcasses from gilts had higher lean meat weight and percentage than barrows which is in agreement with Ellis et al. (1996). Barrows had higher back-fat thickness than gilts which is consistent with previous studies (Choi et al., 2000; Piao et al., 2004; Latorre et al., 2008). In Exp 1, the meat pH in gilts was lower than barrows which is in agreement with Larzul et al. (1997). However, in Exp 2, meat quality parameters were not affect by sex.
In summary, supplementation of 0.12% GAA improved weight gain and feed efficiency in pigs from wean-finish. Finishing pigs fed 0.12% GAA during 60 d before slaughter had higher lean meat content and lower back-fat thickness compared with those supplemented without GAA. In conclusion, GAA could be a promising feed additive as a precursor of CRE in improving growth performance and carcass characteristics in growing-finishing pigs.
Conflict of interest statement. None declared.
ACKNOWLEDGMENTS
We are thankful to Institute of Agricultural Science for Southern Vietnam, Vietnam and financial assistance by Evonik (SEA) Pte. Ltd, Singapore.
LITERATURE CITED
- AOAC 2000. Official methods of analysis. 15th ed K., Helrich, editor. AOAC Int., Assoc. Off. Anal. Chem, Arlington, VA. [Google Scholar]
- Baker D. H. 2009. Advances in protein-amino acid nutrition of poultry. Amino Acids. 37:29–41. doi:10.1007/s00726-008-0198-3 [DOI] [PubMed] [Google Scholar]
- Balsom P. D., Söderlund K., and Ekblom B.. 1994. Creatine in humans with special reference to creatine supplementation. Sports Med. 18:268–280. doi:10.2165/00007256-199418040-00005 [DOI] [PubMed] [Google Scholar]
- Berg E. P., and Allee G. L.. 2001. Creatine monohydrate supplemented in swine finishing diets and fresh pork quality: i. a controlled laboratory experiment. J. Anim. Sci. 79:3075–3080. doi:10.2527/2001.79123075x [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Wijekoon E. P., Warford-Woolgar L., Trottier N. L., Brosnan M. E., Brunton J. A., and Bertolo R. F.. 2009. Creatine synthesis is a major metabolic process in neonatal piglets and has important implications for amino acid metabolism and methyl balance. J. Nutr. 139:1292–1297. doi:10.3945/jn.109.105411 [DOI] [PubMed] [Google Scholar]
- Choi Y. I., Kim Y. T., Lee C. L., and Han I. K.. 2000. Carcass and pork quality characteristics by sex and marketing day. J. Anim. Sci. Technol. (Kor.) 42:933–940 [Google Scholar]
- Dilger R. N., Bryant-Angeloni K., Payne R. L., Lemme A., and Parsons C. M.. 2013. Dietary guanidino acetic acid is an efficacious replacement for arginine for young chicks. Poult. Sci. 92:171–177. doi:10.3382/ps.2012-02425 [DOI] [PubMed] [Google Scholar]
- Ellis M., Webb A. J., Avery P. J., and Brown I.. 1996. The influence of terminal sire genotype, sex, slaughter weight, feeding regime and slaughter-house on growth performance and carcass and meat quality in pigs and on the organoleptic properties of fresh pork. Anim. Sci. 62:521–530. [Google Scholar]
- Heger J., Zelenka J., Machander V., de la Cruz C., Lestak M., and Hampel D.. 2014. Effects of guanidinoacetc acid supplementation to the broiler diets with varying energy content. Acta Universatatis Agriculturae et Silviculturae Mendelianae Brunensis. 62:477–485. doi:10.11118/actaun201462030477 [Google Scholar]
- Honikel K. O. 1998. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49:447–457. doi:10.1016/S0309-1740(98)00034-5 [DOI] [PubMed] [Google Scholar]
- Janicki B., and Buzala M.. 2013. The role of creatine in the organism of pigs and its effect on the quality of pork: a review. Ann. Anim. Sci. 13:207–215. doi:10.2478/aoas-2013-0003 [Google Scholar]
- Larzul C., Lefaucheur L., Ecolan P., Gogué J., Talmant A., Sellier P., Le Roy P., and Monin G.. 1997. Phenotypic and genetic parameters for longissimus muscle fiber characteristics in relation to growth, carcass, and meat quality traits in large white pigs. J. Anim. Sci. 75:3126–3137. doi:10.2527/1997.75123126x [DOI] [PubMed] [Google Scholar]
- Latorre M. A., García-Belenguer E., and Ariño L.. 2008. The effects of sex and slaughter weight on growth performance and carcass traits of pigs intended for dry-cured ham from teruel (Spain). j. Anim. Sci. 86:1933–1942. doi:10.2527/jas.2007-0764 [DOI] [PubMed] [Google Scholar]
- Lemme A., Gobbi R., Helmbrecht A., Van Der Klis J. D., Firman J., Jankowski J., and Kozlowsk K.. 2010. Use of guanidinoacetic acid in all-vegetable diets for turkeys. In: Proc. 4th Turkey Sci. Prod. Conf; March 11 to 12, 2010; Macclesfield, UK Turkeytimes, Tarporley, Cheshire, UK: p. 57–61. [Google Scholar]
- Lemme A., Ringel J., Sterk A., and Young J. F.. 2007. Supplemental guanidinoacetic acid affects energy metabolism of broilers. In: Proceedings of the 16th European Symposium on Poultry Nutrition World’s Poultry Sci. Assoc; August 26 to 30, 2007; Strasbourg, France: p. 26–30. [Google Scholar]
- Liu Y., Li J. L., Li Y. J., Gao T., Zhang L., Gao F., and Zhou G. H.. 2015. Effects of dietary supplementation of guanidinoacetic acid and combination of guanidinoacetic acid and betaine on postmortem glycolysis and meat quality of finishing pigs. Anim. Feed. Sci. Tech. 205:82–89. doi:10.1016/j.anifeedsci.2015.03.010 [Google Scholar]
- Maddock R. T., Bidner B. J., Carr S. N., McKeith F. K., Berg E. P., and Savell J. W.. 2002. Creatine monohydrate supplementation and the quality of fresh pork in normal and halothane carrier pigs. J. Anim. Sci. 80:997–1004. http://jas.fass.org/content/80/4/997 [DOI] [PubMed] [Google Scholar]
- Michiels J., Maertens L., Buyse J., Lemme A., Rademacher M., Dierick N. A., and De Smet S.. 2012. Supplementation of guanidinoacetic acid to broiler diets: effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult. Sci. 91:402–412. doi:10.3382/ps.2011-01585 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Piao J. R., Tian J. Z., Kim B. G., Choi Y. I., Kim Y. Y., and Han I. K.. 2004. Effects of sex and market weight on growth performance, carcass characteristics, and pork quality of market hogs. Asian-Australas. J. Anim. Sci. 10:1452–1458. [Google Scholar]
- Ringel J., Lemme A., Redshaw M. S., and Damme K.. 2008. The effects of supplemental guanidino acetic acid as a precursor of creatine in vegetable broiler diets on performance and carcass parameters. Poult. Sci. 87 (Suppl. 1):72 (Abstr.). [Google Scholar]
- Tossenberger J., Rademacher M., Németh K., Halas V., and Lemme A.. 2016. Digestibility and metabolism of dietary guanidino acetic acid fed to broilers. Poult. Sci. 95:2058–2067. doi:10.3382/ps/pew083 [DOI] [PubMed] [Google Scholar]
- USDA ARS 2014. USDA table of cooking yields for meat and poultry, release 2 Nutrient data [accessed February 15, 2015]. http://www.ars.usda.gov/nutrientdata
- Young J. F., Bertram H. C., Rosenvold K., Lindahl G., and Oksbjerg N.. 2005. Dietary creatine monohydrate affects quality attributes of duroc but not landrace pork. Meat Sci. 70:717–725. doi:10.1016/j.meatsci.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Young J. F., Bertram H. C., Theil P. K., Petersen A. G., Poulsen K. A., Rasmussen M., Malmendal A., Nielsen N. C., Vestergaard M., and Oksbjerg N.. 2007. In vitro and in vivo studies of creatine monohydrate supplementation to duroc and landrace pigs. Meat Sci. 76:342–351. doi:10.1016/j.meatsci.2006.11.015 [DOI] [PubMed] [Google Scholar]
- Wang L. S., Shi B. M., Shan A. S., and Shang Y. Y.. 2012. Effects of guanidinoacetic acid on growth performance, meat quality and antioxidation in growing-finishing pigs. J. Anim. Vet. Adv. 11:631–636. doi:10.3923/javaa.2012.631.636 [Google Scholar]
- Wyss M., and Kaddurah-Daouk R.. 2000. Creatine and creatinine metabolism. Physiol. Rev. 80:1107–1213. doi:10.1152/physrev.2000.80.3.1107 [DOI] [PubMed] [Google Scholar]