Abstract
The use of corn distillers dried grains with solubles (DDGS) in pig diets is limited due to high fiber concentration. Steeping with exogenous fiber-degrading enzymes (FDE) may improve their feeding value. We evaluated apparent ileal digestibility (AID), standardized ileal digestibility (SID), and apparent total tract digestibility (ATTD) of components and DE content in DDGS steeped without or with two commercial FDE (A and B). Mixture of 350 g of DDGS, FDE (none for control), and 1.5 liters of water was incubated at 40 °C for 24 h with 15 min agitation every 40 min. FDE-A (pure combination) supplied 5,500 U of xylanase and 1,050 U of β-glucanase while FDE-B (multienzyme complex) supplied 1,200 U of xylanase, 150 U of β-glucanase, 500 U of cellulase, and 5,000 U of protease per kg of DDGS plus side activities. Samples were taken at time 0, 4, 8, and 24 h for organic acids and pH measurements. Three semi-purified corn starch–based diets were formulated with steeped DDGS as the sole source of CP. The basal mixture contained 0.2% TiO2 as indigestible marker. Six ileal-cannulated pigs (20 kg BW) were fed the three diets in a replicated 3 × 3 Latin square design to give six replicates per diet. Pigs were fed at 2.8× maintenance energy requirements and had free access to water. In each period, pigs were adjusted to diets for 7 d followed by 2 d for grab fecal and 2 d of 8 h continuous ileal digesta collection. There were no (P > 0.05) treatment and sampling time interaction or treatment effects on pH and lactic concentration. Lactic and acetic acids increased, and pH decreased (P < 0.05) over time points. The AID of CP, NDF, and crude fat and SID of CP were not different (P > 0.05) among treatments. Steeping DDGS with FDE-A had lower (P = 0.01) ATTD of NDF than control but higher (P = 0.001) ATTD of crude fat compared with the control or DDGS steeped with FDE-B. Values for DE content in steeped DDGS were not different (P > 0.05) and amounted to 4,095, 4,039, and 3,974 kcal/kg DM for the control, FDE-A, and FDE-B, respectively. In conclusion, under conditions of the study, steeping DDGS with exogenous enzymes did not improve fiber and energy digestibility.
Keywords: corn DDGS, digestibility, fiber, exogenous enzymes, steeping, pigs
INTRODUCTION
Research on the feeding value of corn distiller’s dried grain with solubles (DDGS) which began more than 50 yr ago has commoditized it’s use in swine feed programs (NRC., 2012). However, utilization of DDGS is limited by high concentration of fiber which negatively influences feed intake, nutrients utilization, health, and metabolic processes (Jha and Leterme, 2012; Pedersen et al., 2014). The use of exogenous fiber-degrading enzymes (FDE) in enhancing nutrient and energy utilization in feedstuffs is well documented (Bedford and Schulze, 1998; Adeola and Cowieson, 2011). For example, supplemental FDE enhanced utilization of nutrient and growth performance in complete rations containing cereal coproducts (Agyekum et al., 2016; Kiarie et al., 2016b; Tsai et al., 2017). However, contradicting results have also been reported in multiple studies evaluating FDE (e.g., Diebold et al., 2004; Jacela et al., 2010; Kerr and Shurson, 2013). Therefore, much progress is warranted to achieve consistent efficacy in terms of utilization of fibrous cereal coproducts (Kiarie et al., 2016a; Huntley and Patience, 2018).
Steeping feedstuffs can promote hydrolysis of fiber and improve utilization of fibrous feedstuff (de Lange and Zhu, 2012; Jakobsen et al., 2015). Since FDE are not activated until they reach a liquid medium, a combination of steeping and FDE may have a synergistic effect in improving feeding value of fibrous feedstuffs (Svihus, 2010). Application of FDE in combination with steeping to improve utilization of corn DDGS in pigs has been reported (e.g., Jakobsen et al., 2015; Wiseman et al., 2017). In our previous study, improved feed efficiency was observed when pigs were fed liquid-fermented DDGS treated with xylanase and β-glucanase (Rho et al., 2017a). However, it is unknown whether improved efficiency was due to improved nutrient absorption in the small intestine or hindgut fermentation. Therefore, the aim of the current study was to investigate the effects of steeping corn DDGS with FDE on apparent ileal digestibility (AID), standardized ileal digestibility (SID), apparent total tract digestibility (ATTD) of components, and DE content in growing pigs.
MATERIAL AND METHODS
Animal care and use protocols were approved by the University of Guelph Animal Care and Use Committee, and pigs were cared for in accordance with the Canadian Council on Animal Care guidelines (CCAC, 2009).
Animals
Six barrows (Yorkshire × Landrace ♀ × Duroc ♂, 20 kg BW) were procured from the University of Guelph’s Arkell Swine Research Station (Guelph, ON, Canada). Barrows were individually housed in plexiglass-lined pens with tenderfoot floors in a temperature-controlled room (20–22 °C) for 7 d prior to surgery. Pigs were surgically fitted with a simple T-cannula at the distal ileum (de Lange et al., 1998). After the surgery, a week of post-surgical recovery period was given. The surgical area was cleaned each day with warm water and fully dried. To prevent skin rashes, baby powder and zinc oxide cream was applied on daily basis.
Preparation of Steeped DDGS and Diets
The chemical composition of the conventional DDGS used in the current study was reported previously (Rho et al., 2017b). The preparation and conditions of steeping DDGS was as described by Wiseman (2016). Briefly, DDGS sample was mixed with water (350 g of DDGS and 1.5 liters of water to achieve 16% DM) with or without FDE. The DDGS was used without additional grinding. The mixture was incubated at 40 °C with 15 min of agitation every 40 min for 24 h. The FDE were commercial preparations: 1) FDE-A (Axtra XB, Danisco Animal Nutrition-DuPont Industrial Biosciences, Marlborough, UK) and FDE-B (Superzyme, Canadian Bio-Systems, Calgary, Canada). The targeted activities for FDE-A were 5,500 U of xylanase and 1,050 U of β-glucanase/kg of DDGS. These activities were based on a preliminary in-vitro evaluation that showed this combination had the greatest reduction of pH after 24-h steeping (Rho et al., 2017a). The main target activities for FDE-B (a multienzyme complex) were 1,200, 150, 500, and 5,000 U/kg of DDGS for xylanase, β-glucanase, cellulose, and protease, respectively, plus other-side activities. These activities were based on provider recommendation and test information was not provided.
Fresh DDGS were prepared daily in clean and sterile containers. In each experimental period (explained below), subsamples of steeped DDGS were taken on three random days at time points 0, 4, 8, 24 h for organic acids (lactic and acetic acids) and pH determinations. Containers were vigorously shaken before taking samples. Subsamples were taken in a 10 mL centrifuge tubes, pH read and centrifuged at 30,000 × 10 g for 10 min at room temperature (Wiseman et al., 2017). The supernatant was transferred over to a 2-mL microcentrifuge tube and was kept frozen until organic acids analyses.
Three semi-purified corn starch–based diets were formulated (Table 1) with steeped DDGS as the sole source of CP. The target concentration of CP was 18% based on previous study (Rho et al., 2017b). The inclusion level of steeped DDGS was maintained at 65% to match the inclusion of unsteeped DDGS sample fed in our previous study (Rho et al., 2017b). The basal mixture contained corn starch, minerals, vitamins, and 0.2% TiO2 as indigestible marker. The cornstarch:sucrose:oil ratio was kept constant across diets to allow for calculation of DE content of DDGS by the difference method based using N-free diet estimates reported in our previous study (Rho et al., 2017b). Steeped DDGS was mixed with corn starch basal immediately before feeding.
Table 1.
Diet composition and analyzed nutrient content of complete diet containing steeped DDGS1
| Item | Control2 | FDE-A3 | FDE-B4 |
|---|---|---|---|
| Ingredient, % as fed | |||
| Steeped corn DDGS | 65.0 | 65.0 | 65.0 |
| Corn starch | 28.9 | 28.9 | 28.9 |
| Sucrose | 2.06 | 2.06 | 2.06 |
| Cellulose | 0.69 | 0.69 | 0.69 |
| Corn oil | 0.69 | 0.69 | 0.69 |
| Limestone | 1.35 | 1.35 | 1.35 |
| Mono-calcium phosphate | 0.20 | 0.20 | 0.20 |
| Salt | 0.30 | 0.30 | 0.30 |
| Vitamin and mineral premix5 | 0.60 | 0.60 | 0.60 |
| Titanium dioxide | 0.20 | 0.20 | 0.20 |
| Analyzed | |||
| DM, % DM | 91.0 | 90.9 | 91.2 |
| CP, % DM | 18.7 | 18.5 | 17.1 |
| Ash, % DM | 6.17 | 6.41 | 6.10 |
| Crude fat, % DM | 6.42 | 6.89 | 6.23 |
| NDF, % DM | 24.2 | 22.5 | 23.1 |
| ADF, % DM | 7.10 | 6.86 | 7.07 |
| GE, kcal/kg | 4,434 | 4,411 | 4,377 |
| Calcium, % DM | 0.51 | 0.51 | 0.51 |
| Phosphorus, % DM | 0.60 | 0.60 | 0.60 |
| Sodium, % DM | 0.34 | 0.34 | 0.34 |
DDGS, distillers dried grains with solubles; FDE, fiber-degrading enzymes.
1Steeped (350 g of DDGS and 1.5 liters of water) for 24 h at 40 °C with 15 min of agitation every 40 min. Mixed with other feedstuffs prior to feeding.
2No enzymes.
3Supplied 5,500 U of xylanase and 1,050 U of β-glucanase per kg of DDGS.
4Supplied 1,200 U of xylanase, 150 U of β-glucanase, 500 U of cellulase, and 5,000 U of protease per kg of DDGS.
5Provided per kilogram of diet: vitamin A, 12,000 IU as retinyl acetate; vitamin D3, 1,200 IU as cholecalciferol; vitamin E, 48 IU as dl-α-tocopherol acetate; vitamin K, 3 mg as menadione; pantothenic acid, 18 mg; riboflavin, 6 mg; choline, 600 mg; folic acid, 2.4 mg; niacin, 30 mg; thiamine, 18 mg; pyridoxine, 1.8 mg; vitamin B12, 0.03 mg; biotin, 0.24 mg; Cu, 18 mg from CuSO4 × 5H2O; Fe, 120 mg from FeSO4; Mn, 24 mg from MnSO4; Zn, 126 mg from ZnO; Se, 0.36 mg from Na2SeO3; and I, 0.6 mg from KI (DSM Nutritional Products Canada Inc., Ayr, ON, Canada).
This process was repeated on daily basis with fresh steeped DDGS from previous 24 h. Steeped DDGS prior to mixing and complete feed samples were taken twice in each experimental period and stored frozen for further analyses.
Experimental Procedures
Pigs were fed 2.8 times maintenance energy requirements (NRC, 2012) in two equal meals daily at 0800 and 1600 h and there were no feed refusals. Water was allowed ad libitum throughout the trial. The experiment was designed as repeated 3 × 3 Latin square design (n = 6). Each period lasted for 11 d with 7 d for adaptation followed by 2 d of fresh grab fecal collection and 2 d continuous 8 h ileal digesta collection. For ileal digesta collection, plastic bags were filled with 10 mL 10% formic; the bags were replaced as needed. Digesta samples were kept in the refrigerator during the collection and was pooled at the end of the day and stored at −20 °C. At the end of the study, the pigs were euthanized to examine any intestinal abnormalities.
Laboratory Analyses
Complete feed samples and steeped DDGS samples were pooled and subsamples taken for chemical analyses. The complete feed, steeped DDGS, ileal and fecal samples were freeze dried and finely ground. Samples were analyzed for DM, Ash, N, GE, crude fat, NDF, and ADF. Titanium concentration was analyzed on complete feed, ileal and fecal samples. Complete diets were assayed for Ca (AOAC 985.01), P (AOAC 985.01), and Na (AOAC 969.10) in a commercial laboratory (Agri-Food Laboratories; Guelph, ON, Canada).
DM was determined using method 930.15 (AOAC, 2004) and ash content was determined according to method 942.05 (AOAC, 2004). Nitrogen was determined with a CNS-2000 carbon, N, and sulfur analyzer (Leco Corporation, St. Joseph, MI) according to the combustion method 968.06 (AOAC, 2004). The CP values were calculated by multiplying analyzed N values by 6.25. GE was determined using a bomb calorimeter (IKA Calorimeter System C 5000; IKA Works, Wilmington, NC). Using the Amkom 200 Fiber Analyzer (Ankom Technology, Fairport, NY), NDF and ADF concentrations were determined according to Van Soest et al. (1991). Titanium concentration was measured using the method by Myers et al. (2004). Crude fat content was determined using ANKOM XT 20 Extractor (Ankom Technology).
Organic acids’ (lactic and acetic) concentrations were analyzed by HPLC (Agilent 1100 Series, Agilent Technologies, Santa Clara, CA) as described by Leung et al. (2018). The pH was measured with Fisher Scientific Accumet AB 150 pH meter (Fisher Scientific, Toronto, CA) standardized with certified pH 4 and 7 buffer solutions.
Calculations and Statistical Analysis
The AID and ATTD of components according to Adeola (2001) and the SID of CP was calculated using endogenous N loss values in our previous study (Rho et al., 2017b). The DE content in DDGS samples was calculated using the difference method (Adeola, 2001) using DE values of N-free (corn starch, soy oil, and sucrose) diet from our previous study (Rho et al., 2017b). Data were analyzed using MIXED procedure of SAS (SAS Inst. Inc., Cary, NC). For the pH and organic acids in steeped DDGS, treatment, sampling time, and their interactions were fixed effects. For digestibility data, treatment was considered fixed effect and period was random effect. An alpha level of 0.05 was used to determine statistical significance and means separated using Tukey’s test.
RESULTS AND DISCUSSION
Analyzed chemical composition of steeped DDGS samples are shown in Table 2 and experimental diets are shown in Table 1. Steeped DDGS with (without) FDE had comparable chemical composition (Table 2). The concentration of CP, crude fat, NDF, and ADF in unsteeped DDGS sample was 27.7, 8.57, 28.9, and 11.9% on as-fed basis, respectively (Rho et al., 2017b). Unsteeped DDGS had comparable concentration of CP, NDF, and crude fat to steeped DDGS samples except ADF concentration that was more than 2% unit higher in unsteeped DDGS sample. The explanation for this may be that fermentable fiber were utilized by natural microbial population in DDGS during the 24-h steeping, and the fiber remaining in poststeeping are the insoluble fibers (Jakobsen et al., 2015).
Table 2.
Analyzed chemical composition of steeped DDGS, as-fed basis
| Ingredients | Unsteeped | Steeped DDGS1 | ||
|---|---|---|---|---|
| DDGS2 | Control3 | FDE-A4 | FDE-B5 | |
| DM, % | 92.1 | 96.6 | 96.0 | 96.5 |
| CP, % | 27.7 | 27.1 | 27.0 | 27.2 |
| Ash, % | 4.53 | 6.63 | 6.41 | 6.80 |
| Crude fat, % | 8.57 | 9.30 | 10.2 | 9.60 |
| NDF, % | 28.9 | 32.0 | 30.7 | 32.4 |
| ADF, % | 11.9 | 10.2 | 10.0 | 10.5 |
| GE, kcal/kg | 4,665 | 4,869 | 4,906 | 4,981 |
DDGS, distillers dried grains with solubles; FDE, fiber-degrading enzymes.
1Corn DDGS steeped (350 g of DDGS and 1.5 liters of water) for 24 h at 40 °C with 15 min of agitation every 40 min. Values represent freeze dried samples.
2Adapted from Rho et al. (2017b).
3No enzymes.
4Supplied 5,500 U of xylanase and 1,050 U of β-glucanase per kg of DDGS.
5Supplied 1,200 U of xylanase, 150 U of β-glucanase, 500 U of cellulase, and 5,000 U of protease per kg of DDGS.
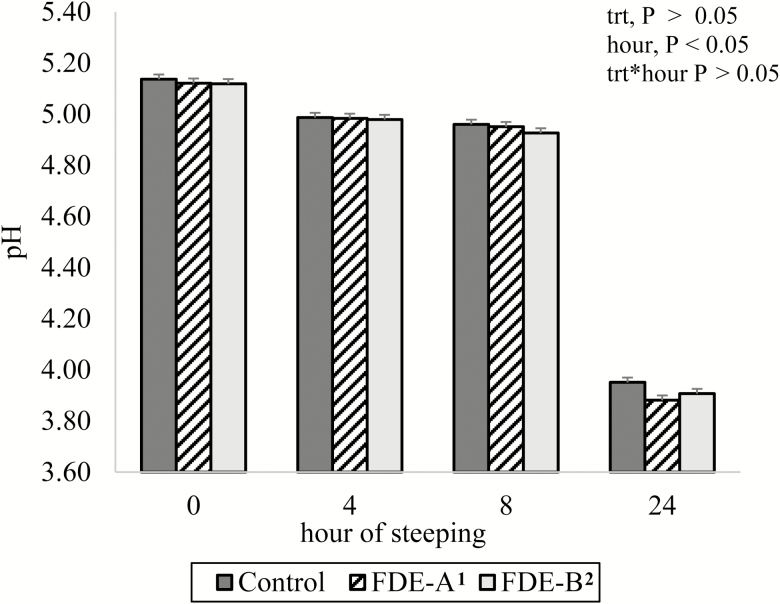
The optimum pH level for liquid feed preparation is suggested to be below 4.5 as low pH environment can prevent pathogens and unwanted organisms (van Winsen et al., 2001). The pH of the three steeped DDGS is shown in Fig. 1. There was neither DDGS treatment effect nor interactive effect of DDGS treatment and sampling time (P > 0.05). A likely explanation for these observations may be due to spontaneous activation of naturally occurring enzymes as other studies have shown reduced pH below 4.5 when liquid feeds were fermented with (without) inoculants (Mikkelsen and Jensen, 2000; Lawlor et al., 2002). However, a time effect (P < 0.05) was observed such that pH values declined over time points. The pH at time 0, 4, 8, 24 h were 5.12, 4.98, 4.93, 3.91, respectively (Fig. 1). After 24 h of steeping all three steeped DDGS reached a pH level below 4.5 which is considered an optimum pH in liquid feed.
Figure 1.
Effects of steeping corn DDGS with (without) fiber-degrading enzyme (FDE) on pH of supernatant sampled at 0, 4, 8 and 24 h. Steeped (350 g of DDGS and 1.5 liters of water) for 24 h at 40 °C with 15 min of agitation every 40 min. 1Control, no enzymes. 2FDE-A, supplied 5,500 U of xylanase and 1,050 U of β-glucanase per kg of DDGS. 3FDE-B, supplied 1,200 U of xylanase, 150 U of β-glucanase, 500 U of cellulase, and 5,000 U of protease per kg of DDGS.
Lactic acid was neither affected by interaction between DDGS treatment and sampling time nor DDGS treatment (P = 0.60). However, a sampling time effect was observed (P < 0.05) as time point 0 and 24 h was different (Fig. 2). For acetic acid, there was an interactive effect of DDGS treatment and sampling time (P = 0.04). At 0, 4, and 24 h, acetic acid concentrations were not different (P > 0.05); however, at 8 h, control had higher acetic acid concentration (P < 0.05) than the FDE treatments, whereas FDE treatments were not different (Fig. 3). The reason for the high acetic acid concentration in the control was not clear, however, given it was only observed in the control, it is plausible FDE may have influenced microbial activity at this time point (Canibe et al., 2007).
Figure 2.
Effects of steeping corn DDGS with (without) fiber-degrading enzyme (FDE) on concentration of lactic acid in the supernatant sampled at 0, 4, 8 and 24 h. Steeped (350 g of DDGS and 1.5 liters of water) for 24 h at 40 °C with 15 min of agitation every 40 min. 1Control, no enzymes. 2FDE-A, supplied 5,500 U of xylanase and 1,050 U of β-glucanase per kg of DDGS. 3FDE-B, supplied 1,200 U of xylanase, 150 U of β-glucanase, 500 U of cellulase, and 5,000 U of protease per kg of DDGS.
Figure 3.
Effects of steeping corn DDGS with (without) fiber-degrading enzyme (FDE) on concentration of acetic acid in the supernatant sampled at 0, 4, 8 and 24 h. Means at time point with different superscripts are different at P < 0.05. Steeped (350 g of DDGS and 1.5 liters of water) for 24 h at 40 °C with 15 min of agitation every 40 min. 1Control, no enzymes. 2FDE-A, supplied 5,500 U of xylanase and 1,050 U of β-glucanase per kg of DDGS. 3FDE-B, supplied 1,200 U of xylanase, 150 U of β-glucanase, 500 U of cellulase, and 5,000 U of protease per kg of DDGS.
The lactic acid concentration increased from 73.5 to 97.5 mM at 24 h and corresponding values for acetic acid concentration were from 16.9 to 20.0 mM. The changes in organic acids in the current study agrees with Wiseman (2016) who fermented corn DDGS up to 6 d with enzymes and silage inoculant at 40 °C. Three parameters are critical in terms of stability and quality of steeping and liquid fermentation (Wiseman, 2016). The pH level below 4.5 to prevent pathogens and unwanted organisms (van Winsen et al., 2001), lactic acid concentrations above 100 mM to reduce Salmonella and Escherichia coli (Brooks et al., 2003), and acetic acid concentrations below 40 mM as acetic acid has a distinct vinegar taste and smell at high concentrations which can reduce the palatability of the feed (Beal et al., 2005). In the current study, lactic acid concentration approached 100 nM and acetic acid was below the 40 mM indicating steeped DDGS were within the parameters. It is noteworthy that recommended lactic acid and acetic acid concentrations vary. For example, suggested lactic acid concentrations ranges from 75, 100 to 150 mmol/L (van Winsen et al., 2001; Beal et al., 2002; Brooks et al., 2003) and acetic acid concentrations ranges from 30 to 40 mmol/L (van Winsen et al., 2001; Brooks et al., 2003; Missotten et al., 2010). Therefore, it is not surprising that a variety of studies have concluded with different criteria for an optimal fermentation.
There were no differences in AID of DM, OM, NDF, ADF, crude fat, and SID of CP across dietary treatments (P > 0.05; Table 3). The values for the SID of CP in steeped DDGS without FDE (67.0%) and with FDE-A (66.7%) were comparable to SID of CP (66.8%) in unsteeped DDGS we reported previously (Rho et al., 2017b). However, the SID of steeped DDGS with FDE-B (64.2%) was numerically lower than unsteeped DDGS. Our previous study showed improved FCR in the first phase after feeding complete corn-soybean meal–based diet containing 30% corn DDGS steeped with xylanase and β-glucanase (Rho et al., 2017a). Tsai et al. (2017) found improvements in ADG and digestibility when pigs were fed dry diets containing 30% corn DDGS with added xylanase or/and β-glucanase. Moran et al. (2016) found improved AID of NDF and GE when corn DDGS was steeped with xylanase. Therefore, it is unclear why we did not see the effects of exogenous enzymes on ileal digestibility of components in the current study. However, unlike the current study, these studies used practical diets containing other feedstuffs (soybean meal, wheat, etc.) which could have impacted on overall digestibility.
Table 3.
Apparent ileal digestibility (AID) of components in experimental diets and standardized ileal digestibility (SID) of crude protein in steeped DDGS fed to growing pigs1
| Item | Control2 | FDE-A3 | FDE-B4 | SEM | P value |
|---|---|---|---|---|---|
| AID, % | |||||
| DM | 59.0 | 58.9 | 60.5 | 2.2 | 0.794 |
| OM | 61.0 | 60.8 | 62.9 | 2.1 | 0.685 |
| NDF | 26.5 | 24.7 | 28.9 | 5.8 | 0.847 |
| ADF | 17.4 | 19.2 | 21.4 | 6.7 | 0.900 |
| Crude fat | 66.1 | 66.2 | 67.1 | 2.5 | 0.945 |
| CP | 59.9 | 59.4 | 56.3 | 2.7 | 0.450 |
| SID, % | |||||
| CP, % | 67.0 | 66.7 | 64.2 | 2.69 | 0.588 |
DDGS, distillers dried grains with solubles; FDE, fiber-degrading enzymes.
1Steeped (350 g of DDGS and 1.5 liters of water) for 24 h at 40 °C with 15 min of agitation every 40 min. Mixed with other feedstuffs prior to feeding.
2No enzymes.
3Supplied 5,500 U of xylanase and 1,050 U of β-glucanase per kg of DDGS.
4Supplied 1,200 U of xylanase, 150 U of β-glucanase, 500 U of cellulase, and 5,000 U of protease per kg of DDGS.
ATTD of DM, OM, ADF, CP, and GE were not influenced by treatments (P > 0.05) (Table 4). In contrast, Villca et al. (2016) observed improved ATTD of DM, NDF, ADF, CP, fat, and GE when a multicarbohydrase mix was included in rye-based liquid feed fed to grower/finisher pigs. The ATTD value for crude fat was greater (P = 0.001) for FDE-A than in the other dietary treatments. However, pigs fed control diet had higher (P < 0.05) ATTD of NDF (55.3%) compared with pigs fed FDE-A (46.7%). Fiber digestibility (NDF and ADF) was numerically higher in steeped DDGS in the current study than the unsteeped DDGS sample which had ATTD of NDF and ADF amounting to 39.3% and 57.6%, respectively (Rho et al., 2017b). Moran et al. (2016) supplemented xylanase in dry or 24-h steeped corn DDGS and did not observe any improvements on ATTD of NDF in growing pigs. Interestingly and in agreement with the current study, steeping wheat middling with xylanase reduced ATTD of NDF (Moran et al., 2016). In the current study, the control DDGS had slightly higher NDF concentration (24.2% vs. 22.5%) than DDGS steeped with FDE-A (Table 1). In this context, it is likely that adding FDE-A during steeping may have led to conversion of some fiber fractions to simple sugars leaving mainly indigestible fiber fractions (Jørgensen et al., 2010). The steeped DDGS DE values were 4,095, 4,039, and 3,974 kcal/kg DM for control, FDE-A, and FDE-B, respectively, which were higher than the DE of unsteeped DDGS (3,614 kcal/kg, DM) (Rho et al., 2017b). This indicated that just steeping corn DDGS improves digestibility of energy as has been observed in other studies (de Lange and Zhu, 2012; Jakobsen et al., 2015).
Table 4.
Apparent total tract digestibility (ATTD) of components in experimental diets and DE content in steeped DDGS fed to growing pigs1
| Item | Control2 | FDE-A3 | FDE-B4 | SEM | P value |
|---|---|---|---|---|---|
| ATTD, % | |||||
| DM | 76.2 | 74.8 | 76.0 | 1.14 | 0.109 |
| OM | 77.6 | 76.3 | 77.4 | 1.17 | 0.132 |
| NDF | 55.3a | 46.7b | 50.7ab | 1.94 | 0.005 |
| ADF | 59.3 | 54.5 | 58.6 | 2.56 | 0.065 |
| Crude fat | 46.8b | 56.4a | 46.4b | 2.44 | 0.001 |
| CP | 73.3 | 71.8 | 70.7 | 2.15 | 0.109 |
| GE | 74.8 | 74.1 | 73.8 | 1.17 | 0.376 |
| Energy5 | |||||
| ATTD, % | 84.0 | 83.2 | 82.8 | 0.98 | 0.680 |
| DE, as-fed basis, kcal/kg | 3,728 | 3,665 | 3,647 | 60.2 | 0.200 |
| DE, DM basis, kcal/kg | 4,095 | 4,039 | 3,974 | 47.7 | 0.230 |
Means within a row with different superscripts are different at P < 0.05. DDGS, distillers dried grains with solubles; FDE, fiber-degrading enzymes.
1Steeped (350 g of DDGS and 1.5 liters of water) for 24 h at 40 °C with 15 min of agitation every 40 min. Mixed with other feedstuffs prior to feeding.
2No enzymes.
3Supplied 5,500 U of xylanase and 1,050 U of β-glucanase per kg of DDGS.
4Supplied 1,200 U of xylanase, 150 U of β-glucanase, 500 U of cellulase, and 5,000 U of protease per kg of DDGS.
5Calculated by difference method (Adeola, 2001).
FDE hydrolyze soluble fiber much easier than insoluble fiber (Urriola et al., 2014; Kiarie et al., 2016a). The high concentration of insoluble vs. soluble fiber is the main challenge of improving utilization of fibrous feedstuff in monogastric animals. In corn DDGS, insoluble fiber can be as high as 31%, whereas soluble fiber content is typically less than 2% (Widyaratne and Zijlstra, 2007). The insoluble DF fractions are much more hydrophobic and crystalline than soluble fiber, and recalcitrant to microbial fermentation (Bach Knudsen, 2011; Knudsen, 2014). Perhaps, the enzymes used in the current study may have not been efficient in hydrolyzing insoluble fiber or the 24 h or steeping was not enough time for the enzymes to degrade non-starch polysaccharide (NSP) as Sørensen et al. (2007) observed NSP degradation continuing after 24-h steeping. The enzymes activities in the mixtures were not measured before and after steeping. Moreover, studies have found inconsistent results when utilizing exogenous enzymes to improve breakdown of indigestible dietary components. The reason for this is partly due to complexity of substrates in feedstuffs and mismatch of activities (Slominski, 2000). Some results have shown that supplementing enzymes in corn-soybean–based diets can improve growth performance and nutrient digestibility in pigs (Omogbenigun et al., 2004; Ji et al., 2008; Jo et al., 2012; Kiarie et al., 2016b). Other studies have shown positive results on pig performance fed corn DDGS pretreated with fiber-degrading enzymes and/or microbial inoculants (Zhu et al., 2011; Jakobsen et al., 2015; Rho et al., 2017a). However, inconsistent results have also been reported in pigs fed diets containing corn DDGS pretreated with enzymes in a liquid environment (Jones et al., 2010; Moran et al., 2016). This may be influenced by the characteristics of arabinoxylans in corn which are largely insoluble (Izydorczyk and Biliaderis, 1995). An in-vitro digestion and fermentation study showed that corn DDGS cell wall structures were barely affected by different processing technologies (unprocessed, wet-milling, extrusion, autoclaving, and hydrothermal acid treatment) and enzymes (de Vries et al., 2013). The complex structure of corn fiber may prevent or delay enzymes to access their targeted backbones (de Vries et al., 2013; Moran et al., 2016). In addition, use of fiber-degrading enzyme in ethanol fermentation processes may reduce easily accessible fiber fractions in DDGS (Pedersen et al., 2014).
In conclusion, steeping corn DDGS with two commercially available fiber-degrading enzymes for 24 h did not improve ileal digestibility of nutrients in pigs. However, steeping DDGS with FDE-A improved apparent total digestibility of crude fat and decreased the ATTD of NDF compared to steeping without exogenous enzyme.
Footnotes
Presented in part at the 2017 ASAS-CSAS Annual Meeting and Trade Show, Baltimore, MD, July 8–11.
LITERATURE CITED
- Adeola O. 2001. Digestion and balance techniques in pigs. In: Lewis A. J., and L. L. Southern, editors. Swine nutrition. 2nd ed; p. 903–916. [Google Scholar]
- Adeola O., and Cowieson A. J.. 2011. Board-Invited Review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 89:3189–3218. doi:10.2527/jas.2010–3715 [DOI] [PubMed] [Google Scholar]
- Agyekum A. K., Regassa A., Kiarie E., and Nyachoti C. M.. 2016. Nutrient digestibility, digesta volatile fatty acids, and intestinal bacterial profile in growing pigs fed a distillers dried grains with solubles containing diet supplemented with a multi-enzyme cocktail. Anim. Feed Sci. Technol. 212:70–80. doi:10.1016/j.anifeedsci.2015.12.006 [Google Scholar]
- AOAC.. 2004. Official methods of analysis AOAC offical methods. AOAC International, Gaithersburg, MD. [Google Scholar]
- Bach Knudsen K. E. 2011. Triennial growth symposium: effects of polymeric carbohydrates on growth and development in pigs. J. Anim. Sci. 89:1965–1980. doi:10.2527/jas.2010–3602 [DOI] [PubMed] [Google Scholar]
- Beal J. D., Niven S. J., Campbell A., and Brooks P. H.. 2002. The effect of temperature on the growth and persistence of Salmonella in fermented liquid pig feed. Int. J. Food. Microbiol. 79:99–104. doi:10.1016/S0168-1605(02)00183-6 [DOI] [PubMed] [Google Scholar]
- Beal J. D., Niven S. J., Brooks P. H., and Gill B. P.. 2005. Variation in short chain fatty acid and ethanol concentration resulting from the natural fermentation of wheat and barley for inclusion in liquid diets for pigs. J. Sci. Food Agric. 85:433–440. doi:10.1002/jsfa.2013 [Google Scholar]
- Bedford M. R. and Schulze H.. 1998. Exogenous enzymes for pigs and poultry. Nutr. Res. Rev. 11:91–114. doi:10.1079/NRR19980007 [DOI] [PubMed] [Google Scholar]
- Brooks P. H., Beal J. D., Niven S., Demeckova V.. 2003. Liquid feeding of pigs II. Potential for improving pig health and food safety. Anim. Sci. Pap. Rep. 21:23–39. doi:10.1080/1828051X.2018.1438214 [Google Scholar]
- Canibe N., O. Højberg J. H. Badsberg, and Jensen B. B.. 2007. Effect of feeding fermented liquid feed and fermented grain on gastrointestinal ecology and growth performance in piglets. J. Anim. Sci. 85:2959–2971. doi:10.2527/jas.2006-744 [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal (CCAC) 2009. The care and use of farm animals in research, teaching and testing. CCAC, Ottawa, ON; p. 12–15. [Google Scholar]
- Diebold G., R. Mosenthin H. P. Piepho, and Sauer W. C.. 2004. Effect of supplementation of xylanase and phospholipase to a wheat-based diet for weanling pigs on nutrient digestibility and concentrations of microbial metabolites in ileal digesta and feces. J. Anim. Sci. 82:2647–2656. doi:10.2527/2004.8292647x [DOI] [PubMed] [Google Scholar]
- Huntley N. F. and Patience J. F.. 2018. Xylose: absorption, fermentation, and post-absorptive metabolism in the pig. J. Anim. Sci. Biotechnol. 9:4. doi:10.1186/s40104-017-0226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izydorczyk M. S., and Biliaderis C. G.. 1995. Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydr. Polym. 28:33–48. doi:10.1016/0144-8617(95)00077-1 [Google Scholar]
- Jacela J., Dritz S., DeRouchey J., Tokach M., Goodband R., and Nelssen J.. 2010. Effects of supplemental enzymes in diets containing distillers dried grains with solubles on finishing pig growth performance. PAS. 26:412–424. doi:10.15232/S1080-7446(15)30623–9 [Google Scholar]
- Jakobsen G. V., Jensen B. B., Knudsen K. B., and Canibe N.. 2015. Impact of fermentation and addition of non-starch polysaccharide-degrading enzymes on microbial population and on digestibility of dried distillers grains with solubles in pigs. Livest. Sci. 178:216–227. doi:10.1016/j.livsci.2015.05.028 [Google Scholar]
- Jha R., and Leterme P.. 2012. Feed ingredients differing in fermentable fibre and indigestible protein content affect fermentation metabolites and faecal nitrogen excretion in growing pigs. Animal. 6:603–611. doi:10.1017/S1751731111001844 [DOI] [PubMed] [Google Scholar]
- Ji F., D. P. Casper P. K. Brown D. A. Spangler K. D. Haydon, and Pettigrew J. E.. 2008. Effects of dietary supplementation of an enzyme blend on the ileal and fecal digestibility of nutrients in growing pigs. J. Anim. Sci. 86:1533–1543. doi:10.2527/jas.2007-0262 [DOI] [PubMed] [Google Scholar]
- Jo J. K., Ingale S. L., Kim J. S., Kim Y. W., Kim K. H., Lohakare J. D., Lee J. H., and Chae B. J.. 2012. Effects of exogenous enzyme supplementation to corn- and soybean meal-based or complex diets on growth performance, nutrient digestibility, and blood metabolites in growing pigs1. J. Anim. Sci. 90:3041–3048. doi:10.2527/jas.2010–3430 [DOI] [PubMed] [Google Scholar]
- Jones C. K., Bergstrom J. R., Tokach M. D., DeRouchey J. M., Goodband R. D., Nelssen J. L., and Dritz S. S.. 2010. Efficacy of commercial enzymes in diets containing various concentrations and sources of dried distillers grains with solubles for nursery pigs 1, 2. J. Anim. Sci. 88:2084–2091. doi:10.2527/jas.2009–2109 [DOI] [PubMed] [Google Scholar]
- Jørgensen H., Sholly D., Pedersen A. Ø., Canibe N., and Knudsen K. E. B.. 2010. Fermentation of cereals—influence on digestibility of nutrients in growing pigs. Livest. Sci. 134:56–58. doi:10.1016/j.livsci.2010.06.096 [Google Scholar]
- Kerr B. J. and Shurson G. C.. 2013. Strategies to improve fiber utilization in swine. J. Anim. Sci. Biotechnol. 4:11. doi:10.1186/2049-1891-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie E., Walsh M., and Nyachoti C.. 2016a. Performance, digestive function, and mucosal responses to selected feed additives for pigs. J. Anim. Sci. 94(suppl_3):169–180. doi:10.2527/jas2015-9835 [Google Scholar]
- Kiarie E., Walsh M., Romero L., and Baidoo S.. 2016b. Digestibility responses of growing pigs fed corn plus corn distiller grains or wheat plus wheat coproduct-based diets without or with supplemental xylanase. J. Anim. Sci. 94(suppl 3):211–214. doi:10.2527/jas.2015–9736 [Google Scholar]
- Knudsen K. E. B. 2014. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 93:2380–2393. doi:10.3382/ps.2014–03902 [DOI] [PubMed] [Google Scholar]
- de Lange C. F. M., Gabert V. M., Gillis D., and Patience J. F.. 1998. Digestible energy contents and apparent ileal amino acid digestibilities in regular or partial mechanically dehulled canola meal samples fed to growing pigs. Can. J. Anim. Sci. 78:641–648. doi:10.4141/A97-114 [Google Scholar]
- de Lange C. F. M., and Zhu C.. 2012. Liquid feeding cornbased diets to growing pigs: practical considerations and use of co-products, Feed efficiency in swine. Wageningen Academic Publishers, Netherlands; p. 63–80. doi:10.3920/978-90-8686=756-1_3 [Google Scholar]
- Lawlor P. G., P. B. Lynch G. E. Gardiner P. J. Caffrey, and O’Doherty J. V.. 2002. Effect of liquid feeding weaned pigs on growth performance to harvest. J. Anim. Sci. 80:1725–1735. [DOI] [PubMed] [Google Scholar]
- Leung H., Arrazola A., Torrey S., and Kiarie E.. 2018. Utilization of soy hulls, oat hulls, and flax meal fiber in adult broiler breeder hens. Poult Sci. 97:1368–1372. doi:10.3382/ps/pex434 [DOI] [PubMed] [Google Scholar]
- Mikkelsen L. L., and Jensen B. B.. 2000. Effect of fermented liquid feed on the activity and composition of the microbiota in the gut of pigs. Pig News and Information. 21:59–66. [Google Scholar]
- Missotten J. A., Michiels J., Ovyn A., De Smet S., and Dierick N. A.. 2010. Fermented liquid feed for pigs. Arch. Anim. Nutr. 64:437–466. doi:10.1080/1745039X.2010.512725 [DOI] [PubMed] [Google Scholar]
- Moran K., de Lange C., Ferket P., Fellner V., Wilcock P., and van Heugten E.. 2016. Enzyme supplementation to improve the nutritional value of fibrous feed ingredients in swine diets fed in dry or liquid form. J. Anim. Sci. 94:1031–1040. doi:10.2527/jas2015-9855 [DOI] [PubMed] [Google Scholar]
- Myers W., Ludden P., Nayigihugu V., and Hess B.. 2004. A procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 82:179–183. [DOI] [PubMed] [Google Scholar]
- NRC.. 2012. Nutrient requirements of swine. 11th revision ed National Academy of Sciences Press, Washington, DC. [Google Scholar]
- Omogbenigun F. O., C. M. Nyachoti, and Slominski B. A.. 2004. Dietary supplementation with multienzyme preparations improves nutrient utilization and growth performance in weaned pigs. J. Anim. Sci. 82:1053–1061. doi:10.2527/2004.8241053x [DOI] [PubMed] [Google Scholar]
- Pedersen M. B., Dalsgaard S., Knudsen K. E. B., Yu S., and Lærke H. N.. 2014. Compositional profile and variation of distillers dried grains with solubles from various origins with focus on non-starch polysaccharides. Anim. Feed Sci. Technol. 197(Suppl C):130–141. doi:10.1016/j.anifeedsci.2014.07.011 [Google Scholar]
- Rho Y., Moran K., Wey D., Zhu C., Walsh M., Kiarie E., van Heugten E., and de Lange C. F. M.. 2017a. 082 Growth performance responses of growing pigs when fed corn-soybean meal diets with corn DDGS treated with fiber degrading enzymes with or without extended steeping. J. Anim. Sci. 95(Suppl 2):38–39. doi:10.2527/asasmw.2017.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho Y., Zhu C., Kiarie E., and de Lange C. F. M.. 2017b. Standardized ileal digestible amino acids and digestible energy contents in high-protein distiller’s dried grains with solubles fed to growing pigs1. J. Anim. Sci. 95:3591–3597. doi:10.2527/jas.2017.1553 [DOI] [PubMed] [Google Scholar]
- Slominski B. A. 2000. A new generation of enzymes for animal feeds. Proc. 21st Western Nutrition Conf, Winnipeg, MB, Canada; p. 1–29. [Google Scholar]
- Sørensen H. R., Pedersen S., and Meyer A. S.. 2007. Synergistic enzyme mechanisms and effects of sequential enzyme additions on degradation of water insoluble wheat arabinoxylan. Enzyme Microb. Technol. 40:908–918. doi:10.1016/j.enzmictec.2006.07.026 [Google Scholar]
- Svihus B. 2010. Effect of digestive tract conditions, feed processing and ingredients on response to NSP enzymes. Enzymes in Farm Animal Nutrition, CAB International, UK; p. 129–159. [Google Scholar]
- Tsai T., Dove C., Cline P., Owusu-Asiedu A., Walsh M., and Azain M.. 2017. The effect of adding xylanase or β-glucanase to diets with corn distillers dried grains with solubles (CDDGS) on growth performance and nutrient digestibility in nursery pigs. Livest. Sci. 197:46–52. doi:10.1016/j.livsci.2017.01.008 [Google Scholar]
- Urriola P., Li M., Kerr B., and Shurson G.. 2014. Evaluation of prediction equations to estimate gross, digestible, and metabolizable energy content of maize dried distillers grains with solubles (DDGS) for swine based on chemical composition. Anim. Feed Sci. Technol. 198:196–202. doi:10.1016/j.anifeedsci.2014.09.006 [Google Scholar]
- Van Soest P. v., Robertson J., and Lewis B.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi:10.3168/jds.S0022-0302(91)78551–2 [DOI] [PubMed] [Google Scholar]
- Villca B., Lizardo R., Broz J., Brufau J., and Torrallardona D.. 2016. Effect of a carbohydrase enzyme complex on the nutrient apparent total tract digestibility of rye-based diets fed to growing-finishing pigs under liquid feeding. J. Anim. Sci. 94(Suppl 3):230–233. doi:10.2527/jas.2015–9747 [Google Scholar]
- de Vries S., A. M. Pustjens M. A. Kabel S. Salazar-Villanea W. H. Hendriks, and Gerrits W. J.. 2013. Processing technologies and cell wall degrading enzymes to improve nutritional value of dried distillers grain with solubles for animal feed: an in vitro digestion study. J. Agric. Food Chem. 61:8821–8828. doi:10.1021/jf4019855 [DOI] [PubMed] [Google Scholar]
- Widyaratne G., and Zijlstra R.. 2007. Nutritional value of wheat and corn distiller’s dried grain with solubles: digestibility and digestible contents of energy, amino acids and phosphorus, nutrient excretion and growth performance of grower-finisher pigs. Can. J. Anim. Sci. 87:103–114. doi:10.4141/A05-070 [Google Scholar]
- van Winsen R. L., Lipman L. J. A., Biesterveld S., Urlings B. A. P., Snijders J., and van Knapen F.. 2001. Mechanism of Salmonella reduction in fermented pig feed. J. Sci. Food Agric. 81:342–346. doi:10.1002/1097-0010(200102)81:3<342::AID-JSFA824>3.0.CO;2–6 [Google Scholar]
- Wiseman M. 2016. Use of enzymes and inoculants to manipulate the feeding value of liquid fed DDGS for young pigs. University of Guelph, Guelph. [Google Scholar]
- Wiseman M., McBride B., Li J., Wey D., Zhu J., and de Lange C. F. M.. 2017. Effects of steeped or fermented distillers dried grains with solubles on growth performance in weanling pigs. J. Anim. Sci. 95:3563–3578. doi:10.2527/jas2017.1478 [DOI] [PubMed] [Google Scholar]
- Zhu C., Rudar D. W., and C. F. M. de Lange. 2011. Glucanase, xylanase and microbial inoculants improve feeding value of DDGS for liquid-fed finishing pigs. J. Anim. Sci. 89(Suppl 1):78. [Google Scholar]