Abstract
Toxoplasma gondii and Neospora caninum are coccidian parasites with a global distribution that cause reproductive failure and production losses in livestock. The seroprevalence of both parasite species in ruminants and Cervidae has been investigated worldwide and found to vary greatly. Studies carried out on mixed flocks with 3 ruminant species (sheep, goats, and fallow deer) living under the same conditions are excellent models for identifying any differences in the rate of infection with the 2 parasites between the animal species. Additionally, the species used in the present study differ in their feeding categories: grazers, browsers, and intermediate feeders. The aim of the study is to identify any variation in the prevalence of the 2 parasites in mixed flocks and to identify any possible relationships with food choice. The seroprevalence against T. gondii and N. caninum in 167 captive fallow deer, 64 sheep, and 39 goats were detected using commercially available ELISA. The seroprevalence for T. gondii achieved 10% in fallow deer, 21% in goats, and 47% in sheep. The seroprevalence for N. caninum achieved 13% in sheep and fallow deer and 21% in goats. Overall, 53% of the sheep, 33% of the goats, and 22% of the fallow deer were seropositive for both infections. Coinfection of T. gondii and N. caninum was detected in 6% of sheep, 8% of goats, and 2% of fallow deer. Statistical analyses of the seroprevalence levels observed between 2 parasites for each animal species revealed that only the results obtained for sheep were significant (P < 0.01). Additionally, the differences in the seroprevalence levels for T. gondii between sheep and goats and between sheep and fallow deer were statistically significant (P < 0.01). The results of the N. caninum seroprevalence levels observed among animal species were not significant. Although the variations in susceptibility to T. gondii and N. caninum infections demonstrated by the examined animals may affect the differences in seropositivity, these appear to be related to the feeding habits of the animal species. Therefore, the risk of infection by agents found close to the ground, such as coccidian oocysts, varies. Sheep as grazers are at a greater risk of infection by T. gondii than goats and fallow deer.
Keywords: Apicomplexan parasites, mixed flock, ruminants, seropositivity
INTRODUCTION
Toxoplasma gondii and Neospora caninum are closely related Coccidian parasites of medical and veterinary importance. Their life cycles involve different definitive carnivorous hosts: Felidae for Toxoplasma and Canidae for Neospora. Both parasites can use a wide range of ungulates as intermediate hosts (Dubey, 2010; Almería, 2013; Donahoe et al., 2015).
Both parasite species are involved in reproductive failure and production losses in livestock.
The seroprevalence of T. gondii and N. caninum in sheep and goats has been investigated worldwide and found to vary greatly (Chikweto et al., 2011; Bartova and Sedlak, 2012; Čobádiová et al., 2013; Guimarăes et al., 2015). In Cervidae, the prevalence of T. gondii varies from 6.6% to 53.5% and N. caninum from 0% to 88% (Dubey et al., 2009; Goździk et al., 2010; Halová et al., 2013).
Ruminants hosts are infected by Apicomplexan parasites by ingesting food or water contaminated with oocysts excreted with the feces of definitive hosts. Several studies have implied that vertical transmission, as occurs in cattle, is also the route of transmission (reviewed by Dubey, 2010; Almería, 2013; Donahoe et al., 2015).
Ruminant species are classified into 3 main feeding categories: those that feed mainly on browse material; those that feed mainly on grass; and those that switch their diet from grasses to browse (Hofmann, 1989). Ruminants are assumed to have morphological and physiological adaptations that allow them to develop more specialized food intake, which are thought to influence all aspects of their life history (Gordon, 2003).
The aim of the study was to determine the seroprevalence of T. gondii and N. caninum infection in sheep, goats, and fallow deer (Dama dama), in a mixed flock farmed on the same area and under the same conditions, and to find possible relationships with food choice.
MATERIALS AND METHODS
Animals
The studies were conducted from 2014 to 2015 in the breeding station in Kosewo Górne, in the Mazurian lake district, north-east Poland (latitude 53°41′ North, longitude 21°25′ East). This area is located on 15 hectares and consists of meadows with several small lakes partially bordered with mixed forest. The animals are kept on pastures from early spring to late autumn and are stabled during the winter months with the possibility to freely range outdoors.
Blood samples were taken from the jugular vein by a veterinary officer during routine examination of 270 living animals breeding together in the same area: 167 captive fallow deer, 64 sheep (Merino), and 39 goats (Polish Fawn Improved). All examined animals were female and aged from 1 to 2 yr old. The samples were left to clot overnight at +4 °C and centrifuged at 1,000 x g for 15 min. The collected sera were stored at −20 °C until assayed by ELISA.
Serology
The presence of antibodies to T. gondii in sheep, goats, and fallow deer was detected using indirect ELISA (ID Screen Toxoplasmosis Indirect Multi-species kit, ID VET, Montpellier, France). Cutoff was calculated based on sample-to-positive (S/P) percentage according to the formula S/P = [optical density (OD) sample – OD negative control (NC)/OD positive control (PC) − OD NC] × 100; samples with S/P ≥ 50% were considered positive.
The presence of antibodies to N. caninum in sheep and goats was detected by competitive ELISA (Neospora caninum Antibody Test Kit, VMRD, Pullman, WA). Percentage inhibition was calculated using the formula %I = 100 [1 − (sample OD/NC OD)]; the samples with %I > 30% were considered as positive.
To detect the presence of antibodies to N. caninum in fallow deer, IDEXX ELISA (IDEXX Laboratories Inc., Westbrook, ME) was performed according to the manufacturer’s instructions, with some modifications according to Bień et al. (2012).
Immunoblot Technique
Antigens were prepared from tachyzoites of T. gondii (RH strain) and N. caninum (NC1 isolate) that were maintained in Vero cells in RPMI 1640 medium supplemented with 1% horse serum, 50 U/mL of penicillin, and 50 μg/mL of streptomycin and incubated at 37 °C in 5% CO2 in a humidified incubator (Bień et al., 2012). The parasites were purified by centrifugation through 30% isotonic Percoll (Pharmacia, Uppsala, Sweden) and washed 3 times in PBS pH 7.2. Next, the tachyzoites were suspended in distilled water (1 × 109 parasites/mL), freeze-thawed 3 times, and sonicated for 3 min. The resulting solution was centrifuged (2,000 × g, for 20 min at 4 °C), the pellet was discarded, and the supernatant was used as antigen. Antigen was prepared according to Cabaj et al. (2005).
The positive results obtained by ELISA examination of the serum samples were verified using an immunoblot technique previously performed for fallow deer serum samples (Bień et al., 2012), with some modifications. Anti-sheep IgG (Bethyl Laboratories, Inc., Montgomery, TX), anti-goat IgG (Sigma–Aldrich, St. Louis, MO), and anti-deer IgG (KPL, Gaithersburg, MD) conjugated to horse-radish peroxidase were used as a second antibody; they were diluted 1:3,000, added to each strip, and incubated for 1 h at room temperature. The immunocomplexes were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Science, Waltham, MA) and visualized using the ChemiDoc MP Imaging System (Bio-Rad Laboratories, Hercules, CA).
Antigens from tachyzoites of T. gondii and N. caninum for Western blot were prepared according to Cabaj et al. (2005). They were prepared from tachyzoites of T. gondii (RH strain) and N. caninum (NC1 isolate) that were maintained in Vero cells in RPMI 1640 medium supplemented with 1% horse serum, 50 U/mL of penicillin, and 50 μg/mL of streptomycin and incubated at 37 °C in 5% CO2 in a humidified incubator (Bień et al., 2012).
Statistical Analysis
T test and chi-square test were used for statistical analysis (http://www.socscistatistics.com). Mc-Callum Layton Confidence Interval Calculation Proportions were used at 95% confidence level (https://www.allto.co.uk/tools/statistic-calculators/confidence-interval-for-proportions-calculator/). A P < 0.05 was considered significant.
The occurrence of antibodies to T. gondii and N. caninum within a single animal species, that is sheep, goats, or fallow deer, was analyzed using binominal analysis: This was performed by calculating the proportion of positive (PA), negative (NA), and overall (po) agreements. The kappa coefficient was calculated to indicate the degree of reliability between rates for each animal species and for the mixed flock (Cicchetti and Feinstein, 1990; Graham and Bull, 1998).
RESULTS
Antibodies to T. gondii were confirmed in 47% of serum samples from the examined sheep, 21% of goats, and 10% of fallow deer (Table 1). Anti-N. caninum antibodies were present in 13% of sheep and fallow deer and 21% of goat serum samples. Coinfection of T. gondii and N. caninum was detected in 6% of sheep, 8% of goats, and 2% of fallow deer.
Table 1.
Toxoplasma gondii and Neospora caninum seropositivity in sera of sheep, goats, and fallow deer
| Animal species | Total number | Toxoplasma gondii positive (%) | Neospora caninum positive (%) | Mixed infection (%) | Overall seroprevalence (%) | P A | N A | p o |
|---|---|---|---|---|---|---|---|---|
| Sheep | 64 | 30 (47) | 8 (13) | 4 (6) | 34 (53) | 0.21 | 0.75 | 0.53 |
| Goats | 39 | 8 (21) | 8 (21) | 3 (8) | 13 (33) | 0.54 | 0.83 | 0.74 |
| Fallow deer | 167 | 17 (10) | 21 (13) | 3 (2) | 35 (22) | 0.16 | 0.92 | 0.80 |
| Total | 270 | 50 (20) | 37 (14) | 10 (4) | 82 (30) | — | — | — |
P A = positive agreement; NA = negative agreement; po = overall agreement.
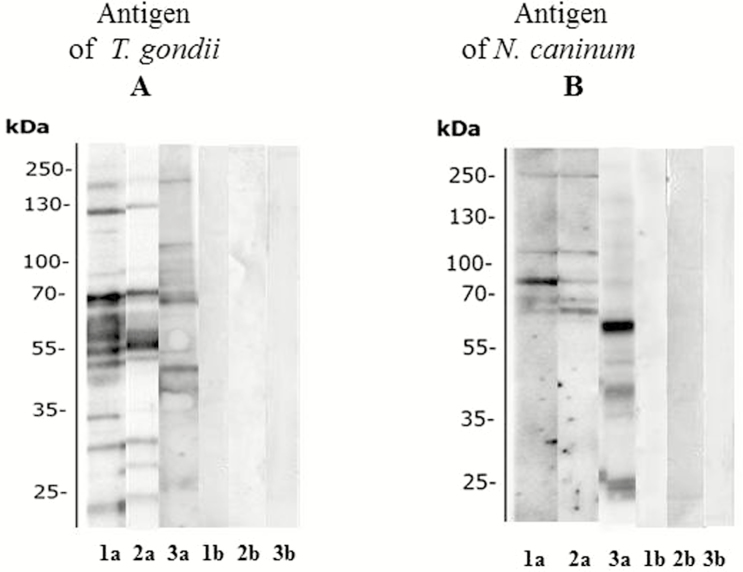
ELISA-positive results revealed seroreactivity to T. gondii and N. caninum antigens in immunoblot; selected results are presented in Fig. 1. Immunoblot analysis of the T. gondii antigen revealed a specific pattern for sera taken from all 3 animal species. The sheep and goat sera gave high reactivity and more bands between 25 and 250 kDa (Fig. 1A, lanes 1 and 2), whereas the fallow deer sera displayed more bands between 35 and 250 kDa (Fig. 1A, line 3).
Figure 1.
Immunoblot analysis of antibody responses of sheep, goats, and fallow deer to Toxoplasma gondii (A) and Neospora caninum (B) infections. Strips were incubated with sera demonstrated as positive (a) and negative (b) by ELISA; serum samples (lines 1: sheep; lines 2: goat; lines 3: fallow deer).
Immunoblot analysis using the N. caninum antigen revealed similar protein recognition patterns between the sera taken from sheep and goats; 5 immunodominant protein bands were located in the area between 55 and 250 kDa (Fig. 1B, lanes 1 and 2). However, the serum from fallow deer revealed seroreactivity to immunodominant N. caninum antigens at 25 and 130 kDa (Fig. 1B, lane 3).
Statistical analysis of the difference in total seroprevalences (20% for T. gondii and 14% for N. caninum) revealed that the result was significant (P < 0.01). However, the differences in the seroprevalence levels observed between 2 parasites for each animal species revealed that only the results obtained for sheep were significant (P < 0.01). Statistical analysis of the seroprevalence levels observed among species for T. gondii revealed statistical significance between the results obtained for the seroprevalence detected in sheep and goats and in sheep and fallow deer (P < 0.01). The results of the N. caninum seroprevalence levels observed among animal species were not significant.
The total seroprevalence of T. gondii for 3 animal species was 20% [55/270; 95% confidence interval (CI) = 9.43 to 30.57] and 14% for N. caninum (37/270; 95% CI = 2.82 to 25.18). The PA value indicates the conditional probability of the examined animal species being infected with both diseases, whereas NA indicates the conditional probability that if the examined animal does not have 1 disease, then it will not have the second. In the present study, low positive PA = 0.21 and higher negative NA = 0.75 indexes were found between the 2 infections in sheep, with median overall agreement po = 0.53. By contrast, median positive PA = 0.54, high negative NA = 0.83, and high overall agreement po = 0.74 were found in goats. Finally, low positive PA = 0.16 and negative NA = 0.92 indexes were found in fallow deer, with a high value of overall agreement po = 0.80 (Table 1). The kappa coefficients calculated for fallow deer, sheep, and goats (0.05, 0.02, and 0.1, respectively) indicate that the degree of reliability between rates is not significant.
DISCUSSION
Earliest studies performed in European countries found that antibodies to T. gondii were detected even in 79% of tested goats and 24% of tested sheep (Misurova et al., 2009; Špilovská et al., 2009). They indicated that goats are associated with higher T. gondii seroprevalence than sheep. Additionally, it has been found that goats are less frequently seropositive to N. caninum (up to 6%; Díaz et al., 2016).
Until now, only one report on the seroprevalence of T. gondii and N. caninum in different animal species kept together in mixed flocks has been available (Diakoua et al., 2013). The authors noted that goats demonstrate higher T. gondii seroprevalence than sheep, whereas sheep had higher N. caninum seroprevalence.
Our findings indicate that although the seroprevalence of both parasite species was similar in goats (21%), the sheep sera demonstrated higher seroprevalence of T. gondii (47%).
Our results are opposite to those published earlier, and the cause may be the breed of sheep identified as an associated factor for the occurrence of anti-T. gondii antibodies (Guimarăes et al., 2015; Deksne et al., 2017).
The study on the seroprevalence of T. gondii and N. caninum in fallow deer in European countries are limited (Bartova et al., 2007; Bień et al., 2012; Cabaj et al., 2017).
In the present study, specific IgG to T. gondii and N. caninum were detected in 10% and 13% of examined fallow deer, respectively, and the difference was not significant. The earlier screening studies on fallow deer from this region found N. caninum seropositivity to be only 3.3% (Bień et al., 2012). The differences may be influenced by the age of animals and number of individuals tested (Halová et al., 2013; De Craeye et al., 2011). Antibodies to T. gondii and N. caninum in fallow deer from the Czech Republic were detected in 17% and 1%, respectively (Bartova et al., 2007).
The study was carried out on mixed flocks, in which 3 animal species live under the same conditions. This is an excellent model for identifying any differences between the animal species with regard to the rate of infection by the 2 parasites. The seroprevalence of T. gondii varied considerably (10% to 47%), although the prevalence of N. caninum was found to be similar in sheep and fallow deer (13%), and higher in goats (21%). However, analysis of the seroprevalence levels observed among 2 parasites for each animal species revealed statistical significance only in the results obtained for sheep. Additionally, the seroprevalence levels for T. gondii observed among species were significant between sheep and goats and between sheep and fallow deer. In case of the N. caninum, seroprevalence levels among animal species were not significant. Vikoren et al. (2004) suggested that the seroprevalence level of parasite species in examined animals is attributed to differences in susceptibility to infections. Low seroprevalence of both parasite species in mixed flock does not indicate a high contamination of the environment by oocysts and high vertical transmission rates, although the animals were not tested for antibodies when they were born.
In the present study, coinfection by both Apicomplexan parasites was found to be rare (4%), being found in only 6% of sheep, 8% of goats, and 2% of fallow deer. Similar results for serum coinfection were found in studies on sheep and goats in Slovakia and the Czech Republic (Špilovská et al., 2009; Bartova and Sedlak, 2012; Čobádiová et al., 2013).
Although binomial analysis revealed that the degree of reliability between rates is not significant, the overall seroprevalence calculated for each of the examined animal species is reflected in the negative agreement NA value. The highest NA, calculated for fallow deer (0.92), related to the lowest overall seroprevalence (22%), whereas the lowest NA, calculated for sheep (0.75), related to the highest overall seroprevalence (53%). In addition, the percentage of mixed infection was reflected in a positive agreement PA value. The highest PA, calculated for goats (0.54), related to the highest percentage of coinfection (8%), whereas the lowest PA, calculated for fallow deer (0.16), related to the lowest percentage of coinfection (2%).
In our study, anti-Toxoplasma IgG antibodies were detected using indirect ELISA (ID VET, Montpellier, France) validated for many animal species (Špilovská et al., 2009; Čobadiová et al., 2013). Due to our earlier experience (Bień et al., 2012; Cabaj et al., 2017), antibodies to N. caninum in fallow deer were detected by modified IDEXX ELISA. Antibodies to N. caninum in sheep and goats were detected by VMRD ELISA. Although it has been validated only for 1 animal species, it has been used in earlier studies to detect antibodies to N. caninum in goats, sheep, and moose (Čobádiová et al., 2013; Moskwa et al., 2014).
In the present study, an immunoblot technique confirmed the positive ELISA results, with a 100% accordance observed between ELISA and immunoblot. The results of earlier (Harkins et al., 1998; Vitor et al., 1999; Bień et al., 2012) and presented studies show that numerous T. gondii or N caninum antigens have been recognized with little consistency within and between animal species. The different numbers of immunoreactive protein bands and their intensity probably reflect the intensity of the infection and time after ingestion.
In the present study, the overall seroprevalence of both coccidian parasites was 53% for sheep, 33% for goats, and 22% for deer. Although the difference in the antibodies level to 2 parasites was significant only for sheep, it appears that the seroprevalence may be related to the feeding habits of the animal species. Sheep are very selective grazers. Goats are more likely to obtain their food by browsing than by grazing. They do not graze monotonically in the pasture but explore the area looking for new plants, typically weeds and herbs (Ekesbo, 2011). Fallow deer predominantly graze on a wide variety of grasses, herbs, forbs, and sedges, but they also commonly browse trees and shrubs (Jensz and Finley, 2013). Thus, it is expected that due to the type of grazing, goats and fallow deer are at less risk of infection by agents found close to the ground, such as coccidian oocysts in general.
CONCLUSION
Although the variations in susceptibility to T. gondii and N. caninum infections demonstrated by the examined animals affect the observed differences in seropositivity, these may also be influenced by the food intake and food choice of the examined animal species.
Footnotes
1The study was partially carried out in the Polish-Slovak Joint Research Project “Observation on serious protozoonoses (neosporosis and toxoplasmosis) in domestic and sylvatic cycle.” B.M. conceived and designed the study area; A.K., A.C., J.B., and K.R. performed experiments; M.B. and Z.S.-B. collected samples; B.M., J.B., K.R., and W.C. analyzed the data and wrote the manuscript. All authors read and approved the final manuscript. The authors declare that they have no competing interests. All animals belong to the Witold Stefański Institute of Parasitology, Polish Academy of Sciences. The Breeding Station is located in Kosewo Górne. Material collection was performed by a veterinary officer during routine examinations.
LITERATURE CITED
- Almería S. 2013. Neospora caninum and wildlife. ISRN Parasitol. 2013:947347. doi:10.5402/2013/947347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartova E., and Sedlak K.. 2012. Toxoplasma gondii and Neospora caninum antibodies in goats in the Czech Republic. Vet. Med. 57:111–114. doi:10.17221/5850 [Google Scholar]
- Bartova E., Sedlak K., Pavlik I., and Literak I.. 2007. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in wild ruminants from the countryside or captivity in the Czech Republic. J. Parasitol. 93:1216–1218. doi:10.1645/GE-1126R.1 [DOI] [PubMed] [Google Scholar]
- Bień J., Moskwa B., Bogdaszewski M., and Cabaj W.. 2012. Detection of specific antibodies anti-Neospora caninum in the fallow deer (Dama dama). Res. Vet. Sci. 92:96–98. doi:10.1016/j.rvsc.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Cabaj W., Bień J., Bogdaszewski M., Steiner-Bogdaszewska Ż., and Moskwa B.. 2017. Potential impact of Neospora caninum infection on farm productivity of fallow deer (Dama dama). Small Ruminants Res. 156:78–81. doi:10.1016/j.smallrumres.2017.09.14 [Google Scholar]
- Cabaj W., Moskwa B., Pastusiak K., and Gill J.. 2005. Antibodies to Neospora caninum in the blood of European bison (Bison bonasus bonasus L.) living in Poland. Vet. Parasitol. 128:163–168. doi:10.1016/j.vetpar.2004.09.033 [DOI] [PubMed] [Google Scholar]
- Chikweto A., Kumthekar S., Tiwari K., Nyack B., Deokar M. S., Stratton G., Macpherson C. N., Sharma R. N., and Dubey J. P.. 2011. Seroprevalence of Toxoplasma gondii in pigs, sheep, goats, and cattle from Grenada and Carriacou, West Indies. J. Parasitol. 97:950–951. doi:10.1645/GE-2811.1 [DOI] [PubMed] [Google Scholar]
- Cicchetti D. V., and Feinstein A. R.. 1990. High agreement but low kappa: II. Resolving the paradoxes. J. Clin. Epidemiol. 43:551–558. doi:10.1016/0895-4356(90)90159-M [DOI] [PubMed] [Google Scholar]
- Čobádiová A., Reiterová K., Derdáková M., Špilovská S., Turčeková L., Hviščová I., and Hisira V.. 2013. Toxoplasma gondii, Neospora caninum and tick-transmitted bacterium Anaplasma phagocytophilum infections in one selected goat farm in Slovakia. Acta Parasitol. 58:541–546. doi:10.2478/s11686-013-0171-5 [DOI] [PubMed] [Google Scholar]
- De Craeye S., Speybroeck N., Ajzenberg D., Dardé M. L., Collinet F., Tavernier P., Van Gucht S., Dorny P., and Dierick K.. 2011. Toxoplasma gondii and Neospora caninum in wildlife: Common parasites in Belgian foxes and cervidae?Vet. Parasitol. 178:64–69. doi:10.1016/j.vetpar.2010.12.016 [DOI] [PubMed] [Google Scholar]
- Deksne G., Ligere B., Šneidere A., and Jokelainen P.. 2017. Seroprevalence and factors associated with Toxoplasma gondii infections in sheep in Latvia: Latvian dark headed sheep breed associated with higher seroprevalence. Vector Borne Zoonotic Dis. 17:478–482. doi:10.1089/vbz.2016.2003 [DOI] [PubMed] [Google Scholar]
- Diakoua A., Papadopoulos E., Panousis N., Karatzias C., and Giadinis N.. 2013. Toxoplasma gondii and Neospora caninum seroprevalence in dairy sheep and goats mixed stock farming. Vet. Parasitol. 198:387–390. doi:10.1016/j.vetpar.2013.09.017 [DOI] [PubMed] [Google Scholar]
- Díaz P., Cabanelas E., Díaz-Cao J. M., Viña M., Béjar J. P., Pérez-Creo A., Prieto A., López C. M., Panadero R., Fernández G.,. et al. 2016. Seroprevalence of Toxoplasma gondii and Neospora caninum in goats from North-Western Spain. Ann. Agric. Environ. Med. 23:587–590. doi:10.5604/12321966.1226851 [DOI] [PubMed] [Google Scholar]
- Donahoe S. L., Lindsay S. A., Krockenberger M., Phalen D., and Šlapeta J.. 2015. A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int. J. Parasitol. Parasites Wildl. 4:216–238. doi:10.1016/j.ijppaw.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J. P. 2010. Toxoplasmosis of animals and humans. 2nd ed CRC Press, Boca Raton, FL. [Google Scholar]
- Dubey J. P., Jenkins M. C., Kwok O. C., Zink R. L., Michalski M. L., Ulrich V., Gill J., Carstensen M., and Thulliez P.. 2009. Seroprevalence of Neospora caninum and Toxoplasma gondii antibodies in white-tailed deer (Odocoileus virginianus) from Iowa and Minnesota using four serologic tests. Vet. Parasitol. 161:330–334. doi:10.1016/j.vetpar.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Ekesbo I. 2011. Farm animal behavior characteristic for assessment of health and welfare. Swedish University of Agricultural Sciences, Sweden. [Google Scholar]
- Gordon I. J. 2003. Browsing and grazing ruminants: Are they different beasts?For. Ecol. Manage. 181:13–21. doi:10.1016/S0378-1127(03)00124-5 [Google Scholar]
- Goździk K., Jakubek E. B., Björkman C., Bień J., Moskwa B., and Cabaj W.. 2010. Seroprevalence of Neospora caninum in free living and farmed red deer (Cervus elaphus) in Poland. Pol. J. Vet. Sci. 13:117–120. [PubMed] [Google Scholar]
- Graham P., and Bull B.. 1998. Approximate standard errors and confidence intervals for indices of positive and negative agreement. J. Clin. Epidemiol. 51:763–771. doi:10.1016/S0895-4356(98)00048-1 [DOI] [PubMed] [Google Scholar]
- Guimarăes A., Raimundo J. M., Morales L. M. B., Silva A. T., Santos H. A., Pieres M. S., Machado R. Z., and Baldani C. D.. 2015. Occurrences of anti-Toxoplasma gondii and anti-Neospora caninum antibodies in sheep from four districts of Tocantins state, Brazilian Legal Amazon Region. Pesq. Vet. Bras. 35:110–114. doi:10.1590/S0100- 736X2015000200002 [Google Scholar]
- Halová D., Mulcahy G., Rafter P., Turčeková L., Grant T., and de Waal T.. 2013. Toxoplasma gondii in Ireland: Seroprevalence and novel molecular detection method in sheep, pigs, deer and chickens. Zoonoses Public Health 60:168–173. doi:10.1111/j.1863-2378.2012.01514.x [DOI] [PubMed] [Google Scholar]
- Harkins D., Clements D. N., Maley S., Marks J., Wright S., Esteban I., Innes E. A., and Buxton D.. 1998. Western blot analysis of the IgG responses of ruminants infected with Neospora caninum and with Toxoplasma gondii. J. Comp. Pathol. 119:45–55. doi:10.1016/S0021-9975(98)80070-4 [DOI] [PubMed] [Google Scholar]
- Hofmann R. R. 1989. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: A comparative view of their digestive system. Oecologia 78:443–457. doi:10.1007/BF00378733 [DOI] [PubMed] [Google Scholar]
- Jensz K., and Finley L.. 2013. Species profile for the fallow deer, Dama dama. Latitude 42 Environmental Consultants Pty Ltd, Hobart, Tasmania. [Google Scholar]
- Misurova L., Svobodova V., Pavlata L., and Dvorak R.. 2009. Titres of specific antibodies against Toxoplasma gondii in goats and their kids. Acta Vet. Brno 78:259–266. doi:10.2754/avb200978020259 [Google Scholar]
- Moskwa B., Goździk K., Bień J., Kornacka A., Cybulska A., Reiterová K., and Cabaj W.. 2014. Detection of antibodies to Neospora caninum in moose (Alces alces): The first report in Europe. Folia Parasitol. 61:34–36. doi:10.14411/fp.2014.012 [PubMed] [Google Scholar]
- Špilovská S., Reiterová K., Kovácová D., Bobáková M., and Dubinský P.. 2009. The first finding of Neospora caninum and the occurrence of other abortifacient agents in sheep in Slovakia. Vet. Parasitol. 164:320–323. doi:10.1016/j.vetpar.2009.05.020 [DOI] [PubMed] [Google Scholar]
- Vikoren T., Tharaldsen J., Fredriksen B., and Handeland K.. 2004. Prevalence of Toxoplasma gondii antibodies in wild red deer, roe deer, moose, and reindeer from Norway. Vet. Parasitol. 120:159–169. doi:10.1016/j.vetpar.2003.12.015 [DOI] [PubMed] [Google Scholar]
- Vitor R. W., Ferreira A. M., and Fux B.. 1999. Antibody response in goats experimentally infected with Toxoplasma gondii. Vet. Parasitol. 81:259–263. doi:10.1016/S0304- 4017(98)00251-9 [DOI] [PubMed] [Google Scholar]