Abstract
The objective of this study was to evaluate the effect of feeding soybean oil (SO) with varying levels of peroxidation on carcass traits and shelf life of loins. Fifty-six barrows were randomly assigned to 1 of 4 diets containing 10% fresh SO (22.5 °C) or thermally processed SO (45 °C for 288 h, 90 °C for 72 h, or 180 °C for 6 h), each infused with air at a rate of 15 liter/min. Individually housed pigs were provided ad libitum access to feed for 81 d. At 82 d, pigs were slaughtered and hot carcass weight and liver weights were recorded. Carcass characteristics and fresh loin quality were evaluated 1 d postmortem. Loin chops from each carcass were overwrap-packaged and subjected to a 10-d simulated retail display. Daily measurements of L*, a*, b*, reflectance, and visual discoloration were conducted, evaluation of cooking loss and Warner–Bratzler shear force (WBSF) was conducted on chops stored 0, 5, and 10 d, and thiobarbituric acid reactive substances (TBARS) were evaluated on chops stored 0 and 10 d. Shelf life–related data were analyzed as a completely randomized design with repeated measures in time, with storage location (shelf) as a random effect. Carcasses of 90 °C pigs weighed 6.0, 8.6, and 6.9 kg less (P < 0.03) than 22.5 °C, 45 °C, and 180 °C carcasses, respectively. Livers of 90 °C and 180 °C pigs were 14.3% and 11.7%, respectively, heavier (P ≤ 0.02) than those from pigs fed 22.5 °C SO, with livers of 45 °C being intermediate. Livers of 90 °C pigs represented 0.12 percentage units less (P = 0.02) of ending live weight than livers of 180 °C pigs, and 180 °C livers were 0.12 percentage units less (P < 0.01) of ending live weight than those from pigs fed 22.5 °C SO, with 45 °C being intermediate. There was no difference (P ≥ 0.19) in back fat depth, loin muscle area, or estimated carcass lean percentage among SO treatments, nor was there an effect (P ≥ 0.13) of SO on any early post-mortem loin quality traits or loin composition. There was no effect (P > 0.14) of SO on cooking loss, WBSF, L*, a*, b*, hue angle, reflectance, discoloration, or TBARS; however, there was a tendency (P = 0.09) for chops of 45 °C pigs to have greater (P < 0.04) chroma than either 22.5 °C or 180 °C, with 90 °C being intermediate. Overall, feeding SO cooked at 90 °C for 72 h resulted in reduced carcass weight and dressing percentage; however, there was no evidence that feeding peroxidized SO was detrimental to shelf life of loin chops.
Keywords: loin, oxidation, oxidized oil, pork quality, shelf life
INTRODUCTION
Interest in dietary lipid quality has focused on the influence of fatty acid profile on carcass and pork product quality. However, it has been increasingly recognized that the oxidative status of the diet is also important and may be of special interest considering rendered fats like white or yellow grease, and soybean oil (SO) is routinely included in swine diets (Lin et al., 2013). Feeding peroxidized lipids has been shown to reduce lipid digestibility (Liu et al., 2014a; Lindblom et al., 2017), growth performance (Rosero et al., 2015), and hot carcass weight (HCW) and back fat (BF) depth (Boler et al., 2012) in growing-finishing pigs.
Although the negative effects of peroxidized lipids on growth and carcass traits have been regularly reported, the influence of feeding peroxidized lipid on the quality and shelf life of pork loin chops has been inconsistent. Buckley et al. (1989) reported feeding corn oil with a peroxide value (PV) of 9 mEq/kg resulted in greater concentrations of thiobarbituric acid reactive substances (TBARS) during display of loin chops compared with chops from pigs fed fresh oil. This is supported in part by Monahan et al. (1992), where there was a tendency for loin chops of pigs fed corn oil (PV = 4.5 mEq/kg) to have greater TBARS than pigs fed fresh oil. Recent studies, however, have reported that feeding peroxidized corn oil does not affect lipid peroxidation or color stability of loin chops during display (Monahan et al., 1994; Boler et al., 2012). The inconsistencies in these results may be due to inconsistencies in oil processing or dosage, which ranged from 4.5 to 9 mEq/kg, with the most significant response in product stability observed at the greater dietary PV.
Therefore, the objective of this study was to determine the effect of feeding divergently thermally processed SO on carcass characteristics and early postmortem loin quality and to determine what role, if any, peroxidized SO in the diet had on the shelf life of pork loin chops, by feeding diets with PV of 0.2 to 12.4 mEq/kg.
MATERIALS AND METHODS
All animal care and use procedures for this experiment were approved by the Institutional Animal Care and Use Committee at Iowa State University.
Dietary Treatments and Experimental design
Fifty-six individually housed barrows (Geneticporc F25 females × B6.0 sires, Hender sonville, TN) were randomly allotted to 1 of 4 diets containing 10% fresh SO (22.5 °C) or SO that was thermally processed at 45 °C for 288 h, 90 °C for 72 h, or 180 °C for 6 h throughout a 2-phase, 81-d finishing trial. Diets were formulated to meet or exceed the nutrient requirements for finishing pigs according to the NRC (2012), and no antioxidants were added to diets before or during their preparation. Oil and diet preparation, as well as design of the feeding trial, was described previously (Overholt et al., 2018).
Slaughter and Carcass Evaluation
At the conclusion of the growth trial, pigs were transported approximately 613 km from Ames, IA to the University of Illinois Meat Science Laboratory in Urbana, IL. One barrow fed the 22.5 °C SO diet was deemed unfit for transport and therefore was removed from further experiments. Once arriving at the University of Illinois Meat Science Laboratory, barrows were held overnight (~16 h) with no access to feed but ad libitum access to water. Barrows were weighed to determine ending live weight (ELW) before being immobilized using the head-to-heart electrical stunning technique immediately followed by exsanguination. Upon evisceration and inspection of internal organs, livers from each carcass were weighed and a section was removed from the right lateral lobe and snap-frozen in liquid nitrogen for subsequent analysis of oxidative status. Hot carcass weight was recorded after each carcass had been inspected and deemed wholesome by an USDA-FSIS inspector. Carcasses were allowed to chill at 4 °C and 24 h after slaughter, the left side of each carcass was ribbed between the 10th and 11th ribs, and carcasses were evaluated fat depth at the 10th rib (BF depth) at a point perpendicular to the skin, 3/4th the length of the LM. Loin muscle area (LMA) was traced onto double-matted acetate paper and the LMA outlines were later traced in duplicate with a digitizer pad (Intuos Pro Digitizer Tablet and Stylus; Wacom Technology Corp., Vancouver, WA), and the mean area of the 2 tracings was reported as LMA. Estimated carcass lean percentage (ECL) was calculated using the equation of Burson and Berg (2001): estimated carcass lean, % = [8.588 + (0.465 × HCW, lbs) − (21.896 × BF, in) + (3.005 × LMA, in2)/HCW, lbs × 100]/HCW, lbs. Adipose tissue cores, measuring 2.54 cm in diameter and consisting of all 3 adipose layers, were collected from the clear plate between the scapula and cervical vertebra near the dorsal midline of the left side of each carcass. Iodine values (IVs) of both adipose depots were determined using a Bruker MPA Multi-Purpose FT-NIR Analyzer (Bruker Optics Inc., Billerica, MA).
Early Postmortem Loin Quality Evaluation
Ultimate pH, loin color, marbling, and firmness were evaluated 30 min after carcasses had been ribbed. Ultimate pH was measured by inserting a pH-meter probe fitted with a glass electrode (MPI pH-Meter, Topeka, KS; calibrated to pH 4 and 7) into the ventral surface of each loin at the posterior termination of the spinalis dorsi. Instrumental color evaluation (L*, a*, b*; CIE, 1978) was conducted with a Minolta CR-400 Chroma-meter (Minolta Camera Co., Ltd., Osaka, Japan) using a D65 light source and a 2° observer with an 8-mm aperture. Calibration of the instrument was conducted using a white tile. Subjective color, marbling (NPPC, 1999), and firmness (NPPC, 1991) were determined by a single trained evaluator. After early postmortem loin quality evaluation, carcasses were fabricated to yield Canadian back loins [North American Meat Processors Association (NAMP) #414]. The anterior portion of Canadian back loins was removed with a cut immediately posterior to the spinalis dorsi and, working from an anterior to posterior direction, three 2.54-cm and two 1.27-cm chops were cut for use in simulated retail display. Additional 2.54-cm and 1.27-cm chops were removed for analysis of moisture and lipid content using the drying and extraction by chloroform: methanol method described by Novakofski et al. (1989) and evaluation of drip loss by the suspension method (Boler et al., 2014), respectively.
Loin Chop Display
There were three 2.54-cm and two 1.27-cm chops saved for the simulated retail display experiment. One chop of each thickness was designated for 0 and 10 d of simulated retail display, with the 2.54-cm chop used for Warner–Bratzler shear force (WBSF) and the 1.27-cm chop to be used for analysis of lipid oxidation. A single 2.54-cm chop was designated for 5 d of display and to also be used for WBSF. Pairs of chops designated for 5 and 10 d of storage were placed on polystyrene trays and overwrapped with polyvinylchloride film (PVC; oxygen transmission rate = 1,627.9 cc/m2/d; moisture vapor transmission rate = 170.5 g/m2/d). Packages were arranged on wire mesh shelves lined with white butcher paper in 2 single-layer rows. Lighting was provided by two 122-cm long 32-W fluorescent bulbs (Ecolux with Starcoat, 3000 K, General Electric, Boston, MA) that were suspended 38 cm above each row of packages. Packages were stored at 4 °C. Color evaluations were conducted through the PVC film on the 1.27-cm chop designated for 10 d of display following the procedure described by Holmer et al. (2009). Daily evaluations of instrumental color (L*, a*, b*, 650/580 ratio) were conducted using a Hunter Lab Miniscan XE Plus (Model 45/0-L, Hunter Associates Laboratory Inc., Reston, VA) using an A illuminant, 10° observer, and a 25-mm aperture. Calibration of the device was conducted prior to each daily evaluation using a black glass and a white ceramic tile covered with the same PVC film used to package the loin chops. Daily visual discoloration scores were determined by a single trained observer using a 10-cm unstructured line scale anchored at 0% and 100% surface discoloration, with each 1-cm increment equal to 10% discoloration of the chop surface. On the designated day of display (0, 5, or 10 d), the chops were removed from display once they had been evaluated (with the exception of the 0-d chops, which were not displayed), and chops were then packaged in individual bags and vacuum sealed. The 1.27-cm chops, which were designated to for analysis of lipid oxidation, were stored at −80 °C until later analysis. The 2.54-cm chops, which were designated for analysis of WBSF, were stored at −20 °C until later analysis.
Warner–Bratzler Shear Force
Vacuum-packaged chops were allowed to thaw at 4 °C for 18 h before analysis, trimmed of excess fat, and weighed before being cooked on a Farberware Open Hearth grill (model 455N; Walter Kidde, Bronx, NY). Chops were flipped once at an internal temperature of 35 °C and then cooked until they reached an internal temperature of 70 °C. Internal temperatures were monitored using copper-constantan thermocouples (Type T; Omega Engineering, Stamford, CT) connected to a digital scanning thermometer (model 92000-00; Barnant Co., Barrington, IL). Cooked chops were cooled to 25 °C and weighed before four 1.25-cm-diameter cores were removed parallel to the orientation of the muscle fibers. Cores were then sheared once through the center using a Texture Analyzer TA.HD Plus (Texture Technologies Corp., Scarsdale, NY/Stable Micro Systems Ltd., Godalming, UK) with a blade speed of 3.3 mm/s and a 100-kg load cell. Shear force was reported as the average peak force of the 4 cores. Cooking loss was calculated as the difference between the pre- and post-cooked chop weights divided by the precooked chop weight (Boler et al., 2011).
Thiobarbituric Acid Reactive Substances
One 1.27-cm chop representing 0 d of display, and another that had been displayed for 10 d, was trimmed of external fat, connective tissue, and any accessory muscles. Chops were cut into cubes and pulverized in a blender (Waring Products, Torrington, CT). Duplicate 5 g samples of pulverized loin muscle were combined with 1 mL of 0.2-mg/mL butylated hydroxytoluene and 45.5 mL of 10% trichloroacetic acid in 0.2 M phosphoric acid, and blended for 30 s (Waring Products, Torrington, CT). The homogenate was filtered through Whatman No. 1 filter paper and collected in flasks. Two 5 mL aliquots of filtrate were transferred from flasks into 15-mL conical tubes. To one tube, 5 mL of 0.02 M thiobarbituric acid was added, and to the other was added 5 mL of deionized water to serve as a blank. An additional 2 pulverized loin muscles were randomly selected, representing 0 and 10 d of display, to serve as spiked samples to estimate percent recovery. The spiked samples were prepared in the same way as described previously, except that 12 mL of 10 μM 1, 1, 3, 3-tetramethoxypropane was added in substitution for an equal volume for 10% trichloroacetic acid in 0.2 M phosphoric acid. A standard curve was made to represent 0, 1.25, 2.5, 5, and 7.5 mg malondialdehyde/mL using 25 μM 1, 1, 3, 3-tetramethoxypropane. An aliquot of the 25 μM 1, 1, 3, 3-tetramethoxypropane was combined with 5 mL of 0.02 M thiobarbituric acid and volumized to 10 mL with 10% trichloroacetic acid in 0.2 M phosphoric acid solution. All tubes were capped, inverted to mix, and then stored in the dark at 23 °C for approximately 16 h to allow pigment development. After 16 h, 150 μL from each tube was transferred onto 96 well round bottom plates and read at 530 nm with a Beckman Du-640 Spectrophotometer (Beckman Coulter Inc., Fullerton, CA). TBARS were reported as mg-MDA/kg tissue and as mg-MDA/kg-extractable lipid.
Statistical Analyses
All analyses were conducted with the MIXED procedure of SAS (SAS v9.4, SAS Inst. Inc., Cary, NC). Because pigs were fed individually, pig carcass served as experimental unit. Carcass and liver data were analyzed as a completely randomized design with the fixed effect of SO treatment, with initial body weight (Overholt et al., 2018) serving as a covariate. There were 2 carcasses determined to be outliers for carcass traits, one each from 45 °C and 180 °C; therefore, they were omitted from related analyses. Early postmortem loin quality data were also analyzed as a completely randomized design, but no covariate was used nor were there any outliers in the data.
Simulated retail display data, including color stability, TBARS, cooking loss, and WBSF, were analyzed as a completely randomized design with repeated measures in time. Therefore, fixed effects were SO treatment, storage time, and the interaction of SO and storage time. Storage location (shelf) served as a random effect. Analysis of repeated measures was conducted using the REPEATED statement of PROC MIXED. Multiple covariance structures were compared using Akaike’s Information-Corrected Criterion for each dependent variable. Based on these comparisons, a heterogenous Toeplitz (TYPE = TOEPH) covariance structure was used for analyses of L*, a*, b*, chroma, hue, reflectance, and discoloration, whereas a heterogeneous compound symmetric [TYPE = CSH] covariance structure was used for analyses of TBARS, cooking loss, and WBSF. Least-square means were separated with the PDIFF option of the MIXED procedure. Main effects and interactions were considered different at P ≤ 0.05 and main effects were considered trending at 0.05 < P ≤ 0.10.
RESULTS AND DISCUSSION
Characteristics of Soybean Oils
A full characterization of the oxidative status and composition of the thermally processed SO are presented in Table 1 and detailed elsewhere (Overholt et al., 2018). However, certain characteristics of the oils merit discussion in the context of the current study. The 4 thermal processing treatments were chosen because they reflected conditions that fat sources and diets routinely experience in the livestock, rendering, and restaurant industries (Meeker and Hamilton, 2006) and because they also represented temperatures evaluated previously (Boler et al., 2012; Liu et al., 2014b; Rosero et al., 2015; Hanson et al., 2016).
Table 1.
Composition and peroxidation analysis of thermally processed soybean oils
| Heating temperature, °C | 22.5 | 45 | 90 | 180 |
|---|---|---|---|---|
| Time heated, h1 | 0 | 288 | 72 | 6 |
| Fatty acids, % of total fat2,3 | ||||
| C8:0, Caprylic | ND4 | ND | ND | 0.07 |
| C16:0, Palmitic | 10.69 | 10.74 | 11.70 | 11.21 |
| C16:1, Palmitoleic | 0.09 | 0.08 | 0.09 | 0.08 |
| C17:0, Margaric | 0.10 | 0.10 | 0.11 | 0.10 |
| C18:0, Stearic | 4.19 | 4.13 | 4.52 | 4.42 |
| C18:1, Oleic | 23.46 | 23.49 | 25.15 | 24.26 |
| C18:2, Linoleic | 53.07 | 52.86 | 50.79 | 51.65 |
| C18:3, Linolenic | 7.14 | 7.15 | 6.14 | 6.43 |
| C19:0, Nonadecanoic | 0.26 | 0.25 | 0.23 | 0.35 |
| C20:0, Arachidic | 0.32 | 0.32 | 0.35 | 0.34 |
| C20:1, Gadoleic | 0.18 | 0.19 | 0.20 | 0.30 |
| C22:0, Behenic | 0.35 | 0.35 | 0.49 | 0.41 |
| C24:0, Lignoceric | ND | 0.12 | ND | 0.13 |
| Other FA | 0.15 | 0.22 | 0.23 | 0.27 |
| UFA:SFA4 | 5.28 | 5.23 | 4.73 | 4.88 |
| IV5 | 131 | 131 | 126 | 127 |
| Free fatty acids, %2 | 0.10 | 0.10 | 0.46 | 0.14 |
| Free glycerin, %2 | 1.04 | 3.72 | 0.82 | 0.82 |
| Moisture, % | 0.02 | 0.02 | 0.04 | 0.04 |
| Insoluble impurities, % | 0.02 | 0.04 | 0.04 | 0.04 |
| Unsaponifiable matter, % | 0.51 | 0.39 | 0.41 | 0.47 |
| Oxidized FA, %2 | 3.0 | 2.8 | 2.5 | 1.9 |
| OSI at 110 C, h2,4 | 6.65 | 4.65 | 2.70 | 3.65 |
| p-Anisidine value2,6 | 1.11 | 1.33 | 121 | 165 |
| Peroxide value, mEq/kg2 | 2.0 | 17.4 | 123.6 | 19.4 |
| Polar compounds, %2 | 3.61 | 3.28 | 20.25 | 11.58 |
| PTAG4, % | ||||
| TBA value2,6 | 0.14 | 1.14 | 0.14 | 0.14 |
| Aldehydes, mg/kg7 | ||||
| 2,4-Decadienal | 2.07 | 1.90 | 912.15 | 915.49 |
| 4-Hydroxynonenal | 0.66 | 1.49 | 170.48 | 82.80 |
| Acrolein | 6.15 | 6.06 | 27.12 | 44.60 |
| 2-Decenal | 0.16 | 0.19 | 55.21 | 81.60 |
| 2,4-Heptadienal | 0.47 | 1.19 | 268.62 | 151.65 |
| 2-Heptenal | 2.33 | 1.82 | 254.48 | 90.68 |
| Hexanal | 2.01 | 2.02 | 33.69 | 6.28 |
| 2-Octenal | 0.40 | 0.67 | 212.60 | 51.96 |
| Pentanal | 5.36 | 1.05 | 10.76 | 2.84 |
| 2,4-Undecadienal | 0.06 | 0.07 | 43.73 | 53.34 |
| 2-Undecenal | 0.19 | 0.19 | 50.29 | 110.38 |
| Ratio8 | 0.12 | 0.21 | 0.58 | 1.14 |
| Total tocopherols, mg/kg2 | 1,328 | 1,331 | 94 | 798 |
| α | 98 | 97 | < 10 | < 10 |
| β | < 10 | < 10 | < 10 | < 10 |
| δ | 196 | 209 | 15 | 169 |
| γ | 1,034 | 1,025 | 79 | 629 |
1Thermally processed oils had constant air flow rate at 15 liter/min.
2Analyzed by Barrow-Agee, Memphis, TN.
3No other FA were detected besides those listed.
4ND = not detected; FA = fatty acid; UFA:SFA = unsaturated:saturated fatty acid ratio; TBA = thiobarbituric acid; OSI = oil stability index; PTAG = polymerized tryacylglycerides; IV = iodine value.
5Iodine values were calculated using the FA profile data following the equation: IV = (16:1 × 0.95) + (18:1 × 0.86) + (18:2 × 1.732) + (18:3 × 2.616) + (20:1 × 0.795) + (20:2 × 1.57) + (20:3 × 2.38) + (20:4 × 3.19) + (20:5 × 4.01) + 22:4 × 2.93) + (22:5 × 3.68) + (22:6 × 4.64); Meadus et al., 2009.
6There are no units for p-anisidine value or TBA value.
7Analyzed by University of Minnesota, St. Paul, MN.
8Ratio of 2-decenal, 2,4-hydroxynonenal, 2,4-undecadienal, and 2-undecenal as a percent of total aldehydes to acrolein, 2,4-heptadienal, and 2-heptenal as a percent of total aldehydes; Wang et al., 2016.
Although statistical comparisons were not made, there are several numerical differences among the SO profiles of interest. Peroxide value has been a common metric by which the oxidative status of lipids used in swine diets has been characterized and therefore is an important measurement available to draw comparisons between the current and previous studies. In these previous experiments, PV ranged from 5.7 to 300 mEq/kg in the oil and 0.2 to 9 mEq/kg in the diets (Liu et al., 2014b, 2014c; Rosero et al., 2015; Dilger et al., 2016). Among the thermally processed oils evaluated in the current study, SO heated at 90 °C had the greatest PV at 123.6 mEq/kg compared with 2, 17.4, and 19.4 mEq/kg of the 22.5 °C, 45 °C, and 180 °C treatments, respectively. The 10% SO inclusion rate used in the experimental diets represents a range of dietary PV of 0.2 to 12.4 mEq/kg. This range fully covers the dietary PV observed in previous studies investigating the effects of peroxidized lipids on swine growth, digestibility and carcass traits, and studies investigating the influence on peroxidized lipid feeding on the shelf life of pork products. Further evidence that the thermal treatments resulted in varying degrees of peroxidative damage was the range in p-anisidine values present in the different oils, ranging from 1.11 to 165. Previous experiments have evaluated oils subjected to varying degrees of thermal abuse, resulting in various oxidative profiles; however, no study to date has investigated such an array of thermal processing treatments under the same experimental conditions, nor have they evaluated the effect such treatments may have on the shelf life of pork products. These previous studies have yielded inconsistent results; therefore, it was an underlying objective of the current study to test a wide battery of thermal processing conditions with the aim of drawing more definitive conclusions to the question of the effects of dietary-peroxidized lipids on pork carcass traits and loin shelf life.
Carcass Characteristics and Early Postmortem Loin Quality
Carcasses of pigs evaluated in the present study were previously part of a feeding trial in which pigs fed 90 °C SO had reduced ADG and G:F as well as reduced DE and acid hydrolyzed ether extract (AEE) digestibility compared with the other SO diets (Overholt et al., 2018). The poorer performance of the pigs fed 90 °C was reflected in the relationships among treatments in regard to carcass characteristics. ELW of pigs fed 90 °C diets was 8.9 and 7.6 kg less (P ≤ 0.03) than pigs fed 45 °C and 180 °C diets, respectively, with ELW of pigs fed 22.5 °C being intermediate (Table 2). This difference in ELW persisted with HCW, as carcasses of pigs fed 90 °C weighed 6.0 to 8.6 kg less than (P ≤ 0.03) carcasses of pigs fed any of the other SO diets, which did not differ (P ≥ 0.36) in HCW. Although the reduced HCW of pigs fed 90 °C diets was largely a function of having reduced ELW, it was exacerbated by a 1.25, 1.26, and 0.76 percentage unit reduction (P ≤ 0.05) in dressing percentage compared with pigs fed 22.5 °C, 45 °C, and 180 °C SO diets, respectively. This observation was in agreement with results of previous studies, in which the reduction in dressing percentage observed in pigs fed peroxidized dried distillers grains with solubles (Song et al., 2014) and corn oil (Boler et al., 2012) was due to reduction in performance and carcass fatness. Although the pigs fed 90 °C in the current study grew less efficiently and at a slower rate than those fed the other SO diets, there was no difference in BF depth (P = 0.44), LMA (P = 0.19), or ECL (P = 0.80) among SO treatments. Therefore, it is unlikely that the reduced dressing percentage of pigs fed 90 °C was due to differences in carcass composition. The reduction in dressing percentage may, in part, be attributable to liver enlargement, as livers of pigs fed 90 °C and 180 °C SO were 14.3% and 11.7%, respectively, larger (P ≤ 0.02) than livers of pigs fed 22.5 °C SO, with livers of pigs fed 45 °C being intermediate. This relationship was even more pronounced when liver weight was expressed as a percentage of ELW, as livers of pigs fed 90 °C accounted for 0.11 percentage units more (P = 0.02) of ELW than did livers of pigs fed 180 °C. Likewise, livers of pigs fed 180 °C accounted for 0.13 percentage units more (P < 0.01) of ELW than livers of pigs fed 22.5 °C SO, with the proportional weight of livers of pigs fed 45 °C being intermediate. Liver enlargement has been previously reported in both pigs (Liu et al., 2014c) and rats (Eder, 1999) fed peroxidized lipids, and is an indication of oxidative stress. Lipid peroxidation products, such as 4-hydroxynonenal (HNE), of which the 90 °C and 180 °C SO had the greatest numerical concentrations in the oil, are the known cytotoxins (Esterbauer et al., 1991; Grootveld et al., 1998). The liver is the primary site of detoxification in the body, and upon prolonged intake of toxins, the liver responds by increasing synthesis of microsomal enzymes (Huang et al., 1988) and increasing hepatocyte proliferation (Dibner et al., 1996), resulting in liver hypertrophy. Compounded with the previously reported increase in F2-isoprostane and TBARS in plasma and urine of pigs fed 90 °C SO (Overholt et al., 2018), the observed liver hypertrophy is a clear sign that feeding 90 °C SO induced oxidative stress to a greater degree than did feeding the other SO treatments (Table 3).
Table 2.
Carcass characteristics of finishing pigs fed peroxidized soybean oil
| Oil treatment | ||||||
|---|---|---|---|---|---|---|
| Item | 22.5 °C | 45 °C | 90 °C | 180 °C | SEM | P-value |
| No. | 13 | 13 | 14 | 13 | ||
| ELW, kg | 127.21ab | 130.48a | 121.59b | 129.15a | 2.37 | 0.05 |
| Lairage loss, % | 3.06 | 3.20 | 3.27 | 3.32 | 0.25 | 0.90 |
| HCW, kg | 101.83a | 104.40a | 95.79b | 102.70a | 1.94 | 0.01 |
| Dressing percentage, % | 79.99a | 80.00a | 78.74b | 79.50a | 0.28 | < 0.01 |
| Liver weight, kg | 1.54b | 1.66ab | 1.76a | 1.72a | 0.05 | 0.03 |
| Liver % ELW | 1.21c | 1.27bc | 1.45a | 1.33b | 0.04 | < 0.0001 |
| 10th rib BF depth, cm | 2.65 | 2.55 | 2.29 | 2.54 | 0.16 | 0.44 |
| LMA, cm2 | 52.46 | 53.01 | 48.51 | 51.38 | 1.59 | 0.19 |
| Estimated carcass lean, % | 51.06 | 51.48 | 52.12 | 51.29 | 0.80 | 0.80 |
| Clear plate IV | 95.68ab | 96.88a | 81.75c | 92.99b | 1.06 | < 0.0001 |
ELW = ending live weight; HCW = hot carcass weight; BF = back fat; LMA = loin muscle area; clear plate IV = iodine value of clear plate fat measured using a Bruker MPA Multi-Purpose FT-NIR Analyzer (Bruker Optics Inc., Billerica, MA).
a-cLS means within a row having different superscripts are statistically different (P ≤ 0.05).
Table 3.
Early postmortem, fresh loin quality characteristics of finishing pigs fed peroxidized soy bean oil
| Item | Oil treatment | SEM | ||||
|---|---|---|---|---|---|---|
| 22.5°C | 45°C | 90°C | 180°C | P-value | ||
| No. | 13 | 14 | 14 | 14 | ||
| 24 h pH | 5.46 | 5.44 | 5.48 | 5.43 | 0.02 | 0.36 |
| L*1 | 49.61 | 49.76 | 50.60 | 49.91 | 0.72 | 0.78 |
| a*1 | 7.60 | 8.48 | 7.96 | 7.82 | 0.42 | 0.49 |
| b*1 | 0.35 | 0.48 | 0.85 | 0.80 | 0.29 | 0.55 |
| Chroma2 | 7.65 | 8.55 | 8.08 | 7.92 | 0.43 | 0.48 |
| Hue angle, °3 | 2.23 | 3.33 | 5.47 | 5.19 | 2.04 | 0.62 |
| NPPC color4 | 2.23 | 2.39 | 2.11 | 2.04 | 0.11 | 0.13 |
| NPPC marbling4 | 1.00 | 1.21 | 1.14 | 1.07 | 0.09 | 0.33 |
| NPPC firmness5 | 2.00 | 2.14 | 1.79 | 1.57 | 0.20 | 0.20 |
| Drip loss, % | 2.70 | 3.18 | 2.92 | 3.55 | 0.30 | 0.20 |
| Moisture, % | 74.11 | 74.22 | 74.11 | 74.44 | 0.31 | 0.84 |
| Extractable lipid, % | 3.36 | 3.20 | 3.28 | 2.93 | 0.26 | 0.65 |
1Measurements conducted with a Minolta CR-400 Chroma-meter (Minolta Camera Co., Ltd., Osaka, Japan) using a D65 light source and a 2° observer with an 8-mm aperture.
2Chroma =
3Hue angle, ° = tan-1(b*/a*) × 57.296.
4 NPPC (1999).
5 NPPC (1991).
IV has become the most accepted metric of determining the technological quality of pork fat (Seman et al., 2013) and is strongly influenced by the qualities of dietary lipids. Thermal processing conditions resulted in divergent IV among SO treatments (22.5 °C = 45 °C > 180 °C ≈ 90 °C), and these differences were reflected in the IV of clear plate adipose tissue. Clear plate IV of carcasses from pigs fed 45 °C SO were 3.4 units greater (P < 0.01) than IV of from carcasses of pigs fed 180 °C SO, with adipose tissue of pigs fed 22.5 °C SO being intermediate. Clear plate IV of pigs fed 90 °C SO drastically reduced compared with the other 3 SO treatments, being 13.9, 15.1, and 11.2 units less than (P < 0.0001) the 22.5 °C, 45 °C, and 180 °C SO treatments, respectively. It is unlikely that the stark reduction in IV observed in the 90 °C was due entirely to the relative reduction in SO IV. This is in spite of the fact that lipid digestibility is reduced when peroxidized lipids are included in the diet (DeRouchey et al., 2004; Rosero et al., 2015; Lindblom et al., 2017), and de novo fatty acid synthesis increases as the amount or digestibility of dietary fat is decreased (Kloareg et al., 2007). Furthermore, the chain length and unsaturation of dietary fatty acids are determinants of the rate of de novo fatty acid synthesis, as palmitic and palmitoleic acids have a greater effect of suppressing de novo fatty acid synthesis than longer chain fatty acids, such as linoleic or oleic acid (Smith et al., 1996). We previously reported that pigs fed 90 °C and 180 °C had the lowest digestibility of AEE of the SO treatments (Overholt et al., 2018). Moreover, the 90 °C SO had, numerically, the greatest concentration of palmitic acid, which may have also contributed to the disproportionate reduction in IV of the 90 °C SO fed pigs compared with the other 3 SO treatments, and especially the IV of pigs fed 180 °C SO.
It is interesting to note that oil composition, carcass weights, or liver weights did not necessarily display a commensurate change with the increasing SO cooking temperatures. When considering this, it is important to note that the SO treatments were specific combinations of time and temperature (selected to represent processing and storage conditions of feeds and feedstuffs). In addition to being thermally processed at different temperatures, the 90 °C and 180 °C treatments were also heated for different lengths of time (72 and 6 h, respectively). The progression of lipid peroxidation, and the production of oxidation products, is dependent on both time and temperature (Labuza and Dugan, 1971).
There was no effect (P ≥ 0.13) of SO on any early postmortem loin quality traits or loin composition, in agreement with the results reported by Boler et al. (2012), but in contrast to the study by Lu et al. (2014). The latter experiment compared loin quality traits of pigs fed either a diet containing 5% oxidized SO and 10% PUFA supplement (diet PV = 9 mEq/kg) or a standard corn–soy diet, and reported that despite there being no difference in NPPC color scores among the 2 treatments, loin chops of pigs fed oxidized SO were less red (reduced a*) than chops from pigs fed a corn–soy diet.
Shelf Life of Loin Chops
Previous studies investigating the effects of diet on pork product shelf life have focused on either the influence of the fatty acid composition of the diet or the supplementation of dietary antioxidants. Few studies have investigated the impact of feeding peroxidized ingredients, and most have done so as a secondary objective to testing the efficacy of dietary antioxidant interventions. The present study was designed with the intent to determine whether there was any effect of feeding peroxidized SO on the shelf life of loin chops, without antioxidant interventions as has been investigated in previous research. Generally, the loins would have been subjected to a “wet-aging” period prior to the initiation of display to best simulate industry-typical conditions. However, it was decided that to best determine whether peroxidized oil feeding affected the shelf life of loin chops, it would be necessary to observe any changes in color beginning on the same day the chops were excised from the carcass (1 d postmortem). Therefore, no “wet-aging” period was employed and chops were packaged, displayed, and baseline color evaluations were recorded 1 d postmortem (approximately 4 h after early postmortem evaluations were conducted).
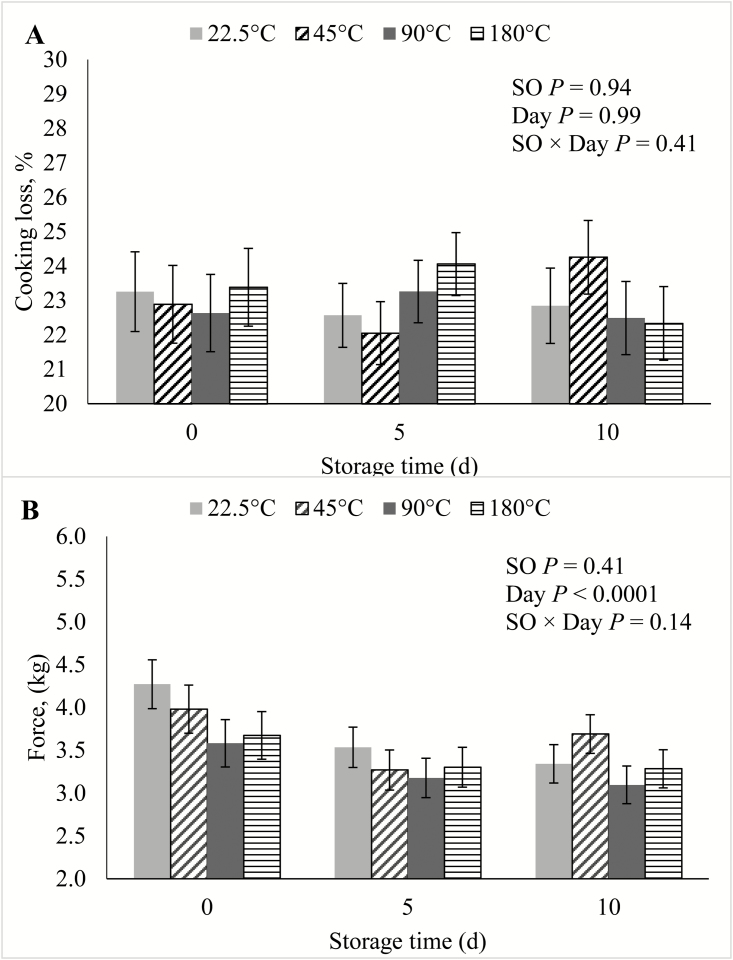
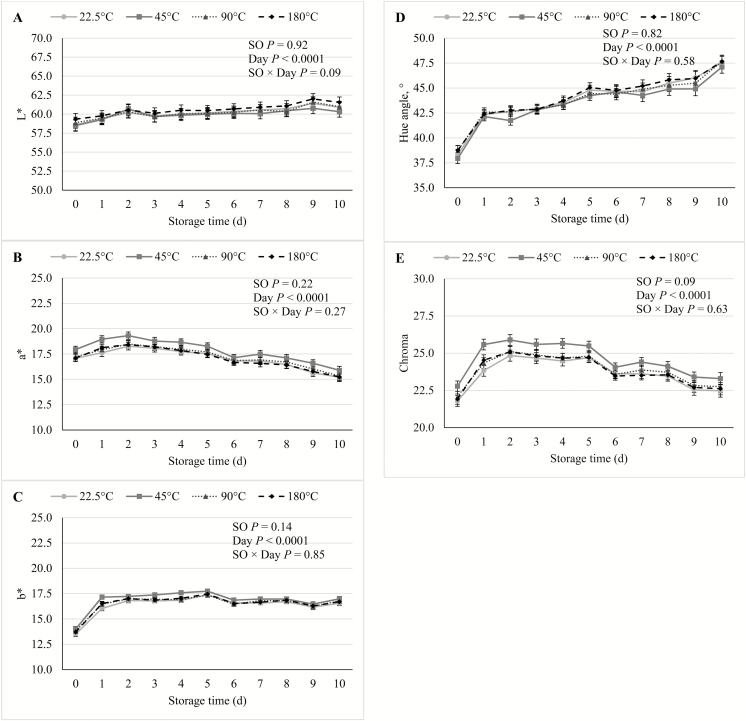
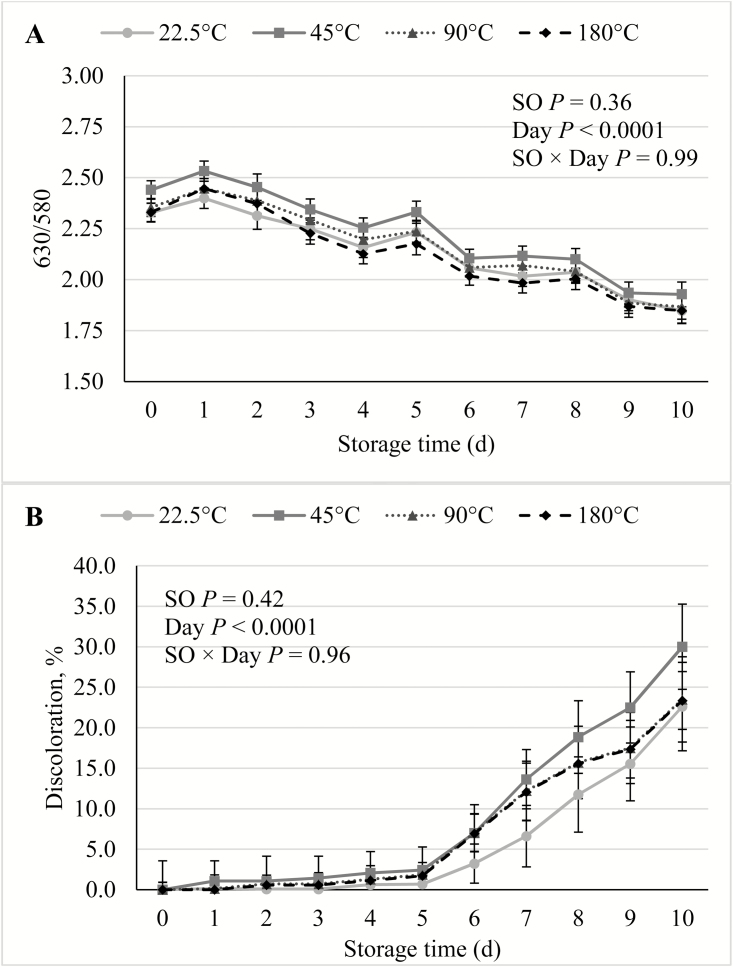
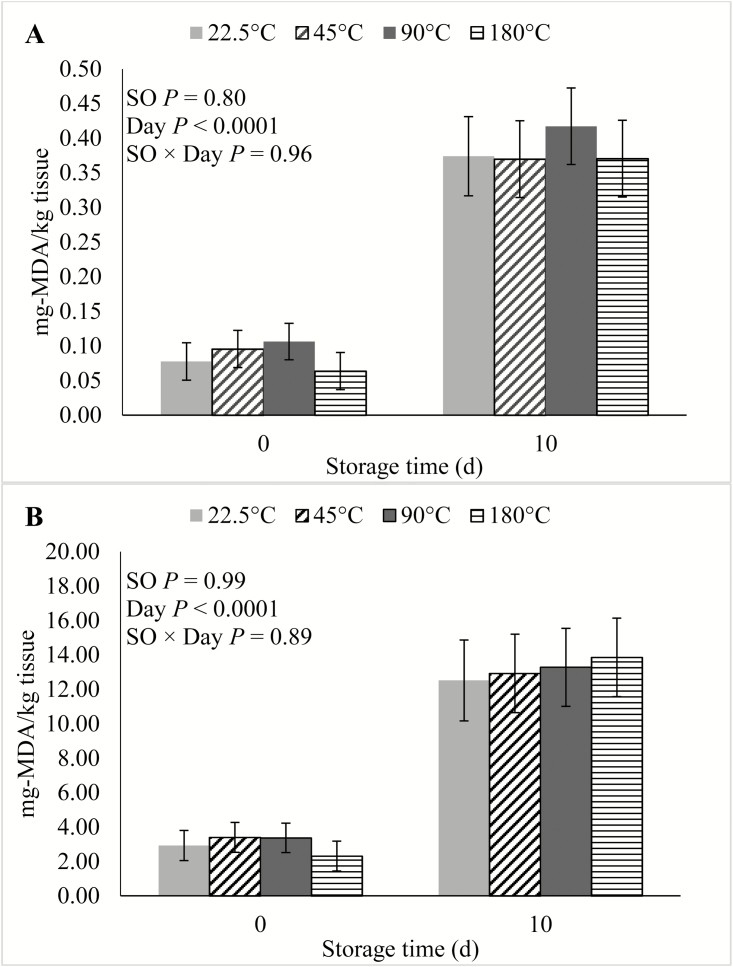
In the current study, there were no SO × Day interactions (P ≥ 0.09) for any of the shelf life traits. During the course of the simulated retail display period, there was no effect (P ≥ 0.14) of SO on either cooking loss, WBSF (Figure 1), lightness, redness, yellowness, hue angle (Figure 2), reflectance, discoloration score (Figure 3), or on TBARS calculated on a tissue or extractable lipid-basis (Figure 4). There was a trend (P = 0.09) for loin chops from pigs fed 45 °C SO to have greater (P ≤ 0.09) chroma than chops from pigs the other 3 SO treatments. Early studies reported that feeding peroxidized corn oil to pigs increased the rate of lipid oxidation, compared with feeding fresh corn oil, in loin chops (Buckley et al., 1989; Monahan et al., 1992) and ground pork patties (Buckley et al., 1989). A similar effect was reported both fresh and cooked loin chops of pigs fed rancid rice bran (Chae and Lee, 2002) and peroxidized SO (Murphy et al., 1991). In agreement with the results of the current study, both Monahan et al. (1994) and Boler et al. (2012) reported that despite having reduced tissue vitamin E concentrations, the color stability and lipid oxidation of loin chops of pigs fed peroxidized corn oil did not differ from chops of pigs fed fresh oil. The penetration of O2 into whole muscle meat is limited to the outermost 1 to 2 mm (O’Keeffe and Hood, 1982), and it is possible that if these outer-most portions of the chops had been evaluated for lipid oxidation, differences would have been detected. However, no differences were detected among SO treatments for any of the color traits, all of which were measured on the surface of the chops. Feeding peroxidized lipids to broilers has routinely elicited a decrease in muscle vitamin E levels and increased susceptibility to lipid oxidation of both breast and thigh meat (Jensen et al., 1997; Tavárez et al., 2011; Zhang et al., 2011). Only one study (Murphy et al., 1991) reported an analogous result in pork. It is not entirely clear why peroxidized lipid feeding results in reduced shelf life in poultry meat, but not pork, despite the intake of peroxidized lipids causing measurable indications of oxidative stress in plasma, liver, and even adipose tissue of both species (Tavárez et al., 2011; Boler et al., 2012). The differential response to diet-induced oxidative stress in muscle may be due to the physiological differences that exist between the species. In general, porcine muscle has a greater proportion of oxidative fibers than chicken breast muscle, which is almost entirely composed of glycolytic fibers. Oxidative muscle fibers have a preference for aerobic metabolism, relying more heavily on β-oxidation of fatty acids and the electron transport chain to produce ATP than glycolytic fibers. Reactive oxygen species (ROS), such as superoxide, are produced as a by-product of aerobic metabolism and because ROS can damage cellular structures, antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidases (GPx) are synthesized by cells to neutralize ROS (Schieber and Chandel, 2014). Activity of catalase (CAT), SOD, and GPx were correlated with the relative number of oxidative fibers in 6 different muscles in rats (Laughlin et al., 1990). This relationship was later confirmed in a comparison of antioxidant enzyme activity in pork psoas major and longissimus dorsi (Lauridsen et al., 1999). The difference in muscle fiber-type composition being a driving force to cause the disparity in the oxidative stress/shelf life relationship between species is further evidenced by the fact that pork has greater concentrations of CAT (Rhee et al., 1996; Pradhan et al., 2000) and SOD (Avanao et al., 2001; Hernández et al., 2004), as well as carnosine (Tian et al., 2007; Mora et al., 2008), than do chickens, regardless of which muscles are evaluated. Although muscle fiber-type differences seem a likely contributor to the differential susceptibility of peroxidized lipid intake to cause reduced oxidative stability of muscle and meat products, further research must be conducted to establish whether a causal link exists.
Figure 1.
Effects of feeding peroxidized soybean oil (SO) to finishing pigs on (A) cooking loss and (B) Warner–Bratzler shear force of loin chops subjected to 0, 5, or 10 d of simulated retail display.
Figure 2.
Effects of feeding peroxidized soybean oil (SO) to finishing pigs on (A) lightness (L*), (B) redness (a*), (C) yellowness (b*), (D) chroma, and (E) hue angle of loin chops during 10 d of simulated retail display. Daily evaluations of instrumental color (L*, a*, b*, 650/580 ratio) were conducted using a Hunter Lab Miniscan XE Plus (Model 45/0-L, Hunter Associates Laboratory Inc., Reston, VA) using an A illuminant, 10° observer, and a 25-mm aperture. Calibration of the device was conducted prior to each daily evaluation using a black glass and a white ceramic tile covered with the same polyvinylchloride (PVC) film used to package the loin chops. Daily visual discoloration scores were determined by a single trained observer using a 10-cm unstructured line scale anchored at 0% and 100% surface discoloration, with each 1-cm increment equal to 10% discoloration of the chop surface.
Figure 3.
Effects of feeding peroxidized soybean oil (SO) to finishing pigs on (A) reflectance and (B) discoloration of loin chops during 10 d of simulated retail display.
Figure 4.
Effects of feeding peroxidized soybean oil (SO) to finishing pigs on thiobarbituric reactive substances (TBARS) of loin chops subjected to 0 or 10 d of simulated retail display calculated on (A) tissue-basis or (B) extractable lipid content-basis.
Lightness, yellowness, hue angle, and discoloration score over storage time increased (P < 0.0001) with storage time, whereas redness, chroma, reflectance, and WBSF all decreased (P < 0.0001) with storage time. These results were consistent with previous studies documenting color stability and deterioration in pork loin (Lindahl et al., 2006; Tikk et al., 2008).
CONCLUSIONS
The extent to which peroxidized SO affected carcass characteristics was dependent on the severity of the thermal processing treatment, with pigs fed the 90 °C SO having reduced HCW and dressing percentage, and feeding SO processed at 45 °C and 180 °C having little effect on carcass characteristics compared with pigs fed the 22.5 °C SO diets. Despite the peroxidized SO feeding inducing signs of oxidative stress, feeding peroxidized SO was not detrimental to the color stability or lipid oxidation of loin chops. Therefore, peroxidized SO feeding is deleterious to carcass value, but appears to be of little concern as it applies to the quality and shelf life of pork loins.
Footnotes
Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the University of Illinois or the United States Department of Agriculture (USDA) and does not imply approval to the exclusion of other products that may be suitable. The USDA is an equal opportunity provider and employer.
LITERATURE CITED
- Avanzo J. L., de Mendonça C. X. Jr, Pugine S. M., and de Cerqueira Cesar M.. 2001. Effect of vitamin E and selenium on resistance to oxidative stress in chicken superficial pectoralis muscle. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 129:163–173. doi:10.1016/S1532-0456(01)00197-1 [DOI] [PubMed] [Google Scholar]
- Boler D. D., Fernández-Dueñas D. M., Kutzler L. W., Zhao J., Harrell R. J., Campion D. R., McKeith F. K., Killefer J., and Dilger A. C.. 2012. Effects of oxidized corn oil and a synthetic antioxidant blend on performance, oxidative status of tissues, and fresh meat quality in finishing barrows. J. Anim. Sci. 90:5159–5169. doi:10.2527/jas.2012-5266 [DOI] [PubMed] [Google Scholar]
- Boler D. D., Kutzler L. W., Meeuwse D. M., King V. L., Campion D. R., McKeith F. K., and Killefer J.. 2011. Effects of increasing lysine on carcass composition and cutting yields of immunologically castrated male pigs. J. Anim. Sci. 89:2189–2199. doi:10.2527/jas.2010-3640 [DOI] [PubMed] [Google Scholar]
- Boler D. D., Puls C. L., Clark D. L., Ellis M., Schroeder A. L., Matzat P. D., Killefer J., McKeith F. K., and Dilger A. C.. 2014. Effects of immunological castration (improvest) on changes in dressing percentage and carcass characteristics of finishing pigs. J. Anim. Sci. 92:359–368. doi:10.2527/jas.2013-6863 [DOI] [PubMed] [Google Scholar]
- Buckley D. J., Gray J. I., Asghar A., Price J. F., Crackel R. L., Booren A. M., Pearson A. M., and Miller E. R.. 1989. Effects of dietary antioxidants and oxidized oil on membranal lipid stability and pork product quality. J. Food Sci. 54:1193–1197. doi:10.1111/j.1365–2621.1989.tb05952.x [Google Scholar]
- Burson D., and Berg E.. 2001. Procedures for estimating pork carcass composition. Pork facts. Natl. Pork Prod. Counc, Des Moines, IA. [Google Scholar]
- Chae B. J. and Lee S. K.. 2002. Rancid rice bran affects growth performance and pork quality in finishing pigs. Asia-Aust. J. Anim. Sci. 15:94–101. doi:10.5713/ajas.2002.94 [Google Scholar]
- CIE 1978. Recommendations on uniform color spaces-color equations, psychometric color terms. Suppl. No. 2 to CIE Publ. No. 15 (E-1.3.L) 1971 (9TC-1–3), Paris, France [Google Scholar]
- DeRouchey J. M., Hancock J. D., Hines R. H., Maloney C. A., Lee D. J., Cao H., Dean D. W., and Park J. S.. 2004. Effects of rancidity and free fatty acids in choice white grease on growth performance and nutrient digestibility in weanling pigs. J. Anim. Sci. 82:2937–2944. doi:10.2527/2004.82102937x [DOI] [PubMed] [Google Scholar]
- Dibner J. J., Atwell C. A., Kitchell M. L., Shermer W. D., and Ivey F. J.. 1996. Feeding of oxidized fats to broilers and swine: effects on enterocyte turnover, hepatocyte proliferation and the gut associated lymphoid tissue. Anim. Feed Sci. Tech. 62:1–13. doi:10.1016/S0377-8401(96)01000-0 [Google Scholar]
- Dilger A. C., Overholt M. F., and Boler D. D.. 2016. Implications of dietary oxidized oils for fresh and further processed pork quality. In: Proc. 16th Annu. Midwest Swine Nutr. Conf, 9 September 2016, Indianapolis, IN. [Google Scholar]
- Eder K. 1999. The effects of a dietary oxidized oil on lipid metabolism in rats. Lipids. 34:717–725. doi:10.1007/s11745-999-0418-0 [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Schaur R. J., and Zollner H.. 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11:81–128. doi:10.1016/0891-5849(91)90192-6 [DOI] [PubMed] [Google Scholar]
- Grootveld M., Atherton M. D., Sheerin A. N., Hawkes J., Blake D. R., Richens T. E., Silwood C. J., Lynch E., and Claxson A. W.. 1998. In vivo absorption, metabolism, and urinary excretion of alpha,beta-unsaturated aldehydes in experimental animals. Relevance to the development of cardiovascular diseases by the dietary ingestion of thermally stressed polyunsaturate-rich culinary oils. J. Clin. Invest. 101:1210–1218. doi:10.1172/JCI1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. R., Urriola P. E., Wang L., Johnston L. J., Chen C., and Shurson G. C.. 2016. Dietary peroxidized maize oil affects the growth performance and antioxidant status of nursery pigs. Anim. Feed Sci. Tech. 216:251–261. doi:10.1016/j.anifeedsci.2016.03.027 [Google Scholar]
- Hernández P., Zomeño L., Ariño B., and Blasco A.. 2004. Antioxidant, lipolytic and proteolytic enzyme activities in pork meat from different genotypes. Meat Sci. 66:525–529. doi:10.1016/S0309-1740(03)00155-4 [DOI] [PubMed] [Google Scholar]
- Holmer S. F., McKeith R. O., Boler D. D., Dilger A. C., Eggert J. M., Petry D. B., McKeith F. K., Jones K. L., and Killefer J.. 2009. The effect of pH on shelf-life of pork during aging and simulated retail display. Meat Sci. 82:86–93. doi:10.1016/j.meatsci.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Huang C. J., Cheung N. S., and Lu V. R.. 1988. Effects of deteriorated frying oil and dietary protein levels on liver microsomal enzymes in rats. J. Am. Oil. Chem. Soc. 65:1796–1803. doi:10.1007/BF02542385 [Google Scholar]
- Jensen C., Engberg R., Jakobsen K., Skibsted L. H., and Bertelsen G.. 1997. Influence of the oxidative quality of dietary oil on broiler meat storage stability. Meat Sci. 47:211–222. doi: 10.1016/S0309-1740(97)00052-1 [DOI] [PubMed] [Google Scholar]
- Kloareg M., Noblet J., and van Milgen J.. 2007. Deposition of dietary fatty acids, de novo synthesis and anatomical partitioning of fatty acids in finishing pigs. Br. J. Nutr. 97:35–44. doi:10.1017/S0007114507205793 [DOI] [PubMed] [Google Scholar]
- Labuza T. P. and Dugan L. R. Jr. 1971. Kinetics of lipid oxidation in foods. CRC Crit. Rev. Food Technol. 2:355–405. doi:10.1080/10408397109527127 [Google Scholar]
- Laughlin M. H., Simpson T., Sexton W. L., Brown O. R., Smith J. K., and Korthuis R. J.. 1990. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J. Appl. Physiol. (1985). 68:2337–2343. doi:10.1152/jappl.1990.68.6.2337 [DOI] [PubMed] [Google Scholar]
- Lauridsen C., Nielsen J. H., Henckel P., and Sørensen M. T.. 1999. Antioxidative and oxidative status in muscles of pigs fed rapeseed oil, vitamin E, and copper. J. Anim. Sci. 77:105–115. doi:10.2527/1999.771105x [DOI] [PubMed] [Google Scholar]
- Lin X, Azain M., and Odle J.. 2013. Lipids and lipid utilization in swine. In: Chiba L., editor, Sustainable swine nutrition. Blackwell Publishing Ltd, Oxford, UK: p. 59–79. [Google Scholar]
- Lindahl G., Karlsson A. H., Lundström K., and Andersen H. J.. 2006. Significance of storage time on degree of bloomin and colour stability of pork loin from different crossbreeds. Meat Sci. 72:603–612. doi:10.1016/j.meatsci.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Lindblom S. C., Gabler N. K., and Kerr B. J.. 2017. Influence of feeding thermally peroxidized soybean oil on growth performance and oxidative status in growing pigs. J. Anim. Sci. 95(Supp. 2):45. doi:10.2527/asasmw.2017.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Kerr B. J., Chen C., Weber T. E., Johnston L. J., and Shurson G. C.. 2014a. Influence of thermally oxidized vegetable oils and animal fats on energy and nutrient digestibility in young pigs. J. Anim. Sci. 92:2980–2986. doi:10.2527/jas.2012–5711 [DOI] [PubMed] [Google Scholar]
- Liu P., Kerr B. J., Chen C., Weber T. E., Johnston L. J., and Shurson G. C.. 2014b. Methods to create thermally oxidized lipids and comparison of analytical procedures to characterize peroxidation. J. Anim. Sci. 92:2950–2959. doi:10.2527/jas.2012-5708 [DOI] [PubMed] [Google Scholar]
- Liu P., Chen C., Kerr B. J., Weber T. E., Johnston L. J., and Shurson G. C.. 2014c. Influence of thermally oxidized vegetable oils and animal fats on growth performance, liver gene expression, and liver and serum cholesterol and triglycerides in young pigs. J. Anim. Sci. 92:9260–9270. doi:10.2527/jas2012-5709 [DOI] [PubMed] [Google Scholar]
- Lu T., Harper A. F., Dibner J. J., Scheffler J. M., Corl B. A., Estienne M. J., Zhao J., and Dalloul R. A.. 2014. Supplementing antioxidants to pigs fed diets high in oxidants: II. Effects on carcass characteristics, meat quality, and fatty acid profile. J. Anim. Sci. 92:5464–5475. doi:10.2527/jas2013-7112 [DOI] [PubMed] [Google Scholar]
- Meadus W. J., Duff P., Uttaro B., Aalhus J. L., Rolland D. C., Gibson L. L., and Dugan M. E. R.. 2009. Production of docosahexaenoic acid (DHA) enriched bacon. J. Agr. Food Chem. 58:465–472. doi: 10.1021/jf9028078 [DOI] [PubMed] [Google Scholar]
- Meeker D. L., and Hamilton C. R.. 2006. An overview of the rendering industry. In: Meeker D. L., editor, Essential rendering. National Renderers Assoc, Alexandria, VA: p. 1–16. [Google Scholar]
- Monahan F. J., Asghar A., Gray J. I., Buckley D. J., and Morrissey P. A.. 1994. Effect of oxidized dietary lipid and vitamin E on the colour stability of pork chops. Meat Sci. 37:205–215. doi:10.1016/0309-1740(94)90081-7 [DOI] [PubMed] [Google Scholar]
- Monahan F. J., Gray J. I., Booren A. M., Miller E. R., Buckley D. J., Morrissey P. A., and Gomaa E. A.. 1992. Influence of dietary treatment on lipid and cholesterol oxidation in pork. J. Agric. Food Chem. 40:1310–1315. doi:10.1021/jf00020a003 [Google Scholar]
- Mora L., Sentandreu M. Á., and Toldrá F.. 2008. Contents of creatine, creatinine and carnosine in porcine muscles of different metabolic types. Meat Sci. 79:709–715. doi:10.1016/j.meatsci.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Murphy T. K., Lynch P. B., Buckley D. J., Monahan F. J., and Morrissey P. A.. 1991. Effect of dietary fat quality and α-tocopheryl acetate supplementation on the susceptibility of porcine tissue to lipid peroxidation. Proc. 37th Int. Congr. Meat Sci. Technol, Kulmbach, Germany p. 1269–1273. [Google Scholar]
- National Pork Producers Council (NPPC) 1991. Procedures to evaluate market hogs. 3rd ed Natl. Pork Prod. Counc, Des Moines, IA. [Google Scholar]
- National Pork Producers Council (NPPC) 1999. Official color and marbling standards. Natl. Pork Prod. Counc, Des Moines, IA. [Google Scholar]
- North American Meat Processors Association 2007. The meat buyer’s guide. North Am. Meat Proc. Assoc, Reston, Va. [Google Scholar]
- Novakofski J., Park S., Bechtel P. J., and McKeith F. K.. 1989. Composition of cooked pork chops—Effect of removing subcutaneous fat before cooking. J. Food Sci. 54:15–17. doi:10.1111/j.1365–2621.1989.tb08556.x [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- O’Brien R. D. 2009. Fats and oils: formulating and processing for application. In: O’Brien R. D., editor, CRC Press, Boca Raton, FL. [Google Scholar]
- O’Keeffe M., and Hood D. E.. 1982. Biochemical factors influencing metmyoglobin formation on beef from muscles of differing colour stability. Meat Sci. 7:209–228. doi: 10.1016/0309-1740(82)90087-0 [DOI] [PubMed] [Google Scholar]
- Overholt M. F., Dilger A. C., Boler D. D., and Kerr B. J.. 2018. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, and gut integrity of finishing pigs. J. Anim. Sci. 96:2789–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan A. A., Rhee K. S., and Hernández P.. 2000. Stability of catalase and its potential role in lipid oxidation in meat. Meat Sci. 54:385–390. doi:10.1016/S0309-1740(99)00114-X [DOI] [PubMed] [Google Scholar]
- Rhee K. S., Anderson L. M., and Sams A. R.. 1996. Lipid oxidation potential of beef, chicken and pork. J. Food Sci. 61:8–12. doi: 10.1111/j.1365-2621.1996.tb14714.x [DOI] [Google Scholar]
- Rosero D. S., Odle J., Moeser A. J., Boyd R. D., and van Heugten E.. 2015. Peroxidised dietary lipids impair intestinal function and morphology of the small intestine villi of nursery pigs in a dose-dependent manner. Br. J. Nutr. 114:1985–1992. doi:10.1017/S000711451500392X [DOI] [PubMed] [Google Scholar]
- Schieber M. and Chandel N. S.. 2014. ROS function in redox signaling and oxidative stress. Curr. Biol. 24:R453–R462. doi:10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seman D. L., Barron W. N., and Matzinger M.. 2013. Evaluating the ability to measure pork fat quality for the production of commercial bacon. Meat Sci. 94:262–266. doi:10.1016/j.meatsci.2013.01.009 [DOI] [PubMed] [Google Scholar]
- Smith D. R., Knabe D. A., and Smith S. B.. 1996. Depression of lipogenesis in swine adipose tissue by specific dietary fatty acids. J. Anim. Sci. 74:975–983. doi:10.2527/1996.745975x [DOI] [PubMed] [Google Scholar]
- Song R., Chen C., Johnston L. J., Kerr B. J., Weber T. E., and Shurson G. C.. 2014. Effects of feeding diets containing highly peroxidized distillers dried grains with solubles and increasing vitamin E levels to wean-finish pigs on growth performance, carcass characteristics, and pork fat composition. J. Anim. Sci. 92:198–210. doi:10.2527/jas.2013-6334 [DOI] [PubMed] [Google Scholar]
- Tavárez M. A., Boler D. D., Bess K. N., Zhao J., Yan F., Dilger A. C., McKeith F. K., and Killefer J.. 2011. Effect of antioxidant inclusion and oil quality on broiler performance, meat quality, and lipid oxidation. Poultry Sci. 90:922–930. doi: 10.3382/ps.2010-01180 [DOI] [PubMed] [Google Scholar]
- Tian Y., Xie M., Wang W., Wu H., Fu Z., and Lin L.. 2007. Determination of carnosine in black-bone silky foul (gallus gallus domesticus Brisson) and common chicken by HPLC. Eur. Food Res. Technol. 226:311–314. doi:10.1007/s00217-006-0528-1 [Google Scholar]
- Tikk K., Lindahl G., Karlsson A. H., and Andersen H. J.. 2008. The significance of diet, slaughter weight and aging time on pork colour and colour stability. Meat Sci. 79:806–816. doi:10.1016/j.meatsci.2007.11.015 [DOI] [PubMed] [Google Scholar]
- Zhang W., Xiao S., Lee E. J., and Ahn D. U.. 2011. Consumption of oxidized oil increases oxidative stress in broilers and affects quality of breast meat. J. Agric. Food. Chem. 59:969–974. doi: 10.1021/jf102918z [DOI] [PubMed] [Google Scholar]