Abstract
The aim of this study was to investigate the effects of dietary supplementation with guanidinoacetic acid (GAA) on the growth performance, creatine and energy metabolism, and carcass characteristics in growing-finishing pigs. In Exp. 1, Duroc × Landrace × Yorkshire pigs (n = 180, 33.61 ± 3.91 kg average BW) were blocked by weight and sex, and allotted to 5 treatments with 6 replicates (3 gilts and 3 barrows per replicate pen). Diets were corn-soybean meal–basal diets supplemented with 0, 300, 600, 900, and 1,200 mg/kg of GAA and fed to the pigs for 98 d. From days 1 to 98, G:F increased (linear, P < 0.05) with increasing addition of dietary GAA. Using a broken-line model, the optimum level of dietary GAA was 300 mg/kg during the overall experimental period (days 1 to 98) to maximize G:F. Hot carcass weight, carcass length, and lean percentage showed a tendency to increase (quadratic, 0.05 < P < 0.10) with increasing addition of dietary GAA. On day 98, serum GAA and liver creatine tended to increase (linear, P = 0.10, 0.07) as dietary GAA increased. In addition, serum ATP on day 98 increased linearly (linear, P < 0.01), and muscle ATP and adenosine monophosphate increased quadratically (quadratic, P = 0.05) with incremental GAA supplementation. In Exp. 2, Duroc × Landrace × Yorkshire pigs (n = 180, 53.19 ± 5.63 kg average BW) were blocked by weight and sex, and allotted to 5 treatments with 6 replicates (3 gilts and 3 barrows per replicate pen). Diets were corn-soybean meal–basal diets supplemented with 0, 150, 300, 600, and 1,200 mg/kg of GAA for 35 d. As dietary GAA increased, final BW, ADG, and G:F increased quadratically (quadratic, P < 0.01), and 300 mg/kg of GAA maximized ADG and final BW (P < 0.05).The results indicate that dietary GAA could increase the creatine and ATP load in the tissues of pigs and accordingly improve growth performance. Dietary supplementation with 300 mg/kg of GAA was suitable to maximize the growth performance of growing-finishing pigs.
Keywords: creatine, energy metabolism, guanidinoacetic acid, performance, pig
INTRODUCTION
Guanidinoacetic acid (GAA; chemical formula C3H7N3O2) is a metabolic intermediate synthesized from the amino acids Gly and Arg, which are subsequently methylated to creatine. Creatine phosphate serves as a dynamic reservoir of high-energy phosphate in exchange with ATP. In animals, approximately 1.7% of total body creatine plus creatine phosphate spontaneously broke down every day (Wyss and Kaddurah-Daouk, 2000). Most growing-finishing pig diets are plant-based which contain little or no dietary creatine. Therefore, there is a need to supplement creatine in pig diets. Reports show that creatine, when used as an additive to pig diets, cannot only improve the body weight gain (Maddock et al., 2002; Young et al., 2005), but also help us to improve the quality of the pork (James et al., 2002). Compared with creatine, GAA is more stable and less expensive, which has led to the idea that perhaps GAA could be a substitute for dietary creatine (Liu, 2015).
Recently, GAA has been used as performance-enhancing agent for animals (Ringel et al., 2007; Michiels et al., 2012; Wang et al., 2012). The Commission Implementing Regulation (EU) 2016/1768 recommended 600 to 1,200 mg/kg of GAA for weaned piglets and pigs for fattening (European Commission, 2016). The European Food Safety Authority (EFSA, 2016) concluded that 1,200 mg of GAA/kg complete feed improved the growth of piglets, and this conclusion was extended to finishing pigs. However, Wang et al. (2012) reported that dietary supplemental GAA from 800 to 2,000 mg/kg in growing pigs (BW = 45 kg for 54 d) did not affect the growth performance. There is a scarcity of data concerning the effect of GAA on growth performance during the entire grow-to-finish period and on the carcass characteristics of pigs. Therefore, the objective of this study was to determine the efficacy of graded dietary levels of GAA on the growth performance, carcass characteristics, biochemical parameters, and energy metabolism in pigs from 30 kg BW until market weight.
MATERIALS AND METHODS
These experiments were conducted at the Fengning Animal Experimental Base of the National Feed Engineering Technology Research Center (Hebei, China) and were approved by and conducted in accordance with the animal care protocols of the Animal Welfare Committee of China Agricultural University (Beijing, China).
General Procedure
Experiment 1 was performed to determine the effective dietary concentration of GAA needed to maximize pig growth. On the basis of Exp. 1, Exp. 2 was designed to further optimize the inclusion level of GAA in growing-finishing pigs. The GAA used in this study was provided by Gendone Agriculture Technology Co., Ltd. (Beijing, China), with purity of more than 98.0%. Crossbred pigs (Duroc × Landrace × Yorkshire) were used in these studies. The corn-soybean meal–basal diets (Table 1) for 3 experimental periods were formulated to meet or exceed NRC (2012) requirement estimates for pigs from 25 to 50 kg BW, 50 to 75 kg BW, and 75 to 100 kg BW, respectively. Experiment 1 included 3 diet formulation phases: Grower (days 1 to 35), Finisher I (days 36 to 70), and Finisher II (days 71 to 98). Experiment 2 used the same diet as Finisher I of Exp. 1.
Table 1.
Ingredient and analyzed composition of experimental diets (as fed basis, %)
| Item | Grower | Finisher I | Finisher II |
|---|---|---|---|
| Ingredient | |||
| Corn | 68.10 | 68.06 | 70.04 |
| Wheat bran | 4.00 | 5.33 | 7.45 |
| Soybean meal (46% CP) | 23.00 | 22.00 | 18.00 |
| Soybean oil | 1.00 | 1.00 | 1.20 |
| Dicalcium phosphate | 1.18 | 1.00 | 0.70 |
| Limestone | 0.85 | 0.77 | 0.80 |
| Sodium chloride | 0.35 | 0.35 | 0.30 |
| Sodium bicarbonate | - | 0.10 | 0.15 |
| Choline chloride (50%) | 0.08 | 0.08 | 0.08 |
| L-Lys HCl (78.8%) | 0.30 | 0.21 | 0.20 |
| DL-Met (98.5%) | 0.14 | 0.1 | 0.08 |
| Premix1 | 1.00 | 1.00 | 1.00 |
| Total | 100 | 100 | 100 |
| Calculated composition | |||
| DE, kcal/kg | 3337 | 3272 | 3267 |
| CP | 16.89 | 15.82 | 14.53 |
| Total Ca | 0.69 | 0.62 | 0.55 |
| Total P | 0.58 | 0.54 | 0.49 |
| Available P | 0.34 | 0.31 | 0.25 |
| SID Lys | 0.90 | 0.81 | 0.71 |
| SID Met | 0.31 | 0.28 | 0.25 |
| SID Met + Cys | 0.56 | 0.51 | 0.46 |
| Analyzed composition2 | |||
| DM | 88.10 | 88.65 | 88.69 |
| CP | 18.79 | 17.16 | 16.42 |
| Total Ca | 0.82 | 0.69 | 0.64 |
| Total P | 0.43 | 0.40 | 0.36 |
| Total Lys | 1.23 | 1.07 | 0.97 |
| Total Met | 0.48 | 0.43 | 0.38 |
| Total Met + Cys | 0.88 | 0.78 | 0.63 |
Within each phase, guanidinoacetic acid was added at 0, 300, 600, 900 and 1,200 mg/kg to create 5 experimental diets.
1Provided per kilogram of diet: 6000 IU of vitamin A; 1500 IU of vitamin D3; 15 IU of vitamin E; 1.5 mg of vitamin K3; 0.9 mg of thiamine; 3 mg of riboflavin; 1.5 mg of pyridoxine; 10 μg of vitamin B12;17 mg of nicotinic acid; 9 mg of pantothenic acid; 0.32 mg of folic acid; 0.02 mg of biotin; 350 mg of choline chloride; 90 mg of Fe; 8 mg of Cu; 50 mg of Zn; 30 mg of Mn; 0.30 mg of Se; 0.32 mg of I.
2The presented nutrient composition is the mean of samples taken from each 5 experimental diets.
Diets were analyzed according to the methods of the Association of Official Analytical Chemists (AOAC, 2000) for DM (AOAC method 930.15), CP (AOAC method 988.05), calcium (AOAC method 927.02), total phosphorus (AOAC method 995.11), and amino acids (AOAC method 994.12). Lysine was analyzed after acid hydrolysis with 6 N HCl at 110 °C for 24 h using an amino acid analyzer (Hitachi L-8900, Tokyo, Japan). For dietary Met and Cys determination, performic acid oxidation was performed prior to acid hydrolysis with 6 M HCl.
Experiment 1
Experimental design.
This experiment was conducted as a randomized complete block design. A total of 180 (average initial BW 33.61 ± 3.91 kg) pigs were blocked by weight and sex and assigned randomly to 5 dietary treatments with 6 replicates (3 gilts and 3 barrows per replicate pen). The dietary treatments were corn-soybean meal–basal diets supplemented with 0, 300, 600, 900, and 1,200 mg/kg GAA for 98 d.
Animal housing and blood collection.
All pigs were fed 2 times per day (900 and 1500 h). Each pen was equipped with a stainless steel feeder and a nipple drinker to allow the pigs to have ad libitum access to feed and water. Room temperature was maintained at approximately 21 °C. Pigs were weighed individually at the start of the experiment as well as on days 35, 70, and 98. Feed intake was recorded, and ADG, ADFI, and G:F were calculated on a pen basis. At the end of each phase (Grower, Finisher I, and Finisher II), 1 pig that was visually approximated to be the average weight of all pigs in a pen was selected from each pen for blood sampling. Blood samples (approximately 10 mL) were drawn through the jugular vein using a 0.9-mm-diameter needle into vacutainer tubes. Blood samples were then centrifuged at 1,500 × g for 10 min at 4 °C in a refrigerated centrifuge. The serum was apportioned into 1.0-mL aliquots and immediately stored at −20 °C for later analysis.
Carcass traits and sampling.
At the end of this experiment, 1 pig that was approximated to be the average weight of all pigs in a pen was selected from each pen. The 30 selected pigs were transported to a commercial abattoir. The pigs were electrically stunned and then killed by exsanguination. The carcasses were dehaired, eviscerated, split longitudinally, and weighed. Dressing percentage was calculated as HCW divided by live weight at harvest ×100. Carcass length was measured as the distance from the first rib to the aitch bone. The left side of the carcass was split at the 10th rib to determine the 10th-rib back fat thickness and longissimus muscle area. The “lean percentage” was calculated according to the equation suggested by the National Pork Producers Council (Berg, 2000). Approximately 5-g samples of the longissimus dorsi muscle and the kidney from the left side of each carcass were collected. Samples of the liver, consisting of the bottom end of each lobule, were collected. The above samples were placed in RNAase-free tubes, then immediately frozen in liquid nitrogen, and stored at −80 °C for subsequent analysis.
Chemical analysis.
The contents of GAA and creatine in plasma and tissues were determined by HPLC (Agilent 1200, Agilent Technologies, Santa Clara, CA, USA) according to the method reported by Buchberger and Ferdig (2004). For sample pretreatment, 1 mL of serum or 1 g of tissue was thoroughly mixed with 3 mL (for serum) or 4 mL (for tissue) of 5% sulfosalicylic acid solution. After centrifugation (214,200 × g, 30 min), the liquid layer was collected as the sample solution. In an HPLC vial, 600 µL of the sample solution was mixed with 450 µL of 1.3 M KOH and 225 µL of a 0.9% aqueous ninhydrin solution. After a reaction time of 15 min at room temperature, 150 µL of 0.5% ascorbic acid solution and 150 µL of 5 M phosphoric acid were added. The closed vial was treated at 90 °C for 30 min. After cooling at room temperature for 5 min, the sample was injected into the HPLC system. Calibration curves were drawn for quantitative determination of GAA and creatine, which showed a range of linearity between 1 and 200 µg/liter for GAA and creatine. The quantitative limits of GAA and creatine were 0.06 and 0.02 µg/mL or µg/g, respectively. The recoveries of GAA and creatine were 88.33% to 95.60%, and 89.58% to 97.73%, respectively. The content of creatinine in serum was measured by commercial kits (Biosino Biotechnology and Science Inc, Beijing, China) according to the manufacturer’s instructions.
Analysis of energy-related metabolites.
The content of ATP in serum was measured by ATP assay kit (Jiancheng Biochemical Reagent Co., Nanjing, China) according to the manufacturer’s instructions. The creatine kinase (CK) activity in the serum and muscle was elevated by assay kits (Biosino Biotechnology and Science Inc., Beijing, China). Phosphorylated compounds including ATP, ADP, adenosine monophosphate (AMP), and inosine monophosphate (IMP) in muscle were analyzed by HPLC in the laboratory of Beijing SINO-UK Institute of Biological Technology (Beijing, China) according to the procedures of Ping et al. (2002).
RNA extraction and real-time quantitative PCR.
The total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA). The RNA concentration was measured using a NanoVue Plus-spectrophotometer (GE Health Care, Piscataway, NJ, USA). After DNase I (Takara Biotechnology Co. Ltd., Dalian, China) treatment, the purified RNA was reverse transcribed with a RevertAid First Strand cDNA Synthesis Kit (Thermo Fischer Scientific, Waltham, MA, USA). Primers for L-arginine:glycine amidinotransferase (AGAT) and N-methyltransferase (GAMT) are described in Table 2. Real-time PCR was performed using an ABI 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and a FastStart Universal SYBR Green Master (Rox) Kit (Roche, Mannheim, Germany) as follows: denaturation step at 95 °C for 30 s, 40 cycles of PCR reaction at 95 °C for 5 s, and 60 °C for 30 s, followed by a dissociation step at 95 °C for 15 s and 60 °C for 30 s. A selected sample was analyzed in triplicate in the same run, and β-actin was used as the internal reference gene.
Table 2.
Forward and reverse primer sequences for real-time quantitative PCR analysis
| Genes1 | Primer sequences (5′-3′) | Product length (bp) | GeneBank accession NO. |
|---|---|---|---|
| AGAT | Forward: TCTCGCTCCTGACTACCG | 271 | NM_001128442.1 |
| Reversed: GGCATCCACCATAACACG | |||
| GAMT | Forward: GCCATCGCAGCCACTAAG | 269 | XM_003353976.2 |
| Reversed: TTCAGCAGGCGGAAAGCA | |||
| β-actin | Forward: TCTGGCACCACACCTTCT | 114 | DQ178122 |
| Reversed: TGATCTGGGTCATCTTCTCAC |
1 AGAT = L-arginine:glycine amidinotransferase; GAMT = N-methyltransferase.
Experiment 2
A total of 180 (average initial BW 53.19 ± 5.63 kg) pigs were blocked by weight and sex and assigned randomly to 5 dietary treatments with 6 replicates (3 gilts and 3 barrows per replicate pen). Diets were corn-soybean meal–basal diets supplemented with 0, 150, 300, 600, and 1,200 mg/kg GAA and were fed to the pigs for 35 d. Growth performance (ADG, ADFI, and G:F) was determined at the end of experiment as described for Exp. 1.
Statistical Analysis
Data were analyzed using the MIXED procedure of SAS (Version 9.2; SAS Inst. Inc., Cary, NC, USA). The pen was the experimental unit for analysis of performance data, and the individual pig was the experimental unit for other parameters. For growth performance and blood parameters, analyses were conducted using repeated measures by sampling day. The model included the fixed effects of dietary GAA, sampling day, and their interaction. Orthogonal polynomial contrast coefficients were used to determine the linear and quadratic effect of increasing level of dietary GAA on the measured traits. Hot carcass weight was used as a covariate in the statistical model for carcass analysis. The PROC NLIN procedure of SAS was used to estimate the optimum level of dietary GAA for growth performance by subjecting the least-squares means of the response traits to the broken-line model [Y = L + U × (R – x), the break point = R]. Tukey’s multiple comparison test was conducted at a significant level of P ≤ 0.05 and tendencies at 0.05 < P ≤ 0.10. Data are presented as least-squares means and SEM.
RESULTS
Experiment 1
Growth performance.
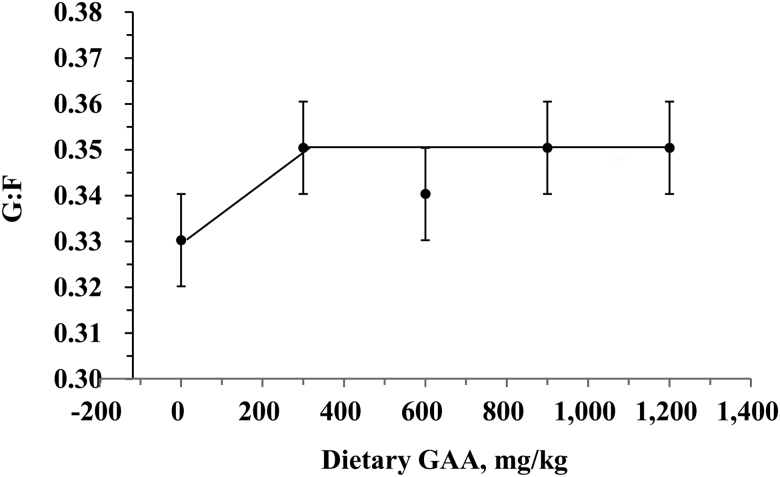
The effects of the graded levels of GAA in diets on growth performance in Exp. 1 are presented in Table 3. The interactions between dietary treatment and sampling day for BW, ADG, ADFI, and G:F were not significant (P > 0.05) (Table 4). During the grower phase, as dietary GAA increased, ADFI decreased (linear, P = 0.05), and ADG and G:F were not affected (P > 0.05). Within the Finisher I phase, as GAA supplementation increased, G:F increased (quadratic, P < 0.05) and pigs supplemented with 300-mg/kg of GAA showed a greater (P < 0.05) G:F than the control group. There was a tendency for an increase in ADG (quadratic, P = 0.08) with increasing dietary GAA during the Finisher II stage. In addition, during the overall period, G:F increased (linear, P < 0.05) with increasing addition of dietary GAA, and G:F was greater (P = 0.05) in 300, 900, and 1,200 mg/kg GAA treatment groups compared with that of the control group. Using a broken-line model, the optimum level of dietary GAA was 300 mg/kg during the overall experimental period (days 1 to 98) to maximize G:F (Figure 1).
Table 3.
Effect of graded levels of guanidinoacetic acid (GAA) on performance of growing-finishing pigs (Exp. 1)
| Item | GAA, mg/kg | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 900 | 1,200 | ANOVA | Linear | Quadratic | ||
| Initial BW, kg | 33.58 | 33.57 | 33.63 | 33.63 | 33.72 | 0.05 | 0.24 | 0.91 | 0.78 |
| day 35 BW, kg | 59.07 | 58.78 | 58.87 | 58.87 | 60.22 | 0.68 | 0.55 | 0.42 | 0.76 |
| day 70 BW, kg | 87.53 | 88.07 | 86.70 | 87.47 | 89.75 | 1.13 | 0.42 | 0.38 | 0.55 |
| day 98 BW, kg | 107.57 | 110.02 | 107.73 | 107.03 | 110.07 | 1.69 | 0.58 | 0.53 | 0.19 |
| Grower, days 1 to 35 | |||||||||
| ADG, g | 729 | 733 | 721 | 721 | 757 | 19 | 0.65 | 0.38 | 0.55 |
| ADFI, kg | 1.91 | 1.83 | 1.86 | 1.82 | 1.90 | 0.03 | 0.26 | 0.05 | 0.80 |
| G:F | 0.38 | 0.40 | 0.39 | 0.40 | 0.41 | 0.01 | 0.45 | 0.66 | 0.35 |
| Finisher I, days 36 to 70 | |||||||||
| ADG, g | 813 | 850 | 796 | 817 | 868 | 24 | 0.24 | 0.40 | 0.27 |
| ADFI, kg | 2.55 | 2.38 | 2.43 | 2.47 | 2.50 | 0.07 | 0.52 | 0.30 | 0.22 |
| G:F | 0.32b | 0.36a | 0.33ab | 0.33ab | 0.34ab | 0.01 | 0.04 | 0.36 | 0.02 |
| Finisher II, days 71 to 98 | |||||||||
| ADG, g | 715 | 817 | 772 | 752 | 769 | 34 | 0.37 | 0.48 | 0.08 |
| ADFI, kg | 2.59 | 2.72 | 2.70 | 2.48 | 2.50 | 0.13 | 0.58 | 0.93 | 0.30 |
| G:F | 0.28 | 0.30 | 0.29 | 0.31 | 0.31 | 0.01 | 0.32 | 0.25 | 0.58 |
| Overall, days 1 to 98 | |||||||||
| ADG, g | 752 | 800 | 763 | 763 | 798 | 16 | 0.14 | 0.30 | 0.85 |
| ADFI, kg | 2.35 | 2.31 | 2.33 | 2.25 | 2.30 | 0.08 | 0.84 | 0.42 | 0.74 |
| G:F | 0.33b | 0.35a | 0.34ab | 0.35a | 0.35a | 0.01 | 0.05 | 0.04 | 0.71 |
Data are the means of 6 replicates per treatment with 6 pigs per pen.
a–cMeans with different superscripts within a row differ (P ≤ 0.05).
Table 4.
Effect of guanidinoacetic acid (GAA) level and sampling day days on growth performance and blood parameters (Exp. 1)
| Item | P- value | ||
|---|---|---|---|
| GAA level | Sampling day | GAA level* Sampling day | |
| Growth performance | |||
| BW | 0.7790 | <0.0001 | 0.9997 |
| ADG | 0.1392 | <0.0001 | 0.7630 |
| ADFI | 0.8446 | <0.0001 | 0.7315 |
| G:F | 0.0492 | <0.0001 | 0.9856 |
| Blood parameters | |||
| GAA | 0.0078 | <0.0001 | 0.4876 |
| creatine | 0.0254 | <0.0001 | 0.7248 |
| creatinine | 0.7610 | 0.6676 | 0.9447 |
| ATP | 0.0106 | 0.9684 | 0.4463 |
| CK1 | 0.0823 | 0.2604 | 0.9148 |
1CK = creatine kinase.
Figure 1.
The broken-line of G:F (the overall period) as a function of dietary guanidinoacetic acid for growing-finishing pigs (Exp. 1). The optimum level of dietary guanidinoacetic acid for overall experimental period was 300 mg/kg [Y = 0.35 – 0.00006 × (300 – x), R2 = 0.77]. Data points (dots) represent least squares mean ± SEM for each dietary treatment (n = 36 pigs per treatment).
Carcass Characteristics
As shown in Table 5, HCW, carcass length, and lean percentage showed a tendency to increase (quadratic, 0.05 < P < 0.10) with increasing addition of dietary GAA. Compared with the control group, HCW tended to increase (P = 0.06) with 300 and 1,200 mg/kg GAA supplementation. Compared with the 900-mg/kg GAA group, carcass length tended to increase (P = 0.07) in the 300 and 1,200 mg/kg GAA groups. Dietary GAA had no effect (P > 0.05) on dressing percentage, leaf fat weight, 10th-rib fat, or longissimus muscle area.
Table 5.
Effect of graded levels of guanidinoacetic acid (GAA) on carcass characteristics of growing-finishing pigs (Exp. 1)
| Item1 | GAA, mg/kg | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 900 | 1,200 | ANOVA | Linear | Quadratic | ||
| HCW, kg | 81.73a | 87.33b | 84.66ab | 86.05ab | 88.68b | 1.71 | 0.06 | 0.42 | 0.07 |
| Dressing, % | 77.12 | 78.82 | 78.17 | 77.39 | 78.68 | 1.10 | 0.75 | 0.63 | 0.82 |
| CL, cm | 93.49ab | 93.83b | 91.25ab | 90.83a | 93.75b | 0.89 | 0.07 | 0.52 | 0.07 |
| LFW, kg | 0.92 | 0.95 | 0.82 | 0.75 | 0.98 | 0.11 | 0.58 | 0.81 | 0.29 |
| 10th rib fat, mm | 22.14 | 20.52 | 19.66 | 19.22 | 20.56 | 1.88 | 0.72 | 0.33 | 0.27 |
| LMA, cm1 | 34.05 | 36.33 | 35.74 | 34.81 | 34.75 | 1.30 | 0.72 | 0.99 | 0.47 |
| Lean, % | 49.32 | 50.54 | 50.90 | 51.54 | 50.12 | 0.72 | 0.29 | 0.27 | 0.08 |
Data are the means of six replicates of one pig per pen.
a,bMeans with different superscripts within a row show a different tendency (0.05 < P ≤ 0.10).
1CL = carcass length; LFW = leaf fat weight; LMA = longissimus muscle area.
Guanidinoacetic Acid and Creatine in Serum and Tissues
Serum GAA, creatine, and creatinine concentrations were measured on days 35, 70, and 98 (Table 6), respectively. Interactions between dietary treatment and sampling day for serum GAA, creatine, and creatinine were not significant (P > 0.05) (Table 4). Dietary GAA had no effect (P > 0.05) on the concentration of GAA or creatinine in serum. However, on day 35, the serum creatine concentration in pigs fed 300 mg/kg of GAA was greater (P < 0.05) than pigs fed 600 mg/kg. On day 98, there was a tendency (linear, P = 0.10) for increased serum GAA content with increasing concentration of GAA in diets. Otherwise, as dietary GAA supplementation increased (Table 7), creatine concentration in the liver showed a tendency to increase (linear, P = 0.07); however, dietary GAA had no influence (P > 0.05) on the concentrations of GAA and creatine in the muscle and the kidney.
Table 6.
Effect of graded levels of guanidinoacetic acid (GAA) on the concentration of GAA and its metabolites in serum (Exp. 1)
| Item | GAA, mg/kg | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 900 | 1,200 | ANOVA | Linear | Quadratic | ||
| day 35 | |||||||||
| GAA, μg/mL | 1.09 | 1.14 | 1.00 | 1.09 | 0.90 | 0.08 | 0.29 | 0.64 | 0.53 |
| Creatine, μg/mL | 22.94ab | 28.03a | 17.06b | 20.21ab | 24.56ab | 2.44 | 0.04 | 0.12 | 0.25 |
| Creatinine, μmol/mL | 93.57 | 91.82 | 95.43 | 92.72 | 97.94 | 5.61 | 0.94 | 0.81 | 0.84 |
| day 70 | |||||||||
| GAA, μg/mL | 0.70 | 0.88 | 0.68 | 0.75 | 0.79 | 0.06 | 0.19 | 0.97 | 0.16 |
| Creatine, μg/mL | 24.00 | 26.22 | 18.85 | 19.11 | 21.22 | 3.45 | 0.51 | 0.28 | 0.63 |
| Creatinine, μmol/mL | 98.51 | 93.06 | 96.91 | 95.70 | 95.58 | 4.48 | 0.94 | 0.77 | 0.56 |
| day 98 | |||||||||
| GAA, μg/mL | 1.00 | 1.36 | 1.09 | 1.25 | 1.03 | 0.10 | 0.12 | 0.10 | 0.41 |
| Creatine, μg/mL | 33.35 | 36.66 | 31.86 | 31.77 | 25.87 | 2.91 | 0.15 | 0.77 | 0.94 |
| Creatinine, μmol/mL | 102.05 | 98.36 | 97.00 | 91.95 | 94.97 | 3.92 | 0.47 | 0.16 | 0.96 |
Data are the means of six replicates of one pig per pen.
a,bMeans with different superscripts within a row differ (P ≤ 0.05).
Table 7.
Effect of graded levels of guanidinoacetic acid (GAA) on tissue metabolite concentrations (Exp. 1)
| Item | GAA, mg/kg | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 900 | 1,200 | ANOVA | Linear | Quadratic | ||
| Muscle | |||||||||
| GAA, mg/kg | 7.77 | 8.17 | 9.15 | 7.95 | 8.88 | 0.83 | 0.78 | 0.82 | 0.46 |
| Creatine, mg/kg | 2.88 | 2.91 | 2.97 | 2.80 | 2.86 | 0.11 | 0.85 | 0.65 | 0.72 |
| Liver | |||||||||
| GAA, mg/kg | 2.36 | 2.22 | 2.14 | 2.04 | 2.27 | 0.29 | 0.95 | 0.42 | 0.98 |
| Creatine, mg/kg | 92.61 | 131.14 | 117.28 | 120.37 | 102.29 | 11.38 | 0.18 | 0.07 | 0.21 |
| Kidney | |||||||||
| GAA, mg/kg | 139.16 | 153.28 | 130.01 | 133.57 | 128.80 | 9.43 | 0.41 | 0.73 | 0.58 |
| Creatine, mg/kg | 210.63 | 240.73 | 234.63 | 235.24 | 240.38 | 21.57 | 0.84 | 0.54 | 0.44 |
Data are the means of 6 replicates of 1 pig per pen.
Energy-Related Metabolites in Serum and Tissues
Interactions between dietary treatment and sampling day for serum ATP and CK were not significant (P > 0.05) (Table 4). As presented in Table 8, with incremental levels of dietary GAA, serum CK activity tended to increase on day 70 (quadratic, P = 0.06), serum ATP on day 98 (linear and quadratic, P < 0.01), and muscle ATP and AMP (quadratic, P = 0.05) increased. Compared with the control group, 300 to 1,200 mg/kg of GAA supplementation increased (P < 0.01) serum ATP on day 98. In addition, dietary GAA had no effect (P > 0.05) on the content of ADP, IMP, and CK activity in muscle.
Table 8.
Effect of graded levels of guanidinoacetic acid (GAA) on energy metabolites in serum and muscle (Exp. 1)
| Item | GAA, mg/kg | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 900 | 1,200 | ANOVA | Linear | Quadratic | ||
| Serum1 | |||||||||
| day 35 | |||||||||
| ATP, mmol/L | 0.79 | 0.87 | 0.69 | 0.88 | 0.91 | 0.08 | 0.36 | 0.88 | 0.96 |
| CK, U/mL | 0.45 | 0.54 | 0.54 | 0.52 | 0.54 | 0.07 | 0.89 | 0.58 | 0.45 |
| day 70 | |||||||||
| ATP, mmol/L | 0.73 | 0.82 | 0.85 | 0.84 | 0.89 | 0.05 | 0.26 | 0.29 | 0.19 |
| CK, U/mL | 0.32 | 0.5 | 0.45 | 0.36 | 0.60 | 0.09 | 0.25 | 0.81 | 0.06 |
| day 98 | |||||||||
| ATP, mmol/L | 0.70e | 0.75d | 0.84c | 0.87b | 0.90a | 0.01 | <0.01 | <0.01 | <0.01 |
| CK, U/mL | 0.31 | 0.49 | 0.33 | 0.45 | 0.56 | 0.12 | 0.53 | 0.89 | 0.46 |
| Muscle2 | |||||||||
| ATP, mg/g | 1.80 | 2.77 | 2.49 | 2.32 | 2.51 | 0.30 | 0.21 | 0.29 | 0.05 |
| ADP, mg/g | 0.77 | 1.03 | 0.95 | 0.95 | 0.93 | 0.10 | 0.39 | 0.23 | 0.17 |
| AMP, mg/g | 0.19 | 0.29 | 0.26 | 0.24 | 0.26 | 0.03 | 0.21 | 0.29 | 0.05 |
| IMP, mg/g | 1.42 | 1.66 | 1.63 | 1.57 | 1.47 | 0.11 | 0.54 | 0.19 | 0.35 |
| CK, U/mg | 6.48 | 9.50 | 7.35 | 6.46 | 5.90 | 1.78 | 0.63 | 0.79 | 0.33 |
Data are the means of 6 replicates of 1 pig per pen.
a–eMeans with different superscripts within a row differ (P ≤ 0.05).
1U is the symbol for one enzyme unit, one U is defined as the amount of the enzyme that produces a certain amount of enzymatic activity.
2mg/g or U/mg means the content in muscle protein; CK = creatine kinase; AMP = adenosine monophosphate; IMP = inosine monophosphate.
The mRNA Expression of AGAT and GAMT
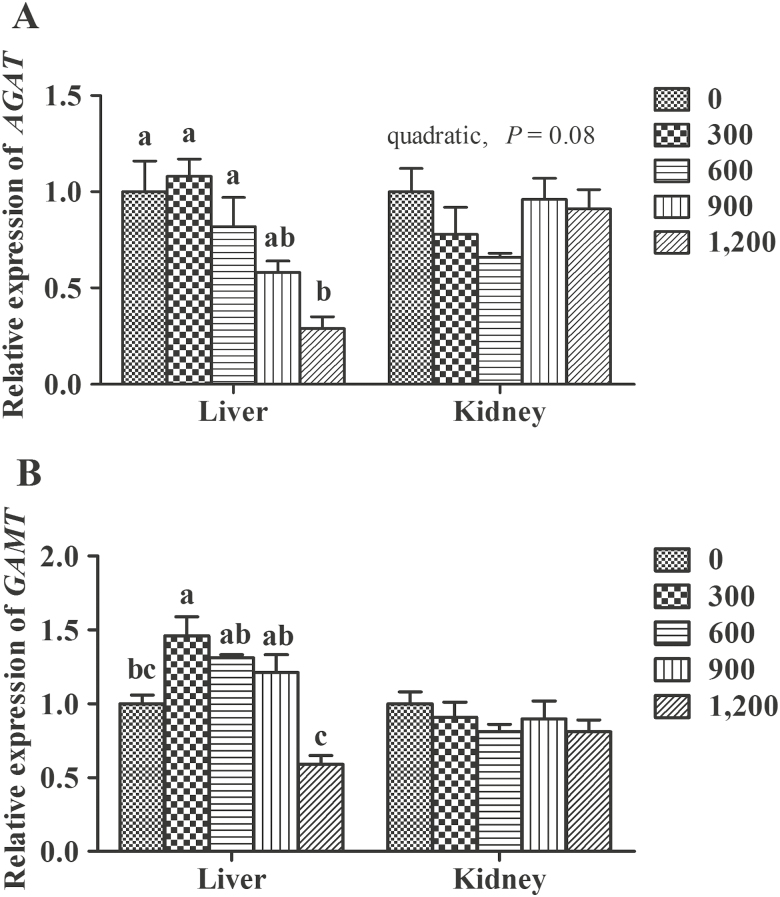
In the liver (Figure 2), compared with the control group, mRNA expression of AGAT decreased (P < 0.05) in the 1,200 mg/kg of GAA treatment group and mRNA level of GAMT increased (P < 0.05) in the 300 mg/kg of GAA treatment group. In the kidney, as dietary GAA increased, mRNA expression of AGAT showed a decreasing tendency (quadratic, P = 0.08) and GAMT was not affected (P > 0.05).
Figure 2.
The mRNA expression of L-arginine:glycine amidinotransferase (AGAT) (A) and N-methyltransferase (GAMT) (B) in liver and kidney of finishing pigs. Guanidinoacetic acid was added at 0, 300, 600, 900, and 1,200 mg/kg to create 5 experimental diets. Values are means ± SEM (n = 6). a–cMean values with different letters are significantly different (P < 0.05).
Experiment 2
The effects of graded levels of GAA in diets on growth performance in Exp. 2 are presented in Table 9. Average daily gain and G:F increased (quadratic, P < 0.05) with increasing concentration of dietary GAA, and the 300-mg/kg GAA treatment groups showed a greater (P < 0.05) ADG compared with the control and 600-mg/kg GAA treatment groups. These observations provided confirmation that dietary 300-mg/kg GAA showed the optimal performance-enhancing effect in growing-finishing pigs.
Table 9.
Effect of graded levels of guanidinoacetic acid (GAA) on performance of growing-finishing pigs (Exp. 2)
| Item | GAA, mg/kg | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 150 | 300 | 600 | 1,200 | ANOVA | Linear | Quadratic | ||
| Initial BW, kg | 53.20 | 53.18 | 53.18 | 53.18 | 53.21 | 0.05 | 0.98 | 0.71 | 0.84 |
| Final BW, kg | 77.18b | 79.13ab | 80.70a | 76.97b | 79.53ab | 0.84 | 0.02 | 0.43 | < 0.01 |
| ADG, g | 707b | 758ab | 808a | 700b | 774ab | 24 | 0.03 | 0.44 | < 0.01 |
| ADFI, kg | 2.31 | 2.28 | 2.31 | 2.16 | 2.32 | 0.06 | 0.27 | 0.05 | 0.45 |
| G:F | 0.30 | 0.34 | 0.35 | 0.32 | 0.34 | 0.01 | 0.13 | 0.51 | 0.02 |
Data are the means of 6 replicates per treatment with 6 pigs per pen.
a,bMeans with different superscripts within a row differ (P ≤ 0.05).
DISCUSSION
GAA is the only immediate precursor for creatine in the body and is a naturally occurring compound in vertebrate animals (Ostojic, 2015). However, there was little information available concerning the dietary supplement GAA on pig performance, especially during a complete grow-to-finish period. The study of Wang et al. (2012) reported that dietary GAA (800 to 2,000 mg/kg) had no influence on the growth performance of growing-finishing pigs. In the current study, during the overall period, dietary supplementation with 300 to 1,200 mg/kg of GAA improved G:F. The greatest impact on G:F was in the Finisher I phase (from 50 to 90 kg BW). Differences among these studies may be attributed to differences in initial pig BW, experimental period, and nutrient levels in the diets, especially CP and Met. In general, the longer the experiment lasted, the more sufficiently the effects of a feed additive can be evaluated. The current study lasted for 98 d (from 30 to 110 kg BW). The experiment done by Wang et al. (2012) only lasted for 54 d (from 45 to 90 kg BW), and the diets contain a relatively lower protein (15%) and Met (0.32%) than that in the present study. Dietary GAA considerably increases the demand of S-adenosyl methionine, a methyl donor synthesized from Met (Stead et al., 2001). Thus, sufficient dietary Met may meet the needs of both protein and creatine synthesis. Compared with Wang et al. (2012), greater protein and Met levels in the current study may contribute to the positive effect of GAA on growth performance.
The broken-line analysis for G:F of the overall period indicated that 300 mg/kg of GAA was optimal for pigs. Moreover, we found that dietary GAA was more effective on the growth performance of pigs in the Finisher I stage (from 50 to 90 kg BW) than that of other 2 stages (Grower, from 30 to 50 kg BW; Finisher II, from 90 to 110 kg BW). The results of Exp. 2 verified those in Exp. 1 in which 300 mg/kg of GAA was more effective than other levels of GAA (150, 600, 900, and 1,200 mg/kg) and maximized growth performance for pigs from 50 to 90 kg BW.
Few studies have reported the effect of GAA on the carcass traits of pigs. However, in broilers, recent studies indicate that supplementation with GAA may increase carcass yield (Metwally et al., 2015; Pradeep et al., 2016) and reduce abdominal fat (Metwally et al., 2015). Consistent with research in broilers, in the current study, supplementation with GAA tended to improve the carcass traits of pigs.
Supplemental GAA is absorbed in the intestine and transported by the blood into the liver where it is methylated to form creatine (Murakami et al., 2014); subsequently, creatine is carried by the blood to creatine-requiring tissues. In this study, supplemental GAA, especially 300 mg/kg GAA, tended to increase serum GAA and creatine, which is consistent with that reported by McBreairty et al. (2015), suggesting that supplementation with GAA (157 mg/kg/d) in pigs led to greater levels of plasma creatine and GAA. Alternatively, decreased serum creatine was found in the 600 mg/kg of GAA group; this phenomenon may be induced by the feedback mechanism on GAA de novo synthesis (Guthmiller et al., 1994). As for tissues, dietary (300 to 1,200 mg/kg) GAA tended to increase liver creatine, which is supported by Liu et al. (2015) where dietary GAA (1,000 mg/kg) increased mRNA expression of creatine transporter in liver. Furthermore, McBreairty et al. (2015) found that supplementation with 157 mg/kg of GAA increased the content of GAA and creatine in tissues (muscle, liver, and kidney). Also, Liu et al. (2015) found that 1,000 mg/kg GAA increased muscle creatine by 7.41% in growing-finishing pigs. However, the present study detected no influence of dietary GAA on the concentrations of GAA and creatine in the muscle and the kidney.
The above analysis suggests that supplemental GAA may increase creatine reserves in pigs. Creatine plays a key role in energy metabolism, and the majority of creatine is stored in skeletal muscle as phosphocreatine (Wyss and Kaddurah-Daouk, 2000). Both creatine and phosphocreatine help replenish ATP from ADP via the CK reaction to maintain ATP at a constant level (Wallimann et al., 1992). In the present study, dietary GAA (300 to 1,200 mg/kg) increased serum ATP. But it only occurred on day 98, probably because the energy metabolism of pigs was affected by age (Heffron and Mitchell, 1975; Thoren, 1982), and the cumulative effect of dietary GAA during the 98-d experiment. Also, the ATP content of the muscle increased with incremental GAA supplementation, which was in accordance with the results of Liu et al. (2015), who demonstrated that 1,000 mg/kg supplementation of GAA increased the concentrations of ATP in the muscle. Therefore, dietary GAA could also improve energy metabolism by increasing ATP reserves.
The process of GAA and creatine biosynthesis occurs naturally in mammals by 2 steps. During the first step, GAA is formed from Arg and Gly by the reaction catalyzed by AGAT. In the second step, GAMT induces the GAA methylation to form creatine (Walker, 1979; da Silva et al., 2009). In pigs, the liver contains high amounts of GAMT and AGAT enzymes (Wyss and Kaddurah-Daouk, 2000). However, the kidney of many species expresses high amounts of AGAT but relatively lower levels of GAMT (Wyss and Kaddurah-Daouk, 2000). It has been suggested that AGAT is a critical rate limiting step in creatine biosynthesis which is subject to feedback inhibition by creatine (Guthmiller et al., 1994). This feedback mechanism may explain the decrease in mRNA levels of AGAT in the liver and kidney with incremental GAA supplementation. However, supplementation with 300-mg/kg GAA increased GAMT mRNA levels in the liver, suggesting an enhanced activity of methylation from GAA to creatine, which was in accordance with the maximum levels of liver creatine in the 300-mg/kg GAA groups. In addition, the lack of an effect of dietary GAA supplementation on kidney mRNA levels of GAMT may be because the liver is the main tissue for GAA methylation, not the kidney (Wyss and Kaddurah-Daouk, 2000).
In conclusion, this study indicated that dietary supplemental GAA may improve growth performance of pigs by increasing creatine content and energy metabolism. Supplementation of 300 to 1,200 mg/kg of GAA in their diet effectively improved growth performance, and probably, the carcass traits for growing-finishing pigs. In addition, supplementing 300-mg/kg GAA increased both creatine content and energy reserve. The broken-line model indicates that 300-mg/kg GAA could maximize G:F. Therefore, supplementing 300-mg/kg GAA for growing-finishing pigs is recommended in this study.
Footnotes
This study was funded by Beijing Science and Technology Program of China (Z151100001215001). We would like to thank Crystal L. Levesque (South Dakota State University, Brookings, USA) for scrutinizing and correcting the language, and thank LetPub (www.letpub.com) for providing linguistic assistance during the preparation of this manuscript.
LITERATURE CITED
- AOAC 2000. Official methods of analysis. 18th ed Assoc. Off. Anal. Chem, Arlington, VA. [Google Scholar]
- Berg E. 2000. Pork composition and quality assessment procedures. Natl. Pork Prod. Counc. (NPPC), Des Monies, IA, USA: 1–38. [Google Scholar]
- Buchberger W. and Ferdig M.. 2004. Improved high-performance liquid chromatographic determination of guanidino compounds by precolumn dervatization with ninhydrin and fluorescence detection. J. Sep. Sci. 27:1309–1312. [DOI] [PubMed] [Google Scholar]
- da Silva R. P., Nissim I., Brosnan M. E., and Brosnan J. T.. 2009. Creatine synthesis: hepatic metabolism of guanidinoacetate and creatine in the rat in vitro and in vivo. Am. J. Physiol. Endocrinol. Metab. 296:E256–E261. doi:10.1152/ajpendo.90547.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission 2016. Commission implementing regulation (EU) No 2016/1768 of 4 October 2016 concerning the authorisation of guanidinoacetic acid as a feed additive for chickens for fattening, weaned piglets and pigs for fattening and repealing Commission Regulation (EC) No 904/2009. Off. J. Eur. Union. L270:4–6. [Google Scholar]
- European Food Safety Authority (EFSA) 2016. Safety and efficacy of guanidinoacetic acid for chickens for fattening, breeder hens and roosters, and pigs. EFSA J. 4394:1–33. [Google Scholar]
- Guthmiller P., Van Pilsum J. F., Boen J. R., and McGuire D. M.. 1994. Cloning and sequencing of rat kidney l-arginine: glycine amidinotransferase. Studies on the mechanism of regulation by growth hormone and creatine. J. Biol. Chem. 269:17556–17560. [PubMed] [Google Scholar]
- Heffron J. J. and Mitchell G.. 1975. Age dependent variation of serum creatine phosphokinase levels in pigs. Experientia 31:657–658. [DOI] [PubMed] [Google Scholar]
- James B. W., Goodband R. D., Unruh J. A., Tokach M. D., Nelssen J. L., and Dritz S. S.. 2002. A review of creatine supplementation and its potential to improve pork quality. J. Appl. Anim. Res. 21:1–16. [Google Scholar]
- Liu Y., Li J. L., Li Y. J., Gao T., Zhang L., Gao F., and Zhou G. H.. 2015. Effects of dietary supplementation of guanidinoacetic acid and combination of guanidinoacetic acid and betaine on postmortem glycolysis and meat quality of finishing pigs. Anim. Feed Sci. Technol. 205:82–89. doi:10.1016/j.anifeedsci.2015.03.010 [Google Scholar]
- Maddock R. J., Bidner B. S., Carr S. N., McKeith F. K., Berg E. P., and Savell J. W.. 2002. Creatine monohydrate supplementation and the quality of fresh pork in normal and halothane carrier pigs. J. Anim. Sci. 80:997–1004. [DOI] [PubMed] [Google Scholar]
- McBreairty L. E., Robinson J. L., Furlong K. R., Brunton J. A., and Bertolo R. F.. 2015. Guanidinoacetate is more effective than creatine at enhancing tissue creatine stores while consequently limiting methionine availability in yucatan miniature pigs. PLoS ONE 10:e0131563. doi:10.1371/journal.pone.0131563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwally A. E., Ibrahim D., and Khater S. I.. 2015. Effects of supplementing broiler diets with CreAMINO on broiler performance, carcass traits and the expression of muscle growth related genes. Res. Opin. Anim. Vet. Sci. 5:435–442. [Google Scholar]
- Michiels J., Maertens L., Buyse J., Lemme A., Rademacher M., Dierick N. A., and De Smet S.. 2012. Supplementation of guanidinoacetic acid to broiler diets: effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult. Sci. 91:402–412. doi:10.3382/ps.2011-01585 [DOI] [PubMed] [Google Scholar]
- Murakami A. E., Rodrigueiro R. J., Santos T. C., Ospina-Rojas I. C., and Rademacher M.. 2014. Effects of dietary supplementation of meat-type quail breeders with guanidinoacetic acid on their reproductive parameters and progeny performance. Poult. Sci. 93:2237–2244. doi:10.3382/ps.2014-03894 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Ostojic S. M. 2015. Advanced physiological roles of guanidinoacetic acid. Eur. J. Nutr. 54:1211–1215. doi:10.1007/s00394-015-1050-7 [DOI] [PubMed] [Google Scholar]
- Ping H., Huwei L., Ganghua J., Yan L., and Kuisheng Y.. 2002. Determination of ATP, ADP, AMP, NAD~+, NADH in skeletal muscle by HPLC. Chin. J. Nat. Med. 1:15. [Google Scholar]
- Pradeep K. R., Radermacher M., and Girish C. K.. 2016. Meeting creatine needs of modern broilers via guanidinoacetic acid supplementation in diets with or without animal protein. In: Proc. 27th Austr. Poult. Sci. Symp, Sydney, New South Wale p. 240. [Google Scholar]
- Ringel J., Lemme A., Knox A., McNab J., and Redshaw M.. 2007. Effects of graded levels of creatine and guanidino acetic acid in vegetable-based diets on performance and biochemical parameters in muscle tissue. Proc. 16th Eur. Symp. Poult. Nutr, Beekbergen, Netherlands p. 387–390. [Google Scholar]
- Stead L. M., Au K. P., Jacobs R. L., Brosnan M. E., and Brosnan J. T.. 2001. Methylation demand and homocysteine metabolism: effects of dietary provision of creatine and guanidinoacetate. Am. J. Physiol. Endocrinol. Metab. 281:E1095–E1100. doi:10.1152/ajpendo.2001.281.5.E1095 [DOI] [PubMed] [Google Scholar]
- Thoren T. K. 1982. Age dependent variation of serum creatine kinase isoenzyme levels in pigs. Zbl. Vet. Med. A. 29:420–428. [DOI] [PubMed] [Google Scholar]
- Walker J. B. 1979. Creatine: biosynthesis, regulation, and function. Adv. Enzymol. Relat. Areas. Mol. Biol. 50:177–242. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Wyss M., Brdiczka D., Nicolay K., and Eppenberger H. M.. 1992. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 281(Pt 1):21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. S., Shi B. M., Shan A. S., and Zhang Y. Y.. 2012. Effects of guanidinoacetic acid on growth performance, meat quality and antioxidation in growing-finishing pigs. J. Anim. Vet. Adv. 11:63–68. doi:10.3923/javaa.2012.631.636 [Google Scholar]
- Wyss M. and Kaddurah-Daouk R.. 2000. Creatine and creatinine metabolism. Physiol. Rev. 80:1107–1213. doi:10.1152/physrev.2000.80.3.1107 [DOI] [PubMed] [Google Scholar]
- Young J. F., Bertram H. C., Rosenvold K., Lindahl G., and Oksbjerg N.. 2005. Dietary creatine monohydrate affects quality attributes of duroc but not landrace pork. Meat Sci. 70:717–725. doi:10.1016/j.meatsci.2005.03.008 [DOI] [PubMed] [Google Scholar]