Abstract
In the current era of genomic selection, there is an increased demand to collect semen from genomically selected sires at a young age. The objective of this study was to assess the effect of bull age, ejaculate number, and season of collection on semen production (ejaculate volume, sperm concentration, and total sperm number; TSN) and sperm motility (prefreeze and post-thaw total and gross motility) parameters in Holstein Friesian bulls in a commercial artificial insemination (AI) center. The study involved the interrogation of a large dataset collected over a 4-yr period, (n = 8,983 ejaculates; n = 176 Holstein Friesian bulls aged between 9 mo and 8 yr). Bulls aged less than 1 yr had the poorest semen production and sperm motility values for all parameters assessed compared with bulls older than 1 yr (P < 0.01). First ejaculates had greater semen production and greater prefreeze motility values than second consecutive ejaculates (P < 0.01), but despite this, there was no difference in post-thaw motility. When subsequent ejaculates were collected from bulls aged less than 1 yr, semen production and sperm motility did not differ compared with mature bulls. Semen collected in winter was poorest in terms of sperm concentration and TSN, but best in terms of post-thaw motility (P < 0.01). In conclusion, second ejaculates can be collected, particularly from bulls aged less than 1 yr, without a significant decrease in post-thaw sperm motility, thus may be a useful strategy to increase semen availability from young genomically selected AI bulls in high demand.
Keywords: artificial insemination, breeding, male fertility, motility, spermatozoa
INTRODUCTION
In recent years, the advent of genomic selection has allowed the dairy industry to reliably select artificial insemination (AI) bulls at a younger age and has thereby hastened genetic progress by reducing the generation interval and increased genetic gain (Goddard and Hayes, 2007). However, the reproductive performance of young bulls varies greatly mainly due to the large variation in the age of onset of puberty among and within breeds (Barth et al., 2008). Thus, there are major challenges to collecting sufficient high-quality semen to meet demand from these young bulls.
Semen quality can be affected by a wide range of genetic and environmental factors including bull age, collection interval, collection frequency, and season (Fuerst-Waltl et al., 2006; Fiaz et al., 2010; Snoj et al., 2013). It is widely acknowledged that the age of a bull at collection affects semen characteristics (Mathevon et al., 1998; Brito et al., 2002a), with older mature bulls having greater semen volume and quality than younger bulls (Brito et al., 2002a; Fuerst-Waltl et al., 2006). This increase is primarily believed to be due to physiological changes such as an increase in body mass (Balić et al., 2012) and the simultaneous development of the testis and accessory glands postpuberty and during sexual maturation which consequently leads to an increase in semen production (Almquist, 1978). However, peak ejaculate volumes and total sperm number are achieved at different ages in different breeds (Snoj et al., 2013).
While the first ejaculate of a bull collected on a given day is typically of greater volume and sperm concentration compared with subsequent collections on the same day (Everett and Bean, 1982), collection of multiple ejaculates on the same day did not affect post-thaw sperm motility (Boujenane and Boussaq, 2013). The reduction in semen production seems to be largely related to the short collection interval between consecutive ejaculates as longer intervals produce greater ejaculate volumes and total sperm number (TSN; Mathevon et al., 1998; Fuerst-Waltl et al., 2006). However, although in general, second ejaculates are of less volume and sperm concentration, the collection of sequential ejaculates increases productivity per unit time, as more insemination doses can be obtained on a given day.
The effect of season on bovine semen production has been widely assessed (Stälhammar et al., 1989; Brito et al., 2002a; Malama et al., 2017); however, data are conflicting, perhaps due to the range of climatic conditions under which these studies have been carried out (Wildeus and Hammond, 1993; Brito et al., 2002b). Studies reporting seasonal variation in semen characteristics have mainly attributed these changes to compromised scrotal thermoregulation and heat dissipation mechanisms (Menegassi et al., 2015) as well as the endocrine profile and the differential response of bull testes to gonadotropins (Jiménez-Severiano et al., 2003). Seasonal variations associated with photoperiod, in particular luteinising hormone, testosterone concentrations, and melatonin levels, can affect spermatogenesis (Godfrey et al., 1990; Lincoln et al., 1996; Tatman et al., 2004). Moreover, the adaptability of a bull to local microclimatic conditions may have consequences for semen quality (Nichi et al., 2006) and therefore could account for differences in their reproductive capacity throughout the year.
Given this background, the aim of this study was to assess the effect of bull age, ejaculate number, and season of collection on semen production and sperm motility parameters in Holstein Friesian AI bulls at a commercial AI center in Ireland.
MATERIALS AND METHODS
Animal Management
Semen collection data from Holstein Friesian bulls (n = 176 bulls, n = 8,983 ejaculates), ranging between 9 mo and 8 yr of age (numbers of bulls in each age category are presented in Figure 1), from Ireland’s largest AI center (National Cattle Breeding Centre, Naas, County Kildare) were used in this study. Bull semen production records over a period of 4 yr (2012 to 2016) were analyzed. Bulls were categorized into years of age ranging from <1, 1 to 2, 2 to 3, 3 to 4, and >4 yr. Data were categorized according to season of collection: spring (February, March, April), summer (May, June, July), autumn (August, September, October), and winter (November, December, January). Mean temperature over the 4 yr during these periods was 7, 14, 13, and 6 °C, respectively (Met Éireann, 2017), with a mean number of collections per day of 33, 25, 23, and 17 for spring, summer, autumn, and winter, respectively. Bulls were individually housed in a barn with ambient (i.e., unregulated) temperature, fed, and maintained under similar management and feeding conditions. Bulls were fed 85% dry matter haylage ad libitum as well as approximately 5 kg of a 14% protein cereal-based ration daily with ad libitum access to water. The mean age of the bulls analyzed in spring, summer, autumn, and winter was 24 ± 0.20, 26 ± 0.25, 29 ± 0.29, and 28 ± 0.31 mo, respectively. Typically, semen was collected from a bull on 1 to 3 d per week, depending on demand. Generally, ejaculates were collected from bulls on consecutive days. In cases where semen from a particular bull was in very high demand, regardless of bull age, a second ejaculate was collected within 1 h of the first collection (as described below).
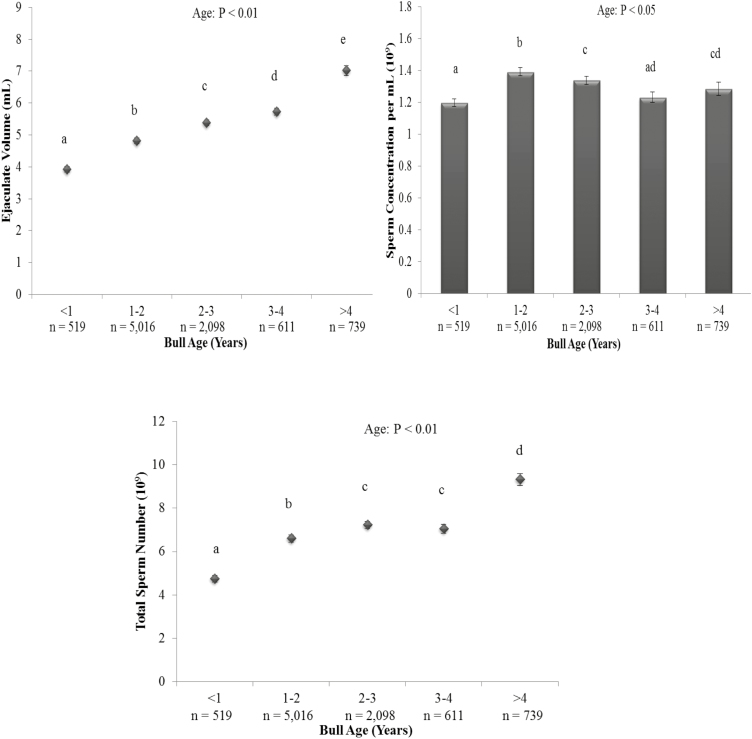
Figure 1.
The effect of bull age on ejaculate volume (upper left panel), sperm concentration (upper right panel), and total sperm number (lower panel). Vertical bars represent s.e.m. abcdeDiffering superscripts differ between bull ages within each parameter (P < 0.01). n = number of ejaculates.
Semen Collection and Processing
All bulls were sexually stimulated using a teaser bull and allowed to false mount a minimum of three times. The time between each false mount varied between individual bulls but was determined by an experienced barn technician and typically took no longer than 2 to 3 min. Semen was collected from all bulls using an artificial vagina, once bulls were deemed to be sufficiently stimulated. This method of stimulation and collection was similar for all collections, regardless of the ejaculate number, and remained constant from year to year. Ejaculates were kept separate throughout and were initially partially diluted in 10-mL prewarmed (37 °C) BullXcell extender (IMV Technologies, L’Aigle, France) and transported in a temperature-regulated box at 18 °C to the laboratory (within 3 h). On arrival, the ejaculate was assessed for weight to determine volume, sperm concentration using a coulter counter (Z Series, Beckman Coulter, Clare, Ireland), total motility (% of the total sperm population both motile and nonmotile), and gross motility (5-point scale: 1 = twitching/no forward progressive motility; 5 = excellent forward progressive motile sperm) to ensure that all semen samples were of a commercial standard. Initial quality control cut-off values were a total and gross motility of ≥70% and a score of ≥3 (at increments of 5% and 0.5), respectively. Any ejaculates failing to meet these criteria were rejected for commercial production but the data were still included in this study.
Following in vitro assessments, the ejaculate was fully extended in prewarmed BullXcell (18 °C) to a final concentration of 15 × 106 sperm per 0.25-mL semen straw (IMV Technologies). Straws were filled, sealed, and printed as per routine procedures using the IS4 instrument (18 °C; IMV Technologies). Straws from each ejaculate were then cooled to 4 °C over 3 h and were frozen to −140 °C as follows: −5 °C per min from +4 to −10 oC, −40 °C per min from −10 to −100 °C, and thereafter −20 °C per min from −100 to −140 °C in a programmable freezer (IMV Technologies), followed by submersion and storage in liquid nitrogen at −196 °C until use (Murphy et al. 2017). Four straws from each ejaculate of each bull were assessed immediately post-thaw via standard microscopic techniques for total and gross motility (as described below). Post-thaw quality control cut-off values were a total and gross motility of ≥50% and a score of ≥3, respectively.
Assessment of sperm motility.
Sperm motility (total and gross) was assessed prefreezing and post-thawing using a phase contrast microscope (CX31; Olympus, Centre Valley, PA, USA) at a magnification of 400×. Frozen straws (n = 4 per ejaculate) were thawed at 37 °C for 30 s and each straw was dried fully to remove any excess water. All straws were cut at the sealed end and separately placed into a prewarmed eppendorf. The plug end of each straw was then cut to expel the contents of the straw into the eppendorf and the semen sample was mixed thoroughly to ensure homogeneity. A droplet (5 µL) of diluted semen was placed on a prewarmed glass slide and covered with a prewarmed coverslip (18 × 18 mm; 37 °C). Total motility was assessed by counting a minimum of 100 sperm over at least five different fields of view, whereas gross motility was evaluated by assessing the swimming pattern of the entire sperm sample on a scale of 1 to 5 as described above. Total motility was expressed as a percentage of the total sperm population (motile and nonmotile).
Statistical Analysis
Data were checked for normality and homogeneity of variance using histograms, QQ plots, and formal statistical tests in the Univariate procedure (version 9.1.3; SAS Institute, Cary, NC, USA). Data that were not normally distributed were transformed by raising the variable to the power of λ. The appropriate λ value was obtained by conducting a Box-Cox transformation analysis using the TRANSREG procedure of SAS. Semen production (ejaculate volume, sperm concentration per mL, and TSN) and sperm motility (prefreeze and post-thaw total and gross motility) parameters were analyzed using the MIXED procedure of SAS with a model that included fixed effect of bull age, season, and ejaculate number. All two- and three-way interactions were tested for among the main factors. Bull was included as a random effect. Differences among means were determined by F-tests using type III sums of squares. The PDIFF option and the Tukey test were applied to evaluate pairwise comparisons between means. Wherever appropriate, Spearman partial correlation analysis was carried out between variables using the PROC CORR accounting for year and GLM procedure of SAS was also used to determine the relationship between the main factors and semen production and sperm motility variables. Values reported for all parameters include data from both first and second ejaculates.
RESULTS
Effect of Bull Age on Semen Production and Sperm Motility
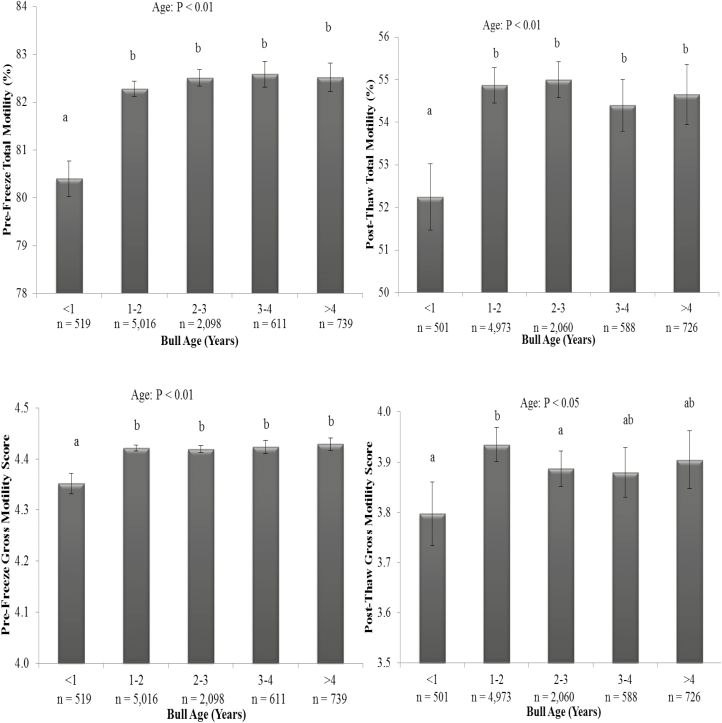
There was an effect of bull age on ejaculate volume, TSN (P < 0.01), and sperm concentration (Figure 1; P < 0.05) as well as pre- and post-thaw total and gross sperm motility (Figure 2; P < 0.01). Sperm motility, as assessed by prefreeze and post-thaw total and gross motility, was lowest for bulls collected at less than 1 yr of age compared with all other age categories; however, the difference in motility scores between bulls aged less than 1 yr and older bulls was small (approximately 2% points) and unlikely to be of biological or commercial importance (Figure 2; P < 0.01). Ejaculate volume was positively correlated with bull age (r = 0.62; P < 0.01) and increased by approximately 0.5 mL per year. As a result, TSN also increased with age, with bulls aged less than 1 yr producing the lowest ejaculate volume and TSN, whereas bulls aged more than 4 yr produced the largest semen volume and TSN (Figure 1; P < 0.01). There was a linear increase in TSN with increasing ejaculate volume (r = 0.71; P < 0.01). Bulls aged between 1 and 2 yr had a greater sperm concentration per ejaculate than bulls aged less than 1 or more than 2 yr old (Figure 1; P < 0.05).
Figure 2.
The effect of bull age on pre-freeze (upper left panel) and post-thaw total motility (upper right panel) and pre-freeze (lower left panel) and post-thaw gross motility (lower right panel). Vertical bars represent s.e.m. abDiffering superscripts differ between bull ages within each parameter (P < 0.01). n = number of ejaculates.
Effect of Ejaculate Number on Semen Production and Sperm Motility
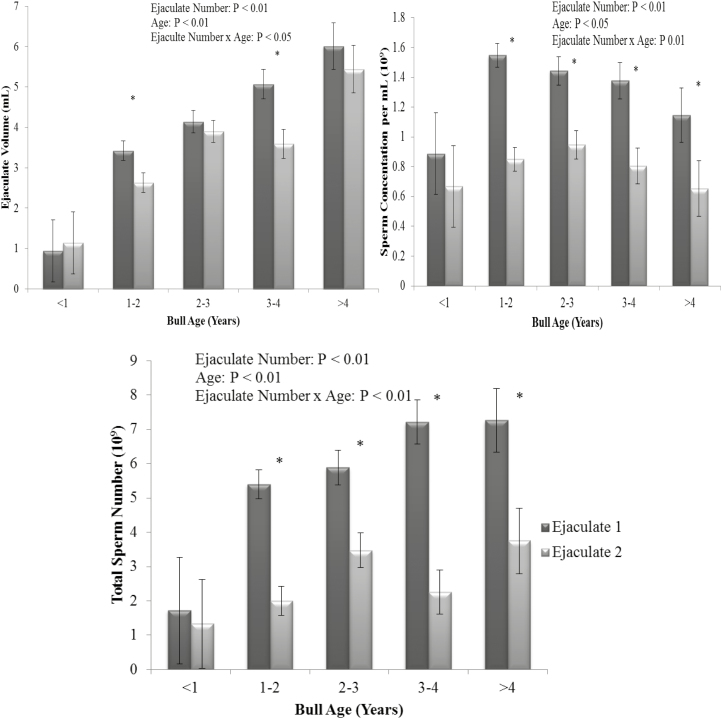
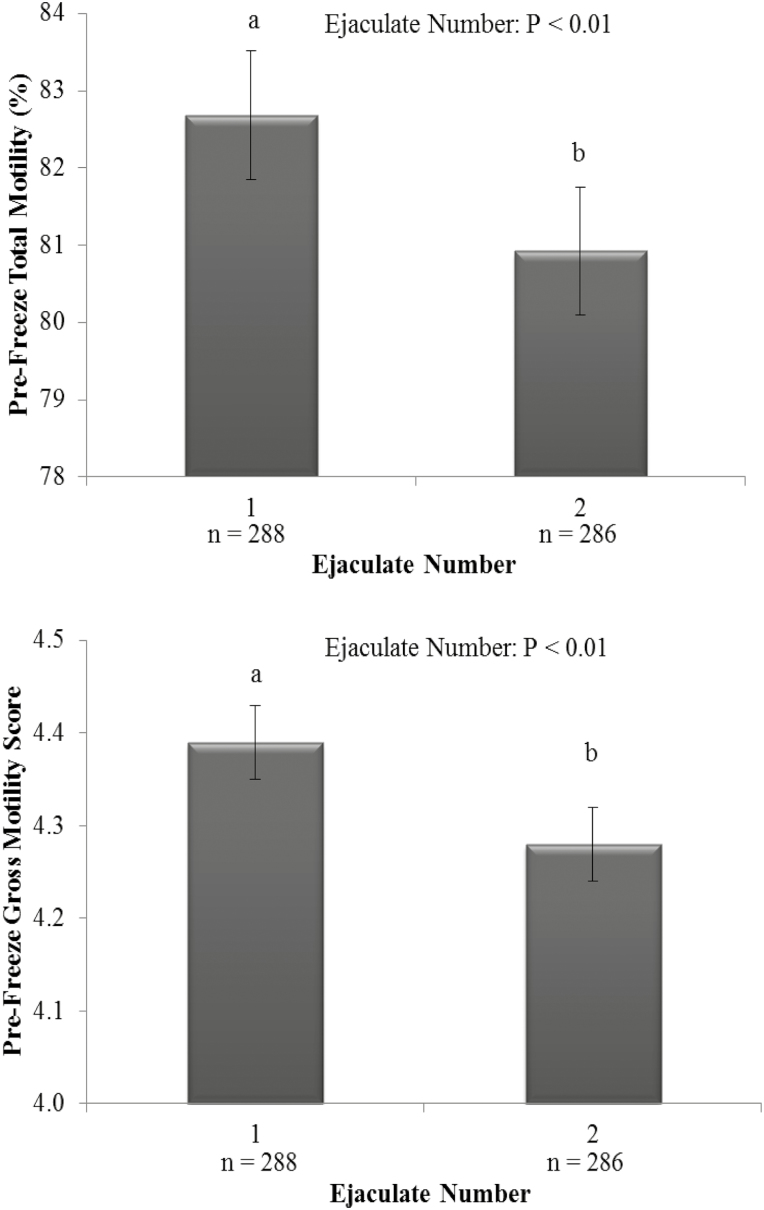
There was an ejaculate number by bull age interaction on volume (P < 0.05), sperm concentration, and TSN (P < 0.01) as bulls aged greater than 1 yr had a reduced ejaculate volume, sperm concentration, and TSN in their second ejaculate (Figure 3; P < 0.01); however, there was no effect of ejaculate number on bulls aged less than 1 yr (P > 0.05). There was an effect of ejaculate number on ejaculate volume, sperm concentration, TSN (Figure 3: P < 0.01) as well as prefreeze total and gross motility (Figure 4; P < 0.01). First ejaculates exhibited greater prefreeze total and gross motility scores (P < 0.01); however, the percentage difference in motility was relatively small and deemed not to be of commercial importance. First ejaculates had a greater ejaculate volume and sperm concentration than second ejaculates, by approximately 15% and 40%, respectively (P < 0.01). However, ejaculate number did not affect post-thaw total and gross motility (Figure 4; P > 0.05). Overall, first ejaculates resulted in more than twice the number of total sperm than a second consecutive collection, with a TSN of 5.5 and 2.6 × 109, respectively, primarily as a result of the large difference in sperm concentration between subsequent ejaculates.
Figure 3.
The interaction of ejaculate number and bull age on ejaculate volume (upper left panel), sperm concentration (upper right panel), and total sperm number (lower panel). Vertical bars represent s.e.m. *Asterisk represents differences between ejaculate number within each parameter (P < 0.01).
Figure 4.
The effect of ejaculate number on pre-freeze total motility (upper panel) and pre-freeze gross motility (lower panel). Vertical bars represent s.e.m. abDiffering superscripts differ between ejaculate numbers within each parameter (P < 0.01). n = number of ejaculates.
Effect of Season of Collection on Semen Production and Sperm Motility
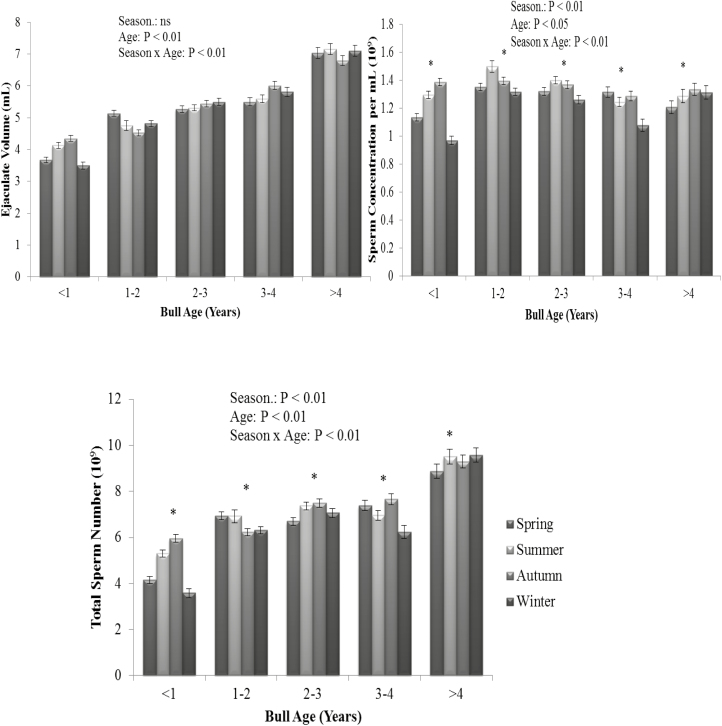
There was a season by bull age interaction for ejaculate volume, sperm concentration, TSN, post-thaw gross motility (P < 0.01), and pre-freeze gross and post-thaw total motility (P < 0.05). Although bulls aged less than 1 yr had a reduced ejaculate volume, sperm concentration, and TSN in winter than any other season, there was no clear biological pattern for any other age category. There was an effect of season on sperm concentration, TSN (Figure 5; P < 0.01), as well as post-thaw total and gross motility (Figure 6; P < 0.01). There was a tendency for season to affect ejaculate volume (P = 0.065) with semen collections in spring having the lowest volume. Semen collections in winter had the greatest post-thaw total and gross motility score compared with semen collections in spring (Figure 6; P < 0.01). Ejaculates collected in summer and autumn had greater sperm concentration and TSN in comparison to spring and winter (Figure 5; P < 0.01). Thus, regardless of the parameter assessed, semen collections in winter resulted in the poorest semen production output, whereas collections in summer and autumn had the best semen production characteristics in terms of sperm concentration and TSN.
Figure 5.
The interaction of season of collection and bull age on ejaculate volume (upper left panel), sperm concentration (upper right panel), and total sperm number (lower panel). Vertical bars represent s.e.m. *Asterisk represents differences between season within each parameter (P < 0.01). ns = not significant.
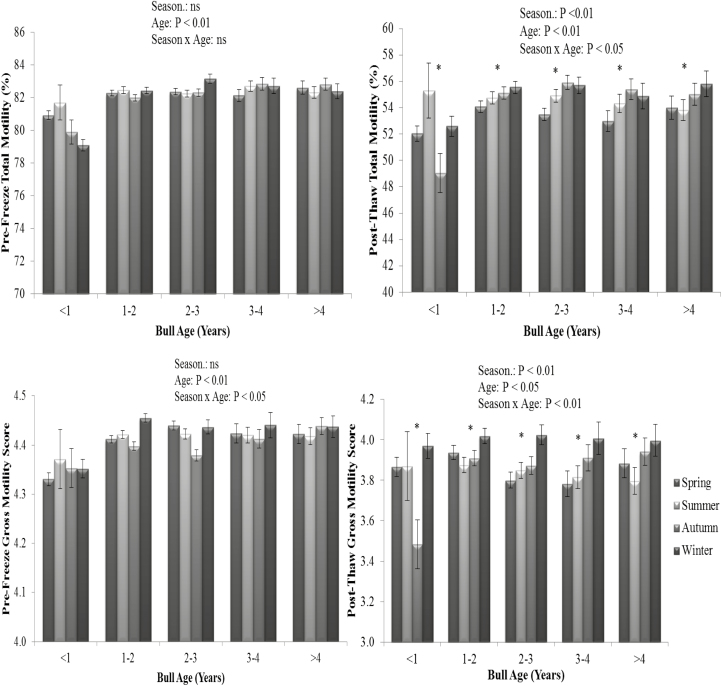
Figure 6.
The interaction of season of collection and bull age on pre-freeze (upper left panel) and post-thaw total motility (upper right panel) and pre-freeze (lower left panel) and post-thaw gross motility (lower right panel). Vertical bars represent s.e.m. *Asterisk represents differences between season within each parameter (P < 0.01). ns = not significant.
DISCUSSION
This study was a retrospective analysis of a large dataset collected over a period of 4 yr, involving a total of 8,983 ejaculates from 176 Holstein Friesian bulls aged between 9 mo and 8 yr of age. Thus, it facilitates an in-depth assessment of sperm motility of AI bulls in a comprehensive attempt to identify factors affecting semen production and sperm motility in a commercial AI setting. The main novel findings of this study were that second ejaculates can be collected from young bulls without a concomitant significant decrease in post-thaw sperm motility and thus may be a useful strategy to increase semen availability from AI bulls in high demand. This study also clearly illustrates the challenges surrounding the collection of ejaculates of sufficient volume and quality from bulls of less than 1 yr of age.
The observed increase in volume and TSN associated with increasing bull age is consistent with a number of other reports (Everett and Bean, 1982; Taylor et al., 1985; Mathevon et al., 1998; Brito et al., 2002a). Since sperm concentration remained constant after 1 yr of age, the increase in TSN with age was being driven by increases in ejaculate volume up to 4 yr of age, consistent with the findings of Everett and Bean (1982). Not surprisingly, bulls aged less than 1 yr had the lowest semen production values (Al-Kanaan et al., 2015). It is widely acknowledged (Karabinus et al., 1990; Mathevon et al., 1998; Perumal, 2014) that peripubertal bulls have reduced ejaculate volumes than mature bulls, which is in agreement with the findings in this study, and that the prepubertal period is generally characterized by rapid increases in both body and testicular weight (Aponte et al., 2005). Therefore, the increase in ejaculate volume with age may be related to an increase in activity of the hypothalamic–pituitary–testicular axis and the concurrent development of the testis and accessory glands with sexual maturity, which are believed to continue to develop for up to 5 yr post-puberty (Almquist, 1978). Following the onset of puberty, the age at which a bull is first able to produce an ejaculate containing 50 × 106 sperm with a minimum of 10% motility (Wolf et al., 1965), at approximately 9 to 11 mo of age in Holstein Friesian bulls (Dance et al., 2015; Byrne et al., 2017), the reproductive capacity of a bull increases for several years until sexually maturity is reached (Amann, 1983).
In the current study, there was an ejaculate number by bull age interaction for ejaculate volume, sperm concentration, and TSN for bulls older than 1 yr of age, with the collection of a subsequent ejaculate resulting in reduced semen production values. First ejaculates had a greater volume, sperm concentration, and TSN for bulls older than 1 yr in comparison to second ejaculates collected on the same day; however, there was no effect of ejaculate number on bulls aged less than 1 yr. This finding is similar to that of Fuerst-Waltl et al. (2006) and Bhakat et al. (2011) as first ejaculates recorded greater semen production values for all age categories; however, these studies did not investigate the effects on bulls aged less than 1 yr with the lowest bull age category for each study of 16 to 18 mo and less than 3 yr, respectively. Conversely, although volume increased with bull age, ejaculate number did not affect ejaculate volume for all ages as bulls aged between 2–3 yr and greater than 4 yr recorded similar volumes for both collections. Surprisingly, the effect of ejaculate number was not significant in bulls aged less than 1 yr for any semen production parameter. This may be primarily due to the lower semen production values associated with young bulls and the large variation within their analysis compared with more mature bulls. Furthermore, although total sperm number decreases with the collection of multiple ejaculates on the same day, the overall sperm number produced increases, resulting in an increase in the number of semen doses produced per bull per day. To place it in perspective, for an average bull, the first and second consecutive ejaculates typically produce approximately 400 and 200 straws, respectively, with a concentration of 15 × 106 sperm per 0.25-mL straw. Therefore, a bull collected twice a day, twice a week (1,200 straws) compared with once a day, twice a week (800 straws) would result in increasing overall production by 400 straws. Thus, a second collection may be justified for bulls which are in high demand, particularly those less than 1 yr of age, as semen production from these young bulls was not negatively affected by the collection of a second ejaculate.
Additionally, in the current study, first ejaculates recorded a greater prefreeze total and gross motility score than second ejaculates which is in agreement with Fuerst-Waltl et al. (2006) who reported a greater percentage of motile sperm in first ejaculates. However, similar to the findings of Boujenane and Boussaq (2013), there was no difference observed in post-thaw motility. One possible explanation behind a reduction in semen production and prefreeze sperm motility associated with second ejaculates may be due to the shortened collection interval of the second ejaculate (although not formally assessed in this study the norm was within 1 h). Longer collection intervals have been reported to result in greater semen production and quality; however, these collection intervals vary from 3–4 to 10 d (Everett and Bean, 1982; Mathevon et al., 1998; Fuerst-Waltl et al., 2006) but are unrealistic in a commercial environment setting. Due to the high demand, it is impractical for AI centers to allow up to 10 d between collections. In the current study, critically there was no effect of ejaculate number on post-thaw sperm motility in which a collection interval of approximately 1 h was implemented. This is important as the ability of sperm to maintain their functional status post-thaw in both first and second ejaculates is essential considering that AI in cattle is primarily implemented with the use of cryopreserved semen (Thibier and Wagner, 2002). Therefore, the results of the current study highlight that semen production and sperm motility can be maintained with a shorter collection interval of approximately 1 h, hence increasing productivity.
The effect of season on semen production has been widely assessed in the bull (Snoj et al., 2013; Bhakat et al., 2014; Al-Kanaan et al., 2015; Malama et al., 2017). Spermatogenesis has been shown to be susceptible to temperature variation (Rahman et al., 2011) and as it takes approximately 61 d in the bull (Johnson et al., 2000), the quality of sperm in an ejaculate may reflect conditions to which the bull was exposed 8 to 9 wk prior to collection. The impact of many environmental factors, however, is reduced when bulls are maintained in temperature-controlled barns (Haugan et al., 2005) as other studies have shown that neither temperature nor humidity affected sperm production or semen quality (Taylor et al., 1985; Brito et al., 2002b). Under the temperate climatic conditions of the current study, there was a season by bull age interaction on semen production; ejaculate volume, sperm concentration, TSN, and sperm motility; and post-thaw total motility and pre- and post-thaw gross motility. Bulls aged less than 1 yr recorded poorest semen production values in terms of volume, sperm concentration, and TSN in winter than any other season; however, there was no clear biological pattern for any other age category. Semen collections in summer recorded the highest values for sperm concentration and TSN; however, this did not differ from collections in autumn. This result is broadly in agreement with Stälhammar et al. (1989) and Snoj et al. (2013) as they observed greater sperm concentration and TSN during the summer months than in any other season in AI centers located in Sweden and Slovenia, respectively. Furthermore, winter collections recorded greater post-thaw sperm motility values; but, while there was a statistical difference in sperm motility, the difference between seasons was relatively small and is unlikely to be of biological importance or have an impact on quality control in a commercial environment as all values recorded were sufficient to pass quality control analysis. The results of the current study are consistent with Sullivan and Elliott (1968) who reported that semen collections in the United States in winter resulted in greater nonreturn rates than those in spring, which may be related to better semen quality in line with the current study. Similarly, Boujenane and Boussaq (2013) reported that semen collected in a Moroccan AI center in winter was of greater quality than summer collections.
CONCLUSION
In conclusion, this study characterized the challenges surrounding the collection of young Holstein Friesian bulls in a commercial AI setting. The low semen ejaculate volume typically associated with young bulls not only reduces sperm numbers but also prefreeze and post-thaw sperm motility. As these young bulls are typically of a higher genomic value compared with older bulls, AI centers require large quantities of their semen in order to meet demand and, therefore, need to minimize the amount of inferior quality semen being handled. The collection of a second consecutive ejaculate does not affect post-thaw sperm motility and, therefore, should be considered, particularly for bulls in high demand.
This work was supported by the Irish Research Council, Department of Agriculture, Food and the Marine as well as Teagasc under the grant number EBPPG/2014/60.
Conflict of interest statement. None declared.
LITERATURE CITED
- Al-Kanaan A., König S., and Brügemann K.. 2015. Effects of heat stress on semen characteristics of Holstein bulls estimated on a continuous phenotypic and genetic scale. Livest. Sci. 177:15–24. doi:10.1016/j.livsci.2015.04.003 [Google Scholar]
- Almquist J. 1978. Bull semen collection procedures to maximize output of sperm Technical Conference on Artif. Insem. Reprod. 33–36. [Google Scholar]
- Amann R. P. 1983. Endocrine changes associated with onset of spermatogenesis in holstein bulls. J. Dairy Sci. 66:2606–2622. doi:10.3168/jds.S0022-0302(83)82135-3 [DOI] [PubMed] [Google Scholar]
- Aponte P. M., de Rooij D. G., and Bastidas P.. 2005. Testicular development in brahman bulls. Theriogenology. 64:1440–1455. doi:10.1016/j.theriogenology.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Balić I. M., S. Milinković-Tur M. Samardžija, and Vince S.. 2012. Effect of age and environmental factors on semen quality, glutathione peroxidase activity and oxidative parameters in simmental bulls. Theriogenology. 78:423–431. doi:10.1016/j.theriogenology.2012.02.022 [DOI] [PubMed] [Google Scholar]
- Barth A. D., L. F. Brito, and Kastelic J. P.. 2008. The effect of nutrition on sexual development of bulls. Theriogenology. 70:485–494. doi:10.1016/j.theriogenology.2008.05.031 [DOI] [PubMed] [Google Scholar]
- Bhakat M., Mohanty T., Gupta A., and Abdullah M.. 2014. Effect of season on semen quality of crossbred (Karan Fries) bulls. Adv. Anim. Vet. Sci. 2:632–637. doi:10.14737/journal.aavs/2014/2.11.632.637 [Google Scholar]
- Bhakat M., Mohanty T. K., Raina V. S., Gupta A. K., Khan H. M., Mahapatra R. K., and Sarkar M.. 2011. Effect of age and season on semen quality parameters in sahiwal bulls. Trop. Anim. Health Prod. 43:1161–1168. doi:10.1007/s11250-011-9817-1 [DOI] [PubMed] [Google Scholar]
- Boujenane I. and Boussaq K.. 2013. Environmental effects and repeatability estimates for sperm production and semen quality of Holstein bulls. Archiv. Tierzucht. 56:1–6. doi:10.7482/0003-9438-56-096 [Google Scholar]
- Brito L. F., Silva A. E., Rodrigues L. H., Vieira F. V., Deragon L. A., and Kastelic J. P.. 2002a. Effect of age and genetic group on characteristics of the scrotum, testes and testicular vascular cones, and on sperm production and semen quality in AI bulls in Brazil. Theriogenology. 58:1175–1186. doi:10.1016/S0093-691X(02)00921-4 [DOI] [PubMed] [Google Scholar]
- Brito L. F., Silva A. E., Rodrigues L. H., Vieira F. V., Deragon L. A., and Kastelic J. P.. 2002b. Effects of environmental factors, age and genotype on sperm production and semen quality in bos indicus and bos taurus AI bulls in Brazil. Anim. Reprod. Sci. 70:181–190. doi:10.1016/S0378-4320(02)00009-X [DOI] [PubMed] [Google Scholar]
- Byrne C. J., Fair S., English A. M., Urh C., Sauerwein H., Crowe M. A., Lonergan P., and Kenny D. A.. 2017. Effect of breed, plane of nutrition and age on growth, scrotal development, metabolite concentrations and on systemic gonadotropin and testosterone concentrations following a GnRH challenge in young dairy bulls. Theriogenology. 96:58–68. doi:10.1016/j.theriogenology.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Dance A., Thundathil J., Wilde R., Blondin P., and Kastelic J.. 2015. Enhanced early-life nutrition promotes hormone production and reproductive development in holstein bulls. J. Dairy Sci. 98:987–998. doi:10.3168/jds.2014-8564 [DOI] [PubMed] [Google Scholar]
- Éireann M. 2017. Monthly Climate Data http://www.met.ie/climate/monthly-data.asp [assessed 12 January 2017]
- Everett R. W. and Bean B.. 1982. Environmental influences on semen output. J. Dairy Sci. 65:1303–1310. doi:10.3168/jds.S0022-0302(82)82344-8 [DOI] [PubMed] [Google Scholar]
- Fiaz M., Usmani R., Abdullah M., and Ahmad T.. 2010. Evaluation of semen quality of Holstein Friesian and Jersey bulls maintained under subtropical environment. Pak. Vet. J. 30:75–78. [Google Scholar]
- Fuerst-Waltl B., H. Schwarzenbacher C. Perner, and Sölkner J.. 2006. Effects of age and environmental factors on semen production and semen quality of Austrian simmental bulls. Anim. Reprod. Sci. 95:27–37. doi:10.1016/j.anireprosci.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Goddard M. E. and Hayes B. J.. 2007. Genomic selection. J. Anim. Breed. Genet. 124:323–330. doi:10.1111/ j.1439-0388.2007.00702.x [DOI] [PubMed] [Google Scholar]
- Godfrey R. W., Lunstra D. D., Jenkins T. G., Berardinelli J. G., Guthrie M. J., Neuendorff D. A., Long C. R., and Randel R. D.. 1990. Effect of season and location on semen quality and serum concentrations of luteinizing hormone and testosterone in Brahman and hereford bulls. J. Anim. Sci. 68:734–749. [DOI] [PubMed] [Google Scholar]
- Haugan T., Reksen O., Gröhn Y. T., Kommisrud E., Ropstad E., and Sehested E.. 2005. Seasonal effects of semen collection and artificial insemination on dairy cow conception. Anim. Reprod. Sci. 90:57–71. doi:10.1016/j.anireprosci.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Jiménez-Severiano H., J. Quintal-Franco V. Vega-Murillo E. Zanella M. E. Wehrman B. R. Lindsey E. J. Melvin, and Kinder J. E.. 2003. Season of the year influences testosterone secretion in bulls administered luteinizing honrmone. J. Anim. Sci. 81:1023–1029. doi:10.2527/2003.8141023x [DOI] [PubMed] [Google Scholar]
- Johnson L., D. D. Varner M. E. Roberts T. L. Smith G. E. Keillor, and Scrutchfield W. L.. 2000. Efficiency of spermatogenesis: a comparative approach. Anim. Reprod. Sci. 60-61:471–480. doi:10.1016/S0378-4320(00)00108-1 [DOI] [PubMed] [Google Scholar]
- Karabinus D. S., D. P. Evenson L. K. Jost R. K. Baer, and Kaproth M. T.. 1990. Comparison of semen quality in young and mature holstein bulls measured by light microscopy and flow cytometry. J. Dairy Sci. 73:2364–2371. doi:10.3168/jds.S0022-0302(90)78919-9 [DOI] [PubMed] [Google Scholar]
- Lincoln G. A., I. J. Clarke, and Sweeney T.. 1996. ‘Hamster-like’ cycles in testicular size in the absence of gonadotrophin secretion in HPD Rams exposed to long-term changes in photoperiod and treatment with melatonin. J. Neuroendocrinol. 8:855–866. doi:10.1046/j.1365-2826.1996.05397.x [DOI] [PubMed] [Google Scholar]
- Malama E., Zeron Y., Janett F., Siuda M., Roth Z., and Bollwein H.. 2017. Use of computer-assisted sperm analysis and flow cytometry to detect seasonal variations of bovine semen quality. Theriogenology. 87:79–90. doi:10.1016/j.theriogenology.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Mathevon M., M. M. Buhr, and Dekkers J. C.. 1998. Environmental, management, and genetic factors affecting semen production in holstein bulls. J. Dairy Sci. 81:3321–3330. doi:10.3168/jds.S0022-0302(98)75898-9 [DOI] [PubMed] [Google Scholar]
- Menegassi S. R., J. O. Barcellos E. A. Dias C. Koetz G. R. Jr Pereira V. Peripolli C. McManus M. E. Canozzi, and Lopes F. G.. 2015. Scrotal infrared digital thermography as a predictor of seasonal effects on sperm traits in braford bulls. Int. J. Biometeorol. 59:357–364. doi:10.1007/s00484-014-0847-z [DOI] [PubMed] [Google Scholar]
- Murphy E. M., Murphy C., O’Meara C., Dunne G., Eivers B., Lonergan P., and Fair S.. 2017. A comparison of semen diluents on the in vitro and in vivo fertility of liquid bull semen. J. Dairy Sci. 100:1541–1554. doi:10.3168/jds.2016-11646 [DOI] [PubMed] [Google Scholar]
- Nichi M., Bols P. E., Züge R. M., Barnabe V. H., Goovaerts I. G., Barnabe R. C., and Cortada C. N.. 2006. Seasonal variation in semen quality in bos indicus and bos taurus bulls raised under tropical conditions. Theriogenology. 66:822–828. doi:10.1016/j.theriogenology.2006.01.056 [DOI] [PubMed] [Google Scholar]
- Perumal P. Scrotal circumference and its relationship with testiscular growth, age, and body weight in Tho Tho (Bos indicus) bulls. Internat, Scholarly Res. Not. 2014:1–6. doi:10.1155/2014/249537. 2014 doi: 10.1155/2014/249537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. B., L. Vandaele T. Rijsselaere D. Maes M. Hoogewijs A. Frijters J. Noordman A. Granados E. Dernelle M. Shamsuddin, et al. 2011. Scrotal insulation and its relationship to abnormal morphology, chromatin protamination and nuclear shape of spermatozoa in holstein-friesian and belgian blue bulls. Theriogenology. 76:1246–1257. doi:10.1016/j.theriogenology.2011.05.031 [DOI] [PubMed] [Google Scholar]
- Snoj T., Kobal S., and Majdic G.. 2013. Effects of season, age, and breed on semen characteristics in different bos taurus breeds in a 31-year retrospective study. Theriogenology. 79:847–852. doi:10.1016/j.theriogenology.2012.12.014 [DOI] [PubMed] [Google Scholar]
- Stälhammar E. M., Janson L., and Philipsson J.. 1989. Genetic studies on fertility in AI bulls. I. Age, season and genetic effects on semen characteristics in young bulls. Anim. Reprod. Sci. 19:1–17. doi:10.1016/0378-4320(89)90042-0 [Google Scholar]
- Sullivan J. and Elliott F.. 1968. Season and fertility in artificial insemination. In: Proceedings of the VI Congr Int Reprod Anim Insem. Artiff, Paris I, 329–332. [Google Scholar]
- Tatman S. R., Neuendorff D. A., Wilson T. W., and Randel R. D.. 2004. Influence of season of birth on growth and reproductive development of Brahman bulls. Theriogenology. 62:93–102. doi:10.1016/j.theriogenology.2003.07.027 [DOI] [PubMed] [Google Scholar]
- Taylor J., Bean B., Marshall C., and Sullivan J.. 1985. Genetic and environmental components of semen production traits of artificial insemination Holstein bulls. J. Dairy Sci. 68:2703–2722. doi:10.3168/jds.S0022-0302(85)81155-3 [Google Scholar]
- Thibier M. and Wagner H. G.. 2002. World statistics for artificial insemination in cattle. Livest. Prod. Sci. 74:203–212. [Google Scholar]
- Wildeus S. and Hammond A. C.. 1993. Testicular, semen and blood parameters in adapted and nonadapted bos taurus bulls in the semi-arid tropics. Theriogenology. 40:345–355. doi:10.1016/0093-691X(93)90272-7 [DOI] [PubMed] [Google Scholar]
- Wolf F. R., J. O. Almquist, and Hale E. B.. 1965. Prepuberal behavior and puberal characteristics of beef bulls on high nutrient allowance. J. Anim. Sci. 24:761–765. [DOI] [PubMed] [Google Scholar]