Abstract
Boer × Spanish kid goats (n = 48) were used to evaluate effects of using ground woody products in feedlot diets on growth performance and blood serum chemistry. A completely randomized study design was used with 2 feeding periods (Period 1 = 70% concentrate, days 0 to 26; Period 2 = 86% concentrate, days 27 to 64). Goats were individually fed 1 of 6 diets that differed only by roughage source (n = 4 wether males and 4 females/treatment; initial BW = 22 ± 2 kg): cottonseed hulls (CSH; control) or ground wood consisting of redberry (RED), blueberry (BLUE), one-seed (ONE), or eastern red cedar (ERC) Juniperus spp., or Prosopis glandulosa (MESQ). Ground woody diets were individually compared with CSH. During Period 1, goats fed CSH had greater (P < 0.05) average daily DMI (DMI), ADG, and G:F than goats fed MESQ and tended to have greater (P < 0.10) ADG and G:F than goats fed BLUE. A Treatment × d interaction (P = 0.008) was observed for goat BW during Period 1 and goats fed CSH tended (P < 0.09) to have greater BW on day 27 than goats fed BLUE or MESQ. During Period 2, Treatment × d interactions were not observed (P > 0.29) for DMI, ADG, G:F, or BW and no differences were observed between goats fed CSH and goats fed any of the treatment diets. Various blood serum variables were different between CSH and goats fed diets containing woody plants (mainly during Period 1); however, blood serum profiles did not indicate hepatotoxicity or any other health issue. Collectively, results suggested that ground Juniperus pinchotii, Juniperus ashei, or Juniperus monosperma can completely replace CSH in goat feedlot diets without negatively affecting growth performance or animal health. During Period 1, feeding diets to goats that contain 30% Juniperus virginiana (ERC) or P. glandulosa (MESQ) may not be economically justifiable in most scenarios, even though goat health, assessed by blood serum profiles, was not negatively affected. However, using 14% J. virginiana (ERC) or P. glandulosa (MESQ) in finishing diets is warranted.
Keywords: feedlot, goats, juniper, mesquite, plant secondary metabolites, serum chemistry
INTRODUCTION
The price volatility of feed ingredients due to normal marketing conditions and other conditions such as drought necessitates investigations into the feasibility of alternatives to common roughage sources. One alternative is the use of ground woody products in mixed diets. Using woody products in livestock diets is not a new concept. For example, Maynard (1920) reported that ground pine wood was used extensively in livestock diets during the first World War; Archibald (1926) discussed the use of various sawdust varieties in livestock diets; and Marion et al. (1957) report that ground Prosopis spp. could replace cottonseed hulls (CSH) in cattle diets without negatively affecting animal health or growth performance.
Invasive Juniperus spp. (Ansley et al., 2006) and Prosopis spp. (Felker, 1996) plants infest millions of acres of rangelands within the United States. Negative effects of juniper encroachment on stocking rates have been documented (Jenkins, 1939). Encroachment can lead to reduced forage production which is essential to livestock and wildlife (Scholes and Archer, 1997). If the demand and economic value of the woody plant material is increased, then selling this material would potentially offset management costs.
Stewart et al. (2015) reported that nutritional and digestive characteristics of 4 Juniperus spp. were comparable to many traditional roughage ingredients. Others have reported that ground Juniperus pinchotii leaves and stems (Whitney et al., 2014) and the entire tree of Juniperus spp. and Prosopis spp. (Whitney et al., 2017) have potential to completely replace ground oat hay or CSH, respectively, in lamb feedlot diets. However, the feeding value of ground mature trees needs to be further evaluated in kid goat diets. It was hypothesized that mature woody plants could replace CSH in kid goat feedlot diets. Therefore, the objective of this experiment was to evaluate effects of ground woody plants in kid goat diets on growth performance and health.
MATERIALS AND METHODS
Animals and Management
The experimental protocol was approved by the Texas A&M AgriLife Research Agriculture Animal Care and Use Committee (number 2014-006; San Angelo, TX). Upon arrival, 48 Boer × Spanish kid goats (approximate age = 6 mo; initial arrival BW = 19.5 ± 1.9 kg) received an ear tag, subcutaneous clostridial vaccine (Vision 7 with SPUR, Intervet Inc., Omaha, NE), and an oral anthelminthic (levamisole; Prohibit, AgriLabs, St. Joseph, MO). Goats had previously been on pasture; thus, there was a 29-d adaptation period before the experiment in which no treatment diets were fed. During the first 24 d of the adaption period, goats were group-fed and had ad libitum access to oat hay, which was supplemented with a 60% concentrate diet (0.22 kg d goat; DM basis).
Each goat was weighed 9 d before study initiation, grouped by gender, stratified by BW, and randomly assigned to an individual, completely covered dirt pen (2.44 × 2.97 m) with automatic watering system and feed bunk. Each goat was also randomly assigned to 1 of 6 treatment diets (n = 4 wether males and 4 females/treatment diet) that differed only by roughage source: CSH (control) or a ground woody product consisting of J. pinchotii (RED), Juniperus ashei (BLUE), Juniperus monosperma (ONE), Juniperus virginiana (ERC), or Prosopis glandulosa (MESQ). One male goat fed ERC died on day 46 (during Period 2) and the veterinary diagnosis revealed a chronic duodenal ulcer with fibrinous exudate. The etiology of the ulcer is unknown and could be attributed to numerous factors such as a change in diet composition (transition between Period 1 and 2) or disease (viral, bacterial, or both).
After the adaptation period, goats (day 0, BW = 22 ± 2 kg) were fed for 2 periods for a total of 64 d. During Period 1 (days 0 to 26), goats were fed their respective 70% concentrate treatment diet (concentrate was composed mainly of corn-dried distillers grains with solubles [DDGS] and ground sorghum grain, Table 1). Goats were then transitioned over 5 d into Period 2 (days 27 to 64) onto their respective 86% concentrate treatment diet (Table 1) by gradual replacement of the Period 1 diet with the Period 2 diet. All mixed diets were nonpelleted, contained 22 g of monensin/t of feed (Rumensin 90, Elanco Animal Health, Indianapolis, IN), and fed ad libitum daily at 0800 h with an approximate allowance of 10% refusal. Goat BW was recorded before the 0800 h feeding on days 0, 27, 41, 55, and 64; goats were not restricted from feed or water before weighing. Goat ADG and average daily DMI were determined between days that BW was recorded. On days 0, 26, and 54, a 10-mL blood sample was collected 4 h after feeding from each goat via jugular venipuncture using a nonheparinized vacutainer collection tube (serum separator tube, gel, and clot activator; Becton Dickenson, Franklin Lakes, NJ).
Table 1.
Ingredient, chemical composition (% DM basis), and digestibility of treatment diets
| Item1 | Diet2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period 1 | Period 2 | |||||||||||
| CSH | RED | BLU | ONE | ERC | MESQ | CSH | RED | BLU | ONE | ERC | MESQ | |
| Cottonseed hulls | 30.0 | – | – | – | – | – | 14.0 | – | – | – | – | – |
| Ground wood | – | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | – | 14.0 | 14.0 | 14.0 | 14.0 | 14.0 |
| DDGS | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| Ground sorghum grain | 21.7 | 21.7 | 21.7 | 21.7 | 21.7 | 21.7 | 37.6 | 37.6 | 37.6 | 37.6 | 37.6 | 37.6 |
| Molasses, cane | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Limestone | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 |
| Ammonium chloride | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Salt | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Mineral and vitamin premix | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Nutrient composition, % | ||||||||||||
| DM | 91.9 | 90.8 | 90.6 | 91.6 | 91.4 | 91.2 | 90.6 | 89.6 | 89.9 | 90.1 | 90.0 | 89.7 |
| CP | 18.2 | 18.5 | 18.6 | 17.3 | 18.5 | 18.8 | 19.1 | 19.8 | 19.1 | 18.5 | 19.3 | 19.7 |
| aNDF | 32.6 | 34.8 | 33.2 | 37.9 | 34.6 | 36.6 | 25.4 | 25.7 | 23.1 | 26.3 | 25.6 | 26.6 |
| ADF | 16.4 | 19.9 | 19.0 | 22.7 | 20.4 | 19.7 | 13.5 | 13.6 | 12.9 | 14.7 | 13.3 | 12.1 |
| Ca | 1.2 | 1.4 | 1.4 | 1.3 | 1.3 | 1.4 | 1.2 | 1.4 | 1.3 | 1.3 | 1.2 | 1.4 |
| P | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Ca:P | 2.3 | 2.7 | 2.9 | 2.8 | 2.9 | 3.0 | 2.4 | 2.7 | 2.8 | 2.7 | 2.5 | 2.9 |
| Ash | 8.1 | 8.0 | 8.5 | 7.7 | 8.5 | 8.4 | 7.8 | 9.1 | 8.3 | 8.0 | 8.1 | 8.5 |
| True IVDMD | 70.8 | 64.4 | 67.1 | 60.8 | 65.0 | 63.6 | 74.6 | 72.6 | 74.7 | 74.8 | 73.2 | 73.8 |
1DDGS = corn-dried distillers grains with solubles are a byproduct of corn ethanol production (POET, Sioux Falls, SD). Mineral premix 3 = NaCl, KCl, S, MnO, ZnO, vitamins A, D, and E, CaCO3, cottonseed meal, cane molasses, and animal fat (Ca = 0.11 to 0.16%; P = 0%; 4 Na 24.6 to 26.5%; Mg = 0%; K = 9.57%; Se = 0%; salt = 64 to 69%); ADICP = acid detergent insoluble CP; True IVDMD = true 48-h IVDMD.
2Goats were transitioned over 29 d onto their respective diets. During Period 1 (days 0 to 26; 48 goats), goats were fed a 70% concentrate ration. Goats were transitioned over 5 d into Period 2 (days 27 to 64; 47 goats) and onto an 86% concentrate ration. Treatment diets were mixed and nonpelleted; ingredient composition differed only by roughage source; either cottonseed hulls (CSH) or ground woody products (RED = Juniperus pinchotii, BLUE = Juniperus ashei, ONE = Juniperus monosperma, ERC = Juniperus virginiana, or MESQ = Prosopis glandulosa). Juniperus spp. (entire above-ground biomass) and P. glandulosa (entire above-ground biomass except for leaves) species were chipped, dried, and hammermilled to pass a 4.76-mm sieve. Monensin (Rumensin 90, Elanco, Indianapolis, IN) was added to each diet at 22 g/t of feed.
Sample Collection and Measurements
Woody plant harvesting and feed processing, collection, and analysis.
During a single collection period, the entire above ground biomass from mature Juniperus spp. (including leaves) and mature P. glandulosa spp. (excluding leaves) trees was harvested separately by species, chipped (Model BC1800, Vermeer Corp., Pella, IA), and dried for 5 h to approximately 93% DM using a drying trailer equipped with a perforated metal bottom sieve and a jet dryer (26 to 31 °C; model 2001; Peerless Manuf. Co., Shellman, GA). Chipped material was hammermilled to pass a 4.76-mm screen (Sentry, model 100; Mix-Mill Feed Processing Systems, Bluffton, IN), bagged, and stored under cover.
Nutritive characteristics of woody plants were evaluated using random subsamples of mechanically dried and hammermilled (4.76-mm screen) woody plants. Subsamples of CSH, sorghum grain, DDGS, and each woody plant species were collected 3 times during each period; subsamples were combined separately by period for analysis (Table 2). In addition, 3 random subsamples of the mixed treatment diets (Table 1) were collected during both periods, combined by period, and analyzed separately. These samples were dried at 55 °C in a forced-air oven (model 630, NAPCO, Portland, OR) for 48 h, ground through a 1-mm screen (Wiley mill, Arthur H. Thomas Co., Philadelphia, PA), and stored at −20 °C. Nitrogen was analyzed by a standard method (Method 990.03; AOAC, 2006) and CP calculated as 6.25 × N. The NDF and ADF were analyzed according to the procedures of Van Soest et al. (1991), which were modified for an Ankom 2000 Fiber Analyzer (Ankom Technol. Corp., Fairport, NY) using α-amylase and Na sulfite. In addition, N was analyzed in residue remaining after the ADF procedure and multiplied by 6.25 to determine acid detergent insoluble CP (ADICP). Standard methods were used to analyze lignin (Method AOAC 973.18; AOAC, 2006), crude fat (Method 2003.05; AOAC, 2006), and ash (Method 942.05; AOAC, 2006). For individual mineral analysis, samples were digested with a Microwave Accelerated Reaction System (MARS6; CEM, Matthews, NC) and then analyzed by a Thermo Jarrell Ash IRIS Advantage HX Inductively Coupled Plasma Radial Spectrometer (Thermo Instrument Systems, Inc., Waltham, MA).
Table 2.
Chemical composition and digestibility (% DM basis) of cottonseed hulls, sorghum grain, and dried distillers grains with solubles (DDGS), and ground Juniperus spp. and Prosopis glandulosa used in the treatment diets
| Item1 | Ingredient2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Cottonseed hulls | Sorghum grain | Corn DDGS | J.pin | J.ash | J.mon | J.vir | P.glan | |
| Nutrient composition | ||||||||
| DM, % | 92.3 | 92.6 | 91.8 | 93.9 | 93.8 | 95.4 | 93.8 | 91.9 |
| CP, % | 3.5 | 11.9 | 30.4 | 2.9 | 2.8 | 2.5 | 3.8 | 5.7 |
| ADICP, % | 3.2 | 1.5 | 1.3 | 1.5 | 1.6 | 1.4 | 1.8 | 2.5 |
| aNDF, % | 85.2 | 7.0 | 30.4 | 62.1 | 65.0 | 71.0 | 68.0 | 74.7 |
| ADF, % | 62.1 | 5.3 | 12.9 | 49.4 | 52.1 | 57.9 | 55.8 | 57.8 |
| Lignin, % | 16.4 | 0.9 | 2.9 | 19.4 | 21.2 | 23.2 | 21.7 | 17.9 |
| Crude fat, % | 0.6 | 3.1 | 8.7 | 3.2 | 3.2 | 4.5 | 4.1 | 6.2 |
| Ash, % | 3.6 | 3.6 | 4.7 | 4.9 | 4.8 | 3.4 | 4.4 | 4.3 |
| Ca, % | 0.12 | 0.04 | 0.07 | 1.31 | 1.53 | 1.27 | 1.37 | 1.42 |
| P, % | 0.04 | 0.21 | 0.88 | 0.04 | 0.04 | 0.03 | 0.06 | 0.03 |
| S, % | 0.06 | 0.14 | .93 | 0.07 | 0.04 | 0.04 | 0.05 | 0.06 |
| K, % | 0.99 | 0.34 | 1.33 | 0.33 | 0.16 | 0.11 | 0.24 | 0.35 |
| Mg, % | 0.14 | 0.12 | 0.38 | 0.06 | 0.04 | 0.03 | 0.1 | 0.02 |
| Na, % | 0.01 | 0.01 | 0.31 | 1.25 | 0.01 | 0.01 | 0.01 | 0.01 |
| Fe, mg/kg | 33 | 48 | 85 | 168 | 98 | 113 | 140 | 112 |
| Zn, mg/kg | 5 | 20 | 64 | 104 | 9 | 6 | 11 | 4 |
| Cu, mg/kg | 3 | 3 | 8 | 3 | 2 | 2 | 2 | 2 |
| Particle distribution, % | ||||||||
| 19 to 8 mm | 68.9 | – | 0.20 | 3.9 | 3.7 | 5.2 | 0.3 | |
| 8 to 1.18 mm | 27.6 | – | – | 69.3 | 68.1 | 68.2 | 63.7 | 70.3 |
| < 1.18 mm | 3.5 | – | – | 30.5 | 28.0 | 28.2 | 31.1 | 29.4 |
| < 8 mm | 31.1 | – | – | 99.8 | 96.2 | 96.3 | 94.8 | 99.7 |
| CT, % | ||||||||
| Extractable | 1.4 | 0 | – | 2.8 | 3.2 | 1.8 | 2.4 | 0.9 |
| Protein-bound | 1.8 | 0 | – | 2.1 | 2.3 | 1.9 | 2.3 | 3.8 |
| Fiber-bound | 0.2 | 0 | – | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Total | 3.2 | 0 | – | 4.9 | 5.5 | 3.7 | 4.7 | 4.7 |
| True IVDMD | 18.9 | 94.3 | 75.6 | 31.3 | 29.0 | 24.6 | 26.9 | 20.9 |
1ADICP = acid detergent insoluble CP; aNDF = neutral detergent fiber, using α-amylase and sodium sulfite; CT = condensed tannins; True IVDMD = true 48-h IVDMD. Particle distribution = ingredient sieved using the manually operated Penn State Particle Separator (Lammers et al., 1996 according to modified procedures of Kononoff et al. (2003).
2J.pin = Juniperus pinchotii; J.ash = Juniperus ashei; J.mon = Juniperus monosperma; and J.vir = Juniperus virginiana (entire above-ground biomass); P.glan = Prosopis glandulosa (entire above-ground biomass excluding leaves); DDGS = corn dried distillers grains with solubles produced from corn ethanol production (POET, Sioux Falls, SD).
To evaluate particle size distribution of CSH and the ground woody plants, as-fed subsamples of these ingredients were sieved with the manually operated Penn State Particle Separator (Lammers et al., 1996) according to the modified procedures of Kononoff et al. (2003). Percentage of material (as-fed) retained on each sieve and under the 8-mm sieve was calculated based on the total sieved material. Condensed tannins (CT) in the juniper, mesquite, CSH, and sorghum grain were assayed for soluble, protein-bound, and fiber-bound fractions by methods described by Terrill et al. (1992); samples were oven dried and standards prepared for each individual ingredient as recommended by Wolfe et al. (2008). Rumen fluid was collected from donor goats using an oral stomach tubing. These goats were grazing native pasture at the time of collection, common to the Edwards Plateau area of Texas, e.g., Nassella leucotricha, Hilaria belangeri, and Bouteloua spp. After collection, ruminal fluid pH was immediately recorded and fluid filtered through 4 layers of cheese-cloth into prewarmed thermoses. An Ankom model DaisyII incubator was used to determine 48-h true IVDMD (tIVDMD) by incubating individual ingredients and each treatment diet in separate F57 bags (3 replicates; Ankom Technol. Corp., Macedon, NY) for 48 h. Each bag contained 0.35 g of material that was ground to pass a 2-mm screen (Wiley mill). Bags were placed into jars containing 400 mL of goat rumen fluid (collected orally from 8 donors and combined) and 1,600 mL of McDougall’s buffer solution (1.0 g of urea/liter; McDougall, 1948). One blank bag per jar was also included and used to adjust for potential residue on the bags. After anaerobic incubation at 39 °C, bags were gently rinsed under cold water for 5 min, subjected to the NDF procedure (using α-amylase and omitting Na sulfite), gently rinsed in acetone, dried at 55 °C in a forced-air oven for 48 h, and weighed.
Blood serum and analysis.
Blood samples were placed on ice, allowed to clot for approximately 30 min, and then centrifuged (Beckman Coulter TJ6 refrigerated centrifuge, Brea, CA) at 970 × g for 25 min at 4 °C. Serum was removed with a bulb pipette and frozen at −20 °C until a chemistry profile was analyzed by The Texas A&M Veterinary Diagnostic Laboratory, Amarillo, using an Olympus AU400E analyzer (Olympus America Inc., Center Valley, PA).
Statistical Analysis
Goat BW, DMI, and blood serum parameters with normal distributions (glucose, β-hydroxybutyrate, serum urea N, creatine, albumin, Ca, P, Mg, Cl, Na, and K) were analyzed by Period using the PROC MIXED procedure of SAS (SAS Inst. Inc., Cary, NC). Within both Periods, goat BW was analyzed with a model that included gender, treatment, day, and a Treatment × d interaction; day was the repeated measure, individual goat was the subject, and Kenward–Rodger degrees of freedom approximation was used. Main effects within period were evaluated when a Treatment × d interaction was not observed (P > 0.10). Goat DMI and blood serum parameters with normal distributions were analyzed with similar procedures, but within Period 1 (DMI) and Periods 1 and 2 (serum), the model included only gender and treatment. Blood serum analyzed on day 0 was not included in the analysis, but reported to provide an initial reference. Goat ADG (beta distribution) and G:F (gamma distribution) were analyzed using PROC GLIMMIX (SAS Inst. Inc.) with the same model as described for DMI, but day was the random effect. Suitable covariance structures (unstructured, compound symmetry, and compound symmetry heterogeneity) were compared for each model and compound symmetry heterogeneity was determined the most appropriate structure according to Akaike information criterion.
Blood serum parameters with non-normal distributions were analyzed using PROC GLIMMIX (SAS Inst. Inc.) with the same model as previously described for the other serum variables. A β (albumin:globulin ratios and Na:K) or lognormal (alanine aminotransferase, total protein, globulin, bilirubin, creatine phophokinase, aspartate aminotransferase, γ-glutamyl transferase, and nonesterified fatty acids) distribution was used. Statistical significance was declared at P ≤ 0.05 and a tendency at 0.05 < P ≤ 0.10. Data are reported as least squares means with greatest SEM. Differences in least squares means between goats fed CSH or each individual ground woody diet were evaluated using the DIFF option with a DUNNETT adjustment.
RESULTS AND DISCUSSION
Animal Growth Performance
Period 1.
Goat DMI, ADG, and G:F data are presented in Table 3. A treatment effect was observed for average daily DMI (P = 0.06), ADG (P = 0.001), and G:F (P = 0.02). Average daily DMI during Period 1 (days 0 to 26) was greater (P = 0.04) for goats fed CSH than goats fed MESQ. However, no differences were observed (P > 0.10) between goats fed CSH and goats fed any of the diets that contained Juniperus spp. Reduced juniper intake in the diet of foraging ruminants has been attributed to the palatability of volatile terpene oils (Provenza et al., 1992) and more recently, CT (Whitney et al., 2017). Since no differences were observed for DMI during Period 1 between goats fed CSH and goats fed Juniperus-based diets, it is unlikely that negative palatability or postingestive feedback was contributing factors for these diets.
Table 3.
Effects of replacing cottonseed hulls with ground woody products on goat growth performance
| Item1 | Diet2 | P-value4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CSH | RED | BLUE | ONE | ERC | MESQ | SEM3 | T | D | T × D | |
| Period 1; day 27 | ||||||||||
| DMI, kg/d | 0.87 | 0.89 | 0.79 | 0.86 | 0.78 | 0.67* | 0.05 | 0.06 | – | – |
| ADG, kg | 0.17 | 0.16 | 0.12† | 0.14 | 0.12 | 0.09* | 0.02 | 0.001 | – | – |
| G:F, kg/kg | 0.20 | 0.18 | 0.15† | 0.16 | 0.17 | 0.12* | 0.02 | 0.02 | – | – |
| Period 2; days 27 to 64 | ||||||||||
| DMI, kg/d | 0.97 | 0.98 | 0.85 | 1.02 | 0.90 | 0.88 | 0.05 | 0.09 | 0.74 | 0.69 |
| ADG, kg | 0.14 | 0.13 | 0.13 | 0.16 | 0.13 | 0.14 | 0.02 | 0.75 | 0.10 | 0.29 |
| G:F, kg/kg | 0.15 | 0.13 | 0.15 | 0.16 | 0.14 | 0.15 | 0.02 | 0.82 | 0.12 | 0.37 |
| Overall (days 0 to 64) | T | P | T × P | |||||||
| DMI, kg/d | 0.92 | 0.95 | 0.81 | 0.93 | 0.85 | 0.77† | 0.05 | 0.05 | < 0.001 | 0.07 |
| ADG, kg | 0.16 | 0.15 | 0.13 | 0.15 | 0.13† | 0.12* | 0.02 | 0.06 | 0.02 | 0.06 |
| G:F, kg/kg | 0.18 | 0.16 | 0.15 | 0.16 | 0.15 | 0.14 | 0.02 | 0.40 | 0.48 | 0.18 |
During Period 1 (days 0 to 26), goats were fed a 70% concentrate ration. Goats were transitioned over 5 d into Period 2 (days 27 to 64) and onto an 86% concentrate ration. Treatments were only compared with cottonseed hulls (CSH), not to each other. Within row, means with a different superscript than the control diet (CSH) differ: †0.05 > P < 0.10; *P < 0.05.
1DMI = average daily DMI.
2Treatment diets were mixed and nonpelleted; ingredient composition differed only by roughage source; either cottonseed hulls (CSH) or ground woody products (RED = Juniperus pinchotii, BLUE = Juniperus ashei, ONE = Juniperus monosperma, ERC = Juniperus virginiana, or MESQ = Prosopis glandulosa). Juniperus spp. (entire above-ground biomass) and P. glandulosa (entire above-ground biomass except for leaves) species were chipped, dried, and hammermilled to pass a 4.76-mm sieve.
3SEM = greatest SEM.
4T = treatment effect; D = day effect; P = period effect.
When compared with goats fed CSH, ADG and G:F were less (P ≤ 0.004) for goats fed MESQ and tended to be less (P ≤ 0.10) for goats fed BLUE. In a companion experiment (Whitney et al., 2017), which evaluated the same treatment diets in feedlot lambs, lambs fed CSH had greater ADG than lambs fed ERC or MESQ, respectively, when averaged across all days. In the current experiment, ground mesquite had relatively similar nutrient and digestive characteristics to CSH, but ground mesquite had a greater percentage of smaller particles than CSH, especially particles less than 8 mm (Table 2). Small particles, especially less than 8 mm (Lammers et al., 1996), can reduce physical effectiveness of NDF (peNDF) and rumen function. Furthermore, the MESQ diet had greater concentrations of structural fiber and less tIVDMD compared with the CSH diet (Table 1), which may have also contributed to the reduced growth performance. It is plausible that the main reason for reduced growth performance of goats fed the MESQ diet was due to differences in physical characteristics (peNDF, particle size, fragility, buoyancy, and rate of hydration) between the mesquite and CSH particles as previously discussed by Whitney et al. (2017).
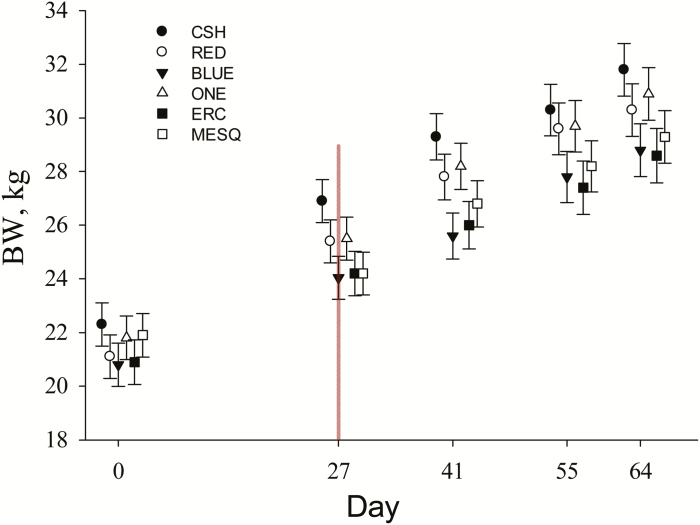
A Treatment × d interaction (P = 0.008) was observed for goat BW (Figure 1). On the final day of Period 1, goats fed the CSH diet tended to have greater (P < 0.09) BW than goats fed BLUE or MESQ diets, which reflects less ADG during Period 1 for these goats. The tIVDMD of the BLUE and MESQ diets was less than the CSH diet, which may also have been a contributing factor in overall growth performance.
Figure 1.
Effects of replacing cottonseed hulls (CSH, control diet) with ground woody plants on goat BW. Treatment diets differed only by roughage source; either CSH or ground woody products (RED = Juniperus pinchotii, BLUE = Juniperus ashei, ONE = Juniperus monosperma, ERC = Juniperus virginiana, or MESQ = Prosopis glandulosa). During Period 1 (days 0 to 26), goats were fed a 70% concentrate ration. Goats were transitioned into Period 2 (days 27 to 64) onto an 86% concentrate ration. Data were analyzed by Period and presented as least square means ± SEM. During Period 1, a Treatment × d interaction (P = 0.008) was observed for BW and goats fed CSH tended to have greater (P < 0.09) BW on day 27 that goats fed BLUE or MESQ. A Treatment × d interaction (P = 0.67) was not observed for goat BW during Period 2.
Period 2.
No Treatment × d interactions were observed for average daily DMI (P = 0.69), ADG (P = 0.29), or G:F (P = 0.37); thus, data were average across all days during Period 2 (Table 3). There tended to be an effect of Treatment (P = 0.09) for DMI, but no differences in DMI were observed (P ≥ 0.25) between lambs fed CSH or any of the ground woody treatment diets. In addition, no differences in ADG or G:F were observed (P ≥ 0.84) between lambs fed CSH or any of the ground woody treatment diets, suggesting that growth performance increased in goats fed MESQ and BLUE after the percentage of roughage in the mixed diet was reduced from 30% to 14%. A Treatment × d interaction (P = 0.67) was not observed for goat BW during Period 2 and final BW were not different (P ≥ 0.21) between goats fed CSH or goats fed any of the ground woody treatment diets.
When data were analyzed across the entire experiment (Table 3), there tended to be a Treatment × Period interaction for DMI (P = 0.07) and ADG (P = 0.06), but not for G:F (P = 0.18). Average DMI tended to be less (P = 0.09) for goats fed MESQ compared with goats fed CSH. Average daily gain was less for goats fed MESQ (P = 0.03) compared with CSH, which was representative of the overall reduced DMI. Also, goat ADG over the entire experiment tended to be less (P = 0.08) for goats fed ERC compared with goats fed CSH. The tIVDMD of the ground P. glandulosa wood was less than all ground Juniperus spp. but was greater than CSH; however, total percentage of protein-bound CT was approximately 2 percentage units greater in P. glandulosa than all the other roughages. Plant CT can form complexes with proteins in the saliva produced by goats and this bond can produce an astringent effect, which could have reduced DMI (Van Soest, 1994).
Blood Serum Profiles
Period 1.
Goat blood serum profiles are presented in Table 4. A Treatment effect was observed for NEFA (P = 0.03), serum urea N (SUN; P < 0.001), bilirubin (P < 0.001), albumin (P = 0.04), γ-glutamyl transferase (GGT; P = 0.02), creatine phophokinase (CPK; P = 0.002), P (P = 0.003), Cl (P = 0.03), and there tended to be an effect for glucose (P = 0.06), β-hydroxy butyrate (BHB; P = 0.08), total protein (TP; P = 0.05), and alanine aminotransferase (ALT; P = 0.05). At the end of Period 1 (day 26), bilirubin, CPK, and Cl were greater (P ≤ 0.02), SUN tended to be greater (P < 0.09), glucose was less (P = 0.02), and NEFA and P tended to be less (P ≤ 0.09) in goats that were fed the MESQ diet compared with goats fed the CSH diet.
Table 4.
Effects of replacing cottonseed hulls with ground woody products on goat blood serum profile
| Item1 | Diet2 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| CSH | RED | BLUE | ONE | ERC | MESQ | SEM3 | Treatment | |
| Period 1 | ||||||||
| Glucose, mg/dL | ||||||||
| day 0 | 71.4 | 71.6 | 74.1 | 68.8 | 74.8 | 73.1 | 2.2 | |
| day 26 | 77.4 | 72.5 | 73.4 | 70.5 | 70.6 | 68.0* | 2.3 | 0.06 |
| NEFA, mEq/liter | ||||||||
| day 0 | 0.15 | 0.15 | 0.18 | 0.16 | 0.22 | 0.15 | 0.04 | |
| day 26 | 0.11 | 0.12 | 0.13 | 0.11 | 0.11 | 0.09† | 0.01 | 0.03 |
| BHB, µmol/liter | ||||||||
| day 0 | 283.8 | 337.9 | 288.4 | 269.5 | 279.3 | 292.3 | 33.7 | |
| day 26 | 264.0 | 362.1† | 375.1* | 334.9 | 322.2 | 330.3 | 28.5 | 0.08 |
| SUN, mg/dL | ||||||||
| day 0 | 15.5 | 17.2 | 17.7 | 20.3 | 19.2 | 17.9 | 1.5 | |
| day 26 | 14.4 | 21.0* | 20.6* | 22.1* | 22.2* | 18.2† | 1.2 | < 0.001 |
| Creatinine, mg/dL | ||||||||
| day 0 | 0.67 | 0.64 | 0.71 | 0.66 | 0.67 | 0.68 | 0.04 | |
| day 26 | 0.64 | 0.58 | 0.61 | 0.58 | 0.61 | 0.63 | 0.04 | 0.79 |
| Bilirubin, mg/dL | ||||||||
| day 0 | 0.11 | 0.13 | 0.12 | 0.13 | 0.13 | 0.14 | 0.05 | |
| day 26 | 0.11 | 0.13* | 0.12 | 0.13* | 0.13* | 0.15* | 0.05 | < 0.001 |
| Albumin, g/dL | ||||||||
| day 0 | 3.1 | 3.2 | 3.2 | 3.3 | 3.1 | 3.2 | 0.06 | |
| day 26 | 3.1 | 3.2 | 3.3 | 3.3 | 3.1 | 3.2 | 0.05 | 0.04 |
| Globulin, g/dL | ||||||||
| day 0 | 2.8 | 2.6 | 2.9 | 2.7 | 2.7 | 2.9 | 0.1 | |
| day 26 | 2.5 | 2.4 | 2.7 | 2.5 | 2.5 | 2.6 | 0.1 | 0.19 |
| A:G | ||||||||
| day 0 | 1.1 | 1.2 | 1.1 | 1.2 | 1.2 | 1.1 | 0.05 | |
| day 26 | 1.3 | 1.3 | 1.2 | 1.3 | 1.2 | 1.3 | 0.04 | 0.26 |
| TP g/dL | ||||||||
| day 0 | 5.8 | 5.8 | 6.1 | 5.9 | 5.8 | 6.1 | 0.1 | |
| day 26 | 5.7 | 5.5 | 5.9 | 5.7 | 5.6 | 5.8 | 0.1 | 0.05 |
| ALT, U/liter | ||||||||
| day 0 | 13.3 | 14.6 | 13.2 | 14.7 | 14.6 | 12.3 | 1.0 | |
| day 26 | 14.8 | 17.6 | 16.0 | 18.3* | 15.2 | 15.2 | 1.0 | 0.05 |
| AST, U/liter | ||||||||
| day 0 | 50.8 | 53.9 | 50.6 | 54.7 | 54.7 | 50.0 | 2.7 | |
| day 26 | 51.6 | 59.8 | 56.2 | 62.2 | 54.6 | 65.3 | 5.5 | 0.34 |
| GGT, U/liter | ||||||||
| day 0 | 36.4 | 40.3 | 36.1 | 39.1 | 37.9 | 39.2 | 2.5 | |
| day 26 | 38.9 | 41.6 | 39.0 | 44.0 | 47.4† | 34.9 | 2.6 | 0.02 |
| CPK, U/liter | ||||||||
| day 0 | 170.1 | 206.3 | 170.6 | 129.3 | 212.7 | 207.3 | 32.7 | |
| day 26 | 79.8 | 101.1 | 96.4 | 119.9* | 78.2 | 127.2* | 11.1 | 0.002 |
| Ca, mg/dL | ||||||||
| day 0 | 9.8 | 10.0 | 9.8 | 10.1 | 9.9 | 9.9 | 0.1 | |
| day 26 | 10.1 | 10.2 | 10.2 | 10.2 | 10.1 | 10.0 | 0.1 | 0.86 |
| P, mg/dL | ||||||||
| day 0 | 12.4 | 11.0 | 11.8 | 11.3 | 11.0 | 10.6 | 0.5 | |
| day 26 | 10.7 | 9.8 | 9.1† | 10.0 | 7.9* | 9.1† | 0.5 | 0.003 |
| Mg, mEq/liter | ||||||||
| day 0 | 2.5 | 2.5 | 2.3 | 2.3 | 2.3 | 2.3 | 0.1 | |
| day 26 | 2.4 | 2.4 | 2.4 | 2.4 | 2.4 | 2.3 | 0.1 | 0.30 |
| Cl, mEq/liter | ||||||||
| day 0 | 113.5 | 115.2 | 114.7 | 113.1 | 113.0 | 113.5 | 1.1 | |
| day 26 | 113.5 | 113.5 | 114.3 | 113.7 | 115.6 | 116.8* | 0.9 | 0.03 |
| Na, mEq/liter | ||||||||
| day 0 | 153.0 | 152.6 | 155.1 | 151.1 | 152.1 | 151.7 | 1.1 | |
| day 26 | 152.9 | 152.8 | 154.0 | 152.8 | 153.0 | 153.2 | 1.0 | 0.93 |
| K, mEq/liter | ||||||||
| day 0 | 7.3 | 7.0 | 6.7 | 6.6 | 6.9 | 6.6 | 0.3 | |
| day 26 | 7.0 | 6.5 | 7.0 | 6.1 | 6.8 | 6.4 | 0.3 | 0.10 |
| Na:K ratio | ||||||||
| day 0 | 20.9 | 22.3 | 23.4 | 23.5 | 22.4 | 23.5 | 1.2 | |
| day 26 | 21.9 | 23.8 | 22.4 | 25.2 | 22.9 | 24.6 | 1.0 | 0.10 |
| Period 2; day 54 | ||||||||
| Glucose, mg/dL | 78.3 | 82.0 | 77.9 | 77.6 | 80.2 | 74.3 | 2.7 | 0.38 |
| NEFA, mEq/liter | 0.10 | 0.08 | 0.08 | 0.09 | 0.07 | 0.09 | 0.01 | 0.77 |
| BHB, µmol/liter | 262.3 | 300.1 | 294.0 | 370.0 | 266.2 | 247.8 | 43.0 | 0.38 |
| SUN, mg/dL | 15.8 | 19.4 | 16.5 | 20.3 | 17.5 | 17.0 | 1.4 | 0.15 |
| Creatinine, mg/dL | 0.64 | 0.58 | 0.59 | 0.54 | 0.54 | 0.61 | 0.04 | 0.32 |
| Bilirubin, mg/dL | 0.11 | 0.12 | 0.12 | 0.13 | 0.12 | 0.14* | 0.006 | 0.04 |
| Albumin, g/dL | 3.2 | 3.2 | 3.2 | 3.3 | 3.1 | 3.2 | 0.05 | 0.31 |
| Globulin, g/dL | 2.6 | 2.4 | 2.6 | 2.5 | 2.5 | 2.6 | 0.1 | 0.70 |
| A:G | 1.2 | 1.3 | 1.3 | 1.3 | 1.2 | 1.3 | 0.05 | 0.54 |
| TP g/dL | 5.8 | 5.6 | 5.8 | 5.8 | 5.6 | 5.7 | 0.1 | 0.58 |
| ALT, U/liter | 15.4 | 17.0 | 15.2 | 16.3 | 16.7 | 14.7 | 0.8 | 0.29 |
| AST, U/liter | 55.2 | 58.1 | 55.0 | 62.6 | 55.7 | 57.1 | 3.5 | 0.56 |
| GGT, U/liter | 41.4 | 41.7 | 39.4 | 43.6 | 43.6 | 38.8 | 2.4 | 0.51 |
| CPK, U/liter | 91.1 | 117.6 | 100.0 | 117.7 | 101.7 | 117.9 | 11.5 | 0.31 |
| Ca, mg/dL | 10.1 | 10.4 | 10.3 | 10.6 | 10.3 | 10.4 | 0.1 | 0.19 |
| P, mg/dL | 10.2 | 9.8 | 9.4 | 10.0 | 8.8 | 9.2 | 0.4 | 0.14 |
| Mg, mEq/liter | 2.5 | 2.3 | 2.3 | 2.5 | 2.3 | 2.4 | 0.1 | 0.21 |
| Cl, mEq/liter | 111.5 | 113.2 | 112.8 | 113.4 | 112.9 | 113.8 | 0.9 | 0.60 |
| Na, mEq/liter | 151.6 | 153.4 | 153.0 | 153.7 | 152.3 | 152.9 | 1.0 | 0.45 |
| K, mEq/liter | 6.2 | 6.6 | 6.1 | 6.4 | 6.3 | 6.7 | 0.3 | 0.45 |
| Na:K ratio | 24.7 | 23.4 | 25.4 | 24.1 | 24.3 | 23.2 | 0.9 | 0.44 |
During Period 1 (days 0 to 26), goats were fed a 70% concentrate ration. Goats were transitioned over 5 d into Period 2 (days 27 to 64) and onto an 86% concentrate ration. Treatments were only compared with cottonseed hulls (CSH), not to each other. Within row, means with a different superscript than the control diet (CSH) differ: †0.05 > P < 0.10; *P < 0.05.
1Day 0 was not included in the analysis and is shown only as a reference; SUN = Serum urea N; ALT = Alanine aminotransferase; AST = Aspartate aminotransferase; GGT = γ-glutamyl transferase; A:G = albumin:globulin ratio; CPK = Creatine phophokinase; BHB = β-hydroxy butyrate; TP = Total protein.
2Treatment diets were mixed and nonpelleted; ingredient composition differed only by roughage source; either cottonseed hulls (CSH) or ground woody products (RED = Juniperus pinchotii, BLUE = Juniperus ashei, ONE = Juniperus monosperma, ERC = Juniperus virginiana, or MESQ = Prosopis glandulosa). Juniperus spp. (entire above-ground biomass) and P. glandulosa (entire above-ground biomass except for leaves) species were chipped, dried, and hammermilled to pass a 4.76-mm sieve.
3SEM = greatest SEM.
Blood serum BHB was greater (P = 0.02) in goats fed BLUE and tended to be greater (P = 0.05) in goats fed RED, but concentrations remained within normal clinical ranges (Kaneko et al., 2008). Blood SUN was greater (P ≤ 0.002) on day 26 in goats fed RED, BLUE, ONE, or ERC compared with goats fed CSH. Circulating SUN can be affected by numerous factors such as total DMI, protein intake, feed protein concentration (more specifically, degradable protein), ruminal ammonia production, starch fermentation, CT, animal health, and catabolism (starvation). Greater starch fermentation can decrease ruminal ammonia concentration (Lana et al., 1998; Rémond et al., 2002), which can then reduce SUN. As reviewed by Patra and Saxena (2011), CT-protein complex formation and the suppression of ruminal proteolytic bacteria can reduce protein degradation, and thus increase the total supply of N to the duodenum and intestinal N absorption, which can increase SUN (Cole and Hutcheson, 1988). Others have reported similar results, where feeding ground juniper in mixed diets increased SUN (Whitney et al., 2014; Whitney, 2017). Considering that diets contained similar CP concentrations, that daily DMI was relatively similar for goats consuming Juniperus spp.-based diets and goats consuming CSH, and that blood serum chemistry did not reveal any negative health issues, the reduced tIVDMD (possibly reduced starch digestion) and greater CT in Juniperus spp.-based diets compared with the CSH diet were likely the main factors affecting SUN.
Serum bilirubin was less (P ≤ 0.02) on day 26 for goats fed CSH than goats fed RED, ONE, or ERC; however, maximum serum bilirubin concentration in any of the treatments was less than 0.15 mg/dL. Concentrations of bilirubin need to get above 2 mg/dL in ruminant animals to be considered symptomatic of liver failure, marked by the impaired uptake and excretion of bilirubin (Carlson, 1996). Serum ALT and CPK were greater in goats fed ONE (P ≤ 0.04) compared with goats fed CSH, but concentrations remained within normal clinical ranges (Carlson, 1996; Kaneko et al., 2008). Serum P tended to be less (P = 0.07) in goats fed BLUE and was less (P < 0.001) in goats fed ERC compared with goats fed CSH.
Serum creatinine, albumin, globulin, A:G, total protein, aspartate aminotransferase (AST), Ca, Mg, Na, K, and Na:K were not different (P ≥ 0.10) between goats fed CSH compared with any of the ground woody diets. Concentrations of serum AST and CPK can be used as indicators of tissue necrosis in ruminant animals. Serum CPK is specific to liver tissue damage and corresponds to a single insult to the liver where serum AST is indicative of a gradual increase in which levels would remain elevated for an extended period of time (Carlson, 1996). Even though goats fed BLUE or MESQ had greater serum CPK than goats fed CSH, the serum concentrations are considered normal for goats (104 to 219 U/liter; Carlson, 1996). Serum profile results indicated that no damage to liver tissue or liver function occurred. In previous studies, no liver lesions or health issues were recorded when utilizing ground juniper leaves, leaves and stems, or whole trees as roughage sources in lamb feedlot diets (Whitney and Muir, 2010; Whitney et al., 2014; Whitney et al., 2017, respectively) or in gestating ewe supplements (Stewart et al. 2017).
Period 2.
A treatment effect was observed for bilirubin (P = 0.04). When compared with goats fed CSH on day 54, bilirubin was greater (P < 0.007) in goats fed MESQ, but concentrations remained within normal clinical range values for goats (Carlson, 1996; Kaneko et al., 2008). No other differences (P > 0.10) were observed in any of the other serum parameters.
CONCLUSIONS
Collectively, results suggested that ground J. pinchotii, J. ashei, or J. monosperma can effectively be used to completely replace CSH in goat feedlot diets. During Period 1, feeding diets to goats that contain 30% J. virginiana (ERC) or P. glandulosa (MESQ) may not be justifiable in most scenarios, even though goat health, assessed by blood serum profiles, was not negatively affected. However, using 14% J. virginiana (ERC) or P. glandulosa (MESQ) in finishing diets is warranted. This experiment, along with numerous others, supports the use of ground J. pinchotii, J. ashei, and J. monosperma in total mixed rations, especially when the price for traditional feed ingredients increases. Harvesting ground woody products to be used in livestock diets provides an additional benefit to livestock production by assisting in the restoration of deteriorating rangelands.
Footnotes
This work was supported by the United States Department of Agriculture National Institute of Food and Agriculture Hatch Project #205866 and funded in part by the National Sheep Industry Improvement Center (NSIIC). Appreciation is expressed to POET Nutrition (Sioux Falls, SD) for donating dried distillers grains with solubles.
LITERATURE CITED
- Ansley R. J., Wiedemann H. T., Castellano M. J., and Schlosser J. E.. 2006. Herbaceous restoration of juniper-dominated grasslands with chaining and fire. Range. Ecol. Manage. 59:171–178. doi:10.2111/05-095R1.1 [Google Scholar]
- AOAC Int 2006. Official methods of analysis. 18th ed AOAC Int, Arlington, VA. [Google Scholar]
- Archibald J. G. 1926. The composition, digestibility, and feeding value of hydrolyzed sawdust. J. Dairy Sci. 9:257–271. doi:10.3168/jds.S0022-0302(26)93896-4 [Google Scholar]
- Carlson G. P. 1996. Clinical chemistry tests. Smith B. P, editor. In: Large animal internal medicine. Mosby-Year Book, Inc, St Louis MO: p. 441–469. [Google Scholar]
- Cole N. A. and Hutcheson D. P.. 1988. Influence of protein concentration in prefast and postfast diets on feed intake of steers and nitrogen and phosphorus metabolism of lambs. J. Anim. Sci. 66:1764–1777. [DOI] [PubMed] [Google Scholar]
- Felker P. 1996. Commercializing mesquite, leucaena, and cactus in Texas. In: J., Janick, editor. Progress in new crops. Amer. Soc. Hort. Sci. Press, Alexandria, VA: p. 133–137. [Google Scholar]
- Jenkins R. B. 1939. Cedars and Poverty. The Cattleman. p. 61–62. [Google Scholar]
- Kaneko J. J., Harvey J. W., and Bruss M. L.. 2008. Clinical biochemistry of domestic animals. 6th rev. ed Academic Press, Burlington, MA. [Google Scholar]
- Kononoff P. J., Heinrichs A. J., and Lehman H. A.. 2003. The effect of corn silage particle size on eating behavior, chewing activities, and rumen fermentation in lactating dairy cows. J. Dairy Sci. 86:3343–3353. doi:10.3168/jds.S0022-0302(03)73937-X [DOI] [PubMed] [Google Scholar]
- Lammers B. P., Buckmaster D. R., and Heinrichs A. J.. 1996. A simple method for the analysis of particle sizes of forage and total mixed rations. J. Dairy Sci. 79:922–928. doi:10.3168/jds.S0022-0302(96)76442-1 [DOI] [PubMed] [Google Scholar]
- Lana R. P., Russell J. B., and Van Amburgh M. E.. 1998. The role of pH in regulating ruminal methane and ammonia production. J. Anim. Sci. 76:2190–2196.doi:10.2527/1998.7682190x [DOI] [PubMed] [Google Scholar]
- Marion P. F., Fisher C. E., and Robinson E. D.. 1957. Ground mesquite wood as a roughage in rations for yearling steers. Progress Rep. 1972. TX Agric. Exp. Sta, College Station. [Google Scholar]
- Maynard L. A. 1920. War-time sources of feeding-stuffs in Germany. J. Anim. Sci. 1920:97–102. [Google Scholar]
- McDOUGALL E. I. 1948. The composition and output of sheep’s saliva. Biochem. J. 43:99–109. doi:10.1042/bj0430099 [PubMed] [Google Scholar]
- Patra A. K. and Saxena J.. 2011. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 91:24–37. doi:10.1002/jsfa.4152 [DOI] [PubMed] [Google Scholar]
- Provenza F. D., Pfister J. A., and Cheney C. D.. 1992. Mechanisms of learning in diet selection with reference to phytotoxicosis in herbivores. J. Range Manage. 45:36−45. doi:10.2307/4002523 [Google Scholar]
- Rémond D., Noziere P., and Poncet C.. 2002. Effect of time of starch supply to the rumen on the dynamics of urea and ammonia net flux across the rumen wall of sheep. Anim. Res. 51:3–13. doi:10.1051/animres:2002002 [Google Scholar]
- Scholes R. J., and Archer S. R.. 1997. Tree–grass interactions in savannas. Ann. Rev. Ecol. Syst. 28:517–544. doi:10.1146/annurev.ecolsys.28.1.517 [Google Scholar]
- Stewart W. C., Whitney T. R., Scholljegerdes E. J., Hallford D. M., Walker J. W., Adams R. P., and Naumann H. D.. 2017. Effects of feeding ground redberry juniper (juniperus pinchotii) to gestating ewes on pre- and postpartum performance, serum metabolites and hormones, milk fatty acid composition, and progeny preweaning performance. J. Anim. Sci. 95:4113–4123. doi:10.2527/jas2016.1090 [DOI] [PubMed] [Google Scholar]
- Stewart W. C., Whitney T. R., Scholljegerdes E. J., Naumann H. D., Cherry N. M., Muir J. P., Lambert B. D., Walker J. W., Adams R. P., Welch K. D., et al. 2015. Effects of juniperus species and stage of maturity on nutritional, in vitro digestibility, and plant secondary compound characteristics. j. Anim. Sci. 93:4034–4047. doi:10.2527/jas.2015-9274 [DOI] [PubMed] [Google Scholar]
- Terrill T. H., Rowan A. M., Douglas G. B., and Barry T. N.. 1992. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals, and cereal grains. J. Sci. Food Agric. 58:321–329. doi:10.1002/jsfa.2740580306 [Google Scholar]
- Whitney T. R. 2017. Ground Juniperus pinchotii and urea in supplements fed to Rambouillet ewe lambs: I. Feedlot growth traits, blood serum parameters, and fecal characteristics. J. Anim. Sci. 95:3676–3686. doi:10.2527/jas2017.1419 [DOI] [PubMed] [Google Scholar]
- Whitney T. R., Glasscock J. L., Muir J. P., Stewart W. C., and Scholljegerdes E. J.. 2017. Substituting ground woody plants for cottonseed hulls in lamb feedlot diets: growth performance, blood serum chemistry, and rumen fluid parameters. J. Anim. Sci. 95:4150–4163. doi:10.2527/jas2017.164 [DOI] [PubMed] [Google Scholar]
- Whitney T. R., Lupton C. J., Muir J. P., Adams R. P., and Stewart W. C.. 2014. Effects of using ground redberry juniper and dried distillers grains with solubles in lamb feedlot diets: growth, blood serum, fecal, and wool characteristics. J. Anim. Sci. 92:1119–1132. doi:10.2527/jas.2013-7007 [DOI] [PubMed] [Google Scholar]
- Whitney T. R., and Muir J. P.. 2010. Redberry juniper as a roughage source in lamb feedlot rations: performance and serum nonesterified fatty acids, urea nitrogen, and insulin-like growth factor-1 concentrations. J. Anim. Sci. 88:1492–1502. doi:10.2527/jas.2009–2410 [DOI] [PubMed] [Google Scholar]
- Wolfe R. M., Terrill T. H., and Muir J. P.. 2008. Drying method and origin of standard affect condensed tannin (CT) concentrations in perennial herbaceous legumes using simplified butanol-HCL CT analysis. J. Sci. Food Agric. 88:1060–1067. doi:10.1002/jsfa.3188 [Google Scholar]
- Van Soest P. J. 1994. Nutritional ecology of the ruminant. 2nd ed Cornell University Press, Ithaca, NY. [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. j. Dairy Sci. 74:3583–3597. doi:10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]