Abstract
We hypothesized that gene expression and fatty acid composition would differ among different muscle depots and over time on a grain-based finishing diet. Additionally, we hypothesized that the concentration of SFA would decrease and the concentration of MUFA would increase proportionately with increases in percent intramuscular lipid (%IML). Ten Yanbian Yellow cattle steers (approximately 8 mo of age) were fed a corn-based diet in which the proportion of corn in the ration was increased at 4-mo intervals. Muscle samples were taken by biopsy from the chuck (trapezius), loin (longissimus dorsi), and round (biceps femoris) muscles at 12, 16, 20, 24, and 28 mo of age. The %IML increased from 12 to 28 mo of age, especially between 24 and 28 mo of age, with loin > round > chuck (age × muscle interaction P < 0.001). The percentage (g/100 g total fatty acids) of oleic acid (18:1n-9), linoleic acid (18:2n-6), and α-linolenic acid (18:3n-3), and the MUFA:SFA ratio increased with age, whereas palmitic (16:0) and stearic acid (18:0) decreased with age in all muscles (age effect P < 0.001). The expression of sterol regulatory element binding protein (SREBP1), adipose tissue fatty acid- binding protein (FABP4), stearoyl-CoA desaturase (SCD), acetyl-CoA carboxylase (ACC1), and lipoprotein lipase (LPL) increased, whereas the expression of peroxisome proliferator-activated receptor gamma (PPARγ) and fatty acid synthase (FASN) decreased with age. Expression of PPARγ, FABP4, SREBP1, SCD, FASN, ACC1, and LPL was greater in the loin than in the chuck or round (age × muscle interaction P < 0.001), although the MUFA:SFA ratio was greater in the chuck than in the loin or round (muscle effect P < 0.001). In conclusion, adipogenic gene expression was greater in the loin than in the chuck or round muscles, consistent with the greater %IML of the loin. However, the greater SCD gene expression in the loin did not result in a greater amount of MUFA in the loin, relative to the chuck and round.
Keywords: age, beef cattle, fatty acid, gene expression, intramuscular lipid, muscle
INTRODUCTION
The fatty acid composition in bovine adipose tissue has been recognized as an important carcass trait affecting meat quality, and has received considerable interest in view of its implications for human health. Although only small changes can be made in the concentrations of n-3 fatty acids in beef, quantitatively large changes are achievable in MUFA by altering the diets of beef cattle. Ground beef naturally enriched with oleic acid (18:1n-9) increases high-density lipoprotein (HDL) cholesterol concentrations and/or increases low-density lipoprotein (LDL) particle size, both of which reduce risk for cardiovascular disease (Adams et al., 2010; Gilmore et al., 2011, 2013; Choi et al., 2018).
The amount of i.m. adipose tissue (i.e., marbling) increases beef palatability traits such as flavor, juiciness, and tenderness (Frank et al., 2016a, 2016b; Hwang and Joo, 2017b), and increasing the amount of marbling in beef is associated with increases in MUFA (Smith et al., 2009). China recently has begun to develop feedlot systems to improve the quality of their beef, but little is known about the genetic capacity of indigenous Chinese cattle to produce highly marbled beef. The Yanbian Yellow breed type is one of the indigenous Chinese beef breeds, and is closely related to Korean Hanwoo cattle (Yoon et al., 2005; Choi et al., 2015a; Yonesaka et al., 2016) and Japanese Black (or Wagyu) cattle (Mei et al., 2017).
We hypothesized that gene expression and fatty acid composition would differ among different muscle depots and over time on a grain-based finishing diet. Additionally, we hypothesized that the concentration of SFA would decrease and the concentration of MUFA would increase proportionately with increases in percent intramuscular lipid (%IML). This information would provide mechanistic insights into the regulation of lipid deposition and fatty acid composition in Yanbian Yellow cattle.
MATERIALS AND METHODS
Animals and Diets
The experimental procedures were approved by the Yanbian University Animal Care and Use Committee, Office of Science and Technology, Yanji City, China. The study was conducted with 10 Yanbian Yellow steers (approximately 8 mo of age; 190.3 ± 1.5 kg, mean ± SEM) at the Yanbian Yellow Cattle Research Center of Yanbian University, Yanji, China. The steers were assigned randomly individual pens. Steers were fed a corn-based diet according to the feeding and management program of Yanbian Yellow cattle (Table 1). The steer calves were early weaned (128 ± 23 d of age) and from weaning to 12 mo of age the steers were given free access to a grain-based diet consisting of 64.00% cracked corn, 3.70% soybean meal, 1.15% wheat bran, 30.15% chopped forage (DM basis), and 1% premix.
Table 1.
Ingredients and chemical composition of the stage diets
| Item | 12–16 mo | 16–20 mo | 20–24 mo | 24–28 mo |
|---|---|---|---|---|
| Ingredient, % | ||||
| Corn | 56.91 | 63.32 | 68.56 | 77.29 |
| Wheat bran | 2.13 | 1.59 | 1.31 | 1.22 |
| Soybean meal | 11.33 | 8.39 | 5.57 | 2.91 |
| Premix1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Corn silage | 23.69 | 20.85 | 18.66 | 12.39 |
| Rice straw | 4.94 | 4.85 | 4.90 | 4.69 |
| Nutrient composition, %DM | ||||
| Dry matter | 85.12 | 86.76 | 86.55 | 89.34 |
| Crude protein | 12.43 | 12.41 | 12.64 | 9.58 |
| Neutral detergent fiber | 14.45 | 15.41 | 14.81 | 14.62 |
| Ether extract | 3.42 | 3.34 | 3.83 | 4.01 |
| Crude ash | 5.17 | 5.71 | 5.74 | 5.71 |
| Calcium | 0.41 | 0.41 | 0.49 | 0.36 |
| Phosphorus | 0.21 | 0.22 | 0.25 | 0.19 |
1Premix (per kg): vitamin A, 5,000 IU; vitamin D, 700 IU; vitamin E, 30 IU; Zn, 100 mg; Fe, 90 mg; Mn, 20 mg; Cu, 10 mg; I, 0.3 mg; Se, 0.2 mg.
Steers were sampled by biopsy at 12, 16, 20, 24, and 28 mo of age. AOAC (1995) procedures were used to determine feed DM (method number 930.15), CP (method number 984.13), ether extract (method number 920.39), and crude ash (method number 942.05) of the diet. The NDF content of the diets was determined using the procedure of Van Soest et al. (1991) with a heat-stable α-amylase and sodium sulfite (Sigma Chemicals A3306; Sigma-Aldrich Co. LLC, St. Louis, MO) and was expressed inclusive of the residual ash. Feed calcium and phosphorus were analyzed by the method of Talapatra et al. (1940).
Biopsy Samples Collection
Muscle biopsy was performed with some modification of the procedure of Meijer et al. (1995). Consecutive biopsy samples (approximately 3 g) were taken from chuck (trapezius), loin (longissimus dorsi; approximately the 12th cervical vertebra), and round (biceps femoris) by surgery by a trained veterinarian at 12, 16, 20, 24, and 28 mo of age. The biopsy procedure entailed shaving a small part of skin (8 × 8 cm) and the skin was then cleaned with iodine. A local anesthetic (20 mg/mL of lidocaine plus 0.8 mg/mL of norepinephrine; Shanghai, China) was applied to the s.c. layer by injection (without penetration into muscle tissue), after which a 3-cm incision was made through the skin. A sterilized biopsy needle (10 mm i.d.) was inserted into the muscle to approximately 5 cm. After sampling, the incision was sutured. Any external (s.c.) adipose tissue removed and muscle samples were stored in liquid N2 within 1 min. Subsequent samples within the same cattle were taken alternately from the left and right sides. The steers were monitored daily for behavioral signs of discomfort and post-surgical care, and no tissue infections were observed after biopsy. All the tissue biopsy samples were snap-frozen in liquid nitrogen immediately and stored at −80 °C for RNA preparation, total lipid extraction, and fatty acid analysis.
Fatty Acid Composition
Total lipid of the muscle samples was extracted by a modification of the method of Folch et al. (1957). Two hundred milligrams of thawed, homogenized muscle tissues was extracted in chloroform:methanol (2:1, vol/vol), and fatty acid methyl esters (FAME) were prepared as described by Lepage and Roy (1986). The FAME were analyzed with a gas chromatograph (model Agilent 7890A fixed with a 7683B autosampler; Agilent Inc., New York, NY). Separation of FAME was accomplished on a fused silica capillary column Supelco SP-2560 (100 m × 0.25 mm i.d.; Sigma-Aldrich Co. LLC, St. Louis, MO). Ultra-high purity helium was used as carrier gas at a flow rate of 45 mL/min. The split ratio was 1:100 and the injector and detector temperatures were 240 and 250 °C, respectively. Oven temperature was maintained at 140 °C for 2 min, increased to 240 °C at a rate of 4 °C/min, and maintained at 240 °C for 40 min. Individual FAME were identified using a FAME analytical standard (18919-1AMP Supelco; Sigma-Aldrich Co. LLC, St. Louis, MO).
RNA Extraction and Real-Time PCR
Total RNA was extracted from 100 mg muscle for each sample using Trizal reagent in accordance with the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Genomic DNA was removed from extracted RNA with DNase (M610A; Promega, Madison, WI) according to the manufacturer’s instructions. The purity, concentration, and integrity of the total RNA from each sample were quantified using a NanoDrop spectrophotometer (2000C; Thermo Scientific, Washington, DC), and the RNA 6000 Nano Assay (Agilent Technologies, Palo Alto, CA) assessed the RNA integrity. The purity of RNA (260:280 absorbance) was greater than 1.85, and the 260:230 absorbance ratio was approximately 2.0 in all samples. Standard cDNA synthesis was achieved by reversing the transcription of the RNA using Oligo dT primer and superscript reverse transcriptase (Invitrogen, Carlsbad, CA). After reverse transcription, analysis of gene expression of peroxisome proliferator-activated receptor gamma (PPARγ), sterol regulatory element binding protein (SREBP), adipocyte fatty acid-binding protein (FABP4), stearoyl-CoA desaturase (SCD), adipose tissue acetyl-CoA carboxylase-1 (ACC1), fatty acid synthase (FASN), and lipoprotein lipase (LPL) in the muscle samples was performed by quantitative real-time reverse-transcription PCR (qRT-PCR) (MX3005P; Agilent, CA). The first-strand cDNA was diluted with deionized water and amplified using gene-specific primers (Table 2). The reactions were conducted according to the protocol of the DyNAmo SYBR Green qRT-PCR kit containing modified Tbr DNA polymerase, SYBR Green, optimized PCR buffer, 1.25 mM MgCl2, and a dNTP mix including dUTP (Finnzymes Oy, Espoo, Finland). The qRT-PCR was carried out with initial denaturation at 95 °C for 5 min, followed by 40 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, after which the samples were heated to 95 °C for 1 min, 55 °C for 1 min, and then held at 4 °C. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a normalizing control. We previously documented the utility of GAPDH as a reference gene for normalizing mRNA levels (Li et al., 2012, 2017). The primers were designed using Primer Express software (Applied Biosystems). The relative quantification of gene expression was analyzed using the 2−ΔΔCT method (Livak and Schmittgen, 2001). All qRT-PCR analyses were repeated at least 3 times.
Table 2.
Forward and reverse primers used for real-time PCR
| Gene symbol1 | Primer sequences | Accession no. |
|---|---|---|
| GAPDH | Forward: 5′-CTGTCCACCTTCCAGCAGAT-3′ | NM_001034 |
| Reverse: 5′-TCACCTTCACCGTTCCAGTT-3′ | ||
| PPARγ | Forward: 5′-GCATTTCCACTCCGCACTAT-3′ | AY137204 |
| Reverse: 5′-GGGATACAGGCTCCACTTTG-3′ | ||
| FABP4 | Forward: 5′-AAACTTAGATGAAGGTGCTCTGG-3′ | AJ4160220 |
| Reverse: 5′-CATAAACTCTGGTGGCAGTGA-3′ | ||
| SREBP1 | Forward: 5′-ACTACCACGCCAAGTTCCTG-3′ | JN790254 |
| Reverse: 5′-CGATGCCAATCTCCTCCTT-3′ | ||
| SCD | Forward: 5′-GCCAACAACTCTGCCTTTATG-3′ | GU947654 |
| Reverse: 5′-CACCAATGACTGACCACCTG-3′ | ||
| ACC1 | Forward: 5′-TATGTCCTCCCAAGCATTCC-3′ | X80045 |
| Reverse: 5′-GCCAATCTCATTTCCTCCTG-3′ | ||
| FASN | Forward: 5′-TTCGGAGATTGTTTCACAGC-3′ | AF479289 |
| Reverse: 5′-GGCAGGAGTTCGCTTCAGTA-3′ | ||
| LPL | Forward: 5′-ACACTTGCCACCTCATTCCT-3′ | X68308 |
| Reverse: 5′-GCACCCAACTCTCATACATTCC-3′ |
1 GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PPARγ, peroxisome proliferator-activated receptor gamma; FABP4, adipocyte fatty acid-binding protein; SREBP1, sterol regulatory element binding protein-1; SCD, stearoyl-CoA desaturase; ACC1, acetyl CoA carboxylase; FASN, fatty acid synthase; LPL, lipoprotein lipase.
Statistical Analysis
Performance characteristics (BW, ADG, and ADI) were compared by 1-factor repeated-measures ANOVA (SuperAnova; Abacus Concepts, Inc., Berkeley, CA). Differences in %IML, fatty acid percentages (g/100 g total fatty acids), and relative gene expression were tested by 2-factor repeated-measures ANOVA (SuperAnova). The first factor was muscle (chuck, loin, or round) and the second factor was time on feed [i.e., age of steers (12, 16, 20, 24, or 28 mo)]. When significant interaction effects (P < 0.05) were detected by ANOVA, means were separated by Fisher’s protected least squares differences. Data are presented as means ± SEM.
RESULTS
Body Weight and %IML
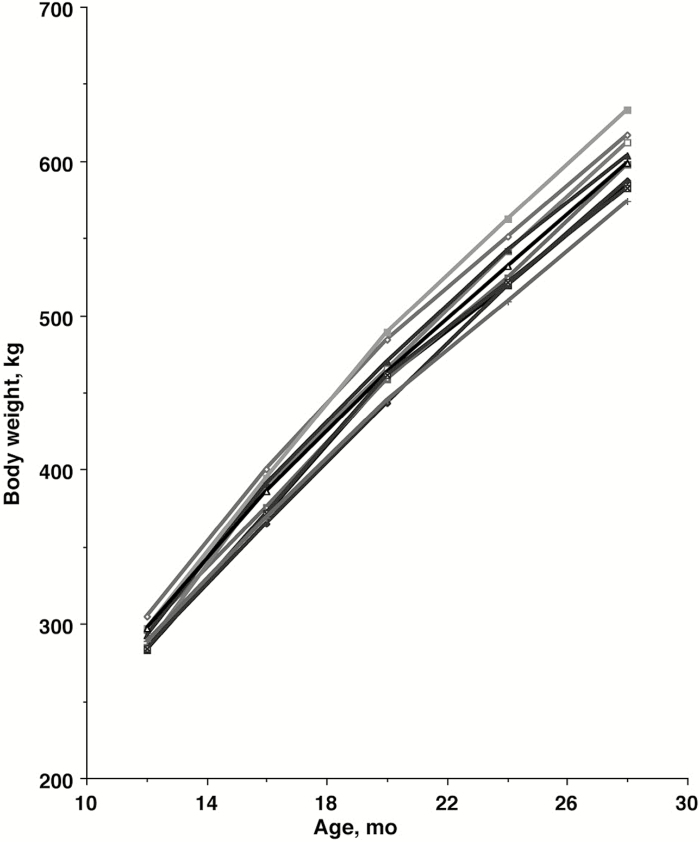
The Yanbian Yellow steers for this study were selected to have similar initial BW (291.4 ± 2.3 kg, mean ± SEM), and had very similar rates of gain over the course of the study (Fig. 1). Steers gained an average 308 kg BW between 12 and 28 mo of age (Table 3). Average daily gain gradually decreased over the 4-mo intervals, whereas ADI increased over time, except for the period between 24 and 28 mo of age. Similarly, the gain:intake ratio declined until 24 mo of age (P < 0.05). The overall CV for ADG was 16.94 (not indicated in Table 3). The %IML increased from 12 to 28 mo of age and the order of %IML was loin > round > chuck (age × muscle interaction P < 0.001) (Fig. 2A).
Figure 1.
Body weights as a function of age. Each line represents data for an individual steer.
Table 3.
Production characteristics of Yanbian Yellow cattle steers at different ages1
| Item | Age, mo | Pooled SEM | ||||
|---|---|---|---|---|---|---|
| 12 | 16 | 20 | 24 | 28 | ||
| Body weight, kg | 291.4e | 381.5d | 463.8c | 532.6b | 599.3a | 15.7 |
| Average daily gain, kg | 0.83a | 0.76b | 0.69c | 0.58d | 0.56d | 0.07 |
| Average daily intake, kg | 8.02d | 8.24d | 9.80c | 10.76b | 11.20a | 0.17 |
| Gain:intake | 0.104a | 0.091b | 0.070c | 0.054d | 0.050d | 0.003 |
a–eMeans within a row with common superscripts do not differ (P > 0.05). Data are means for n = 10 steers.
1Values are interim performance values for each time period.
Figure 2.
Intramuscular lipid (g/100 g muscle) (A) and relative expression for sterol regulatory element binding protein-1 (SREBP1) (B), peroxisome proliferator-activated receptor gamma (PPARγ) (C), and adipocyte fatty acid-binding protein (FABP4) (D) in chuck, loin, and round muscle of steers sampled at 12, 16, 20, 24, and 28 mo of age. Each data point is the mean for n = 10 steers. The pooled SEM for each muscle is affixed to the symbols. The age × muscle interaction was significant for intramuscular lipid, SREBP1, PPARγ, and FABP4 (P < 0.001). a–gMean with common superscripts are not different (P > 0.05).
Fatty Acid Composition of Muscle
The age × muscle interaction was significant (P < 0.01) for the percentage (g/110 g total fatty acids) of stearic acid (18:0), oleic acid (18:1n-9), linoleic acid (18:2n-6), and α-linolenic acid (18:3n-3) during the growing period (Table 4). In all muscles, the percentage of stearic acid decreased by 28 mo of age, but the decline was more rapid in the chuck and round than in the loin. The percentage of oleic acid increased from 16 mo of age in the chuck and round, and from 20 mo of age in the loin. The highest percentage of cis-vaccenic (18:1n-7), linoleic, and α-linolenic acid was observed at 28 mo of age in all muscles. The percentage of palmitoleic acid (16:1n-7) declined over time and the MUFA:SFA ratio was highest at 28 mo of age (age effect P < 0.002).
Table 4.
Percentage of fatty acids (mg/100 mg total fatty acids) in the chuck, loin, and round muscles at increasing ages
| Fatty acid | Muscle | Age, mo | Pooled SEM | Significant differences1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 16 | 20 | 24 | 28 | A | M | A × M | |||
| Palmitic, 16:0 | Chuck | 25.5fg | 24.8g | 28.1ab | 27.4bcd | 24.3g | 0.31 | |||
| Loin | 27.8abcd | 28.2ab | 26.9cde | 26.6de | 25.8g | 0.27 | 0.001 | 0.001 | 0.001 | |
| Round | 28.6a | 27.9abc | 28.8a | 28.0abc | 26.8de | 0.19 | ||||
| Palmitoleic, 16:1n-7 | Chuck | 7.09 | 6.51 | 6.36 | 6.28 | 5.69 | 0.11 | |||
| Loin | 5.96 | 6.54 | 6.31 | 5.51 | 5.77 | 0.12 | 0.002 | 0.08 | 0.22 | |
| Round | 6.71 | 6.17 | 6.44 | 5.99 | 5.73 | 0.14 | ||||
| Stearic, 18:0 | Chuck | 19.7a | 17.3b | 11.9e | 11.6ef | 10.2f | 0.55 | |||
| Loin | 18.9a | 18.0b | 15.0c | 10.8f | 10.2f | 0.54 | 0.001 | 0.009 | 0.001 | |
| Round | 19.3a | 17.3b | 14.2c | 12.9d | 10.1f | 0.49 | ||||
| Oleic, 18:1n-9 | Chuck | 43.4d | 46.6bc | 48.9a | 49.3a | 48.7ab | 0.43 | |||
| Loin | 41.9e | 42.02e | 45.9c | 47.3b | 47.6b | 0.42 | 0.001 | 0.001 | 0.013 | |
| Round | 40.1f | 43.5d | 44.6d | 47.1bc | 46.9bc | 0.42 | ||||
| cis-Vaccenic, 18:1n-7 | Chuck | 1.33abc | 1.24bc | 1.16c | 1.43a | 1.41a | 0.03 | |||
| Loin | 1.27abc | 1.32ab | 1.18c | 1.24bc | 1.36ab | 0.02 | 0.05 | 0.48 | 0.04 | |
| Round | 1.28ab | 1.20bc | 1.36ab | 1.20bc | 1.34ab | 0.03 | ||||
| Linoleic, 18:2n-6 | Chuck | 0.78f | 1.32de | 1.25e | 1.63d | 7.26a | 0.35 | |||
| Loin | 1.91d | 1.52de | 2.51c | 6.46ab | 6.90a | 0.34 | 0.001 | 0.001 | 0.001 | |
| Round | 1.73d | 1.64d | 1.70d | 3.01b | 6.85a | 0.29 | ||||
| α-Linolenic, 18:3n-3 | Chuck | 0.35cd | 0.29de | 0.33cd | 0.29de | 0.64b | 0.02 | |||
| Loin | 0.23fg | 0.27ef | 0.23fg | 0.23fg | 0.64b | 0.03 | 0.001 | 0.001 | 0.001 | |
| Round | 0.25efg | 0.20g | 0.23fg | 0.24efg | 0.86a | 0.04 | ||||
| MUFA:SFA2 | ChuckA | 1.17 | 1.31 | 1.42 | 1.48 | 1.66 | 0.03 | |||
| LoinB | 1.06 | 1.09 | 1.28 | 1.46 | 1.55 | 0.03 | 0.001 | 0.001 | 0.14 | |
| RoundB | 1.02 | 1.13 | 1.23 | 1.34 | 1.49 | 0.03 | ||||
a–gMeans within a fatty acid with common superscripts do not differ (P > 0.05). A,BMuscles with different superscripts differ (muscle main effect P < 0.05). Data are means for n = 10 steers sampled sequentially in each muscle.
1A, age; M, muscle; A × M, age × muscle interaction.
2MUFA:SFA = (14:1n-7 + 16:1n-7 + 18:1n-9 + 18:1n-7)/(14:0 + 16:0 + 18:0). Less abundant fatty acids are not listed in the table.
Gene Expression in Muscle
The age × muscle interaction was significant (P < 0.001) for the relative expression of SREBP1, PPARγ, FABP4, ACC1, FASN, LPL, and SCD (Figs. 2 and 3). Expression of SREBP1 mRNA was higher at 28 mo of age than other ages, and SREBP1 expression increased more rapidly in the loin than in the chuck or round (Fig. 2B). Relative PPARγ expression decreased with age, and the loin had highest level of PPARγ expression during growth (Fig. 2C). Relative FABP4 expression increased with age, and the order of FABP4 gene expression was loin > chuck > round at each age (Fig. 2D). Relative ACC1 expression increased with age, and ACC1 expression increased more rapidly in the loin than in the chuck or round (Fig. 3A). Relative FASN expression declined with age, and FASN expression declined more rapidly in the loin than in the chuck or round (Fig. 3B). Relative LPL expression was higher at 20 to 28 mo of age than at 12 mo of age in the loin, whereas LPL expression increased more gradually and to a lesser extent in the chuck and round (Fig. 3C). Relative SCD expression increased with age more rapidly in the loin than in the chuck or the round (Fig. 3D).
Figure 3.
Relative expression for acetyl-CoA carboxylase-1 (ACC1) (A) fatty acid synthase (FASN) (B), lipoprotein lipase (LPL) (C), and stearoyl-CoA desaturase (SCD) (D) in chuck, loin, and round muscle of steers sampled at 12, 16, 20, 24, and 28 mo of age. Each data point is the mean for n = 10 steers. The pooled SEM for each muscle is affixed to the symbols. The age × muscle interaction was significant for ACC1, FASN, LPL, and SCD (P < 0.001). a–gMean with common superscripts are not different (P > 0.05).
DISCUSSION
China is rapidly developing a feedlot system to increase the amount of marbling in beef in order to compete with more developed beef cattle production systems in Asia. However, there remains a paucity of information about the development of i.m. adipose tissue in Chinese cattle. The Yanbian Yellow breed type is common to the Yanbian Korean Autonomous Prefecture of the Jilin Province of northeastern China. Yanbian Yellow cattle are Bos taurus, and the Yanbian Yellow breed type is closely related to Korean Hanwoo cattle (Yoon et al., 2005; Choi et al., 2015a; Yonesaka et al., 2016). Recent whole genome sequencing and principal component analysis documented that Yanbian Yellow cattle cluster separately from other indigenous Chinese cattle (which have some Bos indicus influence), but cluster tightly with Japanese Black cattle (Mei et al., 2017).
In this study, we documented %IML, fatty acid composition, and specific gene expression in 3 muscles over the period of time when this group of Yanbian Yellow steers was rapidly developing both muscle and i.m. adipose tissue. Age of animal and time on a grain-based diet are the major factors that influence fat deposition in beef (Smith et al., 2009). Growth and fattening of meat animals is associated with an increase in both s.c. and i.m. adipose tissue deposition (Vernon and Flint, 1988). Finishing of Yanbian Yellow cattle typically begins at about 8 to 12 mo of age and continues for about 20 mo, so fattening is completed at about 30 mo of age (over 600 kg BW). In this study, the %IML was different among the chuck, loin, and round muscles and the %IML from the loin muscle was greater than for the chuck or round. Based on the %IML in the loin, the carcasses of the Yanbian Yellow steers would have graded USDA Prime at 28 mo of age as long as they graded A maturity.
As indicated by the small variations in BW, ADG, and ADI over time, the Yanbian Yellow cattle selected for this study were very uniform. This is supported by the low CV for ADG (16.94). The larger population of steers from which we selected steers for this study similarly was uniform in weaning and 12-mo weights. The uniformity among the steers, along with the ability to perform the multiple biopsies at each time point, provided additional power in detecting significant differences across ages for the dependent variables.
From 12 mo of age to 28 mo of age, the major SFA in all muscle depots were palmitic and stearic acid, and the major MUFA in all muscle tissues were oleic and palmitoleic acid. Collectively, these fatty acids accounted for 79% (12 mo), 85% (14 mo), 87% (20 mo), 86% (24 mo), and 85% (28 mo) of total fatty acid percentages (Table 4). Smith et al. (2012) also found similar results from 9 mo of age to 16 mo of age in different adipose tissue depots; palmitic, palmitoleic, stearic, and oleic acid in Angus steers constituted 80% (9 mo), 84% (12 mo), 83% (14 mo), and 83% (16 mo) of total fatty acids. In Korean Hanwoo steers, Lee et al. (2005) reported that these 4 fatty acids constituted 79% (6 mo), 95% (12 mo), 88% (18 mo), and 91% (24 mo) of total fatty acids. Thus, these 4 fatty acids are the major fatty acids in bovine adipose tissue, especially after beef cattle have been fed a grain-based diet for some period. This indicates the major contribution of de novo fatty acid synthesis to the overall fatty acid composition of bovine adipose tissue.
The proportion of stearic acid steadily declined and the proportion of oleic acid increased from 12 mo of age to 24 mo of age across muscles. Similar results were reported by Smith et al. (2012), in that the proportion of stearic acid decreased while proportion of oleic acid markedly increased from 9 mo of age to 16 mo of age in Angus steers. Lee et al. (2005) also reported the proportion of stearic acid was greater in total lipid and triacylglycerol fractions at 6 mo of age than at later growth stages in muscle of Korean Hanwoo steers. The apparent increase in oleic acid and corresponding decrease in stearic acid of muscle as animals grow indicates increased activity of fatty acid ∆9 desaturase (stearoyl-CoA desaturase) with growth age.
Many adipogenic/lipogenic genes, including the all of the genes measured in the current study, have been identified as prospective targets for the regulation of fatty acid synthesis (Kersten, 2001; Sevane et al., 2013). For this reason, these genes were selected as markers of the development and metabolism of i.m. adipose tissue. The expression of ACC1 and FASN is limited to i.m. adipose tissue because the primers were specific for adipose tissue ACC1, and expression of FASN has not been reported in muscle or cultures of primary myoblasts. However, SCD is expressed in both muscle and adipose tissue of beef cattle (Cameron et al., 1994) and LPL is expressed in the parenchymal cells of bovine muscle and adipose tissue (reviewed in Mead et al., 2002), so the level of expression for SCD and LPL represents expression in both muscle and i.m. adipose tissue. Additionally, PPARγ is expressed in bovine i.m. adipose tissue (Choi et al., 2014, 2016) and, to a limited extent, bovine muscle satellite cells (Choi et al., 2015b), so we cannot rule out the possibility that the PPARγ expression measured in this study included mRNA from both muscle and i.m. adipose tissue. We first reported FABP activity in bovine muscle, which increased with age in Angus steers fed a grain-based finishing diet (Moore et al., 1991). Low levels of FABP activity were detected in bovine loin muscle (Kirby Moore et al., 1993). Partial AA sequence indicated that bovine loin muscle FABP was closely related to cardiac FABP and thus was distinct from adipose tissue FABP; this was confirmed for other species subsequently (reviewed in Chmurzynska, 2006). Thus, the FABP4 expression reported in the current study resided primarily in i.m. adipose tissue.
In present study, FABP4, SREBP1, SCD, ACC1, and LPL increased, while PPARγ and FASN gene expression declined between 12 and 28 mo of age. As a member of a subfamily of ligand-activated nuclear hormone receptor, PPARγ is involved in the induction of genes mediating fatty acid uptake, adipocyte differentiation, de novo fatty acid synthesis, and lipolysis (Kersten et al., 1999). The expression of PPARγ plays a pivotal role in adipogenesis in beef cattle (García-Rojas et al., 2010) and PPARγ has been identified as one of the candidate genes related to adipogenesis of bovine i.m. adipose tissue (Lim et al., 2011). In the current study, chuck and round muscles had lower levels of PPARγ expression than the loin over time on the finishing diet. Also, the 12-mo-old steers had high PPARγ gene expression, which declined over time. Smith et al. (2012) reported that relative PPARγ expression was lower at 16 mo of age than at 12 mo of age in s.c. adipose tissue of Angus steers. The decline in PPARγ expression in this and our previous study (Smith et al., 2012) suggests a depression in the differentiation of adipocytes during the fattening period in beef cattle.
The expression of FABP4 is involved in adipocyte differentiation in the PPAR signaling pathway, and FABP4 expression has been examined in many studies with regard to its function in carcass adiposity. Moore et al. (1991) and Jurie et al. (2007) reported that i.m. adipose tissue content is strongly correlated with FABP4 expression in bovine loin muscle. In the present study, the increase in FABP4 expression was much less over time than lipid accumulation in all muscles, although the loin had much higher FABP4 expression than the chuck or round. We interpret this to mean that i.m. adipocytes of the loin have greater capacity to accumulate and metabolize fatty acids than adipocytes in the chuck or round. This is consistent with the greater LPL expression in the loin, which suggests that the adipocytes in the loin have greater capacity for hydrolysis of circulating triacylglycerols in very low-density lipoproteins. The data suggest that uptake and deposition of fatty acids from the circulation contribute significantly to lipid filling in i.m. adipocytes, particularly after being fed grain-based diets for an extended period.
Among transcription factors and nuclear receptors that are involved in lipid metabolism, PPARγ and SREBP1 are considered master regulators of lipid metabolism (Zhao et al., 2010). The expression of SREBP1 regulates fatty acid biosynthesis (Yahagi et al., 1999), and in the current study, SREBP1 expression increased greatly with time on feed and differed across muscles. Only a limited number of studies have reported the regulation of SCD expression by SREBP1 in bovine muscle (Graugnard et al., 2009; Waters et al., 2009), but other studies reported that bovine SREBP1 gene expression was associated with muscle fatty acid composition Japanese Black cattle (Hoashi et al., 2007; Ohsaki et al., 2009) and Korean Hanwoo cattle (Bhuiyan et al., 2009). The increased expression of SREBP1 indicates an elevated capacity for de novo synthesis of fatty acids, leading to increasing lipid accumulation in bovine muscle.
Stearoyl-CoA desaturase catalyzes the synthesis of MUFA from SFA, and SCD activity and SCD expression have been documented in a number of bovine tissues (reviewed in Smith, 2013). In a previous study (Smith et al., 2012), we reported that SCD expression in s.c. adipose tissue overlying the loin was greater than in s.c. adipose tissue from the chuck or the round. Although SCD expression was greatest in the loin in the current study, the percentages of MUFA were higher overall in the chuck than in the loin. Correspondingly, the MUFA:SFA ratio was higher in lipids from the chuck than in the loin. These results are similar to a previous report from our laboratory (Turk and Smith, 2009), in which we demonstrated that the MUFA:SFA ratio of lipids from s.c. adipose tissue overlying the chuck was greater than that of adipose tissue overlying the loin (1.14 vs. 0.98). We concluded that fatty acid composition of bovine adipose tissues reflects not only current SCD activity, but also previous activity during development (Smith et al., 2012). We also concluded that the s.c. adipose tissue overlying the brisket may be an earlier maturing depot than other s.c. adipose tissue sites tested in Smith et al. (2012), based on the lesser expression of genes associated with differentiation in the brisket s.c. adipose tissue (e.g., PPARγ). Therefore, the i.m. adipose tissue of the chuck may have differentiated earlier than i.m. adipose tissues of the loin or round, and gene expression associated with differentiation may have been on the decline even before our initial sampling at 12 mo of age. This hypothesis can only be tested by obtaining samples at earlier ages.
Earlier, we reported that expression of the SCD gene increased steadily between weaning and 12 mo of age, but declined by 18 mo of age, in s.c. adipose tissue of Angus steers (Martin et al., 1999). We subsequently reported that SCD gene expression either decreased (Brooks et al., 2011) or did not change (Chung et al., 2007) in s.c. adipose tissue following extended feeding of a corn-based diet. Conversely, SCD gene expression rose nearly 10-fold in s.c. adipose tissue of Wagyu steers fed a corn-based diet to 26 mo of age (Chung et al., 2007). This is consistent with Lee et al. (2005), who reported that SCD mRNA was not detectable at 6 mo of age in the muscle of Korean Hanwoo steers, but SCD mRNA levels increased 3-fold by 12 mo of age and were maintained to 30 mo of age.
It should be noted that the MUFA:SFA ratio in the loin at 28 mo of age (1.55) exceeded that which we observed in i.m. adipose tissue of American Wagyu steers fed a grain-based diet for 18 mo (MUFA:SFA = 1.38; May et al., 1993), but was similar to values reported for longissimus muscle from Korean Hanwoo steers (MUFA:SFA = 1.50 to 1.55; Lee et al., 2005). This is in spite of the fact that the loin of the Yanbian Yellow cattle contained just over 9% IML, whereas the loin of the Wagyu steers contained nearly 19% IML (Lunt et al., 1993). Thus, although Yanbian Yellow have not yet been selected to accumulate as much i.m. adipose tissue as Wagyu steers (May et al., 1993) or Hanwoo steers (Hwang and Joo, 2017a), they share the genetic capacity to accumulate the relatively large amounts of MUFA in their loin muscle typically seen in their Japanese and Korean relatives. The available evidence suggests that the capacity of these closely related breed types to accumulate MUFA in their tissues is due to increased SREBP1 expression and SCD gene expression during extended periods of being fed a grain-based diet. However, the level of SCD expression does not predict fatty acid composition of i.m. adipose tissue. Instead, we propose that differences in fatty acid composition may indicate that i.m. adipose tissue depots may differ in stage of development across muscles. We further hypothesize that the i.m. adipose tissues in the chuck accumulated MUFA earlier during growth, leading to higher a MUFA:SFA ratio later in growth as compared to i.m. adipose tissue in the round.
LITERATURE CITED
- Adams T. H., Walzem R. L., Smith D. R., Tseng S., and Smith S. B.. 2010. Hamburger high in total, saturated and trans-fatty acids decreases HDL cholesterol and LDL particle diameter, and increases TAG, in mildly hypercholesterolaemic men. Br. J. Nutr. 103:91–98. doi:10.1017/S0007114509991516 [DOI] [PubMed] [Google Scholar]
- AOAC 1995. Official methods of analysis. 15th ed. Association of Official Analytical Chemists, Washington, DC. [Google Scholar]
- Bhuiyan M. S. A., Yu S. L., Jeon J. T., Yoon D., Cho Y. M., Park E. W., Kim N. K., Kim K. S., Lee J. H.. 2009. DNA polymorphisms in SREBF1 and FASN genes affect fatty acid composition in Korean cattle (Hanwoo). Asian-Australas. J. Anim. Sci. 22:765–773. doi:10.5713/ajas.2009.80573 [Google Scholar]
- Brooks M. A., Choi C. W., Lunt D. K., Kawachi H., and Smith S. B.. 2011. Subcutaneous and intramuscular adipose tissue stearoyl-coenzyme A desaturase gene expression and fatty acid composition in calf- and yearling-fed Angus steers. J. Anim. Sci. 89:2556–2570. doi:10.2527/jas.2010-3369 [DOI] [PubMed] [Google Scholar]
- Cameron P. J., Rogers M., Oman J., May S. G., Lunt D. K., and Smith S. B.. 1994. Stearoyl coenzyme A desaturase enzyme activity and mRNA levels are not different in subcutaneous adipose tissue from Angus and American Wagyu steers. J. Anim. Sci. 72:2624–2628. [DOI] [PubMed] [Google Scholar]
- Choi J. W., Choi B. H., Lee S. H., Lee S. S., Kim H. C., Yu D., Chung W. H., Lee K. T., Chai H. H., Cho Y. M., et al. 2015a. Whole-genome resequencing analysis of Hanwoo and Yanbian cattle to identify genome-wide SNPs and signatures of selection. Mol. Cells 38:466–473. doi:10.14348/molcells.2015.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. H., Ghahramany G., Walzem R. L., Meade T., and Smith S. B.. 2018. Ground beef high in total fat and saturated fatty acids decreases X receptor signaling targets in peripheral blood mononuclear cells of men and women. Lipids. 53:279–290. doi:10.1002/lipd.12028 [DOI] [PubMed] [Google Scholar]
- Choi S. H., Park S. K., Choi C. W., Li X. Z., Kim K. H., Kim W. Y., Jeong J., Johnson B. J., Zan L., and Smith S. B.. 2016. The expression of adipogenic genes in adipose tissues of feedlot steers fed supplementary palm oil or soybean oil. Asian-Australas. J. Anim. Sci. 29:404–412. doi:10.5713/ajas.15.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. H., Park S. K., Johnson B. J., Chung K. Y., Choi C. W., Kim K. H., Kim W. Y., and Smith B.. 2015b. AMPKα, C/EBPβ, CPT1β, GPR43, PPARγ, and SCD gene expression in single- and co-cultured bovine satellite cells and intramuscular preadipocytes treated with palmitic, stearic, oleic, and linoleic acid. Asian-Australas. J. Anim. Sci. 28:411–419. doi:10.5713/ajas.14.0598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. H., Silvey D. T., Johnson B. J., Doumit M. E., Chung K. Y., Sawyer J. E., Go G. W., and Smith S. B.. 2014. Conjugated linoleic acid (t-10, c-12) reduces fatty acid synthesis de novo, but not expression of genes for lipid metabolism in bovine adipose tissue ex vivo. Lipids 49:15–24. doi:10.1007/s11745-013-3869-0 [DOI] [PubMed] [Google Scholar]
- Chmurzyńska A. 2006. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J. Appl. Genet. 47:39–48. doi:10.1007/BF03194597 [DOI] [PubMed] [Google Scholar]
- Chung K. Y., Lunt D. K., Kawachi H., Yano H., and Smith S. B.. 2007. Lipogenesis and stearoyl-CoA desaturase gene expression and enzyme activity in adipose tissue of short- and long-fed Angus and Wagyu steers fed corn- or hay-based diets. J. Anim. Sci. 85:380–387. doi:10.2527/jas.2006-087 [DOI] [PubMed] [Google Scholar]
- Folch J., Lee M., and Sloan-Stanley G. H.. 1957. A sample method for the isolation and purification of total lipides from animal tissue. J. Biol. Chem. 226:497–509. [PubMed] [Google Scholar]
- Frank D., Ball A., Hughes J., Krishnamurthy R., Piyasiri U., Stark J., Watkins P., and Warner R.. 2016a. Sensory and flavor chemistry characteristics of Australian beef: influence of intramuscular fat, feed, and breed. J. Agric. Food Chem. 64:4299–4311. doi:10.1021/acs.jafc.6b00160 [DOI] [PubMed] [Google Scholar]
- Frank D., Joo S. T., and Warner R.. 2016b. Consumer acceptability of intramuscular fat. Korean J. Food Sci. Anim. Resour. 36:699–708. doi:10.5851/kosfa.2017.37.2.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rojas P., Antaramian A., González-Dávalos L., Villarroya F., Shimada A., Varela-Echavarría A., and Mora O.. 2010. Induction of peroxisomal proliferator-activated receptor gamma and peroxisomal proliferator-activated receptor gamma coactivator 1 by unsaturated fatty acids, retinoic acid, and carotenoids in preadipocytes obtained from bovine white adipose tissue1,2. J. Anim. Sci. 88:1801–1808. doi:10.2527/jas.2009-2579 [DOI] [PubMed] [Google Scholar]
- Gilmore L. A., Crouse S. F., Carbuhn A., Klooster J., Calles J. A., Meade T., and Smith S. B.. 2013. Exercise attenuates the increase in plasma monounsaturated fatty acids and high-density lipoprotein cholesterol but not high-density lipoprotein 2b cholesterol caused by high-oleic ground beef in women. Nutr. Res. 33:1003–1011. doi:10.1016/j.nutres.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Gilmore L. A., Walzem R. L., Crouse S. F., Smith D. R., Adams T. H., Vaidyanathan V., Cao X., and Smith S. B.. 2011. Consumption of high-oleic acid ground beef increases HDL-cholesterol concentration but both high- and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men. J. Nutr. 141:1188–1194. doi:10.3945/jn.110.136085 [DOI] [PubMed] [Google Scholar]
- Graugnard D. E., Piantoni P., Bionaz M., Berger L. L., Faulkner D. B., and Loor J. J.. 2009. Adipogenic and energy metabolism gene networks in Longissimus lumborum during rapid post-weaning growth in Angus and Angus x Simmental cattle fed high-starch or low-starch diets. BMC Genomics 10:142. doi:10.1186/1471-2164-10-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoashi S., Ashida N., Ohsaki H., Utsugi T., Sasazaki S., Taniguchi M., Oyama K., Mukai F., and Mannen H.. 2007. Genotype of bovine sterol regulatory element binding protein-1 (SREBP-1) is associated with fatty acid composition in Japanese Black cattle. Mamm. Genome 18:880–886. doi:10.1007/s00335-007-9072-y [DOI] [PubMed] [Google Scholar]
- Hwang Y. H. and Joo S. T.. 2017a. Fatty acid profiles of ten muscles from high and low marbled (quality grade 1++ and 2) Hanwoo steers. Korean J. Food Sci. Anim. Resour. 36:679–688. doi:10.5851/kosfa.2016.36.5.679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y. H. and Joo S. T.. 2017b. Fatty acid profiles, meat quality, and sensory palatability of grain-fed and grass-fed beef from Hanwoo, American, and Australian crossbred cattle. Korean J. Food Sci. Anim. Resour. 37:153–161. doi:10.5851/kosfa.2017.37.2.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurie C., Cassar-Malek I., Bonnet M., Leroux C., Bauchart D., Boulesteix P., Pethick D. W., and Hocquette J. F.. 2007. Adipocyte fatty acid-binding protein and mitochondrial enzyme activities in muscles as relevant indicators of marbling in cattle. J. Anim. Sci. 85:2660–2669. doi:10.2527/jas.2006-837 [DOI] [PubMed] [Google Scholar]
- Kersten S. 2001. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2:282–286. doi:10.1093/embo-reports/kve071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S., Seydoux J., Peters J. M., Gonzalez F. J., Desvergne B., and Wahli W.. 1999. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 103:1489–1498. doi:10.1172/JCI6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby Moore K., Cameron P. J., Ekeren P. A., and Smith S. B.. 1993. Fatty acid-binding protein in bovine longissimus dorsi muscle. Comp. Biochem. Physiol. 104B:259–266. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Yoon D. H., Choi N. J., Hwang S. H., Cheong E. Y., Oh S. J., Cheong I. C., and Lee C. S.. 2005. Developmental relationship of unsaturated fatty acid composition and stearoyl-CoA desaturase mRNA level in Hanwoo steers’ muscle. Asian-Australas. J. Anim. Sci. 18:562–566. [Google Scholar]
- Lepage G., and Roy C. C.. 1986. Direct transesterification of all classes of lipid in a one-step reaction. J. Lipid Res. 27:114–221. [PubMed] [Google Scholar]
- Li X. Z., Yan C. G., Lee H. G., Choi C. W., and Song M. K.. 2012. Influence of dietary plant oils on mammary lipogenic enzymes and the conjugated linoleic acid content of plasma and milk fat of lactating goats. Anim. Feed Sci. Technol. 174:26–35. doi:10.1016/j.anifeedsci.2012.02.004 [Google Scholar]
- Li X. Z., Yan C. G., Yu J., Gao Q. S., Choi S. H., Shin J. S., and Smith S. B.. 2017. Dietary whole and cracked linseed increases the proportion of oleic and α-linolenic acids in adipose tissues and decreases stearoyl-coenzyme A desaturase, acetyl-coenzyme A carboxylase, and fatty acid synthase gene expression in the longissimus thoracis muscle of Yanbian Yellow cattle. J. Anim. Sci. 95:718–726. doi:10.2527/jas.2016.1050 [DOI] [PubMed] [Google Scholar]
- Lim D., Kim N. K., Park H. S., Lee S. H., Cho Y. M., Oh S. J., Kim T. H., and Kim H.. 2011. Identification of candidate genes related to bovine marbling using protein-protein interaction networks. Int. J. Biol. Sci. 7:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lunt D. K., Riley R. R., and Smith S. B.. 1993. Growth and carcass characteristics of Angus and American Wagyu steers. Meat Sci. 34:327–334. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Lunt D. K., Britain K. G., and Smith S. B.. 1999. Postnatal development of stearoyl coenzyme A desaturase gene expression and adiposity in bovine subcutaneous adipose tissue. J. Anim. Sci. 77:630–636. [DOI] [PubMed] [Google Scholar]
- May S. G., Sturdivant C. A., Lunt D. K., Miller R. K., and Smith S. B.. 1993. Comparison of sensory characteristics and fatty acid composition between Wagyu crossbred and Angus steers. Meat Sci. 35:289–298. [DOI] [PubMed] [Google Scholar]
- Mead J. R., Irvine S. A., and Ramji D. P.. 2002. Lipoprotein lipase: structure, function, regulation, and role in disease. J. Mol. Med. (Berl). 80:753–769. doi:10.1007/s00109-002-0384-9 [DOI] [PubMed] [Google Scholar]
- Mei C., Wang H., Liao Q., Wang L., Cheng G., Wang H., Zhao C., Song J., Guang X., Liu G. E., et al. 2017. Genetic architecture and selection of Chinese cattle revealed by whole genome resequencing. Mol. Biol. Evol. doi:10.1093/molbev/msx322 [DOI] [PubMed] [Google Scholar]
- Meijer G. A. L., van der Muelen J., Bakker J. G. M., van der Koelen C. J., and van Vuuren A. M.. 1995. Free amino acids in plasma and muscle of high yielding dairy cows in early lactation. J. Dairy Sci. 78:1131–1141. [DOI] [PubMed] [Google Scholar]
- Moore K. K., Ekeren P. A., Lunt D. K., and Smith S. B.. 1991. Relationship between fatty acid-binding protein and marbling scores in bovine longissimus muscle. J. Anim. Sci. 69:1515–1521. [DOI] [PubMed] [Google Scholar]
- Ohsaki H., Tanaka A., Hoashi S., Sasazaki S., Oyama K., Taniguchi M., Mukai F., and Mannen H.. 2009. Effect of SCD and SREBP genotypes on fatty acid composition in adipose tissue of Japanese Black cattle herds. Anim. Sci. J. 80:225–232. doi:10.1111/j.1740-0929.2009.00638.x [DOI] [PubMed] [Google Scholar]
- Sevane N., Armstrong E., Cortés O., Wiener P., Wong R. P., and Dunner S.; GemQual Consortium 2013. Association of bovine meat quality traits with genes included in the PPARG and PPARGC1A networks. Meat Sci. 94:328–335. doi:10.1016/j.meatsci.2013.02.014 [DOI] [PubMed] [Google Scholar]
- Smith S. B. 2013. Functional development of stearoyl-CoA desaturase gene expression in livestock species. In: Ntambi J. M., editor, Stearoyl-CoA desaturase genes in lipid metabolism. Springer Science, New York, NY: p. 141–159. doi:10.1007/978-1-4614-7969-7_12 [Google Scholar]
- Smith S. B., Gill C. A., Lunt D. K., and Brooks M. A.. 2009. Regulation of fat and fatty acid composition in beef cattle. Asian-Australas. J. Anim. Sci. 22:1225–1233. [Google Scholar]
- Smith S. B., Go G. W., Johnson B. J., Chung K. Y., Choi S. H., Sawyer J. E., Silvey D. T., Gilmore L. A., Ghahramany G., and Kim K. H.. 2012. Adipogenic gene expression and fatty acid composition in subcutaneous adipose tissue depots of Angus steers between 9 and 16 months of age. J. Anim. Sci. 90:2505–2514. doi:10.2527/jas.2011-4602 [DOI] [PubMed] [Google Scholar]
- Talapatra S. K., Ray S. C., and Sen K. C.. 1940. The analysis of mineral constituents in biological materials. 1. Estimation of phosphorus, calcium, magnesium, sodium and potassium in feedstuffs. Indian J. Vet. Sci. Anim. Husb. 10: 243–258. [Google Scholar]
- Turk S. N. and Smith S. B.. 2009. Carcass fatty acid mapping. Meat Sci. 81:658–663. doi:10.1016/j.meatsci.2008.11.005 [DOI] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber and non starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. [DOI] [PubMed] [Google Scholar]
- Vernon R. G., and Flint D. F.. 1988. Lipid metabolism in farm animals. Proc. Nutr. Soc. 47:287–293. [DOI] [PubMed] [Google Scholar]
- Waters S. M., Kelly J. P., O’Boyle P., Moloney A. P., and Kenny D. A.. 2009. Effect of level and duration of dietary n-3 polyunsaturated fatty acid supplementation on the transcriptional regulation of delta9-desaturase in muscle of beef cattle. J. Anim. Sci. 87:244–252. doi:10.2527/jas.2008-1005 [DOI] [PubMed] [Google Scholar]
- Yahagi N., Shimano H., Hasty A. H., Amemiya-Kudo M., Okazaki H., Tamura Y., Iizuka Y., Shionoiri F., Ohashi K., Osuga J., et al. 1999. A crucial role of sterol regulatory element-binding protein-1 in the regulation of lipogenic gene expression by polyunsaturated fatty acids. J. Biol. Chem. 274:35840–35844. [DOI] [PubMed] [Google Scholar]
- Yonesaka R., Sasazaki S., Yasue H., Niwata S., Inayoshi Y., Mukai F., and Mannen H.. 2016. Genetic structure and relationships of 16 Asian and European cattle populations using DigiTag2 assay. Anim. Sci. J. 87:190–196. doi:10.1111/asj.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon G. H., Park E. W., Lee S. H., Lee H. K., Oh S. J., Cheong I. C., and Hong K. C.. 2005. Assessment of genetic diversity and relationships between Korean cattle and other cattle breeds by microsatellite loci. J. Anim. Sci. Technol. (Kor.) 47:341–354. [Google Scholar]
- Zhao Y. M., Basu U., Dodson M. V., Basarb J. A., and Guan L. L.. 2010. Proteome differences associated with fat accumulation in bovine subcutaneous adipose tissues. Proteome Sci. 8:14. doi:10.1186/1477-5956-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]