Abstract
Apparent total-tract digestibility (ATTD) of nutrients could be an alternative measure of feed efficiency (FE) when breeding for robust animals that are fed fiber-rich diets. Apparent total-tract digestibility of nutrients requires measuring individual feed intake of a large number of animals which is expensive and complex. Alternatively, ATTD of nutrients and feces chemical composition can be predicted using fecal near-infrared reflectance spectroscopy (FNIRS). The objective of this study was to assess if the feces chemical composition and ATTD of nutrients can be predicted using FNIRS that originate from various pig-experimental datasets. Fecal samples together with detailed information on the feces chemical composition and ATTD of nutrients were obtained from four different pig experiments. Feces near-infrared spectroscopy was analyzed from fecal samples of a complete dataset. The model was calibrated using the FNIRS and reference samples of feces chemical composition and ATTD of nutrients. The robustness and predictability of the model were evaluated by the r2 and the closeness between SE of calibration (SEC) and SE of cross-validation (SECV). Prediction of the feces chemical components and ATTD of nutrients were successful as SEC and SECV were equivalent. Calibration model was developed to estimate the ATTD of nutrients and fecal chemical composition from the FNIRS and worked well for OM (r2 = 0.94; SEC = 48.5; SECV = 56.6), CP (r2 = 0.89; SEC = 18.1; SECV = 18.8), GE (r2 = 0.92; SEC = 1.2; SECV = 1.4), NDF (r2 = 0.94; SEC = 55; SECV = 60.2), OM digestibility (r2 = 0.94; SEC = 5.5; SECV = 6.7), GE digestibility (r2 = 0.88; SEC = 2.3; SECV = 2.6), and fat digestibility (r2 = 0.79; SEC = 6, SECV = 6.8). However, the SE of prediction was slightly higher than what has been reported in another study. The prediction of feces chemical composition for fat (r2 = 0.69; SEC = 11.7, SECV = 12.3), CP digestibility (r2 = 0.63; SEC = 2.3; SECV = 2.7), and NDF digestibility (r2 = 0.64, SEC = 7.7, SECV = 8.8) was moderate. We conclude that the FNIRS accurately predicts the chemical composition of feces and ATTD of nutrients for OM, CP, and GE. The approach of FNIRS is a cost-effective method for measuring digestibility and FE in a large-scale pig-breeding programs.
Keywords: feed efficiency, fecal near-infrared spectroscopy, nutrient digestibility, pig
INTRODUCTION
Intensive pig production systems in Europe are highly dependent on imported feed ingredients, for example, soybean meal (SBM) as a source of protein (FEFAC, 2015). However, access to these feed resources might be limited in the future (Godfray et al., 2010, Hayes et al., 2013). Alternative local feed sources, such as rapeseed meal (RSM) and grain by-products, may provide viable means to improve sustainably and self-sufficiency. The challenge is RSM which is rich in fiber, and antinutritional factors (Mejicanos et al., 2016) reduces feed intake, growth rate, and nutrient utilization (Landero et al., 2011; Seneviratne et al., 2011; Torres-Pitarch et al., 2014). Therefore, breeding robust animals by improving digestibility and feed efficiency (FE) would be beneficial. The present measures of FE aims to increase animal products from a fixed amount of feed intake neglecting the energy requirement of animals to other body functions. This will have a long-term health and fertility-related risks. Another alternative would be to improve the digestive capability of animals and increase the total available of digestible nutrients from a given feed intake. However, quantifying apparent total-tract digestibility (ATTD) of nutrients is challenging because collecting feces samples and feed intake is required (Arthur and Herd, 2008). Alternatively, ATTD of nutrients can be estimated using fecal near-infrared reflectance spectroscopy (FNIRS) which is cost-effective and less technical demanding (Bastianelli et al., 2015; Schiborra et al., 2015). For predicting a fully external dataset, however, a robust calibration equation is necessary. A robust calibration is achieved when the calibration dataset is representative. The objective of this study was to assess the possibility of predicting feces chemical components and ATTD of nutrients using FNIRS that originate from different experiments involving animals with a different genetic background, diets, and age groups.
MATERIALS AND METHODS
Sample Origin and Experimental Design
A total of 198 fecal samples from piglets, young pigs, and growing-finishing pigs were obtained from four experiments. The experiments were conducted at two different locations: experimental farm at the Norwegian University of Life Sciences (NMBU), Ås, Norway, and at the Rørrendegård experimental farm of the University of Copenhagen in Tåstrup, Denmark. The experiments were carried out in accordance with the guidelines of the Norwegian Food Safety Authority and the Animal Experiments Inspectorate, Ministry of Environment and Food, Copenhagen, Denmark, regarding animal experimentation and care. Relevant information about the design of the experiments is presented in Table 1. The detailed description of Exp. 1 is given in Pérez de Nanclares et al. (2017). In Exp. 1, 2, and 3, the pigs were fed a control diet based cereals and SBM, or a treatment diet where SBM was partially or totally replaced by rapeseed co-products. In Exp. 4, the pigs were fed a cereal–SBM control diet or a treatment diet where SBM was partially replaced by increasing levels of yeast. In Exp. 1, 2, and 4, yttrium oxide (Y2O3) was included (0.01%) as internal marker for digestibility calculations. In Exp. 1, 2, and 4, cumulative feces were obtained by grab sampling while the total collection of feces was conducted in Exp. 3. In addition to feces, cumulative samples of all the diets were obtained during the collection period in the three/four experiments for chemical analyses.
Table 1.
Information about the four experiments conducted with young and growing-finishing pigs at two different locations
| Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | |
|---|---|---|---|---|
| Location | Ås, Norway | Ås, Norway | Tåstrup, Denmark | Ås, Norway |
| Type of experiment | Digestibility | Performance | Metabolism | Performance |
| Feces collection method | Grab samples (5–7 d) | Grab samples (7 d) | Total collection (4 d) | Grab samples (5 d) |
| n | 40 | 80 | 32 | 48 |
| Breed | Norwegian Landrace | Norwegian Landrace | Danish Landrace × [Yorkshire × Duroc] | [Norwegian Landrace × Yorkshire] × Duroc and Norwegian |
| Landrace × Duroc | ||||
| Weight | 18–28 kg | 29–110 kg | 22–32 kg | 11–20 kg |
| Sex | Male | Male and Female | Male | Male and female |
| No. of diets | 2 | 2 | 4 | 4 |
| Dietary components | Control: barley, wheat, and SBM1 | Control barley, wheat, oats, and SBM | Control: barley, wheat, oats, and SBM | Control: barley, wheat, oats, PPC5, FM6, RSM, and SBM |
| RSF2: 20% coarse RSM3 and 4% RS4 hulls replacing SBM and wheat | RSM: 20% RSM replacing SBM, barley, and wheat | RSM10,20,30: 10, 20, or 30% RSM replacing SBM and wheat | Y 7108: 3.62% yeast | |
| Y20: 7.26% yeast | ||||
| Y40: 14.6% yeast | ||||
| Feeding level | Restricted (3.5% of BW) | Close to ad libitum | Close to ad libitum | Close to ad libitum |
1SBM = soybean meal.
2RSF = high-fiber rapeseed diet.
3RSM = rapeseed meal.
4RS = rapeseed.
5PPC = potato protein concentrate.
6FM = fishmeal.
7Y = yeast.
8Ratio of CP from yeast, replacing 10, 20, and 40% CP from SBM, PPC, FM, and RSM.
Sample Processing and Chemical Analyses
Feces from each pig over the period of 4- to 7-d collection were pooled, homogenized, and representative samples were frozen at −20 °C until chemical analyses. Upon completion of the experiments, the feces samples were freeze-dried, and diet and fecal samples were ground in a 1-mm sieve. Chemical analyses of diets and feces samples for Exp. 1, 2, and 4 were conducted at the NMBU laboratory, Ås, Norway, while the samples from Exp. 3 were analyzed at the laboratory of the University of Copenhagen. At the NMBU laboratory, all chemical analyses of feces were performed on freeze-dried samples. Dry matter was determined by drying to constant weight at 104 °C (EC, 1971b), ash by incineration at 550 °C (EC, 1971a), CP by Kjeldahl Nitrogen × 6.25 (EC, 1993). Gross energy content was determined with an adiabatic bomb calorimeter (Parr 1281; Parr Instruments, Moline, IL, United States) according to ISO (1998). For determination of yttrium (Y) concentrations in feces and diets, samples were first digested in concentrated ultrapure nitric acid (HNO3) at 250 °C using a microwave (Milestone UltraClave III; Milestone, Sorisole, Italy). Samples were then diluted to 10% HNO3 and Y were analyzed by inductively coupled plasma mass spectrometry using an Agilent 8800 Triple Quadrupole (Agilent Technologies, Santa Clara, CA, United States). At the laboratory of the University of Copenhagen, DM and Nitrogen analyses of feces were performed on fresh material while the rest of the chemical analyses were performed on freeze-dried samples. Dry matter was measured by drying to constant weight at 105 °C, and ash was determined by incineration at 525 °C. Organic matter was the difference between the DM and ash content. Nitrogen was determined by the Kjeldahl method using the Tecator-Kjeltec system 1030 (Tecator AB, Höganäs, Sweden), and CP calculated as nitrogen × 6.25. Fat content was determined by petroleum ether extraction in a Soxtec system 2043 (FOSS Electric A/S, Hillerød, Denmark) after hydrochloric (HCl) hydrolysis. Gross energy was determined using an IKA Calorimeter system (IKA Gmbh and Co. KG, Staufen, Germany).
Determination of Nutrient and Energy Digestibility
Chemical analyses of feces and diets allowed for the determination ATTD coefficients for all the analyzed nutrients and energy. In Exp. 1, 2, and 4, ATTD was calculated by the indirect method, as described by Maynard and Loosli (1969), using Y2O3 as the inert marker (Austreng et al., 2000). In Exp. 3, ATTD of all individual nutrients and energy was calculated according to the equation of McDonald et al. (1998) using the following equation: ATTD (%) = [intake (g) − fecal excretion (g)/intake (g)] × 100%, in which ATTD is percent ATTD for each nutrients, intake, and fecal excretion is the concentration of each nutrients in the feed intake and in the feces, respectively.
Fecal Near-Infrared Spectroscopy Scans
Fecal near-infrared spectroscopy was recorded on the same feces samples that were used for analyzing the chemical components of feces. The feces were scanned at Nofima Laboratory between 400 and 2,500 nm in 2-nm increments using a FOSS monochromatic spectrometer NIRSystem 6500 (FOSS Electric A/S). Fecal near-infrared spectroscopy was analyzed in three replicates and then averaged. Fecal near-infrared spectroscopy between the region of 1,105 and 2,450 was taken for model calibration.
Different mathematic pretreatments such as derivative spectra, extended multiplicative signal correction were applied on the raw FNIRS beforehand to optimize model calibration (Naes et al., 2002).
Statistical Analysis
For both chemical analysis and ATTD of nutrients, calibration models were obtained using partial least-squares regression (Wold et al., 1984) in the Unscrambler × version: 10.4.45271.25 (Camo Software AS, Oslo, Norway). Two types of model validation, internal cross-validation and external-validation, were done on the complete dataset. For the external-validation, the FNIRS dataset was randomly split into two groups, the calibration dataset, which contained 158 samples were used for calibration and the validation dataset (40 samples) were used for validation. In the internal cross-validation, the complete dataset was randomly split into five groups of equal size. Cross-validation was performed on five groups, by sequential calibrating on four groups and then validated on the fifth group. The statistical evaluation during model building was done on calibration and on cross-validation process of r2 of cross-validation (r2cv) and SE of cross-validation (SECV), as stated by Naes et al. (2002). Subsequently, the calibration models were applied to the external-validation database (40 samples), leading to the r2 of validation (r2val) and SE of prediction (SEP). The slope of the regression line was used to describe the magnitude of closeness between the reference datasets and predicted values, and the bias was defined as the mean of the difference between the reference sample and predicted values (Naes et al., 2002).
RESULTS
Variability of the Data
The descriptive statistics for each experimental trials are given in Table 2. For each separate experimental trials, the feces chemical components have higher variability as compared to the ATTD. For feces chemical components, Exp. 3 was more variable in OM (CV = 9%), CP (CV = 9%), and FAT (CV = 29%). Experiment 4 was more variable in NDF (CV = 8%). Experiment 1 was more variable in GE (CV = 17%). For ATTD of nutrients, OM (CV = 15%) was more variable in Exp. 4, NDF (CV = 40%) and FAT (CV = 11%) were more variable in Exp. 2, and CP (CV = 5%) and GE (CV = 4%) were more variable in Exp. 1.
Table 2.
Descriptive statistics of feces chemical components and apparent total-tract digestibility of nutrients for each experimental trial
| Feces chemical components | ATTD1 of nutrients | |||||
|---|---|---|---|---|---|---|
| Mean (g/kg DM) | SD | Range (Min–Max) | Mean (g/kg DM) | SD | Range (Min–Max) | |
| Exp.1 | ||||||
| OM | 791 | 21 | 738.2–822.6 | 84.6 | 3.45 | 78.2–89.4 |
| NDF | 208.5 | 12.1 | 186.9–235.7 | 44.9 | 8.86 | 23–57 |
| CP | 18.5 | 0.25 | 17.5–19.1 | 81.2 | 3.88 | 74.1–87.4 |
| GE | 73.3 | 12.7 | 50.9–96.2 | 82.7 | 3.41 | 75.8–87.7 |
| FAT | 405.1 | 48 | 331–480 | 84.4 | 2.09 | 77–89.4 |
| Exp. 2 | ||||||
| OM | 811.4 | 14.4 | 781.1–84 | 75.3 | 2.5 | 66.9–80.3 |
| NDF | 152.8 | 9.9 | 128.9–173.6 | 23.6 | 9.5 | 0.34–44.2 |
| CP | 18.2 | 0.22 | 17.8–19 | 75.4 | 2.5 | 67.8–80.4 |
| GE | 86.6 | 14.2 | 57.7–117 | 75.2 | 2.7 | 65.7–80.7 |
| FAT | 498.6 | 32.1 | 408.6–561.4 | 61 | 7 | 42.3–74.9 |
| Exp. 3 | ||||||
| OM | 274.4 | 25.1 | 223.3–329 | 79.74 | 2.63 | 74–85.1 |
| CP | 68 | 6.2 | 52.2–78.3 | 79.4 | 3.50 | 72.5–85.9 |
| GE | 6.4 | 0.64 | 5.2–7.8 | 93.4 | 1.08 | 91–95.6 |
| FAT | 25.7 | 7.5 | 26.4–65.2 | 92.52 | 1.70 | 86–95 |
| Exp. 4 | ||||||
| OM | 782 | 22.8 | 658.2–803.7 | 26.4 | 3.93 | 12.3–34.1 |
| NDF | 229 | 19.1 | 190–271.2 | 30.2 | 9.5 | 13.9–60.8 |
| CP | 18.6 | 0.62 | 15.4–20 | 79.3 | 2.92 | 72.6–88.6 |
| GE | 69.5 | 10.2 | 48–96.6 | 82.9 | 1.68 | 79.9–90.5 |
| FAT | 382 | 32.3 | 322.4–443.4 | 71.7 | 4.89 | 63–83.2 |
1ATTD = Apparent total-tract digestibility.
A considerable variation observed in the complete dataset for both chemical components of the feces and ATTD of nutrients (Table 3). For both chemical components and ATTD of nutrients, the combined datasets (Table 3) were more variable as compared to the separate datasets (Table 2). Overall, there was more variation in the chemical components than in the ATTD of nutrients except that the ATTD for OM (dOM) (CV = 34%) was more variable as compared to the feces chemical components of OM (27%). There were no substantial differences in variation between the calibration data and validation datasets (Table 4). For the chemical components, NDF was highly variable (CV = 90%) and the lowest variability was observed with GE and OM (CV = 27%). Apparent total-tract digestibility for NDF was highly variable (CV = 41%) followed by dOM (CV = 34%).
Table 3.
Descriptive statistics of the complete dataset (198 samples)
| Components | Mean (g/kg DM) | SD | CV | Range (Min–Max) |
|---|---|---|---|---|
| Feces chemical components | ||||
| OM | 716 | 192 | 0.27 | 223–838 |
| CP | 169 | 55.3 | 0.33 | 52–71 |
| GE | 17 | 4.4 | 0.27 | 5–20 |
| FAT | 72 | 21.0 | 0.29 | 26–117 |
| NDF | 258 | 232.5 | 0.90 | 14–561 |
| Apparent total-tract digestibility | ||||
| dOM1 | 66 | 22.8 | 0.34 | 12- 89 |
| dCP2 | 78 | 3.8 | 0.05 | 67–89 |
| dGE3 | 81 | 6.8 | 0.08 | 66–96 |
| dFAT4 | 73 | 13.1 | 0.18 | 42–95 |
| dNDF5 | 31 | 12.6 | 0.41 | 0.34–61 |
1dOM = Apparent total-tract digestibility of OM.
2dCP = Apparent total-tract digestibility of CP.
3dGE = Apparent total-tract digestibility of GE.
4dFat = Apparent total-tract digestibility of fat.
5dNDF = Apparent total-tract digestibility of NDF.
Table 4.
Descriptive statistics of calibration and validation datasets
| Calibration datasets (158 samples) | Validation datasets (40 samples) | |||||
|---|---|---|---|---|---|---|
| Components | Mean (g/kg DM) | SD | Range (Min–Max) | Mean (g/kg DM) | SD | Range (Min–Max) |
| Feces chemical components | ||||||
| OM | 712 | 194.5 | 241–838 | 733 | 186.5 | 223–836 |
| CP | 169 | 55.7 | 57–271 | 169 | 54.2 | 52–260 |
| GE | 16 | 4.4 | 6–20 | 17 | 4.2 | 5–19 |
| FAT | 72 | 21.4 | 30 –117 | 71 | 19.7 | 26 –109 |
| Apparent total-tract digestibility | ||||||
| dOM1 | 67 | 23.1 | 12–89 | 66 | 21.6 | 23–88 |
| dCP2 | 78 | 4.0 | 68–89 | 78 | 3.1 | 73–85 |
| dGE3 | 82 | 6.8 | 66–96 | 80 | 6.7 | 71–95 |
| dFAT4 | 74 | 13.2 | 42–94 | 71 | 12.8 | 50–95 |
1dOM = Apparent total-tract digestibility of OM.
2dCP = Apparent total-tract digestibility of CP.
3dGE = Apparent total-tract digestibility of GE.
4dFat = Apparent total-tract digestibility of fat.
Fecal Near-Infrared Treatment
All mathematical pretreatments lead to similar results as the raw spectra. There were little improvements in both the calibration and validation statistical parameters when the derivative spectra procedure was implemented as compared to the others. Therefore, we simply chose and implemented the derivative spectra mathematical pretreatment with the Savitzky-Golay option using 15 smoothing points (Savitzky and Golay, 1964).
Calibration
Descriptive statistics of the complete dataset used for calibration and validation feces chemical composition and ATTD of nutrients are presented in Table 4. The statistical parameters for model calibration are presented for chemical components of the feces and ATTD of nutrients (Table 5).
Table 5.
Fecal near-infrared spectroscopy calibration and cross-validation statistical parameters for feces chemical components and total-tract digestibility of nutrients
| Components | SEC1 | r 2 cal 2 | SECV3 | r 2 cv 4 |
|---|---|---|---|---|
| Feces chemical components | ||||
| OM | 48.5 | 0.94 | 56.5 | 0.92 |
| CP | 18.1 | 0.89 | 18.8 | 0.89 |
| GE | 1.2 | 0.92 | 1.4 | 0.91 |
| FAT | 11.7 | 0.69 | 12.3 | 0.66 |
| NDF | 55.0 | 0.94 | 60.2 | 0.93 |
| Apparent total tract of nutrient digestibility | ||||
| dOM5 | 5.5 | 0.94 | 6.7 | 0.91 |
| dCP6 | 2.3 | 0.63 | 2.7 | 0.51 |
| dGE7 | 2.3 | 0.88 | 2.6 | 0.85 |
| dFAT8 | 6.0 | 0.79 | 6.8 | 0.74 |
| dNDF9 | 7.7 | 0.64 | 8.8 | 0.53 |
1SEC = SE of calibration.
2 r 2 cal = r2 of calibration.
3SECV = SE of cross-validation.
4 r 2 cv = r2 of cross-validation.
5dOM = apparent total-tract digestibility of OM.
6dCP = apparent total-tract digestibility of CP.
7dGE = apparent total-tract digestibility of GE.
8dFat = apparent total-tract digestibility of fat.
9dNDF = apparent total-tract digestibility of NDF.
Calibration and validation equations were successfully developed for all feces chemical components (Table 5). Apart from FAT, a higher r2C observed for most of the feces chemical components. Highest r2C was observed for OM and NDF (r2 = 0.94) followed by GE (r2 = 0.92), and CP (r2 = 0.89) corresponding to a SE of calibration (SEC) of OM (48.5 g/kg DM), NDF (55 g/kg DM), GE (1.2 MJ/kg DM), and CP (18.1 g/kg DM). FAT has the lowest r2C 0.69 and SEC = 11.7 g/kg DM. Despite a highly variable dataset used for calibration, the calibration statistical parameters for most feces chemical components were quite precise. The mean for the calibration dataset (Table 4) was for OM (712 g/kg DM), NDF (405 g/kg DM), CP (169 g/kg DM), GE (16 MJ/kg DM), and FAT (72 g/kg DM).
Apparent total-tract digestibility was expressed relative to the DM of the diet and the calibration and cross-validation statistical parameters are presented in Table 5. Higher to moderate r2C was observation for most of the ATTD of the diet nutrients. Highest r2C was observed for dOM (r2 = 0.94) followed by ATTD of GE (dGE) (r2 = 0.88), ATTD of fat (dFAT) (r2 = 0.79). Moderate r2C was observed for ATTD of NDF (dNDF) (r2 = 0.65) and ATTD of CP (dCP) (r2 = 0.63).
For all ATTD of nutrients, the SEC and SECV were precise (Table 4). The mean of the calibration datasets (40 samples) was dOM (66.49 g/kg DM), dCP (78.26 g/kg DM), dGE (81.68 MJ/kg DM), and dFAT (73.8 g/kg DM).
Validation
Two types of validations were performed. Cross-validation on the complete dataset and validation on a separate external dataset (40 samples) which were not part of the calibration dataset (158 samples). The results for the external-validation are presented in Table 6. Always SECV was higher than the SEC. A close magnitude between the SECV and SEC would mean that the predictability in both datasets is the same.
Table 6.
Fecal near-infrared spectroscopy validation statistical parameters for feces chemical components and apparent total-tract digestibility of nutrients
| Components | SEP1 (g/kg DM) | r 2 val 2 | Bias | Slope3 |
|---|---|---|---|---|
| Feces chemical components | ||||
| OM | 56.5 | 0.91 | 4.4 | 0.83 |
| CP | 17.9 | 0.89 | 3.8 | 0.84 |
| GE | 1.3 | 0.90 | 0.16 | 0.84 |
| FAT | 11.7 | 0.64 | 2.0 | 0.72 |
| Apparent total-tract digestibility | ||||
| dOM4 | 5.5 | 0.94 | -0.60 | 1.0 |
| dCP5 | 1.9 | 0.63 | 0.03 | 0.81 |
| dGE6 | 2.5 | 0.87 | 0.20 | 0.79 |
| dFAT7 | 6.2 | 0.77 | 1.2 | 0.72 |
1SEP = SE of prediction.
2 r 2 val = r2 of cross-validation.
3Slope = characteristics of regression between predicted on measured values.
4dOM = apparent total-tract digestibility of OM.
5dCP = apparent total-tract digestibility of CP.
6dGE = apparent total-tract digestibility of GE.
7dFat = apparent total-tract digestibility of fat.
The differences between SECV and SEC were variable. Some of the chemical components had higher SECV with GE (34%), CP (17%), OM (16%), as compared to the SEC. Overall, the SECV was less than 20% compared to the SEC. This is comparable to what has been reported in another study (Bastianelli et al., 2015).
Higher differences were observed between SECV and SEC for ATTD of nutrients as compared to the feces chemical components (Table 5). For all the ATTD of nutrients, the differences between SECV and SEC were below 22% and the highest difference was observe with dOM (22%). The other ATTD of nutrients had less than 20% difference.
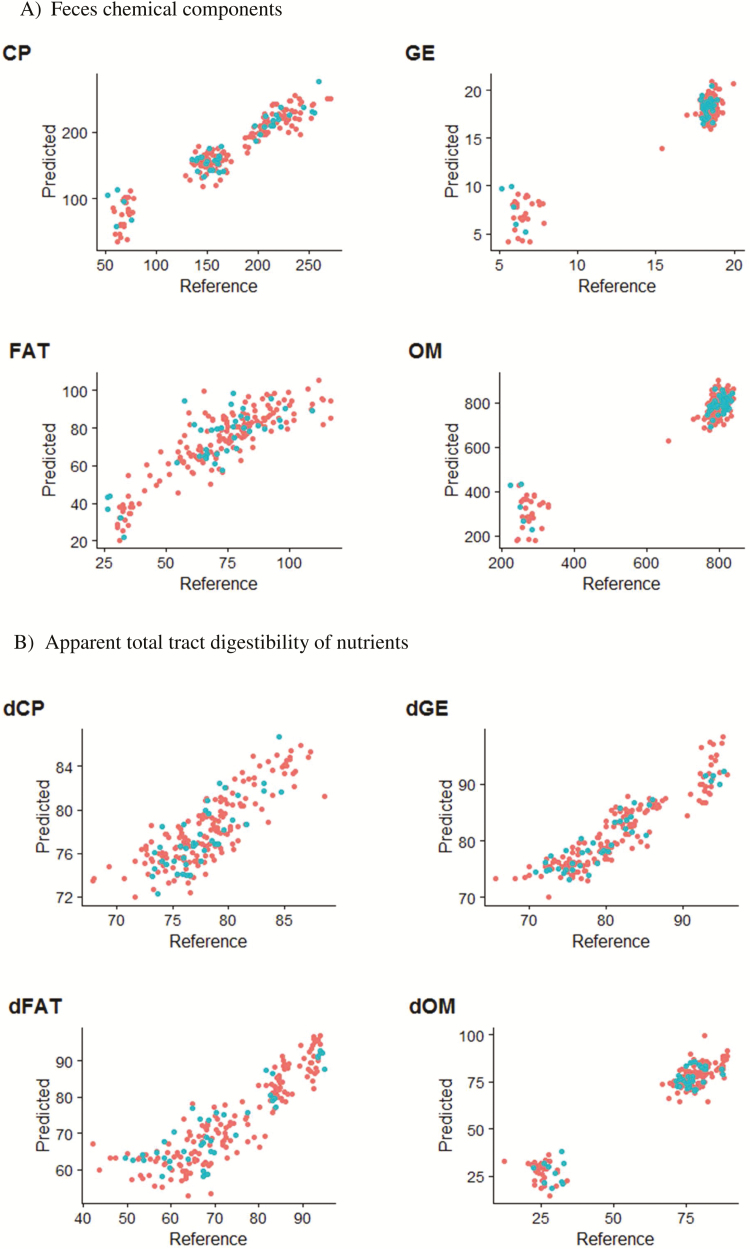
For the second validation, the statistical parameters such as SEP, bias, and the regression coefficients were between the reference, and predicted values are presented in Table 6 for the feces chemical components and for the ATTD of nutrients. Figure 1 shows also that prediction for a completely external dataset (40 samples) for both the feces chemical components and ATTD of nutrients.
Figure 1.
Fecal near-infrared spectroscopy calibration for (A) feces chemical components and (B) apparent total-tract digestibility of OM (dOM), GE (dGE), FAT (dFAT), and CP (dCP). The red circles show for the calibration dataset (158 samples) and green circles show for the external-validation dataset (40 samples).
For both chemical components and ATTD of nutrients (Table 6), the bias was very low but higher than reported in another study (Bastianelli et al., 2015). Apart from FAT, the slope was always close to 1 for all chemical components. Similar to the feces chemical components there was a higher slope for the ATTD of nutrients which were not different from one.
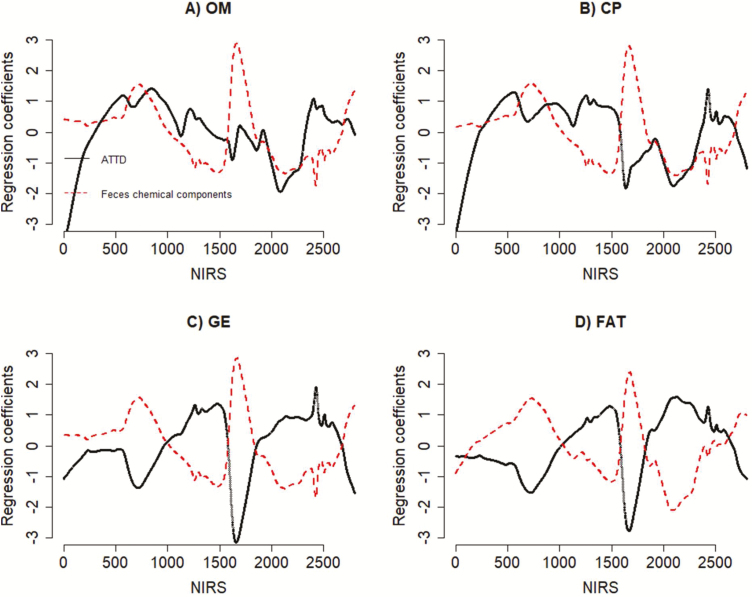
Figure 2 shows partial least-squares regression coefficients for feces chemical components and ATTD of nutrients. For simplicity, the partial least-squares regression was scaled to mean zero and SD equal to 1. The results show that similar trend in regression coefficients were observed for both feces chemical components and ATTD of nutrients. The trend of regression coefficients for feces chemical components and dOM was in the same direction. The trend of regression coefficients of CP, GE, and FAT for ATTD of nutrients and feces chemical composition calibrations was in a different direction.
Figure 2.
Scaled partial least-squares regression coefficients for feces chemical components and apparent total-tract diet digestibility (ATTD) of (A) OM, (B) CP, (C) GE, and (D) Fat.
DISCUSSION
The results of this study show that it is possible to successfully predict the characteristics of feces and ATTD of nutrients using FNIRS. This is because the FNIRS contain information on diet characteristics and ATTD of nutrients even though the diet has been transformed as it passes through the gastrointestinal tract of the animal. It should be noted, however, prediction of diet digestibility or diet and feces characteristics using FNIRS is not novel. Similar findings were observed in many livestock species: in cattle (Robert and Stuth, 1992; Dixon and Coates, 2009;2010), small ruminants (Leite and Stuth, 1995; Landau et al., 2008; Mahipala et al., 2010), donkeys (Kidane et al., 2008), rabbits (Núñez-Sánchez et al., 2012), poultry (Bastianelli et al., 2007). However, the application of FNIRS to predict feces chemical composition and ATTD of nutrients in pigs is novel and few studies have been reported. As per our knowledge, there are only two reports in the literature (Bastianelli et al., 2015; Schiborra et al., 2015). An acceptable accuracy of prediction was reported for ATTD contents of DM, GE, and nitrogen using FNIRS from a digestibility trial conducted using 20 male growing pigs with a variable genetic background fed on the same diet (Bastianelli et al., 2015). The robustness of the calibration equation may be questioned in that study because the calibration datasets were less variable as compared to the results of our study. More recently, the potential of FNIRS in predicting ration composition, ATTD of nutrients and fecal composition was assessed using data that originate from five different experimental trials conducted on different climatic condition and fed 36 different diets (Schiborra et al., 2015). Despite the differences in climatic conditions (Temperate vs. Tropic) which could have an effect on the feed intake of the animals (Renaudeau et al., 2011), FNIRS reported to successfully predict ration composition, diet digestibility, and fecal composition for OM, CP, and fiber fractions.
Using our calibration dataset (158 samples), we observed a higher accuracy of prediction for a completely external dataset (40 samples). The higher accuracy of prediction achieved for the external datasets were because of a robust calibration datasets achieved. Our calibration datasets were obtained after mixing datasets from each of the four experimental trials which were variable in terms of genetic background, diets, and age groups. A robust calibration equation ensures to accurately predict a fully external dataset, and a robust calibration is achieved when the calibration dataset is more variable. This is because a wide range of reference samples is essential for the development of accurate and robust prediction in FNIRS analysis (Zijlstra et al., 2011). Similar findings were observed in growing pigs using a less variable calibration dataset (Schiborra et al., 2015). To our knowledge, this is the first time where a satisfactory accuracy of prediction was obtained using a highly variable calibration dataset. However, these results should be taken with caution because a higher SEP was observed in our study compared to previous findings (Bastianelli et al., 2015). We believe that the relatively higher SEP was observed due to the higher variability of the calibration dataset. It is reasonable to obtain a higher SEP when the calibration datasets are variable or fewer (Valdes and Leeson, 1992; Zijlstra et al., 2011). Therefore, the variability of the calibration dataset could justify for the relatively higher SEP observed in our study. In such cases, it is a common practice to either conduct a pretreatment analysis on the FNIRS; increase the size of calibration dataset; and/or use a combination of feces and diet spectra for calibration to minimize the SEC and improve r2C and r2CV (Decruyenaere et al., 2009). Contrarily, using feces and diet spectra in the calibration dataset did not minimize the SEC (Meineri, 2009). Similarity, our study did not see improvement in the r2C and r2CV using diet and feces spectra in the calibration dataset (results not shown). We conducted different mathematical pretreatment procedures on the FNIRS prior to developing the final calibration equation to determine if calibration equations were more accurate with any of the pretreatments. It was found out that none of the mathematical pretreatments tried increased the r2C and r2CV as compared to another study where a significant improvement in the r2C and r2CV was reported (Bastianelli et al., 2015; Mehtiö et al., 2016). The only way our prediction would have been more precise is if we have had a larger calibration dataset.
In general, a relatively higher to moderate prediction using FNIRS was obtained for nutrient composition and ATTD of nutrients. The advantage of FNIRS technology is that it enables one to measure the chemical and physical properties of diet and feces samples from the analysis of the spectrometer. Obtaining spectral information of the samples only takes a few seconds, which allows large-scale phenotyping at a farm level. Afterward, the fecal near-infrared spectra information can relate to both the chemical composition of feces and ATTD of nutrients. Therefore, once a robust calibration equation is established, only fecal samples are required to assess ATTD of nutrients. This implies a large-scale digestibility prediction using FNIRS could be used to get estimated breeding value and genetic parameters to study the genetics of digestibility in pig-breeding programs. The smallest r2C was 0.63 in our prediction. If we assume the heritability of digestibility is more than 0.3 then approximately 0.18 of the total variation of diet digestibility would be explained by the heritability of the trait. It is obvious that the explained total variance of digestibility by the genetics of the animal would be quite small. However, this would be an acceptable accuracy of prediction given that ATTD of nutrients can be measured on a large number of animals and large-scale measuring of ATTD of nutrients can be done in the field.
Another interesting observation from the present study is that FNIRS relates to the underlying biological components of nutrients when calibrating both feces chemical components and ATTD of nutrients. This is because the trend in partial least square regression coefficients and factor loadings was similar for both feces chemical components and for ATTD of nutrients. This would mean that the FNIRS prediction of feces chemical components and the ATTD of nutrients focuses on the same underlying biological components. Apart from OM, the trend in regression coefficients for the ATTD of nutrients was reversed when calibrating feces chemical components. This is because ATTD of nutrients is calculated as deviations of feces chemical components from the diet chemical components and then weighted by the DM of feed intake. The FNIRS phenotypes are completely dependent on phenotypic variations which implied to the regression coefficients being reversed. In addition, the loadings in partial least regression are not sign dependent. The partial least-squares loadings are considered the same as far as there is a similar trend in both cases irrespective of the direction.
Implications
It is often argued that diet digestibility is influenced by the interaction of feed and genetics of the animal implying that both the characteristics of the diet and genetic potential of the animal contribute to variation in digestibility. There is genetic variability to fiber digestibility (Len et al., 2009a, 2009b; Urriola and Stein, 2012) and medium to higher heritability were reported (Mignon-Grasteau et al., 2004, 2010). Therefore, one can use digestibility to genetically improve FE and robustness. Despite this, there was no emphasis on diet digestibility in FE studies. The common measures of FE include feed conversion ratio (FCR) and residual feed intake (RFI). This could be due to the fact that measuring digestibility at a farm level is very challenging and most digestibility reports have been from an experimental trial point of view. In an experimental trail, ATTD of nutrients is often quantified by providing internal or external markers with the diet, and ATTD is the proportion of chemical components that are not excreted as a form of feces. As observed from the results of this study, the use of FNIRS would make it possible to measure animal-specific digestibility at a farm level with a low cost and technical ease. Therefore, it is possible to use diet digestibility as an alternative measure of FE and robustness in large-scale breeding programs separately or in combination with the existing measures of FE.
Predicted digestibility using FNIRS can be used to measure robustness in pig-breeding programs. This is because most measures of FE, as in the case of FC, consider the digestible nutrients in the feed that is turned into products without taking into consideration the nutrient requirement of the animal into other body functions. However, digestibility can better explain how much the digestible nutrients in the feed are available for all the biological activities of the animal not just for production and maintenance. If required, one can further model the total digestible nutrients into different body functions following the body condition of the animal, physiology, and objective of the breeding program. Therefore, the use of diet digestibility may be a robust alternative to improve FE compared to FCR or RFI. Conversely, digestibility alone might not determine the overall FE of the animal. The overall FE of an animal was associated to feed intake, digestibility, and the metabolic use of the absorbed energy into products (Carré et al., 2008). Therefore, a better measure of FE should be derived which accounts the overall nutrient requirement of the animal.
CONCLUSION
This study demonstrated a cost-effective method using FNIRS to measure ATTD of nutrients which would be useful for genetic studies and breeding value prediction. This is a promising tool specifically for large-scale evaluation of digestibility and chemical composition of diets in pig-breeding programs where controlled digestibility estimation is not possible. Our results were based on a smaller and variable experimental dataset which showed that it is possible to accurately predict ATTD of nutrients using FNIRS. For a commercial application, however, a larger dataset may be required to validate the results of this study and to obtain a more robust calibration dataset. With the present results, it is impossible to say if the predicted ATTD of nutrients is diet specific or animal specific. It is important to further model the FNIRS-predicted digestibility phenotypes to exploit the genetic differences of ATTD of nutrients for each animal. When predicting ATTD of nutrients using FNIRS, it is important to use the ATTD coefficients. It is not necessary to first predict the diet and feces chemical components using FNIRS then calculate the ATTD of nutrients because the FNIRS contain information of the underlying biological components of the ATTD of each nutrient of the diet. The use of diet digestibility as a measure of FE would be used to avoid long-term consequences of FE and robustness as compared to the other measure of FE.
Conflict of interest statement. None declared.
Footnotes
We thank D.-K. Forberg, S. Herlofsen Nes, M. Henne, and L. Andreassen from the pig research facilities and the laboratory staff at NMBU, especially R. Ånestad, for their practical and technical help. This research was financially supported by Feed Mileage-Efficient use of Feed Resources for a sustainable Norwegian Food Production (The Research Council of Norway, OSLO, Norway; grant no. 233685/E50), and Foods of Norway, Center for Research-based Innovation (The Research Council of Norway; grant no. 237841/030).
LITERATURE CITED
- Arthur J. P. F., and Herd R. M.. 2008. Residual feed intake in beef cattle. R. Bras. Zootec. 37:269–279. doi:10.1590/S1516-35982008001300031 [Google Scholar]
- Austreng E., Storebakken T., Thomassen M. S., Refstie S., and Thomassen Y.. 2000. Evaluation of selected trivalent metal oxides as inert markers used to estimate apparent digestibility in salmonids. Aquaculture 188:65–78. doi:10.1016/S0044-8486(00)00336-7 [Google Scholar]
- Bastianelli D., Bonnal L., Jaguelin-Peyraud Y., and Noblet J.. 2015. Predicting feed digestibility from NIRS analysis of pig faeces. Animal 9:781–786. doi:10.1017/S1751731114003097 [DOI] [PubMed] [Google Scholar]
- Bastianelli D., Carré B., Mignon-Grasteau S., Bonnal L., Davrieux F. 2007. Direct prediction of energy digestibility from poultry feces using near infrared spectroscopy. In: Burling-Claridge, G. R., S. E. Holroyd, R. M. W. Sumner, editors. Near infrared spectroscopy. Proc. 12th Int. Conf.; Auckland, New Zealand; April 9–15th, 2005. Chichester: IM Publications; p. 626–629. ISBN: 978-0-473-11646-0|978-0-473-11746-7
- Carré B., Mignon-Grasteau S., and Juin H.. 2008. Breeding for feed efficiency and adaptation to feed in poultry. Worlds Poult. Sci. J. 64:377–390. doi:10.1017/S004393390800010X [Google Scholar]
- Decruyenaere V., Lecomte P., Demarquilly C., Aufrere J., Dardenne P., Stilmant D., and Buldgen A.. 2009. Evaluation of green forage intake and digestibility in ruminants using near infrared reflectance spectroscopy (NIRS): developing a global calibration. Anim. Feed Sci. Technol. 148:138–156. doi:10.1016/j.anifeedsci.2008.03.007 [Google Scholar]
- Dixon R., and Coates D.. 2009. Review: near infrared spectroscopy of feces to evaluate the nutrition and physiology of herbivores. J. Near Infrared Spec. 17:1–31. doi:10.1255/jnirs.822 [Google Scholar]
- Dixon R. M., and Coates D. B.. 2010. Diet quality estimated with fecal near infrared reflectance spectroscopy and responses to N supplementation by cattle grazing Buffel grass pastures. Anim. Feed Sci. Technol. 158:115–125. doi:10.1016/j.anifeedsci.2010.04.002 [Google Scholar]
- European Commission 1971a. European Commission Commission Directive 71/250/EEC of 15 June 1971 establishing community methods of analysis for the official control of feeding-stuffs. Off. J. Eur. Comm. L. 155:13–37. [Google Scholar]
- European Commission 1971b. European Commission Commission Directive 71/393/EEC of 18 November 1971 establishing community methods of analysis for the official control of feeding stuffs. Off. J. Eur. Comm. L. 279:7–18. [Google Scholar]
- European Commission 1993. European Commission Commission Directive 93/28/EEC of 4 June 1993 amending annex I to the third directive 72/199/EEC establishing community methods of analysis for the official control of feeding stuffs. Off. J. Eur. Comm. L. 179:8–10. [Google Scholar]
- European Feed Manufacturers’ Federation (FEFAC) 2015. Feed and food statistical yearbook. FEFAC, Brussels, Belgium. [Google Scholar]
- Godfray H. C. J., Beddington J. R., Crute I. R., Haddad L., Lawrence D., Muir J. F., Pretty J., Robinson S., Thomas S. M., and Toulmin C.. 2010. Food security: the challenge of feeding 9 billion people. Science 327:812–818. doi:10.1126/science.1185383 [DOI] [PubMed] [Google Scholar]
- Hayes B. J., Lewin H. A., and Goddard M. E.. 2013. The future of livestock breeding: genomic selection for efficiency, reduced emissions intensity, and adaptation. Trends Genet. 29:206–214. doi:10.1016/j.tig.2012.11.009 [DOI] [PubMed] [Google Scholar]
- Kidane N. F., Stuth J. W., and Tolleson D. R.. 2008. Predicting diet quality of donkeys via fecal-NIRS calibrations. Rangeland Ecol. Manag. 61:232–239. doi:10.2111/05-193.1 [Google Scholar]
- Landau S., Giger-Reverdin S., Rapetti L., Dvash L., Dorléans M., and Ungar E. D.. 2008. Data mining old digestibility trials for nutritional monitoring in confined goats with aids of Fecal near infra-red spectrometry. Small Rumin. Res. 77:146–158. doi:10.1016/j.smallrumres.2008.03.010 [Google Scholar]
- Landero J. L., Beltranena E., Cervantes M., Morales A., and Zijlstra R. T.. 2011. The effect of feeding solvent-extracted canola meal on growth performance and diet nutrient digestibility in weaned pigs. Anim. Feed Sci. Technol. 170:136–140. doi:10.1016/j.anifeedsci.2011.08.003 [Google Scholar]
- Leite E. R., and Stuth J. W.. 1995. Fecal NIRS equations to assess diet quality of free-ranging goats. Small Rumin. Res. 15:223–230. doi:10.1016/0921-4488(94)00026-4 [Google Scholar]
- Len N. T., Hong T. T., Ogle B., and Lindberg J. E.. 2009a. Comparison of total tract digestibility, development of visceral organs and digestive tract of Mong Cai and Yorkshire x Landrace piglets fed diets with different fibre sources. J. Anim. Physiol. Anim. Nutr. (Berl). 93:181–191. doi:10.1111/j.1439-0396.2007.00804.x [DOI] [PubMed] [Google Scholar]
- Len N. T., Ngoc T. B., Ogle B., and Lindberg J. E.. 2009b. Ileal and total tract digestibility in local (Mong Cai) and exotic (Landrace×Yorkshire) piglets fed low and high-fibre diets, with or without enzyme supplementation. Livest. Sci. 126:73–79. doi:10.1016/j.livsci.2009.06.002 [Google Scholar]
- Mahipala M. B. P. K., Krebs G. L., McCafferty P., Naumovski T., Dods K., and Stephens R.. 2010. Predicting the quality of browse-containing diets fed to sheep using fecal near-infrared reflectance spectroscopy. Anim. Prod. Sci. 50:925–930. [Google Scholar]
- Maynard L. A., and Loosli J. K.. 1969. Animal nutrition. 6th ed McGraw-Hill, New York, NY. [Google Scholar]
- McDonald P. E. R., Greenhalgh J. F. D., and Morgan C. A.. 1998. Animal nutrition. 5th ed Longman Group, Edimburgh, United Kingdom. [Google Scholar]
- Mehtiö T., Rinne M., Nyholm L., Mäntysaari P., Sairanen A., Mäntysaari E. A., Pitkänen T., and Lidauer M. H.. 2016. Cow-specific diet digestibility predictions based on near-infrared reflectance spectroscopy scans of faecal samples. J. Anim. Breed. Genet. 133:115–125. doi:10.1111/jbg.12183 [DOI] [PubMed] [Google Scholar]
- Meineri G., Peiretti P. G., and Masoero G.. 2009. Appraisal of ingestion and digestibility in growing rabbits using near infrared reflectance spectroscopy (NIRS) of feeds and feces. Ital. J. Anim. Sci. 8:75–82. doi:10.4081/ijas.2009.75 [Google Scholar]
- Mejicanos G., Sanjayan N., Kim I. H., and Nyachoti C. M.. 2016. Recent advances in canola meal utilization in swine nutrition. J. Anim. Sci. Technol. 58:7. doi:10.1186/s40781-016-0085-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignon-Grasteau S., Juin H., Sellier N., Bastianelli D., Gomez J., and Carré B.. 2010. Genetic parameters of digestibility of wheat- or corn-based diets in chickens. In: Proc. 9th World Congr. Genet. Appl. to Livest. Prod; Leipzig, Germany : Gesellschaft für Tierzuchtwissenschaften e.V p. 55. [Google Scholar]
- Mignon-Grasteau S., Muley N., Bastianelli D., Gomez J., Péron A., Sellier N., Millet N., Besnard J., Hallouis J. M., and Carré B.. 2004. Heritability of digestibilities and divergent selection for digestion ability in growing chicks fed a wheat diet. Poult. Sci. 83:860–867. doi:10.1093/ps/83.6.860 [DOI] [PubMed] [Google Scholar]
- Naes T., Isaksson T., Fearn T., and Davies T.. 2002. Validation: a user-friendly guide to multivariate calibration and classification. NIR publications, Charlton, Chichester, United Kingdom. [Google Scholar]
- Núñez-Sánchez N., Martínez Marín A. L., Hernández M. P., Carrion D., Castro G. G., and Pérez Alba L. M.. 2012. Fecal near infrared spectroscopy (NIRS) as a tool to assess rabbit’s feed digestibility. Livest. Sci. 150:386–390. doi:10.1016/j.livsci.2012.07.030 [Google Scholar]
- Pérez de Nanclares M., Trudeau M. P., Hansen J. Ø., Mydland L. T., Urriola P. E., Shurson G. C., Piercey Åkesson C., Kjos N. P., Arntzen M. Ø., and Øverland M.. 2017. High-fiber rapeseed co-product diet for Norwegian Landrace pigs: effect on digestibility. Livest. Sci. 203:1–9. doi:10.1016/j.livsci.2017.06.008 [Google Scholar]
- Renaudeau D., Gourdine J. L., and St-Pierre N. R.. 2011. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 89:2220–2230. doi:10.2527/jas.2010-3329 [DOI] [PubMed] [Google Scholar]
- Robert K. L., and Stuth J. W.. 1992. Fecal NIRS equations for predicting diet quality of free-ranging cattle. J. Range Manag. 45:238–244. doi:10.2307/4002970 [Google Scholar]
- Savitzky A., and Golay M. J. E.. 1964. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 36:1627–1639. doi:10.1021/ac60214a047 [Google Scholar]
- Schiborra A., Bulang M., Berk A., Susenbeth A., and Schlecht E.. 2015. Using fecal near-infrared spectroscopy (FNIRS) to estimate nutrient digestibility and chemical composition of diets and feces of growing pigs. Anim. Feed Sci. Technol. 210:234–242. doi:10.1016/j.anifeedsci.2015.10.011 [Google Scholar]
- Seneviratne R. W., Beltranena E., Goonewardene L. A., and Zijlstra R. T.. 2011. Effect of crude glycerol combined with solvent-extracted or expeller-pressed canola meal on growth performance and diet nutrient digestibility of weaned pigs. Anim. Feed Sci. Technol. 170:105–110. doi:10.1016/j.anifeedsci.2011.07.009 [Google Scholar]
- Torres-Pitarch A., Moset V., Ferrer P., Cambra-López M., Hernández P., Coma J., Pascual M., Serrano P., and Cerisuelo A.. 2014. The inclusion of rapeseed meal in fattening pig diets, as a partial replacer of soybean meal, alters nutrient digestion, fecal composition and biochemical methane potential from feces. Anim. Feed Sci. Technol. 198:215–223. doi:10.1016/j.anifeedsci.2014.09.017 [Google Scholar]
- Urriola P. E., and Stein H. H.. 2012. Comparative digestibility of energy and nutrients in fibrous feed ingredients fed to Meishan and Yorkshire pigs. J. Anim. Sci. 90:802–812. doi:10.2527/jas.2010-3254 [DOI] [PubMed] [Google Scholar]
- Valdes E. V., and Leeson S.. 1992. Research note: the use of near infrared reflectance spectroscopy to measure metabolizable energy in poultry feed ingredients. Poult. Sci. 71:1559–1563. doi:10.3382/ps.0711559 [DOI] [PubMed] [Google Scholar]
- Wold S., Ruhe A., Wold H., and Dunn I. W. J.. 1984. The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. SIAM J. Sci. Comput. 5:735–743. doi:10.1137/0905052 [Google Scholar]
- Zijlstra R. T., Swift M. L., Wang L. F., Scott T. A., and Edney M. J.. 2011. Short Communication: near infrared reflectance spectroscopy accurately predicts the digestible energy content of barley for pigs. Can. J. Anim. Sci. 91:301–304. doi:10.4141/cjas10063 [Google Scholar]