Abstract
The effects of feeding different dietary fat sources with modified distillers grains plus solubles (MDGS) on beef display life were evaluated. Steers (n = 256) were fed for 134 d on either a corn, 40% full-fat MDGS, 40% de-oiled MDGS, or 38% de-oiled MDGS plus 2% corn oil diet. Twenty-four United States Department of Agriculture Choice carcasses (3 head/pen) were randomly selected within each dietary treatment and strip loins were collected and aged for 2, 9, 16, or 23 d. Steaks from each aging period were placed under retail display (RD) conditions for 0, 4, and 7 d. Stearic acid was predominant (C18:0; P = 0.03) in beef from the de-oiled MDGS plus oil treatment in comparison with all other dietary treatments. Feeding MDGS increased linoleic acid (C18:2; P < 0.01) and polyunsaturated fatty acids (PUFA; P = 0.01) in comparison to the corn diet. The de-oiled MDGS plus oil group had greater C18:3 content (P = 0.03) when compared to corn, but no differences were observed between all other diets. There were no differences among dietary treatments for L* (P = 0.74) and b* (P = 0.25) values. The de-oiled MDGS group had lower a* values than all other treatments (P < 0.01) at day 5 of RD. The corn treatment had greater a* values (P ≤ 0.05) than de-oiled MDGS and de-oiled MDGS plus oil at day 6 and 7 of RD. Strip loin steaks from cattle fed full-fat MDGS tended to have lower a* values (P = 0.10) than steaks from cattle fed corn at day 7 of RD. Feeding de-oiled MDGS resulted in greater discoloration (P ≤ 0.05) at days 5, 6, and 7 of RD when compared to corn. Steaks from the de-oiled MDGS plus oil and full-fat MDGS groups had greater discoloration scores at day 7 of RD in comparison to corn (P ≤ 0.05). Strip loin steaks from cattle fed corn tended to have lower thiobarbituric acid reactive substance (TBARS) values (P ≤ 0.10) in comparison to de-oiled MDGS and de-oiled MDGS plus oil at day 7 of RD. Results suggest that feeding MDGS to cattle reduces color and lipid stability in addition to increasing C18:2 and PUFA content of beef. Addition of corn oil to de-oiled MDGS decreased redness and increased discoloration and lipid oxidation in comparison to corn diets.
Keywords: beef quality, distillers grains, fatty acid composition, oxidation, shelf life
INTRODUCTION
Over the last 10 years, a significant increase in ethanol production has occurred in the United States, from 6.49 billion gallons in 2006 to 14.80 billion in 2015 (Renewable Fuels Association, 2017). Hence, greater amounts of distillers grains have been available for cattle feeding. Interestingly, even after the fermentation of corn for ethanol production, which removes starch, distillers grains have more energy per kilogram on a dry matter basis than regular corn due to increased protein from about 10% to 30% and fat from 4% to 12% (Klopfenstein et al., 2008). Research has shown that feeding distillers grains to cattle increases the concentration of polyunsaturated fatty acids (PUFA) in meat (Mello et al., 2012a,b). It is well established that beef with higher concentrations of PUFA is more likely to have increased lipid and myoglobin oxidation. This is important because lipid and myoglobin oxidation lead to off-flavor development and discoloration of retail-displayed beef, reducing display life (Faustman et al., 2010). The ethanol industry appears to have evolved in the removal of oils from distillers grains. According to Jolly et al. (2013), approximately 50% of the ethanol plants removed oil from distillers grains in 2012, reducing the amount of energy per kilogram on a dry matter basis. There is an interest in adding the oil back to cattle diets when economically feasible. It is unknown if adding corn oil is equivalent to feeding full-fat or de-oiled distillers grains. A deeper understanding of the dietary fat changes in corn byproducts could help improve beef shelf-life and the way animals are fed. Research was conducted to determine the effect on strip loin steak display life as a result of feeding de-oiled modified distillers grains plus solubles (MDGS) with corn oil added back.
MATERIALS AND METHODS
All procedures related to live animals for this study were approved by the Institutional Animal Care and Use Committee of the University of Nebraska-Lincoln.
Cattle and Dietary Treatments
Initially, 256 Angus crossbreed finishing steers (initial BW = 412 kg, SD = 24) were fed for 134 d on either a corn, 40% full-fat MDGS, 40% de-oiled MDGS, or 38% de-oiled MDGS plus 2% corn oil diet. Cattle were blocked by body weight and randomly assigned 10 per pen for a total of 32 pens (8 pens per treatment). All dietary treatments are presented in Table 1. The fat content of the diets was 3.47, 4.87, 6.71, and 6.33% (for corn, 40% de-oiled MDGS, 38% de-oiled MDGS plus 2% corn oil diet, and 40% full-fat MDGS.
Table 1.
Diet composition (% of DM basis) fed to finishing steers receiving either a corn diet, 40% Full-fat MDGS, 40% De-oiled MDGS, or 38% De-oiled MDGS plus corn oil
| Feed ingredient | Dietary treatment | |||
|---|---|---|---|---|
| Ingredient, % of DM | Corn | 40% MDGS Full-fat | 40% MDGS De-oiled | 38% MDGS Plus Oil |
| DRC1 | 42.75 | 24.00 | 24.00 | 24.00 |
| HMC1 | 42.75 | 24.00 | 24.00 | 24.00 |
| MDGS De-oiled1 | - | - | 40.00 | 38.00 |
| MDGS Full-fat1 | - | 40.00 | - | - |
| Corn Oil | - | - | - | 2.0 |
| Alfalfa | 3.0 | 3.0 | 3.0 | 3.0 |
| Sorghum Silage | 4.0 | 4.0 | 4.0 | 4.0 |
| Supplement2 | 6.165 | 3.66 | 3.66 | 3.66 |
| Tallow | 0.125 | 0.125 | 0.125 | 0.125 |
| FGC | 1.20 | 1.20 | 1.20 | 1.20 |
1DM = dry matter; DRC = dry rolled corn; FGC = fine ground corn; HMC = high moisture corn; MDGS = modified distillers grains plus solubles.
2Formulated to contain 383 mg per head per day of Rumensin and 90 mg per head per day of Tylan.
Sample Collection and Fabrication
At harvest (Greater Omaha Packing, Omaha, NE), 24 United States Department of Agriculture Choice carcasses (3 head per pen) were randomly selected within each treatment (n = 96) and strip loins (Longissimus lumborum) from both sides were collected. Loins were transferred to the University of Nebraska Meat Laboratory. Loins were fabricated anterior to posterior where a 1.27 cm steak was removed at both anterior and posterior ends to remove surfaces with outer exposure. Then, both left and right strip loins were divided in half, and each of the 4 sections per animal were randomly assigned to 1 of the 4 aging periods (2, 9, 16, or 23 d). Loins sections assigned for 2 d of aging were immediately trimmed of subcutaneous fat, and fabricated into 3 steaks (2.54 cm thickness) for proximate composition, fatty acid profile, objective color, visual discoloration, and lipid oxidation [1 steak for objective color and discoloration, 1 steak was split in half for fatty acid profile, proximate composition and lipid oxidation for 0 d retail display (RD), 1 steak was split in half for 4 and 7 d RD lipid oxidation]. At day 2, steaks used for fatty acid profile and proximate composition were vacuum packaged and frozen (−80°C) until further analysis.
After fabrication, steaks used for color analysis and lipid oxidation were placed on foam trays (21.6 × 15.9 × 2.1 cm, Styro-Tech, Denver, CO), overwrapped with oxygen permeable film (Prime Source PSM 18 #75003815, Bunzl Processors Division, North Kansas City, MO; oxygen transmission rate = 2.25 mL/cm2/24 h at 23°C and 0% relative humidity; water vapor transfer rate = 496 g/m2/24 h at 37.8°C and 90% relative humidity) and placed under RD conditions for 7 d (under white fluorescence lighting at 1,000 to 1,800 lux) at 3°C. After RD, samples were frozen in liquid nitrogen, powdered in a metal cup blender (Model 51BL32, Waring Commercial, Torrington, CT) and stored at −80°C until further analysis. The same fabrication scheme was used at 9, 16, and 23 d postmortem, with the exception of proximate composition and fatty acid profile, which were analyzed only at day 2 postmortem.
Proximate Composition
Moisture and ash (%) were quantified with a LECO Thermogravimetric Analyzer in duplicate (Model 604-100-400, LECO Corporation, St. Joseph, MI). Fat content was quantified in triplicate by ether extraction according to the Soxhlet procedure (AOAC, 1990). Protein content was calculated by difference.
Fatty Acid Analysis
Total lipid was extracted following the procedure described by Folch et al. (1957). After extraction, lipids were converted to fatty acid methyl esters according to Morrison and Smith (1964) and Metcalfe et al. (1966). Strip loins samples free of subcutaneous fat were frozen in liquid nitrogen and then powdered in a metal cup blender (Model 51BL32, Waring Commercial, Torrington, CT). Powdered samples were stored at −80°C. Later, 1 g of powered sample was homogenized with 5 mL of 2:1 chloroform:methanol. After 1 h in room temperature (23°C), samples were filtered through filter paper (Whatman #2). The final volume was brought up to 10 mL with 2:1 chloroform:methanol and then vortexed for 5 s with 2 mL of 0.74% KCl. Samples were centrifuged (1,000 × g for 5 min) and the top layer phase was aspirated off. After centrifugation, samples were dried on a heating block at 60°C under nitrogen purge. After drying, 0.5 mL of 0.5 M NaOH in methanol was added to the samples, vortexed and heated for 5 min at 100°C. Subsequently, 0.5 mL of boron trifluoride in 14% methanol was mixed and reheated for 5 min at 100°C. Then, samples were homogenized with 1 mL of saturated NaCl solution and 1 mL of hexane and centrifuged at 1,000 × g for 5 min. The top hexane layer was removed and analyzed using gas chromatography (Hewlett-Packard 6890 FID GC System; Agilent Technologies, Santa Clara, CA). Fatty acids were separated using a Chrompack CP-Sil 88 capillary column (0.25 mm by 100 m; injector temperature: 270°C detector temperature: 300°C; pressure: 40 psi; flow rate: 1.0 mL/min) and identified by their retention times in relation to known commercial standards (NU-Check Prep, Inc., Elysian, MN; # GLC-68D, GLC-79, GLC-87, GLC-455, and GLC-458). The percentage of fatty acids were determined by the peak areas in the chromatograph. Then, values were adjusted according to percent fat and converted to mg/100 g tissue by the following equation:
Instrumental Color Evaluation
Objective color measurements were taken using the L*, a*, b* scales with a Minolta CR-400 colorimeter (Illuminant D65, 8 mm diameter aperture, 2° standard observer angle; Minolta, Osaka, Japan). Six measurements from different areas of the steak surface were taken through the overwrap film once daily at a standardized time from day 0 to 7 of RD.
Subjective Color (Discoloration)
Trained visual color panelists (n = 5) evaluated surface discoloration from day 0 to 7 of RD once daily according to the procedure of Senaratne-Lenagala (2012). Ten steak images ranging from 0% to 100% surface discoloration with increments of 10% were used as a reference for panelist training. Panelists evaluated surface discoloration at 24-h intervals using a percentage scale where 0% meant no discoloration and 100% meant complete surface discoloration. Steaks were randomly rotated daily to minimize any possible location effects within the display.
Lipid Oxidation (TBARS)
Thiobarbituric acid reactive substance (TBARS) values were measured for each aging period at 0, 4, and 7 d of RD according to the procedure of Ahn et al. (1998). From each steak, 5 g of sample was blended with 1 mL of butylated hydroxyanisole (BHA) solution (10%) and 14 mL of distilled water. Samples were homogenized using a Polytron (Kinmatica AG, Lucern, Sui) for 15 s and centrifuged (2,000 × g for 5 min). One milliliter of supernatant was mixed with 2 mL of thiobarbituric acid/trichloroacetic acid (TBA/TCA) solution (15% TCA and 20 mM TBA in deionized distiller water) and placed in a water bath at 70°C for 30 min. Samples were cooled in a water bath at 20°C for 10 min and centrifuged (2,000 × g for 5 min). After centrifugation, duplicate 200 µL of supernatant were transferred to 96-well plates and the absorbance at 540 nm was measured using a microplate spectrophotometer (Model Epoch, Biotek, Winooski, VT). The results were expressed in milligrams of malonaldehyde per kilogram of tissue.
Statistical Analysis
Objective and subjective color data were analyzed as a split-split-plot repeated measures design with dietary fat source as the whole-plot, aging period as the split-plot and RD time as the repeated measures. The PROC MIXED procedure was used for repeated measures of color measurements and the Toeplitz covariance structure was selected based on the best fit model. Lipid oxidation data were analyzed as a split-split-plot design with dietary fat source as the whole-plot, aging period as the split-plot and RD time as the split-split-plot. Fatty acid composition was analyzed as a completely randomized design. In this experiment, pen was considered the experimental unit. The mean of the 3 animals collected within each pen was considered as a replication. For proximate analysis, fatty acid profile, objective color, and lipid oxidation, the fixed effects were dietary treatment, aging time, and RD. For subjective discoloration scores, dietary treatment, aging time, and RD were considered the fixed effects whereas panelist was considered a random effect. Data were analyzed using the PROC GLIMMIX procedure of SAS. All means were separated with the LS MEANS and DIFF functions and the TUKEY adjustment was used with α = 0.05. Alpha values between 0.05 and 0.10 were considered as trends.
RESULTS AND DISCUSSION
Proximate Composition
In the present study, dietary treatments did not affect proximate composition of strip loin steaks (P > 0.05). The overall averages for the nutritional constituents were: 71.91% ± 0.33 moisture, 19.77% ± 0.27 protein, 6.96% ± 0.07 fat, and 1.36% ± 0.01 ash. No differences in marbling scores were found among dietary treatments (P = 0.78).
Fatty Acid Profile
Fatty acid values for all dietary treatments are presented in Table 2. Differences (P < 0.05) were found in the amount of stearic acid (C18:0), linoleic acid (C18:2), α-linolenic acid (C18:3), and PUFA among dietary treatments. Stearic acid (C18:0) was highest for strip loin steaks from cattle fed de-oiled MDGS plus oil (P = 0.03) and lowest for steaks from cattle fed corn. Samples from cattle fed full-fat MDGS and de-oiled MDGS were intermediate and did not differ from de-oiled MDGS plus oil or corn samples for C18:0 content. Van der Pol et al. (2009) indicated that cattle receiving diets supplemented with corn oil had greater proportions of C18:0 reaching the duodenum when compared to cattle receiving wet distillers grains plus solubles (WDGS). Therefore, greater amounts of stearic acid would be available to be deposited in muscle. This could explain the greater C18:0 concentration found in beef from cattle fed de-oiled MDGS supplemented with corn oil in this study.
Table 2.
Amount1 of fatty acids from L. lumborum muscle of steers fed either a corn diet, 40% Full-fat MDGS, 40% De-oiled MDGS, or 38% De-oiled MDGS plus corn oil
| Fatty acid | Corn | Full-fat MDGS | De-oiled MDGS | De-oiled MDGS plus oil | P-value |
|---|---|---|---|---|---|
| C14:0 | 251.39 | 242.10 | 254.82 | 287.61 | 0.37 |
| C14:1 | 70.61 | 65.31 | 70.99 | 75.81 | 0.74 |
| C15:0 | 40.46 | 32.71 | 32.23 | 41.27 | 0.13 |
| C15:1 | 115.99 | 134.60 | 87.21 | 87.10 | 0.14 |
| C16:0 | 1,828.88 | 1,715.35 | 1,868.51 | 2,036.37 | 0.14 |
| C16:1 | 236.26 | 220.69 | 242.30 | 250.14 | 0.70 |
| C17:0 | 81.61 | 66.41 | 61.75 | 68.97 | 0.13 |
| C17:1 | 67.11 | 61.92 | 75.92 | 79.04 | 0.42 |
| C18:0 | 866.31b | 959.23ab | 946.25ab | 1,100.08a | 0.03 |
| C18:1T | 10.23 | 22.44 | 23.77 | 23.93 | 0.07 |
| C18:1 | 2,246.03 | 2,239.03 | 2,229.07 | 2,526.08 | 0.22 |
| C18:1V | 135.11 | 138.57 | 122.14 | 158.95 | 0.07 |
| C18:2 | 406.60b | 549.61a | 555.89a | 565.68a | <0.01 |
| C18:3 | 13.59b | 15.99ab | 14.05ab | 17.97a | 0.03 |
| C20:4 | 104.28 | 96.61 | 98.82 | 91.22 | 0.72 |
| Total | 6,636.87 | 6,693.98 | 6,830.95 | 7,649.29 | 0.12 |
| SFA | 3,139.70 | 3,078.80 | 3,242.24 | 3,627.89 | 0.10 |
| UFA | 3,497.17 | 3,615.18 | 3,588.71 | 4,021.40 | 0.16 |
| MUFA | 2,913.52 | 2,885.56 | 2,856.90 | 3,627.89 | 0.18 |
| PUFA | 577.41b | 729.68a | 731.75a | 751.96a | 0.01 |
a,bMeans in the same row with different superscripts differ (P ≤ 0.05).
1Amount of fatty acid expressed in mg/100 g of tissue.
MDGS = modified distillers grains plus soluble; MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; UFA = unsaturated fatty acids.
In our study, feeding MDGS resulted in greater linoleic acid (C18:2) values in strip loins (P < 0.01). The C18:2 content was lowest for strip loin steaks from cattle fed corn (406.60 mg/100 g) in comparison to all MDGS dietary treatments (Table 2), which did not differ from each other (549.61 mg/100 g for the full-fat MDGS, 555.89 mg/100 g for the de-oiled MDGS, and 565.68 mg/100 g for the de-oiled MDGS plus oil treatment).
The α-linolenic (C18:3) content was least for steaks from cattle fed corn (13.59 mg/100 g) and greatest for the de-oiled MDGS plus oil (17.97 mg/100 g). Full-fat MDGS and de-oiled MDGS had intermediate values (15.99 mg/100 g and 14.05 mg/100 g, respectively) and did not differ from either (Table 2; P = 0.98).
There was a dietary effect on PUFA content of meat (P = 0.01; Table 2). Strip loin steaks from cattle fed corn had the lowest amount of PUFA (577.41 mg/100 g) in comparison to all MDGS dietary treatments (729.68 mg/100 g for the full-fat MDGS, 731.75 mg/100 g for the de-oiled MDGS, and 751.96 mg/100 g for the de-oiled MDGS plus oil group).
These results matches the results from previous studies, which also reported increases of C18:2 and PUFA content in the Longissimus muscle when beef cattle were fed finishing diets containing distillers grains compared with those consuming a diet without distillers grains (Mello et al., 2012a,b; Domenech-Pérez et al., 2017). These results suggest that the fatty acid composition of beef is dependent on the dietary fat source. Particularly, the levels of linoleic acid and PUFA in beef have been recognized to be affected by diet.
In ruminant animals, a high proportion of unsaturated fatty acids are biohydrogenated to saturated fatty acids in the rumen, limiting PUFA deposition in muscle (Jenkins et al., 2008). In a review, Wood et al. (2008) indicated that only a small proportion, around 10% of dietary C18:2 is still available for incorporation into tissue after ruminal biohydrogenation. Increased ruminal biohydrogenation of 18-carbon unsaturated fatty acids for diets with higher lipid content was reported by Duckett et al. (2002). In the same study, authors indicated that feeding higher lipid diets increased duodenal flow of palmitic, stearic, oleic, linoleic, and arachidonic acids by more than 30%. Similarly, Vander Pol et al. (2009) also noted greater amount of PUFA reaching the duodenum when cattle were fed WDGS compared with corn.
Even though ruminant diets contains modest level of fat, the final concentration of fat is increased in diets with high amounts of ethanol byproducts (Klopfenstein et al., 2008). According to Ham et al. (1994), wet distillers grains plus solubles has double the amount of PUFA compared to corn. Therefore, the greater PUFA deposition observed in muscular tissue when corn was substituted for distillers grains in this study could be from increased dietary supply of PUFA in cattle diets, and increased intestinal supply of PUFA due to higher lipid content in cattle rations.
Objective Color
For all 3 color scales, age by RD time interactions were detected (P < 0.01). In general, L* values increased and a* and b* values decreased as aging and RD time increased, regardless the dietary fat source.
There were no significant interactions that included L* and b* among dietary treatments. However, a 2-way interaction between RD and dietary treatment was found for a* values (P < 0.01; Table 3). Specifically, lower a* values (P ≤ 0.05) were found for strip loin steaks from cattle fed de-oiled MDGS in comparison to all other dietary treatments at day 5 of RD. Steaks from steers fed corn had greater a* values than steaks from cattle fed de-oiled MDGS and de-oiled MDGS plus oil at day 6 of RD. However, full-fat MDGS did not differ from corn and de-oiled MDGS plus oil group at day 6 of RD. Greater a* values (P ≤ 0.05) were found for strip loin steaks from cattle fed corn in comparison to steaks from cattle fed de-oiled MDGS and de-oiled MDGS plus oil at day 7 of RD. Moreover, strip loin steaks from cattle fed full-fat MDGS tended to have lower a* values (P = 0.10) than steaks from cattle fed corn at day 7 of RD.
Table 3.
Objective redness (a* values) of strip loin steaks (Longissimus lumborum) from steers fed either a corn diet, 40% Full-fat MDGS, 40% De-oiled MDGS, or 38% De-oiled MDGS plus 2% corn oil through 7 d of retail display (SEM = 0.23)
| Days on retail display | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Corn | 21.24 | 21.40 | 20.71 | 20.08 | 19.37 | 18.13a | 16.20a | 13.79a |
| Full-fat MDGS | 21.60 | 21.87 | 21.14 | 20.38 | 19.52 | 18.15a | 15.57ab | 13.05ab |
| De-oiled MDGS | 21.86 | 21.82 | 21.04 | 20.32 | 18.80 | 17.04b | 14.54c | 12.33b |
| De-oiled MDGS +oil | 22.00 | 21.93 | 21.16 | 20.31 | 19.07 | 17.91a | 15.22bc | 12.48b |
| P-value | 0.20 | 0.34 | 0.47 | 0.81 | 0.13 | <0.01 | <0.01 | <0.01 |
a-cMeans in the same column with different superscripts differ (P ≤ 0.05).
MDGS = modified distillers grains plus soluble.
Meat purchasing decisions are mainly influenced by color because consumers use discoloration as an indicator of freshness. Myoglobin is the protein responsible for the bright cherry red color of beef. When myoglobin is oxidized to metmyoglobin, discoloration occurs, which leads consumers to discriminate against such products in the retail market. This results in product being either discounted or discarded (Mancini and Hunt, 2005).
Many factors affect metmyoglobin formation. These include oxygen partial pressure, temperature, pH, mitochondrial oxygen consumption, metmyoglobin reducing activity, and lipid oxidation. Previous studies have reported that lipid oxidation enhances meat discoloration. The mechanisms by which lipid oxidation enhance myoglobin oxidation have been explained due the propagation of oxidation by PUFA (Faustman et al., 2010). This study showed that finishing diets including distillers grains at high rates (40%; DM basis) significantly decreased color stability of strip loins steaks when compared to a corn diet, as other studies have noted (Roeber et al., 2005; Mello et al., 2012a). Therefore, the reduced color stability of beef from cattle supplemented with MDGS is likely due to the propagation of oxidation caused by higher PUFA content observed in these samples. In general, these results suggest that at the onset of RD, MDGS had no effect on a* values. However, as RD progressed, beef from cattle fed MDGS was less red than beef from cattle fed corn.
Discoloration
A 2-way interaction between aging time and RD for discoloration was observed (P < 0.01). At all aging periods, discoloration increased as RD time increased, regardless the dietary fat source. In general, longer aging periods and RD times increased the amount of browning, extent of discoloration, and percentage of the steak surface that was discolored.
A significant decline in purchasing decisions with 20% surface discoloration on RD beef has been reported by Hood and Riordan (1973). In this study, the 20% discoloration threshold for steaks aged for 9 d was met by steaks from the de-oiled MDGS treatment at day 6 of RD, and at day 7 for the de-oiled MDGS plus oil and full fat MDGS treatments. Steaks from animals fed corn aged for 9 d had 14.72% discoloration at day 7 and therefore did not reach the discoloration threshold.
Steaks aged for 16 d met the 20% discoloration threshold at day 6 of RD for all dietary treatments. Steaks aged for 23 d met the discoloration threshold at day 6 of RD for all dietary treatments, except for de-oiled MDGS, which met the discoloration threshold at day 5 of RD.
A 2-way interaction between dietary treatment and RD for discoloration was found (P < 0.01). Surface discoloration scores of strip loin steaks at prolonged RD are presented in Table 4. No differences were found among dietary treatments over the first 4 d of RD. Samples began to diverge on day 5 of RD. Strip loin steaks from cattle fed de-oiled MDGS had greater discoloration (P ≤ 0.05) than steaks from any other dietary treatment at day 5 and 6 of RD. Strip loin steaks from cattle fed full-fat MDGS tended to have more discoloration than steaks from cattle fed corn (P = 0.06) at day 6 of RD.
Table 4.
Discoloration (%) of strip loins steaks (Longissimus lumborum) from steers fed either a corn diet, 40% Full-fat MDGS, 40% De-oiled MDGS, or 38% De-oiled MDGS plus 2% corn oil through 7 d of retail display (SEM = 1.00)
| Days on retail display | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Corn | 0 | 0 | 0 | 0.15 | 1.11 | 5.70b | 31.39b | 49.82c |
| Full-fat MDGS | 0 | 0.05 | 0.26 | 0.76 | 2.06 | 7.13b | 35.85ab | 58.08b |
| De-oiled MDGS | 0 | 0.02 | 0.10 | 0.54 | 4.04 | 14.63a | 41.32a | 65.16a |
| De-oiled MDGS +oil | 0 | 0 | 0.06 | 0.24 | 1.57 | 7.99b | 33.76b | 58.64b |
| P-value | 1.00 | 1.00 | 0.99 | 0.99 | 0.62 | <0.01 | <0.01 | <0.01 |
a-cMeans in the same column with different superscripts differ (P ≤ 0.05).
MDGS = modified distillers grains plus soluble.
At day 7 RD, discoloration scores were least for cattle fed corn, intermediate for cattle fed full-fat MDGS and de-oiled MDGS plus oil, and greatest for the de-oiled MDGS. In agreement with our results, several studies have shown that feeding distillers grains to cattle increases discoloration of retail-displayed beef (Roeber et al., 2005; Kinman et al., 2011; Segers et al., 2011; Mello et al., 2012a; Senaratne-Lenegala, 2012; Chao, 2015). However, a few studies documented contrasting results, showing no detrimental effects of distillers grains when compared to corn diets on subjective discoloration of retail-displayed beef (Gill et al., 2008; Domenech-Perez et al., 2017). Although there is not a complete consensus in the literature, for the most part it seems as though inclusion of distillers grains, especially at greater concentrations, does have detrimental effects on meat color on RD.
One possible explanation for all MDGS treatments having higher discoloration scores in comparison to the corn diet is the increased PUFA content of beef. The effect of fatty acids on color stability is explained by the propensity of PUFA to oxidize (Wood et al., 2008). It is well documented in the literature that beef with higher concentrations of PUFA is more likely to have increased lipid and myoglobin oxidation (Morrissey et al., 1998; Faustman et al., 2010). According to Faustman et al. (2010) lipid oxidation is a major contributor to metmyoglobin formation and fresh meat discoloration. These authors suggested that meat discoloration occurs due to the oxidation of oxymyoglobin to metmyoglobin, this reaction generally proceeding in parallel to that of lipid oxidation. Thus, lipid oxidation products can promote myoglobin oxidation and vice versa. Gray et al. (1996) suggested that the autoxidation of PUFA produces radical species that can act with myoglobin, promoting pigment oxidation. Nute et al. (2007) reported that meat from animals supplemented with diets enriched in PUFA is more susceptible to lipid oxidation and discoloration. Kinman et al. (2011) suggested that color stability of beef from cattle supplemented with WDGS is usually reduced due to the propagation of oxidation caused by high concentration of PUFA in WDGS.
Interestingly, although no MDGS treatments differed in PUFA content, de-oiled MDGS treatment had higher discoloration scores at the end of RD when compared to the other MDGS treatments (Table 4). The authors have no reasonable explanation for the increased discoloration observed for the de-oiled MDGS treatment at the end of RD. Along with PUFA content, other factors may have also caused an increase in discoloration on de-oiled MDGS steaks displayed aerobically.
Our results suggest that as RD progressed, discoloration progressed at slower rates in beef from cattle fed corn in relation to cattle fed MDGS. Thus, lower PUFA concentration in steaks from cattle fed corn in relation to cattle fed MDGS may have allowed greater color stability during display.
Lipid Oxidation
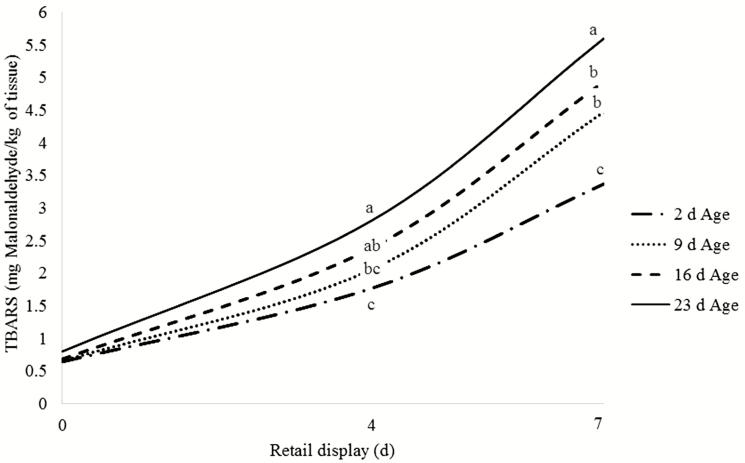
There was an age by RD interaction for lipid oxidation (P < 0.01). As expected, lipid oxidation was favored by long-term storage and increased TBARS values were seen as aging and RD progressed (Fig. 1). Greater lipid oxidation values were observed in samples aged 23 d, followed by samples aged for 16 and 9 d, which did not differ from each other. As expected, samples aged for 2 d had the lowest lipid oxidation values. Within all aging periods, greater TBARS values were seen as RD progressed from day 0 to day 4 and day 7.
Figure 1.
Age by retail display interaction (P < 0.01; SEM = 0.17) for lipid oxidation of samples aged 2, 9, 16, and 23 d with 0, 4, and 7 d retail display. a–cDifferent superscripts indicate differences within the same retail display time (P ≤ 0.05).
There are several factors that can promote lipid oxidation. Substrates necessary for this deteriorative reaction include, but are not limited to: fat content, fatty acid composition, metal ions that accelerate oxidation (e.g., iron), and oxygen (Faustman et al., 2010). In live muscle, enzymes are capable of eliminating free radicals formed during oxidation through the formation of water. However, it is highly unlikely that this mechanism of cell protection still functions postmortem (Morrissey et al., 1998). Hence, the balance of prooxidants and antioxidants of the living muscle is disrupted, favoring oxidation of lipids, nucleic acids, and proteins (Kanner, 1994; Gray et al., 1996; Morrissey et al., 1998). Damage to lipids may be accentuated during meat storage as free radicals continue reacting amongst themselves. Consequently, free radical accumulation increases within cells due to the collapse of intrinsic reactive oxygen species (ROS) preventive mechanisms, resulting in increased lipid oxidation with extended aging (Morrissey et al., 1998; Lobo et al., 2010). A significant interaction between dietary treatment and RD was found for lipid oxidation (P < 0.01; Table 5). No differences in lipid oxidation were found among dietary treatments at 0 and 4 d of RD. Strip loin steaks from cattle fed corn tended to have lower lipid oxidation (P ≤ 0.10) when compared with de-oiled MDGS and de-oiled MDGS plus oil (3.90 vs. 4.94 and 4.90 mg malonaldehyde/kg of meat, respectively) at day 7 of RD. Cattle fed full-fat MDGS had intermediate TBARS values (4.45 mg malonaldehyde/kg of meat) and did not differ from any other dietary treatment at day 7 of RD (Table 5).
Table 5.
Lipid oxidation value (TBARS; mg malonaldehyde/kg of meat) of strip loin steaks (longissimus lumborum) from steers fed either a corn diet, 40% Full-fat MDGS, 40% De-oiled MDGS, or 38% De-oiled MDGS plus 2% corn oil with 0, 4, and 7 d retail display
| d | Dietary treatment | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| Corn | Full-fat MDGS | De-oiled MDGS | De-oiled MDGS + oil | ||||
| TBARS | 0.28 | <0.01 | |||||
| 0 | 0.75a | 0.75a | 0.72a | 0.76a | 0.99 | ||
| 4 | 2.07a | 2.16a | 2.44a | 2.43a | 0.74 | ||
| 7 | 3.98b | 4.45ab | 4.94a | 4.90a | 0.07 | ||
a,bMeans in the same row with different superscripts differ (P ≤ 0.10).
MDGS = modified distillers grains plus soluble; TBARS = thiobarbituric acid reactive substance values.
In this study, feeding MDGS to cattle increased PUFA content of beef (P = 0.01). Fatty acid stability is dependent on the number of double bonds (Horwitt, 1986). Unsaturated fatty acids are more easily oxidized in comparison to saturated fatty acids (Zhang et al., 2007). Therefore, a probable reason for the increase in rancidity could be due to the fact that steers fed de-oiled MDGS and de-oiled MDGS plus oil had greater PUFA content, which are more susceptible to lipid oxidation.
Lipid oxidation that occurs during beef storage can affect beef color, flavor, aroma, and consequently, shelf life (Morrissey et al., 1994; Gray et al., 1996; Ladeira et al., 2014). Campo et al. (2006) suggested that a TBARS value of 2.28 mg/kg could be considered as the limiting threshold for acceptability of oxidation in beef because at this point the perception of rancidity overpowers beef flavor. In this study, beef from cattle fed corn and full fat MDGS remained below this limiting threshold (2.07 and 2.16 mg malonaldehyde/kg, respectively) after 4 d under RD condition. Beef from de-oiled MDGS and de-oiled MDGS plus oil treatments groups overcame this acceptability threshold at day 4 of RD (2.44 and 2.43 mg malonaldehyde/kg, respectively). All dietary treatments overcame this threshold for oxidized beef acceptability at day 7 of RD.
Lipid and myoglobin oxidation in meat often appear to be linked. Oxidation of lipids can lead to the formation of species that accelerate oxidation of myoglobin (Faustman et al., 2010). In our study, no differences in lipid oxidation were found between MDGS treatments (Table 5). However, de-oiled MDGS treatment discolored more than full-fat MDGS and de-oiled MDGS plus corn oil at the end of RD. Therefore, lipid oxidation itself does not fully explain the increased discoloration observed for the de-oiled MDGS treatment as RD progressed. Along with lipid oxidation, other factors may have also caused an increase in discoloration on de-oiled MDGS steaks displayed aerobically. Our results suggest that feeding MDGS to cattle may lead to faster reduction in beef display life when compared to the corn diet.
CONCLUSIONS
Feeding MDGS resulted in increased PUFA content of the meat in comparison to corn finishing diet. Results suggest that with prolonged aging periods and RD, feeding MDGS to cattle has the potential to reduce color and lipid stability compared to corn and thus reduce shelf life. Addition of corn oil to de-oiled MDGS decreased redness and increased discoloration and lipid oxidation in comparison to corn diets.
Conflict of interest statement
None declared.
This research was funded in part by The Beef Checkoff.
LITERATURE CITED
- Ahn D. U., Olson D. G., Jo C., Chen X., Wu C., and Lee J. I.. 1998. Effect of muscle type, packaging, and irradiation on lipid oxidation, volatile production, and color in raw pork patties. Meat Sci. 49:27–39. doi:10.1016/S0309-1740(97)00101-0 [DOI] [PubMed] [Google Scholar]
- AOAC 1990. Official methods of analysis of AOAC (Association of Official Analytical Chemists) international. 18th ed AOAC International, Arlington, VA. [Google Scholar]
- Campo M. M., Nute G. R., Hughes S. I., Enser M., Wood J. D., and Richardson R. I.. 2006. Flavour perception of oxidation in beef. Meat Sci. 72:303–311. doi:10.1016/j.meatsci.2005.07.015 [DOI] [PubMed] [Google Scholar]
- Chao M. D. 2015. Impact of wet distilers grains plus solubles and antioxidants on a basic mechanism of beef tenderization [Diss.]. DigitalCommons, Univ. of Nebr.-Lincoln. [Google Scholar]
- Domenech-Pérez K. I., Calkins C. R., Chao M. D., Semler M. E., Varnold K. A., and Erickson G. E.. 2017. Impact of feeding de-oiled wet distillers grains plus solubles on beef shelf life. J. Anim. Sci. 95:709–717. doi:10.2527/jas.2016.0905 [DOI] [PubMed] [Google Scholar]
- Duckett S. K., Andrae J. G., and Owens F. N.. 2002. Effect of high-oil corn or added corn oil on ruminal biohydrogenation of fatty acids and conjugated linoleic acid formation in beef steers fed finishing diets. J. Anim. Sci. 80:3353–3360. doi:10.2527/2002.80123353x [DOI] [PubMed] [Google Scholar]
- Faustman C., Sun Q., Mancini R., and Suman S. P.. 2010. Myoglobin and lipid oxidation interactions: mechanistic bases and control. Meat Sci. 86:86–94. doi:10.1016/j.meatsci.2010.04.025 [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., and Sloane Stanley G. H.. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226:497–509. [PubMed] [Google Scholar]
- Gray J. I., Gomaa E. A., and Buckley D. J.. 1996. Oxidative quality and shelf life of meats. Meat Sci. 43:S11–S123. doi:10.1016/0309-1740(96)00059-9 [DOI] [PubMed] [Google Scholar]
- Gill R. K., VanOverbeke D. L., Depenbusch B., Drouillard J. S., and Dicostanzo A.. 2008. Impact of beef cattle diets containing corn or sorghum distillers grains on beef color, fatty acid profiles, and sensory attributes. J. Anim. Sci. 86:923–935. doi:10.2527/jas.2007-0244 [DOI] [PubMed] [Google Scholar]
- Ham G. A., Stock R. A., Klopfenstein T. J., Larson E. M., Shain D. H., and Huffman R. P.. 1994. Wet corn distillers byproducts compared with dried corn distillers grains with solubles as a source of protein and energy for ruminants. J. Anim. Sci. 72:3246–3257. doi:10.2527/1994.72123246x [DOI] [PubMed] [Google Scholar]
- Hood D. E. R., and Riordan E. B.. 1973. Discoloration in prepackaged beef: measurement by reflectance spectrophotometry and shopper discrimination. J. Food Technol. 8:333–343. doi:10.1111/j.1365–2621.1973.tb01721.x [Google Scholar]
- Horwitt M. K. 1986. Interpretations of requirements for thiamin, riboflavin, niacin-tryptophan, and vitamin E plus comments on balance studies and vitamin B-6. Am. J. Clin. Nutr. 44:973–985. doi:10.1093/ajcn/44.6.973 [DOI] [PubMed] [Google Scholar]
- Jenkins T. C., Wallace R. J., Moate P. J., and Mosley E. E.. 2008. Board-invited review: recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J. Anim. Sci. 86:397–412. doi:10.2527/jas.2007-0588 [DOI] [PubMed] [Google Scholar]
- Jolly M. L., Nuttelman B. L., Burken D., Schneider C. J., Klopfenstein T. J., and Erickson G. E.. 2013. Effects of modified distillers grains plus solubles and condensed distillers solubles with and without oil extraction on finishing performance. Nebraska Beef Cattle Report MP 98:64–65. [Google Scholar]
- Kanner J. 1994. Oxidative processes in meat and meat products: quality implications. Meat Sci. 36:169–189. doi:10.1016/0309-1740(94)90040-X [DOI] [PubMed] [Google Scholar]
- Kinman L. A., Hilton G. G., Richards C. J., Morgan J. B., Krehbiel C. R., Hicks R. B., Dillwith J. W., and Vanoverbeke D. L.. 2011. Impact of feeding various amounts of wet and dry distillers grains to yearling steers on palatability, fatty acid profile, and retail case life of longissimus muscle. J. Anim. Sci. 89:179–184. doi:10.2527/jas.2010-3137 [DOI] [PubMed] [Google Scholar]
- Klopfenstein T. J., Erickson G. E., and Bremer V. R.. 2008. Board-invited review: use of distillers by-products in the beef cattle feeding industry. J. Anim. Sci. 86:1223–1231. doi:10.2527/jas.2007-0550 [DOI] [PubMed] [Google Scholar]
- Ladeira M. M., Santarosa L. C., Chizzotti M. L., Ramos E. M., Machado Neto O. R., Oliveira D. M., Carvalho J. R., Lopes L. S., and Ribeiro J. S.. 2014. Fatty acid profile, color and lipid oxidation of meat from young bulls fed ground soybean or rumen protected fat with or without monensin. Meat Sci. 96:597–605. doi:10.1016/j.meatsci.2013.04.062 [DOI] [PubMed] [Google Scholar]
- Lobo V., Patil A., Phatak A., and Chandra N.. 2010. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 4:118. doi:10.4103/0973-7847.70902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini R. A., and Hunt M. C.. 2005. Current research in meat color. Meat Sci. 71:100–121. doi:10.1016/j.meatsci.2005.03.003 [DOI] [PubMed] [Google Scholar]
- Mello A. S. Jr, Calkins C. R., Jenschke B. E., Carr T. P., Dugan M. E., and Erickson G. E.. 2012a. Beef quality of calf-fed steers finished on varying levels of corn-based wet distillers grains plus solubles. J. Anim. Sci. 90:4625–4633. doi:10.2527/jas.2010-3239 [DOI] [PubMed] [Google Scholar]
- Mello A. S. Jr, Jenschke B. E., Senaratne L. S., Carr T. P., Erickson G. E., and Calkins C. R.. 2012b. Effects of feeding modified distillers grains plus solubles on marbling attributes, proximate composition, and fatty acid profile of beef. J. Anim. Sci. 90:4634–4640. doi:10.2527/jas.2010-3240 [DOI] [PubMed] [Google Scholar]
- Metcalfe L. D., Schmitz A. A., and Pelka J. R.. 1966. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal. Chem. 38:514–515. doi:10.1021/ac60235a044 [Google Scholar]
- Morrison W. R., and Smith L. M.. 1964. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res. 5:600–608. [PubMed] [Google Scholar]
- Morrissey P. A., Buckley D. J., Sheehy P. J., and Monahan F. J.. 1994. Vitamin E and meat quality. Proc. Nutr. Soc. 53:289–295. doi:10.1079/PNS19940034 [DOI] [PubMed] [Google Scholar]
- Morrissey P. A., Sheehy P. J. A., Galvin K., Kerry J. P., and Buckley D. J.. 1998. Lipid stability in meat and meat products. Meat Sci. 49:S73–S86. doi:10.1016/S0309-1740(98)90039-0 [PubMed] [Google Scholar]
- Nute G. R., Richardson R. I., Wood J. D., Hughes S. I., Wilkinson R. G., Cooper S. L., and Sinclair L. A.. 2007. Effect of dietary oil source on the flavour and the colour and lipid stability of lamb meat. Meat Sci. 77:547–555. doi:10.1016/j.meatsci.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Renewable Fuels Association 2017. Statistics. (accessed 14 March 2017). http://www.ethanolrfa.org/pages/statistics. [Google Scholar]
- Roeber D. L., Gill R. K., and DiCostanzo A.. 2005. Meat quality responses to feeding distiller’s grains to finishing holstein steers. J. Anim. Sci. 83:2455–2460. doi:10.2527/2005.83102455x [DOI] [PubMed] [Google Scholar]
- Segers J. R., Stewart R. L. Jr, Lents C. A., Pringle T. D., Froetschel M. A., Lowe B. K., McKeith R. O., and Stelzleni A. M.. 2011. Effect of long-term corn by-product feeding on beef quality, strip loin fatty acid profiles, and shelf life. J. Anim. Sci. 89:3792–3802. doi:10.2527/jas.2011-4154 [DOI] [PubMed] [Google Scholar]
- Senaratne-Lenagala L. S. 2012. Mechanism and control of beef toughening during retail display in high oxygen modified atmosphere packages [Diss.]. DigitalCommons, Univ. of Nebr.-Lincoln. [Google Scholar]
- Vander Pol K. J., Luebbe M. K., Crawford G. I., Erickson G. E., and Klopfenstein T. J.. 2009. Performance and digestibility characteristics of finishing diets containing distillers grains, composites of corn processing coproducts, or supplemental corn oil. J. Anim. Sci. 87:639–652. doi:10.2527/jas.2008-1036 [DOI] [PubMed] [Google Scholar]
- Wood J. D., Enser M., Fisher A. V., Nute G. R., Sheard P. R., Richardson R. I., Hughes S. I., and Whittington F. M.. 2008. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 78:343–358. doi:10.1016/ j.meatsci.2007.07.019 [DOI] [PubMed] [Google Scholar]
- Zhang W., Shi B., and Shi J.. 2007. A theoretical study on autoxidation of unsaturated fatty acids and antioxidant activity of phenolic compounds. J. Am. Leather Chem. Assoc. 102:99–105. [Google Scholar]