Abstract
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world. A careful multidisciplinary assessment of tumor characteristics, liver function and physical status is required for proper therapeutic management. In recent years, several studies have supported the feasibility and benefit of combined therapy in the treatment of single large HCC, defined as those exceeding 3 cm in size. We present a case of combined treatment using radiofrequency ablation followed by trans-arterial chemoembolization with radiopaque embolic beads. The aim of this technical report was to describe the radiologic findings during combined radiofrequency ablation and radiopaque bead embolization, pointing out the differences and the potential advantages of using radiopaque beads compared with non-radiopaque beads. Furthermore, it is also the first report on using radiopaque beads in combined treatment for HCC.
KEYWORDS : ablation, CB-CT, chemoembolization, combined treatment, follow-up, HCC, mRECIST criteria, radiopaque beads, safety margin, unresectable
Practice points.
Recent literature demonstrated the superiority of combined approach (percutaneous ablation plus intra-arterial chemoembolization) over single modality for the treatment of hepatocellular carcinoma exceeding 3 cm in size in achieving complete tumor necrosis and improving patient survival.
It is not clear which is the best combination of these two procedures. In our opinion, using a single-step ‘combined’ approach makes it possible to obtain and amplify the synergistic effects of radiofrequency ablation (RFA) and trans-arterial chemoembolization (TACE).
First option, TACE followed by RFA, can increase the necrotizing effect of RFA therapy at the tumor level reducing the cooling effect of hepatic blood flow by decreasing hepatic arterial flow.
The second option, RFA followed by TACE, could increase cytotoxic effect acting on the large zones of sublethal heating obtained during RFA application in tissues surrounding the electrode.
The use of DC Bead LUMI™, the first radiopaque embolic bead in Europe for DEE-TACE (drug-eluting embolic TACE), improved therapeutic effect of combined treatment allowing better control of bead placement and tumor coverage, confirmation of location of beads, as well as success of embolization procedure.
When comparing with combined treatments performed with standard beads, the peripheral localization of radiopaque beads around necrotic area, like a hug (called ‘hug sign’) is clearly depicted by intraprocedural non-contrast cone beam computed tomography (CBCT) examination and could predict intraprocedural success.
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide, one of the most common cancers in the world. A careful multidisciplinary therapeutic management is needed, based on tumor characteristics, liver function and patient clinical conditions [1]. Recently, combined therapies have been widely applied in the treatment of HCC. One such approach is based on the combination of the percutaneous approach, such as radiofrequency ablation (RFA), and the intra-arterial locoregional approach, such as trans-arterial chemoembolization (TACE). Several published papers articles have supported the feasibility and benefit of combined therapy in the treatment of single large HCC [2]. However, it is not clear which is the best combination of these two procedures [3]. The more common option is represented by TACE followed by RFA, in order to reduce the cooling effect of hepatic blood flow by decreasing hepatic arterial flow and increasing the necrotizing effect of RFA therapy at the tumor level. However, due to the introduction of new radiopaque embolic bead (DC Bead LUMI™, Biocompatibles UK Limited, a BTG International group company Farnham, UK) for drug-eluting-embolic TACE (DEE-TACE), RFA first and then TACE in the same treatment session could provide synergistic effects of the two therapies and prognostic information regarding the efficacy of the ablation [4].
Based on this background, the aim of this technical report was to describe the radiologic findings during combined RFA and radiopaque bead embolization that may indicate intraprocedural success.
Case report
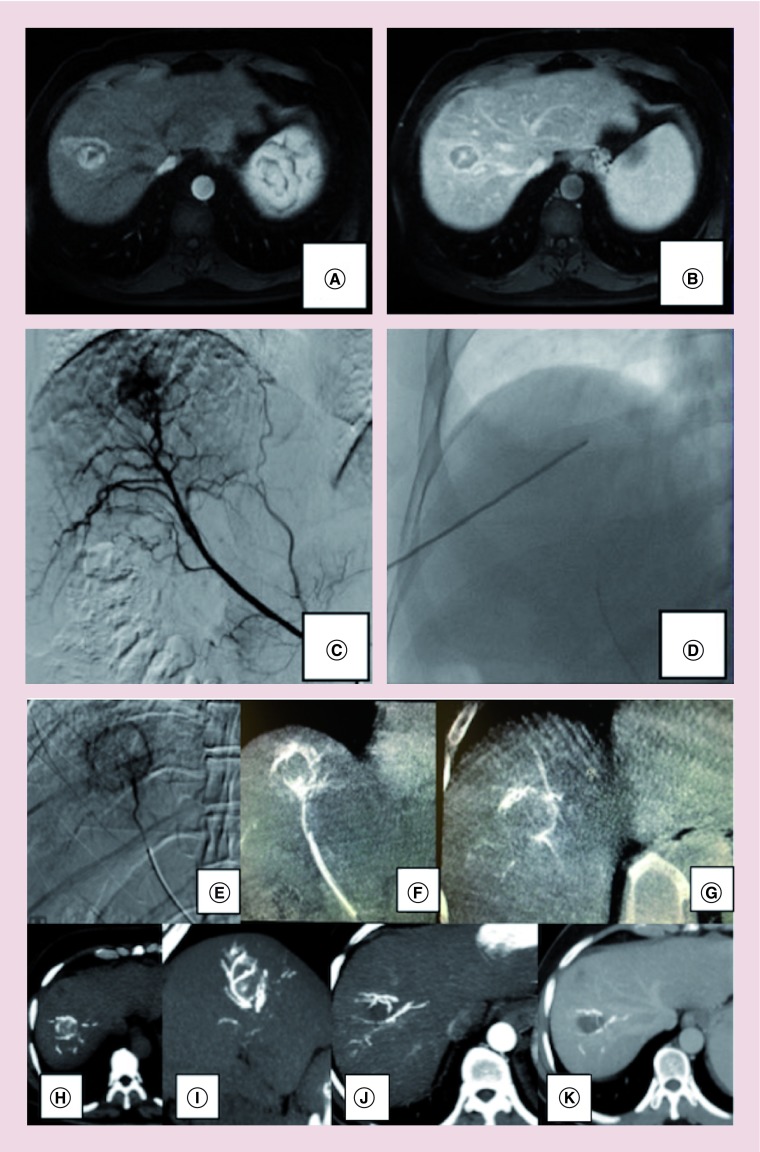
A 60-year-old man with HCV-related cirrhosis complicated by portal hypertension (esophageal varices staged F2, splenomegaly, with decreased platelet count – 49,000/mcL) was referred to our Department for the management of a liver lesion detected on ultrasound (US) examination. Clinical examination and laboratory data showed good clinical conditions and compensated liver function (Child–Pugh score A5, MELD score 6). The patient underwent an abdominal MRI that showed a 3.5 cm nodule at VII-VIII liver segment with a contrast enhancement pattern typical for HCC (arterial hypervascularity and venous phase washout) that was confirmed by US-guided biopsy (Figure 1A & B). The case was discussed in our multidisciplinary tumor board during the weekly meeting concerning liver lesions. Liver resection was judged unfeasible due to portal hypertension and comorbidities and a therapeutic decision of single-step combined treatment, with RFA followed by TACE, was made. In detail, we performed the treatment in an angiographic suite using antibiotic prophylaxis, patient monitoring and anesthesiological assistance. A right common femoral approach was used for performing hepatic artery angiography to depict the vascular pattern of the liver, identifying aberrant vessels and to exclude eventual arteriovenous shunts. Using a coaxial technique, a superselective catheterization was performed by placing a 2.7-Fr microcatheter (Progreat; Terumo, Tokyo, Japan) into the distal segmental hepatic artery feeding the HCC (Figure 1C). Under US-guidance, an internally cooled electrode with a 3-cm exposed tip (RF Medical Ablation System, BVM Medical Ltd, Trinity Ln, UK) was introduced into the nodule, with the patient under sedation with Fentanyl citrate (0.1–0.2 mg, Phentanest; Daiichi Sankyo, Tokyo, Japan) and local anesthesia. RF generator was activated for 10 min (Figure 1D) after that the electrode was withdrawn and selective angiography was obtained for evaluating immediate result (Figure 1E). A superselective drug-eluting-embolic-TACE was then performed using the same 2.7-Fr microcatheter with a slow injection of DC Bead LUMI™ 70-150 micron (Biocompatibles UK Limited, a BTG International group company Farnham, UK) loaded with Doxorubicin (Adriamycin® 50 mg powder, 25 mg/ml of beads), diluted with 10 cc Iomeron 400 mgI/ml per ml (total amount of 20 ml of contrast agent). The full planned dose was 2 ml of beads loaded with 50 mg of Farmarubicin, diluted with 20 ml of contrast agent. A rate of injection of approximately 1 ml of the bead/contrast medium suspension per minute was used, gently suspending the beads in the solution using a three-way stopcock to avoid sedimentation of the beads in the syringe. We used a standard injection technique as usually used also for for non-radiopaque beads. The full planned DEE dose was administered and a complete tumor arterial devascularization was achieved. Post procedure non-contrast 3D cone beam computed tomography (CB-CT) was performed with Philips XPert CT at least 10 min after finishing the bead administration to avoid persistent trapped soluble contrast agent stasis. Non-contrast CB-CT detected DC Bead LUMI™ distribution throughout tumor-feeding vessels with a concentric localization of radiopacity around the necrotic area (Figure 1F & G), also confirmed by unenhanced MDCT-scans (Figure 1H & I) performed 1 h after finishing the procedure. One-month follow-up contrast-enhanced MDCT scans confirmed postprocedural complete response, by using mRECIST criteria, with a central necrotic area and a peripheral complete concentric radiopacity, also concentrated in the arteries surrounding the treated area, without residual vascularized tumor, with a total treated area of 4.2 cm in size (Figure 1J & K).
Figure 1. . Combined treatment and Hug sign.
A 60-year-old man with 3.5 cm hepatocellular carcinoma nodule at SVII-VIII with typical MRI pattern (A & B), as also confirmed by selective digital subtraction angiography (DSA) (C). A combined treatment was performed with RFA followed by TACE using DC Bead LUMI™ (D). Post-RFA hyperemia is clearly detected at immediate DSA (E). After RFA, a superselective DEE-TACE was performed using radiopaque DC Bead LUMI™ 70–50 micron. A concentric localization of radiopacity around necrotic area is easily depicted by immediately postprocedure noncontrast 3D CB-CT performed with Philips XPert computed tomography (F & G), as also confirmed by unenhanced MDCTscans (H & I). This radiological finding is called ‘hug sign’, potentially indicating intraprocedural success, as also confirmed by 1-month follow-up contrast-enhanced MDCT scans (J & K).
RFA: Radiofrequency thermal ablation.
Discussion
The combined ablation therapy is an effective therapy for HCC nodules exceeding 3 cm in size, unsuitable for liver resection and several studies have shown the superiority of multimodal approach over single treatments in achieving complete tumor necrosis of HCC and improving patient survival [2,3]. We treated the lesion with RFA immediately followed by TACE as previously reported [3]. The rationale of this approach is to achieve an increased anticancer effect not only using the lethal heating obtained with RFA but also by taking advantage of the sublethal heating experienced in the large area surrounding the heating zone which is characterized by increased blood flow and increased vascular permeability enhancing the exposure to the chemotherapy. Indeed, the therapeutic effect of TACE performed after RFA was enhanced at the level of the sublethal heating area surrounding the necrotic tumor tissue [3].
In our case, the optimal concentration of chemotherapy drug on the residual post-RFA viable neoplastic tissue could potentially be demonstrated with the use of DC Bead LUMI™, the first radiopaque embolic bead in Europe for DEE-TACE [4]. In detail, drug loaded DC Bead LUMI™ seems to be clearly concentrated in the peripheral portion of the lesion as the central portion is necrotic and avascular due to the prior RFA. To the best of our knowledge, this is the first report on using radiopaque beads in combined treatment for HCC.
The use of radiopaque beads allows better control of bead placement and tumor coverage, highlighting eventual inevitable off-target embolization. They could allow an easy end point determination, allowing confirmation of location of beads, as well as success of the embolization procedure. In detail, when comparing with combined treatments performed with standard beads, the peripheral localization of radiopaque beads around necrotic area, like a hug, is clearly depicted by intraprocedural noncontrast CB-CT examination. We named this radiological sign as ‘hug sign’, showing that radiopaque beads completely surrounded the volume of RFA-related central necrosis, increasing the safety margin of ablation procedure. We know that CB-CT scan can also allow the visualization of standard beads, using trapped contrast agent, but only within a short and limited time gap after embolization. In our case, hug sign seems to be suggestive of radiological complete response, based on mRECIST criteria, as confirmed by 1-month contrast-enhanced CT scans.
The main limitation of our paper is related to only one case reported. Future studies performed on larger populations will be necessary to confirm our assumption on the role of hug sign as early predictive factor of procedural success and to more thoroughly evaluate the role of radiopaque embolic beads. Another potential limitation is the lack of histological complete follow-up to be correlated with radiological evaluation and to demonstrate the tumor-associated effectivity of the combination of RFA and TACE. However, liver resection was judged unfeasible due to portal hypertension and comorbidities, so we could not collect these informations.
Conclusion
Our report seems to demonstrate advantages of using radiopaque beads in combined treatment, allowing a better control of bead placement around post-RFA necrotic area as well as success of embolization procedure, confirming a correct identification and embolization of the feeding arteries. Hug sign, depicted on non-contrast CB-CT, could allow an easily intraprocedural endpoint determination.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
References
- 1.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Deng T, Zeng L, et al. Efficacy and safety of radiofrequency ablation and transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma: A meta-analysis. Hepatol. Res. 2016;46(1):58–71. doi: 10.1111/hepr.12568. [DOI] [PubMed] [Google Scholar]
- 3.Iezzi R, Pompili M, Posa A, et al. Combined locoregional treatment of patients with hepatocellular carcinoma: state of the art. World J. Gastroenterol. 2016;22(6):1935–1942. doi: 10.3748/wjg.v22.i6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashrafi K, Tang Y, Britton H, et al. Characterization of a novel intrinsically radiopaque drug-eluting bead for image-guided therapy: DC Bead LUMI™. J. Control Release. 2017;250:36–47. doi: 10.1016/j.jconrel.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]