SUMMARY
Contrast-enhanced ultrasound (CEUS) is a sure, noninvasive, repeatable imaging technique widely used in the characterization of benign and malignant liver lesions. The European Federation of Societies for Ultrasound in Medicine and Biology guidelines suggest the typical CEUS features of liver lesions as criteria for the noninvasive diagnosis in cirrhotic and not-cirrhotic patients. The clinical application of CEUS in the liver study is summarized in this review; the contrast-enhanced patterns of the most frequent liver lesions are described (hepatocellular and cholangiocellular carcinoma, liver metastases, hemangioma, focal nodular hyperplasia, adenoma). The role of this imaging technique in the diagnostic algorithm of liver malignancy is illustrated and the CEUS application in hepatologic and oncological settings is depicted.
KEYWORDS : cholangiocellular carcinoma, contrast agents, hepatocellular carcinoma, liver lesions, liver metastases, ultrasound
Practice Points.
Contrast-enhanced ultrasound (CEUS) is a contrast-enhanced technology widely used in abdominal imaging, in particular in the characterization of focal lesions in the liver.
CEUS contrast agents offer information about vascular enhancement pattern of liver lesions (Sonovue) and post-vascular enhancement pattern in Kuppfer phase (Sonazoid) in order to obtain a non invasive diagnosis of nodules.
The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines describe the typical and additional enhancement pattern of benign focal liver lesions at CEUS.
The EFSUMB guidelines suggest typical diagnostic noninvasive criteria for the most frequent malignant lesions: hepatocellular carcinoma (HCC), intrahepatic cholangiocellular carcinoma (CCC) and metastatic liver lesions. Moreover, these guidelines report some additional atypical enhancement patterns for these malignant liver lesions.
CEUS is included in the diagnostic algorithm for the management of hepatocellular carcinoma in cirrhosis in Japanese guidelines (Sonazoid) and in the recently published Italian Association for the Study of the Liver position paper (Sonovue); CEUS was excluded by American Association for the Study of Liver Disease guidelines for the risk of misdiagnosis between HCC and CCC.
The problems of misdiagnosis of HCC/CCC, the diagnosis of small liver lesions and hypovascular HCC remain an open issue for clinicians.
Background
Ultrasound (US) is the most commonly used imaging technique worldwide to detect focal lesions in the liver. The introduction of microbubbles contrast agents and the development of new advanced contrast-specific equipments have opened new opportunities in the noninvasive characterization of liver lesions. Contrast-enhanced US (CEUS) is able to assess the contrast enhancement pattern of liver lesions in real time; contrast agents are safe and repeatable, with very low incidence of side effects [1–5].
The published data reported in literature confirm the absence of cardiotoxic, hepatotoxic or nephrotoxic effects related to the use of US contrast agents [1,2]. In a series of more than 23,000 CEUS abdominal examinations with Sonovue, life-threatening anaphylactoid reactions were described in 0.001% of patients, without deaths. Slight adverse reactions were reported in 23 patients itch, dizziness, paresthesias, nausea and vomiting, erythematosus rash, slight dyspnea or heat sensation [3]. A multicentric study was performed in more than 78,000 CEUS examinations with Definity or Optison; severe reactions were described in eight patients (0.01%), with four anaphylactoid reactions without deaths [4]. Deaths in critically ill patients were reported after echocardiography examinations, but without sure evidence of causal relationship [5]. The interaction between US and contrast agents could produce bioeffects. In vitro sonoporation, hemolysis and cell death are described, but nonclinical evidence for bioeffects are reported in the human liver, where CEUS examination is performed at low mechanical index [6]. In a recent review by the Society for Pediatric Radiology and the International Contrast Ultrasound Society the safety of CEUS in children was analyzed for non-cardiac applications. The existing data on CEUS contrast agent safety in children are encouraging in promoting the use of this imaging technique in clinical practice [7]. The low risk for side effects of CEUS contrast agents, compared to the iodinated contrast agents used for computed tomography (CT), represents a strength of this imaging technique in clinical practice, in adults and children.
CEUS is widely used in the characterization of focal liver lesions but some limitations are known. The low diagnostic ability of CEUS in small size nodules (diameter <1 cm) represents a limitation in the correct evaluation and staging of liver tumor. The diagnostic ability in small nodules is problematic even in CT and magnetic resonance (MR). Some studies show that the sensitivity of CT and MR is significantly reduced in nodules with diameter less than 10 mm [8–10]. Moreover, the position of nodules in the liver (subdiaphragmatic lesions) and the characteristics of the surrounding liver parenchyma (liver steatosis) could represent a restriction in the use of CEUS in the diagnostic approach to liver lesions.
In clinical practice, clinicians could analyze the enhanced pattern of liver lesions with CEUS in different groups of patients.
• Detection of focal liver lesions with US in healthy subjects
US examination could show a focal liver lesion in asymptomatic healthy patients; these fortuitously detected lesions in subjects without previous history of cancer or chronic liver disease are prevalently benign liver lesions [11–13]. Malignant lesion could be identified even in a healthy liver, in particular metastatic disease in patients with unknown tumor, hepatocellular carcinoma (HCC) or cholangiocellular carcinoma (CCC).
Celli et al. report the CEUS pattern of 171 liver nodules detected in 125 non-cirrhotic patients. Of these, 87 were liver metastases of different primary tumors; out of the remaining 38 lesions, seven (7/38; 18.4%) were identified as primary liver cancer (one HCC and six CCC) in patients without any previous history of liver diseases, compared with 66 diagnosed with HCC out of the 75 nodules (88%) detected in cirrhotic patients [14]. In a recent publication, Sporea et al. describe the CEUS patterns of 536 de novo focal liver lesions detected on B-mode US in 525 consecutive patients. The indication on performing CEUS was 237 (44.2%) incidental finding in subjects without liver pathology, 207 (38.6%) focal liver lesions in chronic liver disease including cirrhosis, and 86 (16.1%) lesions in patients with oncologic history; in a few cases (1.1%) CEUS was performed in patients with inconclusive results of CT and/or MR [15]. CEUS could represent an efficient tool in the diagnostic framework and management of incidental liver lesions.
• Detection of focal liver lesion with US in cirrhotic patients or patients with a history of chronic liver disease
In this group of patients with a history of chronic liver disease (viral hepatitis, autoimmune liver diseases, liver damage relate to alcohol intake etc.), at risk for development of HCC and CCC, CEUS represents a sure and accurate imaging technique, recommended in the characterization and follow-up of malignant liver nodules.
In the setting of focal lesion in cirrhotic patients, US was firstly included in the guidelines for the management of HCC in cirrhosis, published by the European Association for the Study of the Liver [16]. The noninvasive criteria for the diagnosis of HCC were firstly introduced and accepted in these guidelines. The typical HCC vascular pattern was considered arterial hyper-enhancement at CT, MR, Doppler US or angiography in nodules larger than 2 cm. International guidelines were updated in 2005 by the American Association for the Study of Liver Disease (AASLD) and for the first time CEUS was introduced in the diagnostic algorithm of liver nodules in cirrhosis. The presence of arterial hyper-enhancement was considered typical for HCC only if followed by hypo-enhancement (washout) in late phase [17]. In the 2010 update of AASLD guidelines for the management of HCC, CEUS was excluded due to unavailability of the contrast agent in the USA and because of some false-positive results described in the literature for CEUS, particularly in the differential diagnosis between HCC and CCC [18].
In the recent position paper of the Italian Association for the Study of the Liver (AISF) for the multidisciplinary clinical approach to HCC, the typical vascular pattern for the noninvasive diagnosis of HCC was the presence of homogeneous hyper-enhancement in arterial phase with subsequent hypo-enhancement in late phases in dynamic imaging techniques, including CT, MR and CEUS [19]. The presence of overall arterial enhancement of lesion followed by rapid and marked washout in CEUS was suggested as not entirely typical for HCC but suspicious of non-hepatocellular malignancy (e.g., intrahepatic CCC) [19].
The differential diagnosis between HCC and intrahepatic CCC with dynamic imaging techniques represents an open issue for hepatologists and radiologists. Few studies have recently described the enhancement pattern of intrahepatic CCC in CEUS and the related risk of misdiagnosis [20–22]. Galassi et al. report that CEUS misdiagnoses as HCC a significantly higher number of intrahepatic CCCs in cirrhosis than does CT and MR; however, out of the ten CCCs studied with the three imaging techniques (CEUS, CT & MR), the vascular pattern was different in each of these techniques in 60% of patients [20].
• Detection of focal liver lesions with US in patients with previous history of tumor
The liver is one of the organs most frequently affected by metastatic disease [23,24]. In patients with previous history of cancer, the probability of liver metastasis is higher than in the general population. However, even in patients with known malignancy, small nodules could be benign. Cysts, hemangioma, focal nodular hyperplasia (FNH) and adenoma are described with the same frequency in metastatic liver as in healthy liver; the differential diagnosis is mandatory in the staging of these patients [25]. CEUS represents a sure, noninvasive and accurate technique in the management and follow up of oncological patients. Hepatic metastases are characterized by different patterns according to the histopathological origin of the primitive tumor; in arterial phase, metastases could appear hyper- or hypo-enhanced, but in the late phases the enhancement quickly decreases with complete final hypo-enhancement. In the late phase, even very small metastases, occult on B-mode, could be detected for the appearance of typical 'black focus' against the normally enhanced background liver parenchyma [25,26].
In clinical practice, CEUS could be used not only for qualitative diagnosis of liver tumors, but even for additional applications such as:
To improve the detection of primary and metastatic liver tumors in the intraoperative setting [27–29];
To facilitate radiofrequency ablation electrode placement in hypervascular HCC poorly depicted by B-mode US [27];
To evaluate the response to percutaneous treatments (radiofrequency ablation or ethanol injection);
To experimentally evaluate the early response for chemotherapeutic agents such as Sorafenib, which exhibits anti-neoplastic effect primarily by inhibiting angiogenesis [30].
Contrast agent characteristics & CEUS technique
The CEUS examination is based on the use of different contrast agents, consisting of microbubbles. These are thin-shelled, encapsulated spheres, made of phospholipids, containing different compressible gas. First generation contrast agents consist of air microbubbles (Levovist, Schering, Berlin, Germany); second generation contrast agents are characterized by microbubbles including different gases, sulfur hexafluoride in SonoVue (Bracco, Milan, Italy) and perfluorobutane in Sonazoid (Daiichi-Sankyo, GE Tokio, Japan) [27]. The water solubility of these gases is lower than air, with consequent increased acoustic impedance of contrast agent compared with the surrounding tissues. Microbubbles oscillate on applying US waves, producing strong return signals and suppressing the background echogenicity.
Using first generation contrast agents (Levovist), the goal of improving US images of focal liver lesions was initially pursued by the scan of tissue with high mechanical index and production of signal by collapse of microbubbles. The destructive method produces transient display of contrast; moreover, due to intermittent scanning, series sweeps are required to cover the whole parenchyma. The second generation contrast agents (Sonovue, Sonazoid, Definity) require a lower, nondestructive mechanical index, due to higher harmonic emission capabilities. The continuous real-time imaging obtained with these agents has improved the detection and the characterization of focal liver lesions.
The CEUS examination of liver lesions using the vascular contrast agents consists of a real time study of three different phases after contrast injection: arterial phase (start 10–20 s, end 35–45 s after contrast injection), portal-venous phase (start 30–45 s, end 120 s after contrast injection), and late phase (start >120 s after contrast injection, end complete bubbles disappearance, approximately 4–6 min) [26]. These phases are related to the specific dual blood liver supply; the changing of blood supply in malignant lesions leads to a typical diagnostic vascular pattern of liver tumor.
Furthermore, liver lesions could be analyzed in a delayed additional postvascular phase more than 10 min after injection of Sonazoid. Similarly to superparamagnetic iron oxide MR contrast agent (SPIO-MR), Sonazoid is taken up by reticuloendothelial cells, in particular Kupffer cells [31,32]. The Sonazoid microbubbles could even be detected within cells. The absence of Kupffer cells, typical of malignant hepatic lesions, causes a defect in contrast uptake in the postvascular phase after Sonazoid injection. A lesion characterized by contrast defect in the postvascular phase in a cirrhotic patient should be regarded as highly suggestive of malignancy. The complete study of vascular phases at CEUS is mandatory for hypo-enhancing nodules detected in the postvascular phase in order to confirm the diagnostic suspicion of malignancy. Postvascular phase alone is not sufficient to characterize nodules in cirrhosis.
The Sonovue is an intravascular agent, able to identify vascularization patterns of liver lesions in different phases (arterial, portal and late), with low extravascular distribution and irrelevant phagocytosis by the Kupffer cells [33,34].
The Sonazoid vascular contrast agent is also characterized by retention within hepatic reticuloendothelial cells for about 10–30 min after contrast injection; one of the possible mechanisms of delayed liver-specific image could be considered the phagocytosis of microbubbles by Kupffer cells [31]. The CEUS parenchyma-specific phase adds diagnostic “tissue characterization”, comparable to MR with hepatobiliary contrast agent [26].
Guidelines for the use of contrast-enhanced US in focal liver lesions
The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) and the World Federation of Ultrasound in Medicine and Biology have proposed guidelines for the use of CEUS in clinical practice for the study of the liver. These guidelines were firstly published in 2008 and were updated in 2012 [26,35]. According to these guidelines, CEUS is considered a sure and accurate imaging technique to characterize focal liver lesions; based on clinical experiences of expert centers and evidence-based medicine, the guidelines offer a guide for clinicians to perform and explain CEUS images in the diagnosis and follow-up of focal liver lesions. The guidelines identify the typical vascular pattern of liver lesions in different vascular phases and show possible atypical CEUS features of the same liver lesions.
The characteristics, the time and the intensity of enhancement are described for any benign and malignant liver lesions. In this review we analyze the most frequent benign liver lesions – hemangioma, FNH and adenoma, and the most frequent malignant lesions – HCC, intrahepatic CCC and liver metastases.
Moreover, guidelines underline the difference between liver lesions detected in cirrhotic and non-cirrhotic liver. The most important patterns of appearance of liver malignant nodules with Sonovue and Sonazoid CEUS are summarized in Table 1.
Table 1. . Vascularization enhancement patterns and postvascular Sonazoid patterns of malignant lesions in cirrhotic and non-cirrhotic liver.
| Features | Arterial phase | Portal-venous phase | Delayed phase | Post-vascular phase | |
|---|---|---|---|---|---|
| HCC in cirrhosis | CEUS typical | Hyper-enhancement (complete) | Iso-enhancement, non-enhancement (regions) | Hypo-enhancement | – |
| CEUS additional | Basket pattern, caotic vessels, hypo-, non-enhancement, enhancement tumor thrombus | non-enhancement | Iso-enhancement, non-enhancement | – | |
| Sonazoid | – | – | – | Non-enhancement or hypo-enhancement, iso-enhancement (in well differentiated HCC) | |
| HCC not in cirrhosis | CEUS typical | Hyper-enhancement | Hypo-, non-enhancement | Hypo-, non-enhancement | – |
| CEUS additional | Non-enhancement (regions) | Non-enhancement (regions) | Non-enhancement (regions) | – | |
| CCC | CEUS typical | Rim enhancement | Hypo-, non-enhancement | Hypo-, non-enhancement | – |
| CEUS additional | Non-enhancement | – | – | – | |
| Sonazoid | – | – | – | Non-enhancement or hypo-enhancement (pattern described in cirrhosis) | |
| Metastasis | CEUS typical | Rim enhancement | Hypo-enhancement | Hypo-, non-enhancement | – |
| CEUS additional | Complete enhancement, hyper-enhancement, non-enhancement regions | Non-enhancement (regions) | non-enhancement (regions) | – | |
| Sonazoid | – | – | – | Non-enhancement or hypo-enhancement | |
The CEUS typical and additional patterns are described for HCC, CCC and liver metastasis. The post-vascular patterns (Sonazoid patterns) are described in HCC and CCC in cirrhosis and in liver metastases.
CCC: Cholangiocellular carcinoma; CEUS: Contrast-enhanced ultrasound; HCC: Hepatocellular carcinoma.
Non-malignant liver lesions
• Hemangioma
The typical appearance of hemangioma with CEUS is characterized by peripheral nodular enhancement in arterial phase, progressing in a centripetal direction followed by complete or partial fill in the portal phase and complete enhancement in late phase. Small hemangiomas show complete rapid hyper-enhancement in arterial phase; sometimes, hemangiomas with thrombosed areas can be characterized by non-enhanced portions in late phase [26]. An example of CEUS vascular pattern of hemangioma using Sonovue is illustrated in Figure 1. The pattern of hemangioma in liver cirrhosis in the postvascular phase using Sonazoid is described as a non-enhancing area.
Figure 1. . Contrast-enhanced ultrasound in different vascular phases of hemangioma detected in a patient without liver disease.
(A) Peripheral nodular enhancement in arterial phase; (B) progressive centripetal enhancement; (C) complete enhancement in portal-venous phase; and (D) complete enhancement in late phase.
• Focal nodular hyperplasia
The diagnosis of FNH is strongly suspected when the typical spoke-wheel vascular pattern is detectable at color-Doppler examination [26]. The typical CEUS pattern is characterized by early arterial hyper-enhancement with rapid fill-in from the center outwards. In portal phase, the typical FNH remains hyper-enhancing; in late phase it may remain hyper-enhancing or become iso-enhancing. A central scar may be identified as a hypo-enhancing area in the portal and/or late phase [26]. Figure 2 shows a typical FNH.
Figure 2. . Contrast-enhanced ultrasound in different vascular phases of focal nodular hyperplasia characterized by typical vascular pattern.
(A) Central arterial hyper-enhancement; (B & C) rapid fill-in from the center outwards; (D) iso-enhancement in late phase.
• Hepatocellular adenoma
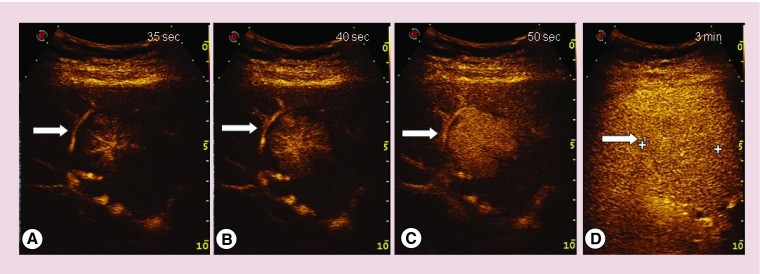
The CEUS pattern of adenoma is characterized by initial peripherical hyper-enhancement in arterial phase with subsequent rapid centripetal filling; in portal and late phase the lesion is iso-enhancing. Non-enhanced regions may appear in all phases and slightly hypo-enhancement could characterize the late phase. Figure 3 describes the CEUS pattern of hepatocellular adenoma.
Figure 3. . Contrast enhanced ultrasound images of hepatocellular adenoma.
The final diagnosis was confirmed after liver resection and pathological examination of focal liver lesion. (A) Peripherical arterial hyper-enhancement; (B & C) rapid centripetal fill-in; and (D) iso-enhancement in late phase.
Malignant liver lesions
• Hepatocellular carcinoma
HCC is the most frequent tumor of the liver and occurs prevalently in patients affected by liver cirrhosis or chronic liver diseases (viral hepatitis, alcohol intake, autoimmune liver diseases, etc.) [36]. According to guidelines for the management of HCC in liver cirrhosis, the diagnosis of HCC could be made according to noninvasive criteria if the appearance of lesions at contrast-enhanced imaging techniques is characterized by hyper-enhancement in arterial phase followed by washout in late phase. The diagnostic algorithm is different in each guideline (AASLD, AISF, Japanese guidelines); CEUS is included in the recommended imaging techniques in the Japanese guidelines for the characterization of hypovascular HCC (Sonazoid) and in the AISF position paper as an adjunctive radiological technique if performed by expert operators [17–19,37].
EFSUMB recommendations report the vascular pattern characterized by arterial hyper-enhancement followed by washout in the late phase as typical of HCC in cirrhosis. Contrast washout may lead to moderate–late hypo-enhancing, which should be regarded as a confirmatory feature of HCC, or to slight hypo-enhancement compared to the surrounding parenchyma, which is consistent, but not as specific as washout for HCC [26]. It is reported that small nodules (diameter between 10 and 30 mm) detected in cirrhosis are characterized by arterial hyper-enhancement followed by complete washout in about 42% of nodules with a final diagnosis of HCC; hyper-vascularization in arterial phase followed by iso-enhancement in late phase was described in 36.2% of finally confirmed HCC [38,39]. The sensitivity and the specificity of CEUS in the diagnosis of HCC were respectively 41 and 96% in this experience, considering the typical vascular pattern; the sensitivity and specificity results were respectively 77 and 79%, considering the complete washout (hypo-enhancement) and iso-enhancement after arterial hyper-vascularization as a diagnostic pattern [39]. Forner et al. demonstrated a sensitivity and specificity of 51.7 and 93.1% of CEUS in the detection of conclusive typical HCC pattern [40]. The results of the multicenter study recently published by Sporea et al., show an overall sensitivity and specificity of CEUS in the diagnosis of HCC respectively of 70.3 and 85.9% [15]. In Table 2 an overview of the results of different studies is reported in order to summarize the diagnostic ability of CEUS compared to other contrast-enhanced imaging techniques in diagnosis of HCC in cirrhosis [41–44].
Table 2. . Comparison between sensitivity and specificity of contrast-enhanced ultrasound, computed tomography and magnetic resonance in the characterization and final diagnosis of hepatocellular carcinoma in cirrhosis.
| Study (year) | Number of nodules | Mean/median diameter (cm) | Sensitivity (%) | Specificity (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| CEUS | CT | MR | CEUS | CT | MR | ||||
| Sangiovanni et al. (2010) | 67 | 1.6 | 26 | 44 | 44 | 100 | 100 | 100 | [41] |
| Di Martino et al (2013) | 163 | 2.3 | 71 | 71 | 71 | 62 | 87 | 87 | [42] |
| Mita et al. (2010) | 34 | 1.27 | 67.6 | 52.9 | 76.5 | – | – | – | [43] |
| Leoni et al. (2010) | 75 | 1.8 | 67.2 | 93.6 | 74.5 | 90 | 95 | 95 | [44] |
Number of nodules included in the study, mean or median diameter, sensitivity and specificity values are reported, where available.
CEUS: Contrast-enhanced ultrasound; CT: Computed tomography; MR: Magnetic resonance.
The published data confirm that more than 97% of liver nodules characterized by typical vascular pattern at CEUS have a final diagnosis of HCC [21,45,46]. The most relevant challenge for clinicians remains the differential diagnosis between HCC and other liver malignancies such as intrahepatic CCC [20,21,47]. The diagnostic limitations of CEUS, as with other imaging techniques, are mostly related to the size of nodules. The early diagnosis of malignancy is crucial for curative treatments and best prognosis; noninvasive diagnosis is difficult in small lesions. When imaging techniques are not sufficiently conclusive to make final diagnosis, liver biopsy is mandatory.
Sonazoid could add some adjunctive diagnostic information, similarly to MR with hepato-specific contrast agents. The absence of Kupffer cells in malignant lesions creates a defect in contrast uptake in the delayed phase, after the late vascular phase.
Sonazoid, as MR, should offer a new diagnostic opportunity in hypovascular HCC, the small group of nodules with final diagnosis of HCC not characterized by typical vascular features; in particular, not characterized by hyper-enhancement in arterial phase. In these HCCs, when vascular pattern is not diagnostic at CT, CEUS and MR performed with vascular contrast agents, Sonazoid or MR with hepato-specific contrast agent could offer cellular information, unrelated to vascular pattern, to improve the noninvasive diagnosis.
Additional features are described in the guidelines; the most frequent vascular patterns of HCC with CEUS and the Sonazoid pattern are summarized in Table 1. Images of HCC vascular patterns with CEUS performed with Sonovue are illustrated in Figure 4.
Figure 4. . Contrast-enhanced ultrasound images of nodules with final diagnosis of hepatocellular carcinoma.
Contrast-enhanced ultrasound images of noduleswith final diagnosis of hepatocellular carcinoma. A typical vascular pattern of hepatocellular carcinoma (A, B-mode) at contrast-enhanced ultrasound is characterized by hyper-enhancement in arterial phase (B) and wash out at hypo- enhancement in late phase (2 min) (C). An atypical vascular pattern of hepatocellular carcinoma (D, B-mode) at contrast-enhanced ultrasound could be characterized by iso-enhancement in arterial phase (E) followed by iso-enhancement in late phase (absence of washout) (F).
• Intrahepatic cholangiocellular carcinoma
Intrahepatic CCC is the second-most-common primary liver tumor in cirrhotic patients after HCC, accounting for 1–2% of all new nodules in cirrhosis [47]. In patients without liver cirrhosis, the detection of intrahepatic CCC usually occurs in advanced stage; the injection of vascular contrast agent shows, with CEUS, different enhancement patterns in arterial phase. The most frequent arterial patterns include peripheral rim-like hyper-enhancement, heterogeneous hyper-enhancement, homogeneous hyper-enhancement and heterogeneous hypo-enhancement [47]. All lesions usually show hypo-enhancement in portal and late phase. The EFSUMB recommendations for the use of CEUS in the liver suggest that the typical enhanced pattern for CCC in the healthy liver could be considered the presence of a rim-like arterial enhancement followed by hypo-/non-enhancement during the portal and delayed phases. In non-cirrhotic patients, large intrahepatic CCCs frequently develop a central fibrotic area, justifying the peripheral rim-like arterial contrast uptake [48,49].
In cirrhotic patients, who undergo surveillance for HCC by US every 6 months, CCC is frequently detected at an earlier stage; tumor size is normally smaller and necrotic areas are infrequent. Guidelines summarize the enhancement patterns and report some additional atypical CEUS features of malignant liver lesions.
Recently Galassi et al. showed that differing CEUS vascular pattern can be used to raise the suspicion of intrahepatic CCC in the cirrhotic liver: a rim-like hyper-enhancement, heterogeneous or very slight hyper-enhancement in arterial phase followed by marked and/or heterogeneous washout in venous phase [20]. Two further studies also reported a very early washout (within 60 s post-injection) in CCC nodules in cirrhosis [21,22]. However, CT and MR are frequently mandatory in these patients to obtain a differential diagnosis of malignancy in cirrhosis. Images of vascular patterns of intrahepatic CCC are reported in Figure 5.
Figure 5. . Contrast-enhanced ultrasound images of nodules with final diagnosis of cholangiocellular carcinoma.
Contrast-enhanced ultrasound images of nodules with final diagnosis of cholangiocellular carcinoma. A vascular pattern of cholangiocellular carcinoma at contrast-enhanced ultrasound is characterized by inhomogeneous hyper-enhancement in arterial phase (A) followed by marked washout (hypo-enhancement) in late phase (B). A different vascular pattern of cholangiocellular carcinoma could be characterized by mild peripheral rim hyper-enhancement in arterial phase (C) and washout (hypo-enhancement) in late phase (D).
• Metastatic liver diseases
Liver metastases are frequent in different tumors; CEUS represents a sure and accurate imaging technique able to detect and characterize secondary liver lesions [50,51]. The late phase is the best vascular phase to identify focal defects corresponding to liver metastases. These lesions appear as punched-out 'black foci' compared with the background liver parenchyma, uniformly enhanced in this phase [26]. Small metastases, sometime occult on B-mode US examination, could be detected in the late phase after contrast injection [25]. In arterial phase the appearances are twofold: hypo-vascular metastases appear as hypo-enhancing lesions, usually with a typical rim enhancement, while hyper-vascular lesions appear as brightly enhancing hyper-enhancing and homogeneous lesions [25]. Both the hyper- and hypo-vascular metastases invariably appear as dark enhancement defects in the delayed phase. The rapid washout (within 75 s), typical of metastases, can be useful in the differentiation of metastasis from HCC [52,53].
Figure 6 reports images of different vascular patterns of liver metastases.
Figure 6. . Contras- enhanced ultrasound images of nodules with final diagnosis of metastasis.
A typical vascular pattern of metastasis is characterized by marked peripheral rim hyper-enhancement in (A) arterial phase followed by marked washout (hypo-enhancement) in (B) late phase; another typical vascular pattern of metastasis on contrast-enhanced ultrasound is represented by hyper-enhancement in (C) arterial phase and washout at hypo-enhancement in (D) late phase.
Conclusion & future prospective
CEUS is recommended for characterizing all nodules detected in the liver during surveillance in cirrhosis and at routine US examination in the non-cirrhotic liver, as suggested in national and international guidelines.
CEUS could be considered when CT and MR are inconclusive or when liver biopsy is not feasible in liver nodules without final diagnosis.
Sonovue and Sonazoid CEUS offer different information about vascular and post-vascular behavior of nodules. Sonazoid CEUS, similarly to MR with hepato-specific contrast agents, is able to add diagnostic value in the diagnostic approach, due to an opportunity to characterize cellular features, and not only the vascular appearance, of nodules.
CEUS, even with some limitations, remains a sure and accurate imaging technique for clinicians for characterizing malignant liver lesions in cirrhotic and non-cirrhotic patients.
Footnotes
Financial & competing interests disclosure
L Bolondi receives consulting and speakers fees from Bayer, Bracco and Roche. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special notes have been highlighted as: • of interest; •• of considerable interest
- 1.Bokor D, Chambers JB, Rees PJ, Mant TG, Luzzani F, Spinazzi A. Clinical safety of SonoVue, a new contrast agent for ultrasound imaging, in healthy volunteers and in patients with chronic obstructive pulmonary disease. Invest. Radiol. 2001;36(2):104–109. doi: 10.1097/00004424-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Torzilli G. Adverse effects associated with SonoVue use. Expert. Opin. Drug Saf. 2005;4(3):399–401. doi: 10.1517/14740338.4.3.399. [DOI] [PubMed] [Google Scholar]
- 3.Piscaglia F, Bolondi L. Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med. Biol. 2006;32(9):1369–1375. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Wei K, Mulvagh SL, Carson L, et al. The safety of deFinity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J. Am. Soc. Echocardiogr. 2008;21(11):1202–1206. doi: 10.1016/j.echo.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Main ML, Goldman JH, Grayburn PA. Ultrasound contrast agents: balancing safety versus efficacy. Expert. Opin. Drug Saf. 2009;8(1):49–56. doi: 10.1517/14740330802658581. [DOI] [PubMed] [Google Scholar]; • Interesting clinical experiences.
- 6.Skyba DM, Price RJ, Linka AZ, Skalak TC, Kaul S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation. 1998;98(4):290–293. doi: 10.1161/01.cir.98.4.290. [DOI] [PubMed] [Google Scholar]
- 7.Darge K, Papadopoulou F, Ntoulia A, et al. Safety of contrast-enhanced ultrasound in children for non-cardiac applications: a review by the Society for Pediatric Radiology (SPR) and the International Contrast Ultrasound Society (ICUS) Pediatr. Radiol. 2013;43(9):1063–1073. doi: 10.1007/s00247-013-2746-6. [DOI] [PubMed] [Google Scholar]; • Interesting clinical experiences.
- 8.Burrel M, Llovet JM, Ayuso C, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38(4):1034–1042. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 9.Bhartia B, Ward J, Guthrie JA, Robinson PJ. Hepatocellular carcinoma in cirrhotic livers: double-contrast thin-section MR imaging with pathologic correlation of explanted tissue. AJR Am. J. Roentgenol. 2003;180(3):577–584. doi: 10.2214/ajr.180.3.1800577. [DOI] [PubMed] [Google Scholar]
- 10.Leoni S, Piscaglia F, Righini R, Bolondi L. Management of small hepatocellular carcinoma. Acta. Gastroenterol. Belg. 2006;69(2):230–235. [PubMed] [Google Scholar]
- 11.Dietrich CF, Sharma M, Gibson RN, Schreiber-Dietrich D, Jenssen C. Fortuitously discovered liver lesions. World J. Gastroenterol. 2013;19:3173–3188. doi: 10.3748/wjg.v19.i21.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Interesting clinical experiences.
- 12.Hirche TO, Russler J, Schröder O, et al. The value of routinely performed ultrasonography in patients with Crohn disease. Scand. J. Gastroenterol. 2002;37:1178–1183. doi: 10.1080/003655202760373399. [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Della Pina C, Crocetti L, Bozzi E, Cioni D. Clinical management of focal liver lesions: the key role of real-time contrast-enhanced US. Eur. Radiol. 2007;17:F73–F79. doi: 10.1007/s10406-007-0231-8. [DOI] [PubMed] [Google Scholar]
- 14.Celli N, Gaiani S, Piscaglia F, et al. Characterization of liver lesions by real-time contrast-enhanced ultrasonography. Eur. J. Gastroenterol. Hepatol. 2007;19(1):3–14. doi: 10.1097/01.meg.0000250585.53608.3c. [DOI] [PubMed] [Google Scholar]
- 15.Sporea I, Badea R, Popescu A, et al. Contrast-enhanced ultrasound (CEUS) for the evaluation of focal liver lesions - a prospective multicenter study of its usefulness in clinical practice. Ultraschall Med. 2014;35(3):259–266. doi: 10.1055/s-0033-1355728. [DOI] [PubMed] [Google Scholar]; • Interesting clinical experiences.
- 16.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J. Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 17.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Guidelines for the management of liver cancer or use of contrast-enhanced ultrasound.
- 19.Italian Association for the Study of the Liver (AISF); AISF Expert Panel; AISF Coordinating Committee. Bolondi L, Cillo U, Colombo M, et al. Position paper of the Italian Association for the Study of the Liver AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig. Liver Dis. 2013;45(9):712–723. doi: 10.1016/j.dld.2013.01.012. [DOI] [PubMed] [Google Scholar]; •• Guidelines for the management of liver cancer or use of contrast-enhanced ultrasound.
- 20.Galassi M, Iavarone M, Rossi S, et al. Patterns of appearance and risk of misdiagnosis of intrahepatic cholangiocarcinoma in cirrhosis at contrast enhanced ultrasound. Liver Int. 2013;33(5):771–779. doi: 10.1111/liv.12124. [DOI] [PubMed] [Google Scholar]
- 21.Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51:2020–2029. doi: 10.1002/hep.23600. [DOI] [PubMed] [Google Scholar]; • Interesting clinical experiences.
- 22.Li R, Zhang X, Ma KS, et al. Dynamic enhancing vascular pattern of intrahepatic peripheral cholangiocarcinoma on contrast-enhanced ultrasound: the influence of chronic hepatitis and cirrhosis. Abdom. Imaging. 2013;38:112–119. doi: 10.1007/s00261-012-9854-x. [DOI] [PubMed] [Google Scholar]
- 23.Schima W, Kulinna C, Langenberger H, Ba-Ssalamah A. Liver metastases of colorectal cancer: US, CT or MR? Cancer Imaging. 2005;5(Spec No A):S149–S156. doi: 10.1102/1470-7330.2005.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page AJ, Weiss MJ, Pawlik TM. Surgical management of noncolorectal cancer liver metastases. Cancer. 2014;120(20):3111–3121. doi: 10.1002/cncr.28743. [DOI] [PubMed] [Google Scholar]
- 25.Dietrich CF, Kratzer W, Strobe D, et al. Assessment of metastatic liver disease in patients with primary extrahepatic tumors by contrast-enhanced sonography versus CT and MRI. World J. Gastroenterol. 2006;12:1699–1705. doi: 10.3748/wjg.v12.i11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver-update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34(1):11–29. doi: 10.1055/s-0032-1325499. [DOI] [PubMed] [Google Scholar]; •• Guidelines for the management of liver cancer or use of contrast-enhanced ultrasound.
- 27.Alzaraa A, Gravante G, Chung WY, et al. Contrast-enhanced ultrasound in the preoperative, intraoperative and postoperative assessment of liver lesions. Hepatol. Res. 2013;43(8):809–819. doi: 10.1111/hepr.12044. [DOI] [PubMed] [Google Scholar]
- 28.Torzilli G, Palmisano A, Del Fabbro D. Contrast-enhanced intraoperative ultrasonography during surgery for hepatocellular carcinoma in liver cirrhosis: is it useful or useless? A prospective cohort study of our experience. Ann. Surg. Oncol. 2007:1347–1355. doi: 10.1245/s10434-006-9278-3. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi M, Hasegawa K, Arita J, et al. Contrast-enhanced intraoperative ultrasonography using perfluorobutane microbubbles for the enumeration of colorectal liver metastases. Br. J. Surg. 2012;99(9):1271–1277. doi: 10.1002/bjs.8844. [DOI] [PubMed] [Google Scholar]
- 30.Shiozawa K, Watanabe M, Kikuchi Y, Kudo T, Maruyama K, Sumino Y. Evaluation of sorafenib for hepatocellular carcinoma by contrast-enhanced ultrasonography: a pilot study. World J. Gastroenterol. 2012;18(40):5753–5758. doi: 10.3748/wjg.v18.i40.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med. Biol. 2007;33:318–325. doi: 10.1016/j.ultrasmedbio.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Hatanaka K, Kudo M, Minami Y, et al. Differential diagnosis of hepatic tumors: value of contrast-enhanced harmonic sonography using the newly developed contrast agent, Sonazoid. Intervirology. 2008;51(Suppl. 1):61–69. doi: 10.1159/000122600. [DOI] [PubMed] [Google Scholar]
- 33.Martie A, Bota S, Sporea I, Sirli R, Popescu A, Danila M. The contribution of contrast enhanced ultrasound for the characterization of benign liver lesions in clinical practice – a monocentric experience. Med. Ultrason. 2012;14(4):283–287. [PubMed] [Google Scholar]
- 34.Strobel D, Bernatik T, Blank W, et al. Diagnostic accuracy of CEUS in the differential diagnosis of small (≤ 20 mm) and subcentimetric (≤ 10 mm) focal liver lesions in comparison with histology. Results of the DEGUM multicenter trial. Ultraschall Med. 2011;32(6):593–597. doi: 10.1055/s-0031-1271114. [DOI] [PubMed] [Google Scholar]
- 35.Claudon M, Cosgrove D, Albrecht T, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 36.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig. Liver Dis. 2010;42(Suppl. 3):S206–S214. doi: 10.1016/S1590-8658(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig. Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]; •• Guidelines for the management of liver cancer or use of contrast-enhanced ultrasound.
- 38.Leoni S, Piscaglia F, Granito A, et al. Characterization of primary and recurrent nodules in liver cirrhosis using contrast-enhanced ultrasound: which vascular criteria should be adopted? Ultraschall Med. 2013;34(3):280–287. doi: 10.1055/s-0033-1335024. [DOI] [PubMed] [Google Scholar]
- 39.Bolondi L, Gaiani S, Celli N, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42(1):27–34. doi: 10.1002/hep.20728. [DOI] [PubMed] [Google Scholar]
- 40.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47(1):97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 41.Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638–644. doi: 10.1136/gut.2009.187286. [DOI] [PubMed] [Google Scholar]
- 42.Di Martino M, De Filippis G, De Santis A, et al. Hepatocellular carcinoma in cirrhotic patients: prospective comparison of US, CT and MR imaging. Eur. Radiol. 2013;23(4):887–896. doi: 10.1007/s00330-012-2691-z. [DOI] [PubMed] [Google Scholar]
- 43.Mita K, Kim SR, Kudo M, et al. Diagnostic sensitivity of imaging modalities for hepatocellular carcinoma smaller than 2 cm. World J. Gastroenterol. 2010;16(33):4187–4192. doi: 10.3748/wjg.v16.i33.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leoni S, Piscaglia F, Golfieri R, et al. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am. J. Gastroenterol. 2010;105(3):599–609. doi: 10.1038/ajg.2009.654. [DOI] [PubMed] [Google Scholar]
- 45.Boozari B, Soudah B, Rifai K, et al. Grading of hypervascular hepatocellular carcinoma using late phase of contrast enhanced sonography - a prospective study. Dig. Liver Dis. 2011;43(6):484–490. doi: 10.1016/j.dld.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Fan ZH, Chen MH, Dai Y, et al. Evaluation of primary malignancies of the liver using contrast-enhanced sonography: correlation with pathology. AJR Am. J. Roentgenol. 2006;186(6):1512–1519. doi: 10.2214/AJR.05.0943. [DOI] [PubMed] [Google Scholar]
- 47.Rimola J, Forner A, Reig M, et al. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50(3):791–798. doi: 10.1002/hep.23071. [DOI] [PubMed] [Google Scholar]
- 48.Xu HX, Chen LD, Liu LN, Zhang YF, Guo LH, Liu C. Contrast-enhanced ultrasound of intrahepatic cholangiocarcinoma: correlation with pathological examination. Br. J. Radiol. 2012;85:1029–1037. doi: 10.1259/bjr/21653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung YE, Kim MJ, Park YN, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009;29:683–700. doi: 10.1148/rg.293085729. [DOI] [PubMed] [Google Scholar]
- 50.Dietrich CF, Kratzer W, Strobe D, et al. Assessment of metastatic liver disease in patients with primary extrahepatic tumors by contrast-enhanced sonography versus CT and MRI. World J. Gastroenterol. 2006;12:1699–1705. doi: 10.3748/wjg.v12.i11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albrecht T, Oldenburg A, Hohmann J, et al. Imaging of liver metastases with contrast-specific low-MI real-time ultrasound and SonoVue. Eur. Radiol. 2003;13:N79–N86. doi: 10.1007/s00330-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 52.Bhayana D, Kim TK, Jang HJ, et al. Hypervascular liver masses on contrast-enhanced ultrasound: the importance of washout. AJR Am. J. Roentdenol. 2010;194(4):977–983. doi: 10.2214/AJR.09.3375. [DOI] [PubMed] [Google Scholar]
- 53.Kong WT, Wang WP, Huang BJ, Ding H, Mao F. Value of wash-in and wash-out time in the diagnosis between hepatocellular carcinoma and other hepatic nodules with similar vascular pattern on contrast-enhanced ultrasound. J. Gastroenterol. Hepatol. 2014;29(3):576–580. doi: 10.1111/jgh.12394. [DOI] [PubMed] [Google Scholar]