Abstract
Lameness is a primary reason for culling and mortality within a sow herd. This study evaluated the impact of feeding organic trace minerals and methionine (Met) to growing gilts (134 d) on lameness, performance, body composition and claw health (to first parity), productivity (to second parity), and reproductive performance through 2 parities. Young gilts (28.8 ± 8.8 kg of body weight [BW], n = 360) were BW blocked (10 gilts/pen) and randomly allotted to 1 of 4 dietary treatments: control (CON, basal diet); CON plus organic minerals (MIN, at 10, 20, and 50 mg/kg of Cu, Mn, and Zn, respectively; Aplomotec Plus, Tecnología & Vitaminas, S.L, Alforja, Spain); additional Met (MET, at 102% Met: Lys); and MET plus MIN (MM). Feed was provided ad libitum. Lameness, BW, and body composition were measured 7 times during rearing, at gilt service, day 109 of gestation, and first weaning. Gilts fed the MM diet had lower average daily feed intake (5.1%) and final BW (2.1%) than CON gilts (P < 0.05), whereas MIN and MET were intermediate and not different from each other. Similarly, final backfat (BF) was greatest in CON (P < 0.05), whereas CON and MIN increased final loin depth compared with MM (P < 0.05) with MET not being different. During rearing, 7.7% of all gilts presented lameness, which appeared between 106.8 and 129.7 kg BW confidence interval. Gilts that had been or were lame had reduced BW and average daily gain compared with never lame gilts (P < 0.05). Lameness during rearing was highest (P < 0.01) in gilts fed CON diet (14.8%), with no differences amongst MIN (2.0%), MET (5.3%), or MM (6.5%). In the sow herd, 21% of sows showed lameness and 24% of those were associated with claw lesions. At weaning, gilts fed CON diet had highest (P < 0.01) prevalence of lameness (20.8%) with no differences amongst MIN (6.5%), MET (11.1%), or MM (7.6%). Over the first 2 parities, 27.3% of gilts were culled. On farm, lameness was associated with 0.7 more stillborn piglets (P < 0.10), 1 mm more BF loss in first lactation (P < 0.05), and increased weaning-to-estrus by 3 d (P < 0.05). In conclusion, lameness during rearing was decreased by supplementing organic trace minerals, methionine, and their combination, which also reduced lameness during lactation.
Keywords: animal welfare, claw health, longevity, osteochondrosis, trace minerals
INTRODUCTION
Adequate gilt development is an essential component to maximize performance and sow longevity. Lameness affects animal welfare (Anil et al., 2007) and is one of the main reasons for gilt failure (Engblom et al., 2007; Jensen et al., 2010). Lameness prevalence on farms is reported near 10% (Boyle et al., 2010; Pluym et al., 2013b), reduces performance (Anil et al., 2009); and generally, locomotive problems are responsible for 20% of the culling over the first parity (Lucia et al., 2000).
Osteochondrosis (OC), arthritis, and claw disorders are the most frequent causes of lameness which affect the bone, articular cartilage, and hoofs (Yazdi et al., 2000; Bradley, 2010). Moreover, several interrelated factors such as genotype, diet composition, growth rate, and mechanical stress are thought to be important in lameness (Nakano and Aherne, 1988; Busch and Wachmann, 2011; de Koning et al., 2014; Quinn et al., 2015; Le et al., 2016). Copper (Cu), manganese (Mn), and zinc (Zn) are involved in cartilage and horn development (Orth, 1999; Tomlinson et al., 2004; Riet et al., 2013). Zinc influences the bone quality and includes catalytic, structural, and regulatory functions for horn hoof growth (Riet et al., 2013).
A reduction of OC severity scores was observed when feeding growing pigs 250 and 100 mg/kg of Cu and Mn (respectively) and 1% extra Met and Thr (Frantz et al., 2008). The sulfur donator capacity of Met might enhance osteoblast differentiation (Ouattara et al., 2016) and increase collagen and bone formation by increasing osteocalcin. Similarly, Quinn et al. (2015) found that additional trace minerals (TM) combined with feed restriction improved locomotor ability and reduced joint lesions. Additional Cu, Mn, and Zn have also shown improvements in claw health with lower horn tubules diameter, higher horn density, and reduced laminitic changes (Lisgara et al., 2016; Varagka et al., 2016).
We hypothesize that supplementing gilt diets with organic TM (Cu, Mn, and Zn), Met, or their combination during rearing would prevent OC and claw lesions resulting in a lower incidence of lameness.
MATERIALS AND METHODS
Animal Care and Use
The experiment was carried out during winter months in north Spain (Lleida) under commercial conditions. Gilts were initially reared in a quarantine facility with capacity for 800 pigs. After rearing, gilts were sent to 2 similar sow sites. The 2 sites chosen had similar performance and management conditions. The Ethical Committee on Animal Experimentation at the Universitat Autònoma de Barcelona reviewed and approved the procedures and protocols for the experiments according to the guidelines of the European Union (Directive 2010/63/EU).
Experimental Design, Housing, and Dietary Treatments
Maternal line gilts (n = 360; DanAvl Dania Hybrid line, Landrace × Yorkshire; DanBred International, Sant Cugat del Vallés, Spain) acquired from a breeding production company were used. At day 0, gilts of 9 to 13 wk of age and 28.8 ± 8.78 kg of body weight (BW) were individually weighed and allocated in 3 different blocks according to BW (heavy [H], medium [M], and light [L], 38.3 ± 4.24 kg, 28.8 ± 3.57 kg, and 19.2 ± 4.24 kg, respectively). Within BW blocks, gilts were distributed into 36 pens (as 10 animals/pen and 0.90 m2/gilt; 60% slatted and 40% solid floor) and pens randomly allotted to 1 of 4 dietary treatments: 1) a control group fed a basal diet (CON); 2) CON plus organic minerals (MIN; 10, 20, and 50 mg/kg of organic Cu, Mn, and Zn, respectively; Aplomotec Plus, Tecnología & Vitaminas, S.L., Alforja, Spain); 3) increased level of Met (MET; calculated to 102% Met: Lys ratio); and 4) MET plus MIN (MM). The experiment had 9 replicates with 3 pens in each of the 3 BW blocks. Gilts had access to enrichment items (biting iron chains and solid plastic balls). Three feeding phases (15, 74, and 45 d) were used during the entire rearing period with dietary treatments maintained for 134 d (Table 1). For each experimental period, feeds were formulated to meet or exceed nutrient requirements (FEDNA, 2013) for a proper gilt growth according to the genetic suppliers’ recommendations (Tybirk, 2015). The experimental diets included 2,550, 2,425, 2,310 kcal net energy (NE)/kg and 1.15%, 0.90%, 0.70% standardized ileal digestible (SID) Lys, respectively, for the 15, 74, and 45 d phases. Feed was offered ad libitum in pelleted form and provided using a single space dry feeder in each pen. The gilts had free access to fresh water provided through drinking nipples.
Table 1.
Composition of the experimental diets (phases I, II, and III) offered to growing gilts (as-fed basis), %
| Ingredient | Phase I (0–14 d) | Phase II (15–91 d) | Phase III (92–136 d) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||||||
| CON / MIN // MET / MM | CON / MIN // MET / MM | CON / MIN // MET / MM | ||||||||||
| Corn | 25.00 | 25.00 // 25.25 | 25.00 | |||||||||
| Wheat | 10.00 | 11.25 // 9.75 | 30.00 // 29.75 | |||||||||
| Barley | 22.40 // 28.25 | 25.00 | 24.05 // 23.30 | |||||||||
| Soybean meal | 23.55 // 23.75 | 19.25 // 19.50 | 10.25 //10.45 | |||||||||
| Sunflower seed meal | – | – | 6.50 | |||||||||
| Bakery byproduct | 10.00 // 2.70 | 10.00 | – | |||||||||
| Wheat middling | – | 2.60 // 2.75 | – | |||||||||
| Fat | 4.00 // 4.25 | 2.10 | 0.30 | |||||||||
| Calcium Carbonate | 0.77 // 0.74 | 1.03 // 0.96 | 1.35 // 1.42 | |||||||||
| Di-calcium phosphate | 1.35 // 1.32 | 1.41 // 1.45 | 1.23 | |||||||||
| Salt | 0.40 | 0.40 | 0.40 | |||||||||
| Hydroxy-analogue Met | 0.18 // 1.16 | 0.03 // 0.86 | // 0.63 | |||||||||
| L-Lys HCl | 0.77 // 0.75 | 0.41 | 0.39 | |||||||||
| L-Thr | 0.21 | 0.08 | 0.04 | |||||||||
| L-Trp | 0.03 | – | – | |||||||||
| Aplomotec Plus1 | 0.10 | 0.10 | 0.10 | |||||||||
| Premix2 | 0.40 | 0.40 | 0.40 | |||||||||
| Calculated composition | ||||||||||||
| NE, kal/kg | 2,550 | 2,425 | 2,310 | |||||||||
| CP, % | 17.70 | 16.51 | 14.01 | |||||||||
| Crude fat, % | 6.69 | 4.74 | 2.12 | |||||||||
| Crude fiber, % | 3.14 | 3.50 | 4.72 | |||||||||
| Lys, % | 1.25 | 1.00 | 0.78 | |||||||||
| Met, % | 0.43 // 1.29 | 0.29 // 1.02 | 0.25 // 0.80 | |||||||||
| Thr % | 0.835 | 0.669 | 0.526 | |||||||||
| Trp, % | 0.243 | 0.202 | 0.168 | |||||||||
| Met: Lys | 0.34 // 1.032 | 0.29 // 1.02 | 0.32 // 1.02 | |||||||||
| Ca, % | 0.75 | 0.89 | 0.96 | |||||||||
| P, % | 0.56 | 0.59 | 0.57 | |||||||||
| Analyzed composition | CON | MIN | MET | MM | CON | MIN | MET | MM | CON | MIN | MET | MM |
| Moisture, % | 11.3 | 11.1 | 11.6 | 11.7 | 11.6 | 11.4 | 12.0 | 11.8 | 12.7 | 12.6 | 12.4 | 12.9 |
| CP, % | 18.1 | 18.0 | 17.8 | 17.7 | 16.5 | 16.6 | 16.2 | 16.3 | 13.9 | 14.1 | 13.7 | 14.0 |
| Crude fat, % | 6.58 | 6.42 | 6.07 | 6.03 | 4.15 | 4.21 | 3.88 | 4.00 | 1.99 | 2.03 | 1.96 | 2.00 |
| Crude fiber, % | 3.39 | 3.34 | 3.23 | 3.30 | 3.59 | 3.40 | 3.50 | 3.58 | 4.53 | 4.49 | 4.52 | 4.55 |
| Lys, % | 1.25 | 1.26 | 1.24 | 1.25 | 1.00 | 0.98 | 0.97 | 1.01 | 0.77 | 0.75 | 0.78 | 0.77 |
| Met, % | 0.43 | 0.41 | 1.30 | 1.28 | 0.27 | 0.25 | 0.98 | 1.02 | 0.26 | 0.24 | 0.51 | 0.54 |
| Hydroxy-analogue Met, % | 0.16 | 0.14 | 1.03 | 1.02 | 0.19 | 0.23 | 0.73 | 0.76 | - | - | 1.02 | 0.99 |
| Cu, mg/kg | 15.6 | 26.3 | 15.2 | 25.1 | 15.5 | 25.4 | 15.9 | 25.8 | 16.4 | 26.1 | 16.0 | 26.5 |
| Mn, mg/kg | 66.4 | 85.2 | 62.1 | 82.7 | 66.1 | 86.0 | 68.5 | 88.2 | 65.3 | 84.4 | 67.1 | 84.7 |
| Zn, mg/kg | 122.5 | 173.4 | 121.1 | 176.3 | 120.4 | 172.8 | 125.1 | 175.5 | 123.4 | 174.1 | 124.1 | 173.9 |
1Aplomotec Plus = only added in MIN and MM treatments and provided the diet with 10, 20, and 50 mg/kg of organic Cu, Mn, and Zn, respectively (Tecnología & Vitaminas, S.L., Alforja, Spain).
2Vitamin-minerals premix provided per kg of feed: vitamin B2, 3.5 mg; vitamin B12, 0.035 mg; nicotinamide, 20 mg; folic acid, 1.25 mg; vitamin D3, 2,000 UI; vitamin A, 10, 000 IU; vitamin E, 30 mg; vitamin K3, 1 mg; vitamin B1, 1 mg; vitamin B6, 2.4 mg; D-calcium pantothenate, 14 mg; biotin, 0.125 mg; choline chloride, 400 mg; Fe (from FeSO4.H2O), 120 mg; I (from Ca(IO3)2), 0.5 mg; Cu (from CuSO4·5H2O), 10 mg; Mn (from MnO2), 40 mg; Zn (from ZnO2), 110 mg; Se (from Na2SeO3), 0.4 mg; phytase EC 3.1.3.26, 1,500 FTU; and butylhydroxytoluene, 25 mg.
At the end of the rearing period, 302 gilts were homogeneously divided into 2 groups according to the 4 dietary treatments and 3 BW blocks; then, transported to 2 destination farms where performance and other measurements were recorded during the first 2 parities. From the initial 360 gilts, 40 gilts were used for other purposes and 18 gilts died (n = 14) or were removed due to a major conformation problem (n = 4). These gilts were distributed similarly across dietary treatments. Gilt conformation was evaluated 4 different times during development using de Koning et al. (2015) methodology. Since there were no differences across treatments nor correlations with lameness, these data are not shown.
Both destination farms were in the same geographical area (20 km distance), similar size (1700 to 1800 sow inventory), integrated within the same company, and fed by the same feed-mill. Equivalent feed and routines were provided, and the farms had the same production and management veterinarian supervisor. During quarantine-adaptation, gilts were penned with 5 to 8 gilts/pen (4 × 3 m; 40% solid floor and 60% concrete slatted floor). Heat detection was routinely performed at 800 h with 10-min boar exposure. After second detected heat, gilts were housed in crates and weekly introduced into production after third detected heat and above 150 kg of BW. Before service, gilts spent 1 wk in a dynamic training pen (40 to 50 gilts) for electronic feeding system (EFS). At 28 d of gestation, gilts and sows were moved (2-min walking distance) to group housing pens of 70 females (as 1.5 m2/female, 4 × 4 m, 30% solid floor, and 70% concrete slatted floor). At day 109 of gestation, gilts and sows were moved (2-min walking distance) to farrowing rooms. All slatted concrete floors had 80-mm width beams and with 20-mm opening between beams.
When first entering the sow farm in the adaptation-quarantine area, the phase III CON diet was provided ad libitum for 30 d to all gilts. Thereafter, feed was switched to gestation feed (2,200 kcal NE/kg and 0.57% SID Lys) and maintained ad libitum until service. From service to day 28 of gestation, feed was individually adjusted according to body composition score (2.2 to 3.2 kg/d). From days 28 to 112 of gestation, feeding level was 2.2 kg/d for gilts and 2.3 kg/d for first parity sows. When entering the lactation barn, sows received 2 kg/d of lactation feed (2,340 kcal NE/kg and 0.93% SID Lys), until farrowing. The day of farrowing, sows were fasted. After farrowing, feed was gradually increased until day 6, and then sows were allowed ad libitum intake until weaning (day 21) through a feeding ball system (Rotecna, S.A., Agramunt, Spain).
In summary, the experimental period included 3 main phases (Table 2): 1) rearing and feeding of dietary treatments, monitored performance, body composition, and lameness; 2) gilt to first parity sow with the body composition, performance, lameness, and claw health measurements; and 3) first parity to second parity sows, with only performance data recorded.
Table 2.
Scheme of measurements and experimental data
| Item | Rearing gilts | Gilts in production | First parity sows |
|---|---|---|---|
| Experimental phase | Dietary treatments and data recording | Data recording | Data recording |
| Measurements | BW1, BF2, LDM3, and lameness; pen ADFI4 | BW, BF, claw health, lameness, performance, culling reasons | Performance and culling reasons |
| Frequency | 0, 24, 41, 67, 96, 117, and 134 d; weekly ADFI | Service, farrowing and weaning | Service, farrowing and weaning |
1BW = body weight.
2BF = backfat.
3LDM = longissimus dorsi muscle depth.
4ADFI = average daily feed intake.
Growth Performance, Body Composition, and Feed Intake
The BW, backfat (BF), and longissimus dorsi muscle (LDM) depth were measured on days 0, 24, 41, 67, 96, 117, and 134 of the experiment using a cage scale to contain the animals. The BF and LDM were measured at 60 mm from the midline and at the level of the last rib (P2) by the same trained person and using an ultrasound scanner (AV-3000V Digital Handheld Electronic B Ultrasound Scanner, AMBISEA Technology Corp., Ltd; Hong Kong, China). During the rearing period, feed intake by pen was measured on a weekly basis. The average daily gain (ADG), average daily feed intake (ADFI), and gain:feed ratio (G:F) were calculated overall.
Once in the sow farm, BW and BF of gilts were measured at service, before farrowing and at weaning. Gilt and sow performance included the number of total born, born alive, number of stillborn, mummified fetuses, and the number of weaned piglets. The breeding performance of sows included returning to service interval and weaning-to-estrus interval. Reproductive disorder was defined as any sow that did not demonstrate a first estrus, had an abortion, or consecutive repeating estrus. Culling reasons and date were recorded during the study.
Lameness
Animals were observed by walking on a hard, solid floor for a distance of 10 m and evaluated to detect lameness at days 0, 24, 41, 67, 96, 117, and 134 of the rearing period, always by the same observer. Lameness was evaluated by using adapted methodology from the work of Mustonen et al. (2011) for scoring lameness as follows: not lame (1); moderate lame (2), from slight limp and shortened stride to obvious limp having some difficulties with exercise; and severe lame, from animal barely weight bearing to incapable of standing (3). Since prevalence was low and severity not different among groups, further nomenclature and evaluation was set as lame and not-lame gilts, including any degree of lameness without visual signs of wound and infection.
The same observer evaluated the presence or absence of lameness in the sow destination farms at 3 phases: 1) early gestation lameness that was measured at service and at day 28 of gestation; 2) late gestation lameness, at day 109 of gestation; and 3) lactation lameness, evaluated at weaning. Lameness evaluation was performed during 2-min observation by walking (6 to 10 m) on a solid floor corridor (moving females to the insemination line at first service and at weaning), or 2-min observation inside the group-housing pens in gestation.
Claw Lesions
The gilts were examined for claw lesions in the insemination area and, due to lack of incidence, all sows were re-evaluated in lactation. Therefore, only data from those gilts that achieved farrowing were used for claw evaluation. Procedure was based on methodology described by Olsson et al. (2016) and simplified to be performed in “in vivo” animals. Health of claws was evaluated by registering lesions from toes: dewclaws, over-growth or cracks on the heel (excessive heel tissue growth extending over the sole), toe-heel transition with cracks, white line, hoof wall cracks, excessive growth of the hooves, and asymmetry between digit claws (Figure 1). Lesions were evaluated as being presented and then by severity. The score grade was as follows: 0 = none, 1 = mild-moderate (without affecting the integrity of the claw), 2 = severe (affecting the integrity of the claw, including gait difficulties or indicating clinics sings of pain); the assessment was on all claws and both outer and inner digits.
Figure 1.
Claw lesions: (a) dewclaws, (b) over-growth on the heel (excessive heel tissue growth extending over the sole), (c) fissure crack in toe-heel transition, (d) white line, (e) hoof wall cracks, (f) excessive growth of the hooves, and (g) asymmetry between digit claws. Adapted and simplified from the work of Olsson et al. (2016) to be performed in “in vivo.” All lesions were evaluated and the score grade was as follows: 0 = none, 1 = mild-moderate (without affecting the integrity of the claw), 2 = severe (affecting the integrity of the claw, including gait difficulties or indicating clinical signs of pain).
The dewclaws, white line, and hoof wall crack are lesions related to the horn properties (Varagka et al., 2016). These lesions were also grouped as hoof lesions, accounting any female that suffered at least 1 dewclaw, or white line, or hoof wall crack.
Biomarkers of Bone and Cartilage Turnover
At the end of rearing, 40 gilts were blood sampled (10 mL) for serum analysis (using siliconized blood-collecting tubes) from the jugular vena cava, whilst standing and restrained by a snout rope. Serum samples were analyzed for concentrations of cartilage biomarkers that may be indicators of OC (Frantz et al., 2010). In this trial, blood was collected from nonlame females (10 blood samples/treatment) with the objective to detect if dietary treatments would influence cartilage and bone turnover biomarkers (Garvican et al., 2010). Blood was centrifuged (3,000 rpm for 15 min at 4 °C), and subsequently, the resulting supernatant was collected for analysis. The biomarkers used were as follows: osteocalcin (Elisa kit, ref YHB0291Po, YH Biosearch Laboratory), C-telopeptide of the β 1-chain of type I collagen of bone and cartilage (ß.CTx; Elisa kit, ref E-EL-P1182, Elabscience), and carboxy-terminal telopeptide of type II collagen (procollagen-II; Elisa kit, ref MBS7215430, Mybiosource).
Porcine Reproductive and Respiratory Syndrome Outbreak
A Porcine Reproductive and Respiratory Syndrome (PRRS) outbreak was detected at day 37 and forced prophylactic measures before gilts were transported to production farms. The decision was made as follows: to 1) immunize all gilts with a live vaccine (AMERVAC PRRSV, Laboratorios Hipra SA, Amer, Spain) and promote the viral diffusion in the barn, 2) prolong the rearing phase for 90 d after vaccination, and 3) have 30 d of quarantine before entering gilts into the sow herd. Serum from 5% population in the facility and saliva pools from each pen were analyzed using a commercial kit (qRT-PCR, LSI VetMAX PRRSV EU/NA Real-Time PCR Kit, Life Technologies, Carlsbad, CA) and confirmed that all pens and analyzed gilts were PRRS positive on day 55.
Statistical Analysis
All experimental procedures were performed using SAS v9.4 (SAS Inst. Inc., Cary, NC). Gilt was the experimental unit for lameness, claw lesions, and number of piglets, whereas pen (10 gilts) was the experimental unit for BW, BF, LDM, ADG, ADFI, G: F ratio, and the parameters from models adjusting BW, BF, and LDM evolution. Normality and homoscedasticity of the different variables were evaluated by using the Shapiro–Wilk test and examining the normal plot (PROC UNIVARIATE). All parametric analysis, Tukey–Kramer adjustment was used to determine significant (P < 0.05) differences among dietary treatment averages.
Growing phase
The BW, LDM, BF, and lameness occurrence over experimental time were analyzed using repeated measures models, including treatment and time (day of experimental study) as fixed effects and block as random effect, using mixed model methods (PROC GLIMMIX). Different models for adjusting data were tested, and finally, linear regression, polynomial regression, and exponential were adjusted for pen BW, LDM, and BF, respectively (PROC REG and PROC NLYN). The equation parameters from regression and exponential equations were tested among dietary treatments using generalized linear model with treatment as the main effect (PROC GLM).
Sow farm phase
The BW and BF at first service, farrowing and weaning, and the reproductive performance were analyzed using generalized linear model (PROC GLM). Specific claw lesions were only described, as insufficient data at some levels invalidated frequency analysis. General claw lesions and grouped hoof lesions and lameness occurrence at different times were analyzed with nonparametric Binomial model (PROC GENMOD). The Fisher’s exact test was used for testing removal reasons (PROC FREQ). The main fixed effects included dietary treatment and farm. Also, the t-test was used to compare BF changes regardless of treatment across different productive phases (PROC TTEST).
RESULTS
Rearing Growth Performance
The gilts fed MM dietary treatment had reduced overall ADFI (5.1%; SE = 0.04, P = 0.042) and final BW (2.1%; SE = 1.72, P = 0.025) compared with those fed the CON diet, with MIN and MET being intermediate. Similarly, CON showed the greatest BF at the end of rearing (P = 0.024) and, together with MIN, had increased LDM compared with MM, with MET being intermediate and not different (P = 0.002; Table 3).
Table 3.
Effect of dietary treatment on growth performance and body composition in rearing gilts (28.8 ± 8.8 to 155.8 ± 14.2 kg of BW)
| Treatment1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|
| CON | MIN | MET | MM | |||
| Observations, n | 9 | 9 | 9 | 9 | ||
| Initial | ||||||
| Body weight, kg | 29.0 | 28.6 | 28.5 | 29.0 | 1.73 | 0.832 |
| Backfat, mm | 5.89 | 5.87 | 5.90 | 5.98 | 0.365 | 0.927 |
| Longissimus dorsi muscle depth, mm | 28.7 | 28.9 | 28.4 | 29.1 | 1.07 | 0.756 |
| Final | ||||||
| Body weight, kg | 157.1a | 156.5ab | 155.4ab | 153.7b | 1.73 | 0.025 |
| Backfat, mm | 15.8a | 14.9b | 14.8b | 15.0b | 0.365 | 0.024 |
| Longissimus dorsi muscle depth, mm | 60.2a | 60.1a | 59.3ab | 58.0b | 1.07 | 0.002 |
| Performance | ||||||
| Average daily feed intake, kg/d | 2.67a | 2.63ab | 2.60ab | 2.54b | 0.046 | 0.042 |
| Average daily gain, g/d | 956 | 955 | 946 | 930 | 11.3 | 0.205 |
| Gain: feed ratio | 0.357 | 0.363 | 0.363 | 0.366 | 0.010 | 0.473 |
a,bValues within a row with different superscripts differ significantly at P < 0.05.
1Treatment = CON, control; MIN, which provided the diet with 10, 20, and 50 mg/kg of organic Cu, Mn, and Zn, respectively (Aplomotec Plus, Tecnología & Vitaminas, S.L., Alforja, Spain) (0.1 %); MET, added Met (102% Met: Lys); or MM, combination of MIN and MET.
Gilts suffered a reduction in growth, LDM, and BF during PRRS outbreak (P < 0.01). In Figure 2, changes in LDM and BF over time are presented. Gilts fed MET and MM had lower LDM depth than those fed CON and MIN on day 67 (−4 mm, SE = 1.074; P < 0.01) and day 96 (−1.8 mm, SE = 1.074; P < 0.01). Similarly, BF depth was on average 0.9 mm lower at day 67 for gilts fed MET and MM than for those CON and MIN (SE = 0.365, P < 0.01). In addition, the first 3 wk after PRRS detection and vaccination, gilts from MM had the lowest ADFI (24% less on average) compared with CON, MIN, or MET; which were not different from each other (SE = 0.04, P < 0.01). No differences were detected thereafter. Finally, the mortality rate during rearing was 3.8% and it was 6 times higher in animals of L BW block than those of H (P = 0.017).
Figure 2.
Evolution of Longissimus dorsi muscle depth (LDM) (a) and backfat (BF) (b) over the experimental time during a Porcine Reproductive and Respiratory Syndrome (PRRS) outbreak in rearing gilts (n = 360) under dietary treatments = CON, control; MIN, which provided the diet with 10, 20, and 50 mg/kg of organic Cu, Mn, and Zn, respectively (Aplomotec Plus, Tecnología & Vitaminas, S.L., Alforja, Spain) (0.1 %); MET, added Met (102% Met: Lys); or MM, combination of MIN and MET. Arrow indicates the PRRS outbreak detection and vaccination (AMERVAC PRRSV, Laboratorios Hipra SA, Amer, Spain). **CON and MIN > MET and MM (P < 0.01).
The BW and body composition evolution of gilts were modeled over age for the rearing period and comparisons among groups are presented in Table 4. The increase of BW was linear, LDM showed a polynomial of second degree form, and BF evolution was exponential. The BW equations indicated that growth was unaffected by the experimental treatments. In a different way, the BF fractional accretion rate (exponent b) of treatment CON tended to be higher than in MIN, MET, or MM dietary treatments, which were not different from each other (P = 0.078).
Table 4.
Effect of dietary treatment on the equation parameters for growing gilts (28.8 ± 8.79 to 155.8 ± 14.2 kg BW) modeled for their BW, Longissimus dorsi muscle depth (LDM), and backfat (BF)
| Treatment1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|
| CON | MIN | MET | MM | |||
| BW2, kg | ||||||
| a2 (BW-Age linear) | −37.9 | −38.2 | −37.0 | −36.6 | 1.69 | 0.902 |
| b2 (BW-Age linear) | 0.912 | 0.909 | 0.912 | 0.889 | 0.0131 | 0.507 |
| LDM depth3, mm | ||||||
| Age at maximum LDM4 | 223.0 | 248.0 | 261.3 | 238.8 | 10.98 | 0.113 |
| Maximum LDM5 | 59.5 | 60.7 | 60.9 | 58.4 | 1.18 | 0.419 |
| BF depth6, mm | ||||||
| a4 (BF-Age exponential) | 4.04 | 4.36 | 4.19 | 4.21 | 0.148 | 0.218 |
| b4 (BF-Age exponential) | 0.00654a | 0.00569b | 0.00558b | 0.00579b | 0.00059 | 0.078 |
a,bValues within a row with different superscripts trend at P < 0.10.
1Treatment = CON, control; MIN, which provided the diet with 10, 20 and 50 mg/kg of organic Cu, Mn and Zn, respectively (Aplomotec Plus, Tecnología & Vitaminas, S.L., Alforja, Spain) (0.1 %); MET, added Met (102% Met: Lys); or MM, combination of MIN and MET.
2Linear: BW, kg = a + b × age, d, R2 = 0.95; adjusted by pen.
3Polynomial: LDM, mm = a + b × age, d + c × age, d ^2, R2 = 0.82; adjusted by pen. The intercepts resulted of LDM polynomial adjustments are not realistic and affected the coefficients; therefore, comparisons are performed only from calculations within experimental ages, which adjusted properly (i.e., age at maximum LDM).
4Calculated from the first derivate equal to zero.
5Calculated from the LDM polynomial equation with given age at maximum LDM.
6Exponential: BF = a EXP ^b × age, d; R2 = 0.72; adjusted by pen.
Sow Farm Performance
There was no carryover effect of dietary treatments to first parity reproductive performance (Table 5). Gilts from MM dietary treatment were inseminated 15 d earlier (and numerically 6 kg lighter) than CON (SE = 4.28, P = 0.045), without evidence of differences for MIN and MET. Across dietary treatments and for each measurement, no differences were detected for BW and BF (P > 0.05). Independently of dietary treatment, BF depth was higher at first service (17.7 mm) than at farrowing (16.7 mm) (SE= 0.94, P = 0.002). Additionally, there was a loss of 13.7% BW and 9.38% of BF between farrowing and weaning with no differences among dietary treatments.
Table 5.
Carry over effects of dietary treatment provided to rearing gilts (134 d), on the performance and culling reasons as gilts and thereafter as first parity sows
| Treatment1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|
| CON | MIN | MET | MM | |||
| Age first service, d | 303a | 289ab | 290ab | 288b | 35.0 | 0.045 |
| Gilts, n | 77 | 74 | 76 | 74 | ||
| Total born | 18.3 | 18.6 | 18.1 | 18.1 | 0.40 | 0.811 |
| Born alive | 15.1 | 15.9 | 15.8 | 14.5 | 0.45 | 0.112 |
| Weaned piglets | 13.4 | 13.4 | 13.8 | 13.3 | 0.25 | 0.567 |
| Culled (including quarantine) | 14 | 11 | 11 | 12 | – | 0.308 |
| First parity sows, n | 52 | 54 | 50 | 52 | ||
| Total born | 17.5xy | 18.0xy | 17.0y | 18.6x | 0.48 | 0.092 |
| Born alive | 16.4xy | 16.5xy | 15.7y | 17.4x | 0.48 | 0.097 |
| Weaned piglets | 14.5 | 14.3 | 14.3 | 14.5 | 0.15 | 0.405 |
| Culled | 10 | 11 | 9 | 3 | – | 0.308 |
| Reasons for culling, n | 77 | 74 | 76 | 74 | Total | P-value |
| Reproductive disorders2 | 9 | 12 | 10 | 6 | 37 | 0.733 |
| Sudden deaths | 5 | 7 | 8 | 2 | 22 | 0.297 |
| Lameness | 7a | 0b | 0b | 3ab | 10 | 0.001 |
| Miscellaneous3 | 3 | 3 | 2 | 4 | 12 | 0.582 |
| Total | 24 | 22 | 20 | 15 | 81 | 0.457 |
a,bValues within a row with different superscripts differ significantly at P < 0.05.
x,yValues within a row with different superscripts trend at P < 0.10.
1Treatment = CON, control; MIN, which provided the diet with 10, 20, and 50 mg/kg of organic Cu, Mn, and Zn, respectively (Aplomotec Plus, Tecnología & Vitaminas, S.L., Alforja, Spain) (0.1 %); MET, added Met (102% Met: Lys); or MM, combination of MIN and MET.
2Includes not demonstrating estrus, abortion, and consecutive returning to service.
3Includes thin-illness, respiratory problems, accidental lesion, fight exhaustion death, and none-lame euthanasia.
Of females that entered the sow farm, 16% were eliminated during the first parity and 11% during the second. The main reasons were the reproductive problems (45.7%). Lack of estrus (33%) and infertility (62%) were the causes in gilts. In sows, 80% of culling was due to farrowing problems and infertility. There were no treatment differences (Table 5). Culling due to lameness was 12.3%, 9 times higher in gilts than in first parity sows, and the second highest reason for removal. Although CON (7 of 24) and MM (3 of 15) dietary treatments had higher incidence of lameness as reason for removal compared with MIN (0 of 22) and MET (0 of 20), there was insufficient incidence and sample size for firm conclusions.
Lameness and Claw Health in Gilts
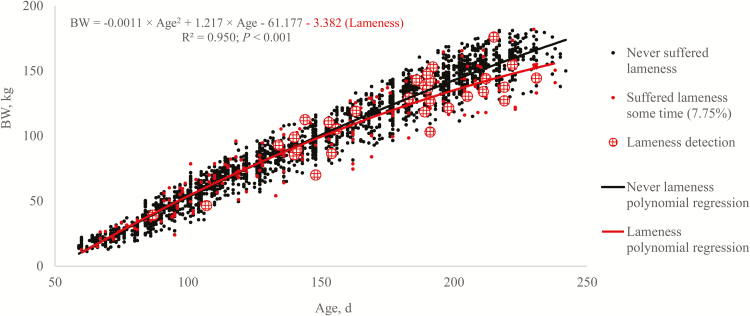
The prevalence of lameness for the entire rearing period was 7.75% and occurrence was first observed in a confidence interval (CI) between 106.8 and 129.7 kg of BW. At the end of rearing, those gilts that had previously shown lameness were 7.06 kg lighter and their ADG was 80.2 g/d lower than the non-lame (SE = 2.9, P = 0.016 and SE = 4.6, P < 0.001, respectively), whereas neither were heavier or lighter before lame problems (Figure 3). The first lame detection was at 39 kg of BW although lameness incidence (> 1.5%) began at 106 kg of BW. Moreover, it was observed that the proportion of lameness at a given moment increased with time and with BW during rearing (P < 0.001).
Figure 3.
Polynomial regression model of body weight (BW) evolution over age for 360 replacement gilts comparing performance of gilts that never suffered lameness with those that suffered lameness and indicating the BW at lameness detection.
Lameness at different productive phases and a summary of claw lesions are presented in Table 6. A greater percentage of gilts fed the CON diet showed lameness during the entire rearing period compared with MIN, MET, or MM dietary treatments (P = 0.006), with no differences amongst MIN, MET, or MM. On the sow farms, 66 of 302 (21%) females showed lameness at some point in time and 37 eventually recovered (56%). Of lameness cases at weaning, 24% were associated with hoof lesions, but whether lesions caused lameness or lameness caused lesions is unknown. There were no differences observed for lameness during gestation. At weaning, the proportion of lameness was highest for CON gilts compared with MIN, MET, or MM dietary treatments (P = 0.010), with no evidence of difference among MIN, MET, and MM. The number of lesions observed per sow or the number of sows with a lesion was not significantly different among dietary treatments (P > 0.05). Grouped hoof lesions were lower in females fed the MET diet vs. those fed CON, MIN, or MM treatments (P = 0.025), which were not different from each other. Moreover, females fed the CON and MET treatments tended to have increased severity for a given lesion compared with MIN and MM (P = 0.089).
Table 6.
Effects and carryover effects of dietary treatment provided to rearing gilts (134 d), on the prevalence of lameness over different productive phases and on claw lesions evaluated in the lactation phase
| Treatment1 | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | MIN | MET | MM | ||||||
| Gilts, n2 | 64 | 61 | 62 | 63 | |||||
| Lameness, % | SE | SE | SE | SE | |||||
| During rearing2 | 14.8a | 57.08 | 2.02b | 68.35 | 5.31b | 63.24 | 6.45b | 62.55 | 0.006 |
| Early gestation3 | 10.9 | 59.68 | 10.1 | 62.83 | 11.3 | 62.69 | 10.9 | 62.69 | 0.994 |
| Late gestation4 | 11.8 | 60.94 | 6.8 | 51.46 | 7.63 | 63.50 | 7.56 | 65.16 | 0.530 |
| Lactation5 | 20.8a | 58.16 | 6.50b | 63.10 | 11.1b | 60.94 | 7.60b | 62.41 | 0.010 |
| Claw lesion6, % | 48.4 | 54.08 | 39.3 | 55.20 | 40.8 | 55.01 | 39.7 | 55.12 | 0.357 |
| Grouped hoof lesions6, % | 20.4a | 58.33 | 15.7a | 60.16 | 8.28b | 61.54 | 14.9a | 60.16 | 0.025 |
| Severe hoof lesions, % | 54.4 | 62.02 | 19.6 | 66.16 | 60.9 | 67.71 | 27.3 | 64.64 | 0.089 |
| Claw lesions7, n | Total | ||||||||
| HeelG (severe) | 10(5) | 10(4) | 9(3) | 10(5) | 39(17) | ||||
| WCV (severe) | 6(4) | 12(7) | 3(2) | 8(5) | 29(18) | ||||
| Asym (severe) | 10(6) | 8(4) | 7(3) | 3(1) | 28 | ||||
| DewB severe | 5 | – | 4 | 1 | 10 | ||||
| WCH severe | 1 | 1 | 1 | 6 | 9 | ||||
| WL moderate | 4 | 2 | 2 | 1 | 9 | ||||
| HoofL (severe) | 6(4) | 2(1) | – | – | 8(5) | ||||
| HeelF severe | – | 2 | 1 | 1 | 4 | ||||
| Total lesions | 42 | 37 | 27 | 30 | 136 | ||||
a,bValues within a row with different superscripts differ significantly at P < 0.05.
1Treatment = CON, control; MIN, which provided the diet with 10, 20, and 50 mg/kg of organic Cu, Mn, and Zn, respectively (Aplomotec Plus, Tecnología & Vitaminas, S.L., Alforja, Spain) (0.1 %); MET, added Met (102% Met: Lys); or MM, combination of MIN and MET.
2During the rearing phase, lameness was evaluated for 90 gilts per dietary treatment; for other measurements, it is indicated in the table.
3Lameness evaluated at service and at 28 d of gestation.
4Lameness evaluated at 109 d of gestation.
5Lameness evaluated at weaning.
6Gilts with at least 1 of 7 claw lesions evaluated (Figure 1); grouped hoof lesion = gilts with at least one dewclaw, or white line, or wall crack lesion.
7As described in Figure 1; HoofL = excessive growth of the hooves; DewB = dewclaw broken or lost; WL: white line lesion; WCV and WCH = wall crack vertical and horizontal; HeelF = fissure crack in transition heel/toe; HeelG = excessive growth of the heel tissue; y Asym = asymmetry between digit claws; The score grade was: 0 = none, 1 = mild-moderate (without affecting the integrity of the claw), 2 = severe (affecting the integrity of the claw, including gait difficulties or indicating clinics sings of pain).
Gilts that showed lameness during gestation lost more BF during lactation (1.8 mm) than never lame sows (SE = 0.49, P = 0.001). Lame gilts had 3.4-d longer weaning to estrus interval compared with never lame gilts (SE = 1.49, P = 0.031). Gilts that showed lameness in the sow farm tended to have 0.7 more stillborn piglets than never lame gilts (SE = 0.32, P = 0.089). Those gilts that showed lameness during rearing weaned 1.2 fewer piglets than never lame gilts (SE = 0.46, P = 0.016). Finally, serum biomarker of bone and cartilage turnover showed a high variability with no differences amongst treatments (Table 7).
Table 7.
Effect of different dietary treatment on serum biomarkers of bone and cartilage turnover of gilts
| Treatment1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|
| CON | MIN | MET | MM | |||
| Gilts, n | 10 | 10 | 10 | 10 | ||
| Osteocalcin, ng/mL | 64.8 | 68.0 | 78.9 | 72.0 | 26.82 | 0.729 |
| ß.CTx 2, pg/mL | 4,280 | 3,763 | 3,822 | 3,210 | 1,117.3 | 0.321 |
| Procollagen-II3, pg/mL | 372 | 261 | 434 | 304 | 185.7 | 0.367 |
1Treatment = CON, control; MIN, which provided the diet with 10, 20, and 50 mg/kg of organic Cu, Mn, and Zn, respectively (Aplomotec Plus, Tecnología & Vitaminas, S.L., Alforja, Spain) (0.1 %); MET, added Met (102% Met: Lys); or MM, combination of MIN and MET.
2ß.CTx = C-telopeptide of the beta 1-chain of type I collagen of bone and cartilage.
3Procollagen-II = porcine cross-linked c-terminal telopeptides of type II collagen.
DISCUSSION
Lameness prevalence during rearing of replacement gilts was 7.75% and within the range (4% to 20%) reported for growing pigs (KilBride et al., 2009). During growth, risk for mechanical stress (BW) increases and can cause lameness (Ewers et al., 2001; Olstad et al., 2014). Incidence of lameness also increased as BW increased in the present experiment (CI of 106 to 129 kg of BW). The primary cause of lameness in growing pigs is severe OC (Ytrehus et al., 2007) with prevalence reported to be 12% and 14% (Busch and Wachmann, 2011; Grevenhof et al., 2012). Occurrence of OC is generally subclinical and starts to appear about 12 wk of age (Grevenhof et al., 2012; Tóth et al., 2016). Its progression is multifactorial (Orth, 1999). We hypothesized that supplementing MIN and MET would enhance joint development and promote healing during the time when 50% to 70% of the OC lesions naturally heal (12 to 25 wk of age). Our results may suggest that dietary supplementation of MIN, MET, and the combination (MM) reduced lameness compared with CON during rearing. Similarly, Frantz et al. (2008) observed a reduction of OC severity when feeding pigs 1% extra Met, and also, with a diet containing 250 and 100 mg/kg of Cu and Mn (base: 14 to 16 mg/kg Cu and 33 to 40 mg/kg Mn).
The content of TM in growing diets is often overdosed. Indeed, the dietary inclusion of TM could even be lowered to 50% of NRC (2012) when feed ingredients contribute enough (Gowanlock et al., 2013). Concerning skeletal development, feeding TM below requirements is known to reduce bone and joint quality (Owen et al., 1973; Ott and Asquith, 1989; Shaw et al., 2006). Above requirements, TM increase excretion in feces and do not improve performance (Creech et al., 2004; Gowanlock et al., 2013). However, it is less clear whether feeding TM above requirements has potential to increase bone mineralization, strength, and joint development (Orth, 1999). A complexity of factors in OC may interact and conflicting results are often observed (Quinn et al., 2015; Tóth et al., 2016). In turkeys, additional Zn, Mn, and Cu at 40, 40, and 20 mg/kg (respectively) above NRC (1994) complexed with Met increased bone strength and health (Ferket et al., 2009). In pigs, bone ash and strength linearly increase with higher levels of Cu, Mn, and Zn, but only with clear beneficial effects to a diet containing at least 100% of NRC (1998) requirements (Veum et al., 2009). In this trial, the basal diet included 10, 40, and 110 mg/kg of Cu, Mn, and Zn (respectively), set as the commercial recommendations baseline (FEDNA, 2013). These levels are considerably higher than the NRC (2012) requirements of 3.0 to 5.0, 2.0 to 3.0, and 50 to 80 mg/kg for Cu, Mn, and Zn, respectively, during the BW ranges in this study. Moreover, the TM values from analyzed diets generally increased between 10% and 50%, which as previous authors reported is likely to be the consequence of feed ingredients’ contribution (Gowanlock et al., 2013).
Controversy among studies may respond to variable conditions. It could be that needs for TM are higher under disease challenge, increased mechanical stress, or to enhance healing of osteochondorsis and longevity under practical conditions (Ferket et al., 2009; Pluym et al., 2013a; Quinn et al., 2015). Furthermore, a re-evaluation of nutritional strategies to minimize lameness is interesting with increasing risk of lameness as genetic improvement for growth advances (Aasmundstad et al., 2013; Le et al., 2016). The dietary treatments MIN and MM (supplemented with organic TM) would increase TM retention (Reese and Hill, 2010; Liu et al., 2014) compared with CON and MET (inorganic nonsupplemented). Feeding organic vs. inorganic sources of Cu, Mn, and Zn also increased absorption and retention of P in finishing pigs, which would enhance bone development (Liu et al., 2014, 2016). The present results suggest potential to reduce lameness during growth when gilts are supplemented with organic TM and Met, either alone or in combination, which is similar to the results of previous authors (Frantz et al., 2008; Quinn et al., 2015).
The gilts fed the combination MM also had reduced final BW (2.16%) compared with CON. Lower ADG and BW could be related to decreased risk of lameness, as some authors have observed (Ewers et al., 2001; Busch and Wachmann, 2011). Conversely, gilts on MIN and MET also had reduced lameness without having reduced performance. Furthermore, other studies observed bone and joint benefits when supplementing Met (Frantz et al., 2008; Ouattara et al., 2016). The slight reduction on performance in gilts fed MM compared with CON may be related to their lower ADFI during PRRS outbreak. During the PRRS outbreak, gilts fed increased Met (MET and MM) had an unexpected decrease of LDM and BF compared with MIN and CON. In mammals, the amino acids Lys and Met compete for the same absorption transport system (Broer, 2008). Under PRRS immune stress, Met excess may cause Lys deficiency because of the lowered feed intake and increased Lys needs (Schweer et al., 2018). Moreover, Met is the most toxic amino acid through its metabolite homocysteine (Benevenga and Steele, 1984). Edmonds and Baker (1987) reported a threshold for Met toxicity between 10 and 20 g/kg in piglets, which lowered feed intake and growth (>40% when supplementing 4% Met). Those are dramatic consequences under higher inclusion levels compared with our data but could explain these short-phase differences.
Gilts that suffered lameness had reduced performance compared with those never lame. Under lameness conditions, it is possible that gilts had increased lying-down time and reduced the number of visits to the feeder; which would explain the lowered growth rate (Bonde et al., 2004). Before lameness detection, the ADG or BW between gilts that become lame and gilts that never become lame was not different. If lameness was caused by OC, the present results are in contrast with previous reports, suggesting a positive relationship between ADG and OC (Busch and Wachmann, 2011; Grevenhof et al., 2012). Conversely, using visual analysis to determine lameness may have resulted in some gilts with OC that did not show lameness (Ytrehus et al., 2007; Etterlin et al., 2015). Other authors have not observed ADG and OC/lameness relationship (Tóth et al., 2016) or observed interactions of ADG and OC/lameness with diet type (fattening, rearing, or gestating), feeding level (ad libitum vs. restriction), or supplements (Ytrehus et al., 2007; Quinn et al., 2015).
The incidence of lameness detected in the sow farms is likely to be influenced by multiple factors. From the end of rearing to day 28 of gestation, lameness increased from 4.0% to 10.8%. The 10.8% lameness during early gestation is double to that observed by Pluym et al. (2013b). Transition conditions to the sow farm are highly variable and it is known that transport, wider slat floors, and formation of new groups are factors that can increase mechanical stress (Bonde et al., 2004; Pluym et al., 2011). In this study, hierarchy fights were observed when regrouping gilts in the training period for EFS, which is likely one reason for increasing lameness (Anil et al., 2007; Olsson et al., 2016). Over gestation, lameness was similar across dietary treatments and in agreement with previous reports (10% to 12%; Pluym, et al., 2013b). Contrarily, lactation lameness was highest for gilts fed the CON diet compared with other treatments, without differences among MIN, MET, or MM. The reason is unclear and could be associated with higher lameness risk already detected for CON during the rearing phase.
Regarding claw health, gilts fed MET diet had fewer grouped hoof lesions. In vivo response to Met is not clear, but in vitro, Met increases protein synthesis in epithelial horn-forming cells (Hepburn et al., 2008). Inconsistently, MM did not present the same response for hoof lesions. This suggests that dietary supplements of organic TM (Cu, Mn, and Zn) and Met did not have a clear effect on claw health, which is in disagreement with previous reports that indicated a positive effect of TM (Lisgara et al., 2016). The carryover approach of this trial was probably insufficient to consistently influence claw lesions. Otherwise, other farm factors such as metal slats under the sow farrowing crate or gilt pastern conformation could have interacted with claw lesions (Bonde et al., 2004; Bradley, 2010). In addition, claw health can be associated with lameness (Pluym et al., 2011), but it is not known if lameness produces claw lesions or vice versa. The claw lesions detected (54.5%) were less frequent than those in the literature (99%, Anil et al., 2007; Pluym et al., 2011). Probably, this is because lesions increase with parity and we only evaluated first parity lesions (Bradley, 2010; Pluym et al., 2013a). In agreement with mentioned authors, the lesions on the heel and horn wall were the most frequent.
In our study, gilts eliminated during first parity were similar to results of Lucia et al. (2000). Lameness was the primary reason for euthanasia as others have also listed (Engblom et al., 2007; Anil et al., 2009). Excluding females euthanized due to lameness, 14% of the other removed females were identified as lame at some point during the first parity, but were listed with another reason for removal. Indeed, reproductive failure is often associated with lameness (Heinonen et al., 2013). High productive genetics, like the present, may require updated research in genetics, nutrition, and management areas to reduce lameness and mortality.
In conclusion, lameness during rearing was decreased by supplementing organic TM (Cu, Mn, and Zn) and methionine. Although dietary treatments may have potential to minimize lameness and claw health, the carryover effects are unclear and inside farm factors are likely to be highly important. Lameness in replacement gilts increased significantly above 106 kg of BW. Before lameness, BW or ADG was not related to future lameness. Gilts identified as lame had reduced future growth performance, fewer weaned piglets, increased BF loss during lactation, and increased the weaning-to-estrus compared with never lame females. Further research including other strategies, such as lowering growth rate, management changes, or genetics, could provide more understanding of the lameness problems.
Footnotes
This study was supported by CDTI (Spanish Innovation Agency, Ministry of Industry Tourism and Trade), Madrid, Spain, project IDI-20150749. We thank the graduate students help, technical support received from the SNIBA staff, and also, the farm owners for the opportunity to work in their facilities. E.V. is employed by the feed supplier for the experiment, but no other authors have a conflict of interest.
LITERATURE CITED
- Aasmundstad T., Kongsro J., Wetten M., Dolvik N. I., and Vangen O.. 2013. Osteochondrosis in pigs diagnosed with computed tomography: heritabilities and genetic correlations to weight gain in specific age intervals. Animal 7:1576–1582. doi: 10.1017/S1751731113001158 [DOI] [PubMed] [Google Scholar]
- Anil S. S., Anil L., and Deen J.. 2009. Effect of lameness on sow longevity. J. Am. Vet. Med. Assoc. 235:734–738. doi: 10.2460/javma.235.6.734 [DOI] [PubMed] [Google Scholar]
- Anil S. S., Anil L., Deen J., Baidoo S., and Walker R.. 2007. Factors associated with claw lesions in gestating sows. J. Swine Health Prod. 15:78–83. [Google Scholar]
- Benevenga N. J., and Steele R. D.. 1984. Adverse effects of excessive consumption of amino acids. Annu. Rev. Nutr. 4:157–181. doi: 10.1146/annurev.nu.04.070184.001105 [DOI] [PubMed] [Google Scholar]
- Bonde M., Rousing T., Badsberg J. H., and Sørensen J. T.. 2004. Associations between lying-down behaviour problems and body condition, limb disorders and skin lesions of lactating sows housed in farrowing crates in commercial sow herds. Livest. Prod. Sci. 87:179–187. doi: 10.1016/j.livprodsci.2003.08.005 [DOI] [Google Scholar]
- Boyle L. A., Bullo E., and Nolan T. C.. 2010. Lameness and limb lesions in replacement gilts on a commercial farm. Adv. Anim. biosci. 1:198. doi: 10.1017/S2040470010003419 [DOI] [Google Scholar]
- Bradley C. L. 2010. Evaluating the impact of dietary inorganic or organic trace mineral supplementation on gilt development and sow reproduction, lameness, and longevity. PhD Diss. Univ. Arkansas, Fayetteville: p. 1–398. [Google Scholar]
- Broer S. 2008. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 88:249–286. doi: 10.1152/physrev.00018.2006 [DOI] [PubMed] [Google Scholar]
- Busch M. E., and Wachmann H.. 2011. Osteochondrosis of the elbow joint in finishing pigs from three herds: associations among different types of joint changes and between osteochondrosis and growth rate. Vet. J. 188:197–203. doi: 10.1016/j.tvjl.2010.03.021 [DOI] [PubMed] [Google Scholar]
- Creech B. L., Spears J. W., Flowers W. L., Hill G. M., Lloyd K. E., Armstrong T. A., and Engle T. E.. 2004. Effect of dietary trace mineral concentration and source (inorganic vs. Chelated) on performance, mineral status, and fecal mineral excretion in pigs from weaning through finishing. J. Anim. Sci. 82:2140–2147. doi: 10.2527/2004.8272140x [DOI] [PubMed] [Google Scholar]
- Edmonds M. S., and Baker D. H.. 1987. Amino acid excesses for young pigs: effects of excess methionine, tryptophan, threonine or leucine. J. Anim. Sci. 64:1664–1671. doi: 10.2134/JAS1987.6461664X [DOI] [PubMed] [Google Scholar]
- Engblom L., Lundeheim N., Dalin A. M., and Andersson K.. 2007. Sow removal in Swedish commercial herds. Livest. Sci. 106:76–86. doi: 10.1016/j.livsci.2006.07.002 [DOI] [Google Scholar]
- Etterlin P. E., Morrison D. A., Österberg J., Ytrehus B., Heldmer E., and Ekman S.. 2015. Osteochondrosis, but not lameness, is more frequent among free-range pigs than confined herd-mates. Acta Vet. Scand. 57:63. doi: 10.1186/s13028-015-0154-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers B. J., Dvoracek-Driksna D., Orth M. W., and Haut R. C.. 2001. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J. Orthop. Res. 19:779–784. doi: 10.1016/S0736-0266(01)00006-7 [DOI] [PubMed] [Google Scholar]
- FEDNA 2013. Necesidades nutricionales para Ganado porcino. 2th ed Fundación Española para el Desarollo de la Nutrición Animal, Madrid, Spain. [Google Scholar]
- Ferket P. R., Oviedo-Rondón E. O., Mente P. L., Bohórquez D. V., Santos A. A. Jr, Grimes J. L., Richards J. D., Dibner J. J., and Felts V.. 2009. Organic trace minerals and 25-hydroxycholecalciferol affect performance characteristics, leg abnormalities, and biomechanical properties of leg bones of turkeys. Poult. Sci. 88:118–131. doi: 10.3382/ps.2008-00200 [DOI] [PubMed] [Google Scholar]
- Frantz N. Z., Andrews G. A., Tokach M. D., Nelssen J. L., Goodband R. D., Derouchey J. M., and Dritz S. S.. 2008. Effect of dietary nutrients on osteochondrosis lesions and cartilage properties in pigs. Am. J. Vet. Res. 69:617–624. doi: 10.2460/ajvr.69.5.617 [DOI] [PubMed] [Google Scholar]
- Frantz N. Z., Friesen K. G., Andrews G. A., Tokach M. D., Yamka R. M., Loughin T. L., Nelssen J. L., and Dritz S. S.. 2010. Use of serum biomarkers to predict the development and severity of osteochondrosis lesions in the distal portion of the femur in pigs. Am. J. Vet. Res. 71:946–952. doi: 10.2460/ajvr.71.8.946 [DOI] [PubMed] [Google Scholar]
- Garvican E. R., Vaughan-Thomas A., Innes J. F., and Clegg P. D.. 2010. Biomarkers of cartilage turnover. Part 1: markers of collagen degradation and synthesis. Vet. J. 185:36–42. doi: 10.1016/j.tvjl.2010.04.011 [DOI] [PubMed] [Google Scholar]
- Gowanlock D. W., Mahan D. C., Jolliff J. S., Moeller S. J., and Hill G. M.. 2013. Evaluating the NRC levels of Cu, Fe, Mn, and Zn using organic and inorganic mineral sources for grower-finisher swine. J. Anim. Sci. 91:5680–5686. doi: 10.2527/jas.2013-6608 [DOI] [PubMed] [Google Scholar]
- Grevenhof E. M., van Heuven H. C. M., van Weeren P. R., and Bijma P.. 2012. The relationship between growth and osteochondrosis in specific joints in pigs. Livest. Sci. 143:85–90. doi: 10.1016/j.livsci.2011.09.002 [DOI] [Google Scholar]
- Heinonen M., Peltoniemi O., and Valros A.. 2013. Impact of lameness and claw lesions in sows on health and production. Livest. Sci. 156:64–70. doi: 10.1016/j.livsci.2013.06.012 [DOI] [Google Scholar]
- Hepburn N., Knight C., Wilde C., Hendry K., and Galbraith H.. 2008. L-methionine uptake, incorporation and effects on proliferative activity and protein synthesis in bovine claw tissue explants in vitro. J. Agric. Sci. 146:103–115. doi: 10.1017/S0021859607007393 [DOI] [Google Scholar]
- Jensen T. B., Bonde M. K., Kongsted A. G., Toft N., Sørensen J. T.. 2010. The interrelationships between clinical signs and their effect on involuntary culling among pregnant sows in group-housing systems. Animal. 4:1922–1928. doi: 10.1017/S1751731110001102 [DOI] [PubMed] [Google Scholar]
- KilBride A. L., Gillman C. E., and Green L. E.. 2009. a cross sectional study of the prevalence, risk factors and population attributable fractions for limb and body lesions in lactating sows on commercial farms in England. BMC Vet. Res. 5:30. doi: 10.1186/1746-6148-5-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning D. B., van Grevenhof E. M., Laurenssen B. F., Hazeleger W., and Kemp B.. 2015. Associations of conformation and locomotive characteristics in growing gilts with osteochondrosis at slaughter. J. Anim. Sci. 93:93–106. doi: 10.2527/jas.2014-8366 [DOI] [PubMed] [Google Scholar]
- de Koning D. B., van Grevenhof E. M., Laurenssen B. F., van Weeren P. R., Hazeleger W., and Kemp B.. 2014. The influence of floor type before and after 10 weeks of age on osteochondrosis in growing gilts. J. Anim. Sci. 92:3338–3347. doi: 10.2527/jas.2014-7902 [DOI] [PubMed] [Google Scholar]
- Le T. H., Madsen P., Lundeheim N., Nilsson K., and Norberg E.. 2016. Genetic association between leg conformation in young pigs and sow longevity. J. Anim. Breed. Genet. 133:283–290. doi: 10.1111/jbg.12193 [DOI] [PubMed] [Google Scholar]
- Lisgara Μ., Skampardonis V., and Leontides L.. 2016. Effect of diet supplementation with chelated zinc, copper and manganese on hoof lesions of loose housed sows. Porcine Health Manag. 2:6. doi: 10.1186/s40813-016-0025-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ma Y. L., Zhao J. M., Vazquez-Añón M., and Stein H. H.. 2014. Digestibility and retention of zinc, copper, manganese, iron, calcium, and phosphorus in pigs fed diets containing inorganic or organic minerals. J. Anim. Sci. 92:3407–3415. doi: 10.2527/jas2013-7080 [DOI] [PubMed] [Google Scholar]
- Liu B., Xiong P., Chen N., He J., Lin G., Xue Y., Li W., and Yu D.. 2016. Effects of replacing of inorganic trace minerals by organically bound trace minerals on growth performance, tissue mineral status, and fecal mineral excretion in commercial grower-finisher pigs. Biol. Trace Elem. Res. 173:316–324. doi: 10.1007/s12011-016-0658-7 [DOI] [PubMed] [Google Scholar]
- Lucia T., Dial G. D., and Marsh W. E.. 2000. Lifetime reproductive performance in female pigs having distinct reasons for removal. Livest. Prod. Sci. 63:213–222. doi: 10.1016/S0301-6226(99)00142-6 [DOI] [Google Scholar]
- Mustonen K., Ala-Kurikka E., Orro T., Peltoniemi O., Raekallio M., Vainio O., and Heinonen M.. 2011. Oral ketoprofen is effective in the treatment of non-infectious lameness in sows. Vet. J. 190:55–59. doi: 10.1016/j.tvjl.2010.09.017 [DOI] [PubMed] [Google Scholar]
- Nakano T., and Aherne F. X.. 1988. Involvement of trauma in the pathogenesis of osteochondritis dissecans in swine. Can. J. Vet. Res. 52:154–155. [PMC free article] [PubMed] [Google Scholar]
- NRC 1994. Nutrient requirements of poultry. 9th rev. ed National Academies Press, Washington, DC. [Google Scholar]
- NRC 1998. Nutrient requirements of swine. 10th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Olsson A. C., Svendsen J., Botermans J., and Bergsten C.. 2016. An experimental model for studying claw lesions in growing female pigs. Livest. Sci. 184:58–63. doi: 10.1016/j.livsci.2015.12.005 [DOI] [Google Scholar]
- Olstad K., Kongsro J., Grindflek E., and Dolvik N. I.. 2014. Consequences of the natural course of articular osteochondrosis in pigs for the suitability of computed tomography as a screening tool. BMC Vet. Res. 10:212. doi: 10.1186/s12917-014-0212-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth M. W. 1999. The regulation of growth plate cartilage turnover. J. Anim. Sci. 77 (Suppl 2):183–189. doi: 10.2527/1999.77suppl_2183x [DOI] [PubMed] [Google Scholar]
- Ott E. A., and Asquith R. L.. 1989. The influence of mineral supplementation on growth and skeletal development of yearling horses. J. Anim. Sci. 67:2831–2840. doi: 10.2527/jas1989.67112831x [DOI] [PubMed] [Google Scholar]
- Ouattara A., Cooke D., Gopalakrishnan R., Huang T. H., and Ables G. P.. 2016. Methionine restriction alters bone morphology and affects osteoblast differentiation. Bone Rep. 5:33–42. doi: 10.1016/j.bonr.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A. A., Peo E. R. Jr, Cunningham P. J., and Moser B. D.. 1973. Chelated trace minerals for G-F swine. J. Anim. Sci. 37:95–103. doi: 10.2527/jas1973.37195x [DOI] [PubMed] [Google Scholar]
- Pluym L. M., Hoorebeke S., Lopez A., and Jeroen R.. 2011. Prevalence and risk factors of lameness and claw lesions in two types of group housing for pregnant sows. Vet. Med. (Praha). 3:2010. [Google Scholar]
- Pluym L., Van Nuffel A., and Maes D.. 2013a. Treatment and prevention of lameness with special emphasis on claw disorders in group-housed sows. Livest. Sci. 156:36–43. doi: 10.1016/j.livsci.2013.06.008 [DOI] [Google Scholar]
- Pluym L. M., Van Nuffel A., Van Weyenberg S., and Maes D.. 2013b. Prevalence of lameness and claw lesions during different stages in the reproductive cycle of sows and the impact on reproduction results. Animal 7:1174–1181. doi: 10.1017/S1751731113000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn A. J., Green L. E., Lawlor P. G., and Boyle L. A.. 2015. The effect of feeding a diet formulated for developing gilts between 70kg and ~140kg on lameness indicators and carcass traits. Livest. Sci. 174:87–95. doi: 10.1016/j.livsci.2014.12.016 [DOI] [Google Scholar]
- Reese D. E., and Hill G. M.. 2010. Trace minerals and vitamins for swine diets. In: Meisinger D. J., editor. National swine nutrition guide National Pork Board. Ames IA: p. 37–52. [Google Scholar]
- Riet M. M. J., Millet S., Aluwé M., and Janssens G. P. J.. 2013. Impact of nutrition on lameness and claw health in sows. Livest. Sci. 156:24–35. doi: 10.1016/j.livsci.2013.06.005 [DOI] [Google Scholar]
- Schweer W. P., Mendoza O. F., Shull C. M., Lehman J., Gaines A. M., Schwartz K. J., and Gabler N. K.. 2018. Increased lysine: ME ratio improves grower pig performance during a PRRSV challenge. J. Anim. Sci. 96 (Suppl.2):24–25. doi: 10.1093/jas/sky073.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. T., Rozeboom D. W., Hill G. M., Orth M. W., Rosenstein D. S., and Link J. E.. 2006. Impact of supplement withdrawal and wheat middling inclusion on bone metabolism, bone strength, and the incidence of bone fractures occurring at slaughter in pigs. J. Anim. Sci. 84:1138–1146. [DOI] [PubMed] [Google Scholar]
- Tomlinson D. J., Mülling C. H., and Fakler T. M.. 2004. Invited review: formation of keratins in the bovine claw: roles of hormones, minerals, and vitamins in functional claw integrity. J. Dairy Sci. 87:797–809. doi: 10.3168/jds.S0022-0302(04)73223-3 [DOI] [PubMed] [Google Scholar]
- Tóth F., Torrison J. L., Harper L., Bussieres D., Wilson M. E., Crenshaw T. D., and Carlson C. S.. 2016. Osteochondrosis prevalence and severity at 12 and 24 weeks of age in commercial pigs with and without organic-complexed trace mineral supplementation. J. Anim. Sci. 94:3817–3825. doi: 10.2527/jas.2015-9950 [DOI] [PubMed] [Google Scholar]
- Tybirk P. E. R. 2015. Nutrient recommendations for pigs in Denmark. 17th ed SEGES-VSP Danis Pig Res. Cent, Copenhague, DK. [Google Scholar]
- Varagka N., Lisgara M., Skampardonis V., Psychas V., and Leontides L.. 2016. Partial substitution, with their chelated complexes, of the inorganic zinc, copper and manganese in sow diets reduced the Laminitic lesions in the claws and improved the morphometric characteristics of the hoof horn of sows from three Greek herds. Porcine Health Manag. 2:26. doi: 10.1186/s40813-016-0040-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veum T. L., Ledoux D. R., Shannon M. C., and Raboy V.. 2009. Effect of graded levels of iron, zinc, and copper supplementation in diets with low-phytate or normal barley on growth performance, bone characteristics, hematocrit volume, and zinc and copper balance of young swine. J. Anim. Sci. 87:2625–2634. doi: 10.2527/jas.2008-1604 [DOI] [PubMed] [Google Scholar]
- Yazdi M. H., Rydhmer L., Ringmar-Cederberg E., Lundeheim N., and Johansson K.. 2000. Genetic study of longevity in Swedish landrace sows. Livest. Prod. Sci. 63:255–264. doi: 10.1016/S0301-6226(99)00133-5 [DOI] [Google Scholar]
- Ytrehus B., Carlson C. S., and Ekman S.. 2007. Etiology and pathogenesis of osteochondrosis. Vet. Pathol. 44:429–448. doi: 10.1354/vp.44-4-429 [DOI] [PubMed] [Google Scholar]