Abstract
This study aimed to determine the influence of finishing diet on beef appearance and lipid oxidation of three beef muscles. A total of 18 Angus steers were selected from three diet treatments: grass-finished (USUGrass), legume-finished (USUBFT), and grain-finished (USUGrain). After processing, longissimus thoracis (LT), triceps brachii (TB), and gluteus medius (GM) steaks were evaluated over a 7-d display period. A muscle × diet interaction was observed for instrumental lightness (L*) and redness (a*) (P ≤ 0.001). Within each combination, USUGrass was considered darker with lower (P < 0.05) L* compared with USUGrain. For USUBFT, L* was similar to USUGrain for the TB and LT, while the L* of USUBFT and USUGrain GM differed (P < 0.05). In terms of redness, LT a* values were elevated (P < 0.05) in USUGrass compared with USUBFT and USUGrain. For GM steaks, a* of USUBFT and USUGrass were each greater (P < 0.05) than USUGrain. Surface a* of TB steaks were greatest (P < 0.05) for USUGrass followed by USUBFT, and with USUGrain, being lowest (P < 0.05). An overall increase in L* was observed throughout display dependent on diet (P = 0.013). During display, USUGrain steaks had the greatest (P < 0.05) L* followed by USUBFT and USUGrass. Additionally, a day × muscle interaction was observed for a* (P = 0.009). Initially, TB steaks had the greatest (P < 0.05) a* values. However, at day 3, a* values were similar (P > 0.05) among muscles. Visual color scores were in agreement with loss of redness (a*) during display, dependent on diet and muscle type (P < 0.001). Similarly, a day × diet × muscle interaction was observed for visual discoloration (P < 0.001). Day and diet interacted to influence thiobarbituric acid reactive substances (TBARS) (P < 0.001). Initial values did not differ (P > 0.05) between USUGrain and USUBFT; however, USUGrass had lower initial (P < 0.05) TBARS than both USUGrain and USUBFT. At days 3 and 7, TBARS were greatest (P < 0.05) in USUGrain steaks, followed by USUBFT, which was greater (P < 0.05) than USUGrass. A diet × muscle interaction was observed for 10 volatile compounds originating from lipid degradation (P ≤ 0.013). These compounds were less (P < 0.05) abundant in USUGrass compared to TB or GM of USUGrain. This study determined grass-finished beef to have a darker more red color and less lipid oxidation in multiple muscles. Possible mechanisms for this may include an increase in endogenous antioxidants in grass-finished beef.
Keywords: beef color, finishing diet, lipid oxidation, retail display, TBARS
INTRODUCTION
Consumers have expressed interest in nonconventionally finished beef due to perceived health benefits (McCluskey et al., 2005). Although forage finishing diets were previously explored for impacts on palatability, less is known about their impacts on beef appearance and shelf life. Meat appearance can greatly impact consumer purchasing decisions (Mancini and Hunt, 2005). Visual appeal decreases during retail display (Jeremiah and Gibson, 2001). Loss of suitable beef color during retail display may have significant economic impacts through price reductions or product loss.
Finishing diet of cattle can affect the final fatty acid composition of beef (Chail et al., 2016). Forage-finished beef has a greater ratio of polyunsaturated fatty acids (PUFA) to saturated fatty acids (French et al., 2000). Previously, PUFA of beef were described to have great susceptibility to oxidation (Wood et al., 2008), which may result in detrimental off-odors and off-flavors in final product (Calkins and Hodgen, 2007). Furthermore, oxidation of lipids has been related to beef color discoloration and accumulation of metmyoglobin (Greene and Price, 1975). Therefore, the greater proportion of PUFA in the fat of forage-finished beef could increase oxidation and color discoloration. However, forage-finished beef has been cited to possess increased antioxidant capacity due to the accumulation of antioxidant species (Wu et al., 2008).
Recently, finishing with the legume birdsfoot trefoil was determined to improve perceived palatability compared with grass finishing and to improve chemical composition in comparison with feedlot finishing (Chail et al., 2016) Furthermore, it was previously demonstrated that finishing diet and muscle type interacted to influence beef composition and quality (Chail et al., 2017). These studies indicated that a legume-finishing diet could improve beef quality relative to grass finishing. They also indicated that this impact had some dependency on muscle type in forage-finished beef. However, it is unclear how these forage diets impact beef color and appearance during retail display. Therefore, the objective of this study was to determine the influence of finishing diet on beef appearance and lipid oxidation of three beef muscles.
MATERIALS AND METHODS
Animal Care and Use
All animal procedures and protocols in this study were approved by the Utah State University (USU) Animal Care and Use committee, IACUC #1493.
Cattle Finishing and Harvest
All cattle production and harvest procedures are described in detail by Chail et al. (2016). A total of 18 Angus steers were selected from the USU beef herd. Diet treatments are described as follows: six Angus steers were finished on tall fescue [Schedonorus arundinaceus (Schreb.) Dumort] grass for 6 wk and then moved on to meadow bromegrass (Bromus riparius Rehmann) (grass-finished; USUGrass); Six steers were fed on birdsfoot trefoil (Lotus corniculatus) (legume-finished; USUBFT); the remaining six steers were feedlot-finished in a single pen on a concentrate diet of high-starch cereal (grain-finished; USUGrain). After 111 d on each finishing diet, animals were slaughtered at the USU Matthew Hillyard Animal Teaching and Research Center (Wellsville, UT), at approximately 18 mo of age and at 416–490 kg of weight. Carcasses were chilled for 24 h at 2–4 °C prior to fabrication.
Product Fabrication
Three different boneless subprimals; ribeye roll (IMPS # 112; NAMP, 2010), top sirloin butt (IMPS # 184, NAMP, 2010), and shoulder clod (IMPS # 114, NAMP, 2010) were collected from each carcass (n = 6 per diet). Subprimals were wet-aged under vacuum for 14 d at 2–4 °C prior to fabrication into retail steaks. After removal of the trapezius, serratus dorsalis and longissimus costarum, and related intermuscular fat, ribeye steaks were produced by hand cutting 2.5-cm-thick steaks, progressing anterior to posterior, and trimming external fat to 0.32 cm thickness. The spinalis dorsi, complexus, and multifidus dorsi muscles remained intact with the longissimus thoracis (LT) of ribeye steaks throughout the trial. However, all analysis was collected only from the LT muscle. Top sirloin steaks of 2.5 cm thickness were prepared following the removal of the biceps femoris, gluteus accessories, and gluteus profundus, leaving only the gluteus medius (GM) muscle. Steaks were hand cut from the GM progressing anterior to posterior. The infraspinatus and teres major muscles were removed from the aged shoulder clod and beef arm steaks were produced from the triceps brachii (TB) muscle only. Prior to cutting steaks, the small elongated side muscle was removed from the center TB. Then the lateral head of the TB was removed at the natural seam between the lateral head and the long head of the TB. After separation, the exposed internal connective tissue previously located between the heads was removed. Finally, 2.5-cm-thick steaks were cut from the long head of the TB by hand cutting perpendicular to the muscle fibers. Steaks intended for simulated retail display were individually packaged on foam trays with absorbent pads and overwrapped with a single layer of PVC film (O2 permeability = 8,400 cm3/(24 h × m2 × atm.) at 23 °C; water vapor transmission = 83 g/(24 h × m2) at 23 °C and 50% relative humidity).
Simulated Retail Display, Instrumental, and Visual Assessment
Three subsets of steaks were utilized to represent initial (0 d), mid (3 d), and late (7 d) stages of retail display. Steaks representing 0 d of retail display were initially packaged, as described above, evaluated for instrumental and visual color, as described below, before being individually vacuum packaged and frozen at −20 °C until further analysis.
Simulated retail display occurred for 7 d in a walk-in cooler (2–4 °C). Packaged steaks representing 3 d and 7 d of display were placed as a single layer on four stainless-steel shelves under continuous fluorescent lighting (3,500 K/75 CRI) at a distance of 35.6 cm between steaks and light source. A completely randomized block design was utilized where equal number of each steak type was randomly assigned to an initial location on each shelf. Within each shelf, steaks were rotated daily to eliminate location/lighting bias within a shelf. Columns of steaks (n = 3) were rotated every 24 h from left to right under the light source. The far right column of packages was rotated to the far left position each time. Additionally, steaks within a row were rotated one position within a column every 24 h. As 3-d steak packages were removed for freezing, empty trays were placed in their positions in order to maintain the rotation scheme. Steaks representing 0 d were evaluated under the same lighting as described below within 2 h of packaging and freezing. Similarly, steaks representing 3 d and 7 d were removed, vacuum packaged, and frozen at the designated interval.
Instrumental and visual attributes of steak surfaces were evaluated according to the AMSA guidelines (AMSA, 2012). Every 24 h, instrumental color (L* = lightness, a* = redness, and b* = yellowness) was measured with a Hunter Colorimeter (Miniscan XE, Hunter Associates Laboratory, Inc., Reston, VA)-Trained panelists (n ≥ 8) evaluated steaks for redness on an 8-point scale (1 = very bright red; 8 = tan to brown) and discoloration (6-point scale, 1 = none, 0% metmyoglobin formation; 6 = extensive discoloration, 81–100% metmyoglobin formation). Only the 7-d packages designated to be within the cooler for the entire duration of display were evaluated for color; additionally, panelists were only trained to evaluate the LT of ribeye steaks.
Sample Preparation for Chemical Assessment
Chemical changes during display were assessed through measurement of thiobarbituric acid reactive substances (TBARS) and volatile compounds on days 0, 3, and 7 of simulated retail display (2–4 °C). Raw steaks were thawed for 24 h at 2–4 °C. External fat and muscle and residual connective tissue were removed, leaving only TB, GM, and LT muscles. Muscle samples were cubed, submerged in liquid nitrogen, placed in a blender (VITA-MIX Corp, Cleveland, OH; model #VM0100A), and pulverized to form a finely powdered homogenate. Powdered samples were packed in VWR sample bags (BPR-4590 VW1, Radnor, PA) and stored at −80 °C for subsequent analysis (Martin et al., 2013).
Thiobarbituric Acid Reactive Substances
Procedures outlined by Buege and Aust (1978) with modifications from Luque et al. (2011) were used to determine TBARS values (mg malondialdehyde/kg meat homogenate). In brief, 10 g of raw meat homogenate was blended with 30 mL of distilled water prior to centrifugation (1,850 × g; room temperature; 10 min). The resulting supernatant was combined with trichloroacetic acid, thiobarbituric acid, and butylated hydroxyanisole (antioxidant). Samples were then heated in a water bath (100 °C) for 15 min before being submerged in an ice water bath for 10 min. The chilled sample was again centrifuged (1,850 × g; room temperature; 10 min), and the absorbance of the final supernatant was determined at 531 nm.
Volatile Compound Analysis
Volatile compound analysis was carried out as outlined by Chail et al. (2016). However, in this study, volatile compounds were evaluated from raw beef homogenates. Five grams of the raw homogenate was weighed into 20-mL glass vials (093640-036-00; Gerstel; Linthicum, MD) and closed with a polytetrafluoroethylene septa and screw cap (093640-092-00; Gerstel). Ten microliters of internal standard (1, 2-dicholorobenzene; 0.801mg/ mL) was added and the vial was loaded by a Gerstel automated sampler (MPS, Linthicum, MD) for a 5-min incubation period at 50 °C in the Gerstel agitator (500 rotations per min) followed by 20 min of extraction where volatile compounds were collected from the headspace of raw samples by solid phase microextraction using an 85-µm film thickness carboxen polydimethylsiloxane fiber (Supelco, Bellefonte, PA). Extracted volatile compounds were injected on a VF-5 ms capillary column (30 m × 0.25 mm × 1.00 µm; Agilent J&W GC Columns, Santa Clara, CA). Authentic standards were purchased from Sigma-Aldrich (St. Louis, MO) and used to validate compound identities through comparison of retention times and ion fragmentation patterns. Quantitation was carried out by an internal standard calibration with the same authentic standards.
Statistical Analysis
Color attributes were analyzed as a 3 × 3 factorial arrangement (finishing diet × muscle) repeated measures (time) design with the SAS MIXED procedure (SAS Inst. Inc., Cary, NC, version 9.4). The Satterthwaite approximation was used to determine denominator degrees of freedom. The Akaike Information Criterion (AIC) was used as the best model for repeated measures. The lowest AIC value among compound symmetry (CS), heterogeneous CS, autoregressive (AR[1]), and heterogeneous AR[1] was utilized.
Chemical attributes were evaluated at days 0, 3, and 7 using the MIXED procedure of SAS with finishing diet, muscle, and day as fixed effects in a factorial arrangement. In all ANOVA analyses, subprimal was considered the experimental unit. Likewise, carcass and display shelf were considered random effects. Following all ANOVA analyses where F-tests were significant (P < 0.05), least squares means were separated using the PDIFF option of LSMEANS. All comparisons were considered significant at α = 0.05 or less.
RESULTS
Instrumental Color
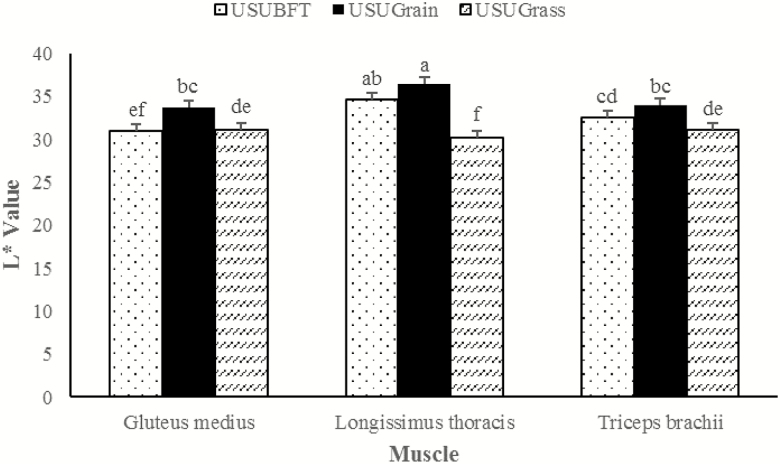
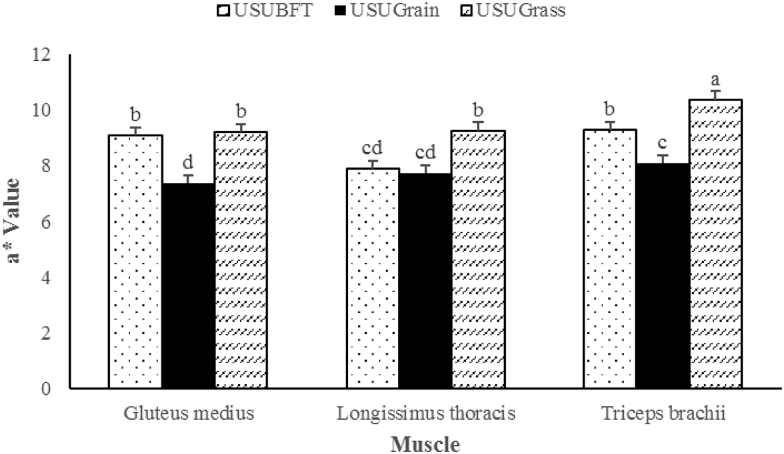
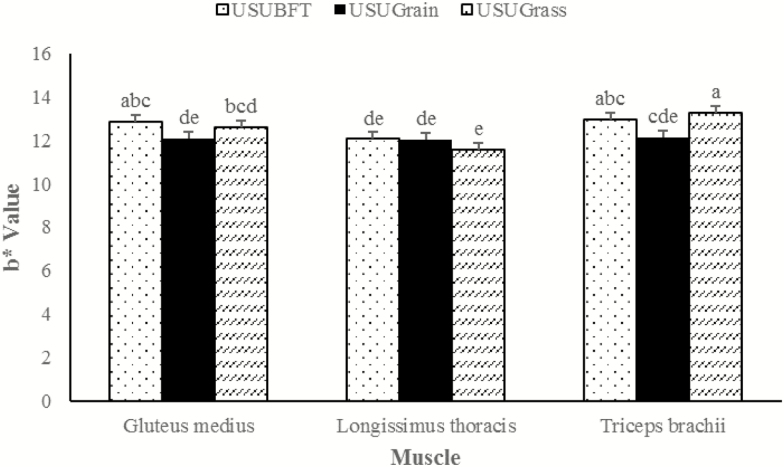
A muscle × diet interaction was observed for instrumental lightness (L*), redness (a*), and yellowness (b*) (P ≤ 0.001), wherein each muscle performed differently relative to diet. For the LT, L* values for USUBFT and USUGrain were greater than USUGrass (P < 0.05) (Figure 1). In the GM, L* values for USUGrain were greater than (P < 0.05) USUBFT and USUGrass. In TB, beef L* values did not differ for USUGrain and USUBFT (P > 0.05) but USUGrain had greater (P < 0.05) L* values than USUGrass. The LT a* values (Figure 2) were elevated (P < 0.05) in USUGrass compared with USUBFT and USUGrain, while a* values for USUBFT and USUGrain did not differ (P > 0.05). For GM steaks, the a* values of USUBFT and USUGrass were similar (P > 0.05) and greater (P < 0.05) than for USUGrain. Surface a* values of TB steaks were greater (P < 0.05) for USUGrass than for USUBFT, while the a* values for USUBFT were greater than USUGrain (P < 0.05). Diet and muscle also influenced b* values (P < 0.001; Figure 3). For the LT, b* did not differ (P > 0.05) among diets. However, b* of GM of USUBFT was greater (P < 0.05) than USUGrain, while b* of GM of USUGrass did not differ from USUBFT or USUGrain (P > 0.05). The TB b* values were greater (P < 0.05) for USUGrass than USUGrain, but b* values for USUBFT and USUGrass did not differ (P > 0.05).
Figure 1.
Lightness (L*) values during retail display of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii, pooled average) were observed from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Two-way interaction (muscle × diet, P < 0.001) was observed. a,b,c,d,e,fLeast squares means lacking a common superscript differ (P < 0.05).
Figure 2.
Redness (a*) values during retail display of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii, pooled average) were observed from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Two-way interaction (muscle × diet, P < 0.001) was observed. a,b,c,dLeast squares means lacking a common superscript differ (P < 0.05).
Figure 3.
Yellowness (b*) values during retail display of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii, pooled average) were observed from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Two-way interaction (muscle × diet, P < 0.001) was observed. a,b,c,d,eLeast squares means lacking a common superscript differ (P < 0.05).
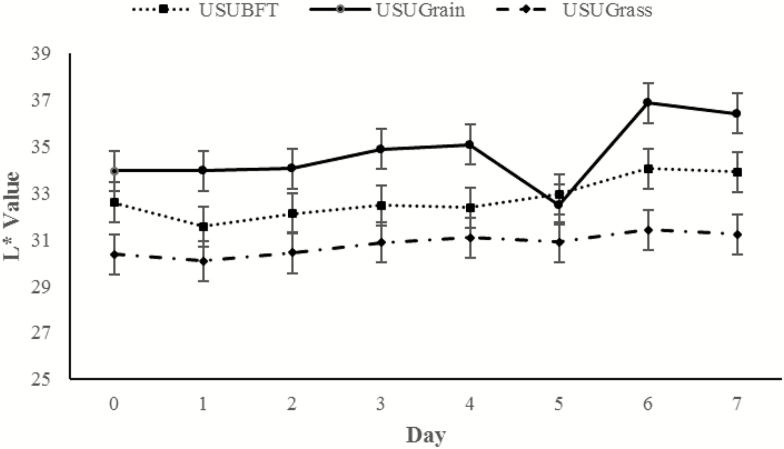
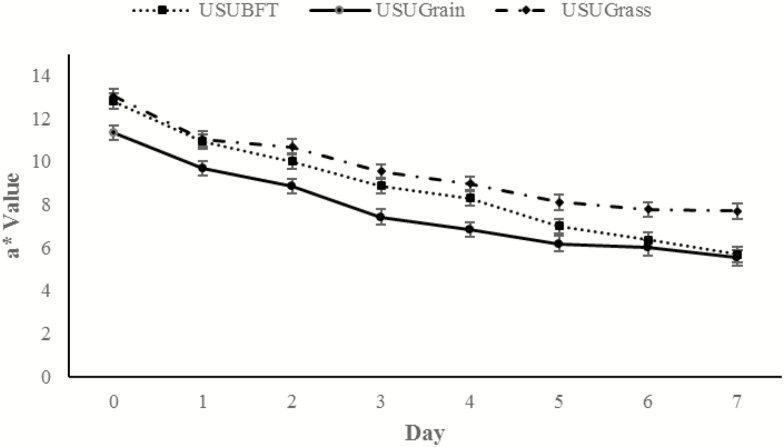
An overall increase in L* was observed throughout display time dependent on diet (P = 0.013; Figure 4). Throughout display time, USUGrain steaks had the greatest (P < 0.05) L* values followed by USUBFT and then USUGrass, with the exception of day 5 measurements. At day 5, the L* of USUGrain did not differ (P > 0.05) from USUBFT. Another day × diet interaction was observed for a*, which decreased overall within each muscle type throughout retail display time (P = 0.014; Figure 5). At day 0, a* values did not differ (P > 0.05) for USUBFT and USUGrass, and the a* values for both USUBFT and USUGrass were greater (P < 0.05) than USUGrain. However, by day 6, a* of USUBFT and USUGrain were similar (P > 0.05) and less than the a* of USUGrass steaks (P < 0.05).
Figure 4.
Lightness (L*) values during retail display of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii; pooled average) from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Two-way interaction (day × diet, P = 0.013) was observed.
Figure 5.
Redness (a*) values for cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass) during retail display for the pooled average of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii). Two-way interaction (day × diet, P = 0.014) was observed.
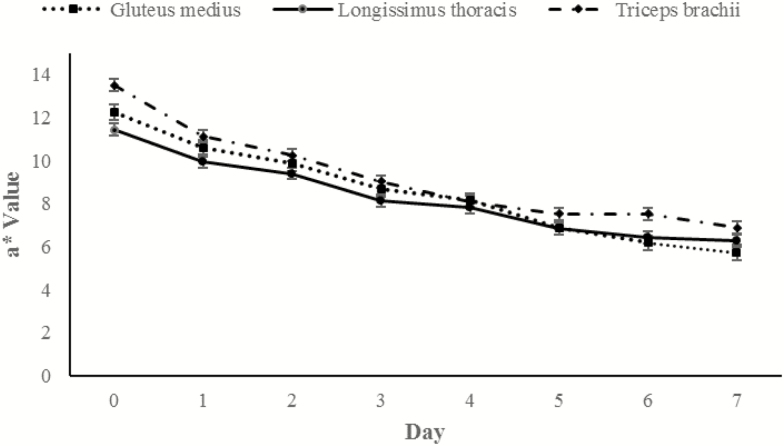
A day × muscle interaction was also observed in data for a* (P = 0.009; Figure 6). For each muscle a* values decreased throughout display time. At day 0, TB steaks had the greatest (P < 0.05) a* values, followed by GM and then LT which did not differ (P > 0.05). However, by day 3, a* values were similar (P > 0.05) for all three muscles. By day 6 of display, TB had the greatest (P < 0.05) a* values, while a* values of LT and GM did not differ (P > 0.05).
Figure 6.
a* Redness (a*) values during retail display of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii) for the pooled average of cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Two-way interaction (day × muscle, P = 0.009) was observed.
Visual Color
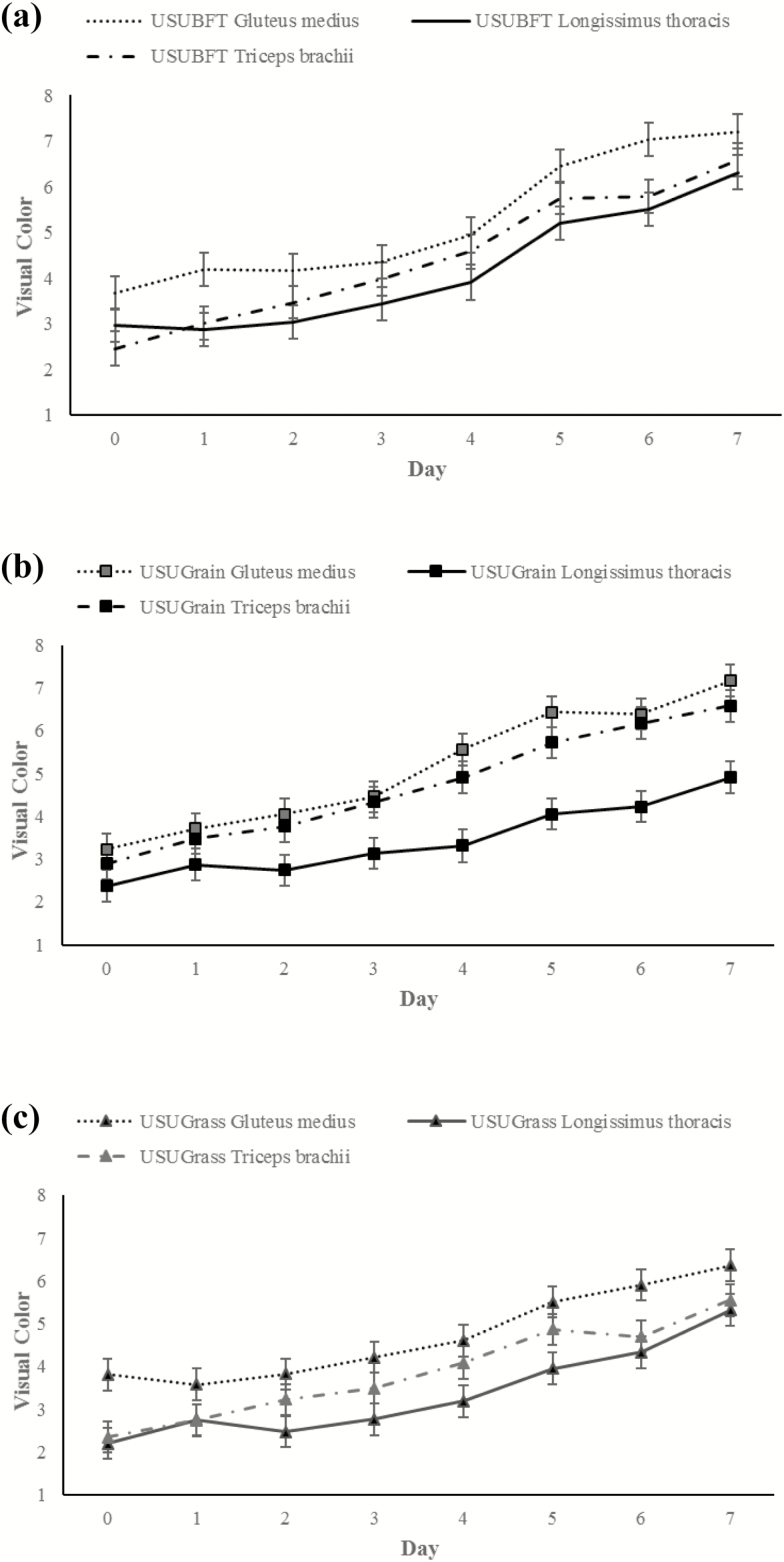
Visual redness was evaluated as a score of lean color redness and a three-way day × diet × muscle interaction was observed (P < 0.001; Figure 7). Visual redness scores increased throughout display time in diet and muscle treatments, with a steeper incline observed between 4 and 5 d of display. For all diets, the LT had consistently lower (P < 0.05) visual redness scores than the GM and TB, while the visual redness for GM and TB did not differ (P > 0.05). For the USUGrain diet, the LT had a consistently lower (P < 0.05) visual redness score than the GM and TB which did not differ (P > 0.05). For USUBFT, visual redness scores of the three muscle types did not differ (P > 0.05) during the display period except for days 1 and 6, when GM was greater (P < 0.05) than LT and TB. For USUGrass beef, the GM was greater (P < 0.05) than LT for the entire display period. The LT and TB visual redness scores were similar (P > 0.05) except for days 4 and 5, where the TB was greater (P < 0.05) than the LT.
Figure 7.
(a–c) Visual color scores (1 = very bright red and 8 = tan to brown, AMSA 2012) during retail display of gluteus medius, longissimus thoracis, and triceps brachii beef steaks from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Three-way interaction (day × diet × muscle, P < 0.001) was observed.
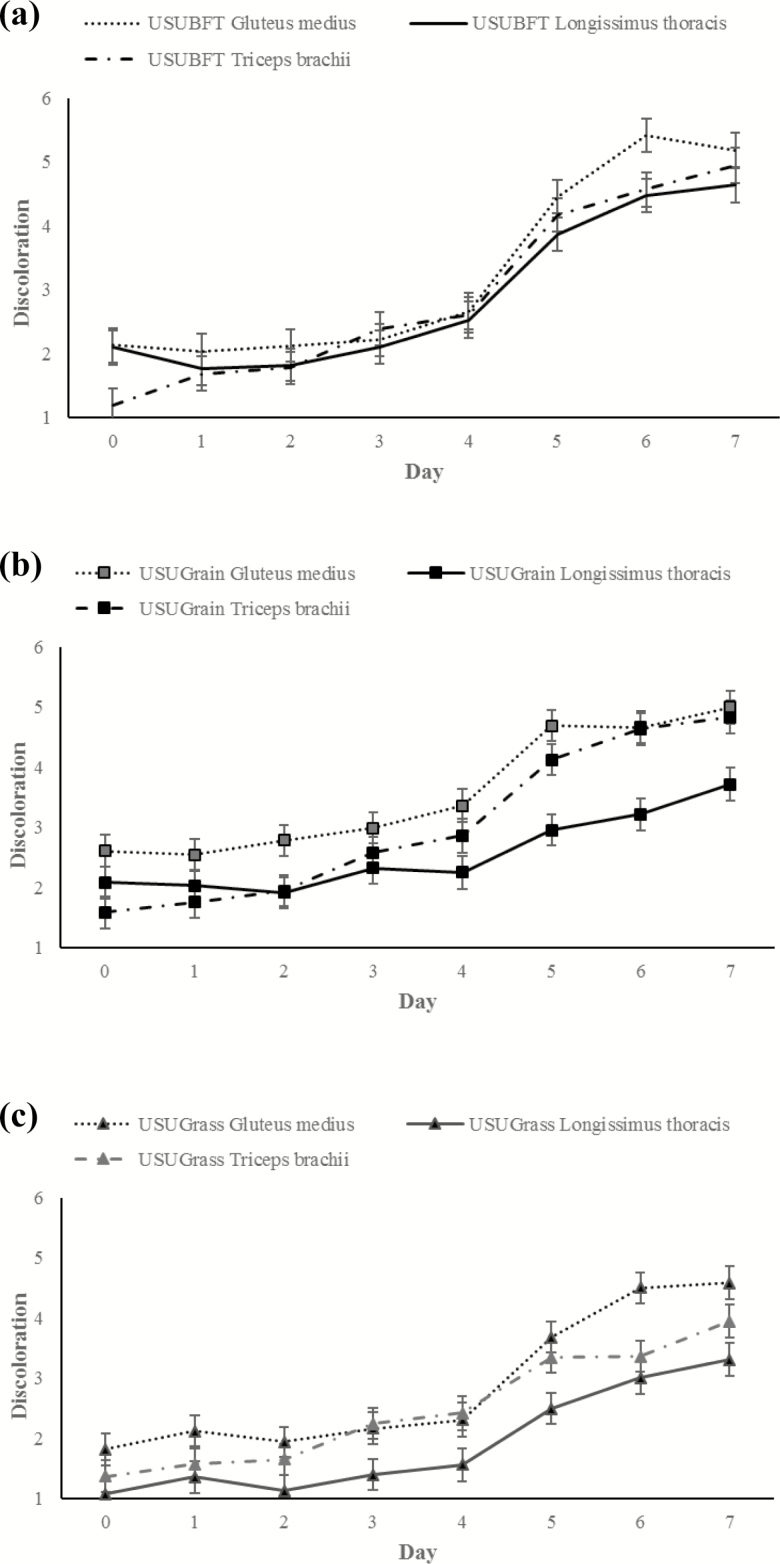
Similarly, a three-way day × diet × muscle interaction was observed for visual discoloration (P < 0.001; Figure 8). Overall, surface discoloration increased between days 0 and 7 within all diets and muscle treatments, and GM discoloration was greater (P < 0.05) than LT, while TB was intermediate. For USUGrain, at day 0 GM had the most (P < 0.05) discoloration followed by LT being greater (P < 0.05) than TB, which was lowest (P < 0.05). At day 4, GM was still the most (P < 0.05) discolored, and discoloration of the TB was greater (P < 0.05) than that of the LT. From days 4 to 7, USUGrain LT had less surface discoloration (P < 0.05) than GM and TB, which did not differ from one another (P > 0.05). In USUBFT at day 0, TB had a lower (P < 0.05) discoloration score than LT and GM, but scores were similar (P > 0.05) among muscles from days 1 to 5. On day 6, USUBFT GM showed greater (P < 0.05) discoloration than TB and LT, which did not differ (P > 0.05). For USUGrass, GM had greater (P < 0.05) discoloration than LT on all dates. The TB of USUGrass was similar (P < 0.05) to LT from days 0 to 2 and on day 6. At day 3, discoloration scores were similar (P > 0.05) for GM and TB and greater (P < 0.05) than for LT. By day 7, GM had the greatest (P < 0.05) surface discoloration in USUGrass, followed by TB and then LT, which was lowest (P < 0.05).
Figure 8.
(a–c) Visual discoloration (6-point scale, 1 = none, 0% metmyoglobin formation; 6 = extensive discoloration, 81–100% metmyoglobin formation) scores during retail display of gluteus medius, longissimus thoracis, and triceps brachii beef steaks from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Three-way interaction (day × diet × muscle, P < 0.001) was observed.
Chemical Assessment
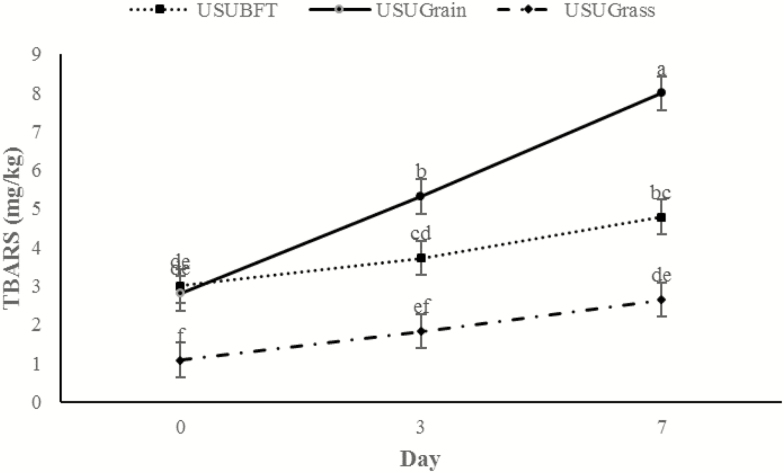
There was a day × diet interaction for TBARS (P < 0.001; Figure 9). Initial values (day 0) did not differ (P > 0.05) for USUGrain and USUBFT; however, USUGrass had lower (P < 0.05) TBARS than both USUGrain and USUBFT on all dates. By day 3, TBARS were greater (P < 0.05) for USUGrain steaks than for USUBFT steaks, which were greater (P < 0.05) than USUGrass. The TBARS of USUBFT and USUGrass steaks were greater (P < 0.05) on day 7 than on day 0. However, for USUGrain, TBARS increased (P < 0.05) throughout the display period.
Figure 9.
TBARS (mg malondialdehyde/kg wet tissue) values during retail display of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii; pooled average) from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Two-way interaction (day × diet, P < 0.001) was observed. a,b,c,d,e,fLeast squares means lacking a common superscript differ (P < 0.05).
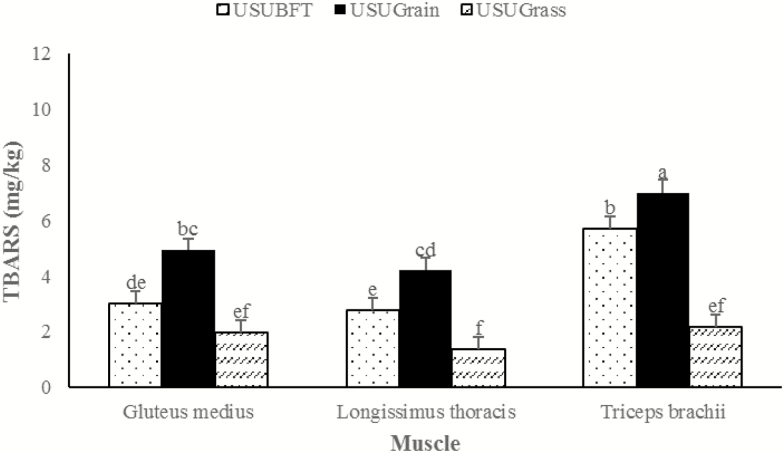
There was also a diet × muscle interaction (P = 0.032) for TBARS values. Within each muscle type, the USUGrain diet resulted in greater (P < 0.05) lipid oxidation than other diets (Figure 10). For the GM, TBAR values of USUBFT and USUGrass did not differ (P > 0.05). For the LT and TB, TBAR values of USUBFT were greater (P < 0.05) than USUGrass.
Figure 10.
Lipid oxidation was TBARS values during retail display of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii; pooled average) from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Two-way interaction (muscle × diet, P = 0.032) was observed. a,b,c,d,e,fLeast squares means lacking a common superscript differ (P < 0.05).
Ten volatile compounds had a diet × muscle interaction (P ≤ 0.013; Table 1). Octane concentration was elevated in TB across each diet, yet the magnitude of this difference was diet dependent (P = 0.006). Hexanal concentrations varied for muscle types within the USUGrain and USUBFT diets (P < 0.001), but for the USUGrass diet, values were similar for all three muscles types. Hexanal concentrations were elevated (P < 0.05) in the TB for USUGrain and USUBFT in comparison to LT and GM steaks. This pattern also occurred for heptanal (P < 0.001) and octanal (P < 0.001). Nonanal and 2-pentylfuran each showed elevated (P < 0.05) concentrations in TB within each diet treatment compared with corresponding LT and GM. However, between diets, nonanal and 2-pentylfuran of USUGrass TB were lower (P < 0.05) than TB of USUGrain or USUBFT. In USUGrain, hexanoic acid did not differ (P > 0.05) between TB and GM, and both were greater (P < 0.05) than LT. In USUBFT, hexanoic acid was greater (P < 0.05) in TB compared with GM and LT. For USUGrass, hexanoic acid did not differ (P < 0.05) between TB and GM while hexanoic acid content of the LT was less (P < 0.05). In USUGrain, 1-hexanol was elevated (P < 0.05) in GM compared with TB and LT. However, in both USUBFT and USUGrass 1-hexanol content did not differ (P > 0.05) among muscles. In each diet treatment, TB had the greatest (P < 0.05) concentrations of 1-heptanol. For USUBFT and USUGrass, 1-heptanol did not differ (P > 0.05) between GM and LT. However, for USUGrain, 1-heptanol content was greater (P < 0.05) in GM compared with LT. For each diet, 1-octen-3-ol content of TB was greater (P < 0.05) than GM and LT, which did not differ (P > 0.05). Across diets, 1-octen-3-ol content of USUGrain TB was greater (P < 0.05) than USUBFT TB, while USUBFT TB was greater (P < 0.05) than USUGrass TB. For the LT, USUGrain 1-octen-3-ol content was greater (P < 0.05) than USUBFT and USUGrass, which did not differ (P > 0.05). Finally, 1-octen-3-ol content of the USUBFT GM did not differ (P > 0.05) from other diet treatments; however, USUGrain GM 1-octen-3-ol was greater (P < 0.05) than USUGrass GM.
Table 1.
Effects of beef finishing diet* and muscle2† on concentrations (ng/g of sample) of volatile compounds from raw steaks during retail display‡
| Volatile compounds | Finishing diet and muscle | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| USUGrain | USUBFT | USUGrass | |||||||||
| GM | LT | TB | GM | LT | TB | GM | LT | TB | SEM§ | P value | |
| Alkane | |||||||||||
| Octane | 0.73bcd | 0.93bc | 2.00a | 0.30d | 0.22d | 2.10a | 0.27d | 0.39cd | 1.09b | 0.28 | 0.006 |
| Aldehydes | |||||||||||
| Hexanal | 22.73cd | 40.78bc | 81.84a | 2.15d | 17.23cd | 62.39ab | 2.02d | 1.04d | 7.29d | 12.97 | <0.001 |
| Heptanal | 1.51c | 1.67c | 5.24b | 0.51c | 1.38c | 7.25a | 0.24c | 0.13c | 1.25c | 0.93 | <0.001 |
| Octanal | 2.94b | 2.59b | 7.57a | 2.16bc | 1.46bc | 7.96a | 1.17bc | 0.55c | 2.34b | 0.77 | <0.001 |
| Nonanal | 5.35b | 4.76b | 12.87a | 2.67bcd | 2.60cd | 11.29a | 1.37d | 1.13d | 4.94bc | 1.01 | 0.002 |
| Furans | |||||||||||
| 2-Pentyl furan | 1.74bcd | 1.97bc | 3.33a | 0.84de | 1.28cde | 2.61ab | 0.72e | 0.89de | 1.30cd | 0.33 | 0.012 |
| Carboxylic acids | |||||||||||
| Hexanoic acid | 4.10a | 2.57c | 3.78ab | 1.43d | 1.06de | 2.98bc | 0.72de | 0.55e | 1.17de | 0.37 | 0.011 |
| Alcohols | |||||||||||
| 1-Hexanol | 33.30a | 10.97b | 11.04b | 11.41b | 4.03bc | 4.43bc | 0.86c | 0.38c | 1.50c | 3.54 | <0.001 |
| 1-Heptanol | 1.57bc | 1.13de | 2.29a | 0.69f | 0.83ef | 1.82b | 0.60f | 0.59f | 1.25cd | 0.13 | 0.013 |
| 1-Octen-3-ol | 3.95cd | 5.03c | 11.52a | 1.88de | 1.79de | 7.69b | 0.74e | 0.96e | 2.91cd | 1.03 | <0.001 |
*Finishing diets (D) included, grain (USUGrain), birdsfoot trefoil (USUBFT), and grass (USUGrass).
†Muscles included; GM, LT, and TB.
‡Display under fluorescent lighting for 7 d with sampling at day 0, 3, and 7. LS means displayed as pooled averages across days due to nonsignificant three-way interaction.
§Pooled (largest) SE of LS mean.
abcdWithin a row, least squares means without a common superscript differ (P < 0.05) due to diet × muscle interaction.
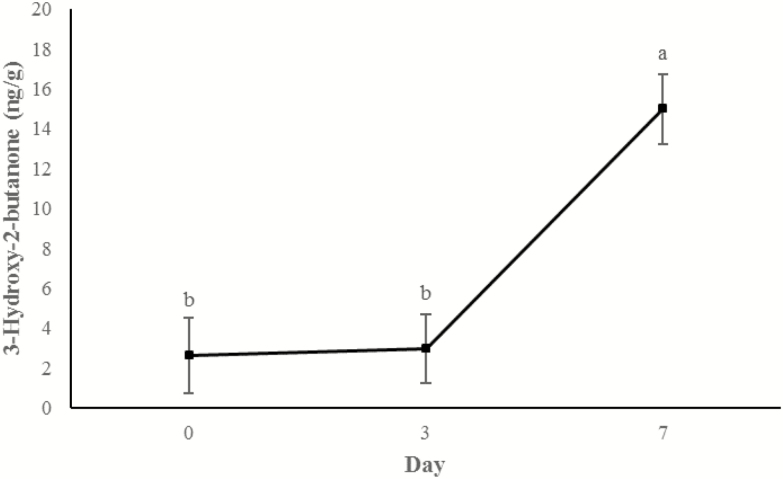
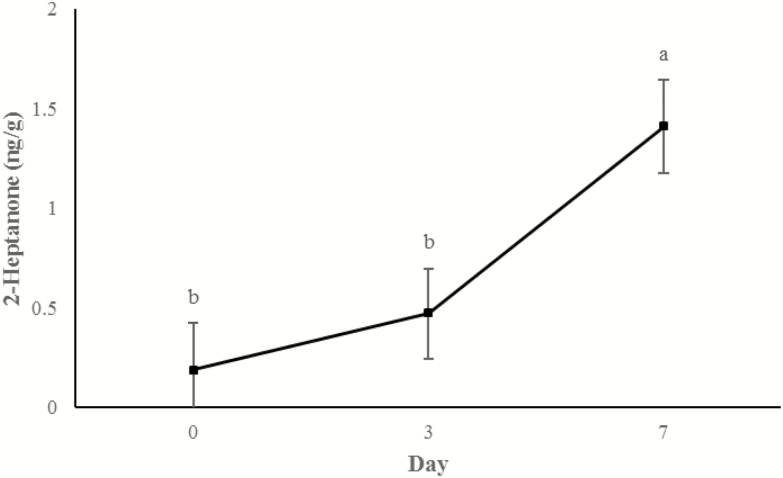
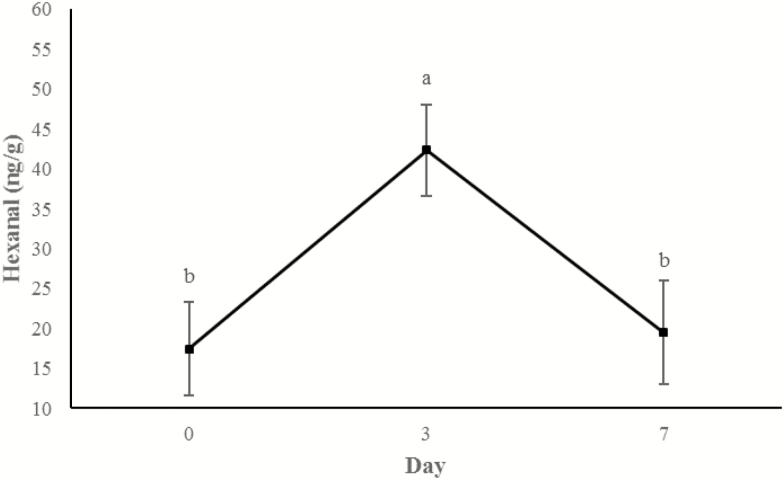
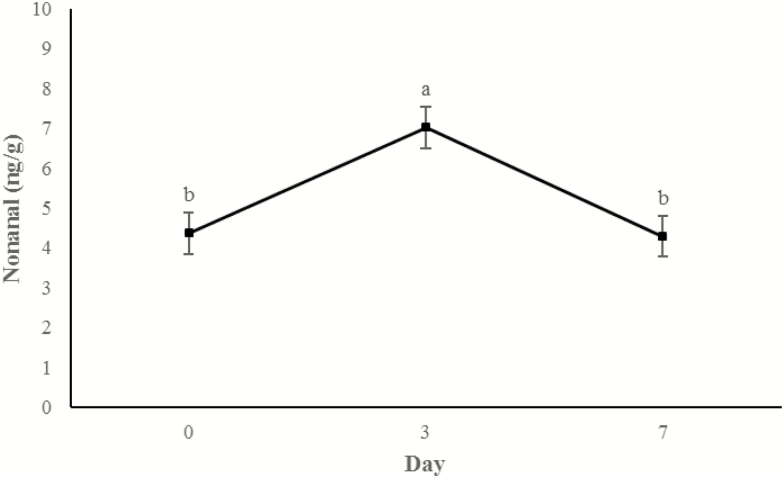
Concentrations of 3-hydroxy-2-butanone (Figure 11) and 2-heptanone (Figure 12) did not change from days 0 to 3, but increased between 3 d and 7 d of storage (P < 0.0001). An additional display time effect was observed for hexanal (P < 0.001; Figure 13) and nonanal (P < 0.001; Figure 14) wherein concentrations increased (P < 0.05) from day 0 to 3 and then decreased (P < 0.05) from days 3 to 7 to day 0 concentrations (P > 0.05).
Figure 11.
Content of 3-hydroxy-2-butanone (ng/g raw wet tissue) values during retail display of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii) from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Main effect of day (P < 0.001) is illustrated. a,bLeast squares means lacking a common superscript differ (P < 0.05).
Figure 12.
Content of 2-heptanone (ng/g raw wet tissue) values during retail display of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii) from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Main effect of day (P < 0.001) is illustrated. a,bLeast squares means lacking a common superscript differ (P < 0.05).
Figure 13.
Content of hexanal (ng/g raw wet tissue) values during retail display of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii) from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Main effect of day (P < 0.001) is illustrated. a,bLeast squares means lacking a common superscript differ (P < 0.05).
Figure 14.
Content of nonanal (ng/g raw wet tissue) values during retail display of three beef muscles (gluteus medius, longissimus thoracis, and triceps brachii) from cattle finished with different diets (grain, USUGrain; birdsfoot trefoil, USUBFT; grass, USUGrass). Main effect of day (P < 0.001) is illustrated. a,bLeast squares means lacking a common superscript differ (P < 0.05).
DISCUSSION
The current investigation found grass-finished beef to have a darker, more red color, with less visual discoloration than grain-finished beef; birdsfoot trefoil (legume) finished beef was intermediate. Previous studies have indicated that grass-finishing produces darker beef with lower lightness (L*) and greater redness (a*) than grain finishing (Bidner et al., 1981; Schroeder et al., 1980; Realini et al., 2004). Schroeder et al. (1980) associated this with a dark cutting defect; however, consumer color preferences may have since evolved. Indeed, instrumental redness measurements have more recently predicted consumer acceptance, with the threshold of a* ≤ 14.5 (Holman et al., 2017). The current study also found TB and GM to be more color labile than LT. Loins are well-known for their color stability throughout postmortem aging and display, especially as compared with color-labile GM (Lanari et al., 1996). However, GM and TB from USUGrass and USUBFT had increased a* compared with their counterparts of USUGrain. Therefore, it could be surmised that both grass and legume forage finishing result in improved color in color-labile cuts compared with grain-finishing. It should be noted that L* values of USUGrain were unexplainably low at day 5 of the trial. All experimental data was confirmed to be measured correctly. However, readers should take care to evaluate the overall trend in L*, as we have done with our interpretation, rather than the unexplained decrease at day 5.
Previous studies have found a decrease in lipid oxidation of raw pasture-finished beef, possibly due to the higher level of endogenous antioxidants such as alpha-tocopherol or beta-carotene (Realini et al., 2004; Descalzo et al., 2007). Additionally, more oxidative muscle types (e.g., GM) are known to be more susceptible to lipid oxidation (Wood et al., 2004; Faustman et al., 2010). The current investigation finds that both grass- and legume-finishing results in less lipid oxidation across muscle types and throughout postmortem display; together with the discoloration scores, this suggests that these diets, rich in antioxidants, may be utilized to improve color and oxidative stability in otherwise labile muscles.
A number of lipid-derived volatile compounds were identified as having differential concentrations between varying muscle type and diet, with LT and/or USUGrass treatments typically having lower concentrations. Although these results are substantiated by our color and TBARS findings, previous literature is inconclusive regarding diet effect on volatile compounds, especially aldehydes. Aldehydes have been identified as a major contributor to the volatile fraction of red meat, which occur due to lipid oxidation (Larick and Turner, 1990; Mottram et al., 1998). Descalzo et al. (2005) found concentrate-fed animals were more likely to have a higher concentration of aldehydes present in the meat, whereas Raes et al. (2003) found pasture-fed animals had increased aldehydes. The volatiles observed in this study could be detrimental to flavor, causing grass-finished beef to have a more pronounced grassy flavor and therefore reduced palatability compared with grain-finished meat (Elmore et al., 2004; Killinger et al., 2004; Calkins and Hodgen, 2007). However, these data from raw beef may not reflect flavor development throughout cooking. A consumer panel reported by Chail et al. (2016) found USUGrain LT to have more preferable flavor compared to the LT of USUBFT or USUGrass. A follow-up panel by Chail et al. (2017) found a trend toward an interaction among the diet treatments studied here (grass, legume, and grain finishing) and GM and TB muscles (P = 0.07), wherein consumers gave lower scores to grass- and BFT-finished TB compared with GM. Muscle variation has also been observed for volatile compounds associated with the Maillard reaction and flavor liking in cooked beef samples (Legako et al., 2015; Hunt et al., 2016), corroborating our findings. However, the extent to which the properties of each muscle type would affect volatile compounds related to lipid oxidation is still unknown.
Of further interest, during storage a select number of volatile compounds changed in concentration over time. Among these, hexanal, a common indicator of lipid oxidation, peaked in concentration at day 3 and then declined. This nonlinear change in concentration is in disagreement with lipid oxidation assessed by TBARS. However, Shahidi and Pegg (1994) suggest that hexanal concentrations may peak at around 5 d of display before hexanal is degraded into smaller lipid oxidation byproducts. Unlike hexanal, the lipid oxidation product 2-heptanone increased with day of display. This finding aligns more closely with the TBARS values of this study. This result may indicate that volatile compounds other than hexanal, may be better utilized as indicators of lipid oxidation. Furthermore, 3-hydroxy-2-butanone, commonly known to originate from the breakdown of sugars during the Maillard reaction, increased with display duration. As these steaks were not cooked, this finding may be an indicator of microbial contributions to the volatile profile of raw beef. Previously, bacterial growth was determined to promote the production of 3-hydroxy-2-butanone by the catabolism of carbohydrates (Joffraud et al., 2001).
CONCLUSIONS
In conclusion, the current investigation finds finishing diet can impact color and oxidative stability of beef that varies across muscle types. Grass-finished produced a darker color than grain- and birdsfoot trefoil-finished beef, although less discoloration and lipid oxidation was observed within this diet. These findings may be in agreement with other studies wherein forage (grass or legume) diets produced beef that has a higher endogenous antioxidant capacity that would counteract oxidation throughout retail display; however, this antioxidative capacity was not directly measured in this study. Additionally, the degree of discoloration and oxidation were each muscle dependent.
Footnotes
This project was supported by the Utah Agricultural Experiment Station, Utah State University, and approved as journal paper number 9058.
LITERATURE CITED
- AMSA 2012. Meat color measurement guidelines. American Meat Science Association, Champaign, IL. [Google Scholar]
- Bidner T. D., Schupp A. R., Montgomery R. E., and Carpenter J. C. Jr. 1981. Acceptability of beef finished on all-forage, forage-plus-grain or high energy diets. J. Anim. Sci. 53:1181–1187. [Google Scholar]
- Buege J. A., and Aust S. D.. 1978. Microsomal lipid peroxidation. Methods Enzymol. 52:302–310. doi:10.1016/S0076-6879(78)52032–6 [DOI] [PubMed] [Google Scholar]
- Calkins C. R., and Hodgen J. M.. 2007. A fresh look at meat flavor. Meat Sci. 77:63–80. doi:10.1016/j.meatsci.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Chail A., Legako J. F., Pitcher L. R., Griggs T. C., Ward R. E., Martini S., and MacAdam J. W.. 2016. Legume finishing provides beef with positive human dietary fatty acid ratios and consumer preference comparable with grain-finished beef. J. Anim. Sci. 94:2184–2197. doi:10.2527/jas.2015-0241 [DOI] [PubMed] [Google Scholar]
- Chail A., Legako J. F., Pitcher L. R., Ward R. E., Martini S., and MacAdam J. W.. 2017. Consumer sensory evaluation and chemical composition of beef gluteus medius and triceps brachii steaks from cattle finished on forage or concentrate diets. J. Anim. Sci. 95:1553–1564. doi:10.2527/jas.2016.1150 [DOI] [PubMed] [Google Scholar]
- Descalzo A. M., Insani E. M., Biolatto A., Sancho A. M., García P. T., Pensel N. A., and Josifovich J. A.. 2005. Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of argentine beef. Meat Sci. 70:35–44. doi:10.1016/j.meatsci.2004.11.018 [DOI] [PubMed] [Google Scholar]
- Descalzo A. M., Rossetti L., Grigioni G., Irurueta M., Sancho A. M., Carrete J., and Pensel N. A.. 2007. Antioxidant status and odour profile in fresh beef from pasture or grain-fed cattle. Meat Sci. 75:299–307. doi:10.1016/j.meatsci.2006.07.015 [DOI] [PubMed] [Google Scholar]
- Elmore J. S., Warren H. E., Mottram D. S., Scollan N. D., Enser M., Richardson R. I., and Wood J. D.. 2004. A comparison of the aroma volatiles and fatty acid compositions of grilled beef muscle from Aberdeen Angus and Holstein-Friesian steers fed diets based on silage or concentrates. Meat Sci. 68:27–33. doi:10.1016/j.meatsci.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Faustman C., Sun Q., Mancini R., and Suman S. P.. 2010. Myoglobin and lipid oxidation interactions: mechanistic bases and control. Meat Sci. 86:86–94. doi:10.1016/j.meatsci.2010.04.025 [DOI] [PubMed] [Google Scholar]
- French P., Stanton C., Lawless F., O’Riordan E. G., Monahan F. J., Caffrey P. J., and Moloney A. P.. 2000. Fatty acid composition, including conjugated linoleic acid, of intramuscular fat from steers offered grazed grass, grass silage, or concentrate-based diets. J. Anim. Sci. 78:2849–2855. doi:10.2527/2000.78112849x [DOI] [PubMed] [Google Scholar]
- Greene B. E., and Price L. G.. 1975. Oxidation-induced color and flavor changes in meat. J. Agric. Food Chem. 23:164–167. doi:10.1021/jf60198a014 [Google Scholar]
- Holman B. W. B., van de Ven R. J., Mao Y., Coombs C. E. O., and Hopkins D. L.. 2017. Using instrumental (CIE and reflectance) measurements to predict consumers’ acceptance of beef colour. Meat Sci. 127:57–62. doi:10.1016/j.meatsci.2017.01.005 [DOI] [PubMed] [Google Scholar]
- Hunt M. R., Legako J. F., Dinh T. T., Garmyn A. J., O’Quinn T. G., Corbin C. H., Rathmann R. J., Brooks J. C., and Miller M. F.. 2016. Assessment of volatile compounds, neutral and polar lipid fatty acids of four beef muscles from USDA choice and select graded carcasses and their relationships with consumer palatability scores and intramuscular fat content. Meat Sci. 116:91–101. doi:10.1016/j.meatsci.2016.02.010 [DOI] [PubMed] [Google Scholar]
- Jeremiah L., and Gibson L.. 2001. The influence of storage temperature and storage time on color stability, retail properties and case-life of retail-ready beef. Food Res. Int. 34:815–826. doi:10.1016/S0963-9969(01)00104-1 [Google Scholar]
- Joffraud J. J., Leroi F., Roy C., and Berdagué J. L.. 2001. Characterisation of volatile compounds produced by bacteria isolated from the spoilage flora of cold-smoked salmon. Int. J. Food Microbiol. 66:175–184. doi:10.1016/S0168-1605(00)00532-8 [DOI] [PubMed] [Google Scholar]
- Killinger K. M., Calkins C. R., Umberger W. J., Feuz D. M., and Eskridge K. M.. 2004. A comparison of consumer sensory acceptance and value of domestic beef steaks and steaks from a branded, argentine beef program. J. Anim. Sci. 82:3302–3307. doi:10.2527/2004.82113302x [DOI] [PubMed] [Google Scholar]
- Lanari M. C., Schaefer D. M., Liu Q., and Cassens R. G.. 1996. Kinetics of pigments in beef from steers supplemented with vitamin E. J. Food Sci. 61:884–889. doi:10.1111/j.1365–2621.1996.tb10895.x [Google Scholar]
- Larick D. K., and Turner B. E.. 1990. Headspace volatiles and sensory characteristics of ground beef from forage- and grain-fed heifers. J. Food Sci. 54:649–654. doi:10.1111/j.1365–2621.1990.tb05198.x [Google Scholar]
- Legako J. F., Brooks J. C., O’Quinn T. G., Hagan T. D., Polkinghorne R., Farmer L. J., and Miller M. F.. 2015. Consumer palatability scores and volatile beef flavor compounds of five USDA quality grades and four muscles. Meat Sci. 100:291–300. doi:10.1016/j.meatsci.2014.10.026 [DOI] [PubMed] [Google Scholar]
- Luque L. D., Johnson B. J., Martin J. N., Miller M. F., Hodgen J. M., Hutcheson J. P., Nichols W. T., Streeter M. N., Yates D. A., Allen D. M.,. et al. 2011. Zilpaterol hydrochloride supplementation has no effect on the shelf life of ground beef. J. Anim. Sci. 89:817–825. doi:10.2527/jas.2010-3317 [DOI] [PubMed] [Google Scholar]
- Mancini R. A., and Hunt M. C.. 2005. Current research in meat color. Meat Sci. 71:100–121. doi:10.1016/j.meatsci.2005.03.003 [DOI] [PubMed] [Google Scholar]
- Martin J. N., Brooks J. C., Thompson L. D., Savell J. W., Harris K. B., May L. L., Haneklaus A. N., Schultz J. L., Belk K. E., Engle T.,. et al. 2013. Nutrient database improvement project: the influence of U.S.D.A. quality and yield grade on the separable components and proximate composition of raw and cooked retail cuts from the beef rib and plate. Meat Sci. 95:486–494. doi:10.1016/j.meatsci.2013.05.031 [DOI] [PubMed] [Google Scholar]
- McCluskey J. J., Wahl T. I., Li Q., and Wandschneider P. R.. 2005. U.S. grass-fed beef: marketing health benefits. J. Food Distribution Res. 36:1–8. [Google Scholar]
- Mottram D. S. 1998. Flavour formation in meat and meat products: a review. Food Chem. 62:415–424. [Google Scholar]
- North American Meat Processors (NAMP) Association 2010. The meat buyer’s guide. 6th ed North American Processors Association, Reston VA. [Google Scholar]
- Raes K., Balcaen A., Dirinck P., De Winne A., Claeys E., Demeyer D., and De Smet S.. 2003. Meat quality, fatty acid composition and flavour analysis in Belgian retail beef. Meat Sci. 65:1237–1246. doi:10.1016/S0309-1740(03)00031-7 [DOI] [PubMed] [Google Scholar]
- Realini C. E., Duckett S. K., Brito G. W., Dalla Rizza M., and De Mattos D.. 2004. Effect of pasture vs. concentrate feeding with or without antioxidants on carcass characteristics, fatty acid composition, and quality of Uruguayan beef. Meat Sci. 66:567–577. doi:10.1016/S0309-1740(03)00160-8 [DOI] [PubMed] [Google Scholar]
- Schroeder J. W., Cramer D. A., Bowling R. A., and Cook C. W.. 1980. Palatability, shelflife and chemical differences between forage- and grain-finished beef. J. Anim. Sci. 50:852–859. [Google Scholar]
- Shahidi F., and Pegg R. B.. 1994. Hexanal as an indicator of meat flavor deterioration. J. Food Lipids 1:177–186. doi:10.1111/j.1745–4522.1994.tb00245.x [Google Scholar]
- Wood J. D., Enser M., Fisher A. V., Nute G. R., Sheard P. R., Richardson R. I., Hughes S. I., and Whittington F. M.. 2008. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 78:343–358. doi:10.1016/j.meatsci.2007.07.019 [DOI] [PubMed] [Google Scholar]
- Wood J. D., Nute G. R., Richardson R. I., Whittington F. M., Southwood O., Plastow G., Mansbridge R., da Costa N., and Chang K. C.. 2004. Effects of breed, diet and muscle on fat deposition and eating quality in pigs. Meat Sci. 67:651–667. doi:10.1016/j. meatsci.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Wu C., Duckett S. K., Neel J. P., Fontenot J. P., and Clapham W. M.. 2008. Influence of finishing systems on hydrophilic and lipophilic oxygen radical absorbance capacity (ORAC) in beef. Meat Sci. 80:662–667. doi:10.1016/j.meatsci.2008.03.003 [DOI] [PubMed] [Google Scholar]