Abstract
Glycine, a component of glutathione (GSH), plays an important role in protection from reactive oxygen species (ROS) and inhibition of apoptosis. The aim of this study was to determine the effect of glycine on in vitro maturation (IVM) of porcine oocytes and their developmental competence after parthenogenetic activation (PA). We examined nuclear maturation, ROS levels, apoptosis, mitochondrial membrane potential (ΔΨm), and ATP concentration, as well as the expression of several genes related to oocyte maturation and development. Our studies found that treatment with glycine in IVM culture medium increased nuclear maturation rate, but varying the concentrations of glycine (0.6, 6, or 12 mM) had no significant effect. Furthermore, 6 mM glycine supported greater blastocyst formation rates and lesser apoptosis after PA than the other concentrations (P < 0.05). All the glycine treatment groups had decreased levels of ROS in both matured oocytes and at the 2-cell stage (P < 0.05). At the 2-cell stage, the 6 mM glycine group had ROS levels that were lesser than the other 2 glycine treatment groups (0.6 and 12 mM). From this, we deemed 6 mM to be the optimal condition, and we then investigated the effects of 6 mM glycine on gene expression. The expression of both FGFR2 and Hsf1 were greater than the control group in mature oocytes. The glycine treatment group had greater levels of expression of an antiapoptotic gene (Bcl2) in mature oocytes and cumulus cells and lesser levels of expression of a proapoptotic gene (Bax) in PA blastocysts (P < 0.05). In addition, mitochondrial ΔΨm and ATP concentration were increased in 6 mM glycine group compared with the control group. In conclusion, our results suggest that glycine plays an important role in oocyte maturation and later development by reducing ROS levels and increasing mitochondrial function to reduce apoptosis.
Keywords: apoptosis, glycine, mitochondrial, porcine oocytes, ROS
INTRODUCTION
Pigs are not only considered important domestic animals but are also an essential animal model for various types of biomedical research (Vodicka et al., 2005). In vitro produced (IVP) porcine embryos are important for embryo transfer, cloning, and transgenesis (Day, 2000; Wu et al., 2011). The demand of high-quality oocytes has increased dramatically with increases in animal embryo production and transgenic cloning. In vitro maturation (IVM) and in vitro culture (IVC) of oocytes and embryos are inevitably different from maturation in vivo; oocyte and embryo qualities are influenced by various factors, including time, temperature, composition of culture media, and so on (Nagai, 2001; Wu et al., 2011). Oxidative damage and apoptosis are also important for porcine oocytes and embryos cultured in vitro. A high concentration of reactive oxygen species (ROS) is known to be the main cause of oxidative damage (Gardiner et al., 1998). Moreover, high levels of ROS have multiple adverse effects on mitochondria and nuclei and cause apoptosis in mammalian oocytes (Chaube et al., 2014). Research has been conducted to study the in vivo conditions of oocytes to improve the quality by reducing the production of ROS and decreasing apoptosis. Several studies have shown that AA added exogenously to an IVC system can affect mammalian embryonic development, and their beneficial effects have been examined in pig (Hong and Lee, 2007), cattle (Rosenkrans and First, 1991), and mouse (Gardner and Lane, 1993) embryos. AA may act as energy substrates, precursors of proteins, pH regulators, and intracellular osmolytes, and they may improve embryonic development and cell number after transfer. Biochemical analysis has shown that glycine is the AA highest in concentration in the oviduct fluid, uterine fluid, and follicular fluid during diestrus in sows (Iritani et al., 1974). Glycine has multiple physiologic functions in animals, including the stimulation of glutathione (GSH) synthesis (Jackson et al., 2004), activation of glycine-gated chloride channels (Qu et al., 2002), and inhibition of apoptosis (Jacob et al., 2003). The role of glycine may be to protect pregnant sows from the potentially harmful effects of the high inorganic ion concentration and apoptosis in oviductal fluid (Van Winkle et al., 1990). However, glycine was traditionally classified as a nutritionally nonessential AA (NEAA) for mammals (Wang et al., 2010, 2014). Thus, few studies have been conducted to determine effects of glycine levels on the maturation and the longer term developmental competence of the porcine oocytes.
The objective of this study was to evaluate the effect of glycine on porcine oocytes. We applied glycine during the IVM and IVC of porcine oocytes to investigate whether it could enhance maturation and developmental competence after parthenogenetic activation (PA). Furthermore, we examined ROS, apoptosis, mitochondrial membrane potential (ΔΨm), and ATP concentration, as well as the expression of several genes related to oocyte function and development.
MATERIALS AND METHODS
Source of Reagents
Unless otherwise specified, all chemicals used in this study were purchased from Sigma–Aldrich (St. Louis, MO).
Oocyte Retrieval and IVM of Oocytes
Ovaries were collected from a local slaughterhouse, incubated in 0.9% NaCl containing 75 µg/mL penicillin G and 50 µg/mL streptomycin sulfate at 25–35 °C, and transported to the laboratory within 2 h of collection. Cumulus–oocyte complexes (COCs) were aspirated from 2- to 5-mm-diameter follicles with a 20-gauge needle attached to a 10-mL disposable syringe. Good-quality COCs were identified that had homogeneous granulated cytoplasm and at least 3 uniform layers of compact cumulus cells. The selected COCs were washed 3 times in Tyrode’s lactate (TL)–HEPES–polyvinyl alcohol (PVA, 0.1%). Approximately 100 COCs were placed into each well of a 4-well multidish containing 500-µL IVM medium and incubated for 44 h at 38.5 °C in an atmosphere of 5% CO2 in air with maximum humidity. The IVM medium was a modified North Carolina State University 37 medium (NCSU-37) (Petters and Wells, 1993) supplemented with 0.1 IU/mL human chorionic gonadotropin and 0.1 IU/mL pregnant mare serum gonadotropin. For this experiment, pig COCs were treated with glycine at 4 different doses (0, 0.6, 6, or 12 mM) for 44 h. The selected COCs were incubated at 38.5 °C under 5% CO2 in 95% humidified air for IVM. Matured oocytes with the first polar body were collected.
Evaluation of Porcine Oocytes Maturation
After 44 h of IVM, cultured oocytes were denuded by gently pipetting with 0.1% hyaluronidase and then denuded oocytes were stained with 10 µg/mL of Hoechst 33342 in TL–HEPES–PVA. The stained oocytes were evaluated using a fluorescence microscope (Nikon Corp., Tokyo, Japan). This experiment was repeated 3 times.
Measurement of Mitochondrial ΔΨ by JC-1 Staining
To monitor ΔΨm changes, porcine Metaphase II (MII) oocytes were incubated with 2 µM JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide). All oocytes were stained in a CO2 incubator at 37 °C for 20 min. The stained oocytes were examined by epifluorescence microscopy. Samples were analyzed using epifluorescence microscopy with fluorescein isothiocyanate (FITC, green) and rhodamine isothiocyanate (RITC, red) channels. Two fluorescent images were recorded and analyzed by confocal software, which allows for quantitative values of the signal intensity of green and red fluorescence. The ratio of RITC to FITC for each oocyte is the point for the ΔΨm. The experiment was replicated 3 times with approximately 20 oocytes each time (Wilding et al., 2001).
Measurement of ATP Concentration in Oocytes
The method used to measure ATP concentration in oocytes is described by Jin et al. (2017). Briefly, denuded oocytes were washed 3 times in PBS (Invitrogen)-PVA, and fixed with 4% paraformaldehyde-PBS for 1 h, washed 3 times, and incubated in PBS supplemented with 500 nM BODIPY FL ATP (BODIPY-ATP; A12410; Molecular Probes, Eugene, OR) for 1 h at room temperature in the dark. Oocytes were washed 3 times in PBS and mounted on cover slips. Images of each oocyte were captured using an epifluorescence microscope.
Parthenogenetic Activation of Oocytes
All matured oocytes with an extruded first polar body were exposed to a direct current pulse (1.5 kV/cm, 60 µs) in 0.28 mol/L mannitol containing 0.1 mM MgSO4, 0.05 mM CaCl2, and 0.1% PVA. After PA, oocytes were washed with NCSU-37 medium supplemented with 4 mg/mL bovine serum albumin and cytochalasin B, incubated in culture medium containing glycine at 4 different doses (0, 0.6, 6, or 12 mM). Activated eggs were cultured in NCSU-37 medium at 38.5 °C in a humidified atmosphere with 5% CO2 for 2 d to record their morphology and cleavage rate. The blastocyst formation rate was recorded on day 7.
The Incidence of Apoptosis in Blastocysts
Apoptotic cells of blastocysts were evaluated by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using an in situ cell death detection kit (Roche, Mannheim, Germany) as described by Jin et al. (2016b). Blastocysts were incubated in TUNEL reaction medium that specifically labeled the broken DNA ends of apoptotic cells with the fluorochrome FITC for 1 h at 38.5 °C in the dark and then were stained with 10 µg/mL Hoechst 33342 for 10 min. After being washed 3 times in PBS, stained blastocysts were mounted on glass slides and observed using an epifluorescence microscope to detect fluorescence intensity.
Measurement of Intracellular ROS Levels in Oocytes and Embryos
The intracellular ROS levels of oocytes at the MII stage and embryos at the 2-cell stage were measured by 2′,7′-dichlorodihdrofluorescein diacetate (DCHFDA) fluorescence assay, as previously described (Ozawa et al., 2002). Briefly, oocytes and embryos from each treatment group were incubated (in the dark) in PBS–PVA containing 10 µM DCHFDA for 20 min at 38.5 °C, washed 3 times with PBS–PVA, oocytes were placed into 10 µL droplets of TL–HEPES–PVA, and fluorescence was observed under an epifluorescence microscope with UV filters. Fluorescence intensities of the oocytes were analyzed using ImageJ software (Nikon, Tokyo, Japan) and normalized to control embryos. The experiment was replicated 3 times with a group of 20 oocytes/embryos in each replicate. To minimize environmental influence, oocytes and embryos were manipulated under low light, and fluorescence intensities were recorded precisely 30 s after exposure to light.
Quantitative Real-Time PCR
For the gene expression study, isolated cumulus cells and matured oocytes derived from 280 COCs, and 90 PA-derived blastocysts from the 6 mM glycine treatment and the control group, were separately sampled using a stereomicroscope. All samples were washed 3 times with PBS–PVA and stored at −80 °C until RNA was extracted. Total mRNA was isolated using the Dynabeads mRNA DIRECT Kit (Life Technologies AS) in accordance with the protocol recommended by the manufacturer, and the concentration was measured using a NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific). Complementary DNA (cDNA) was synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen). Real-time PCR was performed using the Agilent Mx3005P real-time PCR system (Stratagene). Each 20-µL PCR contained 1-µL cDNA, 0.5 µL each of the forward and reverse primers (10 pmol/µL), 10-µL SYBR Premix Ex Taq (Takara), and 8-µL nuclease-free water. The amplification protocol comprised an initial denaturation step at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s, annealing at 60 °C for 30 s and extension at 72 °C for 1 min. All primer information is shown in Table 1. Relative gene expression levels were determined using the 2−∆∆CT∆∆CT method, with Ribosomal protein L19 (RPL19) as the internal control gene. For convenient comparison, the mean expression level of each gene was normalized to the control group.
Table 1.
Primer sequences used for real-time PCR
| Gene | Primers | Primer sequence (5′–3′) | Product size (bp) |
|---|---|---|---|
| RPL19 | Forward | GCTTGCCTCCAGTGTCCTC | 79 |
| Reverse | GGCGTTGGCGATTTCAT | ||
| FGFR2 | Forward | ATTCTGGTGCCGGATGAAGAC | 121 |
| Reverse | GGTGTTGGAGTTCATGGAGG | ||
| Hsf1 | Forward | TTCAAGCACAGCAACATGGC | 190 |
| Reverse | TGGACACGCTGGTCACTTTC | ||
| Hook1 | Forward | GAAGTGTTGGTGTAAGCGGC | 142 |
| Reverse | CATCCTGACAAGGCGCAGTA | ||
| Sox2 | Forward | CGCAGACCTACATGAACG | 103 |
| Reverse | TCGGACTTGACCACTGAG | ||
| Oct4 | Forward | AAGCAGTGACTATTCGCAAC | 136 |
| Reverse | CAGGGTGGTGAAGTGAGG | ||
| Bax | Forward | GAAACCCCTAGTGCCATCAA | 189 |
| Reverse | GGGACGTCAGGTCACTGAAT | ||
| Bcl2 | Forward | CGGGACACGGAGGAGGTTT | 196 |
| Reverse | CGAGTCGTATCGTCGGTTG |
Statistical Analysis
Each experiment was repeated at least 3 times. The data are expressed as the mean values ± SEM. Percentage data (e.g., rates of maturation, blastocyst formation) were arcsine-transformed before analysis to ensure homogeneity of variance. The data were analyzed using univariate analysis of variance followed by Duncan’s multiple range test using IBM-SPSS 23.0 statistical software. Differences in gene expression were compared by Student’s t test. P < 0.05 was considered statistically significant.
RESULTS
Effect of Glycine on Nuclear Maturation
We evaluated the effect of different concentrations (0, 0.6, 6, or 12 mM) of glycine on oocyte nuclear maturation by measuring the rate of the first polar body extrusion (Fig. 1). A total of 2,628 oocytes were assessed in 3 replicates, and nuclear maturation rates among the glycine groups ranged from 75.88% to 82.35% and were greater than control group (P < 0.05). However, there were no significant differences among the glycine treatment groups (Table 2).
Figure 1.
Chromatin configuration of porcine oocytes stained with Hoechst 33342 after 44 h of in vitro maturation. (A) Germinal vesicle. (B) Germinal vesicle breakdown. (C) Metaphase I. (D) Metaphase II.
Table 2.
Effect of glycine treatment during in vitro maturation on nuclear maturation
| Concentration of glycine (mM) | Number of oocyte | Number of maturated oocyte |
|---|---|---|
| Control | 668 | 401 (59.80)a |
| 0.6 | 700 | 531 (75.88)b |
| 6 | 677 | 520 (76.81)b |
| 12 | 583 | 481 (82.35)b |
a,bValues with different superscripts within the same column are significantly different (P < 0.05).
Effects of Glycine During IVC on Embryonic Development and Apoptosis After PA
A total of 652 oocytes were used in 4 replicates to evaluate the effects of glycine at various concentrations on porcine embryo development after PA. As shown in Table 3, there were no significant differences between the glycine treatment groups and control group in 2-4 cell stage; however, the blastocyst rate with 6 mM glycine was greater than other groups (19.5% vs. 8.38%, 7.78% and 8.18%; P < 0.05). According to these results, the 6 mM concentration of glycine for the treatment during IVM and subsequent development of PA embryos was determined to be the optimal concentration. Based on the TUNEL assay, the incidence of apoptosis in blastocysts was decreased when embryos were cultured in IVC medium supplemented with 6 mM glycine compared with the control (Fig. 2).
Table 3.
Effect of glycine supplementation during in vitro culture on embryonic development after parthenogenetic activation
| Concentration of glycine (mM) | Number of embryos cultured | Number of 2-4 cell stage (%) | Number of blastocysts (%) |
|---|---|---|---|
| Control | 167 | 154 (92.21) | 14 (8.38)a |
| 0.6 | 167 | 146 (87.43) | 13 (7.78)a |
| 6 | 159 | 145 (91.19) | 31 (19.50)b |
| 12 | 159 | 150 (94.34) | 13 (8.18)a |
a,bValues with different superscripts in the same column were significantly different (P < 0.05).
Figure 2.
Representative apoptosis images in pig blastocysts after parthenogenetic activation. (A) Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay of blastocysts (green). Each sample was counterstained with Hoechst 33342 to visualize DNA (blue). Original magnification was 200×. Scale bar = 100 µm. (B) Percentage of apoptotic cells in blastocysts. Bars with an asterisk are significantly different (P < 0.05).
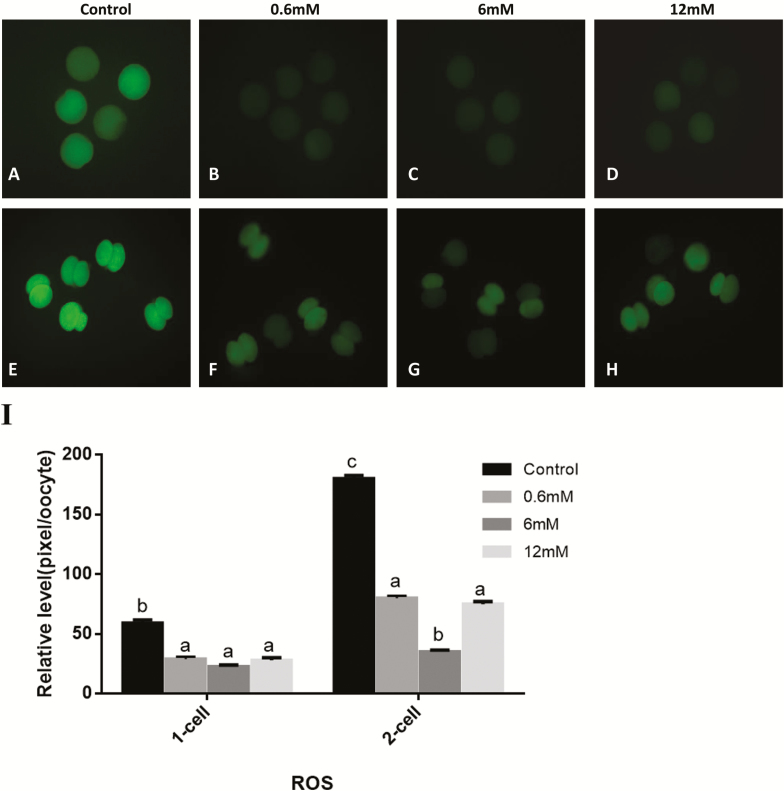
Effects of Glycine During IVM/IVC on the Intracellular ROS Levels in Oocytes/Embryos
The results (Fig. 3) show that supplementing glycine to the IVM/IVC media decreased ROS levels in oocytes or embryos at 2-cell stage compared with the control. Among the 0.6, 6, and 12 mM glycine groups, there were no significant differences in rate of matured oocytes. When 6 mM glycine was added to IVC medium, the ROS levels at 2-cell stage embryos were lesser than the other 2 glycine treatment groups.
Figure 3.
(A–H) Epifluorescent photomicrographic images of in vitro matured porcine oocytes and embryos at the 2-cell stage after parthenogenetic activation. Oocytes and the 2-cell stage embryos derived from control group and various concentration glycine groups were stained with 2′,7′-dichlorodihdrofluorescein diacetate to detect intracellular levels of reactive oxygen species (ROS), respectively. (I) Fluorescence intensities were correlated with intracellular levels of ROS. Bars (a, b, and c) for adjacent pairs of columns, means without a common letter differed (P < 0.05).
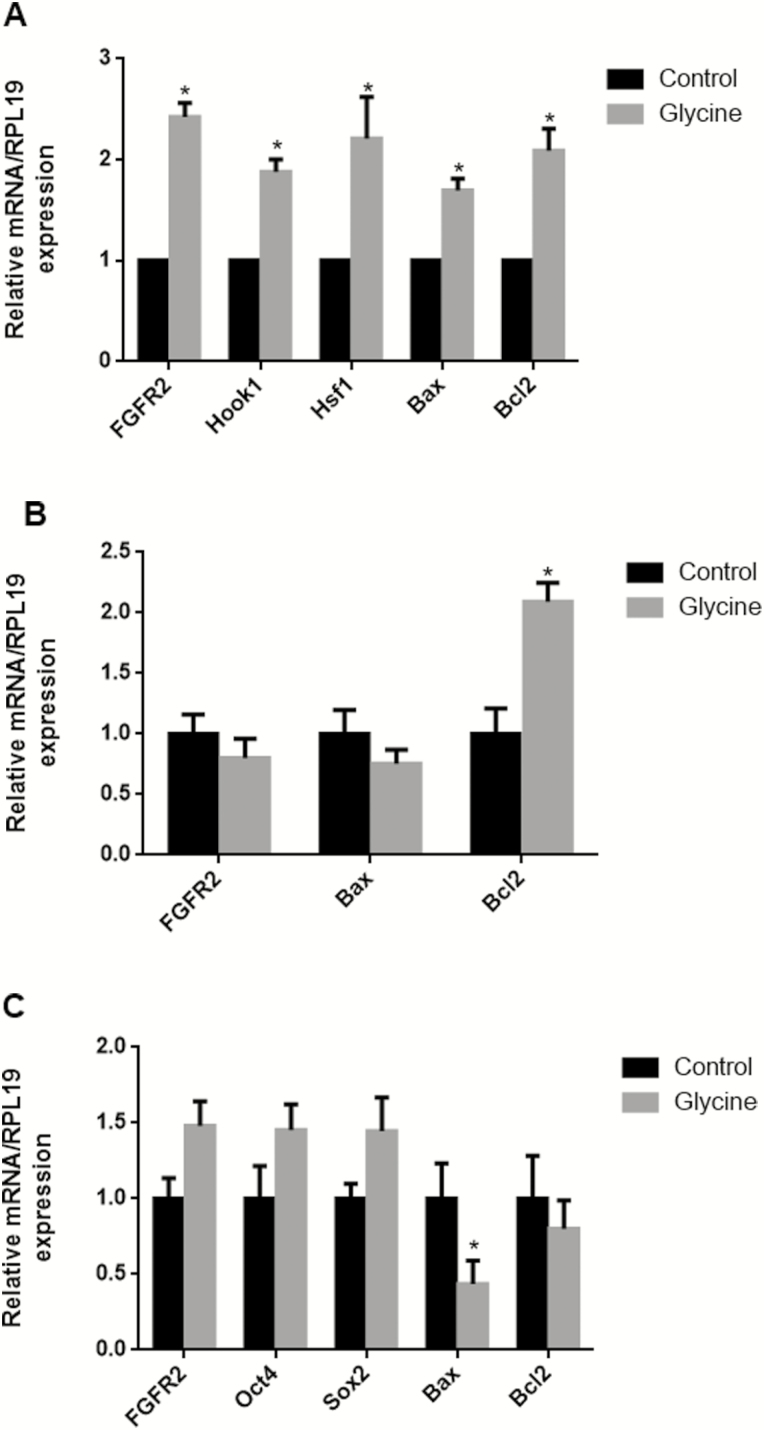
Gene Expression in Mature Oocytes, Cumulus Cells, and PA-Derived Blastocysts After Treatment with Glycine
We evaluated the effect of glycine on FGFR2, Hsf1, Hook1, Oct4, Sox2, Bax, and Bcl2 gene expression in mature oocytes, cumulus cells, and PA-derived blastocysts. As shown in Fig. 4, 6 mM glycine increased Bcl2 mRNA transcript levels significantly in oocytes and in cumulus cells and decreased Bax mRNA transcript levels in PA-derived blastocysts. In mature oocytes treated with 6 mM glycine, expression of FGFR2, Hsf1, and Hook1 were greater than the control group. However, in cumulus cells, FGFR2 mRNA transcript level was lesser than control group, although this was not statistically significant.
Figure 4.
The mRNA expression levels (mean ± SEM) of FGFR2, Hsf1, Hook1, Oct4, Sox2, Bax, and Bcl2 in mature oocytes (A), cumulus cells (B), and PA-derived blastocysts (C) after 6 mM glycine supplementation during in vitro maturation. Within the same mRNA, bars with an asterisk are significantly different (P < 0.05).
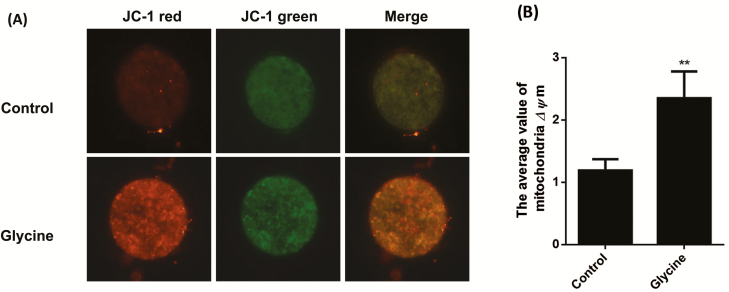
Effects of Glycine on Mitochondria Function
We evaluated the mitochondria ΔΨm and ATP concentration in oocytes with Mitochondria Staining Kit and BODIPY-ATP. A representative image of mitochondria ΔΨm is shown in Fig. 5. Oocytes with high mitochondrial ΔΨm always show orange color. The average value of mitochondria ΔΨm from 6 mM glycine treatment group was greater than that of the control group (P < 0.01). Moreover, the ATP concentration of oocytes treated with 6 mM glycine was also greater than the control group (Fig. 6).
Figure 5.
Effects of glycine on mitochondrial membrane potential (ΔΨm) in porcine in vitro matured oocytes. (A) Oocytes were labeled with JC-1. Scale bar = 100 µm. (B) The average value of mitochondria ΔΨm (red fluorescence/green fluorescence). Bars with an asterisk are significantly different (P < 0.01).
Figure 6.
Glycine treatment enhances ATP content in porcine oocytes. (A) ATP content stained with BODIPY-ATP. Scale bar = 100 µm. (B) Fluorescence intensities indicate ATP content. Bars with an asterisk are significantly different (P < 0.05).
DISCUSSION
ROS play an important role in reproductive physiology, such as oocyte maturation, fertilization, embryo development, and pregnancy (Agarwal et al., 2005). The generation of a tonic level of ROS is beneficial for meiotic maturation, overproduction of ROS leads to oxidative stress, enzyme inactivation, and DNA fragmentation, which are known to have detrimental effects on oocytes and embryos, such as mitochondrial alterations, apoptosis, and embryo cell block. The nonenzymatic system of the antioxidant defense system of ROS is primarily GSH. Glutathione plays a central role as an endogenous antioxidant scavenger that increases porcine oocytes maturation and improves developmental competence by protecting oocytes or embryos from oxidative stress through its defense against ROS. Glutathione is a tripeptide composed of glycine, l-cysteine, and l-glutamic acid. Therefore, at present, the basic culture medium of porcine oocytes for IVM is NCSU-37 medium supplemented with 0.6 mM l-cysteine. It has been reported that adding l-cysteine to IVM medium of porcine oocytes increased GSH and decreased ROS levels and increased porcine oocytes maturation and improved development of parthenogenetic embryos (Abeydeera et al., 1999). l-Cysteine is an EAA as it cannot be synthesized in by animals and must be supplied exogenously. Glycine, however, has traditionally been classified as a NEAA for pigs. In our study, adding 6 mM glycine to IVM medium improved oocytes maturation and development of parthenogenetic embryos, but not in 0.6 mM and 12 mM concentrations. We evaluated the ROS levels within oocytes and 2-cell stage embryos. When 6 mM glycine was added to IVC medium, the ROS levels of 2-cell embryos were lesser than other groups. Therefore, we determined that 6 mM glycine supplement during IVM was the optimum concentration for oocyte maturation and embryo development, and we used that concentration for our further experiments. Recent studies have shown that porcine zygote medium-3 (PZM3) containing 1.69 mM arginine + 10 mM glycine reduces the accumulation of ROS by decrease in thioredoxin reductase 1 transcript abundance in IVF porcine embryos (Redel et al., 2016).
In this study, we examined maturation rates and embryo development after PA both with and without glycine and also compared treatment with 6 mM glycine to 0.6 mM l-cysteine. The results show that oocyte maturation rates (75.89% vs. 76.81%) and blastocyst formation rates after PA (17.88% vs. 19.5%) in IVM/IVC medium containing either 0.6 mM l-cysteine or 6 mM glycine did not differ (data not shown). These results indicated that both 6 mM glycine and 0.6 mM l-cysteine could improve the IVM rate and embryo development in porcine, and this was attributed to glycine and l-cysteine counteracting ROS in porcine matured oocyte. In other species, glycine supplement is beneficial to maturation and longer term developmental competence of the oocytes, in line with our results. In bovine, supplementation of 5 mM alanine and 10 mM glycine in a defined medium synergistically improved developmental competence of IVP embryos (Lee and Fukui, 1996). However, another report in cattle found that supplementation with glycine and alanine enhanced development of in vitro matured and fertilized embryos in the presence of oviductal cells (Moore and Bondioli, 1993). Mouse oocytes contain very high concentrations of glycine (20 mM), which fall by approximately 90% during development from egg to blastocyst in vivo (Schultz et al., 1981), suggesting that glycine metabolism serves as a nutrient source during the progression of oocyte maturation and early embryo development. In pigs, Xia et al. (1995) found that supplement with 1 mM and 3 mM glycine in IVM increased cleavage rate after IVF, but not embryo development in the absence of oviductal cells. Redel et al. (2016) showed that addition of 10 mM glycine to the culture medium (PZM3) containing 1.69 mM arginine enhances IVF porcine embryos cell number and decreases apoptosis (Redel et al., 2016). Another study found that adding glycine and glucose together in porcine zygote medium-5 medium lacking bovine serum albumin resulted in increased pig blastocyst development after IVF, increased cell number, and decreased apoptosis compared with control group (Mito et al., 2012). Our study demonstrates that supplementation of 6 mM glycine during IVM and IVC significantly enhanced oocyte maturation rates and subsequent embryonic development after PA compared with the control group. The demonstration of the beneficial effect of glycine supplementation of IVM and IVC medium on subsequent development of porcine embryos in vitro.
There is a well-described relationship between Bcl2 expression and level of ROS. Kane et al. (1993) found that the overexpression of Bcl2 caused a reduction of intracellular ROS levels. In this study, we measured the apoptotic related genes such as Bax and Bcl2. Bax is a proapoptosis gene and Bcl2 is an antiapoptosis gene (Lowthert et al., 2012). Bax expression was reduced in PA-derived blastocysts, and Bcl2 expression was greater in mature oocytes, cumulus cells derived from the 6 mM glycine treatment group. These results showed that glycine treatment reduced apoptosis in porcine oocytes. It was shown that ROS could reduce apoptosis related to the increase of Bcl2. Moreover, to investigate the effect of glycine on development and apoptosis of oocytes and cumulus cells, we analyzed gene expression of FGFR2, Hsf1, Hook1, Oct4, and Sox2. FGFR2 is the primary receptor partner for the oocyte competent factors FGF10 and FGF7, and its signaling is involved in regulating oocyte maturation, cumulus expansion, and subsequent embryonic development (Zhang et al., 2010; Jin et al., 2016a). Hook1 plays a role in maintaining the normal function of chromosomes by configuring the microtubule cytoskeleton and regulating chromosome segregation. Our study showed that FGFR2 and Hook1 gene expression in the 6 mM glycine treatment group were all greater than the control group in mature oocytes. The maternally derived gene Hsf1, which is essential for early embryonic development, is conserved during oocyte maturation and embryonic progression. In the present study, Hsf1 expression levels were well conserved in the oocytes that were matured in vitro under glycine supplementation. In fact, Hsf1 expression levels were greater in the glycine treatment groups. Oct4 and Sox2, pluripotency genes that affect the in vitro developmental potential of PA embryos, were relatively highly expressed in the 6 mM glycine group compared with the control group. These results showed that 6 mM glycine treatment might reduce apoptosis in pig embryonic development and increase development competence through higher expression of pluripotency genes in porcine embryos.
It is well known that high levels of ROS have adverse effects on mitochondria. Mitochondria, as the energy metabolism center of cells (producing ATP), are also one of the organelles that can report the apoptosis signal. Mitochondria can also activate calcineurin through changes in intracellular calcium ion concentration and activate Bcl2 to promote apoptosis. Mitochondria also play an important role in oocytes and embryos. The numbers of mitochondria in oocyte/embryonic cells are also indicative of the energy and ion requirements associated with oocyte maturation, fertilization, and early embryonic development (Niu et al., 2015). Mitochondria can provide ATP for use as an energy source, which oocytes need for proper embryo development (Dumollard et al., 2007; Van Blerkom, 2011). Therefore, we evaluated ATP concentration and the mitochondrial function within the oocytes using the JC-1 staining method, which can detect mitochondrial ΔΨm. The dissipation of the mitochondrial electrochemical potential gradient is known as an early event in apoptosis. In the present study, a greater percentage of oocytes with high mitochondrial ΔΨm was observed in the glycine group compared with the control. This finding indicates that supplement with 6 mM glycine protects mitochondrial integrity within the oocyte, reduces apoptosis, and allows for the production of the energy needed by the oocyte during maturation and embryo development. Our results indicate that glycine is able to stabilize mitochondrial membranes and increase the supply of energy to the organelle and protect the cell from apoptosis.
In conclusion, the present study shows that 6 mM glycine treatment had a beneficial effect on in vitro oocytes maturation and subsequent blastocyst development after PA through decreased ROS levels and increased mitochondrial function (mitochondrial ΔΨm, ATP concentration) to reduce apoptosis, and regulation of gene expression related to development (FGFR2 and Hsf1) and apoptosis (Bax and Bcl2).
Footnotes
1The study was supported by the project funded by China Postdoctoral Science Foundation (Grant No. 2016M601393-166992), the National Natural Science Foundation of China (Grant No. 31672511), the Research Foundation of Jilin Agricultural University (Grant No. 201602) and the Project of Science and Technology, Education Department of Jilin Province (Grant No. JJKH20180690KJ).
LITERATURE CITED
- Abeydeera L. R., Wang W. H., Cantley T. C., Prather R. S., and Day B. N.. 1999. Glutathione content and embryo development after in vitro fertilisation of pig oocytes matured in the presence of a thiol compound and various concentrations of cysteine. Zygote 7:203–210. doi:10.1017/S0967199499000581 [DOI] [PubMed] [Google Scholar]
- Agarwal S., Sharma S., Agrawal V., and Roy N.. 2005. Caloric restriction augments ROS defense in S. cerevisiae, by a sir2p independent mechanism. Free Radic. Res. 39:55–62. doi:10.1080/10715760400022343 [DOI] [PubMed] [Google Scholar]
- Chaube S. K., Shrivastav T. G., Prasad S., Tiwari M., Tripathi A., Pandey A. N., and Premkumar K. V.. 2014. Clomiphene citrate induces ROS mediated apoptosis in mammalian oocytes. Open J. Apoptosis 3(3):52–58. doi:10.4236/ojapo.2014.33006 [Google Scholar]
- Day B. N. 2000. Reproductive biotechnologies: Current status in porcine reproduction. Anim. Reprod. Sci. 60–61:161–172. doi:10.1016/S0378-4320(00)00079-8 [DOI] [PubMed] [Google Scholar]
- Dumollard R., M. Duchen, and Carroll J.. 2007. The role of mitochondrial function in the oocyte and embryo. Curr. Top. Dev. Biol. 77:21–49. doi:10.1016/S0070-2153(06)77002-8 [DOI] [PubMed] [Google Scholar]
- Gardiner C. S., Salmen J. J., Brandt C. J., and Stover S. K.. 1998. Glutathione is present in reproductive tract secretions and improves development of mouse embryos after chemically induced glutathione depletion. Biol. Reprod. 59:431–436. doi:10.1095/biolreprod59.2.431 [DOI] [PubMed] [Google Scholar]
- Gardner D. K., and Lane M.. 1993. Amino acids and ammonium regulate mouse embryo development in culture. Biol. Reprod. 48:377–385. doi:10.1095/biolreprod48.2.377 [DOI] [PubMed] [Google Scholar]
- Hong J., and Lee E.. 2007. Intrafollicular amino acid concentration and the effect of amino acids in a defined maturation medium on porcine oocyte maturation, fertilization, and preimplantation development. Theriogenology 68:728–735. doi:10.1016/j.theriogenology.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Iritani A., Sato E., and Nishikawa Y.. 1974. Secretion rates and chemical composition of oviduct and uterine fluids in sows. J. Anim. Sci. 39:582–588. doi:10.2527/jas1974.393582x [DOI] [PubMed] [Google Scholar]
- Jackson A. A., Gibson N. R., Lu Y., and Jahoor F.. 2004. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein. Am. J. Clin. Nutr. 80:101–107. doi:10.1093/ajcn/80.1.101 [DOI] [PubMed] [Google Scholar]
- Jacob T., Ascher E., Hingorani A., and Kallakuri S.. 2003. Glycine prevents the induction of apoptosis attributed to mesenteric ischemia/reperfusion injury in a rat model. Surgery 134:457–466. doi:10.1067/S0039-6060(03)00164-8 [DOI] [PubMed] [Google Scholar]
- Jin J. X., Lee S., Khoirinaya C., Oh A., Kim G. A., and Lee B. C.. 2016a. Supplementation with spermine during in vitro maturation of porcine oocytes improves early embryonic development after parthenogenetic activation and somatic cell nuclear transfer. J. Anim. Sci. 94:963–970. doi:10.2527/jas.2015-9761 [DOI] [PubMed] [Google Scholar]
- Jin J. X., Lee S., Taweechaipaisankul A., Kim G. A., and Lee B. C.. 2017. Melatonin regulates lipid metabolism in porcine oocytes. J. Pineal Res. 62: e12388. doi:10.1111/jpi.12388 [DOI] [PubMed] [Google Scholar]
- Jin L., Zhu H. Y., Guo Q., Li X. C., Zhang Y. C., Zhang G. L., Xing X. X., Xuan M. F., Luo Q. R., Yin X. J.,. et al. 2016b. PCI-24781 can improve in vitro and in vivo developmental capacity of pig somatic cell nuclear transfer embryos. Biotechnol. Lett. 38:1433–1441. doi:10.1007/s10529-016-2141-0 [DOI] [PubMed] [Google Scholar]
- Kane D. J., Saratiaa T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S.. 1993. Bcl-2 inhibitior of neural death: Decreased generation of reactive oxygen species. Sci. 262:1274–1277. doi:10.1126/science.8235659 [DOI] [PubMed] [Google Scholar]
- Lee E. S., and Fukui Y.. 1996. Synergistic effect of alanine and glycine on bovine embryos cultured in a chemically defined medium and amino acid uptake by vitro-produced bovine morulae and blastocysts. Biol. Reprod. 55:1383–1389. doi:10.1095/biolreprod55.6.1383 [DOI] [PubMed] [Google Scholar]
- Lowthert L., Leffert J., Lin A., Umlauf S., Maloney K., Muralidharan A., Lorberg B., Mane S., Zhao H., Sinha R.,. et al. 2012. Increased ratio of anti-apoptotic to pro-apoptotic Bcl2 gene-family members in lithium-responders one month after treatment initiation. Biol. Mood Anxiety Disord. 2:15. doi:10.1186/2045-5380-2-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito T., Yoshioka K., Yamashita S., Suzuki C., Noguchi M., and Hoshi H.. 2012. Glucose and glycine synergistically enhance the in vitro development of porcine blastocysts in a chemically defined medium. Reprod. Fertil. Dev. 24:443–450. doi:10.1071/RD11197 [DOI] [PubMed] [Google Scholar]
- Moore K., and Bondioli K. R.. 1993. Glycine and alanine supplementation of culture medium enhances development of in vitro matured and fertilized cattle embryos. Biol. Reprod. 48:833–840. doi:10.1095/biolreprod48.4.833 [DOI] [PubMed] [Google Scholar]
- Nagai T. 2001. The improvement of in vitro maturation systems for bovine and porcine oocytes. Theriogenology 55:1291–1301. doi:10.1016/S0093-691X(01)00483-6 [DOI] [PubMed] [Google Scholar]
- Niu Y., C. Wang Q. Xiong X. Yang D. Chi P. Li H. Liu J. Li, and Huang R.. 2015. Distribution and content of lipid droplets and mitochondria in pig parthenogenetically activated embryos after delipation. Theriogenology 83:131–138. doi:10.1016/j.theriogenology.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Ozawa M., Hirabayashi M., and Kanai Y.. 2002. Developmental competence and oxidative state of mouse zygotes heat-stressed maternally or in vitro. Reproduction 124:683–689. doi:10.1530/rep.0.1240683 [DOI] [PubMed] [Google Scholar]
- Petters R. M., and Wells K. D.. 1993. Culture of pig embryos. J. Reprod. Fertil. Suppl. 48:61–73. [PubMed] [Google Scholar]
- Qu W., Ikejima K., Zhong Z., Waalkes M. P., and Thurman R. G.. 2002. Glycine blocks the increase in intracellular free Ca2+ due to vasoactive mediators in hepatic parenchymal cells. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G1249–G1256. doi:10.1152/ajpgi.00197.2002 [DOI] [PubMed] [Google Scholar]
- Redel B. K., L. D. Spate K. Lee J. Mao K. M. Whitworth, and Prather R. S.. 2016. Glycine supplementation in vitro enhances porcine preimplantation embryo cell number and decreases apoptosis but does not lead to live births. Mol. Reprod. Dev. 83:246–258. doi:10.1002/mrd.22618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkrans C. F. R. Jr, and First N. L.. 1991. Culture of bovine zygotes to the blastocyst stage: effects of amino acids and vitamins ☆. Theriogenology 35:266–266. doi:10.1016/0093-691X(91)90242-6 [Google Scholar]
- Schultz G. A., Kaye P. L., McKay D. J., and Johnson M. H.. 1981. Endogenous amino acid pool sizes in mouse eggs and preimplantation embryos. J. Reprod. Fertil. 61:387–393. doi:10.1530/jrf.0.0610387 [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. 2011. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 11:797–813. doi:10.1016/j.mito.2010.09.012 [DOI] [PubMed] [Google Scholar]
- Van Winkle L. J., Haghighat N., and Campione A. L.. 1990. Glycine protects preimplantation mouse conceptuses from a detrimental effect on development of the inorganic ions in oviductal fluid. J. Exp. Zool. 253:215–219. doi:10.1002/jez.1402530211 [DOI] [PubMed] [Google Scholar]
- Vodicka P., Smetana K. Jr, Dvoránková B., Emerick T., Xu Y. Z., Ourednik J., Ourednik V., and Motlík J.. 2005. The miniature pig as an animal model in biomedical research. Ann. N. Y. Acad. Sci. 1049:161–171. doi:10.1196/annals.1334.015 [DOI] [PubMed] [Google Scholar]
- Wang W., Dai Z., Wu Z., Lin G., Jia S., Hu S., Dahanayaka S., and Wu G.. 2014. Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 46:2037–2045. doi:10.1007/s00726- 014-1758-3 [DOI] [PubMed] [Google Scholar]
- Wang W., Zeng X., Mao X., Wu G., and Qiao S.. 2010. Optimal dietary true ileal digestible threonine for supporting the mucosal barrier in small intestine of weanling pigs. J. Nutr. 140:981–986. doi:10.3945/jn.109.118497 [DOI] [PubMed] [Google Scholar]
- Wilding M., Dale B., Marino M., di Matteo L., Alviggi C., Pisaturo M. L., Lombardi L., and De Placido G.. 2001. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod. 16:909–917. doi:10.1093/humrep/16.5.909 [DOI] [PubMed] [Google Scholar]
- Wu G. Q., Jia B. Y., Li J. J., Fu X. W., Zhou G. B., Hou Y. P., and Zhu S. E.. 2011. l-Carnitine enhances oocyte maturation and development of parthenogenetic embryos in pigs. Theriogenology 76:785–793. doi:10.1016/j.theriogenology. 2011.04.011 [DOI] [PubMed] [Google Scholar]
- Xia P., Rutledge J., and Armstrong D. T.. 1995. Expression of glycine cleavage system and effect of glycine on in vitro maturation, fertilization and early embryonic development in pigs. Anim. Reprod. Sci. 38:155–165. doi:10.1016/0378-4320(94)01345-M [Google Scholar]
- Zhang K., Hansen P. J., and Ealy A. D.. 2010. Fibroblast growth factor 10 enhances bovine oocyte maturation and developmental competence in vitro. Reproduction 140:815–826. doi:10.1530/REP-10-0190 [DOI] [PubMed] [Google Scholar]