Abstract
The objective of this article was to evaluate in vitro effect of grape seed procyanidin extract (GSPE) on differentiation, proliferation, and lipolysis of porcine adipocytes, providing a molecular basis for the use of GSPE in pig fat regulation. Primary preadipocytes isolated from subcutaneous adipose tissue of pigs were used as the in vitro cell model. Treatment of GSPE repressed preadipocyte differentiation, as evidenced by reduced lipid accumulation, decreased mRNA expressions of peroxisome proliferator-activated receptor gamma (PPARγ) and fatty acid–binding protein 4 (FABP4), as well as enhanced expressions of preadipocyte factor-1. Activity of glycerol-3-phosphate dehydrogenase (GPDH), one of the most important enzymes in the pathway for triacylglycerol biosynthesis, was also decreased. Furthermore, GSPE could suppress preadipocyte proliferation by inducing G0/G1 cell cycle arrest and cell apoptosis. In porcine mature adipocytes, treatment with GSPE attenuated lipid content and GPDH activity, and the release of both free fatty acid and glycerol were enhanced; mRNA expressions of key lipolytic transcription factors, including hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL), were elevated in GSPE-treated adipocytes. In summary, our results suggest GSPE inhibits porcine preadipocyte differentiation and proliferation and stimulates lipolysis of mature adipocytes, thus providing novel insights for further exploring the use of GSPE as a fat accumulation inhibitor.
Keywords: procyanidins, pig, adipocyte, proliferation, differentiation, lipolysis
INTRODUCTION
Adipose tissue deposition and distribution are highly correlated with production efficiency of domestic meat animals (Hausman et al., 2014). For pigs (especially Chinese indigenous pig breeds), subcutaneous (SQ) fat accounts for a large amount of body fat (Poulos et al., 2010). Excessive SQ fat accumulation leads to an obvious waste of resources and resultant inefficiency in production (Hausman et al., 2009). Therefore, it is important to develop natural feed additives, which are safe and effective, to reduce undesired body fat and improve feed conversion in pigs.
Adipose tissue mass is basically determined by processes regulating adipocyte number and size (Jo et al., 2009). The increase in adipocyte number results from increased adipogenesis, that is, proliferation and differentiation of preadipocytes, whereas the size of adipocyte varies because of lipogenesis and lipolysis (Hausman et al., 2014). Seeking strategies and/or potential natural products to control preadipocyte adipogenesis and lipid metabolism of adipocytes is valuable to combat excessive fat in animals. Procyanidins, a type of bioflavonoids, are composed of catechins and/or epicatechins with different degrees of polymerization (Esatbeyoglu et al., 2015). There is accumulating evidence that grape seed procyanidin extract (GSPE) possesses the ability to regulate metabolic signaling pathways and exerts beneficial effects against dyslipidemia (Del Bas et al., 2008; Quesada et al., 2009) and insulin resistance (Montagut et al., 2010). We hypothesized that GSPE is involved in lipid synthesis and metabolism of porcine adipocytes, which may affect fat development of pigs. The objective of this study was to investigate GSPE effect on preadipocyte proliferation, differentiation, and lipolysis of pig adipocytes in vitro as well as key signaling pathways involved in these processes, expected to provide insights into mechanisms for the use of GSPE in pig fat management.
MATERIALS AND METHODS
Experimental Animals and Chemicals
Five small Meishan (Chinese indigenous obese pig breed) male piglets (3 d old) used in this study were provided by the Small Meishan Pig Breeding Center (Jurong, Jiangsu, China). All animal procedures were approved by the Experimental Animal Care and Use Committee of Nanjing Agricultural University.
Grape seed procyanidin extract was provided by Tianjin Jianfeng Natural Product R&D Co., Ltd (Tianjin, China). According to the manufacturer, the purity is over 99.9%, and oligomeric procyanidins (procyanidins containing 2 to 10 monomeric units) account for 67.68%. Dulbecco’s modified Eagle medium:Nutrient Mixture F-12 (DMEM/F12) and PBS were obtained from Hyclone (Logan, UT). Fetal bovine serum (FBS) was from Sciencell (San Diego, CA). Type I collagenase, penicillin, and streptomycin were from Gibco (Grand Island, NY). TRIzol reagent was from Ambion (Austin, TX). DNase I was from Thermo (Carlsbad, CA).
Cell Isolation and Culture
Subcutaneous adipose tissue was collected from neck and back of the sacrificed piglets and soaked in PBS. The isolation and culture of preadipocytes were performed as previously described with minor alterations (Wei et al., 2015). Briefly, adipose tissue was rinsed with PBS and cut with scissors into approximately 1 mm3 sections under sterile-free condition, followed by digestion with type I collagenase at 37 °C for 60 min in a shaking water bath. Then, DMEM/F12 + 10% FBS media (complete media) was added to stop further digestion. The mixture was filtered through a 100 µm and then a 40-µm cell strainer, and filtrate was centrifuged at 1,200 rpm for 10 min. The pellet containing stromal vascular cells was resuspended in DMEM/F12 + 10% FBS media and incubated at 37 °C in a humidified atmosphere containing 5% CO2. Phosphate-buffered saline and DMEM/F12 used in this study were supplemented with 100 IU/mL penicillin and 100 µg/mL streptomycin. All cell culture experiments were carried out in triplicate per pig.
Morphological Observation and Lactate Dehydrogenase Assay
Pig preadipocytes were seeded in 96-well culture plates for 24 h. Then different concentrations (5 to 300 µg/mL) of GSPE (dissolved in media) were added to each well and incubated for 24 h. The control group was added equal amount of complete media (carrier; 0 µg/mL GSPE). Morphological observation was performed under a CKX41 inverted microscope (Olympus, Japan), followed by lactate dehydrogenase (LDH) assay. The LDH assay is based on the measurement of LDH release and has been used as a reliable and simple indicator of cytotoxicity (Decker and Lohmann-Matthes, 1988). Lactate dehydrogenase release from cells after treatment with GSPE was evaluated via a commercial cytotoxicity detection kit (Applygen Technologies, Beijing, China) according to the manufacturer’s protocol. Lactate dehydrogenase activities in culture medium and cell lysate were measured by optical density at 440 nm by a microplate reader (Thermo). Cell viability index is defined as a percentage of LDH leakage in medium compared with total LDH (LDH in medium and cell lysate) activity. Data are expressed as a percentage of the cell viability index in GSPE-treated cells relative to that in control cells.
Cell Differentiation
Preadipocytes were seeded in 24-well culture plates and separated into several groups. Two days after confluence (day 0), cells were stimulated with a standard DMI (inductive differentiation medium) cocktail [DMEM/F12 + 10% FBS supplemented with insulin (1 µg/mL), dexamethasone (1 µM), and isobutylmethylxanthine (0.5 mM)] for 6 d and then maintained in insulin (1 µg/mL) supplemented complete medium (DMEM/F12 + 10% FBS) for 5 more days. Different concentrations (0 to 100 µg/mL) of GSPE were added into cell cultures at day 0 of cell differentiation and cultured for 24 h. Cells cultured with complete media alone were used as the blank group to monitor the adipogenic effect of DMI cocktail. All media were changed every 2 or 3 d.
Lipid Quantification
Oil Red-O staining was performed as previously described (Wei et al., 2015). To quantify lipid droplets, Oil Red-O dye retained in adipocytes was extracted with isopropanol and quantified based on the absorbance values at 510 nm by a microplate reader. In addition, triglyceride (TG) contents were measured using a commercial TG assay kit (Applygen, Beijing, China) according to its protocol. Protein content was quantified using a bicinchoninic acid (BCA) protein assay kit (Cell Signaling, Boston, MA), and the TG contents were normalized against the protein. Data are expressed as a percentage of the TG contents in GSPE-treated cells relative to that in control (0 µg/mL GSPE) cells.
Glycerol-3-Phosphate Dehydrogenase Activity Assay
Glycerol-3-phosphate dehydrogenase activity was measured by determining the decrease of dihydronicotinamide-adenine dinucleotide during glycerol-3-phosphate dehydrogenase (GPDH)-catalyzed reduction of dihydroxyacetone phosphate using a GPDH activity assay kit (Takara, Shiga, Japan). Briefly, differentiated adipocytes were rinsed with PBS, scraped into enzyme extraction buffer and sonicated. After centrifugation at 10,000 rpm for 5 min, GPDH activity in supernatants was determined spectrophotometrically at 340 nm with the GPDH assay kit, according to the manufacturer’s protocol. Protein concentration was measured using a BCA protein assay kit to correct the GPDH assay results. Data are expressed as a percentage of GPDH activity in GSPE-treated cells relative to that in control cells.
Cell Proliferation and Cell Cycle Analysis
Pig preadipocytes were seeded in 96-well culture plates for 24 h. Then, different concentrations of GSPE were added to each well and incubated for 24 h. The effect of GSPE on cell proliferation was assessed using a cell counting kit-8 (Vazyme, Nanjing, China) according to the manufacturer’s protocol, followed by absorbance measurement at 450 nm by a microplate reader.
For cell cycle phase determination, pig preadipocytes were seeded in 6-well culture plates and incubated with 100 µg/mL GSPE for 36 h. Then, cells were trypsinized and fixed in 75% ethanol overnight at −20 °C, followed by propidium iodide (PI)-RNase staining for 15 min. The cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and the Modfit software (Verity Software House, Topsham, ME). The proliferative index stands for the proportion of cells undergoing mitosis from 10,000 cells examined. In addition, after GSPE incubation, cells were collected by TRIzol reagent to investigate GSPE effect on cell cycle–related gene expressions.
Lipolysis Assay
Preadipocytes were induced to differentiate as previously described. At day 11 of differentiation, adipocytes were cultured in serum-free DMEM/F12 media and treated by GSPE for 12 h. Glycerol contents released in culture media were determined using a glycerol assay kit (Applygen, Beijing, China). Briefly, 50-µL medium was incubated at 37 °C for 10 min with 150 µL of reaction buffer, and the absorbance at 550 nm was read and calculated using a glycerol standard curve. Free fatty acid contents released in culture media were quantified by an FFA assay kit (Jiancheng, Nanjing, China), and the absorbance at 550 nm was read as described by the supplier.
Apoptosis Assay
Cell apoptosis was quantified by flow cytometry using the Annexin V-FITC apoptosis detection kit (KeyGEN, Nanjing, China). Briefly, preadipocytes were treated with different concentrations of GSPE for 24 h. Then, cells were harvested and resuspended in 500-µL binding buffer. The mixture was incubated with 5 µL of Annexin V-FITC and 5 µL of PI for 15 min in darkness at room temperature. Cells were analyzed within 1 h by a FACS Caliber flow cytometer (Becton Dickinson). Normal cells showed Annexin V-FITC and PI double negative, whereas early apoptotic cells showed Annexin V-FITC-positive alone, and late apoptotic and necrotic cells showed Annexin V-FITC and PI double positive. Cell Quest software was used to calculate percentages of cells undergoing apoptosis.
Real-Time Quantitative PCR
Total RNA was extracted using TRIzol reagent and treated with DNase I to remove genomic DNA. Then, RNA was reverse transcribed into cDNA by PrimeScript RT Master Mix (Takara, Dalian, China). Real-time quantitative PCR (RT-qPCR; 15-µL volume per reaction) was performed with the StepOne Real-time PCR system (Applied Biosystems, Foster, CA) using the SYBR Premix Ex Taq (Takara, Dalian, China). The following PCR conditions were used: 95 °C for 30 s, 40 cycles at 95 °C for 5 s and 60 °C for 30 s. Primers were designed via Primer-BLAST (NCBI) and synthesized by Shanghai Sangon Biological Engineering Technology Services Co., Ltd (Shanghai, China). Primer sequence and product size are shown in Table 1. The amplification efficiency was 95% to 105%. Dissociation (melting) curves (0.01 °C/s) were used to check the specificity of amplification, followed by agarose gel electrophoresis to confirm the targeted sizes. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene. Real-time quantitative PCR data were analyzed using the 2−ΔΔCT method to compare the relative quantity.
Table 1.
Primer sequences for real-time quantitative PCR
| Gene | Accession number | Forward primer sequence (5′ to 3′) | Reverse primer sequence (5′ to 3′) | Product size (bp) |
|---|---|---|---|---|
| PPARγ | NM_214379.1 | GATTTCTCCAGC ATTTCCA | GCTCTTCGTGG GTTTGTT | 184 |
| C/EBPα | XM_003127015.3 | CAAAGCCAAGAAGTCGGTAGA | ATTGTCACTGGTCAGCTCCA | 148 |
| Pref-1 | DQ309458.1 | CCCATGGAGCTGAATGCCT | TTGCAAATGCACTGCCAGGG | 169 |
| FABP4 | NM_001002817.1 | AATTGGGCCAGGAATTTGAT | TCTTTCCATCCCACTTCTGC | 108 |
| HSL | NM_214315.1 | GAAATGCCACTGACTGCTGA | CATAGGAGATGAGCCTGACGA | 100 |
| ATGL | NM_001098605.1 | AGGACAGCTCCACCAACATC | TTGCACATCTCTCGAAGCAC | 130 |
| p21 | XM_013977858.2 | CATGTGGACCTGTTGCTGTC | AAATCTGTCATGCTGGTCTGC | 122 |
| p16 | AJ242787.1 | GAGGCTTCACAGTCCTGACC | AGGACCACCAAAGTGTCCAG | 179 |
| CDK2 | NM_001285465.1 | AAATTCATGGATGCCTCTGC | GGTTTAAGGTCTCGGTGCAA | 125 |
| CDK4 | NM_001123097.1 | TGGTTACAAGTGGTGGGACA | CTGGAGCACGGTACCAGAGT | 111 |
| CDK6 | XM_013979690.1 | TGTTTCAGCTTCTCCGAGGT | ACTGTAGATGCGGGCAAGAC | 137 |
| Cyclin A | GQ265874.1 | GCAGCAGCCTTTCATTTAGC | GGTGAAGGTCCAGGAGACAA | 118 |
| Cyclin D1 | XM_013994006.1 | AAGTGCGTGCAGAAGGAAAT | AGGAAGCGGTCCAGGTAGTT | 131 |
| GAPDH | NM_001206359.1 | ATGGTCCACATGGCCTCCAA | GAAGTCAGGAGATGCTCGGTG | 142 |
Western Blot Analysis
Proteins were extracted with cell lysis buffer (pH 8.0) containing 10 mM Tris–HCl, 10 mM NaCl, 3 mM MgCl2, 1% SDS, 0.5% NP-40, and 1% protease inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL). After quantification by BCA method, equal amounts (30 mg) of proteins were separated by SDS–PAGE and transferred to polyvinylidene fluoride membranes. After blocking in defatted 5% milk, the membranes were immunoblotted with rabbit anti-cleaved caspase-3 (Santa Cruz Biotechnology, Santa Cruz, CA) or β-actin (Cell Signaling) antibody (1:1,000) overnight at 4 °C, followed by incubation with anti-rabbit IgG secondary antibody (1:10,000) for 1 h at room temperature. Then protein bands were visualized using an enhanced chemiluminescence detection system (Amersham, Piscataway, NJ). Protein band density was quantified and normalized to the β-actin content.
Statistical Analysis
All data were obtained from at least 3 animals. For each test animal, triplicate measurements were performed. Results were expressed as the mean ± SEM. Statistics were calculated with SPSS software (version 20.0). The 2-tailed t test was used to analyze differences between groups after checking normal distribution. For comparisons among groups, 1-way ANOVA was used, followed by Duncan test. Differences with P values less than 0.05 and P values less than 0.01 were deemed statistically significant and very significant, respectively.
RESULTS
Cytotoxicity Assay for Porcine Preadipocytes After Treatment With GSPE
To obtain the optimal concentration of GSPE for porcine preadipocyte duration of the treatment study, cell morphology was monitored after cells were treated with GSPE at various concentrations for 24 h. Results showed that porcine preadipocytes had no morphological changes incubated with relatively low concentrations of GSPE (5 to 100 µg/mL), which showed fibroblast-like cell shape and adhered to the culture dish firmly, whereas high concentrations of GSPE (especially 300 µg/mL) made a large number of cells turn round and float (Fig. 1A). In addition, LDH assay indicated no change for LDH leakage into culture medium from cells during a 24-h incubation with GSPE at 100 µg/mL (Fig. 1B). Therefore, based on the morphological observation and LDH assay results, 5 to 100 µg/mL GSPE is used for further treatment study.
Figure 1.
Effect of grape seed procyanidin extract (GSPE) on cell morphology and lactate dehydrogenase (LDH) leakage of porcine preadipocytes. Porcine preadipocytes were treated with GSPE at indicated concentrations for 24 h, and cell morphology was observed (A) by a microscope (100× magnification), followed by LDH leakage assessment (B).
Grape Seed Procyanidin Extract Attenuates Lipid and TG Accumulation During Porcine Preadipocyte Differentiation
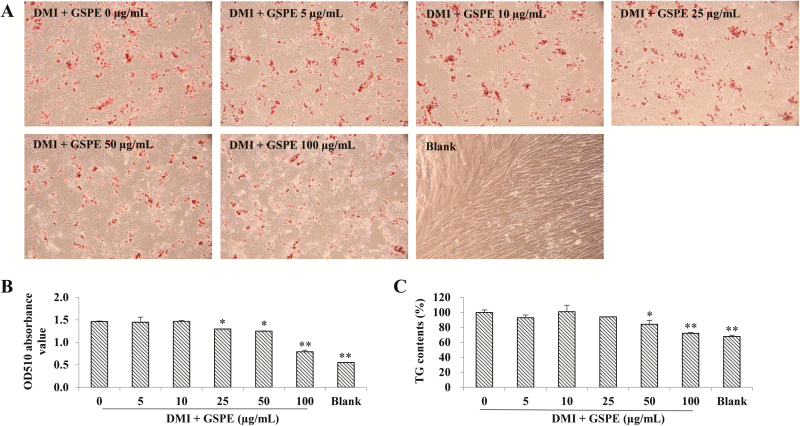
To investigate effect of GSPE on adipocyte differentiation, intracellular lipids were observed (Fig. 2A) and quantified (Fig. 2B) after Oil Red-O staining. Triglyceride contents in mature adipocytes were measured in parallel (Fig. 2C). Results showed that treatment of GSPE inhibited lipid and TG accumulation in a dose-dependent manner in porcine adipocytes. Specifically, 25 µg/mL GSPE inhibited lipid accumulation in adipocytes (P < 0.05), and 50 and 100 µg/mL GSPE inhibited both lipid and TG accumulation in adipocytes (50 µg/mL, P < 0.05; 100 µg/mL, P < 0.01).
Figure 2.
Effect of grape seed procyanidin extract (GSPE) on lipid and triglyceride (TG) accumulation of porcine preadipocytes. Porcine preadipocytes were induced to differentiate with DMI media and treated with GSPE at indicated concentrations for 24 h at day 0 of differentiation. At day 11 of differentiation, lipid accumulation was monitored with Oil Red-O staining by phase contrast image (A) under a microscope (100× magnification), and lipid contents were quantified based on the absorbance values at 510 nm of destained Oil Red-O extracted from the cells (B). Meanwhile, TG contents were measured at day 11 of differentiation (C). Blank group was cells cultured with complete media alone. These data were compared with the control cells (0 µg/mL GSPE). *P < 0.05; **P < 0.01.
Grape Seed Procyanidin Extract Changes Expressions of Adipogenic-Related Genes in Porcine Adipocytes
As above data indicated that 100 µg/mL GSPE maximally inhibited lipid accumulation of porcine preadipocytes without toxicity, the concentration of 100 µg/mL was used to evaluate GSPE effects on key adipogenic-related factors in vitro. Results show that GSPE treatment in porcine adipocytes decreased mRNA expressions of peroxisome proliferator-activated receptor gamma (PPARγ) and fatty acid–binding protein 4 (FABP4; P < 0.05) and enhanced preadipocyte factor-1 (Pref-1) expression (P < 0.01), whereas no difference was observed for CCAAT/enhancer-binding protein alpha (C/EBPα) expression (Fig. 3A). Next, functional activity of GPDH in porcine adipocytes was measured at day 11 of differentiation, which showed GSPE downregulated GPDH activity compared with that in control cells (Fig. 3B; P < 0.01).
Figure 3.
Relative expressions of adipogenic-related genes and glycerol-3-phosphate dehydrogenase (GPDH) activity in porcine adipocytes. Porcine preadipocytes were induced to differentiate and treated with 100 µg/mL grape seed procyanidin extract (GSPE) at day 0 of differentiation for 24 h. At day 11 of differentiation, cells were harvested for real-time quantitative PCR (A) and GPDH activity assay (B). These data were compared with the control cells (0 µg/mL GSPE). *P < 0.05; **P < 0.01.
Grape Seed Procyanidin Extract Stimulates Lipolysis and Changes Expressions of Lipolytic-Related Genes in Porcine Adipocytes
In pig mature adipocytes, treatment with GSPE reduced lipid (Fig. 4A; P < 0.01) and TG (Fig. 4B; P < 0.01) contents in adipocytes, and both glycerol (Fig. 4C; P < 0.01) and FFA (Fig. 4D; P < 0.01) released in cell media were increased. Treatment with GSPE downregulated functional activity of GPDH (Fig. 4E; P < 0.01). Furthermore, mRNA expression levels of hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) in porcine adipocytes were increased compared with control cells. (Fig. 4F; P < 0.05).
Figure 4.
Effect of grape seed procyanidin extract (GSPE) on lipolysis and relative expressions of lipolytic-related genes in porcine adipocytes. Porcine adipocytes were treated with 100 µg/mL GSPE for 12 h, and lipolysis assay, real-time quantitative PCR, and glycerol-3-phosphate dehydrogenase (GPDH) activity test were performed. Lipid contents quantified by Oil Red-O method (A). Triglyceride (TG) contents measured by a TG assay kit (B). Amount of glycerol released in culture medium (C). Amount of FFA released in culture medium (D). Changes in GPDH activity with GSPE treatment (E). Relative mRNA expressions of lipolytic-related genes including hormone-sensitive lipase and adipose triglyceride lipase were examined by real-time quantitative PCR (F). These data were compared with the control cells (0 µg/mL GSPE). *P < 0.05; **P < 0.01.
Grape Seed Procyanidin Extract Suppresses Proliferation and Induces Cell Cycle Arrest in Porcine Preadipocytes
Grape seed procyanidin extract suppressed preadipocyte proliferation in a dose-dependent manner. As shown in Fig. 5A, 25 to 300 µg/mL GSPE resulted in a decrease in cell number (P < 0.01), and the half maximal inhibitory concentration is around 166 µg/mL. No change was observed for cells treated with lower concentrations (≤10 µg/mL) of GSPE. Next, 100 µg/mL GSPE effect on cell cycle distribution was determined by flow cytometry. The data showed that 100 µg/mL GSPE markedly induced the accumulation of porcine preadipocytes in G0/G1 phase, and the percentage of S-phase cells was decreased in GSPE-treated group (Fig. 5B). In addition, the proliferation index was lower in GSPE-treated cells than in control cells (Fig. 5C). These findings indicated that cell cycle distribution was blocked in the G0/G1 phase when porcine preadipocytes were treated with GSPE. Quantitative RT-qPCR analysis was therefore conducted to detect key cell cycle–related gene expressions, including p21, p16, CDK2, CDK4, CDK6, Cyclin A, and Cyclin D1. The results showed that mRNA expressions of p21 and p16 were upregulated by GSPE, accompanied with decreased CDK2, CDK4, CDK6, Cyclin A, and Cyclin D1 compared with those in control cells (Fig. 5D).
Figure 5.
Effect of grape seed procyanidin extract (GSPE) on cell proliferation and cell cycle of porcine preadipocytes. Porcine preadipocytes were treated with GSPE at indicated concentrations for 24 h, and cell proliferation was assessed using a cell counting kit-8 assay (A). In addition, porcine preadipocytes were treated with 100 µg/mL GSPE for 36 h, and cell cycle phase (B) and proliferation index (C) were analyzed by flow cytometry, followed by real-time quantitative PCR (D). These data were compared with the control cells. *P < 0.05; **P < 0.01.
High Concentrations of GSPE Induces Cell Apoptosis in Porcine Preadipocytes
Effect of different concentrations of GSPE on cell apoptosis was detected with flow cytometry. Results indicated 200 and 300 µg/mL of GSPE increased cell apoptosis rate, with decreased viable cells and increased early and late apoptotic cell numbers (Fig. 6A; P < 0.01). Necrotic cells were increased by 300 µg/mL GSPE. No difference was detected for cells treated with 100 µg/mL GSPE regarding cell populations of viable cells, early apoptotic cells, late apoptotic cells, and necrotic cells. Protein expressions of cleaved caspase-3 were increased by 200 µg/mL GSPE (Fig. 6B).
Figure 6.
Effect of high concentrations of grape seed procyanidin extract (GSPE) on cell apoptosis of porcine preadipocytes. Porcine preadipocytes were treated with GSPE at indicated concentrations for 24 h, and cell apoptosis was assessed using flow cytometry (A). Furthermore, protein expressions of cleaved caspase-3 were detected and quantified after treatment with 200 µg/mL GSPE (B). These data were compared with the control cells. **P < 0.01.
DISCUSSION
The management of adipose tissue development in farm animals has been an important topic in the field of animal production sciences (Dodson et al., 2015; Louveau et al., 2016). As both increased number and expanded volume of adipocytes contribute to fat accumulation, seeking natural products against adipocyte proliferation, differentiation, and/or lipolysis is an important approach to develop potential feed additives that regulate fat development in local pig breeds. The present study provides the first evidence that grape seed-derived GSPE inhibits both number increase and lipid accumulation of porcine adipocytes in vitro. In addition, our data indicate that the inhibitory effect of GSPE on cell proliferation is mediated by G0/G1 cell cycle arrest and apoptosis, whereas the decreased differentiation and enhanced lipolysis were through PPARγ signaling pathway and ATGL and HSL regulation, respectively.
Glycerol-3-phosphate dehydrogenase is an enzyme that plays a major role in lipid biosynthesis pathway and lipid content of fat cells (Wise and Green, 1979). It has been shown that GSPE reduced GPDH activity and lipid content in 3T3-L1 adipocytes (Pinent et al., 2005). Similarly, we found that GPDH activity in porcine primary adipocytes was decreased after GSPE incubation. Also, as shown by morphological and quantitative data of the amount of triglyceride, our study indicated treatment of GSPE inhibited lipid and TG accumulation in a dose-dependent manner in porcine adipocytes. Several adipogenic genes, including PPARγ, C/EBPα, and FABP4, and an adipogenesis inhibitor Pref-1, were used to define the progress of adipocyte differentiation. Peroxisome proliferator-activated receptor gamma, a master regulator of adipogenesis (Tontonoz and Spiegelman, 2008), is induced and necessarily required during differentiation (Chawla et al., 1994; Lefterova et al., 2014); C/EBPα is a key transcription factor regulating adipogenesis (Lefterova and Lazar, 2009); FAPB4 is highly regulated during adipogenesis (Hunt et al., 1986) and functions as a lipid chaperone in adipocytes (Cristancho and Lazar, 2011); and Pref-1, a molecular gatekeeper of adipogenesis (Hudak and Sul, 2013), acts by maintaining the preadipocyte state and preventing adipogenesis (Smas and Sul, 1993). The data showed that GSPE decreased expressions of PPARγ and FABP4 and simultaneously increased Pref-1 expression at the final phase of differentiation. These results are consistent with the reduced lipid accumulation observed in the present study, indicating that GSPE represses differentiation of porcine preadipocyte through altering transcriptional activity of key genes in adipocytes.
Lipolysis is defined as the breakdown of lipids and involves hydrolysis of TG into glycerol and FFA (Dijk and Kersten, 2014). Adipose triglyceride lipase and HSL are the 2 major enzymes that catabolize TG during lipolysis (Zimmermann et al., 2004). In the present study, treatment with GSPE reduced lipid and TG content in porcine adipocytes, and both glycerol and FFA released in cell media were increased. Correspondingly, the expressions of the key lipolysis genes ATGL and HSL were increased, implying that GSPE stimulates lipolysis of porcine mature adipocytes. This result is consistent with observations in 3T3-L1 adipocytes, in which GSPE inhibit TG synthesis and favor TG hydrolysis (Ardévol et al., 2000; Pinent et al., 2004).
As proliferative ability of preadipocytes also contributes to adipose tissue expansion, we further detected GSPE effect on proliferation of porcine preadipocytes. There is evidence that some flavonoids inhibit both differentiation and proliferation in adipose cell lines (Harmon and Harp, 2001; Cao et al., 2013; Yang et al., 2015). Consistent with these findings, for the first time, we show that GSPE in porcine preadipocytes inhibits cell differentiation and proliferation in a dose-dependent manner. Also, we found that 100 µg/mL GSPE effectively induced cell cycle arrest at the G0/G1 phase, accompanied by enhanced mRNA expressions of p21 and p16. It is known that cell proliferation is controlled by the activation of cyclin-dependent kinases (CDKs), which associate with cyclins and drive cells through the G1 phase of the cell cycle (Atanasoski et al., 2006). Cyclin-dependent kinase inhibitors including p21 and p16 play an important role in inhibiting cell cycle progress in response to various signals (Johnson and Walker, 1999). In addition to the inhibition of their kinase activity, upregulation of p21 and p16 favors the binding to Cyclin E-CDK2 and Cyclin D-CDK4/CDK6 complex, respectively; thus, these complexes cannot contribute to Rb phosphorylation (Lim and Kaldis, 2013). Unphosphorylated pRb restricts cell cycle progression by restraining E2F (Dyson, 1998), which regulates several genes (such as Cyclin A) required for the subsequent transitions through the cell cycle (Bracken et al., 2004). Concomitantly to the upregulation of p21 and p16 by GSPE, we also observed suppressed expressions of CDK2, CDK4, CDK6, Cyclin A, and Cyclin D1, suggesting GSPE inhibits cell cycle arrest at the G0/G1 phase by upregulating p21 and p16 in porcine preadipocytes. Moreover, 200 µg/mL GSPE induced apoptosis of porcine preadipocytes, with enhanced protein expressions of cleaved caspase-3. Cleaved caspase-3 is the active form of caspase-3, which plays a central role in the execution phase of cell apoptosis. These results imply that GSPE suppresses preadipocyte proliferation through G0/G1 cell cycle arrest and apoptosis, providing a basis for further exploring growth arrest effect by GSPE. Taken together, the antiproliferation and antidifferentiation effects in preadipocytes, coupled with pro-lipolysis activity in adipocytes, suggesting GSPE might decrease adipose tissue mass in pigs. However, more evidences from in vivo studies are needed to further elucidate the role of GSPE potential use as a fat mass inhibitor of pigs.
In summary, this study shows that GSPE inhibits proliferation and differentiation of porcine preadipocytes and promotes lipolysis in mature adipocytes. Altered expressions of adipogenic-related transcription factors such as PPARγ and Pref-1 participated in the antiadipogenic effects of GSPE. Grape seed procyanidin extract also decreased GPDH activity during preadipocyte differentiation and in mature adipocytes. Moreover, GSPE inhibited proliferation of porcine preadipocytes through G0/G1 cell cycle arrest and cell apoptosis. The antilipid accumulation and antiproliferation activity of GSPE on adipocytes suggest GSPE impedes signals for the formation of adipose cells, indicating the applicable potential of GSPE for the management of adipose tissue in pigs.
Footnotes
This project was supported by the National Natural Science Foundation of China (#31501930), the Natural Science Foundation of Jiangsu Province (#BK20150656), and the Fundamental Research Funds for the Central Universities of China (#KJQN201606). The authors have declared that no conflict of interest exists.
LITERATURE CITED
- Ardévol A., Bladé C., Salvadó M. J., and Arola L.. 2000. Changes in lipolysis and hormone-sensitive lipase expression caused by procyanidins in 3T3-L1 adipocytes. Int. J. Obes. Relat. Metab. Disord. 24:319–324. doi:10.1038/sj.ijo.0801130 [DOI] [PubMed] [Google Scholar]
- Atanasoski S., Boller D., De Ventura L., Koegel H., Boentert M., Young P., Werner S., and Suter U.. 2006. Cell cycle inhibitors p21 and p16 are required for the regulation of Schwann cell proliferation. Glia 53:147–157. doi:10.1002/glia.20263 [DOI] [PubMed] [Google Scholar]
- Bracken A. P., Ciro M., Cocito A., and Helin K.. 2004. E2F target genes: Unraveling the biology. Trends Biochem. Sci. 29:409–417. doi:10.1016/j.tibs.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Cao Z. H., Yang H., He Z. L., Luo C., Xu Z. Q., Gu D. H., Jia J. J., Ge C. R., and Lin Q. Y.. 2013. Effects of aqueous extracts of raw pu-erh tea and ripened pu-erh tea on proliferation and differentiation of 3T3-L1 preadipocytes. Phytother. Res. 27:1193–1199. doi:10.1002/ptr.4831 [DOI] [PubMed] [Google Scholar]
- Chawla A., Schwarz E. J., Dimaculangan D. D., and Lazar M. A.. 1994. Peroxisome proliferator-activated receptor (PPAR) gamma: Adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 135:798–800. doi:10.1210/endo.135.2.8033830 [DOI] [PubMed] [Google Scholar]
- Cristancho A. G., and Lazar M. A.. 2011. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 12:722–734. doi:10.1038/nrm3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T., and Lohmann-Matthes M. L.. 1988. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 115:61–69. doi:10.1016/0022-1759(88)90310-9 [DOI] [PubMed] [Google Scholar]
- Del Bas J. M., Ricketts M. L., Baiges I., Quesada H., Ardevol A., Salvadó M. J., Pujadas G., Blay M., Arola L., Bladé C., et al. 2008. Dietary procyanidins lower triglyceride levels signaling through the nuclear receptor small heterodimer partner. Mol. Nutr. Food Res. 52:1172–1181. doi:10.1002/mnfr.200800054 [DOI] [PubMed] [Google Scholar]
- Dijk W., and Kersten S.. 2014. Regulation of lipoprotein lipase by Angptl4. Trends Endocrinol. Metab. 25:146–155. doi:10.1016/j.tem.2013.12.005 [DOI] [PubMed] [Google Scholar]
- Dodson M. V., Allen R. E., Du M., Bergen W. G., Velleman S. G., Poulos S. P., Fernyhough-Culver M., Wheeler M. B., Duckett S. K., Young M. R., et al. 2015. Invited review: Evolution of meat animal growth research during the past 50 years: Adipose and muscle stem cells. J. Anim. Sci. 93:457–481. doi:10.2527/jas.2014-8221 [DOI] [PubMed] [Google Scholar]
- Dyson N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245–2262. doi:10.1101/gad.12.15.2245 [DOI] [PubMed] [Google Scholar]
- Esatbeyoglu T., Wray V., and Winterhalter P.. 2015. Isolation of dimeric, trimeric, tetrameric and pentameric procyanidins from unroasted cocoa beans (Theobroma cacao L.) using countercurrent chromatography. Food Chem. 179:278–289. doi:10.1016/j.foodchem.2015.01.130 [DOI] [PubMed] [Google Scholar]
- Harmon A. W., and Harp J. B.. 2001. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am. J. Physiol. Cell Physiol. 280:C807–C813. doi:10.1152/ajpcell.2001.280.4.C807 [DOI] [PubMed] [Google Scholar]
- Hausman G. J., Basu U., Wei S., Hausman D. B., and Dodson M. V.. 2014. Preadipocyte and adipose tissue differentiation in meat animals: Influence of species and anatomical location. Annu. Rev. Anim. Biosci. 2:323–351. doi:10.1146/annurev-animal-022513-114211 [DOI] [PubMed] [Google Scholar]
- Hausman G. J., Dodson M. V., Ajuwon K., Azain M., Barnes K. M., Guan L. L., Jiang Z., Poulos S. P., Sainz R. D., Smith S., et al. 2009. Board-invited review: The biology and regulation of preadipocytes and adipocytes in meat animals. J. Anim. Sci. 87:1218–1246. doi:10.2527/jas.2008-1427 [DOI] [PubMed] [Google Scholar]
- Hudak C. S., and Sul H. S.. 2013. Pref-1, a gatekeeper of adipogenesis. Front. Endocrinol. (Lausanne) 4:79. doi:10.3389/fendo.2013.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. R., Ro J. H., Dobson D. E., Min H. Y., and Spiegelman B. M.. 1986. Adipocyte P2 gene: Developmental expression and homology of 5′-flanking sequences among fat cell-specific genes. Proc. Natl. Acad. Sci. U.S.A. 83:3786–3790. doi:10.1073/pnas.83.11.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J., Gavrilova O., Pack S., Jou W., Mullen S., Sumner A. E., Cushman S. W., and Periwal V.. 2009. Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLoS Comput. Biol. 5:e1000324. doi:10.1371/journal.pcbi.1000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. G., and Walker C. L.. 1999. Cyclins and cell cycle checkpoints. Annu. Rev. Pharmacol. Toxicol. 39:295–312. doi:10.1146/annurev.pharmtox.39.1.295 [DOI] [PubMed] [Google Scholar]
- Lefterova M. I., Haakonsson A. K., Lazar M. A., and Mandrup S.. 2014. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 25:293–302. doi:10.1016/j.tem.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterova M. I., and Lazar M. A.. 2009. New developments in adipogenesis. Trends Endocrinol. Metab. 20:107–114. doi:10.1016/j.tem.2008.11.005 [DOI] [PubMed] [Google Scholar]
- Lim S., and Kaldis P.. 2013. Cdks, cyclins and CKis: Roles beyond cell cycle regulation. Development 140:3079–3093. doi:10.1242/dev.091744 [DOI] [PubMed] [Google Scholar]
- Louveau I., Perruchot M. H., Bonnet M., and Gondret F.. 2016. Invited review: Pre- and postnatal adipose tissue development in farm animals: From stem cells to adipocyte physiology. Animal 10:1839–1847. doi:10.1017/S1751731116000872 [DOI] [PubMed] [Google Scholar]
- Montagut G., Bladé C., Blay M., Fernández-Larrea J., Pujadas G., Salvadó M. J., Arola L., Pinent M., and Ardévol A.. 2010. Effects of a grapeseed procyanidin extract (GSPE) on insulin resistance. J. Nutr. Biochem. 21:961–967. doi:10.1016/j.jnutbio.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Pinent M., Bladé M. C., Salvadó M. J., Arola L., Hackl H., Quackenbush J., Trajanoski Z., and Ardévol A.. 2005. Grape-seed derived procyanidins interfere with adipogenesis of 3T3-L1 cells at the onset of differentiation. Int. J. Obes. (Lond) 29:934–941. doi:10.1038/sj.ijo.0802988 [DOI] [PubMed] [Google Scholar]
- Pinent M., Blay M., Bladé M. C., Salvadó M. J., Arola L., and Ardévol A.. 2004. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology 145:4985–4990. doi:10.1210/en.2004-0764 [DOI] [PubMed] [Google Scholar]
- Poulos S. P., Hausman D. B., and Hausman G. J.. 2010. The development and endocrine functions of adipose tissue. Mol. Cell. Endocrinol. 323:20–34. doi:10.1016/j.mce.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Quesada H., del Bas J. M., Pajuelo D., Díaz S., Fernandez-Larrea J., Pinent M., Arola L., Salvadó M. J., and Bladé C.. 2009. Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int. J. Obes. (Lond) 33:1007–1012. doi:10.1038/ijo.2009.136 [DOI] [PubMed] [Google Scholar]
- Smas C. M., and Sul H. S.. 1993. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 73:725–734. doi:10.1016/0092-8674(93)90252-L [DOI] [PubMed] [Google Scholar]
- Tontonoz P., and Spiegelman B. M.. 2008. Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 77:289–312. doi:10.1146/annurev.biochem.77.061307.091829 [DOI] [PubMed] [Google Scholar]
- Wei S., Fu X., Liang X., Zhu M. J., Jiang Z., Parish S. M., Dodson M. V., Zan L., and Du M.. 2015. Enhanced mitogenesis in stromal vascular cells derived from subcutaneous adipose tissue of wagyu compared with those of Angus cattle. J. Anim. Sci. 93:1015–1024. doi:10.2527/jas.2014-7923 [DOI] [PubMed] [Google Scholar]
- Wise L. S., and Green H.. 1979. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J. Biol. Chem. 254:273–275. [PubMed] [Google Scholar]
- Yang Y., Qiao L., Zhang X., Wu Z., and Weng P.. 2015. Effect of methylated tea catechins from Chinese oolong tea on the proliferation and differentiation of 3T3-L1 preadipocyte. Fitoterapia 104:45–49. doi:10.1016/j.fitote.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386. doi:10.1126/science.1100747 [DOI] [PubMed] [Google Scholar]