Abstract
The objective was to evaluate the influence of varying amounts of Brahman genetics on body temperature under pasture conditions during hot weather. Vaginal temperatures were measured at 5-min intervals for 3 to 5 d on four occasions during August and September from a total of 190 pregnant cows that were either Angus, 2/8 Brahman (remainder Angus), Brangus (3/8 Brahman), 4/8 Brahman, 6/8 Brahman or Brahman. Vaginal temperature was higher for the first two replicates than for the second two replicates. In the first two replicates, average vaginal temperature did not differ between genetic groups, but average vaginal temperature from 1500 to 1900 h was lower for Brahman than other groups. In the second two replicates, average vaginal temperature was lower for cows that were 4/8 or higher Brahman than for cows that were 2/8 Brahman or Angus. Average vaginal temperature from 1500 to 1900 h was lower for cows that were 4/8 or higher Brahman than for cows that were 2/8 Brahman or Angus. In addition, Brahman cows had lower vaginal temperatures than cows that were 4/8 Brahman or 3/8 Brahman (i.e., Brangus). In one replicate, a tracking device was used to map cow location. At 1200 to 1300 h, cows that were 6/8 Brahman or Brahman had fewer observations near the tree line (i.e., in shade) than cows that were 4/8 Brahman or less. At 1500 to 1600 h, cows that were 4/8 or higher Brahman experienced fewer observations near the tree line than cows that contained a lower fraction of Brahman genetics. In summary, a minimum of 4/8 Brahman genetics was required to increase the ability to regulate body temperature and at least 6/8 Brahman when heat stress was severe. It is likely, therefore, that using Brahman genetics to optimize adaptation to thermal stress under conditions of severe heat stress requires a preponderance of Brahman genes.
Keywords: heat stress, thermoregulation, body temperature, cattle, Angus, Brahman
INTRODUCTION
Heat stress can compromise physiology of cattle to reduce feed intake (Mitlöhner et al., 2001; Brown-Brandl et al., 2005; Rhoads et al., 2009), growth rate (Mitlöhner et al., 2001; Sullivan et al., 2011), milk yield (Igono et al., 1987; Smith et al., 2006), and fertility (Roman-Ponce et al., 1981; Putney et al., 1988). Populations of cattle that were developed in hot regions of the world often possess genes that confer resistance to heat stress. For examples, see comparisons of Angus and Hereford vs. Brahman, Romosinuano, and Senepol heifers for beef cattle (Hammond et al., 1996) and Carora vs. Holstein cows (Olson et al., 2003) for dairy cattle. Bos indicus cattle are particularly adapted to hot climates and possess superior ability to regulate body temperature (Hansen, 2004).
One approach to mitigate effects of heat stress is to introduce genes from thermotolerant breeds into more thermally sensitive breeds through crossbreeding. This strategy can result in cattle with increased adaptability to hot climates (Hammond et al., 1996, 1998; Olson et al., 2003) but also with reduced performance for production and reproductive traits (Dow et al., 1982; Freetly and Cundiff, 1997; Olson et al., 2003; Elzo et al., 2012a, 2012b). It is likely that an optimal admixture of genes from adapted and nonadapted breeds can be found to optimize economic returns. For example, carcass characteristics were not adversely affected by introduction of genes from Brahman cattle into an Angus founder population until the Brahman contribution was more than 50% (Elzo et al., 2012a).
The objective of the current study was to evaluate the influence of various amounts of Brahman genetics in a multibreed herd formed from Angus founder animals on regulation of body temperature under pasture conditions during hot weather. The goal was to identify the minimal amount of Brahman genetics required to achieve optimal regulation of body temperature during heat stress for cows maintained on pasture.
MATERIALS AND METHODS
Animals
Animal use was approved by the University of Florida Institutional Animal Care and Use Committee (Approval No. 201203578). Animals were from the multibreed beef cattle herd maintained at the University of Florida Beef Research Unit (Fairbanks, FL; 29°44′38.2″N +82°15′55.2″W). The description of the creation of the herd and its reproductive management was described by Elzo et al. (2009). The herd was produced in a diallel mating system involving mating sires and cows from six breed groups: Angus, 3/4 Angus and 1/4 Brahman, Brangus (5/8 Angus and 3/8 Brahman), 1/2 Angus and 1/2 Brahman, 1/4 Angus and 3/4 Brahman, and Brahman. Sires from each breed group were mated to cows of all breed groups each year. Sires were used for 2 yr to create connectedness across years.
A total of 190 spring-bred pregnant cows were used for the current study. Animals were categorized into groups based on the fraction Brahman as follows: group 1 = Angus (0/32 to 6/32 Brahman; designated as AN; n = 37); group 2 = 1/4 Brahman (7/32 to 11/32 Brahman; designated as 2/8; n = 23); group 3 = Brangus (12/32 Brahman; n = 23); group 4: 50% Brahman (13/32 to 19/32 Brahman; designated as 4/8; n = 25), group 5 = 3/4 Brahman (20/32 to 25/32 Brahman; designated as 6/8; n = 21); and group 6 = Brahman: (26/32 to 32/32 Brahman; n = 61). In all cases, the fraction of genetics that was not Brahman was Angus. Average age (SD) of animals (mo) was 51 (22), 55 (24), 63 (39), 47 (21), 45 (23), and 46 (23) for groups 1 to 6, respectively, while average days pregnant (SD) at initiation of measurements was 131 (27), 138 (21), 137 (25), 129 (22), 136 (29), and 106 (42).
Measurements
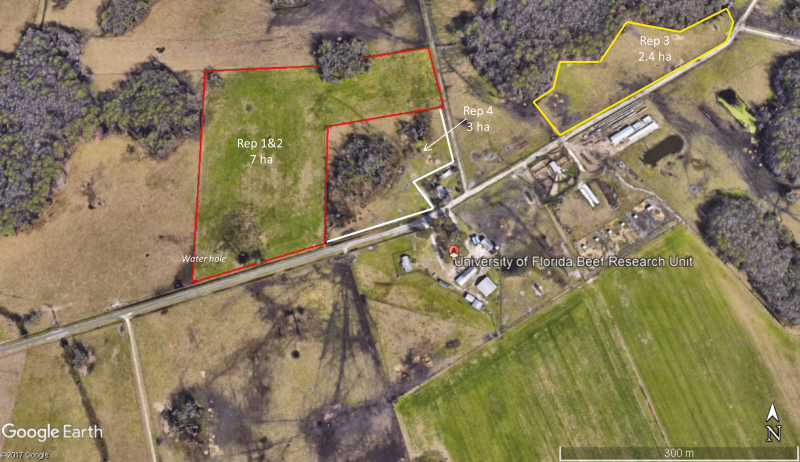
The experiment was conducted in four replicates using 45–50 cows per replicate. An individual cow was used in one replicate only (i.e., no cow had records in more than one replicate). Replicate 1 (n = 50) was conducted from 18 August 2015 to 21 August 2015, replicate 2 (n = 49) was conducted from 24 August 2015 to 27 August 2015, replicate 3 (n = 46) was conducted from 28 August to 31 August 2015, and replicate 4 (n = 45) was conducted from 1 September 2015 to 3 September 2015. Cows in replicates 1 and 2 were maintained in a single 7 ha pasture, cows in replicate 3 were maintained in a second 2.4 ha pasture, and cows in replicate 4 were maintained in a third 3 ha pasture (Figure 1). Each pasture included access to shade from extensive stands of trees. The pasture for replicates 1 and 2 also contained a water hole that cows could enter. During each replicate, only those cows under study were located in the pasture.
Figure 1.
Satellite view of pastures from the University of Florida Beef Research Unit used in the study.
At the beginning of each replicate, a blank (i.e., without progesterone) controlled internal release device (CIDR, Pfizer Animal Health, New York, NY) to which was attached an iButton temperature logger (model 1922L; Maxim Integrated, San Jose, CA; accuracy of ±0.0625 °C at 11-bit resolution) was placed within the vagina of each cow to record vaginal temperature at 5-min intervals. Details of assembly of the device are found in Dikmen et al. (2014). In addition, each cow was fitted with a portable global positioning system (GPS) device (Super Trackstick, Telespial Systems Inc, Burbank, CA) to track geographical position. The device was attached to a collar by means of two zip ties and the collar placed around the neck (Figure 2, top and middle panels). The device recorded latitude, longitude, altitude, coordinates for mapping location on Google MapLink (Google, Mountain View, CA), dry-bulb temperature, and whether the device was moving or stationery. Data were recorded each time the device moved. When moving, the direction and speed of movement was recorded. The device also recorded the duration of stationery periods.
Figure 2.
Tracking of location of individual cows using a global positioning system device. The top panel is a photograph of the placement of the device on a collar around the neck of the animal, and the middle panel shows a cow wearing a collar during the experiment. The bottom panel is an example of positional information (yellow circles indicate the cow was moving and red circles indicate the cow was stationery) mapped using Google MapLink. Data were recorded each time the device changes course or becomes stationery. At each position, data were collected for latitude, longitude, altitude, coordinates for mapping location on Google MapLink, dry-bulb temperature, and whether the device was moving or stationery. When moving, the direction and speed of movement was recorded. The device also recorded the duration of stationery periods.
Because of technical problems, sufficient GPS data for statistical analysis were only available for 27 h of replicate 2. The location of each cow at each reading was plotted on a map of the pasture using Google MapLink (Figure 2, bottom panel). Recorded positions of the cow during specific time intervals [0600 to 0700 h (n = 28 cows), 0900 to 1000 h (n = 31 cows), 1200 to 1300 h (n = 41 cows), 1500 to 1600 h (n = 40 cows), and 1800 to 1900 h (n = 34 cows)] were assessed to determine whether the cow was within 10 m of the tree line. This measurement was considered an estimate of whether cows were in shade because trees were the only source of shade in the pasture. Within a given time interval, the percent of readings in which the cow was located within 10 m of the tree line was calculated.
Environmental variables of dry-bulb temperature, relative humidity, dew point temperature, and black globe temperature were also recorded at a single location within ~50 m of each pasture at 5-min intervals. Dry-bulb temperature, relative humidity, and dew point temperature were measured with a HOBO-U23 data logger (Onset Computer Corp., Bourne, MA) placed in a shaded area 3 m above the ground, and black globe temperature was measured with a HOBO-U22 data logger placed in direct sunlight at a position 3 m above the ground under direct sunlight. The temperature–humidity index (THI) was subsequently calculated using the following formula (NRC, 1971):
where T = dry-bulb temperature (°C) and RH = relative humidity (percent).
Statistical Analysis
All data analysis was conducted using SAS v.9.4 (SAS Inst., Cary, NC). Initial statistical analyses were conducted using a repeated-measures design with the GLIMMIX procedure and with genetic group, time of day, and genetic group × time as fixed effects and with cow nested within genetic group as a random variable. There were significant genetic group × time interactions and additional analyses were performed using average temperatures to clarify the nature of the interactions. Treatment effects on average vaginal temperatures of individual cows were calculated three ways—as the average of all vaginal temperatures collected during the 3 to 5 d of recording, as the average of vaginal temperatures during 1500 to 1900 h, a time that corresponds to the highest environmental temperatures, and as the average of vaginal temperatures during 0300 to 0700 h, a time that corresponds to lower vaginal temperatures. For statistical analysis of genetic group effect on average vaginal temperature, replicates 1 and 2 (cows were maintained in pasture 1 during the first half of the study) were defined as phase 1, while replicates 3 and 4 (cows were maintained in pastures 2 and 3 during the second half of the study) were defined as phase 2. Vaginal temperature and percent of recorded locations within 10 m of the tree line at each time interval were analyzed using generalized linear models with the GLM procedure of SAS. The model for vaginal temperature included the fixed effects of genetic group, phase, replicate nested within phase, and genetic group by phase interaction, whereas the model for percent of recorded locations within 10 m of the tree line included only the fixed effect of genetic group. The pdiff procedure of SAS was used to determine differences between least squares means of genetic groups for each trait overall or within each phase of the experiment. Analysis of residuals of average vaginal temperatures using the Univariate procedure of SAS, and the Kolmogorov–Smirnov, Cramer–von Mises, and Anderson–Darling tests indicated that distribution of residuals did not deviate significantly from normality.
RESULTS AND DISCUSSION
Meteorological Conditions
Conditions were obtained at 5-min intervals at times coincident with measurements of vaginal temperature. Average values, based on all measurements as well as measurements from 1500 to 1900 h (the hottest time of the day), are presented in Table 1. Cows were subjected to environmental conditions characteristic of heat stress in all four replicates. For example, average dry-bulb temperature varied from 25.6 and 27.7 °C and average dry-bulb temperature from 1500 to 1900 h ranged from 27.4 to 32.5 °C among replicates. Average THI fluctuated between 76.4 and 78.8 °C, whereas average THI from 1500 to 1900 h ranged from 78.0 and 83.6 °C among replicates. Beef cows subjected to similar conditions of dry-bulb and black globe temperature have previously been reported to experience hyperthermia (Hammond et al., 1996, 1998; Mitlöhner et al., 2001). In addition, average relative humidity was high, being always greater than 62% between 1500 and 1900 h. High humidity impedes heat loss from cows to the surrounding environment (McLean, 1963; Maia et al., 2005).
Table 1.
Meteorological conditions during the experiment
| Replicate 1 | Replicate 2 | Replicate 3 | Replicate 4 | |
|---|---|---|---|---|
| Average, 0000 to 2400 ha | ||||
| Dry-bulb temperature, °C | 27.3 (4.1) | 27.7 (4.1) | 25.6 (3.1) | 27.0 (2.7) |
| Black globe temperature, °C | 29.3 (6.9) | 30.6 (7.8) | 27.8 (6.4) | 29.3 (5.9) |
| Relative humidity, % | 83.2 (17.0) | 81.9 (16.9) | 88.1 (13.4) | 88.3 (11.5) |
| Dew point temperature, °C | 23.7 (0.8) | 23.7 (0.8) | 23.1 (0.7) | 24.6 (0.7) |
| Temperature–humidity index | 78.4 (4.3) | 78.8 (4.3) | 76.4 (3.6) | 78.8 (3.0) |
| Average, 1500 to 1900 ha | ||||
| Dry-bulb temperature, °C | 31.0 (2.8) | 32.5 (1.2) | 27.4 (2.2) | 30.1 (0.6) |
| Black globe temperature, °C | 33.1 (4.6) | 38.1 (2.9) | 29.8 (4.3) | 36.1 (2.1) |
| Relative humidity, % | 67.1 (11.3) | 62.2 (5.9) | 78.3 (8.8) | 74.0 (2.7) |
| Dew point temperature, °C | 24.0 (0.8) | 24.3 (0.5) | 22.9 (0.4) | 25.0 (0.4) |
| Temperature–humidity index | 82.2 (2.4) | 83.6 (0.9) | 78.2 (2.2) | 82.1 (0.7) |
aMean (SD) of data collected at 5 min intervals for 3 to 5 d.
Meteorological conditions were similar for all replicates with the exception of replicate 3. Dry-bulb temperature, black globe temperature, dew point temperature, and THI were lower, and relative humidity higher for this replicate than for the other replicates.
Breed Group Effect on Vaginal Temperatures
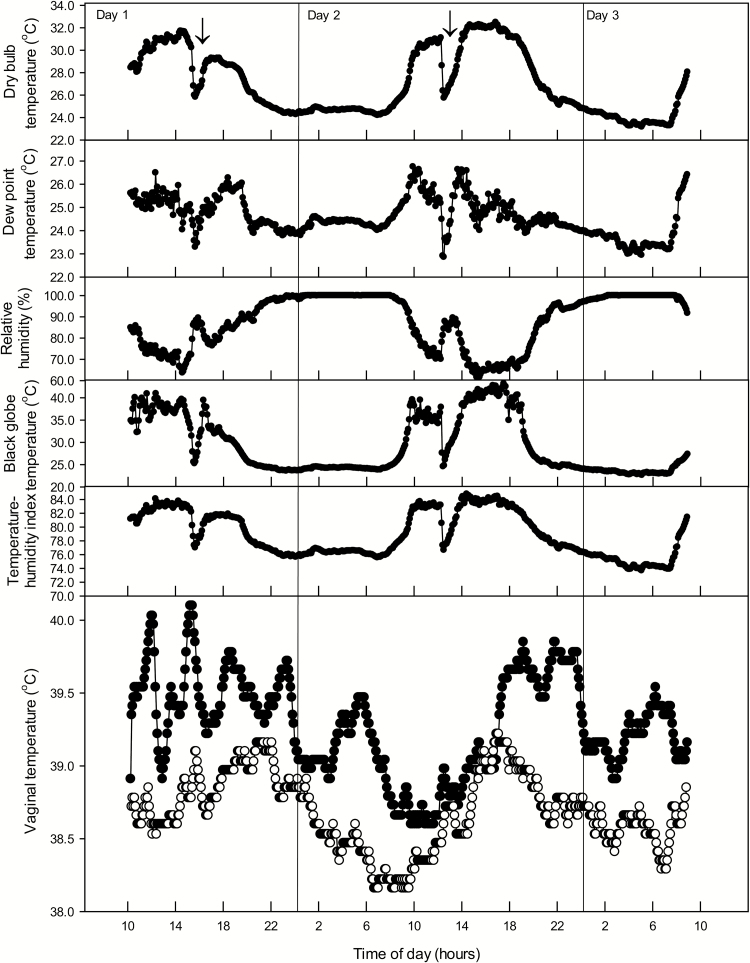
Representative examples of daily patterns of meteorological variables and associated changes in vaginal temperature are shown in Figure 3. In general, vaginal temperatures were low in the morning before dawn and rose thereafter to peak between ~1400 and 1900 h. In many cases, multiple peaks of vaginal temperature occurred during the day. It is likely that some of the multiple peaks represented behavioral responses of cows to seek shade or, for replicates 1 and 2, the water hole. Other peaks were likely caused by occasional rain events of short duration. Vaginal temperatures remained high after sunset and gradually declined to a nadir before sunset.
Figure 3.
Representative examples of daily variation in meteorological conditions and associated changes in vaginal temperatures. Vaginal temperature is plotted for an individual Angus cow (filled circles) and Brahman cow (open circles). Arrows represent rain events.
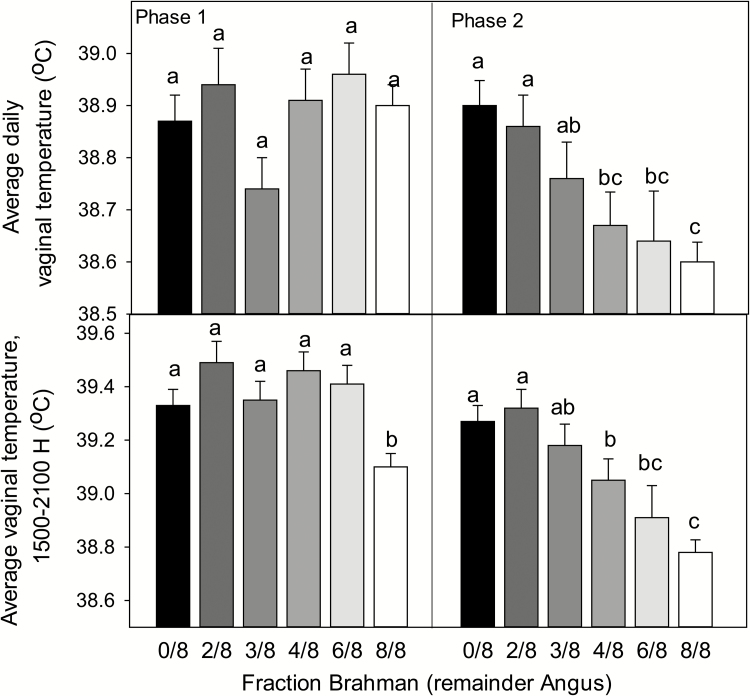
Data on average daily vaginal temperatures and average vaginal temperatures from 1500 to 1900 H for each genetic group during phases 1 and 2 are presented in Figure 4. Average daily vaginal temperatures were affected by genetic group (P = 0.0162), phase (P < 0.0001), and the interaction between genetic group and phase (P = 0.0015). In phase 1, there were no differences among genetic groups in average daily vaginal temperature. In phase 2, in contrast, vaginal temperature was significantly lower for cows that were 4/8 or higher Brahman than for cows that were 2/8 Brahman or Angus. In addition, Brahman cows had significantly lower vaginal temperatures than cows that were 3/8 Brahman (i.e., Brangus).
Figure 4.
Effect of genetic group on average daily vaginal temperatures and average vaginal temperatures from 1500 to 1900 h in phase 1 (replicates 1 and 2) and phase 2 (replicates 3 and 4). Average daily vaginal temperatures were affected by genetic group (P = 0.0162), phase (P < 0.0001), and the interaction between genetic group and phase (P = 0.0015). Average vaginal temperatures from 1500 to 1900 h were affected by genetic group (P < 0.0001), phase (P < 0.0001), and the interaction between genetic group and phase (P = 0.0374). Means with different subscripts differ (P < 0.05).
Results were generally similar for average vaginal temperatures from 1500 to 1900 h (Figure 4). This measurement of vaginal temperature was affected by genetic group (P < 0.0001), phase (P < 0.0001), and the interaction between genetic group and phase (P = 0.0374) (Figure 4). In phase 1, vaginal temperature from 1500 to 1900 h was generally unaffected by genetic group except that temperatures were significantly lower for Brahman than other groups. In phase 2, vaginal temperature from 1500 to 1900 h was significantly lower for cows that were 4/8 or higher Brahman than for cows that were 2/8 Brahman or Angus. In addition, Brahman cows had significantly lower vaginal temperatures than cows that were 4/8 Brahman or 3/8 Brahman (i.e., Brangus).
Vaginal temperatures were also analyzed for the period from 0300 to 0700 h when vaginal temperatures were at a nadir. Average vaginal temperature during this time was affected by genetic group (P = 0.001) and phase (P < 0.0001) but not by the interaction between genetic group and phase. Vaginal temperature was slightly higher in phase 1 than phase 2 (38.6 ± 0.02 vs. 38.4 ± 0.03 °C). Vaginal temperature was lower for 3/8 Brahman than 0/8 (P = 0.0027), 2/8 (P = 0.0212), or 8/8 Brahman (P < 0.001) and lower for 4/8 Brahman than 8/8 Brahman (P = 0.0335). Least squares means ± SEM were 38.6 ± 0.3, 38.6 ± 0.05, 38.4 ± 0.05, 38.5 ± 0.05, 38.5 ± 0.06, and 38.6 ± 0.03 °C for 0/8, 2/8, 3/8, 4/8, 6/8, and 8/8 Brahman, respectively. Despite the differences between genetic groups, average vaginal temperatures from 0300 to 0700 h were within the range of temperatures considered as homeothermy. There are several differences between phases that could explain differences between genetic groups in vaginal temperature. Overall, vaginal temperatures were higher for phase 1 than phase 2, which could reflect differences in meteorological conditions between the phases as well as particular features of the different pastures in which cows were maintained for each phase. It is likely that increasing the proportion of genes from Brahman increases capacity of cows to regulate body temperature, but this superior thermoregulatory capacity is not expressed when heat stress is severe for animals that are not almost 100% Brahman. Present results are similar to those of Gaughan et al. (1999) who found that differences between Brahman and crosses with Brahman increased as the magnitude of heat stress increased.
As shown in Figure 4, the average vaginal temperatures during 1500 to 1900 h usually exceeded the value for uterine temperature of 39.0 °C associated with a reduction in fertility in dairy cows (Gwazdauskas et al., 1973). Thus, if beef cows are affected by hyperthermia similarly as for dairy cows, it is likely that cows in the current study were experiencing heat stress of sufficient magnitude to reduce conception rates. However, genetic group affected the magnitude of the hyperthermia. In replicates 1 and 2, average vaginal temperatures were only slightly above 39.0 °C for Brahman cattle. In replicates 3 and 4, when heat stress was of reduced magnitude, both Brahman and 6/8 Brahman cattle maintained average vaginal temperatures below the 39 °C threshold. Thus, cattle with a high percent of Brahman genetics are less likely to experience body temperatures during heat stress that are associated with reduced reproductive function than cattle with a lower fraction of Brahman genetics.
Shade Seeking Behavior
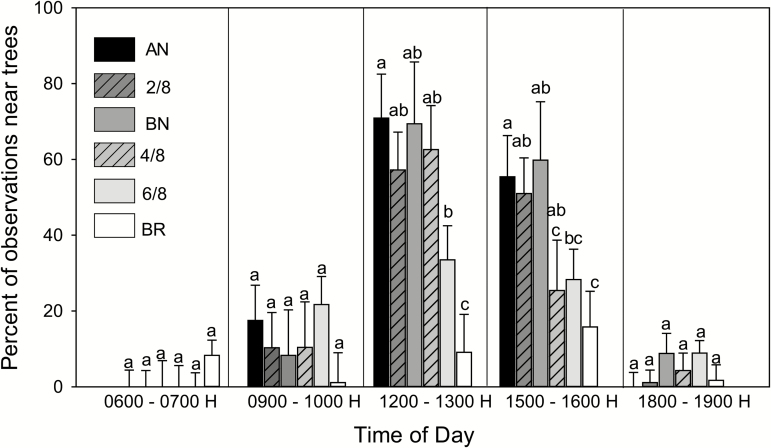
During heat stress, cattle seek shade to reduce radiant heat load (Veissier et al., 2018). Cows in the current study were capable of movement throughout the pasture and could attempt to regulate body temperature by seeking shade. To evaluate differences in behavior among genetic groups, cows in replicate 2 were evaluated for the percent of recorded locations within 10 m of the tree line at various 60-min intervals throughout daylight hours. Results are shown in Figure 5. There were no effects of genetic group on percent observations near the tree line from 0600 to 0700 h, 0900 to 1000 h, or 1800 to 1900 h. During each of these time intervals, few cows were near the tree line. However, genetic group affected percent observations near the tree line at 1200 to 1300 h (P = 0.0013) and 1500 to 1600 h (P = 0.0296), which represent times when many cows were near the tree line. At 1200 to 1300 h, cows that were 6/8 Brahman or Brahman had fewer percent of observations near the tree line than cows that were 4/8 Brahman or less. At 1500 to 1600 h, cows that were 4/8 or higher Brahman experienced fewer observations near the tree line than cows that contained a lower fraction of Brahman genetics. Thus, the superior thermoregulatory ability of cattle with a high fraction of Brahman genetics was associated with altered behavior during heat stress. Specifically, cattle were more likely to leave shade during the hot period of the day. It remains to be determined whether grazing activity, either during the day or in a 24-h period, was greater for cattle with a high fraction of Brahman genetics. It is possible that cattle with a low fraction of Brahman genetics compensated for time spent near the tree line during the day by increased grazing in the evening, night, or early morning.
Figure 5.
Effect of genetic group on the proportion of observations in which cows were within 10 m of the tree line. Data were calculated for 1-h intervals at various times of the day for cows in replicate 2. Genetic group affected percent observations near the tree line at 1200 to 1300 h (P = 0.0013) and 1500 to 1600 h (P = 0.0296). Means with different subscripts differ (P < 0.05).
Under the conditions cattle experienced in the current study, incorporation of Brahman genetics did not increase ability to regulate body temperature until cattle were at least 4/8 Brahman or, when heat stress was severe, when cattle were greater than 6/8 Brahman. It is likely, therefore, that using Brahman genetics to optimize adaptation to thermal stress under conditions of severe heat stress requires a preponderance of Brahman genes. A challenge for using Brahman genetics in this manner is that other, less beneficial traits, for example, delayed age at puberty (Dow et al., 1982; Freetly and Cundiff, 1997) and reduced beef quality (Elzo et al., 2012a), are also conferred by introduction of Brahman genetics. Genetic selection within the Brahman breed for traits such as early puberty and meat quality should make it possible to produce a tropically adapted breed that does not possess negative traits common in the Brahman breed. Progress in selection for these traits is possible because the heritability for age at puberty (Vargas et al., 1988) and carcass characteristics (Riley et al., 2002; Smith et al., 2007; Elzo et al., 2017) are high. Identification of allelic variants of genes responsible for thermotolerance in Brahman is another strategy for increasing thermotolerance because such alleles could be introduced into less thermotolerant breeds by crossbreeding or gene editing. Such a strategy has already been employed for introduction of the slick mutation of the PRLR gene present in Senepol cattle into the Holstein breed (Dikmen et al., 2014).
Research was supported as supported by Competitive Grants no. 2013-68004-20365 and 2017-67007-26143 from the Agriculture and Food Research Initiative of USDA-NIFA, a grant from the Southeast Milk Inc. Milk Checkoff Program and funds from the L.E. “Red” Larson Endowment. The authors are grateful for the outstanding assistance from Danny Driver and the crew at the University of Florida Beef Research Unit, and the technical help from Sofia Ortega and Dae Young Kim. Thanks are also extended to Zoetis, for donation of Blank CIDRs.
LITERATURE CITED
- Brown-Brandl T. M., Eigenberg R. A., Hahn G. L., Nienaber J. A., Mader T. L., Spiers D. E., and Parkhurst A. M.. 2005. Analyses of thermoregulatory responses of feeder cattle exposed to simulated heat waves. Int. J. Biometeorol. 49:285–296. doi:10.1007/s00484-004-0250-2 [DOI] [PubMed] [Google Scholar]
- Dikmen S., Khan F. A., Huson H. J., Sonstegard T. S., Moss J. I., Dahl G. E., and Hansen P. J.. 2014. The SLICK hair locus derived from Senepol cattle confers thermotolerance to intensively managed lactating Holstein cows. J. Dairy Sci. 97:5508–5520. doi:10.3168/jds.2014-8087 [DOI] [PubMed] [Google Scholar]
- Dow J. S. Jr, Moore J. D., Bailey C. M., and Foote W. D.. 1982. Onset of puberty in heifers of diverse beef breeds and crosses. J. Anim. Sci. 55:1041–1047. [DOI] [PubMed] [Google Scholar]
- Elzo M. A., Johnson D. D., Wasdin J. G., and Driver J. D.. 2012a. Carcass and meat palatability breed differences and heterosis effects in an Angus-Brahman multibreed population. Meat Sci. 90:87–92. doi:10.1016/j.meatsci.2011.06.010 [DOI] [PubMed] [Google Scholar]
- Elzo M. A., Lamb G. C., Johnson D. D., Thomas M. G., Misztal I., Rae D. O., Martinez C. A., Wasdin J. G., and Driver J. D.. 2012b. Genomic-polygenic evaluation of Angus-Brahman multibreed cattle for feed efficiency and postweaning growth using the illumina 3K chip. J. Anim. Sci. 90:2488–2497. doi:10.2527/jas.2011-4730 [DOI] [PubMed] [Google Scholar]
- Elzo M. A., Mateescu R. G., Johnson T. L., Scheffler J. M., Carr C., Rae D. O., Wasdin J. G., Driver M. D., and Driver J. D.. 2017. Genomic-polygenic and polygenic predictions for nine ultrasound and carcass traits in Angus-Brahman multibreed cattle using three sets of genotypes. Livest. Sci. 202:58–66. doi:10.1016/j.livsci.2017.05.027 [Google Scholar]
- Elzo M. A., Rae D. O., Lanhart S. E., Hembry F. G., Wasdin J. G., and Driver J. D.. 2009. Association between cow reproduction and calf growth traits and ELISA scores for paratuberculosis in a multibreed herd of beef cattle. Trop. Anim. Health Prod. 41:851–858. doi:10.1007/s11250-008-9262-y [DOI] [PubMed] [Google Scholar]
- Freetly H. C. and Cundiff L. V.. 1997. Postweaning growth and reproduction characteristics of heifers sired by bulls of seven breeds and raised on different levels of nutrition. J. Anim. Sci. 75:2841–2851. [DOI] [PubMed] [Google Scholar]
- Gaughan J. B., T. L. Mader S. M. Holt M. J. Josey, and Rowan K. J.. 1999. Heat tolerance of Boran and Tuli crossbred steers. J. Anim. Sci. 77:2398–2405. [DOI] [PubMed] [Google Scholar]
- Gwazdauskas F. C., Thatcher W. W., and Wilcox C. J.. 1973. Physiological, environmental, and hormonal factors at insemination which may affect conception. J. Dairy Sci. 56:873–877. doi:10.3168/jds.S0022-0302(73)85270-1 [DOI] [PubMed] [Google Scholar]
- Hammond A. C., Chase C. C. Jr, Bowers E. J., Olson T. A., and Randel R. D.. 1998. Heat tolerance in Tuli-, Senepol-, and Brahman-sired F1 Angus heifers in Florida. J. Anim. Sci. 76:1568–1577. [DOI] [PubMed] [Google Scholar]
- Hammond A. C., Olson T. A., Chase C. C. Jr, Bowers E. J., Randel R. D., Murphy C. N., Vogt D. W., and Tewolde A.. 1996. Heat tolerance in two tropically adapted Bos taurus breeds, Senepol and Romosinuano, compared with Brahman, Angus, and Hereford cattle in Florida. J. Anim. Sci. 74:295–303. [DOI] [PubMed] [Google Scholar]
- Hansen P. J. 2004. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci. 82-83:349–360. doi:10.1016/j.anireprosci.2004.04.011 [DOI] [PubMed] [Google Scholar]
- Igono M. O., Johnson H. D., Steevens B. J., Krause G. F., and Shanklin M. D.. 1987. Physiological, productive, and economic benefits of shade, spray, and fan system versus shade for Holstein cows during summer heat. J. Dairy Sci. 70:1069–1079. doi:10.3168/jds.S0022-0302(87)80113-3 [DOI] [PubMed] [Google Scholar]
- Maia A. S., daSilva R. G., and Battiston Loureiro C. M.. 2005. Sensible and latent heat loss from the body surface of Holstein cows in a tropical environment. Int. J. Biometeorol. 50:17–22. doi:10.1007/s00484-005-0267-1 [DOI] [PubMed] [Google Scholar]
- Mclean J. A. 1963. The partition of insensible losses of body weight and heat from cattle under various climatic conditions. J. Physiol. 167:427–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitlöhner F. M., Morrow J. L., Dailey J. W., Wilson S. C., Galyean M. L., Miller M. F., and McGlone J. J.. 2001. Shade and water misting effects on behavior, physiology, performance, and carcass traits of heat-stressed feedlot cattle. J. Anim. Sci. 79:2327–2335. [DOI] [PubMed] [Google Scholar]
- NRC 1971. A guide to environmental research on animals. Natl. Acad. Sci, Washington, DC. [Google Scholar]
- Olson T. A., Lucena C., Chase C. C. Jr, and Hammond A. C.. 2003. Evidence of a major gene influencing hair length and heat tolerance in Bos taurus cattle. J. Anim. Sci. 81:80–90. [DOI] [PubMed] [Google Scholar]
- Putney D. J., Drost M., and Thatcher W. W.. 1988. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between days 1 to 7 post insemination. Theriogenology. 30:195–209. [DOI] [PubMed] [Google Scholar]
- Rhoads M. L., Rhoads R. P., VanBaale M. J., Collier R. J., Sanders S. R., Weber W. J., Crooker B. A., and Baumgard L. H.. 2009. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 92:1986–1997. doi:10.3168/jds.2008-1641 [DOI] [PubMed] [Google Scholar]
- Riley D. G., Chase C. C. Jr, Hammond A. C., West R. L., Johnson D. D., Olson T. A., and Coleman S. W.. 2002. Estimated genetic parameters for carcass traits of Brahman cattle. J. Anim. Sci. 80:955–962. [DOI] [PubMed] [Google Scholar]
- Roman-Ponce H., Thatcher W. W., and Wilcox C. J.. 1981. Hormonal interelationships and physiological responses of lactating dairy cows to a shade management system in a subtropical environment. Theriogenology. 16:139–154. [DOI] [PubMed] [Google Scholar]
- Smith T. R., Chapa A., Willard S., Herndon C. Jr, Williams R. J., Crouch J., Riley T., and Pogue D.. 2006. Evaporative tunnel cooling of dairy cows in the southeast. II: impact on lactation performance. J. Dairy Sci. 89:3915–3923. doi:10.3168/jds.S0022-0302(06)72434-1 [DOI] [PubMed] [Google Scholar]
- Smith T., Domingue J. D., Paschal J. C., Franke D. E., Bidner T. D., and Whipple G.. 2007. Genetic parameters for growth and carcass traits of Brahman steers. J. Anim. Sci. 85:1377–1384. doi:10.2527/jas.2006-653 [DOI] [PubMed] [Google Scholar]
- Sullivan M. L., Cawdell-Smith A. J., Mader T. L., and Gaughan J. B.. 2011. Effect of shade area on performance and welfare of short-fed feedlot cattle. J. Anim. Sci. 89:2911–2925. doi:10.2527/jas.2010-3152 [DOI] [PubMed] [Google Scholar]
- Vargas C. A., Elzo M. A., Chase C. C. Jr, Chenoweth P. J., and Olson T. A.. 1998. Estimation of genetic parameters for scrotal circumference, age at puberty in heifers, and hip height in Brahman cattle. J. Anim. Sci. 76:2536–2541. [DOI] [PubMed] [Google Scholar]
- Veissier I., Van Laer E., Palme R., Moons C. P. H., Ampe B., Sonck B., Andanson S., and Tuyttens F. A. M.. 2018. Heat stress in cows at pasture and benefit of shade in a temperate climate region. Int. J. Biometeorol. 62:585–595. doi:10.1007/s00484-017-1468-0 [DOI] [PubMed] [Google Scholar]