Abstract
The objective of the study was to investigate the effect of feeding reduced CP, AA-supplemented diets on meat quality in growing and finishing pigs as well as the related mechanism. In experiment 1, 18 growing pigs (36.5 kg BW) were assigned randomly and fed 1 of 3 corn-soybean meal diets containing either 18% CP (normal protein, NP), 15% CP (low protein, LP), or 12% CP (very low protein, VLP). In experiment 2, 18 finishing pigs (62.3 kg BW) were allotted randomly into 1 of the following diets: 16% CP (NP), 13% CP (LP), or 10% CP (VLP). In both experiments, the LP and VLP diets were supplemented with crystalline AA to achieve equal content of standardized ileal digestible lysine, methionine, threonine, and tryptophan. At the end of each experiment, all pigs were slaughtered to collect longissimus dorsi muscle (LM) samples. Samples were used for determining meat quality, intramuscular fat (IMF) content, fatty acid composition, free AA profile, and expression of genes for myosin heavy chain isoforms. Results showed that growing and finishing pigs fed the LP diets increased (P < 0.05) redness value of LM, while finishing pigs fed the LP and VLP diets decreased (P < 0.05) the shear force values. Compared with the NP diet, growing and finishing pigs fed lower CP diets had higher (P < 0.05) contents of IMF and MUFA, and lower (P < 0.05) contents of PUFA. Besides, higher (P < 0.05) expression levels of type I and/or IIa muscle fibers were observed in LP diet-fed growing and finishing pigs, and greater concentrations of taurine and tasty AA in VLP diet-fed growing and finishing pigs. Taken together, our results indicate that low-protein diets could positively affect meat quality of growing and finishing pigs, and likely through regulation of IMF content and fatty acid composition, fiber characteristics, and free AA profile in the muscle.
Keywords: amino acids, lipid metabolism, low protein, meat quality, pigs
INTRODUCTION
Due to the growing focus on the relationship between nutrition and health, consumers are increasingly demanding high-quality meat products. It is generally assumed that nutritional strategies are the major influential factors in meat quality of pigs, as seen with protein-deficient diets that dramatically enhance the content of intramuscular fat (IMF) in the growing or finishing phases (Wood et al., 2004a; Tous et al., 2014). Production of pork with higher amounts of IMF, termed marbling fat in muscle and implicated in improvement of eating quality, would be advantageous for the industry (Wood et al., 2004a). In this sense, the low-protein strategy is expected to be back on the front burner. Numerous studies have indicated an inextricable connection between the fatty acid composition of IMF and several aspects of meat quality, including tissue firmness, shelf life, and eating quality (Wood et al., 2004b), but the effect of low-protein diets on the related lipid metabolism of IMF is less known.
Longissimus dorsi muscle (LM), the most frequently used indicator muscle in pig studies of meat quality, is a mixture of fiber types. Proportion of muscle fibers can be regulated by diets and is believed to be of particular importance, because of the various fiber types that having different contractile, metabolic, biochemical, and biophysical properties play an essential role in meat quality (Klont et al., 1998; Karlsson et al., 1999). In addition, we know that inadequate protein provision in the diet is bound to cause corresponding changes in the concentrations of free AA in the muscle (Davila et al., 2013). Earlier studies pointed out that oxidative muscles are tastier than glycolytic ones and this has been partly attributed to the contents of free AA (Valin et al., 1982; Cornet and Bousset, 1999). Indeed, free AA of muscle have been evaluated as potential biochemical markers of pork quality (Flores et al., 2000), but so far, few reports describing how protein level affects these small molecules as well as their transporters in the muscle.
In view of the foregoing, we hypothesized that the low-protein diets may change meat quality traits of pigs through influencing lipid metabolism, fiber characteristics, and free AA profile of the muscle. The 2 experiments in the present study were performed using growing and finishing pigs respectively to test this hypothesis.
MATERIALS AND METHODS
Animals and Experimental Diets
Two experiments that involved a total of 36 pigs were conducted according to the guidelines of the Animal Care and Use Committee of the Institute of Subtropical Agriculture, the Chinese Academy of Sciences (2013020). Crossbred pigs (Duroc × Landrace × Yorkshire) were randomly assigned into 3 dietary treatments (6 pigs per treatment): 1) a normal protein (NP) diet; 2) a low protein (LP) diet (3% units reduction); 3) a very low protein (VLP) diet (6% units reduction). In experiment 1, there were 18 growing pigs with the average BW of 36.47 ± 0.20 kg were assigned randomly into 1 of the 3 dietary CP levels: NP diet containing 18% CP, LP diet containing 15% CP, and VLP diet containing 12% CP. The experiment lasted 30 days. In experiment 2, there were 18 finishing pigs with the average BW of 62.30 ± 0.07 kg were assigned randomly into 1 of the 3 dietary CP levels: NP diet containing 16% CP, LP diet containing 13% CP, and VLP diet containing 10% CP. This experiment lasted for 50 days. In both experiments, the diets were based on corn-soybean meal and supplemented with the limiting AA including lysine, methionine, threonine, and tryptophan to the LP and VLP diets to meet the nutrient requirements for growing or finishing pigs (NRC, 2012), and their ingredients are shown in Table 1. Pigs were individually housed in stainless steel metabolism pens (0.8 by 1.8 m) in an environmentally controlled room with an average temperature of 28 °C and had free access to feed and drinking water throughout the total trial period. Each pig was weighed at the beginning and the termination of the 2 experimental periods for calculation of ADG. Feed intake and feed refusals per pen were recorded weekly and at the end of the each experimental period for calculation of ADFI and G:F.
Table 1.
Ingredients and nutrient levels of diets for 30- to 60-kg growing and 60- to 100-kg finishing pig (as-fed basis, %)
| Item | Growing pig, dietary treatmenta | Finishing pig, dietary treatment | ||||
|---|---|---|---|---|---|---|
| NP | LP | VLP | NP | LP | VLP | |
| Ingredient composition, % | ||||||
| Corn | 58.60 | 67.50 | 77.60 | 67.00 | 78.36 | 87.40 |
| Soybean meal | 29.00 | 19.50 | 10.00 | 23.70 | 15.00 | 5.50 |
| Whey bran | 7.80 | 6.94 | 5.06 | 6.00 | 3.00 | 2.00 |
| Soybean oil | 1.55 | 2.38 | 3.00 | 0.88 | 0.90 | 1.71 |
| Lysine | 0.18 | 0.46 | 0.74 | 0.01 | 0.27 | 0.55 |
| Methionine | 0.00 | 0.09 | 0.17 | 0.00 | 0.00 | 0.09 |
| Threonine | 0.01 | 0.14 | 0.26 | 0.00 | 0.06 | 0.19 |
| Tryptophan | 0.00 | 0.02 | 0.07 | 0.00 | 0.01 | 0.06 |
| CaHPO4 | 0.69 | 0.78 | 0.90 | 0.50 | 0.55 | 0.65 |
| Limestone | 0.87 | 0.89 | 0.90 | 0.55 | 0.55 | 0.55 |
| NaCl | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| 1% premixb | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient levels, % | ||||||
| DE,c MJ/kg | 14.20 | 14.20 | 14.20 | 14.20 | 14.20 | 14.20 |
| CP | 18.27 | 15.16 | 12.35 | 16.30 | 13.17 | 10.26 |
| Lysine | 0.97 | 0.97 | 0.94 | 0.72 | 0.72 | 0.73 |
| Methionine + cysteine | 0.57 | 0.56 | 0.55 | 0.40 | 0.42 | 0.43 |
| Threonine | 0.61 | 0.61 | 0.60 | 0.51 | 0.50 | 0.49 |
| Tryptophan | 0.17 | 0.17 | 0.17 | 0.14 | 0.13 | 0.13 |
| Calcium | 0.60 | 0.63 | 0.61 | 0.52 | 0.50 | 0.51 |
| Total phosphorus | 0.51 | 0.48 | 0.45 | 0.45 | 0.40 | 0.38 |
aDietary treatment: NP = normal protein; LP = low protein; VLP = very low protein.
bSupplied per kg of diet: vitamin A, 10,800 IU; vitamin D3, 4,000 IU; vitamin E, 40 IU; vitamin K3, 4 mg; vitamin B1, 6 mg; vitamin B2, 12 mg; vitamin B6, 6 mg; vitamin B12, 0.05 mg; biotin, 0.2 mg; folic acid, 2 mg; niacin, 50 mg; D-calcium pantothenate, 25 mg; Fe, 100 mg as ferrous sulfate; Cu, 150 mg as copper sulfate; Mn, 40 mg as manganese oxide; Zn, 100 mg as zinc oxide; I, 0.5 mg as potassium iodide; and Se, 0.3 mg as sodium selenite.
cCalculated values; other values are measured.
Sample Collection
At the end of each experiment, blood (10 mL) from the overnight fasting pigs was collected by vein puncture, centrifuged at 3,000 × g at 4 °C for 15 min. Serum aliquots were then stored at −20 °C until analysis. After blood sampling, all pigs sacrificed by jugular puncture under general anesthesia via an i.v. injection of 4% sodium pentobarbital solution (50 mg/kg BW). Then, the LM muscle from the left side carcass was collected and refrigerated at 4 °C for meat quality data collection. Within 20 min of slaughter, approximate 100 g of LM sample from the right side of the carcass was taken anterior to the 10th rib and frozen at −20 °C until lyophilization for IMF, fatty acid, and free AA analysis. Meanwhile, approximate 1.0-cm-thick LM sample obtained posterior to the 10th rib was rapidly frozen in liquid nitrogen and stored at −80 °C until used for RNA extraction.
Meat Quality Measurements
The LM samples anterior to the 13th rib from the left side carcass were used in the following order: 1) 3.0-cm-thick chop used for pH measurement; 2) 4.0-cm-thick chop used for meat color measurement; 3) 2.5-cm-thick chop used for drip loss measurement; 4) 2.54-cm-thick chop used for cooking loss measurement; and 5) 2.54-cm-thick chop used for shear force measurement.
The pH values were recorded at 45 min and 24 h postmortem using a portable pH meter (Matthaus pH Star, Germany) which was calibrated with pH 4.6 and 7.0 buffers equilibrated at 35 °C. Meat color attributes including lightness (L*), redness (a*), and yellowness (b*) were determined using a hand-held colorimeter (CR-410, Kinica Minolta Sensing Inc., Osaka, Japan) calibrated against a standard white plate (8-mm diameter aperture, d/0 illumination system). Drip loss was determined as previously described (Honikel et al., 1986). Briefly, a 4-cm-diameter core (approximate 55 g) was manually trimmed and weighed at about 45 min postmortem. The sample was then suspended on a fishhook surrounded by an inflated plastic bag and suspended for 24 h at 4 °C, after which sample was removed from the fishhook, blotted dry on filter paper, and reweighed. Drip loss was expressed as the weight change percentage. For cooking loss determination, approximate 100 g of LM sample was weighed and then covered in a container before cooking. The temperature of water bath was set at 80 °C and meat samples were cooked for 1 h to an internal temperature of 70 °C. Afterwards, the samples were cooled at room temperature and reweighed. The difference between pre- and post-cooking weights was used to calculate the percentage loss during cooking. Cooking loss measurement was made in duplicate. The shear force values were measured using a Warner–Bratzler shear force device (TA.XT Plus, Stable Micro Systems, Godalming, UK). Briefly, LM samples were packaged in polyethylene bags at 72 h postmortem, and then were heated in 80 °C water until the inner temperature reached 70 °C. After cooling to 4 °C, 3 cores were removed from each sample parallel to the longitudinal orientation of muscle fiber, and then were sheared perpendicular to the long axis of the cores.
Serum Analysis
The concentrations of total cholesterol (TC) and triglyceride (TG) were measured using the Biochemical Analytical Instrument (Beckman CX4 Chemistry Analyzer; Beckman Coulter, Inc., Brea, CA) and commercial kits (Sino-German Beijing Leadman Biotech Ltd, Beijing, China). The concentrations of leptin and adiponectin were analyzed with corresponding commercial ELISA kits (Cusabio Biotech Co., Ltd, Wuhan, China) following the recommended procedures. All samples were measured in 6 replicates.
Intramuscular Fat Content and Fatty Acid Analysis
The IMF content was measured using the methods described previously (Liu et al., 2015). The total lipids were extracted from each homogenized LM sample and used for IMF determination.
The analysis of fatty acid methyl esters was determined with an Agilent 7890A gas chromatographer equipped with SP-2560 column (100 m × 250 μm × 0.2 μm) (Agilent Technologies Inc., Palo Alto, CA) according to the method described previously (Martin et al., 2011). Individual fatty acid peaks were identified by matching their retention times with those of the authentic standards (Sigma Chemicals, St. Louis, MO). The concentration of individual fatty acid was quantified according the peak area, and expressed as a percentage of total fatty acids. The average amount of each fatty acid was used to calculate the sum of the SFA, MUFA, and PUFA.
Free AA Profile
Approximate 100-mg LM sample (within 1 wk after slaughter) was dissolved in a solution of water and methanol (1:1, vol/vol) at 4 °C for 10 min, and centrifuged at 10,000 × g for 10 min at 4 °C. The 40 μL of the supernatant was labeled with iTRAQ reagents (AA 45/32 kit, Applied Biosystems, Forest City, CA) as recommended by the manufacturer and analyzed on an Applied Biosystems 3200 Q TRAP LC/MS/MS System equipped with a RP-C18-column (150-mm length, 4.6-mm diameter, 5-mm particle size). The identity and quantity of the AA were determined by comparison with the retention times and peak areas of each AA standard.
Quantitative Real-Time PCR Analysis
Total RNA was isolated from LM sample using TRIzol Reagent (Invitrogen-Life Technologies, Carlsbad, CA). The RNA quality was checked by spectrophotometry using NanoDrop ND2000 (NanoDrop Technologies Inc., Wilmington, DE), and purity of RNA was assessed at the optical density absorbance ratio of 260:280 nm, which ranged from 1.8 to 2.0. Approximately 1.0 μg of total RNA was incubated with DNase I (Fermentas Inc., Glen Burnie, MD) and later using First-Strand cDNA Synthesis Kit (Fermentas Inc.) according to the manufacturer’s protocol. Quantitative Real-Time PCR (RT-PCR) was conducted with ABI 7900HT RT-PCR system (Applied Biosystems, Branchburg, NJ) to measure mRNA expression levels of the selected genes as we have described in detail previously (Li et al., 2014). The primers for myosin heavy chain (MyHC) I, IIa, IIx, and IIb are given in Li et al. (2016a), while acetyl-CoA carboxylase alpha (ACCα), hormone-sensitive lipase (HSL), sodium-coupled neutral amino acid transporter 2 (SNAT2), system L amino acid transporter 1 (LAT1), proton-assisted amino acid transporter 1 (PAT1), and PAT2 are given in Li et al. (2015). The RT-PCR cycling conditions used were 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 60 s, then 1 cycle at 72 °C for 30 s. A pooled control sample representative of all treatment groups was run on each plate as an internal standard. Normalized expression (ΔΔCt; Ct = threshold cycle) for each sample was determined using β-actin as an endogenous control gene. The average normalized expression of the pooled control sample was used as the calibrator to calculate relative gene expression. For each sample, relative expression was calculated as 2–ΔΔCt, in which ΔΔCt represents ΔCt sample − ΔCt calibrator (Livak and Schmittgen, 2001).
Statistical Analysis
An individual pig was the experimental unit for analysis of all data. The data were analyzed using SAS software 9.2 (SAS Inst. Inc., Cary, NC). An analysis of variance (1-way ANOVA by Duncan) was used to assess the differences between the treatments. The data were expressed as the means ± SEM. Mean values were considered to be significantly different when P < 0.05 and supposed as tendency when 0.05 ≤ P < 0.10.
RESULTS
Growth Performance
Growth performance is shown in Table 2. For growing pigs, decreasing the CP level from 18% to 12% resulted in decreased (P < 0.05) final BW, ADG, and G:F, whereas there were no significant differences between the LP and VLP treatments. For finishing pigs, in comparison with the NP treatment, the VLP treatment exhibited an approximate 20% reduction (P < 0.05) in ADG. Finishing pigs fed the NP diet or the LP diet had a similar ratio of gain to feed, which was greater (P < 0.01) than that for pigs fed the VLP diet.
Table 2.
Effect of low-protein diet on growth performance of growing and finishing pig
| Item | Dietary treatmenta | SEM | P value | ||
|---|---|---|---|---|---|
| NP | LP | VLP | |||
| Growing pig | |||||
| Initial BW, kg | 36.60 | 36.43 | 36.53 | 1.47 | 0.983 |
| Final BW, kg | 64.33a | 58.73b | 57.30b | 2.32 | <0.01 |
| ADG, g/d | 924a | 743b | 692b | 124 | <0.01 |
| ADFI, g/d | 2,009 | 1,849 | 1,755 | 192 | 0.069 |
| G:F | 0.46a | 0.40b | 0.39b | 0.02 | <0.01 |
| Finishing pig | |||||
| Initial BW, kg | 62.33 | 62.30 | 62.28 | 2.02 | 0.990 |
| Final BW, kg | 101.43a | 97.88ab | 94.01b | 4.57 | <0.01 |
| ADG, g/d | 782a | 712b | 634c | 73 | <0.01 |
| ADFI, g/d | 2,819 | 2,615 | 2,548 | 212 | 0.059 |
| G:F | 0.28a | 0.27a | 0.25b | 0.01 | <0.01 |
Adapted from He et al. (2016). The same pigs were used in He et al.’s experiment and the present experiment.
a,bWithin a row, values with different superscript letters differ (P < 0.05).
aDietary treatment: NP = normal protein; LP = low protein; VLP = very low protein.
Meat Quality
The effects of low-protein diets on meat quality are summarized in Table 3. For growing pigs, no effects of low-protein diets were detected on values of pH, L*, drip loss, cooking loss, and shear force. In comparison with the NP diet, the LP and VLP diets tended to reduce (P = 0.07) the b* value of growing pigs, while there was no obvious difference between the pigs fed the LP and VLP diets. Growing pigs fed the LP diet had a higher (P < 0.05) a* value than those fed the NP or VLP diets, and the same impact on the a* value was also observed in finishing pigs. The value of pH24 h in finishing pigs fed the VLP diet was greater (P < 0.01) compared to those fed the NP or LP diets. Finishing pigs fed the LP and VLP diets decreased (P < 0.05) the shear force value compared to those fed the NP diet. However, low-protein diets failed to influence other detected parameters involved in meat quality of finishing pigs.
Table 3.
Effect of low-protein diet on meat quality in growing and finishing pig
| Itema | Dietary treatmentb | SEM | P value | ||
|---|---|---|---|---|---|
| NP | LP | VLP | |||
| Growing pig | |||||
| pH45 min | 6.87 | 6.88 | 6.83 | 0.03 | 0.482 |
| pH24 h | 6.32 | 6.34 | 6.35 | 0.02 | 0.541 |
| L* | 48.81 | 47.32 | 48.98 | 0.48 | 0.259 |
| a* | 13.31b | 14.14a | 13.53b | 0.19 | 0.020 |
| b* | 6.40a | 5.75b | 5.96ab | 0.19 | 0.073 |
| Drip loss, % | 2.14 | 2.12 | 1.97 | 0.21 | 0.818 |
| Cooking loss, % | 46.74 | 46.27 | 46.66 | 0.66 | 0.872 |
| Shear force, N | 31.44 | 30.64 | 27.61 | 1.36 | 0.143 |
| Finishing pig | |||||
| pH45 min | 6.39 | 6.60 | 6.41 | 0.08 | 0.169 |
| pH24 h | 5.37b | 5.32b | 5.46a | 0.02 | <0.01 |
| L* | 46.36 | 45.17 | 46.12 | 0.60 | 0.351 |
| a* | 13.16b | 14.42a | 13.23b | 0.35 | 0.044 |
| b* | 4.90 | 4.66 | 4.80 | 0.26 | 0.763 |
| Drip loss, % | 2.23 | 2.36 | 2.68 | 0.16 | 0.162 |
| Cooking loss, % | 46.12 | 45.48 | 47.46 | 0.73 | 0.192 |
| Shear force, N | 44.07a | 31.74b | 32.49b | 2.42 | 0.014 |
a,bWithin a row, values with different superscript letters differ (P < 0.05).
a L* = lightness; a* = redness; b* = yellowness.
bDietary treatment: NP = normal protein; LP = low protein; VLP = very low protein.
Lipid Metabolism
Table 4 shows that in comparison to the NP and LP diets, the VLP diet increased (P < 0.01) serum concentration of leptin, while it decreased (P < 0.01) that of adiponectin in growing pigs. However, serum concentrations of TC and TG in growing pigs and those of TC, TG, leptin, and adiponectin in finishing pigs were not affected by the low-protein diets.
Table 4.
Effect of low-protein diet on lipid metabolism in growing and finishing pig
| Itema | Dietary treatmentb | SEM | P value | ||
|---|---|---|---|---|---|
| NP | LP | VLP | |||
| Growing pig | |||||
| Serum TC, mmol/L | 2.10 | 1.66 | 1.76 | 0.20 | 0.154 |
| Serum TG, mmol/L | 0.34 | 0.24 | 0.30 | 0.04 | 0.300 |
| Serum leptin, ng/mL | 0.50b | 0.41b | 0.66a | 0.03 | <0.01 |
| Serum adiponectin, μg/mL | 3.15a | 3.27a | 2.69b | 0.10 | <0.01 |
| IMF in muscle, % | 1.35b | 1.59ab | 2.02a | 0.20 | 0.082 |
| ACCα mRNA level in muscle | 0.85b | 1.09ab | 1.37a | 0.19 | 0.078 |
| HSL mRNA level in muscle | 1.33a | 1.32a | 0.88b | 0.17 | 0.053 |
| Finishing pig | |||||
| Serum TC, mmol/L | 2.30 | 2.42 | 2.56 | 0.14 | 0.440 |
| Serum TG, mmol/L | 0.46 | 0.42 | 0.50 | 0.05 | 0.544 |
| Serum leptin, ng/mL | 0.41 | 0.50 | 0.51 | 0.05 | 0.323 |
| Serum adiponectin, μg/mL | 3.42 | 3.26 | 3.06 | 0.13 | 0.231 |
| IMF in muscle, % | 1.59b | 2.21a | 2.49a | 0.19 | 0.018 |
| ACCα mRNA level in muscle | 0.89b | 1.32a | 1.39a | 0.21 | 0.041 |
| HSL mRNA level in muscle | 1.56a | 1.07b | 0.91b | 0.14 | 0.027 |
a,bWithin a row, values with different superscript letters differ (P < 0.05).
aTC = total cholesterol; TG = triglyceride; IMF = intramuscular fat; ACCα = acetyl-coenzyme A carboxylase alpha; HSL = hormone-sensitive lipase.
bDietary treatment: NP = normal protein; LP = low protein; VLP = very low protein.
The IMF content is an important indicator of meat quality. As protein decreased in the diet, the IMF content tended to increase (P = 0.082) in growing pigs and notably, it also increased (P < 0.05) in finishing pigs, but there was no difference between pigs consumed the LP and VLP diets (Table 4).
The changes in mRNA abundance of critical genes implicated in regulating lipid metabolism of the muscle are listed in Table 4. It can be seen that decreasing protein levels in the diet showed a tendency for an upregulation (P = 0.078) of ACCα mRNA expression in the muscle of growing pigs. Meanwhile, HSL mRNA expression tended to downregulate (P = 0.053) in growing pigs fed the VLP diet compared to those fed the NP and LP diets. Likewise, compared to finishing pigs fed the NP diet, mRNA expression of ACCα was upregulated (P < 0.05), and that of HSL was downregulated (P < 0.05) in those fed the LP and VLP diets which were not different.
Also, data on fatty acid profile of total fat from the muscle of growing and finishing pigs by the dietary CP level are summarized in Table 5. For growing pigs, we noticed that concentrations of the most of fatty acids were altered by the low-protein diets, main effects were observed that MUFA concentration increased (P < 0.05), whereas PUFA concentration tended to decrease (P = 0.071) and PUFA/SFA ratio decreased (P < 0.05) as protein decreased in the diet. The tendency was similar in finishing pigs, but those differences among the diets were not as significant as in growing pigs. In finishing pigs, only concentrations of C14:1, C17:1, C18:1, and C18:2 fatty acids were notably influenced by the low-protein diets, which resulted in a lower (P < 0.05) concentration of MUFA and higher of PUFA in the NP group than those in the LP and VLP groups. In short, the fatty acid profile in growing pigs was much more affected by the dietary CP level than that in finishing pigs.
Table 5.
Effect of low-protein diet on fatty acid profile of longissimus dorsi muscle in growing and finishing pig
| Item | Growing pig, dietary treatmenta | Finishing pig, dietary treatment | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NP | LP | VLP | SEM | NP | LP | VLP | SEM | Growing | Finishing | |
| Fatty acid composition, % | ||||||||||
| C12:0 | 0.084 | 0.088 | 0.095 | 0.004 | 0.093 | 0.087 | 0.093 | 0.004 | 0.182 | 0.527 |
| C14:0 | 1.26b | 1.30b | 1.41a | 0.03 | 1.43 | 1.40 | 1.42 | 0.03 | 0.024 | 0.853 |
| C14:1 | 0.032b | 0.042ab | 0.046a | 0.004 | 0.037b | 0.042ab | 0.064a | 0.007 | 0.091 | 0.082 |
| C15:0 | 0.056b | 0.066ab | 0.075a | 0.004 | 0.052 | 0.048 | 0.061 | 0.008 | 0.024 | 0.523 |
| C16:0 | 24.21b | 24.77ab | 25.48a | 0.38 | 25.12 | 24.77 | 25.04 | 0.31 | 0.087 | 0.753 |
| C16:1 | 2.57 | 2.94 | 3.08 | 0.21 | 3.31 | 3.49 | 3.56 | 0.14 | 0.241 | 0.493 |
| C17:0 | 0.29 | 0.30 | 0.27 | 0.02 | 0.24 | 0.24 | 0.22 | 0.02 | 0.593 | 0.782 |
| C17:1 | 0.19 | 0.16 | 0.19 | 0.03 | 0.06b | 0.08ab | 0.12a | 0.01 | 0.784 | 0.034 |
| C18:0 | 12.47 | 12.56 | 13.03 | 0.47 | 12.65 | 12.14 | 12.09 | 0.37 | 0.682 | 0.584 |
| C18:1 | 40.76b | 41.71ab | 42.70a | 0.55 | 44.07b | 46.17a | 46.27a | 0.40 | 0.076 | 0.037 |
| C18:2 | 13.00 | 12.60 | 11.76 | 0.46 | 10.62a | 8.56b | 8.92b | 0.35 | 0.193 | 0.014 |
| C18:3 | 0.77a | 0.74ab | 0.67b | 0.04 | 0.53 | 0.47 | 0.43 | 0.04 | 0.083 | 0.314 |
| C20:0 | 0.08b | 0.16b | 0.27a | 0.03 | 0.13 | 0.14 | 0.15 | 0.04 | <0.01 | 0.983 |
| C20:1 | 0.61b | 0.67ab | 0.74a | 0.03 | 0.78 | 0.79 | 0.83 | 0.04 | 0.042 | 0.771 |
| C20:2 | 0.57a | 0.54a | 0.44b | 0.01 | 0.41 | 0.40 | 0.38 | 0.02 | <0.01 | 0.572 |
| C20:3 | 0.36a | 0.29ab | 0.27b | 0.02 | 0.22 | 0.24 | 0.20 | 0.01 | 0.066 | 0.360 |
| C20:4 | 1.15a | 0.85b | 0.77b | 0.08 | 0.77 | 0.79 | 0.71 | 0.07 | 0.020 | 0.744 |
| C21:0 | 0.021b | 0.022b | 0.034a | 0.003 | 0.021 | 0.029 | 0.027 | 0.003 | 0.013 | 0.312 |
| C22:0 | 0.048b | 0.053b | 0.078a | 0.007 | 0.039 | 0.039 | 0.039 | 0.003 | 0.009 | 0.993 |
| C22:2 | 0.029 | 0.027 | 0.021 | 0.003 | 0.026 | 0.027 | 0.023 | 0.003 | 0.203 | 0.669 |
| C22:6 | 0.057 | 0.067 | 0.053 | 0.008 | 0.025 | 0.033 | 0.027 | 0.004 | 0.414 | 0.518 |
| C24:1 | 0.023 | 0.032 | 0.036 | 0.006 | 0.022 | 0.022 | 0.028 | 0.003 | 0.567 | 0.422 |
| Partial sums of fatty acid, % | ||||||||||
| SFA | 38.52 | 39.32 | 40.74 | 0.75 | 39.77 | 38.89 | 39.14 | 0.63 | 0.140 | 0.646 |
| MUFA | 44.18b | 45.55ab | 46.80a | 0.60 | 48.27b | 50.60a | 50.87a | 0.50 | 0.034 | 0.028 |
| PUFA | 15.92a | 15.13ab | 13.99b | 0.54 | 12.61a | 10.52b | 10.69b | 0.43 | 0.071 | 0.023 |
| PUFA/SFA | 0.41a | 0.39ab | 0.35b | 0.02 | 0.32 | 0.27 | 0.27 | 0.01 | 0.043 | 0.104 |
a,bWithin a row, values with different superscript letters differ (P < 0.05).
aDietary treatment: NP = normal protein; LP = low protein; VLP = very low protein.
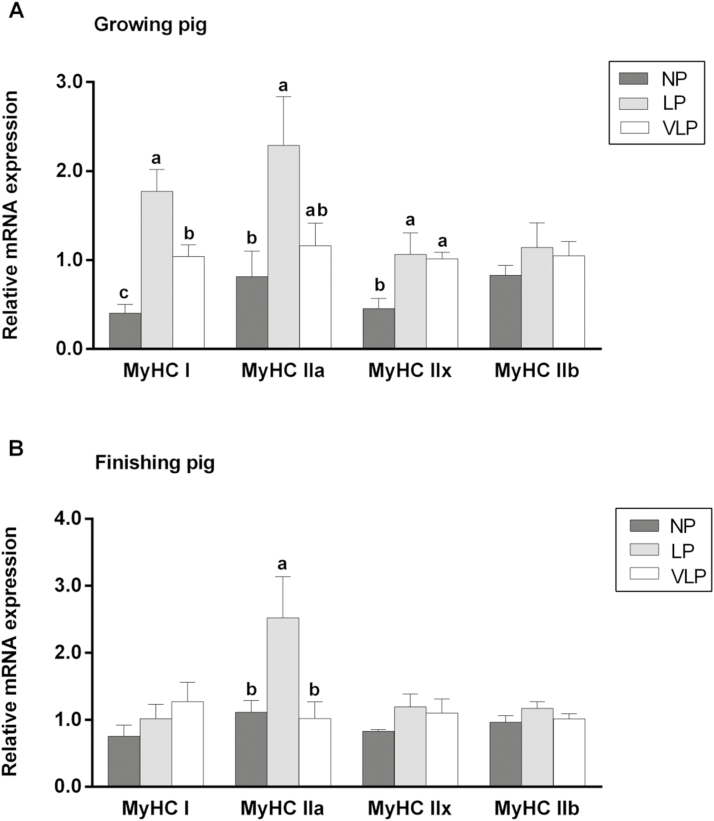
Muscle MyHC Expression Abundance
As indicated in Fig. 1, the low-protein diets obviously affected mRNA expression abundance of MyHC isoforms in the muscle of growing pigs except for that of MyHC IIb. Growing pigs fed the LP diet exhibited significantly or numerically higher expression levels of MyHC I and IIa than those fed the NP and VLP diets, and of note, feeding the NP diet had the numerically lowest expression levels of those 2 genes. A drop of expression level for MyHC IIx was also achieved for growing pigs fed the NP diet compared to those fed the LP and VLP diets. In addition, dietary protein level did not affect mRNA expression levels of MyHC I, IIx, and IIb in finishing pigs, but that of MyHC IIa on the LP diet was greater (P < 0.05) than the NP and VLP diets.
Figure 1.
Effect of low-protein diet on relative mRNA expression levels of myosin heavy chain (MyHC) isoforms of longissimus dorsi muscle in growing (A) and finishing (B) pig. Dietary treatment: normal protein (NP), low protein (LP), and very low protein (VLP). Data are expressed as means ± SEM (n = 6). a–cBars with different letters are different among dietary protein treatments (P < 0.05).
Muscle Free AA Profile and Transporters Expression Abundance
Wide modifications of AA profile in the muscle of growing and finishing pigs by the low-protein diets were observed, as shown in Table 6. The effect of treatment on AA concentration of the muscle basically depended on whether the AA was supplemented in the diet. In general, concentrations of the limiting AA that were supplemented (lysine, methionine, and threonine) increased (P < 0.05 or P = 0.073) in both growing and finishing pigs as protein was reduced in the diet, while tryptophan concentration was not affected by dietary CP treatment. As expected, concentrations of other essential AA (EAA) that were not supplemented declined in the pigs as dietary protein level decreased, which was particularly true in growing pigs. An intriguing observation was that concentrations of isoleucine and valine in either growing or finishing pigs fed the LP and VLP diets were lower (P < 0.05 or P = 0.080) compared to those fed the NP diet, and there were no differences between the LP and VLP groups. Less consistent effects were observed on concentrations of non-essential AA (NEAA) and other AA. In growing pigs, concentrations of alanine, glutamic acid, glutamine, serine, and taurine in the VLP group were greater (P < 0.05 or P = 0.073) than those in the NP or LP groups which were not different, whereas concentrations of arginine, tyrosine, anserine, and carnosine in the NP group were significantly or numerically greater than those in the LP or VLP groups. In finishing pigs, however, most of NEAA concentrations were not affected by the dietary CP level, except for alanine and glutamic acid, concentrations of which enhanced (P < 0.05) in the VLP group compared to the NP and LP groups. As with in growing pigs, taurine was at higher concentration in finishing pigs fed the VLP diet but this was not true for anserine and carnosine. Overall, concentrations of tasty AA (TAA) and NEAA in both growing and finishing pigs fed the VLP diet were higher (P < 0.05) than those fed the NP and LP diets. It should be noted that the AA profile in finishing pigs was less influenced by the dietary CP level than that in growing pigs.
Table 6.
Effect of low-protein diet on free AA profile (μg/100 mg) of longissimus dorsi muscle in growing and finishing pig
| Itema | Growing pig, dietary treatmentb | Finishing pig, dietary treatment | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NP | LP | VLP | SEM | NP | LP | VLP | SEM | Growing | Finishing | |
| Essential AA, μg/100 mg | ||||||||||
| Histidine | 0.99a | 0.81b | 0.81b | 0.05 | 1.19 | 1.01 | 1.07 | 0.09 | 0.034 | 0.433 |
| Isoleucine | 1.33a | 1.08b | 1.05b | 0.06 | 1.24a | 0.97b | 1.02b | 0.07 | 0.012 | 0.022 |
| Leucine | 2.21a | 2.14ab | 1.85b | 0.11 | 1.84 | 1.77 | 1.64 | 0.14 | 0.087 | 0.630 |
| Lysine | 2.47b | 3.36ab | 3.95a | 0.42 | 1.14b | 1.27b | 2.63a | 0.31 | 0.073 | 0.008 |
| Methionine | 1.42b | 1.44b | 1.82a | 0.10 | 1.25b | 1.36b | 2.00a | 0.14 | 0.037 | <0.01 |
| Phenylalanine | 1.76a | 1.84a | 1.39b | 0.11 | 1.61 | 1.40 | 1.63 | 0.14 | 0.018 | 0.434 |
| Threonine | 1.39b | 1.72ab | 1.95a | 0.14 | 1.35b | 1.39b | 1.82a | 0.13 | 0.044 | 0.032 |
| Tryptophan | 0.47 | 0.44 | 0.45 | 0.03 | 0.49 | 0.53 | 0.65 | 0.07 | 0.711 | 0.264 |
| Valine | 1.98a | 1.34b | 1.35b | 0.14 | 1.93a | 1.61ab | 1.48b | 0.13 | 0.007 | 0.080 |
| Non-essential AA, μg/100 mg | ||||||||||
| Alanine | 12.32b | 11.99b | 15.01a | 0.62 | 9.34b | 10.25b | 12.16a | 0.52 | 0.014 | <0.01 |
| Arginine | 2.91a | 2.40ab | 2.16b | 0.22 | 1.90 | 1.52 | 1.71 | 0.14 | 0.069 | 0.211 |
| Asparagine | 0.89 | 0.69 | 0.95 | 0.14 | 0.65 | 0.58 | 0.81 | 0.09 | 0.387 | 0.158 |
| Aspartic acid | 0.54 | 0.57 | 0.52 | 0.08 | 0.60 | 0.55 | 0.59 | 0.08 | 0.921 | 0.866 |
| Glutamic acid | 2.85b | 3.42ab | 3.79a | 0.26 | 1.40b | 1.46b | 2.13a | 0.19 | 0.073 | 0.028 |
| Glutamine | 13.84b | 13.30b | 18.86a | 1.57 | 10.95 | 9.87 | 12.77 | 1.29 | 0.046 | 0.300 |
| Glycine | 10.12 | 10.79 | 11.01 | 0.80 | 5.34 | 5.64 | 6.83 | 0.61 | 0.723 | 0.224 |
| Proline | 1.59 | 1.47 | 1.67 | 0.13 | 1.29 | 1.27 | 1.28 | 0.14 | 0.536 | 0.907 |
| Serine | 2.56b | 2.33b | 3.50a | 0.19 | 1.88 | 1.98 | 2.06 | 0.23 | < 0.01 | 0.863 |
| Tyrosine | 2.36a | 2.03ab | 1.77b | 0.14 | 2.16 | 1.95 | 2.12 | 0.15 | 0.024 | 0.604 |
| Others, μg/100 mg | ||||||||||
| Anserine | 13.73a | 11.27b | 12.67ab | 0.66 | 18.58 | 17.24 | 16.10 | 1.04 | 0.072 | 0.269 |
| Carnosine | 411.88a | 351.99ab | 324.43b | 19.89 | 484.59 | 493.35 | 473.95 | 16.19 | 0.023 | 0.704 |
| Taurine | 14.81b | 15.04b | 18.99a | 1.08 | 15.35b | 16.23b | 19.69a | 1.00 | 0.027 | 0.032 |
| TAA, μg/100 mg | 25.83b | 26.77b | 30.33a | 1.12 | 16.68b | 17.90b | 21.72a | 0.92 | 0.028 | 0.013 |
| EAA, μg/100 mg | 14.04 | 14.15 | 14.64 | 0.59 | 12.03b | 11.32b | 13.95a | 0.61 | 0.829 | <0.01 |
| NEAA, μg/100 mg | 49.98b | 48.99b | 59.24a | 2.42 | 35.52b | 35.07b | 42.47a | 1.47 | 0.023 | 0.038 |
a,bWithin a row, values with different superscript letters differ (P < 0.05).
aTAA = tasty AA, including alanine, aspartic acid, glutamic acid, and glycine; EAA = essential AA; NEAA = non-essential AA.
bDietary treatment: NP = normal protein; LP = low protein; VLP = very low protein.
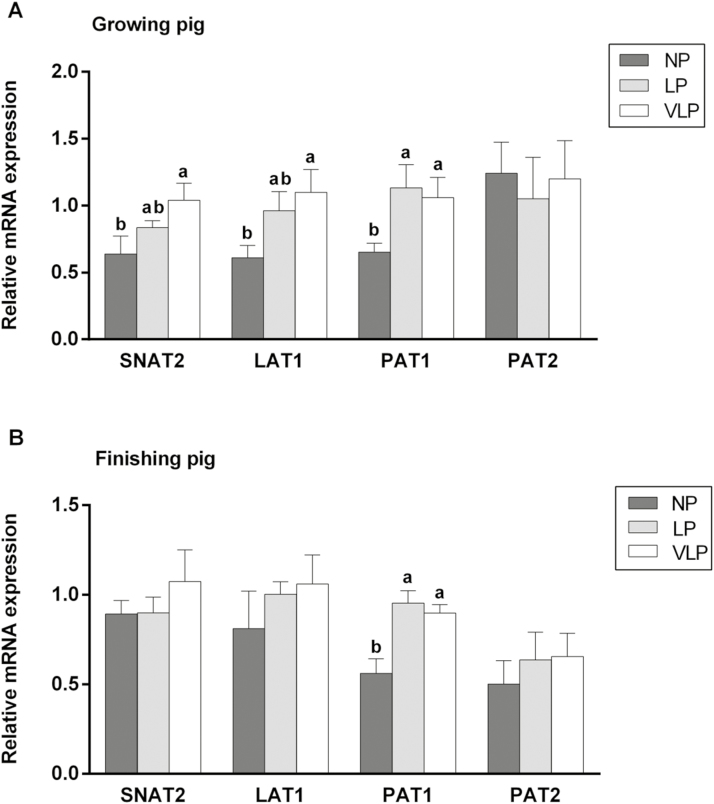
As shown in Fig. 2, the low-protein diets had a large effect on mRNA levels of the AA transporters, especially for those growing pigs, in which mRNA expression for SNAT2 and LAT1 upregulated (P < 0.05) as the CP level of the diet was reduced. In both growing and finishing pigs, mRNA expression levels of PAT1 on the LP and VLP diets were much higher (P < 0.05) than on the NP diet. However, there were no evident effects obtained for mRNA expression level of PAT2 in growing pigs and those of SNAT2, LAT1, and PAT2 in finishing pigs.
Figure 2.
Effect of low-protein diet on relative mRNA expression levels of sodium-coupled neutral amino acid transporter 2 (SNAT2), system L amino acid transporter 1 (LAT1), proton-assisted amino acid transporter 1 (PAT1), and PAT2 of longissimus dorsi muscle in growing (A) and finishing (B) pig. Dietary treatment: normal protein (NP), low protein (LP), and very low protein (VLP). Data are expressed as means ± SEM (n = 6). a,bBars with different letters are different among dietary protein treatments (P < 0.05).
DISCUSSION
An enormous amount of research effort goes into the growth response of pigs fed the low-protein diets with supplementation of the limiting AA, however, up to date the data about the extent to which the protein content of the diets can be reduced are still conflicting. Apparently, there was a tendency in the low-protein diets toward lower growth rate and the poorer feed conversion in those previous studies, despite in some of which the effect was insignificant (Hansen et al., 1993; Kerr, 1995; Tuitoek et al., 1997; Gomez et al., 2002), these findings are also in agreement with our growth performance results. But it is worthwhile to note that the increased marbling fat in the muscle on the low-protein diets (Kerr et al., 1995; Wood et al., 2004a; Teye et al., 2006; Tous et al., 2014), which agrees with the result of the present study. This effect of the diet is predictable because provision of insufficient protein for growing and finishing pigs resulted in an inhibition of protein synthesis and muscle growth to some extent with the redundant energy transferred to fat accumulation in the muscle (Wood et al., 2004a). Early work on lipid metabolism concentrated on adipose tissue that the main depot of fatty acids. Nevertheless, there has been growing emphasis on muscle tissue recently owing to increasing significance of IMF in meat quality.
To further reveal the molecular mechanism of dietary protein level implicated in regulating lipid metabolism of the muscle, we performed relative gene expression analysis and found that mRNA abundance of the lipogenic-related factor ACCα was higher while that of the lipolytic-related gene HSL was lower in pigs fed the low-protein diets. The inverse regulation of ACCα and HSL in the muscle by protein level is expected to play a dominant influence on deposition of IMF (Zechner et al., 2012), and indeed, here, the effects of low-protein diets on variations of 2 genes expression and increase of IMF content were highly consistent in growing and finishing pigs, respectively, which verify and provide compelling evidence that a critical function of protein restriction on regulation of key genes related to lipid metabolism, thereby promoting fat deposition in the muscle. Also, we noticed that in the present study there was a remarkable increase in serum concentration of leptin in growing pigs fed the VLP diet compared to those fed higher level of protein diets, whereas the opposite was true for serum concentration of adiponectin, and of note, this effect was not pronounced in finishing pigs. It is well known that leptin and adiponectin are 2 important adipocytokines, and their production is primarily regulated by adipocyte metabolism (Havel, 2004). Moreover, previous studies reported that leptin level of serum is strongly proportional to adiposity (Takahashi et al., 1996), while adiponectin level of serum is believed to be negatively correlated with body fat (Weyer et al., 2001). In line with this view, we confirmed once again that low-protein supply changed lipid metabolism of growing pigs, and our data also imply a greater content of fat mass (including IMF) in the VLP group.
Besides, an important observation was that the low-protein diets improved meat tenderness (decreased the shear force value) in finishing pigs of this study. As a key indicator of improved eating quality, meat tenderness is believed to the most important aspect of meat quality (Wood et al., 2004a). Based on the current findings, several possible reasons may be involved in improvement of meat tenderness. First and foremost, meat tenderness is more readily influenced by increased content of IMF (Alonso et al., 2010). In other words, the shear force value was generally higher in group with lower IMF (Karlsson et al., 1993), and particularly occurring in the later stages of growth (Wood et al., 2008). Accordingly, in the present study, reduction of shear force value was accompanied by alteration of IMF content from 1.59% to 2.49% by reducing protein level from 16% to 10% in the diet for finishing pigs. Along with the enhanced IMF content due to the lowering of dietary protein, there was a concurrent increase in MUFA concentration and decrease in PUFA of the muscle in the pigs. This result has also been found before (Alonso et al., 2010) and is likely another reason for improving meat tenderness. According to Wood et al. (2004b), variation in fatty acid composition of IMF for reasons of diet changes lipid melting points and so that it results in the change of fat firmness, which means with the increased unsaturation, melting point and firmness decrease as well. Therefore, higher amounts of oleic acid (c18:1) and some other MUFA obtained in the low-protein diets of this study indicate lowering of meat firmness and accounting for reduction of shear force. Our study also confirms previous other studies which suggest that there is a strongly inverse relationship between MUFA concentration and shear force, while the proportion of PUFA is positively correlated with shear force (Alonso et al., 2010). An additional explanation for the improvement of meat tenderness by the low-protein diets lies in the protein composition of the muscle. Possibly that muscle becomes less cohesive due to collagen reduction, after all, protein synthesis has been proposed to be inhibited in the muscle when insufficient protein is provided (Li et al., 2016b).
In general, skeletal muscle is a mosaic collection of muscle fiber types, of which 4 major isoforms are known in the muscle of pigs (type I, IIa, IIx, and IIb) (Chang, 2007). Eggert et al. (2002) reported that relative abundance of individual fiber types was significant in the prediction of classic meat quality. And there is a general perception that favorable redder meat contains greater abundance of type I and IIa fibers which have a higher oxidative capacity than type IIb fiber (Chang, 2007). With these in mind, then we detected mRNA abundance of MyHC isoforms in this study, and found that the LP diet supply to either growing or finishing pigs resulted in higher expression levels of MyHC I and/or IIa in their muscles, it might be a prominent contribution to increase of their a* values. In terms of pork quality, a* value reflects redness of the meat, and normally the higher for a* value the more desirable for meat. On the other hand, histochemical staining has revealed in pigs that fat is deposited predominantly in type I and IIa fibers (Essen-Gustavsson et al., 1994). Consequently, it is possible that the variations in muscle fiber characteristics also explain differences in IMF content brought about by low-protein intake.
Actually, this feeding strategy could contribute greatly to various aspects of meat quality other than tenderness and color, such as juiciness and flavor (Wood et al., 2004a; Alonso et al., 2010), although these aspects have not being investigated here. It is widely acknowledged that free AA in the muscle are responsible for nutritional value of meat and are involved in many reactions affecting the flavor as taste enhancers or precursors of aroma compounds (Kato et al., 1989; Toldrá et al., 1997). Not surprisingly, free AA profile in the present study showed a large variation in the muscle of the pigs in response to low-protein supply. Especially in growing pigs fed the VLP diet, the concentrations of taurine and TAA including alanine and glutamic acid elevated while that of carnosine declined. There is evidence that taurine and TAA are pleasant tasting AA and are positively associated with meat flavor, while carnosine has been reported to give a bitter taste (Koga et al., 1985; Troy, 2006). As a result, we might expect a tastier meat in growing pigs consumed a low-protein diet. The variation of taurine and TAA concentrations in the muscle was observed alike in finishing pigs, from the flavor point of view, this result further provided a good prediction of favorable meat on low-protein diet. However, somewhat surprisingly, we found that much smaller variation of free AA profile was in the muscle of finishing pigs than that of growing pigs. The AA transporters including SNAT2, LAT1, PAT1, and PAT2 are now acknowledged to serve as gatekeepers of muscle cells to sense AA availability and control uptake and efflux of AA (Nicklin et al., 2009). In this regard, it is noteworthy that expression levels of the most of those typical AA transporters varied with dietary protein level in growing pigs, but only a little effect on them was observed in finishing pigs, which are in general agreement with the variation of free AA profile in growing and finishing pigs. Meanwhile, we noticed that PAT1 expression was influenced by low-protein intake in both growing and finishing pigs, implying its vital function in the uptake and metabolism of muscle AA.
In conclusion, the data reported here indicate that lower level of protein in the diet decreased shear force (more tenderness), probably through increase in total fat content and modification in fatty acid composition in the muscle of growing pigs, but it failed to obviously influence shear force in the muscle of finishing pigs. Additionally, increase of a* value in both growing and finishing pigs fed the LP diet was partly caused by upregulation of type I and/or IIa fibers expression in the muscle. Meanwhile, VLP diet supply to growing and finishing pigs resulted in higher concentrations of taurine and TAA, which, therefore, likely produce meat with a better flavor. These results provide valuable information for understanding the underlying mechanisms of high meat quality of pigs in response to low-protein diet (especially a reduction of dietary CP by a 3% value) treatment.
Conflict of interest statement. None declared.
ACKNOWLEDGMENTS
This research was supported by the National Basic Research Program of China (2013CB127305), the Youth Innovation Promotion Association CAS (2016326), the Science and Technology Projects of Hunan Province (2016SK3022, 2017RS3058), the Major Project of Hunan Province (2015NK1002), the Key Project of Research and Development Plan of Hunan Province (2016NK2170) and Youth Innovation Team Project of ISA, CAS (2017QNCXTD_ZCS), the Earmarked Fund for China Agriculture Research System (CARS-35), and the Plant Germplasm Resources Innovation Project of Strategic Biological Resources Service Network Plan from the Chinese Academy of Sciences (ZSZC-011).
LITERATURE CITED
- Alonso V., Campo M. D. E. L. M., Provincial L., Roncalés P., and Beltrán J. A.. 2010. Effect of protein level in commercial diets on pork meat quality. Meat Sci. 85:7–14. doi:10.1016/j.meatsci.2009.11.015 [DOI] [PubMed] [Google Scholar]
- Chang K. C. 2007. Key signalling factors and pathways in the molecular determination of skeletal muscle phenotype. Animal 1:681–698. doi:10.1017/S1751731107702070 [DOI] [PubMed] [Google Scholar]
- Cornet M. and Bousset J.. 1999. Free amino acids and dipeptides in porcine muscles: differences between ‘red’ and ‘white’ muscles. Meat Sci. 51:215–219. doi:10.1016/S0309-1740(98)00104-1 [DOI] [PubMed] [Google Scholar]
- Davila A. M., Blachier F., Gotteland M., Andriamihaja M., Benetti P. H., Sanz Y., and Tomé D.. 2013. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol. Res. 68:95–107. doi:10.1016/j.phrs.2012.11.005 [DOI] [PubMed] [Google Scholar]
- Eggert J. M., Depreux F. F., Schinckel A. P., Grant A. L., and Gerrard D. E.. 2002. Myosin heavy chain isoforms account for variation in pork quality. Meat Sci. 61:117–126. [DOI] [PubMed] [Google Scholar]
- Essen-Gustavsson B., Karlsson A., Lundström K., and Enfält A. C.. 1994. Intramuscular fat and muscle fibre lipid contents in halothane-gene-free pigs fed high or low protein diets and its relation to meat quality. Meat Sci. 38:269–277. doi:10.1016/0309-1740(94)90116-3 [DOI] [PubMed] [Google Scholar]
- Flores M., Moya V.-J., Aristoy M.-C., and Toldrá F.. 2000. Nitrogen compounds as potential biochemical markers of pork meat quality. Food Chem. 69:371–377. [Google Scholar]
- Gomez R. S., Lewis A. J., Miller P. S., and Chen H. Y.. 2002. Growth performance, diet apparent digestibility, and plasma metabolite concentrations of barrows fed corn-soybean meal diets or low-protein, amino acid-supplemented diets at different feeding level. J. Anim. Sci. 80:644–653. [DOI] [PubMed] [Google Scholar]
- Hansen J. A., Knabe D. A., and Burgoon K. G.. 1993. Amino acid supplementation of low-protein sorghum-soybean meal diets for 20- to 50-kilogram swine. J. Anim. Sci. 71:442–451. [DOI] [PubMed] [Google Scholar]
- Havel P. J. 2004. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 53:S143–S151. [DOI] [PubMed] [Google Scholar]
- He L., Wu L., Xu Z., Li T., Yao K., Cui Z., Yin Y., and Wu G.. 2016. Low-protein diets affect ileal amino acid digestibility and gene expression of digestive enzymes in growing and finishing pigs. Amino Acids 48:21–30. doi:10.1007/s00726-015-2059-1 [DOI] [PubMed] [Google Scholar]
- Honikel K. O., Kim C. J., Hamm R., and Roncales P.. 1986. Sarcomere shortening of prerigor muscles and its influence on drip loss. Meat Sci. 16:267–282. doi:10.1016/0309-1740(86)90038-0 [DOI] [PubMed] [Google Scholar]
- Karlsson A., Enfält A. C., Essén-Gustavsson B., Lundström K., Rydhmer L., and Stern S.. 1993. Muscle histochemical and biochemical properties in relation to meat quality during selection for increased lean tissue growth rate in pigs. J. Anim. Sci. 71:930–938. doi:10.2527/1993.714930x [DOI] [PubMed] [Google Scholar]
- Karlsson A. H., Klont R. E., and Fernandez X.. 1999. Skeletal muscle fibres as factors for pork quality. Livest. Prod. Sci. 60:255–269. doi:10.1016S0301-6226(99)00098-6 [Google Scholar]
- Kato H., Rhue M. R., and Nishimura T.. 1989. Role of free amino acids and peptides in food taste. In: ACS Symposium Series-American Chemical Society (USA) Chapter 13, pp. 158–174. doi:10.1021/bk-1989-0388.ch013. [Google Scholar]
- Kerr B. J. 1995. Nutritional strategies for waste reduction management: nitrogen. In: Longenecker, J. B. and J. W. Speers, editors. News horizons in animal nutrition and health. University of North Carolina, Chapel Hill, NC. [Google Scholar]
- Kerr B. J., McKeith F. K., and Easter R. A.. 1995. Effect on performance and carcass characteristics of nursery to finisher pigs fed reduced crude protein, amino acid-supplemented diets. J. Anim. Sci. 73:433–440. doi:10.2527/1995.732433x [DOI] [PubMed] [Google Scholar]
- Klont R. E., Brocks L., and Eikelenboom G.. 1998. Muscle fibre type and meat quality. Meat Sci. 49:S219–S229. doi:10.1016/S0309-1740(98)90050-X [PubMed] [Google Scholar]
- Koga K., Fukunaga T., Ohki Y., and Kawaida H.. 1985. Free amino acids and carnosine contents in the lean meats (Longissimus dorsi and Biceps femoris) from the strain and the strain-cross pigs. Bull. Fac. Agric. Kagoshima Univ. (Japan) 35:65–73. [Google Scholar]
- Li F., Duan Y., Li Y., Tang Y., Geng M., Oladele O. A., Kim S. W., and Yin Y.. 2015. Effects of dietary n-6:n-3 PUFA ratio on fatty acid composition, free amino acid profile and gene expression of transporters in finishing pigs. Br. J. Nutr. 113:739–748. doi:10.1017/S0007114514004346 [DOI] [PubMed] [Google Scholar]
- Li Y., Li F., Lin B., Kong X., Tang Y., and Yin Y.. 2014. Myokine IL-15 regulates the crosstalk of co-cultured porcine skeletal muscle satellite cells and preadipocytes. Mol. Biol. Rep. 41:7543–7553. doi:10.1007/s11033-014-3646-z [DOI] [PubMed] [Google Scholar]
- Li F., Li Y., Tan B., Wang J., Duan Y., Guo Q., Liu Y., Kong X., Li T., Tang Y., et al. 2016a. Alteration of inflammatory cytokines, energy metabolic regulators, and muscle fiber type in the skeletal muscle of postweaning piglets. J. Anim. Sci. 94:1064–1072. doi:10.2527/jas.2015-9646 [DOI] [PubMed] [Google Scholar]
- Li Y., Li F., Wu L., Wei H., Liu Y., Li T., Tan B., Kong X., Yao K., Chen S., et al. 2016b. Effects of dietary protein restriction on muscle fiber characteristics and mtorc1 pathway in the skeletal muscle of growing-finishing pigs. J. Anim. Sci. Biotechnol. 7:47. doi:10.1186/s40104-016-0106-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li F., He L., Tan B., Deng J., Kong X., Li Y., Geng M., Yin Y., and Wu G.. 2015. Dietary protein intake affects expression of genes for lipid metabolism in porcine skeletal muscle in a genotype-dependent manner. Br. J. Nutr. 113:1069–1077. doi:10.1017/S0007114514004310 [DOI] [PubMed] [Google Scholar]
- Livak K. J. and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Martin D., Antequera T., Muriel E., Perez-Palacios T., and Ruiz J.. 2011. Effect of dietary conjugated linoleic acid in combination with monounsaturated fatty acids on the composition and quality traits of cooked loin. Food Chem. 124:518–526. doi:10.1016/j.foodchem.2010.06.063 [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC). 2012. Nutrient requirement of swine. 11th rev. ed. Natl. Acad. Sci., Washington, DC. [Google Scholar]
- Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., Yang H., Hild M., Kung C., Wilson C., et al. 2009. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136:521–534. doi:10.1016/j.cell.2008.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Funahashi T., Shimomura I., Miyaoka K., and Matsuzawa Y.. 1996. Plasma leptin levels and body fat distribution. Horm. Metab. Res. 28:751–752. doi:10.1055/s-2007-979893 [DOI] [PubMed] [Google Scholar]
- Teye G. A., Sheard P. R., Whittington F. M., Nute G. R., Stewart A., and Wood J. D.. 2006. Influence of dietary oils and protein level on pork quality. 1. Effects on muscle fatty acid composition, carcass, meat and eating quality. Meat Sci. 73:157–165. doi:10.1016/j.meatsci.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Toldrá F., Flores M., and Sanz Y.. 1997. Dry-cured ham flavour: enzymatic generation and process influence. Food Chem. 59:523–530. doi:10.1016/S0308-8146(97)00013-7 [Google Scholar]
- Tous N., Lizardo R., Vilà B., Gispert M., Font-I-Furnols M., and Esteve-Garcia E.. 2014. Effect of reducing dietary protein and lysine on growth performance, carcass characteristics, intramuscular fat, and fatty acid profile of finishing barrows. J. Anim. Sci. 92:129–140. doi:10.2527/jas.2012-6222 [DOI] [PubMed] [Google Scholar]
- Troy D. 2006. 52nd International Congress of Meat Science and Technology: Harnessing and Exploiting Global Opportunities Wageningen Academic Pub, The Netherlands.. [Google Scholar]
- Tuitoek K., Young L. G., de Lange C. F., and Kerr B. J.. 1997. The effect of reducing excess dietary amino acids on growing-finishing pig performance: an elevation of the ideal protein concept. J. Anim. Sci. 75:1575–1583. [DOI] [PubMed] [Google Scholar]
- Valin C., Touraille C., Vigneron P., and Ashmore C. R.. 1982. Prediction of lamb meat quality traits based on muscle biopsy fibre typing. Meat Sci. 6:257–263. doi:10.1016/0309-1740(82)90036-5 [DOI] [PubMed] [Google Scholar]
- Weyer C., Funahashi T., Tanaka S., Hotta K., Matsuzawa Y., Pratley R. E., and Tataranni P. A.. 2001. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 86:1930–1935. doi:10.1210/jcem.86.5.7463 [DOI] [PubMed] [Google Scholar]
- Wood J. D., Enser M., Fisher A. V., Nute G. R., Sheard P. R., Richardson R. I., Hughes S. I., and Whittington F. M.. 2008. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 78:343–358. doi:10.1016/j.meatsci.2007.07.019 [DOI] [PubMed] [Google Scholar]
- Wood J. D., Nute G. R., Richardson R. I., Whittington F. M., Southwood O., Plastow G., Mansbridge R., da Costa N., and Chang K. C.. 2004a. Effects of breed, diet and muscle on fat deposition and eating quality in pigs. Meat Sci. 67:651–667. doi:10.1016/j.meatsci.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Wood J. D., Richardson R. I., Nute G. R., Fisher A. V., Campo M. M., Kasapidou E., Sheard P. R., and Enser M.. 2004b. Effects of fatty acids on meat quality: a review. Meat Sci. 66:21–32. doi:10.1016/S0309-1740(03)00022-6 [DOI] [PubMed] [Google Scholar]
- Zechner R., Zimmermann R., Eichmann T. O., Kohlwein S. D., Haemmerle G., Lass A., and Madeo F.. 2012. FAT SIGNALS–lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15:279–291. doi:10.1016/j.cmet.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]