Abstract
Intrauterine growth restriction (IUGR) is the second leading cause of perinatal mortality and predisposes offspring to metabolic disorders at all stages of life. Muscle-centric fetal adaptations reduce growth and yield metabolic parsimony, beneficial for IUGR fetal survival but detrimental to metabolic health after birth. Epidemiological studies have reported that IUGR-born children experience greater prevalence of insulin resistance and obesity, which progresses to diabetes, hypertension, and other metabolic disorders in adulthood that reduce quality of life. Similar adaptive programming in livestock results in decreased birth weights, reduced and inefficient growth, decreased carcass merit, and substantially greater mortality rates prior to maturation. High rates of glucose consumption and metabolic plasticity make skeletal muscle a primary target for nutrient-sparing adaptations in the IUGR fetus, but at the cost of its contribution to proper glucose homeostasis after birth. Identifying the mechanisms underlying IUGR pathophysiology is a fundamental step in developing treatments and interventions to improve outcomes in IUGR-born humans and livestock. In this review, we outline the current knowledge regarding the adaptive restriction of muscle growth and alteration of glucose metabolism that develops in response to progressively exacerbating intrauterine conditions. In addition, we discuss the evidence implicating developmental changes in β adrenergic and inflammatory systems as key mechanisms for dysregulation of these processes. Lastly, we highlight the utility and importance of sheep models in developing this knowledge.
Keywords: catecholamines, cytokines, glucose oxidation, intrauterine growth restriction
INTRODUCTION
Intrauterine growth restriction (IUGR) diminishes perinatal survival rates and encumbers long-term health in a substantial portion of the global population (Berghella, 2007; Saleem et al., 2011). It is second only to prematurity as a cause of perinatal mortality and morbidity (Alisi et al., 2011) and predisposes affected persons to metabolic dysfunction throughout life (Hales and Barker, 1992, 2001). As early as 3 yr of age, IUGR-born children exhibit greater rates of insulin resistance, high blood pressure, and obesity than their average for gestational age (AGA) counterparts (Flanagan et al., 2000; Ong et al., 2000; Mericq et al., 2005). By adulthood, their risk for developing metabolic disorders such as type 2 diabetes, obesity, and hypertension is increased by 18-fold (Barker et al., 1993; Gatford et al., 2010). In fact, IUGR is believed to be a predisposing factor in as many as half of all clinical cases of metabolic syndrome (Vo and Hardy, 2012). Statistics confirm that IUGR is a global health issue, afflicting approximately 25% of all infants worldwide (10% of U.S. infants; Berghella, 2007; Saleem et al., 2011). The most recent perinatal data indicate that premature births in the United States have declined steadily for the last decade and the total rate of infant mortality is at a historic low (Murphy et al., 2017), yet the rate of IUGR births has remained steady since 2002; 20% greater than in the mid-1980s (Arias et al., 2003). Improved neonatal care has caused worldwide mortality rates for IUGR infants to decline considerably over the last several decades (Goldenberg and Culhane, 2007), which increases the need for a better understanding of how IUGR fetal adaptations lead to metabolic deficiencies that shorten life span and decrease quality of life.
Postnatal consequences of IUGR are not limited to humans and occur in most mammalian species. In livestock, IUGR-induced low birthweight greatly increases perinatal mortality rates due to reduced energy reserves and lack of vigor at birth (Reynolds and Caton, 2012; Dwyer et al., 2016). Consequently, the offspring are at a disadvantage during the first few months of life. The lack of available information regarding how to manage these animals costs the U.S. livestock industry ~8% of its annual product (Wu et al., 2006). This equates to a loss of about 3 million beef calves, 930,000 dairy calves, and 530,000 lambs each year. Starvation deaths of low birthweight livestock are a serious animal welfare issue for the industry and a barrier to financial sustainability for many producers. In addition, IUGR-born offspring that survive exhibit reduced efficiency and performance (Gondret et al., 2005; De Blasio et al., 2007) as demonstrated by reduced feed conversion, lighter carcasses, and reduced carcass merit (Hegarty and Allen, 1978; Powell and Aberle, 1980; Greenwood et al., 1998, 2000; Gondret et al., 2005). Greater metabolic dysfunction and perinatal mortality rates have also been documented in IUGR-born companion animals including dogs (Kliegman, 1989) and horses (Peugnet et al., 2014) and in managed wildlife species such as fallow deer (English and Mulley, 1992) and baboons (Pantham et al., 2015; Li et al., 2017).
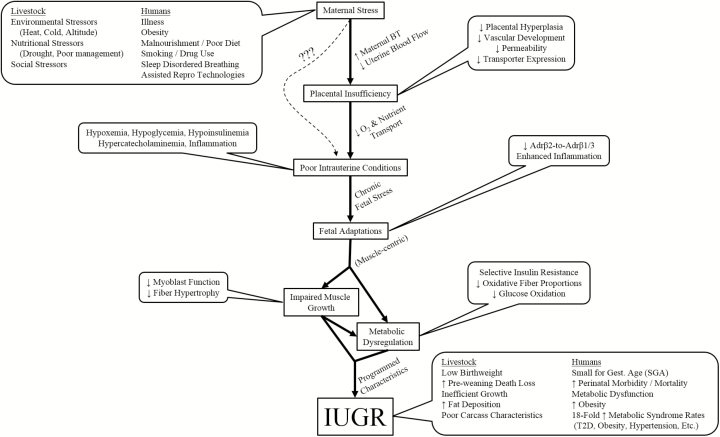
Barker’s thrifty phenotype hypothesis (Hales and Barker, 2001) states that metabolic deficits are the result of adaptive fetal programming aimed at sparing nutrients in response to placental insufficiency or other nutrient-restricting stressors. These adaptations create metabolic thrift in nonessential tissues (i.e., skeletal muscle) that is critical to in utero survival but detrimental after birth when nutrients are no longer restricted (Yates et al., 2011a, 2012b; Brown, 2014). Despite the well-established epidemiological links between IUGR and life-long metabolic dysfunction, the underlying adaptive mechanisms are not well understood. In this review, we discuss the existing knowledge regarding metabolic programming in the IUGR fetus, the essential contributions of sheep models to these discoveries, and the potential roles for adaptive changes in stress-system regulation of IUGR muscle growth and metabolism (Fig. 1).
Figure 1.
Working model for the mechanistic link between maternofetal stress and the postnatal consequences of intrauterine growth restriction (IUGR).
PLACENTAL INSUFFICIENCY AND THE IUGR FETAL CONDITION
Placental Stunting
Clinical reports indicate that the majority of IUGR cases in humans are the result of placental insufficiency (Cox and Marton, 2009). Maternal conditions such as illness, stress, obesity, smoking, drug use, malnourishment, sleep disordered breathing, and even assisted reproduction technologies can stunt placental development (Carnelio et al., 2017; Woo et al., 2017). Placental insufficiency resulting from any of these maternal etiologies reduces nutrient and oxygen delivery and causes predictably poor intrauterine conditions for the fetus. Similar placental stunting can occur in pregnant livestock as the result of environmental stress (i.e., heat, cold, altitude), undernourishment, illness, and ingestion of noxious plants (Greenwood and Cafe, 2007). Stress conditions often elevate maternal body temperature, redistributing blood flow away from the uterus to peripheral tissues (Alexander et al., 1987; Wallace et al., 2005). An increase of as little as 1 °C can reduce uterine blood flow up to 30% (Dreiling et al., 1991), which if sustained will reduce placental hyperplasia (Regnault et al., 2002) and disrupt vascular endothelial growth factor-regulated vascular development (Regnault et al., 2002). The reduced number, diameter, and branching of placental arteries (Regnault et al., 2002) increases blood flow resistance 4-fold (Galan et al., 2005; Regnault et al., 2007), which in turn decreases placental O2 permeability (Regnault et al., 2003; de Vrijer et al., 2004). Transport of glucose and amino acids is also reduced by decreased placental expression of transporters for these nutrients (Malandro et al., 1996; Wallace et al., 2005; Jansson et al., 2006). Consequently, birthweight is strongly correlated with placental vascularization and mass (Pantham et al., 2015; Poudel et al., 2015).
IUGR Fetal Pathophysiology
As the fetus outgrows its stunted placenta, typically in the early third trimester, it begins to exhibit hypoglycemia and hypoxemia that progressively worsen until birth (Macko et al., 2013). Umbilical O2 and glucose concentrations are reduced by as much as 50% near term (Limesand et al., 2007; Macko et al., 2016) despite normal concentrations in uterine blood. Placental transfer of amino acids is also markedly reduced (de Vrijer et al., 2004) by deficient active transport across the microvillous membrane (Malandro et al., 1996; Pantham et al., 2016). These deficits necessitate reductions in protein utilization by fetal tissues and in severe cases increase protein mobilization (Limesand et al., 2007; Rozance et al., 2018). To illustrate, circulating leucine concentrations are maintained in the IUGR fetus because hindlimb utilization is reduced by 32% (Rozance et al., 2018). The greatest reductions are in utilization for protein synthesis and muscle growth, as leucine oxidation rates are comparable to normal fetuses.
In concert with worsening nutrient states, the IUGR fetus exhibits progressive increases in circulating norepinephrine and epinephrine (Macko et al., 2013; Macko et al., 2016). These catecholamines are secreted from the fetal adrenal in response to hypoxemia (Yates et al., 2012a), which inactivates O2-sensing potassium channels on medullary chromaffin cells that normally block catecholamine exocytosis (Adams and McMillen, 2000). Chronic hypoxemia also stimulates greater circulating inflammatory cytokines (Guo et al., 2010; Kelly et al., 2017). At delivery, fetal cord blood from IUGR infants has greater concentrations of tumor necrosis factor α (TNFα), interleukin-6 (IL-6), and interleukin-18 (IL-18), as well as the chemokine C-C motif ligand 16 (CCL16) and the acute phase protein C-reactive protein (CRP) (Makikallio et al., 2012; Krajewski et al., 2014; Visentin et al., 2014). In IUGR animal models, TNFα and IL-6 are elevated in late gestation (Bertucci et al., 2011), and we recently reported that maternofetal inflammation due to maternal bacterial endotoxin injection at mid-gestation in rats results in smaller fetuses with increased circulating TNFα at term, well after maternal biomarkers of inflammation had subsided (Cadaret et al., 2017a). Reduced delivery of nutrients can also increase fetal biomarkers of inflammation late in gestation (Jones et al., 2018). The adrenergic and inflammatory systems mediate many of the physiological changes affecting growth and metabolism in the IUGR fetus, and one prominent mode of action is inhibition of growth factors. Insulin, insulin-like growth factor (IGF)-I, IGF-II, and placental growth factor concentrations are suppressed in the IUGR fetus by the mid-third trimester (Thorn et al., 2009; Leos et al., 2010), as thoroughly reviewed by Laura Brown (2014). Catecholamines are particularly strong inhibitors of insulin secretion, as norepinephrine infusion into otherwise uncompromised fetuses substantially reduces circulating concentrations (Chen et al., 2014, 2017b), and fetal adrenal demedullation recovers glucose-stimulated insulin secretion in acutely hypoxemic fetuses (Yates et al., 2012a) and in IUGR fetuses (Macko et al., 2016). Moreover, inflammatory cytokines disrupt the ability of IGF-I to interact with its receptor (Hashimoto et al., 2010) and increase the levels of IGF-binding proteins (IGFBP) (Street et al., 2006), which impedes the activity of IGF through high-affinity binding (Hoeflich et al., 1999).
The conditions described above result in redistributed fetal blood flow. The IUGR fetus directs more blood to the brain, heart, adrenal glands, and pancreas compared to the normal fetus (Alexander et al., 1987; Poudel et al., 2015). At the same time, catecholamines mediate femoral vascular resistance, resulting in an almost 50% decrease in hindlimb blood flow (Poudel et al., 2015; Rozance et al., 2018). Redirected blood flow prioritizes nutrient and O2 delivery to vital organs at the cost of restricted muscle growth, resulting in the hallmark asymmetric body composition of IUGR fetuses (Galan et al., 1999). Fetal growth restriction progressively worsens during the later stages of gestation in parallel with its causative conditions (Carr et al., 2012). Divergent growth starts early in the third trimester, just after characteristics of placental insufficiency begin to appear (Limesand et al., 2013; Macko et al., 2013). By midway through the third trimester, IUGR fetuses are ~28% lighter than uncompromised counterparts (Bubb et al., 2007), and by term, growth restriction can exceed 50% (Leos et al., 2010; Rozance et al., 2015; Kelly et al., 2017).
Ovine Models of IUGR
A great deal of knowledge regarding IUGR fetal and neonatal pathology has been ascertained from pregnant sheep models. The fetal sheep’s tolerance of experimental conditions provides a means to characterize IUGR conditions in utero and has helped identify the nature and timing of developmental adaptations (Anthony et al., 2003; Morrison, 2008; Yates et al., 2011a, 2012b). Sheep are the ideal ruminant model for low birthweight, as IUGR lambs share developmental characteristics with IUGR calves and other ruminant species but experience reduced rates of prenatal and perinatal death (Anthony et al., 2003). The smaller size, greater survival rates, and shorter gestation lengths allow IUGR lambs to be produced for research more consistently and at about one-tenth the expense of IUGR calves. Low birthweight due to IUGR is a particularly common problem in swine due to the natural process of runting, which results from intrauterine crowding (Foxcroft et al., 2006). It should be noted that information learned from IUGR sheep is largely applicable to IUGR swine due to similar fetal pathophysiological conditions, despite noteworthy differences in litter size, relative myogenic milestones, and perinatal mortality rates between the two species (Wu et al., 2010; Oksbjerg et al., 2013). Fetal sheep also share developmental milestones for muscle growth and metabolic responsiveness with humans (Yates et al., 2012b), making them an appropriate model for biomedical research as well. Effective methods for creating or mimicking placental insufficiency in sheep include maternal hyperthermia, uterine artery ligation, uteroplacental embolization, carunclectomy, maternal nutrient restriction, maternal overnutrition, fetal hormone infusion, and maternofetal hypoxia. Detailed comparisons among models are available (Anthony et al., 2003; Wallace et al., 2005; Vuguin, 2007; Morrison, 2008; Green et al., 2010), but maternal heat stress is perhaps the most effective method to naturally induce placental stunting and IUGR. This model originated from observations in the early 1950s of decreased birthweights and greater mortality in summer-born compared to winter-born lambs in northern Australia (Morley, 1954; Moulk, 1954). Controlled-environment studies soon confirmed that chronic exposure to high ambient temperatures was indeed the impetus for smaller, less vigorous, summer-born offspring (Yeates, 1956; Shelton and Huston, 1968). Placental insufficiency is naturally produced when timed-pregnant ewes are exposed to high ambient temperatures and humidity (typically 40 °C and 35% relative humidity) from mid-first trimester to late-second trimester, resulting in 0.7 °C to 1.5 °C increase in maternal body temperature (Yates et al., 2011a). In response, the ewe’s body increases heat expulsion by rerouting blood flow from the uterus and other deep organs to body surfaces (Alexander et al., 1987; Thureen et al., 1992). The reduced blood supply stunts placental growth and development, which consistently reproduces the classic IUGR phenotype. The information obtained from almost three decades of work in this model has helped define IUGR fetal pathophysiology and adaptive programming (Table 1).
Table 1.
Studies showing parallels in IUGR fetal pathology between sheep and humans
| Pathology | Sheep | Human | ||
|---|---|---|---|---|
| O2 | ~28% ↓ fetal arterial O2 at 0.7 gestation; 30–50% ↓ fetal arterial O2 near term | Limesand et al., 2007; Limesand et al., 2013; Macko et al., 2013; Macko et al., 2016 | ~33% ↓ fetal arterial O2 at ~0.75 gestation;15–40% ↓ cord blood O2 at delivery | Pardi et al., 1993; Roberts et al., 1999; Marconi et al., 2006 |
| Glucose | ~23% ↓ fetal arterial O2 at 0.7 gestation; ~40% ↓ fetal arterial O2 near term | Limesand et al., 2013; Macko et al., 2013; Macko et al., 2016 | ~33% ↓ umbilical vein glucose in 3rd trimester; ~50% ↓ cord blood glucose at delivery | Economides et al., 1991; Roberts et al., 1999; Lee et al., 2010 |
| Catecholamines | 5 to 5.7-fold ↑ fetal arterial norepinephrine near term; ~2.7-fold ↑ fetal arterial epinephrine near term | Limesand et al., 2007; Leos et al., 2010; Macko et al., 2013; Macko et al., 2016; Rozance et al., 2018 | 7.4-fold ↑ fetal arterial norepinephrine near term; 3.1-fold ↑ amniotic fluid norepinephrine in third trimester | Divers et al., 1981; Greenough et al., 1990 |
| Inflammation | 5-fold ↑ fetal arterial IL-6 near term; 10-fold ↑ fetal arterial Activin A near term | Bertucci et al., 2011; Kelly et al., 2017; Jones et al., 2018 | 1.3 to 2.4-fold ↑ cord blood TNFα, IL-6, and IL-18 at delivery | Makikallio et al., 2012; Krajewski et al., 2014; Visentin et al., 2014 |
| Insulin, IGF | ~40% (basal) and 52% (glucose-stim.) ↓ fetal arterial insulin near term; ~33% ↓ fetal arterial IGF-I near term | Bauer et al., 2003; Leos et al., 2010; Macko et al., 2016 | ~50% ↓ cord blood insulin at delivery; ~38% ↓ cord blood IGF-I at delivery; ~13% ↓ cord blood insulin at delivery | Hubinont et al., 1991; Lee et al., 2010 |
| Insulin action | 1.6 to 2.4-fold ↑ perinatal insulin sensitivity; ~19% ↓ neonatal insulin sensitivity | Camacho et al., 2016a; Camacho et al., 2017; Merrick et al., 2017 | 3.7-fold ↑ perinatal insulin sensitivity; ~7% ↓ insulin responsive in neonatal muscle | Ozanne et al., 2005; Meral et al., 2011; Milovanovic et al., 2014 |
| Glucose oxidation | 17–30% ↓ fetal glucose oxidation near term; ~33% ↓ neonatal glucose oxidation | Limesand et al., 2007; Brown et al., 2015; Merrick et al., 2017 | ~23% ↓ juvenile glucose oxidation | Jornayvaz et al., 2004 |
| Myoblast function | ~13% ↓ fetal myoblast proliferation; ~46% ↓ fetal myoblast differentiation; ~12% ↓ neonatal myoblast proliferation | Yates et al., 2014; Camacho et al., 2016b; Riley et al., 2016 | ↓ Myonuclear content of gastrocnemius muscle | Widdowson et al., 1972 |
IGF, insulin-like growth factor; IL, interleukin; IUGR, intrauterine growth restriction; TNFα, tumor necrosis factor α.
PANCREATIC ISLET DEVELOPMENT AND β CELL FUNCTION
Pancreatic β cell dysfunction is a hallmark of IUGR-born offspring and is a product of disrupted prenatal islet development (Green et al., 2010; Boehmer et al., 2017). Human IUGR fetuses develop smaller and less vascularized islets that contain fewer β cells (Van Assche and De Prins, 1981), impairing insulin secretion during late gestation (Nicolini et al., 1990), infancy (Bazaes et al., 2003), childhood (Li et al., 2001), and adulthood (Jensen et al., 2002). Underlying islet pathologies have been characterized in detail in IUGR fetal sheep, which exhibit substantially less whole-pancreas and islet vascularity, basal insulin secretion, and glucose-stimulated insulin secretion near term (Limesand et al., 2006; Rozance et al., 2015). In addition to reduced size, IUGR islets exhibit massive reductions in insulin content and glucose oxidation, the latter of which mediates signaling-secretion coupling (Limesand et al., 2006; Brown et al., 2016a). Interestingly, β cell development is disproportionally reduced compared to other islet endocrine cells. Islets from IUGR fetal sheep islets have less insulin content, insulin-positive (β cell) area, and β cells per islet but have normal glucagon content and α and δ cell metrics (Limesand et al., 2005; Limesand et al., 2013; Brown et al., 2016a). This is due to disruption of β cell proliferative capacity, as IUGR β cells undergo normal rates of apoptosis but exhibit substantial reductions in mitosis (Limesand et al., 2005; Limesand et al., 2013; Brown et al., 2016a). Nutrient restriction is only indirectly responsible for islet pathologies, as fetal infusion of nutrients or insulin does little to correct islet function. An exception is the partial restoration of islet size and β cell sensitivity after sustained infusion of high amount of branched-chain amino acids (Brown et al., 2016a). Rather, hypoxemia and hypercatecholaminemia appear to be the primary contributors to islet dysfunction. Elevated circulating catecholamines in fetal sheep inhibit glucose-stimulated insulin secretion during both acute (Yates et al., 2012a) and chronic exposure (Chen et al., 2014; Macko et al., 2016). Moreover, sustained infusion of norepinephrine into uncompromised fetal sheep recapitulates the IUGR phenotype for pancreatic islets, insulin concentrations, and fetal growth rates (Bassett and Hanson, 1998; Chen et al., 2014, 2017b). Surgical adrenal demedullation of IUGR fetal sheep prior to the third trimester circumvented hypercatecholaminemia and partially recovered β cell sensitivity, islet function, and fetal growth near term despite presence of fetal hypoxemia and hypoglycemia (Davis et al., 2015; Macko et al., 2016). In fact, adrenergic desensitivity after chronic exposure can cause β cells to oversecrete insulin when the elevated adrenergic stimulation is reduced pharmaceutically (Leos et al., 2010; Macko et al., 2013; Chen et al., 2014, 2017a) or by birth of the fetus (Camacho et al., 2017). Paradoxically, basal adrenergic stimulation appears important for islet development; adrenal demedullation reduces basal and glucose-stimulated insulin secretion in control fetuses (Yates et al., 2012a; Davis et al., 2015; Macko et al., 2016) and fails to recover β cell mitosis rates in IUGR fetuses (Davis et al., 2015). In addition, correction of fetal hypoxemia improves glucose-stimulated insulin secretion in both intact and adrenal demedullated IUGR fetal sheep, indicating catecholamine-independent as well as catecholamine-mediated effects (Macko et al., 2016).
Heightened inflammatory activity also appears to mediate β cell function and fetal growth restriction. In an unpublished preliminary study in sheep, maternal inflammation induced by serial bacterial endotoxin injection from days 100 to 113 of gestation reduced glucose-stimulated insulin secretion, semitendinosus fiber size, and fetal weights at day 125 of gestation, which is similar to rodent models of maternal inflammation (Cotechini et al., 2014; Chen et al., 2015). Incubation of normal islets with inflammatory cytokines reduces glycolysis and glucose-stimulated insulin secretion (Oleson et al., 2015), and RNA-seq data from IUGR fetal sheep islets indicate that they are desensitized to cytokine activity and binding (Kelly et al., 2017). Adrenergic and inflammatory suppression of insulin secretion helps explain the asymmetry of fetal growth restriction, as hypoinsulinemia only affects insulin-responsive tissues like skeletal muscle.
MUSCLE GROWTH CAPACITY AND METABOLIC FUNCTION
Skeletal muscle accounts for 40% of total body mass and consumes 65% of the body’s total glucose (DeFronzo et al., 1981). With metabolic rates highly responsive to endocrine regulation, muscle is a key factor in metabolic homeostasis as well as a primary target for nutrient sparing in the IUGR fetus (Brown, 2014). Consequently, reduced muscle growth capacity and altered metabolic function are key developmental adaptations that help reapportion limited nutrient and O2 supplies during late gestation (Yates et al., 2011a, 2012b). Although critical for fetal survival, these programmed mechanisms of nutrient sparing continue to reduce nutrient utilization by muscle in offspring despite sufficient postnatal nutrient availability.
Skeletal Muscle Mass and Growth Efficiency
Throughout life, IUGR-born humans have less skeletal muscle (Kensara et al., 2005; Yliharsila et al., 2007) that strongly correlates with metabolic dysfunction due to the tissue’s role in glucose homeostasis (Kim et al., 2014). Ultrasonography shows that slower muscle growth is present well before birth (Padoan et al., 2004; Larciprete et al., 2005) and continues throughout infancy and childhood (Hediger et al., 1998). Dietary nutrients that would normally be used for muscle growth are instead stored in central fat deposits that manifest in abdominal obesity in children as young as 10 yr of age (Zarrati et al., 2013). Increased body fat can lead to secondary pathologies such as systemic low-grade inflammation and hyperlipidemia (Ampem et al., 2016; Veiga-Lopez et al., 2016) that further disrupt metabolic function. Similar adaptive programming in livestock results in poor feed conversion and body composition (De Blasio et al., 2007; Madsen and Bee, 2015), as catch-up growth is driven by fat deposition rather than muscle growth (De Blasio et al., 2010; Liu et al., 2015). Consequently, carcasses of IUGR livestock have reduced merit (decreased weight, excessive 12th-rib fat thickness, and decreased subprimal weight) (Hegarty and Allen, 1978; Powell and Aberle, 1980; Greenwood et al., 1998, 2000; Gondret et al., 2005), which substantially affects profit. P.L. Greenwood has comprehensively documented the detriment of IUGR to carcass quality; longissimus dorsi muscles that comprise the loin are substantially lighter and smaller in diameter in IUGR heifers at 30 mo of age (Greenwood and Cafe, 2007). For each kilogram less that an animal weighs at birth, its HCW, LM area, and total retail yield at slaughter are reduced by an average of 2.71 kg, 0.52 cm2, and 1.97 kg, respectively (Robinson et al., 2013). In fact, birthweight differences explain up to 37% of variation in carcass weight and yield independently of postnatal diet. Similar deficits occur in IUGR-born lambs, as carcass yield strongly correlates with birthweight and crown-rump length (De Blasio et al., 2007). Likewise, IUGR-born pigs produce carcasses with less meat and more omental and subcutaneous fat regardless of their diet (Madsen and Bee, 2015). Current USDA-AMS Daily Beef Reports (April 20, 2018) indicate the potential reduction in final value that an IUGR steer carcass might receive. Discounts for decreased USDA Yield Grade (reduction of ~$10.71/45.4 kg/animal for USDA Yield Grade 4) and reduced intramuscular fat deposition (USDA Select vs. Choice) would result in a combined reduction of $23.13/45.4 kg/animal. Per current USDA-AMS reported average dressed steer HCW (403 kg) and negotiated prices for domestic steers ($189.78/45.4 kg), this would equate to a $205.40 reduction in carcass value. Additionally, a reduction in HCW of 45.4 kg would equate to a loss of $189.78/animal. The combination of reduced HCW and decreased carcass merit would equate to a loss of $395.18/animal. Clearly, the financial implications of IUGR livestock are not trivial.
In ruminants and humans, muscle fiber hyperplasia ceases early in the third trimester (Robelin et al., 1991; Wilson et al., 1992). Subsequent muscle growth occurs by fiber hypertrophy that is facilitated by increased myonuclear content, which amplifies protein synthesis capacity (Davis and Fiorotto, 2009; Ten Broek et al., 2010). However, muscles in IUGR infants and lambs contain fewer myonuclei per fiber at birth, resulting in less DNA, RNA, and protein per fiber (Widdowson et al., 1972; Greenwood et al., 1999; Greenwood et al., 2000). Myonuclei are postmitotic and accumulation requires incorporation of muscle stem cells called myoblasts that proliferate, terminally differentiate, and then fuse with adjacent fibers, effectively donating their nuclei to the fiber (Davis and Fiorotto, 2009; Ten Broek et al., 2010). Myoblast function is rate limiting for muscle growth (Pavlath et al., 1989; Allen et al., 1999), and myoblasts isolated from IUGR fetal sheep exhibit intrinsically reduced functional capacity (Yates et al., 2014). Specifically, IUGR myoblast cultures contained fewer myogenic differentiation 1 (MyoD)-positive cells and reduced replication rates whether supplemented with fetal bovine serum, control fetal sheep serum, or IUGR fetal sheep serum. This coincided with reduced proliferating cell nuclear antigen (PCNA)-positive myoblasts in IUGR semitendinosus cross-sections near term and at 28 d of age (Yates et al., 2014; Camacho et al., 2016b). Moreover, IUGR myoblasts cultured in differentiation media (2% fetal bovine serum) for 4 d had fewer myogenin-positive and desmin-positive cells than control fetal myoblasts (Yates, unpublished data). This explains altered myoblast populations and reduced fiber sizes in IUGR hindlimb muscles near term (Yates et al., 2014, 2016) and at 28 d of age (Camacho et al., 2016b), as postnatal muscle stem cells (satellite cells) originate from fetal myoblast precursors (Greenwood et al., 1999; Messina and Cossu, 2009). Insulin promotes proliferation and differentiation of myoblasts (Allen et al., 1985) via AKT-mediated signaling pathways (Sumitani et al., 2002), and insulin infusion into uncompromised sheep fetuses increases myoblast proliferation (Brown et al., 2016b). However, skeletal muscle AKT content is reduced in IUGR sheep fetuses (Thorn et al., 2009) and adult rats (Camm et al., 2011). Also, insulin activation of AKT is impaired in muscle from IUGR-born men (Jensen et al., 2008) and rats despite greater insulin-stimulated insulin receptor substrate 1 phosphorylation (Rueda-Clausen et al., 2011). Impaired insulin–AKT coupling is particularly noteworthy, as insulin is an important promoter of muscle growth and glucose metabolism.
Skeletal Muscle Metabolism and Glucose Homeostasis
Fetal metabolic adaptations predispose IUGR-born offspring to metabolic diseases that lower quality of life (Vickers et al., 2000; Newsome et al., 2003). Glucose intolerance and obesity rates are profoundly higher in IUGR-born individuals even at very young ages (Flanagan et al., 2000; Ong et al., 2000; Mericq et al., 2005). Poor insulin sensitivity and reduced glucose oxidation, which are hallmark characteristics of diabetics (Vind et al., 2012), are present in IUGR-born children by 12 yr of age even at normal bodyweights (Jornayvaz et al., 2004; Dulloo, 2006). A pair of pivotal studies in IUGR fetal sheep indicates that deficits in systemic glucose oxidation arise prenatally despite normal rates of whole-body glucose utilization (Limesand et al., 2007; Brown et al., 2015). Fractional reductions in glucose oxidation also coincide with greater lactate production, which indicates a metabolic shift from oxidation to anaerobic glycolysis. Interestingly, when hypoinsulinemia is corrected in IUGR fetal sheep via infusion, glucose utilization increases while O2 consumption (an indirect measure of oxidative metabolism) does not (Thorn et al., 2013). Instead, circulating lactate concentrations further increase, indicating that the metabolic shift is not dictated by hypoinsulinemia but rather by selectively impaired sensitivity of glucose oxidation to insulin. Impaired proximal insulin signaling in muscle from IUGR fetal sheep and adult humans (Ozanne et al., 2005; Thorn et al., 2009) may be explained in part by altered fiber type proportions.
Skeletal muscle fiber types differ in their metabolic rates, mitochondrial densities, and responsiveness to insulin and other metabolic regulators (Henriksen et al., 1990; Mackrell and Cartee, 2012). Specifically, insulin sensitivity and glucose oxidation rates are greatest in type I or slow oxidative fibers, intermediate in type IIa or fast oxidative and/or glycolytic fibers, and lowest in type IIx or fast glycolytic fibers. Semitendinosus and biceps femoris muscles frosm IUGR fetal sheep express proportionally fewer type I fibers and total oxidative fibers (types I and IIa) near term (Yates et al., 2016). Flexibility in fiber type composition throughout postnatal life is critical to maintaining metabolic homeostasis through changing physiological states (Costagliola et al., 2016). Our results indicate that fiber type plasticity is reduced by IUGR adaptations (Yates et al., 2016), which likely contributes to metabolic dysfunction in offspring. In fact, we report that the shift in glucose metabolism is muscle-centric and is retained in IUGR-born lambs independent of adipose-driven catch-up growth and despite normal circulating glucose, insulin, cortisol, epinephrine, and norepinephrine (Camacho et al., 2016a). In pregnant sheep, we recently observed that sustained periods of maternofetal inflammation in the early third trimester (days 100 to 115 of gestation, term = 150 d) induced by serial maternal administration of bacterial endotoxin recapitulates fetal growth restriction, deficient skeletal muscle glucose oxidation, and greater lactate production (Merrick et al., 2017). These results were consistent with altered metabolic regulation by stress components (Cadaret et al., 2017a, 2017b).
ADRENERGIC AND INFLAMMATORY ADAPTATIONS
Adrenergic and Inflammatory Regulation of Muscle Growth
Catecholamines and inflammatory cytokines are important regulators of myoblast function and muscle growth (Barnes et al., 2017; Merrick et al., 2017). Adrenergic regulation is primarily facilitated by β2 receptors which are the most highly expressed isoform in skeletal muscle, although β1 receptors and to a lesser extent β3 and α1D receptors are also present (Kim et al., 1991; Shi et al., 2017). Growth studies in animals have led to the use of isoform-specific β adrenergic agonists as growth-promoting feed supplements for finishing livestock, which can increase meat yield of feedlot cattle by up to 40% (Johnson et al., 2014). In a preliminary study, proliferation of primary myoblasts from steers was increased after 48-h incubation with the β2 agonist zilpaterol HCl but not with the β1 agonist ractopamine HCl (Yates, unpublished data). Our preliminary findings in L6 myoblasts show that acute (4 h) incubation with epinephrine in the absence of insulin reduces proliferation rates but longer exposure (48 to 96 h) increases proliferation (Riley et al., 2016).
Myoblast regulation by inflammatory cytokines is quite complex (Kumar et al., 2012; Pillon et al., 2013). For example, TNFα and TNF-related weak inducer of apoptosis (TWEAK) stimulate proliferation in cultured myoblasts but strongly suppress differentiation and fusion (Kumar et al., 2012; Trendelenburg et al., 2012; Otis et al., 2014). Furthermore, muscle fiber size is reduced by both the knockout of and overexposure to inflammatory cytokines (Dogra et al., 2007; Kumar et al., 2012; Pillon et al., 2013). In an unpublished preliminary study with myoblasts isolated from mature cows, we observed 10% fewer differentiated (myogenin-positive) myoblasts after 4 d when media was spiked with TNFα.
Adrenergic and Inflammatory Regulation of Muscle Metabolism
Catecholamines and inflammatory cytokines also regulate muscle metabolism (Fernandes et al., 2014; Barnes et al., 2017; Cadaret et al., 2017b; Merrick et al., 2017). Acute stimulation of primary rat muscle and differentiated bovine myoblasts with the β2 agonist zilpaterol HCl increased basal and insulin-stimulated glucose oxidation with little impact on glucose uptake (Cadaret et al., 2017b; Merrick et al., 2017). Conversely, acute stimulation with the β1 agonist ractopamine HCl did not increase glucose oxidation and antagonized insulin-stimulated AKT phosphorylation. Zilpaterol supplementation to wethers for 21 d prior to harvest increased glucose oxidation in isolated hindlimb muscle, but ractopamine did not (Barnes et al., 2017).
Like β2 agonists, inflammatory cytokines directly stimulate acute muscle glucose oxidation. Primary rat and fetal sheep muscle incubated with TNFα or IL-6 in the absence of insulin exhibited greater glucose oxidation rates (Cadaret et al., 2017b; Merrick et al., 2017). However, the inflammatory cytokines impaired insulin-stimulated AKT phosphorylation, and thus insulin did not increase glucose oxidation in muscle incubated with either TNFα or IL-6. Sustained inflammation created substantial insulin resistance in ewes administered a large intravenous bolus of bacterial endotoxin; insulin-to-glucose ratios were comparable to controls after 2 h but were increased after 4 h and remained greater through 24 h (Yates et al., 2011b).
Adaptive Changes in Adrenergic and Inflammatory Regulation
We previously postulated that deficient growth and glucose metabolism in IUGR skeletal muscle are due to adaptive changes in adrenergic and inflammatory regulation. Preliminary findings indicate that differences in adrenergic regulation may be due to changes in relative proportions of β receptor isomers. Gene expression for β2 receptors is reduced relative to that of β1 and β3 receptors in IUGR fetal and neonatal muscle and in fetal myoblasts (Yates et al., 2012b). Since β2 activity stimulates growth and metabolism, the reduction in relative β2-to-β1/β3 signaling may represent the intrinsic deficit underlying impaired IUGR myoblast function and muscle hypertrophy (Yates et al., 2014, 2016). Moreover, β2 adrenergic activity enhances insulin action, whereas β1 and β3 adrenergic activity disrupt insulin signaling (Jost et al., 2005; Cadaret et al., 2017b). Less relative β2 activity is also consistent with the metabolic phenotype of IUGR muscle, as β2 stimulation increases glucose oxidation and insulin activity (Cadaret et al., 2017b), likely via mammalian target of rapamycin complex (mTORC)-mediated pathways (Sato et al., 2014; Posont et al., 2017). Conversely, β1 stimulation diminishes glucose oxidation and insulin signaling (Hoeks et al., 2003; Cadaret et al., 2017b). The shift in β adrenergic activity may also have indirect implications on muscle glucose metabolism, including the loss of β2-mediated anti-inflammatory effects (Silva et al., 2014) and greater β1-mediated disruption of IGF activity (Walker et al., 2010).
In addition to adrenergic changes, basal inflammation status is greater in IUGR-born offspring. In rats, IUGR increased circulating TNFα concentrations in 21-d-old male offspring, despite substantial catch-up growth (Riddle et al., 2014). Circulating TNFα and toll-like receptor 9 (TLR9) concentrations were also higher in IUGR-born rats at 8 wk (Oliveira et al., 2017) and 9 mo of age (Desai et al., 2009), and hepatic TNFα and IL-6 concentrations were greater at 12 mo (Tarry-Adkins et al., 2016). In IUGR-born mice, catch-up growth had normalized body weights by 2 wk of age, yet IL-6, IL-1β, and TNFα were all increased at 10 wk (Chisaka et al., 2016). Chronic low-grade inflammation almost certainly contributes to muscle dysregulation due to the effects of inflammatory cytokines on myoblast function (Kumar et al., 2012; Trendelenburg et al., 2012; Otis et al., 2014) and muscle hypertrophy (Dogra et al., 2007; Bhatnagar et al., 2012). Preliminary results in rats show amplified inflammatory activity prenatally, as maternal inflammation at mid-gestation increased fetal plasma TNFα by ~67% at term (Cadaret et al., 2017a). These pups also exhibited evidence of inflammatory adaptations with skeletal muscle tissues; total resident macrophages and growth-promoting M2 macrophages were reduced in hindlimb muscles. This coincided with fewer MyoD-positive and more myogenin-positive hindlimb myoblasts, indicating precocious differentiation (Cadaret et al., 2017a). Unpublished preliminary results in IUGR fetal sheep indicate enhanced cytokine stimulus-response coupling as muscle content of the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB)-sequestering protein, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor (IκB), is reduced. In addition, gene expression for TNFα and TNF receptor 1 (TNFR1) is increased in muscle of IUGR fetal sheep and white blood cells of IUGR fetal rats. The combination of greater basal inflammation and enhanced responsiveness to cytokines along with adrenergic changes likely mediate muscle dysregulation.
SUMMARY AND CONCLUSIONS
Intrauterine growth restriction is the result of fetal adaptations to poor intrauterine conditions. This adaptive programming benefits fetal survival but leads to a multitude of problems after birth. Humans and animals that experience IUGR have much greater rates of perinatal and/or neonatal morbidity and mortality, and those that survive have reduced muscle mass, increased fat deposition, and are at much greater risk for metabolic dysfunction throughout life. In livestock, IUGR-born animals exhibit low birthweight, inefficient growth, reduced carcass quality, and less lean product. In humans, IUGR is associated with reduced muscle mass and strength, greater childhood obesity, and increased risk for type-2 diabetes, hypertension, and other conditions of metabolic syndrome in adulthood. Maternofetal stress caused by any of a number of different environmental, nutritional, and toxicity stressors diverts maternal blood flow away from the gravid uterus, thus stunting placental development. These placentas, characterized by poor vascularity and reduced permeability, fail to provide the fetus with sufficient O2 and nutrients during the later stages of gestation. As the fetus progressively outgrows its stunted placenta, it becomes increasingly hypoxemic and hypoglycemic, resulting in sustained fetal stress responses that include hypercatecholaminemia and inflammation. As these conditions become chronic, the fetus develops two key muscle-centric adaptations to restrict nonvital use of nutrients. First, skeletal muscle growth is disproportionally reduced via impaired myoblast function, which results in the hallmark asymmetric fetal growth restriction. Second, muscle glucose metabolism is reduced via inhibition of insulin secretion and reduction of glucose oxidation. Our recent findings indicate that reduced responsiveness to β2 adrenergic regulation and enhanced responsiveness to inflammatory regulation in IUGR skeletal muscle may be key mechanisms underlying the IUGR metabolic phenotype. Moreover, these systems may provide unique targets for interventions and treatments to improve outcomes in IUGR-born offspring.
Footnotes
Based on a presentation entitled “Fetal origins of impaired muscle growth and metabolic dysfunction: Lessons from the heat-stressed pregnant ewes,” presented at the ASAS-SSR Triennial Reproductive Symposium, July 13, 2017, Washington, DC. This project is based on research that was partially supported by the National Institute of General Medical Sciences Grant 1P20GM104320 (J. Zempleni, Director), the Nebraska Agricultural Experiment Station with funding from the Hatch Act (NEB-26–224) and Hatch Multistate Research capacity funding program (NEB-26–226, NEB-26–225) through the USDA National Institute of Food and Agriculture.
LITERATURE CITED
- Adams M. B. and McMillen I. C.. 2000. Actions of hypoxia on catecholamine synthetic enzyme mRNA expression before and after development of adrenal innervation in the sheep fetus. J. Physiol. 529 (Pt 3):519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G., Hales J. R., Stevens D., and Donnelly J. B.. 1987. Effects of acute and prolonged exposure to heat on regional blood flows in pregnant sheep. J. Dev. Physiol. 9:1–15. [PubMed] [Google Scholar]
- Alisi A., Panera N., Agostoni C., and Nobili V.. 2011. Intrauterine growth retardation and nonalcoholic fatty liver disease in children. Int. J. Endocrinol. 2011:269853. doi:10.1155/2011/269853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. E., Luiten L. S., and Dodson M. V.. 1985. Effect of insulin and linoleic acid on satellite cell differentiation. J. Anim. Sci. 60:1571–1579. [DOI] [PubMed] [Google Scholar]
- Allen D. L., Roy R. R., and Edgerton V. R.. 1999. Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22:1350–1360. [DOI] [PubMed] [Google Scholar]
- Ampem G., Azegrouz H., Bacsadi Á., Balogh L., Schmidt S., Thuróczy J., and Röszer T.. 2016. Adipose tissue macrophages in non-rodent mammals: a comparative study. Cell Tissue Res. 363:461–478. doi:10.1007/s00441-015-2253-1 [DOI] [PubMed] [Google Scholar]
- Anthony R. V., Scheaffer A. N., Wright C. D., and Regnault T. R.. 2003. Ruminant models of prenatal growth restriction. Reprod. Suppl. 61:183–194. [PubMed] [Google Scholar]
- Arias E., MacDorman M. F., Strobino D. M., and Guyer B.. 2003. Annual summary of vital statistics–2002. Pediatrics 112(6 Pt 1):1215–1230. [DOI] [PubMed] [Google Scholar]
- Barker D. J., Hales C. N., Fall C. H., Osmond C., Phipps K., and Clark P. M.. 1993. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36:62–67. [DOI] [PubMed] [Google Scholar]
- Barnes T., Kubik R., Cadaret C., Beede K., Merrick E., Chung S., Schmidt T., Petersen J., and Yates D.. 2017. Identifying hyperthermia in heat-stressed lambs and its effects on β agonist–stimulated glucose oxidation in muscle. Proc. West. Sect. Am. Soc. Anim. Sci. 68:106–110. [Google Scholar]
- Bassett J. M. and Hanson C.. 1998. Catecholamines inhibit growth in fetal sheep in the absence of hypoxemia. Am. J. Physiol. 274(6 Pt 2):R1536–R1545. [DOI] [PubMed] [Google Scholar]
- Bauer M. K., Breier B. B., Bloomfield F. H., Jensen E. C., Gluckman P. D., and Harding J. E.. 2003. Chronic pulsatile infusion of growth hormone to growth-restricted fetal sheep increases circulating fetal insulin-like growth factor-I levels but not fetal growth. J. Endocrinol. 177:83–92. [DOI] [PubMed] [Google Scholar]
- Bazaes R. A., Salazar T. E., Pittaluga E., Peña V., Alegría A., Iñiguez G., Ong K. K., Dunger D. B., and Mericq M. V.. 2003. Glucose and lipid metabolism in small for gestational age infants at 48 hours of age. Pediatrics 111(4 Pt 1):804–809. [DOI] [PubMed] [Google Scholar]
- Berghella V. 2007. Prevention of recurrent fetal growth restriction. Obstet. Gynecol. 110:904–912. doi:10.1097/01.AOG.0000267203.55718.aa [DOI] [PubMed] [Google Scholar]
- Bertucci M. C., Loose J. M., Wallace E. M., Jenkin G., and Miller S. L.. 2011. Anti-inflammatory therapy in an ovine model of fetal hypoxia induced by single umbilical artery ligation. Reprod. Fertil. Dev. 23:346–352. doi:10.1071/RD10110 [DOI] [PubMed] [Google Scholar]
- Bhatnagar S., Mittal A., Gupta S. K., and Kumar A.. 2012. TWEAK causes myotube atrophy through coordinated activation of ubiquitin-proteasome system, autophagy, and caspases. J. Cell. Physiol. 227:1042–1051. doi:10.1002/jcp.22821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer B. H., Limesand S. W., and Rozance P. J.. 2017. The impact of IUGR on pancreatic islet development and β-cell function. J. Endocrinol. 235:R63–R76. doi:10.1530/JOE-17-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. D. 2014. Endocrine regulation of fetal skeletal muscle growth: impact on future metabolic health. J. Endocrinol. 221:R13–R29. doi:10.1530/JOE-13-0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. D., Davis M., Wai S., Wesolowski S. R., Hay W. W. Jr, Limesand S. W., and Rozance P. J.. 2016a. Chronically increased amino acids improve insulin secretion, pancreatic vascularity, and islet size in growth-restricted fetal sheep. Endocrinology 157:3788–3799. doi:10.1210/en.2016-1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. D., Rozance P. J., Bruce J. L., Friedman J. E., Hay W. W. Jr, and Wesolowski S. R.. 2015. Limited capacity for glucose oxidation in fetal sheep with intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309:R920–R928. doi:10.1152/ajpregu.00197.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. D., Wesolowski S. R., Kailey J., Bourque S., Wilson A., Andrews S. E., Hay W. W. Jr, and Rozance P. J.. 2016b. Chronic hyperinsulinemia increases myoblast proliferation in fetal sheep skeletal muscle. Endocrinology 157:2447–2460. doi:10.1210/en.2015-1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb K. J., Cock M. L., Black M. J., Dodic M., Boon W. M., Parkington H. C., Harding R., and Tare M.. 2007. Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J. Physiol. 578(Pt 3):871–881. doi:10.1113/jphysiol.2006.121160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadaret C. N., Beede K., Merrick E., Barnes T., Loy J., and Yates D.. 2017a. Maternal inflammation at mid-gestation in pregnant rats impairs fetal muscle growth and development at term. Proc. West. Sect. Am. Soc. Anim. Sci. 68:213–218. [Google Scholar]
- Cadaret C. N., Beede K. A., Riley H. E., and Yates D. T.. 2017b. Acute exposure of primary rat soleus muscle to zilpaterol HCl (β2 adrenergic agonist), TNFα, or IL-6 in culture increases glucose oxidation rates independent of the impact on insulin signaling or glucose uptake. Cytokine 96:107–113. doi:10.1016/j.cyto.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho L. E., Chen X., Hay W. W. Jr, and Limesand S. W.. 2017. Enhanced insulin secretion and insulin sensitivity in young lambs with placental insufficiency-induced intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 313:R101–R109. doi:10.1152/ajpregu.00068.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho L. E., Yates D. T., Allen R. E., and Limesand S. W.. 2016a. Reduced insulin-stimulated glucose uptake in skeletal muscle strips from intrauterine growth restricted lambs. Reprod. Sci. 23:312A. [Google Scholar]
- Camacho L. E., Yates D. T., Davenport H. M., Allen R. E., and Limesand S. W.. 2016b. Decreased satellite cell proliferation rates contribute to small fibers in the semitendinosus muscle of intrauterine growth restricted lambs. Reprod. Sci. 23:316A. [Google Scholar]
- Camm E. J., Martin-Gronert M. S., Wright N. L., Hansell J. A., Ozanne S. E., and Giussani D. A.. 2011. Prenatal hypoxia independent of undernutrition promotes molecular markers of insulin resistance in adult offspring. Faseb J. 25:420–427. doi:10.1096/fj.10-158188 [DOI] [PubMed] [Google Scholar]
- Carnelio S., Morton A., and McIntyre H. D.. 2017. Sleep disordered breathing in pregnancy: the maternal and fetal implications. J. Obstet. Gynaecol. 37:170–178. doi:10.1080/01443615.2016.1229273 [DOI] [PubMed] [Google Scholar]
- Carr D. J., Aitken R. P., Milne J. S., David A. L., and Wallace J. M.. 2012. Fetoplacental biometry and umbilical artery Doppler velocimetry in the overnourished adolescent model of fetal growth restriction. Am. J. Obstet. Gynecol. 207:141.e6–141.15. doi:10.1016/ j.ajog.2012.05.008 [DOI] [PubMed] [Google Scholar]
- Chen X., Green A. S., Macko A. R., Yates D. T., Kelly A. C., and Limesand S. W.. 2014. Enhanced insulin secretion responsiveness and islet adrenergic desensitization after chronic norepinephrine suppression is discontinued in fetal sheep. Am. J. Physiol. Endocrinol. Metab. 306:E58–E64. doi:10.1152/ajpendo.00517.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Kelly A. C., Yates D. T., Macko A. R., Lynch R. M., and Limesand S. W.. 2017a. Islet adaptations in fetal sheep persist following chronic exposure to high norepinephrine. J Endocrinol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Kelly A. C., Yates D. T., Macko A. R., Lynch R. M., and Limesand S. W.. 2017b. Islet adaptations in fetal sheep persist following chronic exposure to high norepinephrine. J. Endocrinol. 232:285–295. doi:10.1530/JOE-16-0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Yu Z., Fu L., Wang H., Chen X., Zhang C., Lv Z. M., and Xu D. X.. 2015. Vitamin D3 inhibits lipopolysaccharide-induced placental inflammation through reinforcing interaction between vitamin D receptor and nuclear factor kappa B p65 subunit. Sci. Rep. 5:10871. doi:10.1038/srep10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisaka T., Mogi M., Nakaoka H., Kan-No H., Tsukuda K., Wang X. L., Bai H. Y., Shan B. S., Kukida M., Iwanami J., et al. 2016. Low-protein diet-induced fetal growth restriction leads to exaggerated proliferative response to vascular injury in postnatal life. Am. J. Hypertens. 29:54–62. doi:10.1093/ajh/hpv072 [DOI] [PubMed] [Google Scholar]
- Costagliola A., Wojcik S., Pagano T. B., De Biase D., Russo V., Iovane V., Grieco E., Papparella S., and Paciello O.. 2016. Age-related changes in skeletal muscle of cattle. Vet. Pathol. 53:436–446. doi:10.1177/0300985815624495 [DOI] [PubMed] [Google Scholar]
- Cotechini T., Komisarenko M., Sperou A., Macdonald-Goodfellow S., Adams M. A., and Graham C. H.. 2014. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J. Exp. Med. 211:165–179. doi:10.1084/jem.20130295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P. and Marton T.. 2009. Pathological assessment of intrauterine growth restriction. Best Pract. Res. Clin. Obstet. Gynaecol. 23:751–764. doi:10.1016/ j.bpobgyn.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Davis T. A. and Fiorotto M. L.. 2009. Regulation of muscle growth in neonates. Curr. Opin. Clin. Nutr. Metab. Care 12:78–85. doi:10.1097/MCO.0b013e32831cef9f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. A., Macko A. R., Steyn L. V., Anderson M. J., and Limesand S. W.. 2015. Fetal adrenal demedullation lowers circulating norepinephrine and attenuates growth restriction but not reduction of endocrine cell mass in an ovine model of intrauterine growth restriction. Nutrients 7:500–516. doi:10.3390/nu7010500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blasio M. J., Blache D., Gatford K. L., Robinson J. S., and Owens J. A.. 2010. Placental restriction increases adipose leptin gene expression and plasma leptin and alters their relationship to feeding activity in the young lamb. Pediatr. Res. 67:603–608. doi:10.1203/PDR.0b013e3181dbc471 [DOI] [PubMed] [Google Scholar]
- De Blasio M. J., Gatford K. L., Robinson J. S., and Owens J. A.. 2007. Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292:R875–R886. doi:10.1152/ajpregu.00430.2006 [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., and Felber J. P.. 1981. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30:1000–1007. [DOI] [PubMed] [Google Scholar]
- Desai M., Gayle D. A., Casillas E., Boles J., and Ross M. G.. 2009. Early undernutrition attenuates the inflammatory response in adult rat offspring. J. Matern. Fetal. Neonatal Med. 22:571–575. doi:10.1080/14767050902874105 [DOI] [PubMed] [Google Scholar]
- Divers W. A., Wilkes M. M., Babaknia A., Hill L. M., Quilligan E. J., and Yen S. S.. 1981. Amniotic fluid catecholamines and metabolites in intrauterine growth retardation. Am. J. Obstet. Gynecol. 141:608–610. [DOI] [PubMed] [Google Scholar]
- Dogra C., Changotra H., Wedhas N., Qin X., Wergedal J. E., and Kumar A.. 2007. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. Faseb J. 21:1857–1869. doi:10.1096/fj.06-7537com [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiling C. E., Carman F. S. III, and Brown D. E.. 1991. Maternal endocrine and fetal metabolic responses to heat stress. J. Dairy Sci. 74:312–327. doi:10.3168/jds.S0022-0302(91)78175-7 [DOI] [PubMed] [Google Scholar]
- Dulloo A. G. 2006. Regulation of fat storage via suppressed thermogenesis: a thrifty phenotype that predisposes individuals with catch-up growth to insulin resistance and obesity. Horm. Res. 65Suppl 3:90–97. doi:10.1159/000091512 [DOI] [PubMed] [Google Scholar]
- Dwyer C. M., Conington J., Corbiere F., Holmøy I. H., Muri K., Nowak R., Rooke J., Vipond J., and Gautier J. M.. 2016. Invited review: improving neonatal survival in small ruminants: science into practice. Animal 10:449–459. doi:10.1017/S1751731115001974 [DOI] [PubMed] [Google Scholar]
- Economides D. L., Nicolaides K. H., and Campbell S.. 1991. Metabolic and endocrine findings in appropriate and small for gestational age fetuses. J. Perinat. Med. 19:97–105. [DOI] [PubMed] [Google Scholar]
- English A. W. and Mulley R. C.. 1992. Causes of perinatal mortality in farmed fallow deer (Dama dama). Aust. Vet. J. 69:191–193. [DOI] [PubMed] [Google Scholar]
- Fernandes G. W., Ueta C. B., Fonseca T. L., Gouveia C. H., Lancellotti C. L., Brum P. C., Christoffolete M. A., Bianco A. C., and Ribeiro M. O.. 2014. Inactivation of the adrenergic receptor β2 disrupts glucose homeostasis in mice. J. Endocrinol. 221:381–390. doi:10.1530/JOE-13-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan D. E., Moore V. M., Godsland I. F., Cockington R. A., Robinson J. S., and Phillips D. I.. 2000. Fetal growth and the physiological control of glucose tolerance in adults: a minimal model analysis. Am. J. Physiol. Endocrinol. Metab. 278:E700–E706. doi:10.1152/ajpendo.2000.278.4.E700 [DOI] [PubMed] [Google Scholar]
- Foxcroft G. R., Dixon W. T., Novak S., Putman C. T., Town S. C., and Vinsky M. D.. 2006. The biological basis for prenatal programming of postnatal performance in pigs. J. Anim. Sci. 84Suppl:E105–E112. [DOI] [PubMed] [Google Scholar]
- Galan H. L., Anthony R. V., Rigano S., Parker T. A., de Vrijer B., Ferrazzi E., Wilkening R. B., and Regnault T. R.. 2005. Fetal hypertension and abnormal Doppler velocimetry in an ovine model of intrauterine growth restriction. Am. J. Obstet. Gynecol. 192:272–279. doi:10.1016/ j.ajog.2004.05.088 [DOI] [PubMed] [Google Scholar]
- Galan H. L., Hussey M. J., Barbera A., Ferrazzi E., Chung M., Hobbins J. C., and Battaglia F. C.. 1999. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am. J. Obstet. Gynecol. 180:1278–1282. [DOI] [PubMed] [Google Scholar]
- Gatford K. L., Simmons R. A., De Blasio M. J., Robinson J. S., and Owens J. A.. 2010. Review: placental programming of postnatal diabetes and impaired insulin action after IUGR. Placenta 31Suppl:S60–S65. doi:10.1016/j.placenta.2009.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg R. L. and Culhane J. F.. 2007. Low birth weight in the United States. Am. J. Clin. Nutr. 85:584S–590S. doi:10.1093/ajcn/85.2.584S [DOI] [PubMed] [Google Scholar]
- Gondret F., Larzul C., Combes S., and de Rochambeau H.. 2005. Carcass composition, bone mechanical properties, and meat quality traits in relation to growth rate in rabbits. J. Anim. Sci. 83:1526–1535. doi:10.2527/2005.8371526x [DOI] [PubMed] [Google Scholar]
- Green A. S., Rozance P. J., and Limesand S. W.. 2010. Consequences of a compromised intrauterine environment on islet function. J. Endocrinol. 205:211–224. doi:10.1677/JOE-09-0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A., Nicolaides K. H., and Lagercrantz H.. 1990. Human fetal sympathoadrenal responsiveness. Early Hum. Dev. 23:9–13. [DOI] [PubMed] [Google Scholar]
- Greenwood P. L. and Cafe L. M.. 2007. Prenatal and pre-weaning growth and nutrition of cattle: long-term consequences for beef production. Animal 1:1283–1296. doi:10.1017/S175173110700050X [DOI] [PubMed] [Google Scholar]
- Greenwood P. L., Hunt A. S., Hermanson J. W., and Bell A. W.. 1998. Effects of birth weight and postnatal nutrition on neonatal sheep: I. Body growth and composition, and some aspects of energetic efficiency. J. Anim. Sci. 76:2354–2367. [DOI] [PubMed] [Google Scholar]
- Greenwood P. L., Hunt A. S., Hermanson J. W., and Bell A. W.. 2000. Effects of birth weight and postnatal nutrition on neonatal sheep: II. Skeletal muscle growth and development. J. Anim. Sci. 78:50–61. [DOI] [PubMed] [Google Scholar]
- Greenwood P. L., Slepetis R. M., Hermanson J. W., and Bell A. W.. 1999. Intrauterine growth retardation is associated with reduced cell cycle activity, but not myofibre number, in ovine fetal muscle. Reprod. Fertil. Dev. 11:281–291. [DOI] [PubMed] [Google Scholar]
- Guo R., Hou W., Dong Y., Yu Z., Stites J., and Weiner C. P.. 2010. Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reprod. Sci. 17:540–548. doi:10.1177/1933719110364061 [DOI] [PubMed] [Google Scholar]
- Hales C. N. and Barker D. J.. 1992. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35:595–601. [DOI] [PubMed] [Google Scholar]
- Hales C. N. and Barker D. J.. 2001. The thrifty phenotype hypothesis. Br. Med. Bull. 60:5–20. [DOI] [PubMed] [Google Scholar]
- Hashimoto R., Sakai K., Matsumoto H., and Iwashita M.. 2010. Tumor necrosis factor-alpha (TNF-alpha) inhibits insulin-like growth factor-I (IGF-I) activities in human trophoblast cell cultures through IGF-I/insulin hybrid receptors. Endocr. J. 57:193–200. [DOI] [PubMed] [Google Scholar]
- Hediger M. L., Overpeck M. D., Kuczmarski R. J., McGlynn A., Maurer K. R., and Davis W. W.. 1998. Muscularity and fatness of infants and young children born small- or large-for-gestational-age. Pediatrics 102:E60. [DOI] [PubMed] [Google Scholar]
- Hegarty P. V. and Allen C. E.. 1978. Effect of pre-natal runting on the post-natal development of skeletal muscles in swine and rats. J. Anim. Sci. 46:1634–1640. [DOI] [PubMed] [Google Scholar]
- Henriksen E. J., Bourey R. E., Rodnick K. J., Koranyi L., Permutt M. A., and Holloszy J. O.. 1990. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am. J. Physiol. 259(4 Pt 1):E593–E598. doi:10.1152/ajpendo.1990.259.4.E593 [DOI] [PubMed] [Google Scholar]
- Hoeflich A., Wu M., Mohan S., Föll J., Wanke R., Froehlich T., Arnold G. J., Lahm H., Kolb H. J., and Wolf E.. 1999. Overexpression of insulin-like growth factor-binding protein-2 in transgenic mice reduces postnatal body weight gain. Endocrinology 140:5488–5496. doi:10.1210/endo.140.12.7169 [DOI] [PubMed] [Google Scholar]
- Hoeks J., van Baak M. A., Hesselink M. K., Hul G. B., Vidal H., Saris W. H., and Schrauwen P.. 2003. Effect of beta1- and beta2-adrenergic stimulation on energy expenditure, substrate oxidation, and UCP3 expression in humans. Am. J. Physiol. Endocrinol. Metab. 285:E775–E782. doi:10.1152/ajpendo.00175.2003 [DOI] [PubMed] [Google Scholar]
- Hubinont C., Nicolini U., Fisk N. M., Tannirandorn Y., and Rodeck C. H.. 1991. Endocrine pancreatic function in growth-retarded fetuses. Obstet. Gynecol. 77:541–544. [PubMed] [Google Scholar]
- Jansson N., Pettersson J., Haafiz A., Ericsson A., Palmberg I., Tranberg M., Ganapathy V., Powell T. L., and Jansson T.. 2006. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J. Physiol. 576(Pt 3):935–946. doi:10.1113/jphysiol.2006.116509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C. B., Martin-Gronert M. S., Storgaard H., Madsbad S., Vaag A., and Ozanne S. E.. 2008. Altered PI3-kinase/Akt signalling in skeletal muscle of young men with low birth weight. Plos One 3:e3738. doi:10.1371/journal.pone.0003738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C. B., Storgaard H., Dela F., Holst J. J., Madsbad S., and Vaag A. A.. 2002. Early differential defects of insulin secretion and action in 19-year-old Caucasian men who had low birth weight. Diabetes 51:1271–1280. [DOI] [PubMed] [Google Scholar]
- Johnson B. J., Smith S. B., and Chung K. Y.. 2014. Historical overview of the effect of β-adrenergic agonists on beef cattle production. Asian-Australas. J. Anim. Sci. 27:757–766. doi:10.5713/ajas.2012.12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. K., Hoffman M. L., Pillai S. M., McFadden K. K., Govoni K. E., Zinn S. A., and Reed S. A.. 2018. Gestational restricted- and over-feeding promote maternal and offspring inflammatory responses that are distinct and dependent on diet in sheep. Biol. Reprod. 98:184–196. doi:10.1093/biolre/iox174 [DOI] [PubMed] [Google Scholar]
- Jornayvaz F. R., Selz R., Tappy L., and Theintz G. E.. 2004. Metabolism of oral glucose in children born small for gestational age: evidence for an impaired whole body glucose oxidation. Metabolism. 53:847–851. [DOI] [PubMed] [Google Scholar]
- Jost M. M., Jost P., Klein J., and Klein H. H.. 2005. The beta3-adrenergic agonist CL316,243 inhibits insulin signaling but not glucose uptake in primary human adipocytes. Exp. Clin. Endocrinol. Diabetes 113:418–422. doi:10.1055/s-2005-865771 [DOI] [PubMed] [Google Scholar]
- Kelly A. C., Bidwell C. A., McCarthy F. M., Taska D. J., Anderson M. J., Camacho L. E., and Limesand S. W.. 2017. RNA sequencing exposes adaptive and immune responses to intrauterine growth restriction in fetal sheep islets. Endocrinology 158:743–755. doi:10.1210/en.2016-1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensara O. A., Wootton S. A., Phillips D. I., Patel M., Jackson A. A., and Elia M.; Hertfordshire Study Group 2005. Fetal programming of body composition: relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am. J. Clin. Nutr. 82:980–987. doi:10.1093/ajcn/82.5.980 [DOI] [PubMed] [Google Scholar]
- Kim K. S., Park K. S., Kim M. J., Kim S. K., Cho Y. W., and Park S. W.. 2014. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr. Gerontol. Int. 14Suppl 1:115–121. doi:10.1111/ggi.12189 [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Sainz R. D., Molenaar P., and Summers R. J.. 1991. Characterization of beta 1- and beta 2-adrenoceptors in rat skeletal muscles. Biochem. Pharmacol. 42:1783–1789. [DOI] [PubMed] [Google Scholar]
- Kliegman R. M. 1989. Alterations of fasting glucose and fat metabolism in intrauterine growth-retarded newborn dogs. Am. J. Physiol. 256(3 Pt 1):E380–E385. doi:10.1152/ajpendo.1989.256.3.E380 [DOI] [PubMed] [Google Scholar]
- Krajewski P., Sieroszewski P., Karowicz-Bilinska A., Kmiecik M., Chudzik A., Strzalko-Gloskowska B., Kwiatkowska M., Pokrzywnicka M., Wyka K., Chlapinski J., et al. 2014. Assessment of interleukin-6, interleukin-8 and interleukin-18 count in the serum of IUGR newborns. J. Matern. Fetal. Neonatal Med. 27:1142–1145. doi:10.3109/14767058.2013.851186 [DOI] [PubMed] [Google Scholar]
- Kumar A., Bhatnagar S., and Paul P. K.. 2012. TWEAK and TRAF6 regulate skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 15:233–239. doi:10.1097/MCO.0b013e328351c3fc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larciprete G., Valensise H., Di Pierro G., Vasapollo B., Casalino B., Arduini D., Jarvis S., and Cirese E.. 2005. Intrauterine growth restriction and fetal body composition. Ultrasound Obstet. Gynecol. 26:258–262. doi:10.1002/uog.1980 [DOI] [PubMed] [Google Scholar]
- Lee M. H., Jeon Y. J., Lee S. M., Park M. H., Jung S. C., and Kim Y. J.. 2010. Placental gene expression is related to glucose metabolism and fetal cord blood levels of insulin and insulin-like growth factors in intrauterine growth restriction. Early Hum. Dev. 86:45–50. doi:10.1016/j.earlhumdev.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Leos R. A., Anderson M. J., Chen X., Pugmire J., Anderson K. A., and Limesand S. W.. 2010. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am. J. Physiol. Endocrinol. Metab. 298:E770–E778. doi:10.1152/ajpendo.00494.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Jenkins S., Mattern V., Comuzzie A. G., Cox L. A., Huber H. F., and Nathanielsz P. W.. 2017. Effect of moderate, 30 percent global maternal nutrient reduction on fetal and postnatal baboon phenotype. J. Med. Primatol. 46:293–303. doi:10.1111/jmp.12290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Johnson M. S., and Goran M. I.. 2001. Effects of low birth weight on insulin resistance syndrome in Caucasian and African-American children. Diabetes Care 24:2035–2042. [DOI] [PubMed] [Google Scholar]
- Limesand S. W., Jensen J., Hutton J. C., and Hay W. W. Jr. 2005. Diminished beta-cell replication contributes to reduced beta-cell mass in fetal sheep with intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288:R1297–R1305. doi:10.1152/ajpregu.00494.2004 [DOI] [PubMed] [Google Scholar]
- Limesand S. W., Rozance P. J., Macko A. R., Anderson M. J., Kelly A. C., and Hay W. W. Jr. 2013. Reductions in insulin concentrations and β-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am. J. Physiol. Endocrinol. Metab. 304:E516–E523. doi:10.1152/ajpendo.00435.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand S. W., Rozance P. J., Smith D., and Hay W. W. Jr. 2007. Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am. J. Physiol. Endocrinol. Metab. 293:E1716–E1725. doi:10.1152/ajpendo.00459.2007 [DOI] [PubMed] [Google Scholar]
- Limesand S. W., Rozance P. J., Zerbe G. O., Hutton J. C., and Hay W. W. Jr. 2006. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147:1488–1497. doi:10.1210/en.2005-0900 [DOI] [PubMed] [Google Scholar]
- Liu H., Schultz C. G., De Blasio M. J., Peura A. M., Heinemann G. K., Harryanto H., Hunter D. S., Wooldridge A. L., Kind K. L., Giles L. C., et al. 2015. Effect of placental restriction and neonatal exendin-4 treatment on postnatal growth, adult body composition, and in vivo glucose metabolism in the sheep. Am. J. Physiol. Endocrinol. Metab. 309:E589–E600. doi:10.1152/ajpendo.00487.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macko A. R., Yates D. T., Chen X., Green A. S., Kelly A. C., Brown L. D., and Limesand S. W.. 2013. Elevated plasma norepinephrine inhibits insulin secretion, but adrenergic blockade reveals enhanced β-cell responsiveness in an ovine model of placental insufficiency at 0.7 of gestation. J. Dev. Orig. Health Dis. 4:402–410. doi:10.1017/S2040174413000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macko A. R., Yates D. T., Chen X., Shelton L. A., Kelly A. C., Davis M. A., Camacho L. E., Anderson M. J., and Limesand S. W.. 2016. Adrenal demedullation and oxygen supplementation independently increase glucose-stimulated insulin concentrations in fetal sheep with intrauterine growth restriction. Endocrinology 157:2104–2115. doi:10.1210/en.2015-1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackrell J. G. and Cartee G. D.. 2012. A novel method to measure glucose uptake and myosin heavy chain isoform expression of single fibers from rat skeletal muscle. Diabetes 61:995–1003. doi:10.2337/db11-1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen J. G. and Bee G.. 2015. Compensatory growth feeding strategy does not overcome negative effects on growth and carcass composition of low birth weight pigs. Animal 9:427–436. doi:10.1017/S1751731114002663 [DOI] [PubMed] [Google Scholar]
- Mäkikallio K., Kaukola T., Tuimala J., F Kingsmore S., Hallman M., and Ojaniemi M.. 2012. Umbilical artery chemokine CCL16 is associated with preterm preeclampsia and fetal growth restriction. Cytokine 60:377–384. doi:10.1016/j.cyto.2012.07.008 [DOI] [PubMed] [Google Scholar]
- Malandro M. S., Beveridge M. J., Novak D. A., and Kilberg M. S.. 1996. Rat placental amino acid transport after protein-deprivation-induced intrauterine growth retardation. Biochem. Soc. Trans. 24:839–843. [DOI] [PubMed] [Google Scholar]
- Marconi A. M., Paolini C. L., Zerbe G., and Battaglia F. C.. 2006. Lactacidemia in intrauterine growth restricted (IUGR) pregnancies: relationship to clinical severity, oxygenation and placental weight. Pediatr. Res. 59(4 Pt 1):570–574. doi:10.1203/01.pdr.0000205477.70391.3e [DOI] [PubMed] [Google Scholar]
- Meral C., Cekmez F., Pirgon O., Asya Tanju I., Metin Ipcioglu O., Karademir F., and Gocmen I.. 2011. The relationship between serum visfatin, adiponectin, and insulin sensitivity markers in neonates after birth. J. Matern. Fetal. Neonatal Med. 24:166–170. doi:10.3109/14767058.2010.482604 [DOI] [PubMed] [Google Scholar]
- Mericq V., Ong K. K., Bazaes R., Peña V., Avila A., Salazar T., Soto N., Iñiguez G., and Dunger D. B.. 2005. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia 48:2609–2614. doi:10.1007/s00125-005-0036-z [DOI] [PubMed] [Google Scholar]
- Merrick E. M., Cadaret C. N., Barnes T. L., Beede K. A., and Yates D. T.. 2017. Metabolic regulation by stress systems in bovine myoblasts and ovine fetal skeletal muscle. Proc. West. Sect. Am. Soc. Anim. Sci. 68:87–91. [Google Scholar]
- Messina G. and Cossu G.. 2009. The origin of embryonic and fetal myoblasts: a role of Pax3 and Pax7. Genes Dev. 23:902–905. doi:10.1101/gad.1797009 [DOI] [PubMed] [Google Scholar]
- Milovanovic I., Njuieyon F., Deghmoun S., Chevenne D., Levy-Marchal C., and Beltrand J.. 2014. SGA children with moderate catch-up growth are showing the impaired insulin secretion at the age of 4. Plos One 9:e100337. doi:10.1371/journal.pone.0100337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley F. 1954. Prenatal loss in Merino sheep. Aust. Vet. J. 30:125–128. [Google Scholar]
- Morrison J. L. 2008. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin. Exp. Pharmacol. Physiol. 35:730–743. doi:10.1111/j.1440-1681.2008.04975.x [DOI] [PubMed] [Google Scholar]
- Moulk G. 1954. Observations on mortality amongst lambs in Queensland. Aust. Vet. J. 30:153–171. [Google Scholar]
- Murphy S. L., Mathews T. J., Martin J. A., Minkovitz C. S., and Strobino D. M.. 2017. Annual summary of vital statistics: 2013–2014. Pediatrics 139:e20163239. [DOI] [PubMed] [Google Scholar]
- Newsome C. A., Shiell A. W., Fall C. H., Phillips D. I., Shier R., and Law C. M.. 2003. Is birth weight related to later glucose and insulin metabolism?–A ystematic review. Diabet. Med. 20:339–348. [DOI] [PubMed] [Google Scholar]
- Nicolini U., Hubinont C., Santolaya J., Fisk N. M., and Rodeck C. H.. 1990. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm. Metab. Res. 22:426–430. doi:10.1055/s-2007-1004939 [DOI] [PubMed] [Google Scholar]
- Oksbjerg N., Nissen P. M., Therkildsen M., Møller H. S., Larsen L. B., Andersen M., and Young J. F.. 2013. Meat science and muscle biology symposium: in utero nutrition related to fetal development, postnatal performance, and meat quality of pork. J. Anim. Sci. 91:1443–1453. doi:10.2527/jas.2012-5849 [DOI] [PubMed] [Google Scholar]
- Oleson B. J., McGraw J. A., Broniowska K. A., Annamalai M., Chen J., Bushkofsky J. R., Davis D. B., Corbett J. A., and Mathews C. E.. 2015. Distinct differences in the responses of the human pancreatic β-cell line endoc-βh1 and human islets to proinflammatory cytokines. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309:R525–R534. doi:10.1152/ajpregu.00544.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira V., Silva Junior S. D., de Carvalho M. H., Akamine E. H., Michelini L. C., and Franco M. C.. 2017. Intrauterine growth restriction increases circulating mitochondrial DNA and toll-like receptor 9 expression in adult offspring: could aerobic training counteract these adaptations?J. Dev. Orig. Health Dis. 8:236–243. doi:10.1017/S2040174416000714 [DOI] [PubMed] [Google Scholar]
- Ong K. K., Ahmed M. L., Emmett P. M., Preece M. A., and Dunger D. B.. 2000. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 320:967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis J. S., Niccoli S., Hawdon N., Sarvas J. L., Frye M. A., Chicco A. J., and Lees S. J.. 2014. Pro-inflammatory mediation of myoblast proliferation. Plos One 9:e92363. doi:10.1371/journal.pone.0092363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne S. E., Jensen C. B., Tingey K. J., Storgaard H., Madsbad S., and Vaag A. A.. 2005. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 48:547–552. doi:10.1007/s00125-005-1669-7 [DOI] [PubMed] [Google Scholar]
- Padoan A., Rigano S., Ferrazzi E., Beaty B. L., Battaglia F. C., and Galan H. L.. 2004. Differences in fat and lean mass proportions in normal and growth-restricted fetuses. Am. J. Obstet. Gynecol. 191:1459–1464. doi:10.1016/j.ajog.2004.06.045 [DOI] [PubMed] [Google Scholar]
- Pantham P., Rosario F. J., Nijland M., Cheung A., Nathanielsz P. W., Powell T. L., Galan H. L., Li C., and Jansson T.. 2015. Reduced placental amino acid transport in response to maternal nutrient restriction in the baboon. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309:R740–R746. doi:10.1152/ajpregu.00161.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantham P., Rosario F. J., Weintraub S. T., Nathanielsz P. W., Powell T. L., Li C., and Jansson T.. 2016. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in maternal nutrient restricted baboons. Biol. Reprod. 95:98. doi:10.1095/biolreprod.116.141085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi G., Cetin I., Marconi A. M., Lanfranchi A., Bozzetti P., Ferrazzi E., Buscaglia M., and Battaglia F. C.. 1993. Diagnostic value of blood sampling in fetuses with growth retardation. N. Engl. J. Med. 328:692–696. doi:10.1056/NEJM199303113281004 [DOI] [PubMed] [Google Scholar]
- Pavlath G. K., Rich K., Webster S. G., and Blau H. M.. 1989. Localization of muscle gene products in nuclear domains. Nature 337:570–573. doi:10.1038/337570a0 [DOI] [PubMed] [Google Scholar]
- Peugnet P., Wimel L., Duchamp G., Sandersen C., Camous S., Guillaume D., Dahirel M., Dubois C., Jouneau L., Reigner F., et al. 2014. Enhanced or reduced fetal growth induced by embryo transfer into smaller or larger breeds alters post-natal growth and metabolism in pre-weaning horses. Plos One 9:e102044. doi:10.1371/journal.pone.0102044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon N. J., Bilan P. J., Fink L. N., and Klip A.. 2013. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am. J. Physiol. Endocrinol. Metab. 304:E453–E465. doi:10.1152/ajpendo.00553.2012 [DOI] [PubMed] [Google Scholar]
- Posont R. J., Cadaret C. N., Barnes T. L., and Yates D. T.. 2017. A potential role for mTORC1/2 in β2 adrenergic regulation of skeletal muscle glucose oxidation in models of intrauterine growth restriction. Diabesity 3:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel R., McMillen I. C., Dunn S. L., Zhang S., and Morrison J. L.. 2015. Impact of chronic hypoxemia on blood flow to the brain, heart, and adrenal gland in the late-gestation IUGR sheep fetus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308:R151–R162. doi:10.1152/ajpregu.00036.2014 [DOI] [PubMed] [Google Scholar]
- Powell S. E. and Aberle E. D.. 1980. Effects of birth weight on growth and carcass composition of swine. J. Anim. Sci. 50:860–868. [DOI] [PubMed] [Google Scholar]
- Regnault T. R., de Vrijer B., Galan H. L., Davidsen M. L., Trembler K. A., Battaglia F. C., Wilkening R. B., and Anthony R. V.. 2003. The relationship between transplacental O2 diffusion and placental expression of PIGF, VEGF and their receptors in a placental insufficiency model of fetal growth restriction. J. Physiol. 550(Pt 2):641–656. doi:10.1113/jphysiol.2003.039511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault T. R., de Vrijer B., Galan H. L., Wilkening R. B., Battaglia F. C., and Meschia G.. 2007. Development and mechanisms of fetal hypoxia in severe fetal growth restriction. Placenta 28:714–723. doi:10.1016/j.placenta.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Regnault T. R., Orbus R. J., de Vrijer B., Davidsen M. L., Galan H. L., Wilkening R. B., and Anthony R. V.. 2002. Placental expression of VEGF, PIGF and their receptors in a model of placental insufficiency-intrauterine growth restriction (PI-IUGR). Placenta 23:132–144. doi:10.1053/plac.2001.0757 [DOI] [PubMed] [Google Scholar]
- Reynolds L. P. and Caton J. S.. 2012. Role of the pre- and post-natal environment in developmental programming of health and productivity. Mol. Cell. Endocrinol. 354:54–59. doi:10.1016/j.mce.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle E. S., Campbell M. S., Lang B. Y., Bierer R., Wang Y., Bagley H. N., and Joss-Moore L. A.. 2014. Intrauterine growth restriction increases TNF α and activates the unfolded protein response in male rat pups. J. Obes. 2014:829862. doi:10.1155/2014/829862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley H. E., Beede K. A., and Yates D. T.. 2016. Impact of stress hormones and IUGR fetal conditions on myoblast function. UCARE Res. Prod. 2016:67(Abstr). [Google Scholar]
- Robelin J., Lacourt A., Béchet D., Ferrara M., Briand Y., and Geay Y.. 1991. Muscle differentiation in the bovine fetus: a histological and histochemical approach. Growth. Dev. Aging 55:151–160. [PubMed] [Google Scholar]
- Roberts A., Nava S., Bocconi L., Salmona S., and Nicolini U.. 1999. Liver function tests and glucose and lipid metabolism in growth-restricted fetuses. Obstet. Gynecol. 94:290–294. [DOI] [PubMed] [Google Scholar]
- Robinson D. L., Cafe L. M., and Greenwood P. L.. 2013. Meat science and muscle biology symposium: developmental programming in cattle: consequences for growth, efficiency, carcass, muscle, and beef quality characteristics. J. Anim. Sci. 91:1428–1442. doi:10.2527/jas.2012-5799 [DOI] [PubMed] [Google Scholar]
- Rozance P. J., Anderson M., Martinez M., Fahy A., Macko A. R., Kailey J., Seedorf G. J., Abman S. H., Hay W. W. Jr, and Limesand S. W.. 2015. Placental insufficiency decreases pancreatic vascularity and disrupts hepatocyte growth factor signaling in the pancreatic islet endothelial cell in fetal sheep. Diabetes 64:555–564. doi:10.2337/db14-0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozance P. J., Zastoupil L., Wesolowski S. R., Goldstrohm D. A., Strahan B., Cree-Green M., Sheffield-Moore M., Meschia G., Hay W. W. Jr, Wilkening R. B., et al. 2018. Skeletal muscle protein accretion rates and hindlimb growth are reduced in late gestation intrauterine growth-restricted fetal sheep. J. Physiol. 596:67–82. doi:10.1113/JP275230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda-Clausen C. F., Dolinsky V. W., Morton J. S., Proctor S. D., Dyck J. R., and Davidge S. T.. 2011. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes 60:507–516. doi:10.2337/db10-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem T., Sajjad N., Fatima S., Habib N., Ali S. R., and Qadir M.. 2011. Intrauterine growth retardation–small events, big consequences. Ital. J. Pediatr. 37:41. doi:10.1186/1824-7288-37-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Dehvari N., Oberg A. I., Dallner O. S., Sandström A. L., Olsen J. M., Csikasz R. I., Summers R. J., Hutchinson D. S., and Bengtsson T.. 2014. Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes 63:4115–4129. doi:10.2337/db13-1860 [DOI] [PubMed] [Google Scholar]
- Shelton M. and Huston J. E.. 1968. Effects of high temperature stress during gestation on certain aspects of reproduction in the ewe. J. Anim. Sci. 27:153–158. [DOI] [PubMed] [Google Scholar]
- Shi T., Papay R. S., and Perez D. M.. 2017. The role of α1-adrenergic receptors in regulating metabolism: increased glucose tolerance, leptin secretion and lipid oxidation. J. Recept. Signal Transduct. Res. 37:124–132. doi:10.1080/10799893.2016.1193522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Wensing L. A., Brum P. C., Câmara N. O., and Miyabara E. H.. 2014. Impaired structural and functional regeneration of skeletal muscles from β2-adrenoceptor knockout mice. Acta Physiol. (Oxf). 211:617–633. doi:10.1111/apha.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street M. E., Seghini P., Fieni S., Ziveri M. A., Volta C., Martorana D., Viani I., Gramellini D., and Bernasconi S.. 2006. Changes in interleukin-6 and IGF system and their relationships in placenta and cord blood in newborns with fetal growth restriction compared with controls. Eur. J. Endocrinol. 155:567–574. doi:10.1530/eje.1.02251 [DOI] [PubMed] [Google Scholar]
- Sumitani S., Goya K., Testa J. R., Kouhara H., and Kasayama S.. 2002. Akt1 and Akt2 differently regulate muscle creatine kinase and myogenin gene transcription in insulin-induced differentiation of C2C12 myoblasts. Endocrinology 143:820–828. doi:10.1210/endo.143.3.8687 [DOI] [PubMed] [Google Scholar]
- Tarry-Adkins J. L., Fernandez-Twinn D. S., Hargreaves I. P., Neergheen V., Aiken C. E., Martin-Gronert M. S., McConnell J. M., and Ozanne S. E.. 2016. Coenzyme Q10 prevents hepatic fibrosis, inflammation, and oxidative stress in a male rat model of poor maternal nutrition and accelerated postnatal growth. Am. J. Clin. Nutr. 103:579–588. doi:10.3945/ajcn.115.119834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Broek R. W., Grefte S., and Von den Hoff J. W.. 2010. Regulatory factors and cell populations involved in skeletal muscle regeneration. J. Cell. Physiol. 224:7–16. doi:10.1002/jcp.22127 [DOI] [PubMed] [Google Scholar]
- Thorn S. R., Brown L. D., Rozance P. J., Hay W. W. Jr, and Friedman J. E.. 2013. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes 62:65–73. doi:10.2337/db11-1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn S. R., Regnault T. R., Brown L. D., Rozance P. J., Keng J., Roper M., Wilkening R. B., Hay W. W. Jr, and Friedman J. E.. 2009. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 150:3021–3030. doi:10.1210/en.2008-1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thureen P. J., Trembler K. A., Meschia G., Makowski E. L., and Wilkening R. B.. 1992. Placental glucose transport in heat-induced fetal growth retardation. Am. J. Physiol. 263(3 Pt 2):R578–R585. doi:10.1152/ajpregu.1992.263.3.R578 [DOI] [PubMed] [Google Scholar]
- Trendelenburg A. U., Meyer A., Jacobi C., Feige J. N., and Glass D. J.. 2012. TAK-1/p38/nNFκB signaling inhibits myoblast differentiation by increasing levels of Activin A. Skelet. Muscle 2:3. doi:10.1186/2044-5040-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche F. A. and De Prins F. A.. 1981. Endocrine pancreas in intrauterine growth retardation. Am. J. Obstet. Gynecol. 141:233. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A., Moeller J., Sreedharan R., Singer K., Lumeng C., Ye W., Pease A., and Padmanabhan V.. 2016. Developmental programming: interaction between prenatal BPA exposure and postnatal adiposity on metabolic variables in female sheep. Am. J. Physiol. Endocrinol. Metab. 310:E238–E247. doi:10.1152/ajpendo.00425.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers M. H., Breier B. H., Cutfield W. S., Hofman P. L., and Gluckman P. D.. 2000. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am. J. Physiol. Endocrinol. Metab. 279:E83–E87. doi:10.1152/ajpendo.2000.279.1.E83 [DOI] [PubMed] [Google Scholar]
- Vind B. F., Birk J. B., Vienberg S. G., Andersen B., Beck-Nielsen H., Wojtaszewski J. F., and Højlund K.. 2012. Hyperglycaemia normalises insulin action on glucose metabolism but not the impaired activation of AKT and glycogen synthase in the skeletal muscle of patients with type 2 diabetes. Diabetologia 55:1435–1445. doi:10.1007/s00125-012-2482-8 [DOI] [PubMed] [Google Scholar]
- de Vrijer B., Regnault T. R., Wilkening R. B., Meschia G., and Battaglia F. C.. 2004. Placental uptake and transport of ACP, a neutral nonmetabolizable amino acid, in an ovine model of fetal growth restriction. Am. J. Physiol. Endocrinol. Metab. 287:E1114–E1124. doi:10.1152/ajpendo.00259.2004 [DOI] [PubMed] [Google Scholar]
- Visentin S., Lapolla A., Londero A. P., Cosma C., Dalfrà M., Camerin M., Faggian D., Plebani M., and Cosmi E.. 2014. Adiponectin levels are reduced while markers of systemic inflammation and aortic remodelling are increased in intrauterine growth restricted mother-child couple. Biomed Res. Int. 2014:401595. doi:10.1155/2014/401595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo T. and Hardy D. B.. 2012. Molecular mechanisms underlying the fetal programming of adult disease. J. Cell Commun. Signal. 6:139–153. doi:10.1007/s12079-012-0165-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuguin P. M. 2007. Animal models for small for gestational age and fetal programming of adult disease. Horm. Res. 68:113–123. doi:10.1159/000100545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. K., Titgemeyer E. C., Baxa T. J., Chung K. Y., Johnson D. E., Laudert S. B., and Johnson B. J.. 2010. Effects of ractopamine and sex on serum metabolites and skeletal muscle gene expression in finishing steers and heifers. J. Anim. Sci. 88:1349–1357. doi:10.2527/jas.2009-2409 [DOI] [PubMed] [Google Scholar]
- Wallace J. M., Regnault T. R., Limesand S. W., Hay W. W. Jr, and Anthony R. V.. 2005. Investigating the causes of low birth weight in contrasting ovine paradigms. J. Physiol. 565(Pt 1):19–26. doi:10.1113/jphysiol.2004.082032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson E. M., Crabb D. E., and Milner R. D.. 1972. Cellular development of some human organs before birth. Arch. Dis. Child. 47:652–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. J., McEwan J. C., Sheard P. W., and Harris A. J.. 1992. Early stages of myogenesis in a large mammal: formation of successive generations of myotubes in sheep tibialis cranialis muscle. J. Muscle Res. Cell Motil. 13:534–550. [DOI] [PubMed] [Google Scholar]
- Woo I., Hindoyan R., Landay M., Ho J., Ingles S. A., McGinnis L. K., Paulson R. J., and Chung K.. 2017. Perinatal outcomes after natural conception versus in vitro fertilization (IVF) in gestational surrogates: a model to evaluate IVF reatment versus maternal effects. Fertil. Steril. 108:993–998. doi:10.1016/j.fertnstert.2017.09.014 [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Burghardt R. C., Johnson G. A., Kim S. W., Li X. L., Satterfield M. C., and Spencer T. E.. 2010. Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J. Anim. Sci. 88(13 Suppl):E195–E204. doi:10.2527/jas.2009-2446 [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Wallace J. M., and Spencer T. E.. 2006. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J. Anim. Sci. 84:2316–2337. doi:10.2527/jas.2006-156 [DOI] [PubMed] [Google Scholar]
- Yates D. T., Cadaret C. N., Beede K. A., Riley H. E., Macko A. R., Anderson M. J., Camacho L. E., and Limesand S. W.. 2016. Intrauterine growth-restricted sheep fetuses exhibit smaller hindlimb muscle fibers and lower proportions of insulin-sensitive type I fibers near term. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310:R1020–R1029. doi:10.1152/ajpregu.00528.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. T., Clarke D. S., Macko A. R., Anderson M. J., Shelton L. A., Nearing M., Allen R. E., Rhoads R. P., and Limesand S. W.. 2014. Myoblasts from intrauterine growth-restricted sheep fetuses exhibit intrinsic deficiencies in proliferation that contribute to smaller semitendinosus myofibres. J. Physiol. 592:3113–3125. doi:10.1113/jphysiol.2014.272591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. T., Green A. S., and Limesand S. W.. 2011a. Catecholamines mediate multiple fetal adaptations during placental insufficiency that contribute to intrauterine growth restriction: lessons from hyperthermic sheep. J. Pregnancy 2011:740408. doi:10.1155/2011/740408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. T., Löest C. A., Ross T. T., Hallford D. M., Carter B. H., and Limesand S. W.. 2011b. Effects of bacterial lipopolysaccharide injection on white blood cell counts, hematological variables, and serum glucose, insulin, and cortisol concentrations in ewes fed low- or high-protein diets. J. Anim. Sci. 89:4286–4293. doi:10.2527/jas.2011-3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. T., Macko A. R., Chen X., Green A. S., Kelly A. C., Anderson M. J., Fowden A. L., and Limesand S. W.. 2012a. Hypoxaemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinaemia in fetal sheep. J. Physiol. 590:5439–5447. doi:10.1113/jphysiol.2012.237347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. T., Macko A. R., Nearing M., Chen X., Rhoads R. P., and Limesand S. W.. 2012b. Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism. J. Pregnancy 2012:631038. doi:10.1155/2012/631038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates N. 1956. The effect of high air temperature on pregnancy and birth weight in Merino sheep. Aust. J. Agric. Res. 7: 435–9. [Google Scholar]
- Ylihärsilä H., Kajantie E., Osmond C., Forsén T., Barker D. J., and Eriksson J. G.. 2007. Birth size, adult body composition and muscle strength in later life. Int. J. Obes. (Lond). 31:1392–1399. doi:10.1038/sj.ijo.0803612 [DOI] [PubMed] [Google Scholar]
- Zarrati M., Shidfar F., Razmpoosh E., Nezhad F. N., Keivani H., Hemami M. R., and Asemi Z.. 2013. Does low birth weight predict hypertension and obesity in schoolchildren?Ann. Nutr. Metab. 63:69–76. doi:10.1159/000351869 [DOI] [PubMed] [Google Scholar]