Abstract
A meta-analysis was performed on eight trials, which included a total of 992 parity 1 to 8 lactating sows, to evaluate the effects of feeding xylanase which is the main enzyme activity present in the enzymatic complex (Rovabio Excel, Adisseo, France) supplement throughout lactation on the following sow performance factors: BW loss, feed intake, backfat depth, and piglet growth. Even a short period of enzyme supplementation during lactation led to a reduction in BW loss of approximately 3 kg per sow (P = 0.003). This reduction represented 1–2% of the BW of sows. This effect could be explained by an increase in feed energy intake and enhanced feed digestibility. Sows fed enzyme-supplemented diets exhibited greater DM, OM, and GE digestibilities (3.4, 3.9, and 4.2% increases, respectively; P < 0.001) than sows fed control diets. During lactation, sows lost from 19 to 25 kg of BW (i.e., approximately 10% of their BW), with a difference between parity groups (P < 0.001). Body reserve mobilization was decreased in sows fed enzyme-supplemented diets (−2.9 kg, P = 0.003), with a more pronounced effect in primiparous than multiparous sows when BW loss is expressed relative to total BW (−2.27 vs. −0.59%, respectively; P = 0.058). Enzyme supplementation also increased litter weight gain up to weaning, with a greater effect in litters from multiparous sows than those from primiparous sows (5.4 vs. 0.6 kg, respectively; P = 0.009). These results could be explained in part by the relationship between their NE intake and either variations in BW or litter weight gain (R2 = 0.51 and 0.49, respectively; P < 0.001). Finally, the meta-analysis suggests that there are differences in the partitioning of the NE intake between growth and milk production and in relation to the sow’s parity or physiological status. Extra energy released by enzyme is used for one of these functions (i.e., body mobilization reduction or greater milk export for litter gain).
Keywords: body condition, nonstarch polysaccharide-hydrolyzing enzyme, litter weight, sow
INTRODUCTION
Sows face drastic metabolic changes during farrowing and lactation. During the lactation phase, which lasts from 18 to 28 d, the sow’s increased energy requirements normally lead to an enormous mobilization of body reserves (Dourmad et al., 1994). Moreover, due to changes in gut physiology, feed intake is often limited during the first days after farrowing which is followed by a large increase throughout lactation (Revell et al., 1998; Eissen et al., 2000). However, this increase in feed intake does not prevent lactating sows from losing up to 25 kg BW during the 3–4 first weeks of lactation, which could represent 10% of their total BW.
Most studies and surveys on this topic, conducted in different countries, have shown that sows, especially young sows at their first and second parities, do not consume enough feed during lactation to meet their energy and amino acid requirements. Moreover, it has been extensively demonstrated that excessive weight loss during lactation compromises subsequent reproductive performance (Hughes, 1993; Tantasuparuk et al., 2001). Clowes et al. (2003) and Thaker and Bilkei (2005) reported that sows mobilizing more than 12% of their overall protein mass or more than 10% of their BW during lactation subsequently face reproductive difficulties and produce litters with reduced weights. Some authors recommend the use of backfat thickness as an indicator of body reserve mobilization, and Tantasuparuk et al. (2001) reported a correlation between delays in sows returning to oestrus and backfat thicknesses of less than 14 mm. As a consequence, increase the weaning-to-service delay are often associated with increase weight and body condition loss during the preceding lactation period (Brooks et al., 1975; Trotter and Johnston, 2001).
According to the NRC (2012), a 175-kg sow should eat approximately 5.6 kg/d (i.e., 118 kg of feed) during a 3-wk lactation period. However, sows generally do not eat during the first few days after farrowing, while their feed intake might reach up to 9 kg/d by peak lactation. As a consequence, most producers search for solutions to promote increased feed intake and avoid weight loss, especially during the initial days of lactation.
Cereals, including wheat and barley, and some legumes, such as soybeans, used in diets for lactating sows contain up to 15% nonstarch polysaccharides (NSP) in their cell walls (Sterk et al., 2007). These NSP are poorly digested by pigs as they lack the proper enzymes for their degradation (Bach Knudsen and Jorgensen, 2001). Many NSP affect feed transit, thus increasing water-holding capacity and digesta viscosity. This change subsequently results in reduction in feed intake. They can also decrease nutrient digestibility by entrapping starches and proteins (Kim et al., 2005). The addition of enzyme, such as carbohydrase, to pig diets facilitates the hydrolysis of such NSP, increasing the utilization of available raw materials (Diebold et al., 2004; Bindelle et al., 2011). Enzyme supplementation to break down NSP has been well documented for use in piglet feed and in growing–finishing diets for pigs (Barrera et al., 2004; Nortey et al., 2008; Emiola et al., 2009). However, due to the difficulties in conducting experiments on a large number of sows, data on the impact of such additives for use in sow diets are limited. Exogenous enzymes with xylanase activity have been shown to increase the digestibility of nutrients during lactation in sows; however, a similar response was not observed during gestation (de Souza et al., 2007). The present study analyses the data from eight trials performed on lactating sows that either did or did not receive a supplementary enzymatic complex in their diet. It provides new insights into energy partitioning among body reserves and the effect of diet on litter growth, improving our knowledge of the precise nutritional requirements of lactating sows.
MATERIALS AND METHODS
Animal Welfare
This study complied with European Union Council Directive 91/630/EEC, which outlines minimum standards for the protection of pigs, and European Union Council Directive 98/58/EC, which relates to the protection of animals kept for farming purposes. All experimental facilities selected were approved by their organizational or national ethics committees.
Source of Data
A database was compilated with eight studies performed between 2004 and 2010, dealing with the effects of enzyme supplementation on the performance of lactating sows (Table 1). These eight studies have been selected on two criteria: 1) the report of both sow and piglet growth performance and 2) the use of an NSP enzyme derived from Talaromyces versatilis. Among these different studies, some of them evaluate only enzyme effects (trials 1, 2, 4, 5, 6, and 7) or other additional points, such as: 1) effect of graded enzyme dose (Exp. 3), 2) enzyme effect over two successive reproductive cycles (Exp.6), 3) enzyme effect on different dietary energy density (Exp. 3 and 8), and 4) enzyme effect on nutrient digestibility (Exp. 3). Indeed, trials 1, 2, 5, 6, and 7 compared one experimental diet with and without enzyme addition. In trial 4, there were three treatments with two densities and enzymes have been tested only on the less dense diet (control 2). Trial 8 has been well described by Walsh et al. (2012) and consisted in factorial design with two diets densities and two enzyme doses (with or without). Finally, trial 3 compared five experimental treatments with two diet densities with and without enzyme supplementation. The lowest density diet (control 2) was supplemented with two different doses of enzymes (50 and 500 g/ton). This resulted in a database of eight experiments, with a total of 22 treatments.
Table 1.
Experimental details for each trial included in the meta-analysis
| Experiment | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Facility | Shur gain/maple leaf | Prairie Swine Centre | Prairie Swine Centre | Universidade Federal |
| Location | Canada | Canada | Canada | Brazil |
| Year | 2006 | 2004 | 2007 | 2006 |
| Number of sows | 132 | 100 | 125 | 141 |
| Treatments | Control | Control | Control 1 | Control 1 |
| Control + enzyme | Control + enzyme | Control 1 + enzyme (1×) | Control 2 | |
| Control 2 + enzyme | ||||
| Control 1 + enzyme (10×) | ||||
| Control 2 | ||||
| Control 2 + enzyme (1×) | ||||
| Diet type | Corn–soybean meal | Wheat–barley–soybean meal | Wheat–barley–soybean meal | Corn–soybean meal |
| Form of diet | Pellets | Dry mash | Dry mash | Pellets |
| Additional goals | Enzyme dose (0, 1 or 10×) | Two dietary energy levels | ||
| Two dietary energy levels | ||||
| Experiment | 5 | 6 | 7 | 8 |
| Facility | Primex Languidic | Euronutrition | IRTA | Teagasc |
| Location | France | France | Spain | United Kingdom |
| Year | 2005 | 2010 | 2006 | 2008 |
| Number of sows | 124 | 46 | 74 | 204 |
| Treatments | Control | Control | Control | Control 1 |
| Control + enzyme | Control + enzyme | Control + enzyme | Control 1 + enzyme | |
| Control 2 | ||||
| Control 2 + enzyme | ||||
| Diet type | Wheat–soybean meal | Wheat–barley–soybean sunflower meal | Wheat–soybean meal | Wheat–barley–soybean meal |
| Diet form | Wet mash | Pellets | Wet mash | Pellets |
| Additional goals | Effect of enzyme supplementation over two reproductive cycles | Two dietary energy levels |
Descriptive statistics for each variable in the selected dataset are reported in Table 2. For experimental purposes, a total of 992 sows, ranging from 46 to 204 sows per experimental farm, were selected and randomly allocated to one of the dietary treatments at farrowing and for up to 4 wk of lactation. Average parity and BW was maintained constant between dietary treatments.
Table 2.
Descriptive statistics for each trial included in the meta-analysis
| Experiment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Cycle | 1 | 2 | |||||||
| Number of sows per treatment | 66 | 50 | 25 | 47 | 62 | 23 | 23 | 37 | 51 |
| Number of sows per parity group | |||||||||
| 1st | 40.5 | 9.0 | — | 15.7 | — | 5.0 | 1.0 | 2.0 | — |
| 2nd | 9.5 | 6.0 | 5.8 | 9.0 | 14.0 | 5.0 | 5.0 | 8.0 | 2.5 |
| 3rd | 7.0 | 6.8 | 6.7 | 13.0 | 13.0 | 5.0 | 8.0 | 18.0 | |
| 4th and higher | 16.0 | 28 | 12.4 | 15.6 | 35.0 | 12.0 | 19.0 | 30.5 | |
| Average parity | 1.9 | 3.1 | 2.5 | 2.4 | 3.3 | 2.3 | 3.2 | 3.2 | 3.5 |
| Average sows BW, kg | 214 | 278 | 296 | 268 | 281 | 289 | 289 | 252 | 248 |
| Backfat thickness initial, mm | 20.1 | 20.4 | 18.4 | 15.4 | 16.1 | 16.9 | 13.9 | ||
| Litter weight after cross-fostering, kg | 15.5 | 17.6 | 17.6 | 16.4 | 18.5 | 21.6 | 20.7 | 19.3 | 17.6 |
| Litter weaning weight, kg | 64.6 | 76.4 | 62.2 | 59.3 | 93.1 | 71.4 | 64.7 | 71.1 | 80.8 |
| Weaning age, day | 19 | 21 | 21 | 21 | 28 | 28 | 28 | 28 | 28 |
In all experiments, the diets were conformed with standard practices and NE reformulation were applied in some experiment. The diets were described in Table 3. Average (min–max) of NE and digestible lysine were 10.21 (9.68–10.95) MJ/kg and 0.84 (0.74–0.90) %, respectively. The composition of the diets and the nutritional specifications of each experiments are summarized in Table 3.
Table 3.
Diet composition and nutrient content for each trial included in the meta-analysis
| Experiment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Density1 | Low | High | Low | High | Low | High | |||||
| Composition, % | |||||||||||
| Wheat | 10.0 | 25.0 | 50.2 | 53.2 | 47.4 | 42.0 | 25.0 | 42.3 | 36.4 | ||
| Barley | 30.2 | 27.0 | 21.0 | 7.0 | 15.0 | 35.8 | 42.3 | 36.4 | |||
| Corn | 54.7 | 53.1 | 50.3 | ||||||||
| Oat | 4.9 | ||||||||||
| Wheat middling | 5.0 | 8.0 | 10.0 | ||||||||
| Wheat bran | 4.1 | 3.6 | 5.0 | ||||||||
| Peas | 15.0 | 5.0 | 5.0 | ||||||||
| Flaxseed | 5.0 | ||||||||||
| Sunflower seed | 7.7 | 8.5 | |||||||||
| Soybean meal | 25.3 | 18.7 | 14.0 | 15.0 | 29.0 | 29.8 | 10.7 | 10.8 | 25.4 | 12.3 | 18.6 |
| Palm meal expeller | 4.0 | ||||||||||
| Soybean by-product | 2.9 | 3.6 | |||||||||
| Sugar beet pulp | 4.9 | ||||||||||
| Sugar | 5.0 | 5.0 | |||||||||
| Animal fat white grease | 5.8 | ||||||||||
| Vegetable oil | 0.7 | 1.9 | 0.0 | 2.0 | 4.9 | 6.0 | 1.2 | 0.8 | 0.0 | 6.0 | |
| Dicalcium phosphate | 1.0 | 1.6 | 1.2 | 1.2 | 0.4 | 0.4 | 1.1 | 0.8 | 0.8 | ||
| Calcium carbonate | 2.0 | 0.8 | 0.8 | 0.8 | 1.2 | 1.2 | 0.9 | 1.2 | 1.2 | ||
| Salt | 0.5 | 0.6 | 0.5 | 0.5 | 0.5 | 0.4 | 0.4 | ||||
| Lysine | 0.2 | 0.1 | 0.3 | 0.3 | 0.4 | 0.3 | 0.1 | 0.3 | 0.1 | ||
| Methionine | 0.1 | 0.1 | |||||||||
| Threonine | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||||||
| Premix | 0.4 | 1.1 | 1.0 | 1.0 | 5.3 | 5.3 | 2.0 | 3.4 | 0.4 | 0.2 | 0.2 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Calculated nutrient content, % as fed | |||||||||||
| DM | 87.4 | 87.9 | 87.5 | 87.8 | 88.4 | 88.6 | 88.4 | 88.5 | 88.2 | 87.3 | 88.1 |
| CP | 17.9 | 18.4 | 15.8 | 15.9 | 17.8 | 18.1 | 15.0 | 15.2 | 18.6 | 14.7 | 16.2 |
| Crude fat | 3.5 | 5.3 | 1.5 | 3.5 | 7.5 | 8.5 | 6.4 | 6.5 | 7.5 | 1.6 | 7.2 |
| Crude fiber | 3.3 | 4.2 | 3.4 | 3.3 | 3.8 | 4.1 | 5.6 | 5.3 | 4.2 | 3.6 | 3.6 |
| NDF | 11.6 | 13.8 | 13.6 | 13.0 | 10.6 | 10.9 | 18.4 | 19.0 | 14.9 | 14.7 | 13.6 |
| ADF | 4.0 | 5.0 | 4.3 | 4.2 | 4.6 | 4.9 | 7.0 | 7.5 | 5.2 | 4.5 | 4.5 |
| NE, MJ/kg | 10.20 | 10.10 | 9.74 | 10.16 | 10.81 | 10.95 | 9.68 | 9.76 | 10.44 | 9.76 | 10.77 |
| Digestible lysine | 0.90 | 0.90 | 0.85 | 0.87 | 0.84 | 0.86 | 0.84 | 0.76 | 0.90 | 0.77 | 0.74 |
1Density refers to NE content and digestible lysine content.
Experimental Procedure
Sows were selected and assigned to blocks according to BW and parity, from 1 to 8 parity, to represent a parity distribution similar to that found in pig herds. Animals were randomly assigned to one of the treatments within the experiment. Diets were formulated using raw materials, taking into account the local context and the typical constraints on locally available raw materials (Table 3). The energy content of the diets was modulated by varying the quantity of vegetable oil added.
Sows were housed in dry sow accommodation and were fed a dry (Exp. 1, 2, 3, 4, 6, and 8) or liquid (Exp. 5 and 7) diet. Daily feed allocations were not changed relative to classical practices. The feeding period in all experiments started at farrowing and continued to weaning. Sows had unlimited access to water through nipple waterers. Feed wastage and refusal were collected daily and analyzed for their DM content.
At farrowing, litters were weighed before and after standardization to homogenize litter sizes within the treatments. Litter weaning weight reported in Table 2 was the weight after standardization. Cross-fostering in the 24-h period following farrowing was applied. The duration of lactation was in agreement with the standard procedures used in the experimental facilities. Within the eight experiments, the duration of the lactation period varied from 19 to 28 d, with one experiment at 19 d, three at 21 d, and four at 28 d.
Enzyme Product
The enzymatic complex used in these experiments (Rovabio Excel) is produced via fermentation of T. versatilis and consists mainly of enzyme with carbohydrase activities, including at least 20 different identified enzymatic activities, with a minimum of 5,500 visco-units of endo-β-1.4-xylanase and 500 units of endo-1.3(4)-β-glucanase per mL of product. It provided 1,100 visco-units of endo-β-1.4-xylanase and 100 units of endo-1.3(4)-β-glucanase per ton of finished feed at the recommended dose of 50 g/ton. Enzyme was evaluated as described by Francesch and Geraert (2009). One visco-unit of endo-1,4-β-xylanase activity is defined as the amount of enzyme required to reduce the viscosity of a solution (0.25% wheat arabinoxylan) by 1 arbitrary unit of relative fluidity per minute per mL under the conditions of the assay (pH 5.5 and 30 °C). One azo-β-glucanase unit of endo-1.4-β-glucanase activity is defined based on the release of oligomers from a barley-derived β-glucan solution containing a bound ethanol-soluble chromophore and is the amount of enzyme required to produce an absorbance of 0.820 units at 590 nm under the conditions of the assay (20 min at pH 4.6 and 30 °C). Enzyme activity recoveries in the diets were estimated based on xylanase viscosity activity as described for the pure enzyme preparation above, except that the pH was maintained at 3 during the procedure.
Measured Parameters
Sow feed intake was recorded weekly, from farrowing to weaning. Sow BW and backfat thickness (average of six ultrasonic measurements taken on each side of the spine at the shoulder, the mid back, and the hip joint) were recorded during the week before farrowing and on the day of weaning to quantify sow body composition and backfat mobilization. Similar measurements were taken for dorsal muscle depth. Piglets were individually weighed at birth and at weaning.
Total tract digestibility of the DM, OM, and DE contents of the diets were measured in gilts in study 3. The diets were supplemented with Celite S (Imerys Minerals California, Inc; San Jose; CA), which was used as an indigestible marker. Fecal samples were collected for 3 d after a 10-d adaptation period. At the end of the collection period, excreta were freeze-dried and then ground and homogenized. Finally, excreta and feed were analyzed for their DM and GE content using standardized methodologies (AOAC, 1990).
Calculations and Statistical Analyses
Data were analyzed using a meta-analytical approach (SAS, 2000; St-Pierre, 2001). Trials were coded as random effects and were considered to be a random sample of a large population. The PROC MIXED (for linear functions) and PROC NLMIXED (for nonlinear functions) were used for data treatment.
For clarification, sows were grouped based on parity (P) group. The three groups were defined as young sows (P1), intermediate sows (P2 and P3), and old sows (P4 to P8). The factorial mixed model fitted to the data considered the treatments (n = 2; enzyme and control), parity groups (n = 3), and their interaction (n = 6) as fixed effects and the trial as a random effect. The model used was as follows:
Where µ is the overall mean, α is the enzyme effect, β the parity-group effect, αβ the interaction, C the random effect of trial, and ε the error term (Greek letters denote fixed effects and Roman letters denote random effects). The least square means were used for the comparison of means and are presented in Tables.
Digestibility measurements from Exp. 3 were analyzed via variance analysis. PROC GLM (SAS, 2000) was used, and the model included diet (n = 2; with and without enzyme), enzyme (n = 2; doses 1 and 2), and their interaction as fixed effects. Dose response was estimated using PROC REG (SAS, 2000).
RESULTS
Nutrient Digestibility in Lactating Sows
The main effects of diet and enzyme supplementation on nutrient digestibility in the sows are outlined in Table 4. Digestibilities averaged 57, 47, and 58% for DM, OM, and GE, respectively. Consequently, the average DE content was 10.41 MJ/kg, which is lower than the expected value. No interaction was observed between diet and enzyme inclusion (P > 0.100). Diet digestibility in the present experiment was similar between the high- and low-density diets (P > 0.325), whereas DE content differed by 0.33 MJ DE/kg (P = 0.056). The difference between the positive and negative control diets was 0.71 MJ DE/kg, which is in line with the expected value of 0.42 MJ/kg. The inclusion of the enzyme supplement resulted in an absolute increase in nutrient digestibility of 5, 5, and 4% for DM, OM, and GE, respectively (P < 0.001), which represents 0.72 MJ DE/kg in the diet (P < 0.001).
Table 4.
Exp. 3: determination of total tract feed digestibility of DM, OM, and GE in lactating sows
| Density1 | High | Low | Statistical analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Enzyme (g/ton)2 | 0 | 50 | 500 | 0 | 50 | SEM | Diet | Enzyme3 | Inter4 |
| DM intake, kg | 125 | 125 | 119 | 126 | 126 | 5.4 | 0.845 | 0.976 | 0.940 |
| Digestibility, % | |||||||||
| DM | 52.9 | 59.4 | 58.1 | 55.3 | 58.7 | 1.1 | 0.445 | 0.001 | 0.145 |
| OM | 42.6 | 50.7 | 48.6 | 46.0 | 49.9 | 1.2 | 0.325 | 0.001 | 0.100 |
| GE | 55.2 | 60.7 | 57.6 | 55.6 | 59.8 | 1.0 | 0.793 | 0.001 | 0.526 |
| DE, MJ/kg | 10.26 | 11.28 | 10.70 | 9.55 | 10.26 | 0.17 | 0.056 | 0.001 | 0.720 |
1Density refers to NE content and digestible lysine content.
2Levels of enzyme in the diets.
3Comparisons between diet (high and low) and enzyme (0 and 50 g/t).
4Comparisons between enzyme dose (0, 50, and 500 g/t; tolerance study).
Growth Performance Parameters of Lactating Sows
Descriptive statistics are reported in Table 2. An average of 47 sows was used per treatment, but there was a high degree of variability in the number of animals used across the studies (range: 23 to 76 sows per treatment). On average, sow BW and parity (min–max) were 248 (133–387) kg and 2.9 (1–8) events, respectively. There was a high number of primiparous sows in Exp. 1 (62%), whereas in Exp. 5, 58% of the animals had a parity value higher than 4. Husbandry practices and experimental facilities also differed between experiments. Large differences were observed in backfat thickness initial and post-farrowing. The feeding programs and lactation durations ranged from 19 to 28 d, with the majority of piglets weaned at 28 d (50%). In all experiments, all animals remained healthy throughout the duration of the experiment and no abnormal sow or piglet mortalities were observed among the experimental treatments.
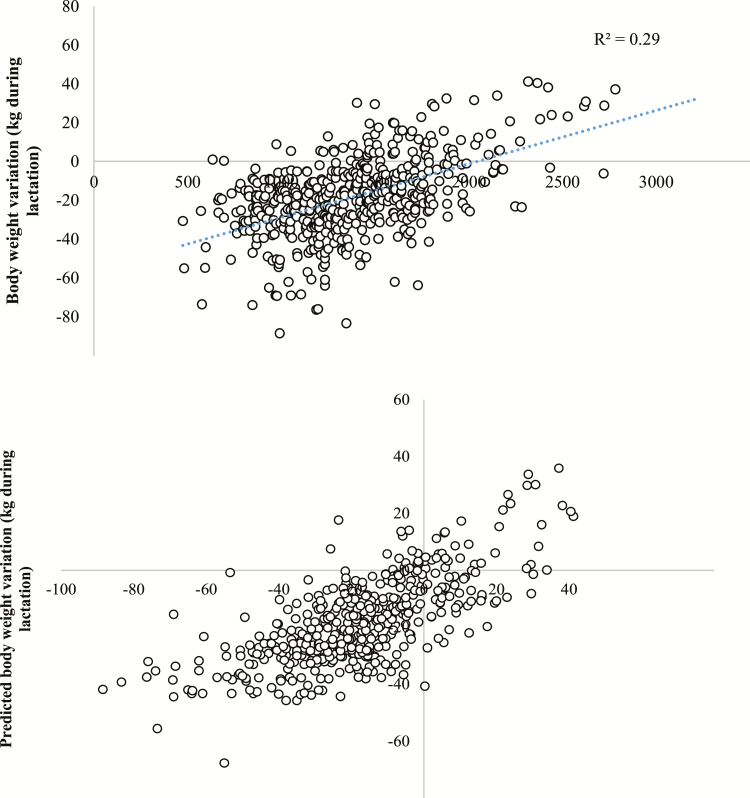
Sow performance characteristics are reported in Table 5. Daily feed intake remained unchanged by treatment (P = 0.150), and no interactions were observed between treatment and parity (P = 0.965). Animal feed intake and NE intake increased with parity (P < 0.001) in relation with sow BW during the lactation period (R2 = 0.29, P < 0.01; Fig. 1) and body growth. As suggested by the different intercepts for the different parity groups reported by the prediction equation, energy partitioning changed in relation to parity (P < 0.001). The coefficients suggest a lesser effect of NE intake on the variation in BW of primiparous and intermediate parity sows than that of older sows (Fig. 1).
Table 5.
Effect of enzyme supplementation on sow BW and reserves
| Parity group | Enzyme | Statistical analysis1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme | 1 | 2 | 3 | − | + | SEM2 | Parity | Enzyme | Interaction | R 2 |
| Number of observation | 146 | 353 | 485 | 492 | 492 | |||||
| Feed intake, kg/d | 5.3c | 5.6b | 5.9a | 5.6 | 5.5 | 1.2 | < 0.0001 | 0.150 | 0.965 | 0.37 |
| NE intake, MJ/lactation | 1,251b | 1,313a | 1,445a | 300 | < 0.0001 | 0.44 | ||||
| BW, kg | ||||||||||
| Initial | 206.1c | 263.7b | 271.2a | 248.5 | 245.5 | 28.7 | < 0.0001 | 0.192 | 0.435 | 0.47 |
| Farrowing | 210.5c | 263.2b | 273.0a | 249.0 | 248.7 | 26.8 | < 0.0001 | 0.861 | 0.259 | 0.58 |
| Weaning | 190.6c | 236.3b | 254.2a | 226.7 | 227.7 | 24.4 | < 0.0001 | 0.674 | 0.371 | 0.59 |
| BW loss3 | -19.9a | -26.9b | -18.8a | -22.8 | -19.9 | 15.4 | < 0.0001 | 0.003 | 0.158 | 0.36 |
| Corrected BW loss, %4 | -9.5b | -10.2b | -6.8a | -8.8 | -7.8 | 6.2 | < 0.0001 | 0.003 | 0.058 | 0.35 |
| Backfat thickness, mm | ||||||||||
| Farrowing | 18.3a | 18.2a | 17.1b | 17. 9 | 17.8 | 4.3 | 0.004 | 0.702 | 0.248 | 0.36 |
| Weaning | 15.5 | 15.4 | 15.0 | 15.2 | 15.2 | 3.7 | 0.250 | 0.770 | 0.330 | 0.39 |
| Change | -2.8b | -2.8b | -2.1a | -2.7 | -2.6 | 2.5 | 0.020 | 0.508 | 0.500 | 0.23 |
| Dorsal muscle thickness, mm | ||||||||||
| Initial | 59.0 | 57.2 | 57.8 | 58.4 | 57.6 | 5.4 | 0.479 | 0.467 | 0.085 | 0.16 |
| Weaning | 55.4 | 54.4 | 56.7 | 55.5 | 55.6 | 5.0 | 0.183 | 0.894 | 0.257 | 0.01 |
| Change | -3.6 | -2.8 | -1.1 | -3.0 | -1.9 | 4.0 | 0.056 | 0.245 | 0.278 | 0.3 |
1From the analysis of variance of 992 results including parity group (n = 3), assay (n = 8), and enzyme (n = 2). Means with different subscript letter were significantly different P < 0.05.
2SEM for the overall model.
3Determination coefficient. BW losses are calculated relative to sow weight at farrowing.
4Corrected BW losses relative to farrowing BW.
Figure 1.
Effect of NE intake during the lactation period on sow BW change. Data (n = 486) were subject to variance analysis including parity group (n = 3) and trial (n = 8) as fixed effects, NE intake (NEI, MJ per lactation) as covariates and the interactions NEI × trial, NEI × parity. Body weight change = −65.7 × group 3 − 44.4 × group 1 − 51.0 × group 2 + 3.6E−02 × NEI × group 3 + 2.3E−02 × NEI × group 1 + 2.4E−02 × NEI × group 2; R2 = 0.51, SEM = 14.7 kg. (group 1 includes primiparus, group 2 includes parity 2 and parity 3 and group 3 includes parity 4 and older, respectively).
Body weight losses were lower in primiparous and high-parity sows than in multiparous sows of parity group 2 (P < 0.001). Body weight losses averaged 19 kg per sow of parity groups 1 and 3. Moreover, intermediate parity sows (P2 and P3) lost more weight (−26.9 kg, P < 0.001) than the other two groups. Enzyme supplementation reduced BW loss (−2.9 kg, P = 0.003), but there was no interaction enzyme and parity effect (P = 0.158). Relative to BW at farrowing, the percentage of body stores mobilized tended to be improved by enzyme supplementation and to be affected by parity (P = 0.058). Primiparous sows fed diets supplemented with enzyme mobilized a smaller proportion of their BW (−2.3%, P = 0.058; Fig. 2), whereas no differences were observed for the other two groups.
Figure 2.
Effect of enzyme supplementation on BW loss in relation to parity (group 1 includes primiparus, group 2 includes parity 2 and parity 3, and group 3 includes parity 4 and older).
The backfat thickness of sows decreased during the lactation period (from 17.9 to 15.2 mm at farrowing and weaning, respectively; Table 5) and with parity group (P = 0.004). The differences between backfat thickness at farrowing and weaning varied depending on sow parity: the difference was greater for the youngest and intermediate sows than for the older sows (P = 0.020). Data suggested a greater backfat mobilization by youngest animals. Moreover, changes in sow backfat thickness were not affected by the inclusion of enzyme in the diet (P > 0.50). In addition, dorsal muscle thickness tended to decrease with age (P = 0.056).
Litter Performance
Litter performance is summarized in Table 6. Litter size and piglet BW (min–max) at farrowing averaged 11.3 (2–27) piglets and 1.5 (0.5–2.8) kg, respectively. Differences were observed in the number of piglets born and weaned between the parity groups, with the most prolific sows found in the intermediate parity group (group 2; P = 0.001) compared to groups 1 and 3. In addition, the lightest litters were obtained from the low parity group, averaging 17 kg, whereas the litters from the P2 and P3 and the P4 to P8 groups averaged 18.4 kg (P < 0.0001). No effect of enzyme addition was observed on the numbers of born or weaned animal (P > 0.802). Litter weight gain was positively influenced by NE intake, as shown in Fig. 3. In addition, as mentioned by the coefficient of the prediction equation, parity affected the ability of the sows to convert NE intake into litter growth (P = 0.008). The efficiency of transferring energy to their litters increased with the age of the sow (P < 0.01, Fig. 3). The low parity group had litters with the lowest weight gains (48.4 kg; Table 6), whereas higher weight gains were achieved by the sows of group 2 (57.1 kg, P = 0.0001). Enzyme supplementation increased litter weight gain (+3.8 kg, P = 0.001; Table 6). Moreover, the effect of enzyme supplementation was higher for the sows from groups 2 to 3 than for the primiparous sows. Body weight gains of litters from sows fed enzyme-supplemented diets were 0.6, 5.3, and 5.4 kg/litter higher than for control for groups 1, 2, and 3 (Fig. 4; P = 0.009). The average effect of enzyme supplementation observed for sows with two or more parities was an 11% increase in total litter BW.
Table 6.
Effect of enzyme supplementation for sows on litter and piglet performance
| Parity group | Enzyme | Statistical analysis1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme | 1 | 2 | 3 | − | + | SEM2 | Parity | Enzyme | Interaction | R 2 |
| Number of observation | 146 | 353 | 485 | 492 | 492 | |||||
| Litter size | ||||||||||
| Birth | 11.3ab | 11.8a | 10.0b | 11.7 | 11.7 | 1.5 | 0.001 | 0.802 | 0.801 | 0.27 |
| Weaning | 10.4a | 10.3a | 10.0b | 10.3 | 10.3 | 1.5 | 0.001 | 0.947 | 0.219 | 0.27 |
| Piglet BW, kg | ||||||||||
| Birth | 1.5 | 1.6 | 1.5 | 1.5 | 1.5 | 0.2 | 0.068 | 0.727 | 0.929 | 0.16 |
| Weaning | 6.5b | 7.0a | 6.9a | 6.7 | 6.9 | 0.9 | <0.0001 | 0.009 | 0.025 | 0.49 |
| Weight gain | 5.0b | 5.3a | 5.4a | 5.2 | 5.4 | 0.8 | <0.0001 | 0.120 | 0.008 | 0.53 |
| Litter weight, kg | ||||||||||
| Birth | 17.0b | 18.7a | 18.1a | 17.8 | 17.9 | 3.3 | <0.0001 | 0.657 | 0.583 | 0.25 |
| Weaning | 65.7c | 75.8a | 69.1b | 67.8 | 70.6 | 13.0 | <0.0001 | 0.001 | 0.122 | 0.44 |
| Weight gain | 48.4b | 57.1a | 50.3ab | 48.4 | 52.2 | 10.7 | <0.0001 | 0.001 | 0.009 | 0.40 |
1From the analysis of variance of 992 results including group parity (n = 3; group 1 includes primiparus, group 2 includes parity 2 and parity 3 and group 3 includes parity 4 and older), assay (n = 8), and enzyme (n = 2). Means with different subscript letter were significantly different P < 0.05.
2SEM for the overall model.
Figure 3.
Effect of NE intake during the lactation period on litter weight gain. Data (n = 389) were subject to a variance analysis including trial (n = 8) as a fixed effect and NE intake (NEI, MJ per lactation) as a covariate. Litter weight gain (kg) = 25.0 * group 3 + 34.8 * group 1 + 35.8 * group 2 + 1.6E−02 * NEI * group 3 − 9.0E−03 * NEI * group 1 − 10.9E−03 * NEI * group 2; R2 = 0.49, SEM = 10.8 kg (group 1 includes primiparus, group 2 includes parity 2, and parity 3 and group 3 includes parity 4 and older, respectively).
Figure 4.
Effect of enzyme supplementation on litter weight gain in relation to parity (group 1 includes primiparus, group 2 includes parity 2 and parity 3, and group 3 includes parity 4 and older).
Body weight gains of individual piglets during the lactation period averaged 5.0, 5.3, and 5.4 kg for litters from the young, intermediate, and old sows, respectively. Consequently, the heaviest piglets were weaned from the oldest sows (the P4 to P8 group) (P < 0.0001). There was no effect of enzyme supplementation on individual piglet BW gain (P = 0.120). However, there was an interaction between parity group and enzyme supplementation (P = 0.008, Table 6).
DISCUSSION
Data from these experiments suggest a negative relationship between NE intake and BW loss during the lactation period, as has been previously reported by O’Grady and Lynch (1978). Body weight loss in sows during lactation occurs as a result of high milk yield and a relatively small appetite (Aherne and Williams, 1992; Lawlor and Lynch, 2007). Increasing energy intake in lactating sows reduces backfat and BW losses, and it increases litter weight gain (Eissen et al., 2003). One way to increase energy intake during lactation is to increase the energy density of the diet by adding fat (O’Grady and Lynch, 1978). Other methods include improving diet digestibility using exogenous enzyme as described by Nortey et al. (2008) and Bindelle et al. (2011) for pigs. In Exp. 3, improving diet digestibility via enzyme supplementation gave results that are consistent with previously reported values for growing pigs. Similarly, the estimated improvement in NE intake, based on the relationship with BW loss (Fig. 1), averaged 0.61 MJ/kg, which is consistent with the improved DE values (0.72 MJ/kg in the diet) measured in association with enzyme supplementation. However, very low DM, OM, and GE digestibility values were obtained in study 3. Discrepancies in the results might be associated with the use of a methodology based on Celite recovery. The addition of NSP-hydrolyzing enzymes to sow diets, especially xylanases, has been shown to increase the total tract and ileal digestibilities of proteins and DM during lactation but has not been shown to have a significant effect during gestation (de Souza et al., 2007). The additional NE intake was partitioned either for growth or reproduction associated with animal age and development. In our study, animal groupings based on energy partitioning distinguished group 2 sows as intermediate between the primiparous (group 1) sows and the high-parity sows (group 3). When fed the control diet, primiparous sows had a lower energy intake, lost more weight and tissue reserves than multiparous sows. These findings agreed with previous results obtained by Eissen et al. (2003) using diet with graded densities. Similar observations were performed for sows fed enzyme-supplemented diets. Primiparous (group 1) and high-parity sows (group 3) fed enzyme-supplemented diets presented significant BW losses reduction, whereas P2 and P3 sows fed diets added with enzymes had similar losses than those fed control diets. Inadequate energy intake during lactation can potentially lead to impaired future performance of sows (Gueblez et al., 1985; Gaughan et al., 1995; Challinor et al., 1996). A greater daily energy intake could ensure the development of primiparous animals, as suggested by the low levels of mobilized body reserves (i.e., muscle and backfat) observed in the present meta-analysis. It might also reduce the weaning-to-service interval, as shown by Koketsu and Dial (1997) and Koketsu et al. (1997). Weight gain of litters from primiparous sows was lower than the corresponding values for multiparous sows. In several previous experiments (Reese et al., 1982; Prunier et al., 1993; Zak et al., 1997), litter weight gain has been shown to be affected by feed intake, especially for primiparous sows. This could be due to the high-nutrient requirements of primiparous sows for growth and to insufficient or nonmobilized body reserves (Reese et al., 1982; Prunier et al., 1993; Zak et al., 1997). Using over-fed sows (+38% vs. ad libitum intake), Pluske et al. (1998) found that the extra feed given to primiparous sows was preferentially used to improve the sow’s body condition rather than for milk production. Indeed, these authors observed that the growth of litters from over-fed to ad libitum-fed sows was similar. The correlations of NE intake with BW change and litter weight gain observed in the present meta-analysis support these previous results. In our current study, enzyme supplementation caused a large decrease in body reserve mobilization in young sows, as has been suggested by Pluske et al. (1998), which could be crucial for subsequent reproductive cycles. No difference in litter weight gain for primiparous animal fed enzyme-supplemented diets was also in agreement with previous results suggested (Reese et al., 1982; Prunier et al., 1993; Zak et al., 1997).
Many studies have reported the potential of multiparous sows to produce larger and heavier litters than primiparous sows (French et al., 1979). Multiparous sows had greater body reserves, especially of backfat, and had less BW loss than did primiparous sows. As a consequence, the effect of enzyme supplementation was less pronounced for multiparous sows than it was for primiparous sows in terms of BW and backfat change. However, feeding sows with a NSP-hydrolyzing enzyme supplement with a broad enzymatic spectrum, as this is the case of studies reported in the meta-analysis, resulted in enhanced litter weights in multiparous sows, which could be explained by increased levels of nutrient available for milk production. Indeed, Matzat et al. (1990) found that lactating multiparous sows fed excess feed via the gastric infusion of nutrients produced more milk and grew their litters at a faster rate than sows fed ad libitum. The authors concluded that multiparous sows responded to an increase in nutrient intake by increasing nutrient output via greater milk production. The differences in the way gilts and older sows partition additional energy expenditures above the level required to maintain a zero-energy balance is most likely due to the continuing growth of gilts, whereas multiparous sows have already reached their mature body size. A previous study showed that feeding high-energy and high-protein diets to multiparous lactating sows increased their protein intake but not their energy intake and that it increased litter weight at weaning (Brendemuhl et al., 1987). However, in two previous experiments (Prunier et al., 1993; Zak et al., 1997), litter weight gains have not resulted from increased feed intake. This can be explained by the fact that in these studies, sows were restrictively fed and, that during gestation, they tended to mobilize more body reserves to compensate for the shortfall in nutrients arising from reduced feed intake during lactation. These results are in agreement with those of the present work. In these previous studies, feed intake did not appear to be the primary limiting factor, whereas digestibility did seem to be limiting. Increased nutrient release was associated with an increase in the energy available to support litter weight gain.
In conclusion, feeding enzyme-supplemented diets to sows increased their energy intake during lactation. Enzyme supplementation during lactation attenuated the loss of body reserves, resulting in higher levels of backfat at weaning in primiparous sows. In multiparous sows, the extra energy intake as a result of enzyme supplementation was reallocated to increasing litter weight gain, resulting in an increase in piglet BW at weaning.
LITERATURE CITED
- Aherne F. X. and Williams I. H.. 1992. Nutrition for optimizing breeding herd performance. Vet. Clin. North Am. Food Anim. Pract. 8:589–608. doi: 10.1016/S0749-0720(15)30706-4 [DOI] [PubMed] [Google Scholar]
- AOAC 1990. Official methods of analysis. 15th ed Association of Official Analytical Chemists, Arlington, VA. [Google Scholar]
- Bach Knudsen K. E., and Jorgensen H.. 2001. Intestinal degradation of dietary carbohydrates from birth to maturity. In: L. E. Lindberg, and B. Ogle, editors. Digestive physiology of pigs. CABI Publishing, Wallingford: p 109–121. [Google Scholar]
- Barrera M., Cervantes M., Sauer W. C., Araiza A. B., Torrentera N., and Cervantes M.. 2004. Ileal amino acid digestibility and performance of growing pigs fed wheat-based diets supplemented with xylanase. J. Anim. Sci. 82:1997–2003. doi: 10.2527/2004.8271997x [DOI] [PubMed] [Google Scholar]
- Bindelle J., Pieper R., Montoya C. A., Van Kessel A. G., and Leterme P.. 2011. Nonstarch polysaccharide-degrading enzymes alter the microbial community and the fermentation patterns of barley cultivars and wheat products in an in vitro model of the porcine gastrointestinal tract. FEMS Microbiol. Ecol. 76:553–563. doi: 10.1111/j.1574-6941.2011.01074.x [DOI] [PubMed] [Google Scholar]
- Brendemuhl J. H., Lewis A. J., and Peo E. R. Jr. 1987. Effect of protein and energy intake by primiparous sows during lactation on sow and litter performance and sow serum thyroxine and urea concentrations. J. Anim. Sci. 64:1060–1069. [DOI] [PubMed] [Google Scholar]
- Brooks P. H., Cole D. J. A., and Rowlinson P., Croxson V. J., and Luscombe J. R.. 1975. Studies in sow reproduction. 3. The effect of nutrition between weaning and remating on the reproductive performance of multiparous sows. Anim. Prod. 20:407–412. doi: 10.1017/S0003356100041209 [DOI] [Google Scholar]
- Challinor C. M., Dams G., Edwards B., and Close W. H.. 1996. The effect of body condition of gilts at first mating on long-term sow productivity. Anim. Sci. 62:660. (Abstr.) [Google Scholar]
- Clowes E. J., Aherne F. X., Schaefer A. L., Foxcroft G. R., and Baracos V. E.. 2003. Parturition body size and body protein loss during lactation influence performance during lactation and ovarian function at weaning in first-parity sows. J. Anim. Sci. 81:1517–1528. doi: 10.2527/2003.8161517x [DOI] [PubMed] [Google Scholar]
- Diebold G., Mosenthin R., Piepho H. P., and Sauer W. C.. 2004. Effect of supplementation of xylanase and phospholipase to a wheat-based diet for weanling pigs on nutrient digestibility and concentrations of microbial metabolites in ileal digesta and feces. J. Anim. Sci. 82:2647–2656. doi: 10.2527/2004.8292647x [DOI] [PubMed] [Google Scholar]
- Dourmad J. Y., Etienne M., Prunier A., and Noblet J.. 1994. The effect of energy and protein intake of sows on their longevity: a review. Livest. Prod. Sci. 40:87–97. doi:10.1016/0301-6226(94)90039-6 [Google Scholar]
- Eissen J. J., Apeldoorn E. J., Kanis E., Verstegen M. W., and de Greef K. H.. 2003. The importance of a high feed intake during lactation of primiparous sows nursing large litters. J. Anim. Sci. 81:594–603. doi: 10.2527/2003.813594x [DOI] [PubMed] [Google Scholar]
- Eissen J. J., Kanis E., and Kemp B.. 2000. Sow factors affecting voluntary feed intake during lactation. Liv. Prod. Sci. 64:147–165. doi:10.1016/S0301-6226(99)00153-0 [Google Scholar]
- Emiola I. A., Opapeju F. O., Slominski B. A., and Nyachoti C. M.. 2009. Growth performance and nutrient digestibility in pigs fed wheat distillers dried grains with solubles-based diets supplemented with a multicarbohydrase enzyme. J. Anim. Sci. 87:2315–2322. doi: 10.2527/jas.2008-1195 [DOI] [PubMed] [Google Scholar]
- Francesch M. and Geraert P. A.. 2009. Enzyme complex containing carbohydrases and phytase improves growth performance and bone mineralization of broilers fed reduced nutrient corn-soybean-based diets. Poult. Sci. 88:1915–1924. doi: 10.3382/ps.2009-00073 [DOI] [PubMed] [Google Scholar]
- French L. R., Rutledge J. J., and First N. L.. 1979. Effect of age and parity on litter size in pigs. J. Reprod. Fertil. 57:59–60. [DOI] [PubMed] [Google Scholar]
- Gaughan J. B., Cameron R. D. A., Dryden G. McL., and Josey M. J.. 1995. Effect of selection for leanness on overall reproductive performance in Large White sows. Anim. Sci. 61:561–564. doi: 10.1017/S1357729800014144 [DOI] [Google Scholar]
- Gueblez R., Gestin J. M., and Le Henaff G.. 1985. Incidence de l’age et de l’epaisseur de lard dorsal a 100kg sur la carriere reproductive des truies Large White. Jour. Rech. Porc. Fr. 17:113–120. [Google Scholar]
- Hughes P. E. 1993. The effects of food level during lactation and early gestation on the reproductive performance of mature sows. Anim. Prod. 57:437–445. doi:10.1017/S1357729800042776 [Google Scholar]
- Kim J. C., Simmins P. H., Mullan B. P., and Pluske J. R.. 2005. The digestible energy value of wheat for pigs, with special reference to the post-weaned animal [Review]. Anim. Feed Sci. Technol. 122:257–287. doi: 10.1016/j.anifeedsci.2006.09.016 [DOI] [Google Scholar]
- Koketsu Y. and Dial G. D.. 1997. Factors influencing the postweaning reproductive performance of sows on commercial farms. Theriogenology. 47:1445–1461. [DOI] [PubMed] [Google Scholar]
- Koketsu Y., Dial G. D., and King V. L.. 1997. Influence of various factors on farrowing rate on farms using early weaning. J. Anim. Sci. 75:2580–2587. doi:10.1016/S0093-691X(97)00135-0 [DOI] [PubMed] [Google Scholar]
- Lawlor P. G. and Lynch P. B.. 2007. A review of factors influencing litter size in Irish sows. Ir. Vet. J. 60:359–366. doi: 10.1186/2046-0481-60-6-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzat P. D., Hogberg M. G., Fogwell R. L., and Miller E. R.. 1990. Lactation performance in high producing gilts fed in excess of ad libitum. In: Report of swine research, AS-SW-8904. Michigan State University, East Lansing, MI: p. 36–40. [Google Scholar]
- Nortey T. N., Patience J. F., Sands J. S., Trottier N. L., and Zijlstra R. T.. 2008. Effects of xylanase supplementation on the apparent digestibility and digestible content of energy, amino acids, phosphorus, and calcium in wheat and wheat by-products from dry milling fed to grower pigs. J. Anim. Sci. 86:3450–3464. doi: 10.2527/jas.2007-0472 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. National Academic Press, Washington, DC. [Google Scholar]
- O’Grady J. F., and Lynch P. B.. 1978. Voluntary feed intake by lactating sows: influence of system of feeding and nutrient density of the diet. Ir. J. Agri. Res. 17:1–5. [Google Scholar]
- Pluske J. R., Williams I. H., Zak L. J., Clowes E. J., Cegielski A. C., and Aherne F. X.. 1998. Feeding lactating primiparous sows to establish three divergent metabolic states: III. Milk production and pig growth. J. Anim. Sci. 76:1165–1171. doi:10.2527/1998.7641165x [DOI] [PubMed] [Google Scholar]
- Prunier A., Dourmad J. Y., and Etienne M.. 1993. Feeding level, metabolic parameters and reproductive performance of primiparous sows. Liv. Prod. Sci. 37:185–196. doi: 10.1016/0301-6226(93)90071-O [DOI] [Google Scholar]
- Reese D. E., Moser B. D., Peo E. R. Jr, Lewis A. J., Zimmerman D. R., Kinder J. E., and Stroup W. W.. 1982. Influence of energy intake during lactation on the interval from weaning to first estrus in sows. J. Anim. Sci. 55:590–598. [DOI] [PubMed] [Google Scholar]
- Revell D. K., Williams I. H., Mullan B. P., Ranford J. L., and Smits R. J.. 1998. Body composition at farrowing and nutrition during lactation affect the performance of primiparous sows: II. Milk composition, milk yield, and pig growth. J. Anim. Sci. 76:1738–1743. doi:10.2527/1998.7671738x [DOI] [PubMed] [Google Scholar]
- SAS 2000. SAS user’s guide: statistics. SAS Institute Inc, Cary, NC. [Google Scholar]
- de Souza A. L. P., Lindemann M. D., and Cromwell G. L.. 2007. Supplementation of dietary enzymes has varying effects on apparent protein and amino acid digestibility in reproducing sows. Livest. Sci. 109:122–124. doi: 10.1016/j.livsci.2007.01.113 [DOI] [Google Scholar]
- St-Pierre N. R. 2001. Invited review: integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 84:741–755. doi: 10.1017/S1751731108002280 [DOI] [PubMed] [Google Scholar]
- Sterk A., Verdonk A. J., Mul A. J., Soenen B., Bezençon M. L., Frehner M., and Losa R.. 2007. Effect of xylanase supplementation to a cereal-based diet on the apparent faecal digestibility in weanling piglets. Liv. Sci. 108:269–271. Doi: 10.1016/j.livsci.2007.01.077. [DOI] [Google Scholar]
- Tantasuparuk W., Lundeheim N., Dalin A.-M., Kunavongkrit A., and Einarsson S.. 2001. Weaning-to-service interval in primiparous sows and its relationship with longevity and piglet production. Liv. Prod. Sci. 69:155–162. doi: 10.1016/S0301-6226(00)00256-6 [DOI] [Google Scholar]
- Thaker M. Y. and Bilkei G.. 2005. Lactation weight loss influences subsequent reproductive performance of sows. Anim. Reprod. Sci. 88:309–318. doi: 10.1016/j.anireprosci.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Trotter P. J., and Johnston L. J.. 2001. Feeding gilts during development and sows during gestation and lactation. In: Lewis A. J., and Southern L. L., editors. Swine nutrition. CRC Press, Washington, DC. [Google Scholar]
- Walsh M. C., Geraert P. A., Maillard R., Kluess J., and Lawlor P. G.. 2012. The effect of a non-starch polysaccharide-hydrolysing enzyme (Rovabio® Excel) on feed intake and body condition of sows during lactation and on progeny growth performance. Animal 6:1627–1633. doi: 10.1017/S1751731112000237 [DOI] [PubMed] [Google Scholar]
- Zak L. J., Cosgrove J. R., Aherne F. X., and Foxcroft G. R.. 1997. Pattern of feed intake and associated metabolic and endocrine changes differentially affect postweaning fertility in primiparous lactating sows. J. Anim. Sci. 75:208–216. doi:10.2527/1997.751208x [DOI] [PubMed] [Google Scholar]