Abstract
The objective of this study was to investigate the effects of Lactobacillus reuteri LR1, a new strain isolated from the feces of weaned pigs, on the growth performance, intestinal morphology, immune responses, and intestinal barrier function in weaned pigs. A total of 144 weaned pigs (Duroc × Landrace × Yorkshire, 21 d of age) with an initial BW of 6.49 ± 0.02 kg were randomly assigned to 3 dietary treatments with 8 replicate pens, each of per treatment and 6 pigs. Pigs were fed a basal diet (CON, controls), the basal diet supplemented with 100 mg/kg olaquindox and 75 mg/kg aureomycin (OA) or the basal diet supplemented with 5 × 1010 cfu/kg L. reuteri LR1 for a 14-d period. At the end of study, the ADG, ADFI, and G:F were calculated, and 1 randomly selected pig from each pen was euthanized for sample collection. The LR1 increased ADG (22.73%, P < 0.05) compared with CON. The villus height of the ileum was increased (P < 0.05) and crypt depth in duodenum was reduced (P < 0.05), along with increased (P < 0.05) villus height to crypt depth ratio of the jejunum and ileum by LR1 compared with CON and OA. LR1 increased (P < 0.05) ileal mucosal content of IL-22 and transforming growth factor-β compared with OA. Compared with CON, LR1 increased (P < 0.05) and OA decreased (P < 0.05) the ileal content of secretory immunoglobulin A (sIgA), and the abundance of transcripts of porcine β-defensin 2 and protegrin 1-5. Compared with CON, LR1 increased (P < 0.05) tight junction protein zonula occludens-1 and occludin transcripts in the mucosa of the jejunum and ileum, and those of mucin-2 in ileal mucosa. The relative expression of toll-like receptor 2 (TLR2) and TLR4 were increased (P < 0.05) in ileal mucosa in pigs fed LR1 compared with CON. In conclusion, these data indicated that dietary LR1 supplementation at 5 × 1010 cfu/kg improved growth performance, intestinal morphology, and intestinal barrier function in weaned pigs.
Keywords: antibiotics, intestinal barrier function, Lactobacillus reuteri LR1, weaned pig
INTRODUCTION
Weaning stress causes intestinal inflammation and intestinal barrier disruptions in pigs, leading to diarrhea and decreased growth performance (Smith et al., 2010; Hu et al., 2013). Although antibiotics have long been used to prevent diarrhea or to promote growth in pigs, increasing bacterial resistance and public health concerns is limiting their use (Ashiru-Oredope and Hopkins, 2015). Additionally, recent studies have showed that antibiotics disrupt the intestinal barrier function by decreasing the expression of tight junction (TJ) proteins, antimicrobial peptides, and mucins in the intestine (Wlodarska et al., 2011b; Yi et al., 2016). However, antibiotics reduction increases postweaning diarrhea, mortality, and feed costs in swine production. There is an urgent need to find alternatives to antibiotics. Lactobacilli reuteri strains are important members of the intestinal commensal microbiota (Wegmann et al., 2015). Previous studies in mice showed that L. reuteri enhanced the intestinal barrier function by improving expression of TJ proteins (Ahl et al., 2016). In addition, L. reuteri I5007 enhanced intestinal barrier function in newborn pigs via improving gene expression of TJ proteins (Hou et al., 2014; Yang et al., 2015). Previous studies in this laboratory have shown that a new strain of L. reuteri isolated from the feces of healthy pigs (LR1) showed notable acid and bile resistance, antibacterial activity, and adherence to intestinal porcine epithelial cells, and increased the expression of zonula occludens 1 (ZO-1) in intestinal epithelial cells (Wang et al., 2016). However, the possible effects of L. reuteri LR1 on growth performance and intestinal barrier function in weaned pigs in vivo remain unknown. Thus, the objective of this study was to investigate the effects of L. reuteri LR1 on the growth performance, intestinal morphology, immune responses, and intestinal barrier function in weaned pigs.
MATERIALS AND METHODS
All animal protocols used in this study were in accordance with the Guidelines for the Care and Use of Animals for Research and Teaching and approved by the Animal Care and Use Committee of Guangdong Academy of Agricultural Sciences.
Animals and Experimental Design
A total of 144 weaned pigs (Duroc × Landrace × Yorkshire, 21 d of age) with an initial BW of 6.49 ± 0.02 kg were balanced for sex and then randomly assigned to 3 dietary treatments with 8 replicate pens per treatment, each pen containing 3 barrows and 3 gifts with a complete randomized experimental design. Control pigs (CON) were fed a basal diet, and the others were fed the basal diet supplemented with 100 mg/kg olaquindox plus 75 mg/kg aureomycin (OA), or the basal diet supplemented with 5 × 1010 cfu/kg L. reuteri LR1 for a 14-d period. The basal diet was formulated to meet nutritional requirements based on pig BW (NRC, 2012), and the ingredients and composition of the basal diet are listed in Table 1. All pigs were allowed ad libitum access to feed and water throughout. At the end of study, the ADG, ADFI, and G:F were calculated. One randomly selected pig from each pen within a treatment was sacrificed after anesthesia by i.m. injection with sodium pentobarbital (40 mg/kg BW). Samples of the middle duodenum, the middle jejunum, and distal ileum were carefully collected, rinsed with PBS, and fixed in 4% paraformaldehyde. Other segments of the middle jejunum and distal ileum were opened and thoroughly rinsed with sterile normal saline, and then mucosa was collected by scraping with glass slides and immediately snap-frozen in liquid nitrogen and stored at −80 oC.
Table 1.
The formulations and chemical composition of the basal diet (as-fed basis)
| Ingredient | % |
|---|---|
| Corn, 8% | 35.31 |
| Extrude corn | 15.00 |
| Fermented soybean meal, 54% | 9.00 |
| Expanded soybean | 10.00 |
| Fish meal | 4.00 |
| Whey powder | 11.00 |
| Soybean hull | 5.00 |
| Soybean oil | 1.20 |
| Blood plasma | 3.00 |
| Sucrose | 2.00 |
| Choline chloride | 0.20 |
| Salt | 0.45 |
| Monocalcium phosphate | 0.62 |
| Limestone | 0.65 |
| L-Lys HCl | 0.60 |
| DL-Met | 0.22 |
| L-Thr | 0.21 |
| L-Trp | 0.04 |
| Premix1 | 1.50 |
| Total | 100 |
| Energy and nutrient composition | |
| DE, kcal/kg | 3550 |
| CP (analyzed), % | 19.30 |
| Ca (analyzed), % | 0.70 |
| Total P (analyzed), % | 0.56 |
| STTD of P, % | 0.39 |
| Lys (analyzed), % | 1.55 |
| Met + Cys, % | 0.89 |
| Thr, % | 0.97 |
| Trp, % | 0.26 |
1Provided vitamin and mineral premix per kg of diet: vitamin A = 2400 IU; vitamin D3 = 2800 IU; vitamin E = 200 IU; vitamin K3 = 5 mg; vitamin B12 = 40 μg; vitamin B1 = 3 mg; vitamin B2 = 10 mg; niacin = 40 mg; pantothenic acid = 15 mg; folic acid = 1 mg; vitamin B6 = 8 mg; biotin = 0.08 mg; Fe (FeSO4•H2O) = 120 mg; Cu (CuSO4•5H2O) = 16 mg; Mn (MnSO4•H2O) = 70 mg; Zn (ZnSO4•H2O) = 120 mg; I (CaI2O6) = 0.7 mg; Se (Na2SeO3) = 0.48 mg.
Analysis of Intestinal Morphology
The fixed intestinal segments were dehydrated, embedded in paraffin, and sectioned for intestinal morphology as described before (Gao et al., 2013). Briefly, sections of 5-μm thickness were deparaffinized in xylene, rehydrated, and stained with hematoxylin and eosin (H&E). Images were obtained using an Axio Scope A1 microscope (Zeiss, Germany). The villous height and crypt depth of each segment were measured with Image-Pro software (Media Cybernetics, Rockville, MD). A minimum of 9 villi from each sample were measured for each treatment. Mean villus height, crypt depth, and villus height to crypt depth ratio for each pig were used for analysis.
Intestinal Cytokines Determined by ELISA
After grinding in liquid nitrogen, total protein was extracted from the jejunal and ileal mucosa samples with a lysis buffer (KeyGEN, Nanjing, China), followed by clarification by centrifugation; protein content in supernatants was determined using a BCA protein assay kit (KeyGEN). The concentrations of IL-6, IL-8, IL-10, and IL-22 were determined in duplicate using porcine ELISA kits (Raybiotech, Norcross, GA) as described before (Yi et al., 2016). In addition, the level of transforming growth factor-β (TGF-β) and secretory IgA (sIgA) was detected in duplicate using porcine ELISA kits (Cusabio Biotech Co., Ltd, Wuhan, China) as described previously (Gao et al., 2013). These data were expressed as pg/mg protein.
Real-Time PCR for Relative Measurement of Transcripts
Total RNA was extracted from mucosal tissue powders using TRIzol reagent (Invitrogen, Carlsbad, CA). The purity and the yield of the RNA were evaluated using NanoDrop 1000 (Thermo Fisher Scientific, Waltham, MA). The integrity of RNA was determined by electrophoresis in 1% agarose gels, and 1 μg of RNA was used to generate cDNA in a volume of 20 μL with a PrimeScript RT Reagent Kit (Takara, Dalian, China). Real-time PCR was performed in duplicate with SYBR Green master mix in a CFX Connect Detection system (Bio-Rad, Hercules, CA). Each 10-μL reaction included 5-μL iTaq Universal SYBR Green Supermix (Bio-Rad), 0.5-μL forward primer (10 μM), 0.5-μL reverse primer (10 μM), and 4-μL 20-fold diluted cDNA. The thermocycler protocol consisted of 10 min at 95 °C followed by 40 cycles of 30 s at 95 °C, 30 s at 60 °C, and 20 s at 72 °C. GAPDH and β-actin were used as housekeeping genes, and the primers for the real-time PCR in this study are listed in Table 2. The relative mRNA expression of the target genes was determined using the 2–ΔΔCt method, and data for each target transcript were normalized to the control pigs (1.0).
Table 2.
Primers for real-time PCR in this study
| Gene1 | Sequence (5ʹ–3ʹ) | Size(bp) | Accession number |
|---|---|---|---|
| ZO-1 | Forward: AGCCCGAGGCGTGTTT Reverse: GGTGGGAGGATGCTGTTG |
147 | XM_013993251 |
| Occludin | Forward: GCACCCAGCAACGACAT Reverse: CATAGACAGAATCCGAATCAC |
144 | XM_005672525 |
| Mucin-2 | Forward: CTGCTCCGGGTCCTGTGGGA Reverse: CCCGCTGGCTGGTGCGATAC |
100 | XM_007465997.1 |
| pBD2 | Forward: CCAGAGGTCCGACCACTACA Reverse: GGTCCCTTCAATCCTGTTGAA |
88 | AY506573.1 |
| PG1-5 | Forward: GTAGGTTCTGCGTCTGTGTCG Reverse: CAAATCCTTCACCGTCTACCA |
166 | XM_005669497.2 |
| NOD1 | Forward: ACCGATCCAGTGAGCAGATA Reverse: AAGTCCACCAGCTCCATGAT |
140 | NM_001114277.1 |
| NOD2 | Forward: CCTTTTGAAGATGCTGCCTG Reverse: GATTCTCTGCCCCATCGTAG |
100 | NM_001105295.1 |
| TLR2 | Forward: TCACTTGTCTAACTTATCATCCTCTTG Reverse: TCAGCGAAGGTGTCATTATTGC |
162 | NM_213761.1 |
| TLR4 | Forward: GCCATCGCTGCTAACATCATC Reverse: CTCATACTCAAAGATACACCATCGG |
108 | NM_001113039.1 |
| β-actin | Forward: CTGCGGCATCCACGAAACT Reverse: AGGGCCGTGATCTCCTTCTG |
380 | XM_003124280 |
| GAPDH | Forward: ACTCACTCTTCCACTTTTGATGCT Reverse: TGTTGCTGTAGCCAAATTCA |
100 | NM_001206359 |
1ZO-1 = zonula occludens-1; pBD2 = porcine β defensin 2; PG1-5 = protegrin 1-5; NOD = nucleotide binding oligomerization domain; TLR = toll-like receptor; GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
Immunofluorescence
Paraformaldehyde-fixed and paraffin-embedded sections of the ileum were used for immunofluorescence examination, as described (Yi et al., 2017). Briefly, sections of 5-μm thickness were deparaffinized and rehydrated and then processed for antigen retrieval. The sections were incubated in the dark for 20 min with 3% hydrogen peroxide. The sections were then incubated sequentially with each primary antibody (rabbit polyclonals, 1:200, anti-ZO-1, Abcam Inc., Cambridge, MA and anti-mucin-2, GeneTex Inc., Irvine, CA) and then with 1:50 CY3-labeled goat antirabbit IgG (Abcam). Nuclei were stained with DAPI. Images were obtained using an ECLIPSE TI-SR microscope with a DS-U3 Image-Pro system (Nikon, Tokyo, Japan).
Statistical Analyses
Replicate (n = 8) served as the experimental unit. Data were analyzed using the general linear model (GLM) with SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The effect of dietary treatment was assessed by one-way ANOVA followed by Tukey’s test. All data are expressed as the means ± SEM. Differences were considered to be significant at P < 0.05.
RESULTS
Effects of LR1 on the Growth Performance in Weaned Pigs
The LR1 treatment increased ADG (22.7%, P < 0.05) above CON (Table 3), similar to the effect of OA on ADFI (17.8%, P < 0.05) and ADG (29.6%, P < 0.05) compared with CON.
Table 3.
Effects of LR1 on the growth performance in weaned pigs
| Item | CON | OA | LR1 | SEM | P value |
|---|---|---|---|---|---|
| Initial BW, kg | 6.50 | 6.48 | 6.49 | 0.01 | 0.611 |
| Finial BW, kg | 9.27b | 10.07a | 9.89a | 0.11 | 0.002 |
| ADFI, g/d | 310b | 365a | 358ab | 11 | 0.002 |
| ADG, g/d | 198b | 256a | 243a | 8 | 0.065 |
| G:F | 0.64 | 0.70 | 0.68 | 0.01 | 0.148 |
CON = a basal diet; OA = a basal diet supplemented with 100 mg/kg olaquindox + 75 mg/kg aureomycin; LR1 = a basal diet supplemented with 5 × 1010 cfu/kg L. reuteri LR1.
a bMeans lacking common superscript letter indicated significant differences (P < 0.05) within a row.
Effects of LR1 on Intestinal Morphology of Weaned Pigs
The LR1 reduced (P < 0.05) jejunal crypt depth and both LR1 and OA increased (P < 0.05) villus height to crypt depth ratio compared with CON (Table 4). In addition, LR1 increased (P < 0.05) both the villus height and the villus height to crypt depth ratio of the ileum over those in CON and OA pigs.
Table 4.
Effects of LR1 on intestinal morphology in weaned pigs
| Item | CON | OA | LR1 | SEM | P value |
|---|---|---|---|---|---|
| Duodenum | |||||
| Villus height, μm | 378 | 378 | 413 | 13 | 0.447 |
| Crypt depth, μm | 229 | 204 | 217 | 10 | 0.392 |
| Villus height/crypt depth | 1.68 | 1.86 | 1.94 | 0.07 | 0.354 |
| Jejunum | |||||
| Villus height, μm | 377 | 412 | 421 | 14 | 0.403 |
| Crypt depth, μm | 239a | 199ab | 167b | 11 | 0.012 |
| Villus height/crypt depth | 1.62c | 2.13b | 2.54a | 0.11 | 0.000 |
| Ileum | |||||
| Villus height, μm | 316b | 340b | 405a | 13 | 0.004 |
| Crypt depth, μm | 166 | 186 | 154 | 6 | 0.140 |
| Villus height/crypt depth | 1.95b | 1.85b | 2.71a | 0.14 | 0.011 |
CON = a basal diet; OA = a basal diet supplemented with 100 mg/kg olaquindox + 75 mg/kg aureomycin; LR1 = a basal diet supplemented with 5 × 1010 cfu/kg L. reuteri LR1.
a b cMeans lacking common superscript letter indicated significant differences (P < 0.05) within a row.
Effects of LR1 on Intestinal Cytokines of Weaned Pigs
To evaluate the LR1’s effects on immune responses in the intestine of weaned pigs, the levels of cytokine IL-6, IL-8, IL-10, IL-22, and TGF-β in the mucosa of jejunum and ileum were determined (Table 5). The LR1 increased (P < 0.05) ileal contents of TGF-β, compared with CON and OA. Additionally, LR1 increased (P < 0.05) ileal contents of IL-22 compared with OA.
Table 5.
Effects of LR1 on cytokines production in intestines in weaned pigs
| Item1 | CON | OA | LR1 | SEM | P value |
|---|---|---|---|---|---|
| Jejunal mucosa | |||||
| IL-6 | 5.49 | 5.36 | 3.67 | 0.68 | 0.499 |
| IL-8 | 72.80 | 61.43 | 42.71 | 6.57 | 0.171 |
| IL-10 | 6.62 | 7.02 | 5.36 | 0.59 | 0.514 |
| IL-22 | 4.96 | 4.17 | 2.99 | 0.43 | 0.170 |
| TGF-β | 18.10 | 13.84 | 11.01 | 1.41 | 0.115 |
| Ileal mucosa | |||||
| IL-6 | 4.59 | 4.49 | 4.99 | 0.46 | 0.905 |
| IL-8 | 51.62 | 55.41 | 65.97 | 4.80 | 0.472 |
| IL-10 | 6.76 | 6.82 | 9.37 | 0.77 | 0.300 |
| IL-22 | 4.30a,b | 3.95b | 6.53a | 0.49 | 0.058 |
| TGF-β | 15.51b | 15.91b | 37.61a | 3.87 | 0.019 |
CON = a basal diet; OA = a basal diet supplemented with 100 mg/kg olaquindox + 75 mg/kg aureomycin; LR1 = a basal diet supplemented with 5 × 1010 cfu/kg L. reuteri LR1. Data are normalized as pg/1 mg protein of tissues.
1TGF-β = transforming growth factor-β.
a,bMeans lacking common superscript letter indicated significant differences (P < 0.05) within a row.
Effects of LR1 on Intestinal Barrier Function in Weaned Pigs
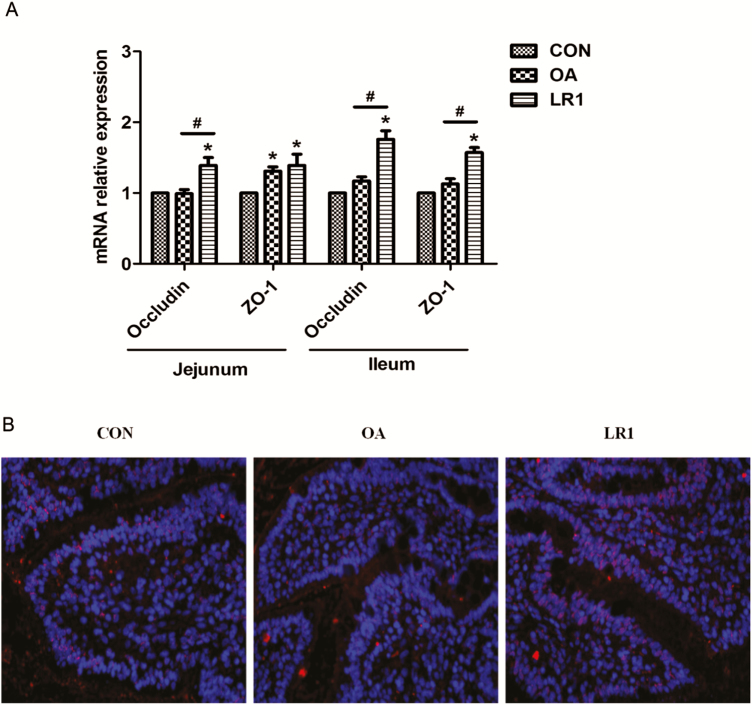
Given the roles of IL-22 and TGF-β in intestinal barrier function, the effects of LR1 on sIgA and the expression of genes encoding TJ proteins, antimicrobial peptides, and mucins in the mucosa of the jejunum and ileum were examined. For TJ-related components, LR1 increased (P < 0.05) ZO-1 and occludin transcripts in jejunal and ileal mucosa compared with CON (Figure 1A) and OA increased jejunal mucosal expression of ZO-1. When the 2 supplemented diets were compared, LR1 had more (P < 0.05) ZO-1 and occludin transcripts in ileal mucosa and occludin transcripts in jejunal mucosa. The protein expression of ZO-1 in the ileum was confirmed by immunofluorescence (Figure 1B).
Figure 1.
LR1 increased the tight junction protein expression in the intestine of weaned pigs. (A) Relative mRNA expression levels of zonula occludens-1 (ZO-1) and occludin in the jejunal and ileal mucosa determined by real-time PCR. (B) The ZO-1 expression in the ileal mucosa confirmed using immunofluorescence. Red, ZO-1; blue, DAPI; 200×. All data are expressed as the mean ± SEM (n = 8). Differences were determined by one-way ANOVA followed by Tukey’s test. *P < 0.05 compared with CON, #P < 0.05 LR1 compared with OA. CON, a basal diet; OA, a basal diet supplemented with 100 mg/kg olaquindox + 75 mg/kg aureomycin; LR1, a basal diet supplemented with 5 × 1010 cfu/kg L. reuteri LR1.
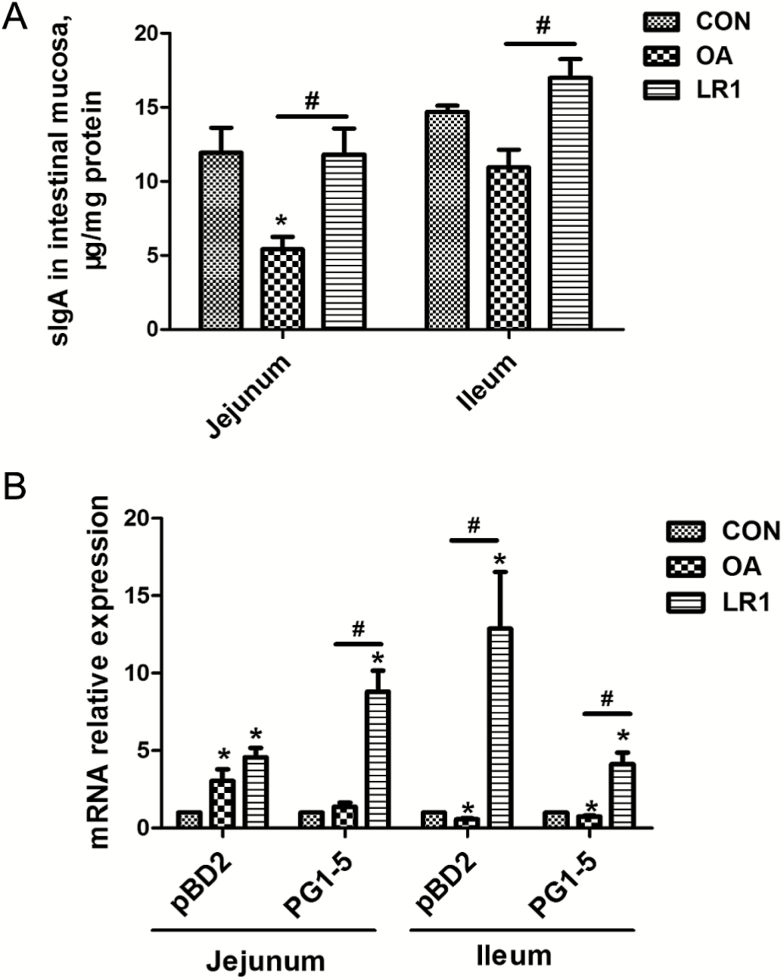
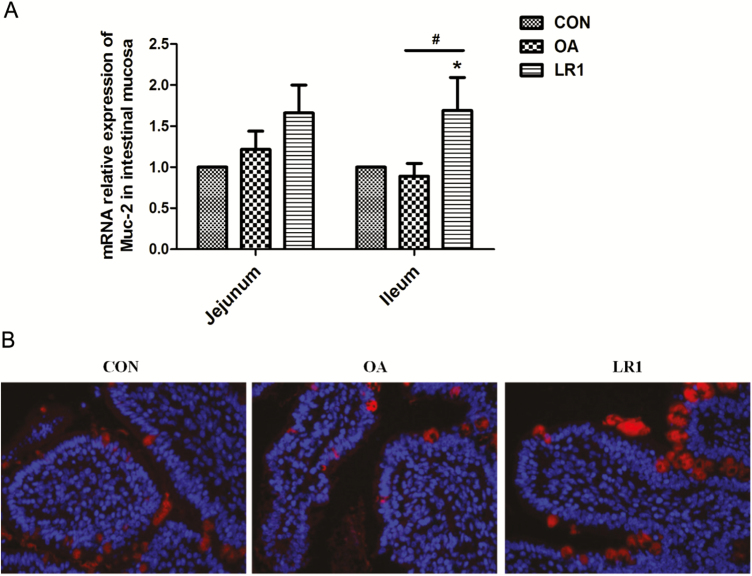
With regard to antimicrobial protein, OA, but not LR1, decreased (P < 0.05) the sIgA content of the jejunal mucosa from that in CON (Figure 2A); concentrations of sIgA in jejunal and ileal mucosa in LR1 pigs exceeded (P < 0.05) those of OA and were the same or greater than in CON. Transcripts of porcine β defensin 2 (pBD2) and protegrin 1-5 (PG1-5) in jejunal and ileal mucosa were increased (P < 0.05) in pigs fed LR1, compared with CON (Figure 2B). However, OA decreased (P < 0.05) pBD2 and PG1-5 in ileal mucosa compared with CON. Mucin-2 transcripts in ileal mucosa were greatest (P < 0.05) in pigs fed LR1 compared with both CON and OA pigs (Figure 3A). These results on ileal mucin-2 with LR1 were confirmed at the protein level by immunofluorescence (Figure 3B).
Figure 2.
LR1 improved the antimicrobial proteins expression in the intestine of weaned pigs. (A) Levels of sIgA in the jejunal and ileal mucosa determined by ELISA. (B) Relative mRNA expression levels of pBD2 and PG1-5 in the jejunal and ileal mucosa determined by real-time PCR. All data are expressed as the mean ± SEM (n = 8). Differences were determined by one-way ANOVA followed by Tukey’s test. *P < 0.05 compared with CON, #P < 0.05 LR1 compared with OA. CON, a basal diet; OA, a basal diet supplemented with 100 mg/kg olaquindox + 75 mg/kg aureomycin; LR1, a basal diet supplemented with 5 × 1010 cfu/kg L. reuteri LR1.
Figure 3.
LR1 increased mucin-2 expression in the intestine of weaned pigs. (A) Relative mRNA expression level of mucin-2 in the jejunal and ileal mucosa determined by real-time PCR. All data are expressed as the mean ± SEM (n = 8). Differences were determined by one-way ANOVA followed by Tukey’s test. *P < 0.05 compared with CON, #P < 0.05 LR1 compared with OA. (B) The mucin-2 expression in the mucosa of the jejunum was confirmed using immunofluorescence. Red, mucin-2; blue, DAPI; 200×. CON, a basal diet; OA, a basal diet supplemented with 100 mg/kg olaquindox + 75 mg/kg aureomycin; LR1, a basal diet supplemented with 5 × 1010 cfu/kg L. reuteri LR1.
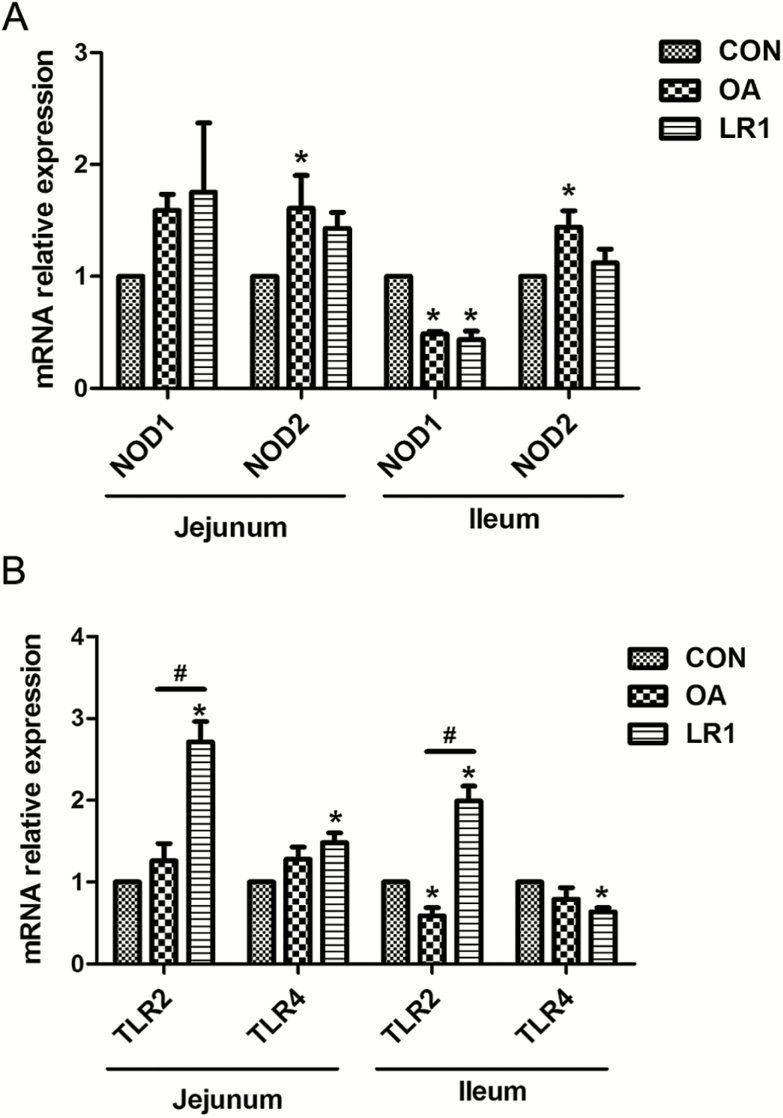
Effects of LR1 on toll-like receptors (TLRs) and nucleotide binding oligomerization domain (NODs) in Intestines of Weaned Pigs
The possible mechanism through which LR1 influenced intestinal barrier functions was examined using relative transcript abundance of genes encoding toll-like receptor-2 and -4 (TLR2, TLR4), and nucleotide binding oligomerization domains 1 and 2 (NOD1, NOD2) in the intestines of weaned pigs (Figure 4). The expression of NOD2 in jejunal and ileal mucosa was increased (P < 0.05) by OA, and both OA and LR1 decreased (P < 0.05) NOD1 expression in jejunal mucosa compared with CON (Figure 4A). In terms of TLRs expression, relative abundance of TLR2 and TLR4 transcripts in jejunal mucosa was increased (P < 0.05) in pigs fed LR1 compared with CON (Figure 4B). Comparing the supplements, TLR2 in the jejunal mucosa was greater (P < 0.05) in those given LR1 than OA, whereas OA decreased (P < 0.05) TLR2 transcripts in ileal mucosa. Compared with CON, TLR2 expression in ileal mucosa increased (P < 0.05) in LR1 pigs but that of TLR4 decreased (P < 0.05).
Figure 4.
Effects of LR1 on the expression of NODs and TLRs in the intestine of weaned pigs. (A) Relative mRNA expression levels of nucleotide binding oligomerization domain 1 (NOD1) and NOD2 in the mucosa of the jejunum and ileum. (B) Relative mRNA expression levels of toll-like receptor 2 (TLR2) and TLR4 in the mucosa of the jejunum and ileum. All data are expressed as the mean ± SEM (n = 8). Differences were determined by one-way ANOVA followed by Tukey’s test. *P < 0.05 compared with CON, #P < 0.05 LR1 compared with OA. CON, a basal diet; OA, a basal diet supplemented with 100 mg/kg olaquindox + 75 mg/kg aureomycin; LR1, a basal diet supplemented with 5 × 1010 cfu/kg L. reuteri LR1.
DISCUSSION
In this study, dietary LR1 supplementation at 5 × 1010 cfu/kg could improve the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. Antibiotics increased the growth performance of weaned pigs, but reduced the sIgA production and antimicrobial peptides expression in the intestine.
Aureomycin and olaquindox have been used to improve the growth performance of pigs during weaning in the past years in China. In this study, dietary supplementation with 75 mg/kg aureomycin plus 100 mg/kg olaquindox increased the ADG and ADFI in weaned pigs. However, the use of the antimicrobial growth promoter olaquindox has been banned in the European Union since September 1999 and in China since August 2017 because of its side effects on animals. To overcome the increased rate of mortality, morbidity, and post-weaning diarrhea because of this ban, a number of alternatives have been proposed. Lactobacillus reuteri LR1 is a new strain of L. reuteri isolated from the feces of healthy weaned pig in our previous study (Wang et al., 2016). Surprisingly, the LR1’s improvements of ADG of weaned pigs are similar to the antibiotics in this study. These data indicate that the LR1 may be an effective alternative to antibiotics for the improvement of growth performance in weaned pigs.
Lactobacillus reuteri is an important member of the commensal microbiota in intestines of both humans and animals. Lactobacillus reuteri has exhibited to regulate immune response and improve the gut health (Li et al., 2008; Liu et al., 2013). A recent study showed that L. reuteri together with a tryptophan-rich diet could reprogram intraepithelial CD4+ T cells into immunoregulatory T cells (Cervantes-Barragan et al., 2017). In addition, L. reuteri could reduce intestinal inflammation via inhibiting TLR4-nuclear factor κB (NF-κB) signaling pathway (Liu et al., 2012). In this study, LR1 increased the production of cytokines IL-22 and TGF-β associated with inhibiting TLR4 expression in the ileum of weaned pigs. Previous studies showed that TGF-β down-regulates inflammatory cytokine production in monocytes and macrophages (Laine et al., 2004) and plays a role in the induction from CD4+ T cells of induced immune regulatory T cells (Stifter et al., 2016). IL-22 is produced by activated NK and T cells, and initiates innate immune responses against pathogens invasion especially in the intestinal epithelial cells (Trevejo-Nunez et al., 2016). Importantly, TGF-β and IL-22 also play important roles in maintaining the integrity of intestinal barrier and wound healing in the intestine (Howe et al., 2005; Hainzl et al., 2015; Xiao et al., 2017). Thus, LR1’s effects on the intestinal barrier function were determined in the next.
The intestinal epithelial barrier is the first line of restricting the invasion of microbes, food antigens, and toxins. Weaning stress causes intestinal barrier damage associated with decreasing TJ proteins expression in pigs (Hu et al., 2013; Yi et al., 2016). Tight junction proteins create semipermeable barriers that can separate different substances in the epithelial cells of the intestine (Dokladny et al., 2016). ZO-1 and occludin are the most important components of TJ proteins (Han et al., 2015). In this study, dietary supplementation with LR1 increased the expression of ZO-1 and occludin in the intestine of weaned pigs. A previous study showed that L. reuteri LR1 attenuated enterotoxigenic Escherichia coli (ETEC) K88-induced decreases of the expression of ZO-1 and occludin in porcine intestinal epithelial cells (Wang et al., 2016). In addition, L. reuteri I5007, another strain of porcine L. reuteri, increased TJ proteins expression in the intestinal epithelial cells in vitro and in the intestine of newborn pig in vivo (Hou et al., 2014; Hou et al., 2015; Yang et al., 2015). Lactobacillus reuteri has also shown to improve intestinal epithelial barrier function by promoting cell migration (Preidis et al., 2012). These data indicated that LR1 could improve TJ protein ZO-1 and occludin expression in the intestine.
The mucins and antimicrobial proteins that are secreted by intestinal epithelial cells provide defenses against pathogen invasion into the underlying lamina propria (Wlodarska et al., 2011a; Gallo and Hooper, 2012). In this study, LR1 increased pBD2, PG1-5, and mucin-2 expression in the intestine of weaned pigs. Consistently, previous studies showed that L. reuteri I5007 also increased intestinal antimicrobial peptides in pigs (Yang et al., 2015). By contrast, the antibiotics reduced the expression of sIgA, pBD2, and PG1-5 in the intestine of weaned pigs. A previous study reported that administration of the antibiotics neomycin, vancomycin, and metronidazole decreased the expression levels of antimicrobial proteins in intestinal epithelial cells (Brandl et al., 2008). A recent study demonstrated that antibiotics compromised goblet cell function and inner mucus layer production in the intestine (Wlodarska et al., 2011a). All these data indicate that the intestinal barrier function could be improved by LR1 in weaned pigs.
To explore possible mechanisms of intestinal barrier function improved by LR1, the expression of NODs and TLRs in the intestine of weaned pigs was examined. The NODs and TLRs play important roles in recognizing bacteria and signaling translocation, and then initiating the immune responses in the intestine. The TLR4 recognizes lipopolysaccharide (LPS) from Gram-negative bacteria, and the TLR2 detects lipoproteins and peptidoglycans in both Gram-positive and Gram-negative bacteria, as well as lipoteichoic acid from Gram-positive bacteria (Kawai and Akira, 2011). In this study, LR1 increased the TLR2 expression but decreased TLR4 expression in the ileum, whereas antibiotics decreased the TLR2 expression. A previous study exhibited that L. plantarum increased the mRNA expression of ZO-1 and occludin associated with increasing the TLR2 expression in the intestine of ETEC K88-induced pigs (Yang et al., 2014). Previous studies have showed that the activation of TLR2 could enhance the expression of antimicrobial peptides and TJ proteins (Cario et al., 2004; Lai et al., 2010). Thus, these data suggested that LR1’s improvements of antimicrobial peptides and TJ proteins may be via the activation of TLR2. However, the underlying mechanisms are still needed to be addressed in the future.
In this study, both LR1 and antibiotics improved growth performance and intestinal morphology in weaned pigs. Additionally, LR1 increased the expression of TJ proteins, mucin-2, antimicrobial peptides, and TLR2 in the intestine, whereas antibiotics decreased the expression of sIgA, antimicrobial peptides, and TLR2 expression in the intestine of weaned pigs. These results may come from the differential mode of action between probiotics and antibiotics. Antibiotics are known to modify the composition of the intestinal microbiota including both pathogenic and commensal bacteria, and their improvements of growth performance may come from reduced total intestinal biota biomass and elimination of the harmful bacteria, which compete for nutrients with the host animal (Heo et al., 2013). In this study, the decreased sIgA production and antimicrobial peptide expression may come from reduced pathogenic and total bacterial content in the intestine of pigs fed OA. Although their mode of action has not been fully elucidated, probiotics are known to inhibit pathogen adhesion by competitive exclusion, produce bacteriosins and organic acids against pathogens, and modulate the immune system in the intestine (Gresse et al., 2017). The different regulation of TLR2, sIgA, and antimicrobial peptides may be due to the changes of intestinal microbiota in pigs fed LR1 and antibiotics. Previous studies showed that probiotics improved intestinal barrier function through regulating intestinal microbiome (Preidis et al., 2012). The LR1 could affect intestinal microbiome and then improve intestinal barrier function. However, the limitation of the current study is lacking the microbiome data in the intestine in pigs fed LR1 and antibiotics. The effects and underlying mechanisms of LR1 and antibiotics on intestinal microbiome will be further addressed in our next studies.
In conclusion, these data suggested that dietary LR1 supplementation at 5 × 1010 cfu/kg could improve the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. The underlying mechanisms of the differential regulation of intestinal barrier function by LR1 and antibiotics are needed to be explored in the future.
This study was funded by Science and Technology Planning Project of Guangzhou (201607020035), the Special Fund for Agro-scientific Research in the Public Interest (201403047), China Agriculture Research System (CARS-35), Science and Technology Program of Guangdong Province (2015A010107010), National Natural Science Foundation of China (31201815), and the Special Foundation of President of the Guangdong Academy of Agricultural Sciences (201710). The authors declare no competing financial interests.
LITERATURE CITED
- Ahl D., H. Liu O. Schreiber S. Roos M. Phillipson, and Holm L.. 2016. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol. (Oxf). 217:300–310. doi:10.1111/apha.12695 [DOI] [PubMed] [Google Scholar]
- Ashiru-Oredope D. and Hopkins S.. 2015. Antimicrobial resistance: moving from professional engagement to public action. J. Antimicrob. Chemother. 70:2927–2930. doi:10.1093/jac/dkv297 [DOI] [PubMed] [Google Scholar]
- Brandl K., G. Plitas C. N. Mihu C. Ubeda T. Jia M. Fleisher B. Schnabl R. P. DeMatteo, and Pamer E. G.. 2008. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 455:804–807. doi:10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario E., Gerken G., and Podolsky D. K.. 2004. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 127:224–238. doi:10.1053/j.gastro.2004.04.015 [DOI] [PubMed] [Google Scholar]
- Cervantes-Barragan L., Chai J. N., Tianero M. D., Di Luccia B., Ahern P. P., Merriman J., Cortez V. S., Caparon M. G., Donia M. S., Gilfillan S.,. et al. 2017. Lactobacillus reuteri induces gut intraepithelial CD4+CD8alphaalpha+ T cells. Science. 357:806–810. doi:10.1126/science.aah5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny K., M. N. Zuhl, and Moseley P. L.. 2016. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. (1985). 120:692–701. doi:10.1152/japplphysiol.00536.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. L. and Hooper L. V.. 2012. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 12:503–516. doi:10.1038/nri3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., F. Han X. Huang Y. Rong H. Yi, and Wang Y.. 2013. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with escherichia coli K88: a comparative study. J. Anim. Sci. 91:5614–5625. doi:10.2527/jas.2013-6528 [DOI] [PubMed] [Google Scholar]
- Gresse R., F. Chaucheyras-Durand M. A. Fleury T. Van de Wiele E. Forano, and Blanquet-Diot S.. 2017. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 25:851–873. doi:10.1016/j.tim.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Hainzl E., S., Stockinger I., Rauch S., Heider D., Berry C., Lassnig C., Schwab F., Rosebrock G., Milinovich M., Schlederer et al. 2015. Intestinal epithelial cell tyrosine kinase 2 transduces IL-22 signals to protect from acute colitis. J. Immunol. 195:5011–5024. doi:10.4049/jimmunol.1402565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F., Zhang H., Xia X., Xiong H., Song D., Zong X., and Wang Y.. 2015. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J. Immunol. 194:1882–1893. doi:10.4049/jimmunol.1402300 [DOI] [PubMed] [Google Scholar]
- Heo J. M., F. O. Opapeju J. R. Pluske J. C. Kim D. J. Hampson, and Nyachoti C. M.. 2013. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. (Berl). 97:207–237. doi:10.1111/j.1439-0396.2012.01284.x [DOI] [PubMed] [Google Scholar]
- Hou C., H. Liu J. Zhang S. Zhang F. Yang X. Zeng P. A. Thacker G. Zhang, and Qiao S.. 2015. Intestinal microbiota succession and immunomodulatory consequences after introduction of lactobacillus reuteri I5007 in neonatal piglets. Plos One. 10:e0119505. doi:10.1371/journal.pone.0119505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C., Q. Wang X. Zeng F. Yang J. Zhang H. Liu X. Ma, and Qiao S.. 2014. Complete genome sequence of lactobacillus reuteri I5007, a probiotic strain isolated from healthy piglet. J. Biotechnol. 179:63–64. doi:10.1016/j.jbiotec.2014.03.019 [DOI] [PubMed] [Google Scholar]
- Howe K. L., Reardon C., Wang A., Nazli A., and McKay D. M.. 2005. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic escherichia coli o157:h7-induced increased permeability. Am. J. Pathol. 167:1587–1597. doi:10.1016/S0002-9440(10)61243-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. H., Xiao K., Luan Z. S., and Song J.. 2013. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 91:1094–1101. doi:10.2527/jas.2012-5796 [DOI] [PubMed] [Google Scholar]
- Kawai T. and Akira S.. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 34:637–650. doi:10.1016/j.immuni.2011.05.006 [DOI] [PubMed] [Google Scholar]
- Lai Y., Cogen A. L., Radek K. A., Park H. J., Macleod D. T., Leichtle A., Ryan A. F., Di Nardo A., and Gallo R. L.. 2010. Activation of TLR2 by a small molecule produced by staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J. Invest. Dermatol. 130:2211–2221. doi:10.1038/jid.2010.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine T., M. Yliaho V. Myllys T. Pohjanvirta M. Fossi, and Anttila M.. 2004. The effect of antimicrobial growth promoter withdrawal on the health of weaned pigs in finland. Prev. Vet. Med. 66:163–174. doi:10.1016/j.prevetmed.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Li X. J., Yue L. Y., Guan X. F., and Qiao S. Y.. 2008. The adhesion of putative probiotic lactobacilli to cultured epithelial cells and porcine intestinal mucus. J. Appl. Microbiol. 104:1082–1091. doi:10.1111/j.1365-2672.2007.03636.x [DOI] [PubMed] [Google Scholar]
- Liu Y., Fatheree N. Y., Dingle B. M., Tran D. Q., and Rhoads J. M.. 2013. Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PloS ONE. 8:e56547. doi:10.1371/journal.pone.0056547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Fatheree N. Y., Mangalat N., and Rhoads J. M.. 2012. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-kappaB signaling in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 302:G608–617. doi:10.1152/ajpgi.00266.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. Natl. Acad. Press, Washington, DC.
- Preidis G. A., D. M., Saulnier S. E., Blutt T. A., Mistretta K. P., Riehle A. M., Major S. F., Venable M. J., Finegold J. F., Petrosino M. E., Conner et al. 2012. Probiotics stimulate enterocyte migration and microbial diversity in the neonatal mouse intestine. Faseb J. 26:1960–1969. doi:10.1096/fj.10-177980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F., J. E. Clark B. L. Overman C. C. Tozel J. H. Huang J. E. Rivier A. T. Blikslager, and Moeser A. J.. 2010. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 298:G352–G363. doi:10.1152/ajpgi.00081.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifter K., C. Schuster M. Schlosser B. O. Boehm, and Schirmbeck R.. 2016. Exploring the induction of preproinsulin-specific foxp3(+) CD4(+) treg cells that inhibit CD8(+) T cell-mediated autoimmune diabetes by DNA vaccination. Sci. Rep. 6:29419. doi:10.1038/srep29419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevejo-Nunez G., W. Elsegeiny P. Conboy K. Chen, and Kolls J. K.. 2016. Critical role of IL-22/IL22-RA1 signaling in pneumococcal pneumonia. J. Immunol. 197:1877–1883. doi:10.4049/jimmunol.1600528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang L., Chen Z., Ma X., Yang X., Zhang J., and Jiang Z.. 2016. In Vitro evaluation of swine-derived lactobacillus reuteri: probiotic properties and effects on intestinal porcine epithelial cells challenged with enterotoxigenic escherichia coli K88. J. Microbiol. Biotechnol. 26:1018–1025. doi:10.4014/jmb.1510.10089 [DOI] [PubMed] [Google Scholar]
- Wegmann U., D. A. MacKenzie J. Zheng A. Goesmann S. Roos D. Swarbreck J. Walter L. C. Crossman, and Juge N.. 2015. The pan-genome of lactobacillus reuteri strains originating from the pig gastrointestinal tract. BMC Genomics. 16:1023. doi:10.1186/s12864-015-2216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarska M., Willing B., Keeney K. M., Menendez A., Bergstrom K. S., Gill N., Russell S. L., Vallance B. A., and Finlay B. B.. 2011. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated citrobacter rodentium-induced colitis. Infect. Immun. 79:1536–1545. doi:10.1128/IAI.01104-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., S. Cao L. Jiao Z. Song J. Lu, and Hu C.. 2017. tgf-β1 protects intestinal integrity and influences smads and mapk signal pathways in ipec-j2 after tnf-α challenge. Innate Immun. 23:276–284. doi:10.1177/1753425917690815 [DOI] [PubMed] [Google Scholar]
- Yang K. M., Jiang Z. Y., Zheng C. T., Wang L., and Yang X. F.. 2014. Effect of lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic escherichia coli K88. J. Anim. Sci. 92:1496–1503. doi:10.2527/jas.2013-6619 [DOI] [PubMed] [Google Scholar]
- Yang F., Wang A., Zeng X., Hou C., Liu H., and Qiao S.. 2015. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 15:32. doi:10.1186/s12866-015-0372-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H., Hu W., Chen S., Lu Z., and Wang Y.. 2017. Cathelicidin-WA improves intestinal epithelial barrier function and enhances host defense against enterohemorrhagic escherichia coli O157:H7 infection. J. Immunol. 198:1696–1705. doi:10.4049/jimmunol.1601221 [DOI] [PubMed] [Google Scholar]
- Yi H., Zhang L., Gan Z., Xiong H., Yu C., Du H., and Wang Y.. 2016. High therapeutic efficacy of cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci. Rep. 6:25679. doi:10.1038/srep25679 [DOI] [PMC free article] [PubMed] [Google Scholar]