SUMMARY
Surgical resection is the only curative modality for colorectal liver metastases (CLM) and 5-year overall survival after resection is about 40%. Nonresectable CLM is not curable and 5-year overall survival is currently about 10%. Before 1995, several liver transplantations for CLMs were performed, but outcome was poor (5-year survival rate: 18%). Liver transplantation for CLMs was abandoned and CLMs were even considered a contraindication to the procedure. Since then, the survival rate after liver transplantation in general has improved by almost 30%. In a prospective pilot study of liver transplantation for nonresectable CLM, a 5-year overall survival rate of 60% was demonstrated, however 19 of 21 patients experienced recurrence of disease. Here, current knowledge and ongoing research in this field is reviewed, and the potential role for liver transplantation as one of several treatment modalities for CLM discussed.
KEYWORDS: : cancer, chemotherapy, colorectal cancer, colorectal liver metastases, liver resection, liver transplantation, outcome, survival
Practice points.
Historically, liver transplantation for colorectal liver metastases (CLM) has been performed until 1995 when it was abandoned because of poor results and organ shortage. Since then, survival after liver transplantation has increased with 20–30%, oncological diagnostic tools and treatment has improved. Current experience is limited to a Norwegian pilot study (SECA study, n = 21), which is followed by the SECA 2 study. Practice points drawn from current experience:
Liver transplantation for CLM is safe.
The 5-year overall survival was 60%, disease-free survival 35% at 1 year.
Five-year survival of about 60% for nonresectable CLM seems superior to best option oncological treatment which is about 10% after start of first-line chemotherapy. This is not established in a randomized controlled trial, but a retrospective comparison with matched liver only metastases from a chemotherapy study supports this view.
Even for the six patients in the SECA study that were liver transplanted on progressive disease after all standard lines of chemotherapy and unfortunate characteristics, survival appears better than chemotherapy. Retrospective comparison with matched liver only metastases from a chemotherapy study supports this view.
Established clinical factors known from liver resection after CLM such as largest tumor load, Carcinoembryonic atigen (CEA) level, time from primary colorectal cancer (CRC) to liver transplantation and response to chemotherapy is significantly associated with survival. This could be used to patient selection in order to improve survival.
The potential for cure needs to be established by a plateau in survival curve, but from previous experience there have been long-time survivors.
Current studies include: The SECA 2 study, which involves implementation of selection factors in two uncontrolled study arms and an RCT between liver transplantation and liver resection for CLM with large tumor load [1]. The RAPID study, exploring left lateral liver transplantation and an ALLPS-like resection in CLM patients [2]. A European multicenter RCT on liver transplantation versus chemotherapy for nonresectable disease is planned.
Background
Colorectal cancer (CRC) is the third most common cancer in men and the second in women [3]. Hepatic metastases develop in more than 50% of CRC patients [4]. Among those with liver only metastases, 10–30% are reported to have potentially resectable disease that can be treated with curative intent [4]. The current treatment for patients with colorectal liver metastases (CLM) is multimodal, including liver resection, chemotherapy, targeted therapies (monoclonal antibodies), interventional radiology and radiotherapy.
Surgical treatment of colorectal liver metastases (CLM) is the only measure with curative potential. In a recent comprehensive meta-analysis on 116 peer-reviewed papers published between 1999 and 2010, 86 studies reported a 5-year overall survival that was median 38% (range 16–74%) [4]. A report from the Memorial Sloan-Kettering Cancer Center, demonstrated a plateau at 17% in disease-specific survival at about 10 years post hepatic resection [5]. This strongly indicates that the procedure is potentially curative. The 10-year survival is in the range of 12–36% in recent studies [6]. For nonresectable CLM, the overall survival has doubled from 12 to 24 months after start of first-line chemotherapy the last decade. However, oncological treatment alone is not curative and the 5-year overall survival after start of first-line chemotherapy is currently at best 12% [7], after start of second-line chemotherapy the overall survival is in the range of 10–12 months [8], and about 6–8 months after third line [9].
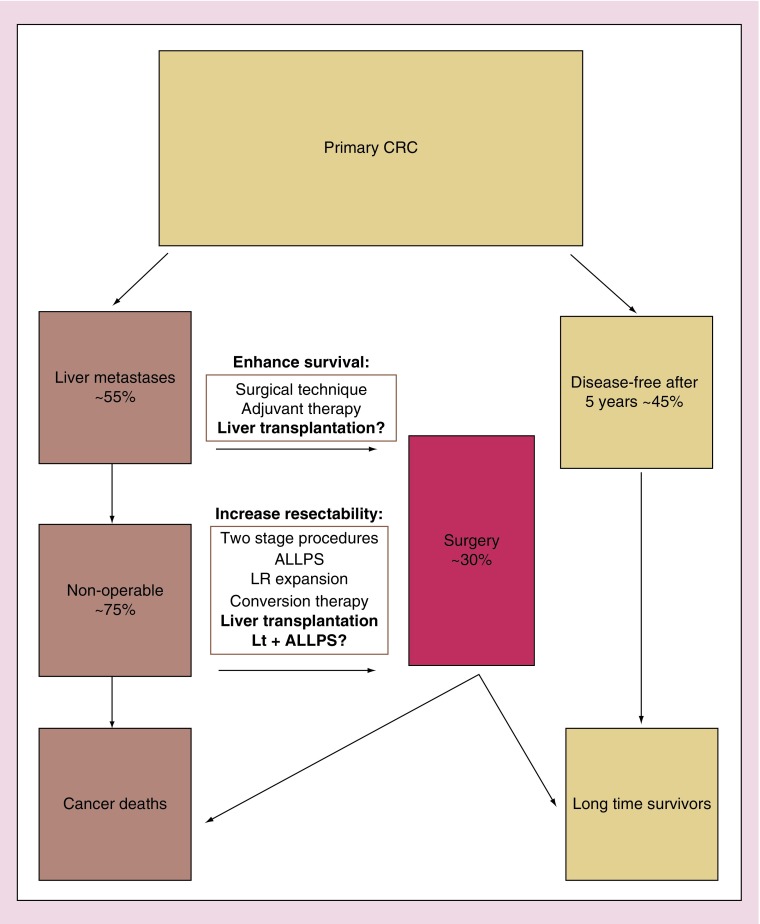
The primary goal in the treatment of the CLM patient is thus to remove the metastases surgically. This will offer a potential of cure and enhance survival regardless of tumor stage. The criteria for resectability has evolved from tumor-specific factors to retaining adequate liver volume after resection; and from emphasis on formal resections toward a more aggressive approach where providing a sufficient liver remnant (usually 20–30% in normal liver tissue) is the key element [10]. In addition, several strategies have been developed in order to enhance amount of patients available for surgery (Figure 1). These involve downstaging of the metastases in conversion therapy [11], or expansion of the future liver remnant in two-stage hepatectomy and portal vein embolization [10,12]. A novel approach is the Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALLPS) technique which includes portal vein ligation in combination with liver partition. This induces rapid hypertrophy of the liver remnant followed by an early stage 2 procedure [13,14]. Taken together, the expansion of resectability criteria, the novel strategies to increase resectability and conversion therapy have increased the number of patients that are considered surgical candidates for resection from less than 10% to more than 20% during the last decade, and further growth is anticipated [10]. At the same time, the 5-year survival has improved from 30% in a comprehensive meta-analysis on studies published before 2001 [15] to 38% in a recent report [10].
Figure 1. . Treatment options for colorectal cancer.
The only curative measure is surgical removal of metastases, and the current goal is to expand the fraction of patients eligible for surgical treatment. Liver transplantation is currently being explored both for surgical removal of nonresectable colorectal liver metastases, and to enhance survival in patients with resectable disease and large tumor load.
ALLPS:Associating liver partition and portal vein ligation for staged hepatectomy; CRC: Colorectal cancer; LR:Liver remnant; Lt+ALLPS:Combined associating liver partition and portal vein ligation for staged hepatectomy and segment 2+3 split liver transplantation (RAPID procedure).
Nevertheless, about 75% of the CLM patients currently end up with nonresectable disease and is only offered palliative care. For these patients, liver transplantation could be a treatment option. Conceptually, liver transplantation would offer a R0 procedure and have the possibility to expand the pool of surgically removable CLM. In this review, previous experiences, publications and ongoing studies in for liver transplantation for CLM are reported.
• Previous experience with liver transplantation for CLM
Cancer constitutes 14% of primary diseases leading to liver transplantation in Europe. Liver transplantation for hepatocellular carcinoma (HCC) was hampered with poor results until the development of robust patient selection strategies was initiated by the Milan group in the 1990s which transformed HCC to one of the major indications for liver transplantation [16,17]. For cholangiocarcinoma, a combination of carefully selected patients and a multimodal aggressive oncological treatment developed by the Mayo Clinic has rendered excellent results with overall survival rates of about 70% for nonresectable cholangiocarcinoma [18,19]. Also, metastases from neuroendocrine tumors have emerged as an established indication for liver transplantation [20].
Liver transplantations for CLM were not uncommon in the early phase of liver transplantation. In two of the first seven liver transplantations performed in 1963 and 1964, the indication was colon metastases [21]. The majority of procedures were performed before 1995. In the European Liver Transplant Registry (ELTR), 50 cases were reported from 1968 to 1995. Based on ELTR data, the 1- and 5-year survival prior to 1995 was 62 and 18%, respectively [22]. Twenty four of the procedures were performed as part of a program in Vienna that lasted from 1984 until 1994, the remaining performed as sporadic procedures at several centers. The Vienna group reported 30 days perioperative mortality of 30% in the initial parts of the series that included the learning phase of liver transplantation [23]. In 44% of the cases, graft loss was not related to tumor recurrence [24]. They soon restricted the procedure to patients with histologically lymph node negative primary tumor (pN0), which improved the results. However the program was abandoned in 1994 due to a high rate of recurrence and decline in available donor livers [25]. A subgroup of three pN0 patients that were without genetically detectable micrometastases achieved long-term survival, one more than 22 years after liver transplantation [25]. Further, there are case reports with recurrence-free survival after 5 and 10 years [26,27]. These cases demonstrate that long-term survival is possible after liver transplantation for CLM.
Liver transplantation for nonresectable CLM in Norway – The SECA study
Due to traditionally low prevalence of hepatitis C and a fortunate donor situation, the average waiting time for donor livers in Norway until recently has been less than 1 month and few die on the waiting list [28,29]. This made it possible to explore new indications for liver transplantation, including malignancies. In the early part of the millennium, CLM were considered a contraindication for liver transplantation [30]. Data from The European Liver Transplant Registry (ELTR) showed 5-year survival of 18% after liver transplantation for CLM prior to 1995. However, survival after liver transplantation has improved with 20 to 30% since then. In addition, there have been improvements in oncological treatment and imaging techniques, as well as the introduction of the mammalian target of rapamycin (mTOR) inhibitors as immunosuppressive drugs with antioncogenic properties. Based on these factors, 5-year overall survival after liver transplantation for CLM of 50% was anticipated [22].
The SECA study was a pilot study for nonresectable CLM that was initiated at Oslo University Hospital in 2006. A total of 21 patients underwent liver transplantation in this study. The 5-year overall survival was 60% (95% CI: 34–85%). Median follow-up was 27 months (range 8–60 months). Six of 21 patients died due to disseminated cancer after median 26 months (range 6–41 months) after liver transplantation. Disease-free survival was 35% at 1 year, and 19 of the 21 patients experienced recurrences, however, a significant proportion of these were accessible for surgery and at follow up 33% of the patients had no evidence of disease [31].
The initial study protocol was quite strict regarding extent of disease and response to chemotherapy; but after 11 months without included patients, a protocol amendment with wider inclusion criteria was approved. These consisted broadly of minimum six weeks of chemotherapy, good performance status and absence of extrahepatic disease. So, principally nonresectability was the only criterion for patient selection. Accordingly, the study population ended up heterogeneous regarding T and N stage, previous exposure and response to oncological treatment. At time of liver transplantation, 16 patients had progressed on first or later lines of chemotherapy, six patients had progressed on all standard lines of chemotherapy, and 38% of the patients had received second-line chemotherapy. The hepatic tumor load was extensive; median number of metastatic lesions was eight (range 4–40 metastases), and median diameter of the largest lesion was 4.5 cm (range 2.8–13.0 cm) [31].
Since the survival benefit from hepatic resection for CLM is extensively documented, the prognostic factors and the corresponding clinical scoring systems are not used in the decision-making on whether to perform hepatic resection or not. However, regarding liver transplantatio n for CLM the selection of patients is important because of the scarcity of donor livers. Outcome for a new indication must prove to be comparable or better than outcome for established indications for liver transplantation. The heterogeneity of the SECA population made it likely that there were differences in prognostic profiles of the patients. In both the patients with shortest survival, breaching of the liver capsule and cancer infiltration of diaphragm were found after vital structures were divided and transplantation unavoidable [31]. Four factors were found to be significantly associated with survival: maximal hepatic tumor diameter above 5.5 cm, time from primary cancer surgery less than two years, carcinoembryonic antigen of more than 80 µg/l and progressive disease at time of liver transplantation. These are established clinical prognostic factors known from studies on liver resection. Five of the six deceased patients had all of these factors present. Very cautious interpretation on these findings is stressed because of the small study population, but the findings demonstrate a potential for selecting patients based on clinical parameters [31]. Since the original report the SECA data has matured with more than two years. At 65 months (range 19-85 months) post liver transplantation, the four prognostic factors were still significantly associated with survival, and if excluding the five patients with all four factors present, the survival at 6 and 7 years was 60%. [32].
All patients in the SECA study that was observed for more than 11 months experienced recurrence of disease. The median time to recurrence was 6 months (range 2–24 months), 17 patients experienced lung metastases and 7 patients received metastases to their new liver. At end of follow up, seven patients were alive with no evidence of disease, eight patients were alive with recurrence and six patients were deceased (Box 1).
Box 1. . Recurrences after liver transplantation for colorectal liver metastases, experiences from the SECA study.
Recurrence of disease was almost universal. Almost all patients develop pulmonary metastases. These are slow growing and several are accessible to surgical treatment.
In the SECA study, pulmonary metastases at time of - or even prior to liver transplantation were found without affecting survival negatively.
Thirty three percent of the patients developed metastases to the new liver. These were only seen as part of disseminated disease and were devastating prognostically.
The initial recurrence pattern was: 68% lung metastases, 11% liver and lung metastases, 11% lymph node metastases, 5% liver and ovarian metastasis and 5% experienced local recurrence of rectal tumor as first recurrence. No patient had metastases to the new liver only as first site, and liver metastases developed exclusively as part of disseminated disease. At end of follow-up, six of the seven patients that developed liver metastases were dead. Median time from diagnosis of liver metastases to death was 14 months (range 4–21 months). In contrast, all the 12 patients with recurrences that did not include the liver were alive at end of follow up, and patients with pulmonary first site recurrence had a 5-year overall survival of 72% [33].
Because the pulmonary metastases were slow growing, reassessment of CT scans by one experienced radiologist was performed. Tracing back from evident metastases in all the 17 patients with pulmonary manifestations, revealed that seven of them had pulmonary metastases appearing as small nodules at time of liver transplantation. Four of them had pulmonary deposits on earlier CT scans as well (2, 2, 3 and 12 months prior to liver transplantation, respectively). The survival analysis showed that the presence of these metastases at the time of liver transplantation did not have negative impact on survival [32,33].
The SECA study was an uncontrolled pilot study. In order to compare survival after liver transplantation with outcome from a modern chemotherapy study, data from a similar cohort of patients included in the NORDIC-VII study were obtained [34]. The NORDIC-VII study was a three-arm, multicenter Phase III trial on Nordic FLOX and two different regimens containing cetuximab and FLOX as first-line treatment of metastatic CRC [35]. Patients that had nonresectable CLM, no extrahepatic disease, no BRAF mutation and similar age were extracted from the NORDIC-VII database. The study population characteristics ended up comparable to the SECA population; however 5-year overall survival was 9% after start of first-line chemotherapy (n = 47), significantly shorter than the 5-year overall survival in the SECA study [34]. Six of the patients in the SECA study had progressive disease on all standard lines of chemotherapy, three after three lines of chemotherapy and three with mutation of Kras after second line of chemotherapy. For these patients, the median overall survival was 41 months and 5-year overall survival was 41%. In the Nordic-VII study, the median overall survival for a similar cohort was significantly shorter, 5.6 months, and all patients were dead before 2 years. [36].
Discussion & future perspective
The implementation of liver transplantation for CLM in modern clinical practice is novel and its place in treatment of CLM is far from established. There is only one study currently reported, the size was small, the frequency of recurrences almost universal and the data need to mature (Box 2) [37]. Principally, demarcations between liver transplantation and chemotherapy for CLM, between liver transplantation and liver resection for CLM, as well as between liver transplantation for CLM and established indications for CLM need to be established.
Box 2. . Key concerns for liver transplantation for colorectal liver metastases.
The main obstacle to further investigation of liver transplantation for colorectal liver metastases is global shortage of donor livers. Long-term survival after most established indications for liver transplantation is excellent and new indications must prove similar outcome.
Modern experience is restricted to one pilot study so far. Lack of randomized controlled trial for liver transplantation versus chemotherapy.
Selection criteria are not established and there are currently no available effect measures. This makes assessment of benefit and evidence-based donor liver allocation for this condition impossible at the moment.
Recurrence of disease in the pilot was almost universal. Curative potential is still uncertain.
Liver transplantation for nonresectable CLM versus chemotherapy
The 5-year overall survival of 60% from the SECA study and the preliminary update data seem better than chemotherapy which currently offer median survival of about 24 months, and 5-year overall survival up to 12% after start of first-line chemotherapy [7]. None of the patients in the SECA study were at start of first-line chemotherapy. Sixteen of the 21 SECA patients had progressed on first or later lines of chemotherapy. Six patients had progressed on all standard lines of chemotherapy; the 5-year overall survival for these six patients was 44% and median overall survival was 41 months [36]. In comparison, the only drug that has demonstrated prolonged survival in treatment of metastatic CRC after progression on all lines of chemotherapy is Regorafenib. In a multicenter trial on this drug, the median survival was enhanced from median 5.0 to 6.4 months [38]. Also, the comparison with the matched group of CLM only in the NORDIC-VIII study strengthens the impression that liver transplantation for nonresectable CLM is superior to chemotherapy alone [34,36]. However, efficacy measures and potential benefit, as well as validation of the SECA data should preferably be obtained in a randomized controlled trial. For this purpose, a European multicenter study initiated in Paris and comprising 20 centers is under evaluation. The plan is to conduct a randomized controlled trial between liver transplantation and chemotherapy.
A major concern for adapting the modality internationally is the short waiting time for donor livers in the SECA study. The mean time on waiting list in the study population was 17 days, which reflects the short waiting list at the time in Norway. In any other deceased donor program, the time on waiting list would probably be considerably longer. How longer waiting list figures will affect patient survival and thus the survival benefit, is a key question if the procedure should be adapted in a deceased donor program outside Norway. One could speculate on whether longer waiting time will improve outcome due to a selection effect. Oslo University Hospital directly adapted the protocol for liver transplantation for nonresectable hilar cholangiocarcinoma developed in the Mayo Clinic, Rochester. For the six patients that have been treated by this protocol in Oslo since 2009, the results seem inferior to the US results, with five cancer deaths and a median overall survival of 26 months [39]. This contrasts the finding that 5-year survival after liver transplantation in general in Oslo is 83% which is high compared with most centers [40]. The time on waiting list for cholangiocarcinoma in Oslo was 15 days, while in a report from the US experience; the median time on waiting list was 6.9 months and the dropout rate 31% [41]. A possible reason for the inferior results could be the absence of such patient selection effect in Norway [19].
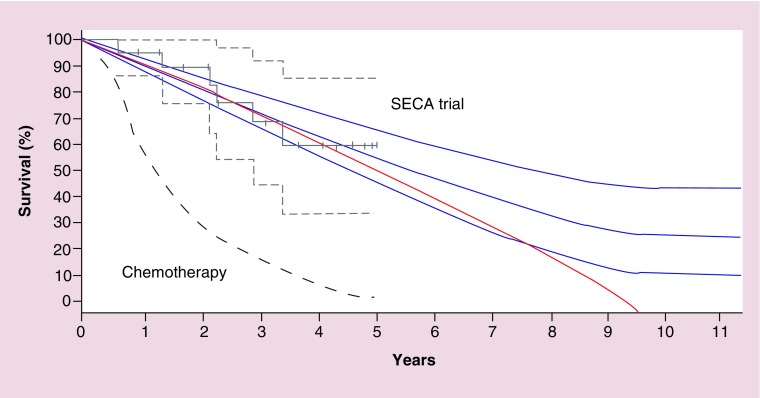
Another concern that has been addressed is whether the procedure has curative potential considering the short disease-free survival and almost universal recurrence of disease [37]. Whether the procedure is curative or not, will be determined if a plateau in the survival curve occurs at some point. If one can extrapolate experiences from liver resection for CLM, this plateau should be established at about 10 years after the procedures (Figure 2) [5].
Figure 2. . Possible future development of the overall survival after liver transplantation for colorectal liver metastases in the SECA trial.
Gray lines are Kaplan Meier plots of overall survival in the SECA study with 95% confidence interval. Projected curative curves in blue with plateaus at 10 years post procedure. Red line is a projected palliative curve in the case of no long-time survivors. Lower stapled line is typical overall survival for nonresectable colorectal liver mestastses patients treated with modern chemotherapy.
Adapted with permission from [31] © Walters Kluwer Health (2013).
Liver transplantation for CLM versus established indications for liver transplantation
The main current challenge in liver transplantation is the scarcity of donor livers. In United States, more than 16,000 patients are awaiting donor livers, while only 5–6000 procedures are performed yearly and about 2000 die on waiting list each year [42]. Although, the donor situation in Norway in comparison is favorable, the waiting lists are getting longer and ultimately the donor organ shortage needs to be also addressed here.
With the precaution of small sample size in the SECA study, the outcome of 60% overall survival at 5-year post liver transplantation is superior to the survival for retransplantations, and especially after recurrent hepatitis C cirrhosis [43]. Retransplantations constitute 5–10% of transplantations and patients tend to routinely be put on waiting list for this condition. Further, the inclusion criteria in the SECA trial were wide, and the common clinical factors that could be used for patient selection were identified. Implementing some of these factors as selection criteria could potentially improve survival [32]. In the ongoing SECA II study, the aim in two of the arms is to achieve 5-year overall survival of 60 and 80% by application of selection criteria identified in the pilot study [1].
Further, comparison of overall survival curves only partly reflects the truth when it comes to the justice of introducing a new indication for liver transplantation. Another approach would be comparison of the potential transplant benefit of the procedures [44]. As pointed out by Martins et al., the justice over utility ratio in the SECA study is 50% [45]. The low survival for the alternative treatment, which for nonresectable CLM is chemotherapy, will yield high benefit at 5 years when overall survival rates are in the range of 60%. Again with caution of small numbers and no direct effect measures, the transplant survival benefit appears to be in the same range or better than after resectable HCC within the Milan criteria [44].
An aspect which is pointed to by Martins et al. is that the patients with CLM acceptable for liver transplantation are in general good condition [45]. In the SECA study the requirement was Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Eligible CLM patients do not exhibit portal hypertension, coagulopathy or renal failure associated with liver failure in the cirrhotic patient, and is as such at lower risk for surgical complications postoperatively. Primary nonfunction is associated with higher MELD scores and the absence of liver failure makes the CLM patient more likely to tolerate a lesser quality allograft. Extended criteria graft, such as grafts with steatosis and prolonged ischemia time, that might otherwise be discarded because of the excessive risk for primary nonfunction in a patient deteriorated with liver failure could successfully be used in the setting of CLM [46]. Further, the prognosis of nonresectable CLM is so poor that one could probably accept grafts with slightly higher risk of transferring malignancies or infections such as seropositive hepatitis B patients or patients with previous cancer. About 3% of procured deceased donor grafts are discarded because of concerns regarding quality or other risk factors [45].
The use of living donor donation is another way of expanding the donor pool. In parts of the world, such as several Asian countries, this is the main source of donor livers. The main concern for living donor liver transplantation is the donor mortality between 0.2 and 0.5% and morbidity of up to 40% [47]. This makes weighing the risk to donor and outcome of recipient important. Recipients should be carefully selected to avoid exposing the donor to an unacceptable risk in order to donate the liver to a recipient with poor outcome. No such selection criteria have so far been developed for CLM, making this a potentially highly experimental strategy. Nevertheless, a case report from Zagreb showed 5-year recurrence-free survival after a right lobe living donor liver transplantation for nonresectable CLM [26].
Another possibility to expand the pool of accessible organs is the use of split-liver grafts. A novel approach being explored in our hospital is a hybrid technique combining an ALLPS-like procedure and a small left lateral (segment 2–3) split liver transplantation. The transplantation of left lateral segments is safe, but the volume of the left lateral donor liver graft is almost never sufficient for an adult recipient. However, the accelerated hypertrophy of the left lateral lobe following right portal vein ligation and in situ splitting of the liver could potentially be utilized in this setting [13]. The concept is that resection of the patients left liver, followed by orthotopic placement of a left lateral segment graft facilitates hypertrophy within few weeks and the remaining metastatic liver would be removed before further cancer development. The remaining split liver is used for another patient leaving the impact waiting list minimal. If feasible, this modality could also pave the way for utilization of left lateral living donor graft for this purpose, which carries minimal risk for the donor [48]. The first patient is included and successfully treated in this study [2].
Liver transplantation versus resection of CLM
Resectability in liver surgery is an evolving concept that differs between centers. From an oncological perspective, whether CLMs are technically resectable or not might not be the optimal criterion for selection of CLM patients to liver transplantation. The 5-year overall survival of 60% in the SECA study is in the range as the best of the liver resection studies. Consequently, there are several subgroups of patients with resectable CLM that exhibit inferior survival than the patients in the SECA study. In patients with large tumor load, reported survival is lower after resection than in the SECA-study. In patients with six or more CLMs, the 5-year overall survival is only 19–27% [49,50]. In the following SECA II trial, one of the arms involves randomization to liver transplantation or resection in selected patients with more than six metastases [1].
Conclusion
Liver transplantation has the potential to be a part of the armamentarium of treatment modalities for CLM, but to what extent is yet to be established (Figure 1). The most obvious group that probably would benefit from the procedure is CLM patients with favorable prognostics and nonresectable disease despite modern surgical or oncosurgical approaches. Only a fraction of this group would be transplantable because of old age and comorbidity.
Future perspective
The main challenge for further exploration of liver transplantation for CLM is the scarcity of donor livers worldwide. Strategies such as improving patient selection, utilization of extended criteria or high-risk liver grafts, priority over established indications with inferior outcome and the possible utilization of small left lateral donor grafts in combination with ALLPS-like resection is currently being investigated in order to meet this challenge. In a longer perspective, improvement in resectability and oncological treatment might diminish the population that will benefit from liver transplantation for CLM. On the other hand, the recent introduction of antiviral drugs with capacity to eradicate hepatitis C might reduce the huge impact this condition has on the waiting list and hopefully expand the pool of available donor livers for other indications. Also, living donor liver transplantation might be part of the future strategies for nonresectable CLM. However, outcome and robust selection criteria should be established before this modality is explored.
Acknowledgement
The author would like to thank his supervisor, transplant surgeon A Foss, oncologist S Dueland, and transplant surgeon P-D Line for initiating and developing the projects on liver transplantation for CLM in Norway.
Footnotes
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as •• of considerable interest
- 1.ClinicalTrials. NCT01479608. www.ClinicalTrials.gov
- 2.ClinicalTrials. www.ClinicalTrials.gov NCT02215889.
- 3.American Cancer Society. Global Cancer Facts & Figures (2nd Edition). American Cancer Society; GA, USA: 2011. [Google Scholar]
- 4.Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin. Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J. Clin. Oncol. 2007;25(29):4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 6.Abbas S, Lam V, Hollands M. Ten-year survival after liver resection for colorectal metastases: systematic review and meta-analysis. ISRN Oncol. 2011 doi: 10.5402/2011/763245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht JR, Mitchell E, Chidiac T, et al. A randomized Phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J. Clin. Oncol. 2009;27(5):672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 8.Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised Phase 3 trial. Lancet Oncol. 2013;14(1):29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 9.Broadbridge VT, Karapetis CS, Price TJ. Cetuximab in metastatic colorectal cancer. Expert. Rev. Anticancer Ther. 2012;12(5):555–565. doi: 10.1586/era.12.25. [DOI] [PubMed] [Google Scholar]
- 10.Alberts SR, Poston GJ. Treatment advances in liver-limited metastatic colorectal cancer. Clin. Colorectal Cancer. 2011;10(4):258–265. doi: 10.1016/j.clcc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Beppu T, Miyamoto Y, Sakamoto Y, et al. Chemotherapy and targeted therapy for patients with initially unresectable colorectal liver metastases, focusing on conversion hepatectomy and long-term survival. Ann. Surg. Oncol. 2014;21(Suppl. 3):405–413. doi: 10.1245/s10434-014-3577-x. [DOI] [PubMed] [Google Scholar]
- 12.van Lienden KP, van den Esschert JW, de GW, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc. Intervent. Radiol. 2013;36(1):25–34. doi: 10.1007/s00270-012-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012;255(3):405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 14.Gauzolino R, Castagnet M, Blanleuil ML, Richer JP. The ALPPS technique for bilateral colorectal metastases: three “variations on a theme”. Updates Surg. 2013;65(2):141–148. doi: 10.1007/s13304-013-0214-3. [DOI] [PubMed] [Google Scholar]
- 15.Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br. J. Cancer. 2006;94(7):982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 17.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 18.Masuoka HC, Rosen CB. Transplantation for cholangiocarcinoma. Clin. Liver Dis. 2011;15(4):699–715. doi: 10.1016/j.cld.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Darwish MS, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143(1):88–98. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Treut YP, Gregoire E, Klempnauer J, et al. Liver transplantation for neuroendocrine tumors in europe-results and trends in patient selection: a 213-case European liver transplant registry study. Ann. Surg. 2013;257(5):807–815. doi: 10.1097/SLA.0b013e31828ee17c. [DOI] [PubMed] [Google Scholar]
- 21.Starzl TE, Fung JJ. Themes of liver transplantation. Hepatology. 2010;51(6):1869–1884. doi: 10.1002/hep.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foss A, Adam R, Dueland S. Liver transplantation for colorectal liver metastases: revisiting the concept. Transpl. Int. 2010;23(7):679–685. doi: 10.1111/j.1432-2277.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- 23.European Liver Transplant Registry. Data Analysis Booklet. www.eltr.org
- 24.Muhlbacher F, Huk I, Steininger R, et al. Is orthotopic liver transplantation a feasible treatment for secondary cancer of the liver? Transplant Proc. 1991;23(1 Pt 2):1567–1568. [PubMed] [Google Scholar]; •• Report from the Vienna group on initial experience with liver transplantation for colorectal liver metastases (CLM).
- 25.Kappel S, Kandioler D, Steininger R, et al. Genetic detection of lymph node micrometastases: a selection criterion for liver transplantation in patients with liver metastases after colorectal cancer. Transplantation. 2006;81(1):64–70. doi: 10.1097/01.tp.0000189711.98971.9c. [DOI] [PubMed] [Google Scholar]; •• Identification of three patients with long survival after liver transplantation for CLM from the early phase of liver transplantation.
- 26.Kocman B, Mikulic D, Jadrijevic S, et al. Long-term survival after living-donor liver transplantation for unresectable colorectal metastases to the liver: case report. Transplant Proc. 2011;43(10):4013–4015. doi: 10.1016/j.transproceed.2011.09.065. [DOI] [PubMed] [Google Scholar]
- 27.Honore C, Detry O, De RA, Meurisse M, Honore P. Liver transplantation for metastatic colon adenocarcinoma: report of a case with 10 years of follow-up without recurrence. Transpl. Int. 2003;16(9):692–693. doi: 10.1007/s00147-003-0605-3. [DOI] [PubMed] [Google Scholar]
- 28.www.scandiatransplant.org/data Scandiatransplant waiting list figures 2012.
- 29.Scandiatransplant. The Nordic Liver Transplant Registry Annual report 2009. www.scandiatransplant.org/ANNUAL_REPORT_2009_FINAL.pdf
- 30.Hoti E, Adam R. Liver transplantation for primary and metastatic liver cancers. Transpl. Int. 2008;21(12):1107–1117. doi: 10.1111/j.1432-2277.2008.00735.x. [DOI] [PubMed] [Google Scholar]
- 31.Hagness M, Foss A, Line PD, et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann. Surg. 2013;257(5):800–806. doi: 10.1097/SLA.0b013e3182823957. [DOI] [PubMed] [Google Scholar]; •• Report from the SECA study. The first prospective study on liver transplantation for CLM. Shows KM estimate of 5-year overall survival at 60% and identifies prognostic factors.
- 32.Hagness M, Solheim JM, Line PD, Dueland S, Foss A. The Joint International Congress of ILTS, ELITA and Licage. London, UK: 6 June 2014.. An update on liver transplantation for non-resectable liver metastases. Presented at. [Google Scholar]
- 33.Hagness M, Foss A, Egge TS, Dueland S. Patterns of recurrence after liver transplantation for nonresectable liver metastases from colorectal cancer. Ann. Surg. Oncol. 2014;21(4):1323–1329. doi: 10.1245/s10434-013-3449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Detailed report on the metastatic recurrence after Lt for CLM. Shows difference in survival after liver recurrence and pulmonary recurrence.
- 34.Dueland S, Guren TK, Hagness M, et al. Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann. Surg. 2014 doi: 10.1097/SLA.0000000000000786. Epub ahead of print. [DOI] [PubMed] [Google Scholar]; •• A comparison between the SECA study population and a subgroup of equivalent patients with liver only metastases from a modern chemotherapy study.
- 35.Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J. Clin. Oncol. 2012;30(15):1755–1762. doi: 10.1200/JCO.2011.38.0915. [DOI] [PubMed] [Google Scholar]
- 36.Dueland S, Hagness M, Line PD, Guren TK, Tveit KM, Foss A. Is liver transplantation an option in colorectal cancer patients with nonresectable liver metastases and progression on all lines of standard chemotherapy? Ann. Surg. Oncol. 2014 doi: 10.1245/s10434-014-4137-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]; •• A report on the patients in the SECA study that were transplanted after progression on all lines of chemotherapy. 5- year overall survival was 41% which is a significantly better outcome than a subgroup of equivalent patients with liver only metastases from a modern chemotherapy study.
- 37.Chapman WC. Liver transplantation for unresectable metastases to the liver: a new era in transplantation or a time for caution? Ann. Surg. 2013;257(5):816–817. doi: 10.1097/SLA.0b013e3182908c8d. [DOI] [PubMed] [Google Scholar]
- 38.Grothey A, Van CE, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, Phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 39.Solheim JM, Foss A, Line PD, Dueland S, Hagness M. The Joint International Congress of ILTS, ELITA and Licage. London, UK: 7 June 2014. Adapting liver transplantation for Hilar non resectable Stage I-II cholangio carcinomas according to mayo clinic's protocol to a single center with low waiting list time – the Oslo experience. Presented at. [Google Scholar]
- 40.Scholz T, Karlsen TH, Sanengen T, et al. Liver transplantation in Norway through 25 years. Tidsskr. Nor Laegeforen. 2009;129(24):2587–2592. doi: 10.4045/tidsskr.09.1106. [DOI] [PubMed] [Google Scholar]
- 41.Darwish MS, Kim WR, Therneau T, et al. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012;56(3):972–981. doi: 10.1002/hep.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilarinho S, Lifton RP. Liver transplantation: from inception to clinical practice. Cell. 2012;150(6):1096–1099. doi: 10.1016/j.cell.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 43.Rowe IA, Barber KM, Birch R, Curnow E, Neuberger JM. Retransplantation for graft failure in chronic hepatitis C infection: a good use of a scarce resource? World J. Gastroenterol. 2010;16(40):5070–5076. doi: 10.3748/wjg.v16.i40.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitale A, Volk M, Cillo U. Transplant benefit for patients with hepatocellular carcinoma. World J. Gastroenterol. 2013;19(48):9183–9188. doi: 10.3748/wjg.v19.i48.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martins PN, Movahedi B, Bozorgzadeh A. Liver transplantation for unresectable colorectal cancer liver metastases: a paradigm change? Ann. Surg. 2014 doi: 10.1097/SLA.0000000000000483. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Harring TR, O'Mahony CA, Goss JA. Extended donors in liver transplantation. Clin. Liver Dis. 2011;15(4):879–900. doi: 10.1016/j.cld.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Lauterio A, Di SS, Giacomoni A, De CL. The role of adult living donor liver transplantation and recent advances. Expert. Rev. Gastroenterol. Hepatol. 2014:1–15. doi: 10.1586/17474124.2015.967762. [DOI] [PubMed] [Google Scholar]
- 48.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N. Engl. J. Med. 2007;356(15):1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 49.Wicherts DA, de Haas RJ, Andreani P, et al. Short- and long-term results of extended left hepatectomy for colorectal metastases. HPB (Oxford) 2011;13(8):536–543. doi: 10.1111/j.1477-2574.2011.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pulitano C, Bodingbauer M, Aldrighetti L, et al. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann. Surg. Oncol. 2011;18(5):1380–1388. doi: 10.1245/s10434-010-1459-4. [DOI] [PubMed] [Google Scholar]