Abstract
The best results in surgical resection are obtained in patients with solitary tumors without clinically significant portal hypertension (hepatic venous pressure gradient >10 mmHg). In such settings, 5-year survival rates exceed 70%. When portal hypertension exceeds this cut-off value, 5-year survival decreases to 55%, as is also the case in patients with more than one nodule. Surgery may be technically feasible, in other words, with acceptable 30-day mortalities although the clinically relevant survival outcome is significantly reduced. In such instances, patients may be better served by liver transplantation. If this option is not available, the outcome may not differ to that obtained by ablation for small solitary hepatocellular carcinoma or for chemoembolization for those patients with multifocal hepatocellular carcinoma within the Milan criteria. This philosophy is the backbone for the Barcelona Clinic Liver Cancer decision-making process.
KEYWORDS : BCLC classification, expanded criteria, hepatocellular carcinoma, HVPG, liver resection, liver transplantation, Milan criteria, portal hypertension, vascular invasion
Practice points.
In the majority of patients, the presence of underlying cirrhosis increases surgical risk.
Hepatic resection is a curative treatment from the oncological point of view and, at the same time must maintain the maximum quantity of functioning liver parenchyma.
The most commonly used methods allowing us to satisfactorily assess the condition of the liver are: the liver stiffness measurement by transient elastography, the indocyanine green clearance rate and model for end-stage liver disease.
In cases of hepatocellular carcinoma with portal hypertension operative risk is increased and long-term survival is less than optimal.
Measurement of hepatic venous pressure gradient is the gold standard in assessing the presence of clinically significant portal hypertension.
Vascular invasion is strongly related to the size of the tumor and presence of multicentricity. In these cases long-term survival is reduced.
Knowledge of the natural history of the hepatocellular carcinoma, is a very important factor when determining the therapeutic benefits of various options.
Feasibility should not be the main factor dictating the choice of treatment. Instead, the potential impact of the various options on chance of survival should guide the decisions.
For most groups, over a very long time, surgical treatment, either resection or liver transplantation, has been the first therapeutic option to be considered in cases of hepatocellular carcinoma (HCC) [1,2]. Hepatic resection should be a curative treatment from the oncological point of view and, at the same time must maintain the maximum quantity of functioning parenchyma. From a historical perspective hepatic resection has been associated with a high surgical morbidity–mortality rate [3]. However, over the last two decades, the prognosis of patients with HCC has changed dramatically. Some large studies have documented better perioperative results after resection. The decline in operative mortality is attributed to improvements in refined patient selection, and improved surgical techniques and perioperative care [3].
Furthermore, during the follow-up period, the tumor recurrence rate is over 50% at 3 years, and to date there is no efficient treatment to reduce its incidence [4]. Liver transplantation not only rules out tumor recurrence but also treats the underlying illness. In this case the oncogenic potential is eliminated. Over recent years, many published studies have shown that hepatic transplant has a good survival outcome, and reduces recurrence rate by up to 5 years in patients with HCC diagnosed at the early phase of its development [4]. However, in Spain, where we have the highest rate of donation in the world, the disproportion between donors and recipients makes little difference because a growing waiting list of patients worsen the transplantation results when analyzed according to the intention of treatment [5]. Realistically speaking, it is important to recognize that hepatic resection maintains its standing as a viable treatment for HCC by reducing waiting list growth for those cases that could undergo resection, or by acting as a ‘bridge’ to later transplant [6].
In the vast majority of HCC patients, the presence of underlying cirrhosis, increases the risk and, in some cases, can limit postoperative indications for resection [2,3]. Hepatic resection, while a curative treatment from the oncological point of view, must at the same time, endeavor to maintain the maximum quantity of functioning parenchyma. For this reason, both a preoperative assessment of liver reserve capacity and careful selection of patients is of particular importance for success. There is the possibility of preoperative biopsy to assess liver steatosis [7] or the degree of fibrosis [8], using the SAF score or Metavir score. Postoperative risk correlates with increased fibrosis. A preoperative biopsy could increase the risk of complications that could otherwise be avoided through the use of other satisfactory assessment methods [9].
The most common methods employed are the liver stiffness measurement by transient elastography, the indocyanine green clearance rate and model for end-stage liver disease (MELD).
Transient elastography has been proposed as a new method for noninvasive diagnose of liver fibrosis. Recently, it has been suggested that patients with liver stiffness values higher than 12 kPa have an increased risk of postoperative liver failure [10,11]. Elastography was used together with noninvasive methods to identify clinically significant portal hypertension and esophageal varices in patients with compensated cirrhosis [12]. However, in real-life scenarios half of the patients with potential liver nodules can not be classified as having or not having clinically significant portal hypertension, because of nonapplicability (obese patients) or inaccuracy [13].
The indocyanine green (ICG) clearance rate represents the most common test for predicting mortality, especially in Eastern countries [9,14–15]. In healthy patients ICG-R15 is approximately 8–14%. Large resection (e.g., right hepatectomy) was judged to be feasible when ICG-R15 was less than 20% [14]. However, it has been suggested that ICG clearance test has not shown reliable results because the ICG test was influenced by hepatic blood flow [16]. Additionally, test results may vary between examiners. Nevertheless, in a recent Korean study the attempt to replace ICG by the MELD proved unsuccessful [17].
The MELD score was originally developed to evaluate the survival rate of patients undergoing transjugular intrahepatic portosystemic shunt procedures, and was thereafter modified to evaluate patients with liver disease undergoing surgery [18]. MELD scores perform well in predicting death within 3 months in patients awaiting liver transplantation and have been applied in the allocation of donors’ livers [9,17–18]. In addition, the MELD score shows a significant correlation with the degree of metabolic liver functional impairment, and has also been used to predict the postoperative mortality risk of patients undergoing hepatic resection. Patients with a MELD score higher than 10 have a significantly increased risk of morbidity and poor outcome after surgery [19,20].
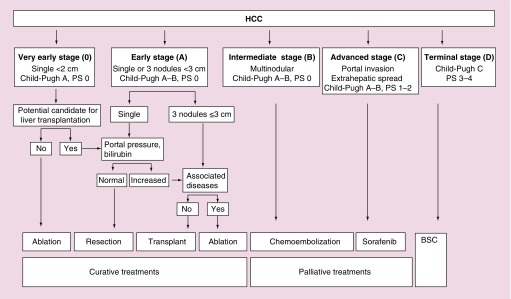
Over the last few years many classifications have been reported [14–28]. The Barcelona Clinic Liver Cancer staging and treatment strategy (BCLC) has gained wide acceptance and endorsement because of its stratification capacity and its linkage of staging with treatment indication [22] (Figure 1). It is important that selection of candidates and surgical techniques be optimal in order to reduce at maximum the postsurgical morbidity–mortality rate and assure adequate long-term patient survival [29]. From 1996, in our center, selection of optimal candidates for resection has usually been based on the assessment of the presence of portal hypertension, as assessed by hepatic vein catheterization [30]. Studies have shown that a normal bilirubin concentration and the absence of any clinically significant portal hypertension measured by hepatic vein catheterization (hepatic vein pressure gradient [HVPG] <10 mmHg) are the best predictors of an excellent postsurgery outcomes, with almost no risk for postoperative liver failure [5]. Therefore, since 1996 measurement of portal pressure has been a key step in the evaluation of our candidates for resection.
Figure 1. . BCLC classification system (2012).
HCC: Hepatocellular carcinoma.
The BCLC algorithm is a prospective, externally validated [31–33] system, based on the results of a cohort of untreated patients corresponding to the aim of no treatment in randomized control trials [34]. Knowledge of patient history is a very important fact when determining the therapeutic benefits of various options. The BCLC classification system can predict prognosis on the basis on variables related to: tumor characteristics (number, size, extrahepatic spread and vascular invasion); the degree of liver-function impairment according to the Child-Pugh classification; plasma bilirubin levels and presence of portal hypertension, and finally, to the patient's general health status [35,36].
According to BCLC classification, candidates for liver resection are those patients at an early stage (single nodule, Child-Pugh A-B, PS 0, normal bilirubin value and no portal hypertension). Patients in a very early stage are also considered for inclusion if they are suitable for liver transplantation [36].
A new staging system with treatment stratification has been recently reported, employing Hong Kong-based data from 3856 patients [37]. In the study, it was suggested that their aggressive treatment system, based on liver resection, provides better survival than the treatments recommended by the BCLC. However, major concerns have been made manifested by Sherman [38]. Briefly, the Hong-Kong staging is retrospective while the BCLC is prospective and based on the natural history of the patients. Furthermore, the retrospective studies introduces a bias risk, in other words, selecting for surgery only patients with a highly favorable condition. Further to the above study design considerations, these findings should be validated in other populations (mainly in hepatitis C virus disease) in order to confirm the Hong Kong study.
Portal hypertension
Portal hypertension has been involved as a physiopathological mechanism in the majority of processes that affect decompensated cirrhotic patients [39,40]. In cirrhotic patients with increased portal pressure, the development of related pathologies such as ascites and oesophageal varices further reduce life expectancy. The meta-analysis from D'Amico demonstrated that patients in Child-Pugh B have a spontaneous (without surgery) reduction of their 2-year survival rate. When these patients presented decompensation, the survival rate decreased significantly [41].
These patients are therefore not prime candidates for liver resection because of their increased incidence of morbidity and mortality. Moreover, their long-term survival rate is also reduced. Resection although feasible, is not advisable because, if portal hypertension predicts outcome in cirrhosis, why should this not be the case after surgical resection? [42].
However, some authors argue in favor of liver resection in the presence of portal hypertension. It is important to note though that patients with preoperative portal hypertension underwent a lower rate of major resection [43–45].
Capussotti et al. [43], reported his results on cirrhotic patients with portal hypertension undergoing liver resection for hepatocellular carcinoma. He concluded that portal hypertension should not be considered an absolute contraindication for hepatectomy in cirrhotic patients. Nevertheless, his results demonstrate a higher operative mortality in patients with portal hypertension when comparing to patients without (11.1 vs 5.1%). Moreover, a higher number liver related complications and a higher incidence of blood transfusions (51.5 vs 32.2%) were also reported. In addition, significant 3- and 5-year survival differences were reported when comparing patients with or without portal hypertension (62.1 and 39.8 vs 44.8 and 28.9%, respectively).
Ishizawa, from Tokyo University, recently published their liver resection results in their series of cirrhotic patients with HCC [44]. They concluded that neither the presence of multiple tumors nor portal hypertension is a contraindication to liver resection. However, their 5-year survival rate was poorer in their group with portal hypertension than in their group with no portal hypertension, either in Child A or Child B patients. Actually, the findings by Tokyo University confirm our data published some years ago [5] in that the results are almost the same. Our group has never maintained that portal hypertension is a contraindication to surgery. Obviously, whilst it is possible to perform liver resection in HCC patients with portal hypertension, it is also necessary to know the price to be paid. At our institute, when a liver transplant is viable, patients with HCC and portal hypertension are given a 5-year overall posttransplant survival rate of more than 70%, with a recurrence rate less than 10%. The disease-free survival rate is significantly higher than the rate obtained with liver resection. Thus, it is ethically questionable in our strategy of treatment, not to include patients with portal hypertension on the liver transplant list. However, liver transplantation is well established in the USA and Europe, but in some areas of the world, transplantation is not available or has very limited applicability [4]. In these cases, availability of resources also has to be considered in developing treatment strategies. In addition, the limited number of donations means careful selection of candidates for transplantation (Milan criteria). This approach might exclude some candidates with acceptable outcomes, unless there were more donors. According to the BCLC classification (Figure 1), percutaneous ablation is the best treatment option for patients with early stage HCC, who are not suitable for resection or transplantation. Transarterial chemoembolization is recommended for nonsurgical patients with large/multifocal HCC who do not have vascular invasion or extrahepatic spread [4].
Recently, Cucchetti published his study results on a series of 241 cirrhotic patients [45]. From the results it was inferred that portal hypertension should not be considered as a contraindication for hepatic resection. However, the first analysis demonstrated that the patients with portal hypertension had a significant major incidence of liver failure, and a significant low 3- and 5-year survival rate. These data also confirm our results published some years ago. To overcome bias arising from the variation of severity of liver function impairment throughout the two groups, a one-to-one match was created using propensity score analysis [45], thus obtaining two groups comparable for baseline variables. Propensity score reduces selection bias by equating groups on the basis of the covariates. Patients in whom the propensity score was not matched were excluded for further analysis. The 3- and 5-year survival rates of the matched study were similar, suggesting that the presence of portal hypertension had no impact on intraoperative course and postoperative outcome.
However, it is worthy to remark that, in the 11 patients excluded in the group of portal hypertension, the incidence of liver failure was nearly 50%, with a significantly lower 3- and 5-year survival rate. Despite the aim of the study from Cuchetti was reduce confounding factor by exclusion of outliers, these data suggest that the exclusion of the worse patients in the group of portal hypertension had improved the 3- and 5-year rate of this group in the matched study.
To try to clarify this controversial issue the BCLC group performed a systematic review and meta-analysis to explore in detail the impact of clinically significant portal hypertension on the postoperative outcomes either survival and clinical decompensation in a published series of compensated cirrhotic patients with preserved liver function without extrahepatic disease or macrovascular invasion [46]. The primary analysis focused on mortality (at 3 and 5 years) after surgery. The second analysis addressed postoperative clinical decompensation of liver disease, defined as the onset of complications related to cirrhosis such as ascites, variceal bleeding, hepatic encephalopaty, progressive jaundice, spontaneous bacterial peritonitis and hepatorrenal syndrome.
The above search identified 272 citations, of which 54 potential articles were selected. Eleven articles amounting to a total of 1737 patients were finally included in the analysis.
The presence of clinically significant portal hypertension was associated with a higher risk of death at 3 and 5 years. On meta-analysis of eight studies, the presence of clinically significant portal hypertension was associated with an increased risk of developing decompensation of cirrhosis. The odds ratios were maximal in studies using the gold standard measurement of HVPG to estimate the presence of portal hypertension.
Large tumors
Since the first report in 1999 [22], the BCLC classification has never considered size as a limiting factor to offer resection as first-line treatment [4,35–36,47].
However, some publications have erroneously stated that, in the BCLC classification, resections were contraindicated in tumors larger than 5 cm in diameter [48]. In this study the authors challenge the BCLC strategy while at the same time highlighting the need to update the most recent Clinical Practice Guidelines produced by AASLD [4] and EASL [47]. As acknowledged by them, the study is retrospective and the analysis of the data offered shows that there is a large proportion of patients excluded or with data missing in relevant parameters. This may be a major flaw when applying subgroup analysis. In addition, the staging of the patients is based on the examination of the resected tumors and this is information that is obviously not available at the time of treatment indication. Thus, such a study will never serve to inform clinical decisions about treatment indication. These decisions should be based on adequate imaging and state-of-the-art techniques. Using the data of Torzilli et al., patients with vascular invasion discovered at the time of surgery are classified as BCLC C although when according to intention to treat they would correspond to BCLC A at the time of treatment indication [49]. Clearly, authors confused Milan criteria for liver transplantation [50] with the BCLC criteria for resection. There are now no statements suggesting that BCLC classification does not recommend resection for solitary HCC greater than 5 cm [51].
However, although tumor size is not a contraindication for resection it plays a very important role in treatment decision making. Some publications analyzed the results obtained by experienced surgeons in patients for large HCC [52–55]. Although the number of patients with cirrhosis varied in these series, the long-term results are disappointing. Even though operative mortality is low and it can be argued that the intervention is safe, the presence of risk factors such as a macroscopic vascular invasion, additional lesions or underlying cirrhosis, get the benefit of the surgery in question.
Multiple tumors
The BCLC classification recommended hepatic resection for single tumors. In cases of multiple nodules, liver transplantation is advised if the patients meet Milan criteria and no significant comorbidities are present [35]. However, Ishizawa concluded than the presence of multiple tumors is not a contraindication to liver resection [44]. However, the patient 5-year survival rate was significantly poorer in the group with multiple tumors than in the group with a single nodule, both in Child A or Child B patients.
Poon et al. in their paper concerning the strategy of salvage transplantation [56], reported that the survival results of patients with concomitant two or three nodules and cirrhosis were particularly poor compared with other subgroups of patients, and suggest that primary transplantation may be a more appropriate option for this subset of patients.
The presence of multiple resectable lesions should probably not be considered as absolute contraindications even though curative resection cannot be accomplished [57–60]. The reported 5-year survival (36–58%) and disease-free survival (11–30%) questions the indication of resection.
As Llovet has demonstrated in a series of patients from the Mount Sinai Hospital, vascular invasion is strongly related to the size of the main nodule. The percentage of vascular invasion increases according to size [61]. These data have been recently confirmed by Pawlik in a multicenter international study in more than one thousand patients [62]. Pawlik demonstrated that the tumor size and multinodularity appears in the multivariate analysis as a prognostic factor that can predict vascular invasion. The intent to extend the indications beyond BCLC could impair the results of resection.
Nevertheless, there are other alternative treatments that can be offered to patients with multinodularity. In a series, with unresectable HCC treated by transarterial chemoembolization, the overall three and 5-year survival rate after transarterial chemoembolization were 55 and 34%, respectively. While these results are not exactly the same, they are comparable to those obtained with the surgical option in a similar population of patients [63].
The study of Malagari [64] with chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma shows overall survival rates of 93, 62 and 22% at 1, 3 and 5 years after sequential treatment, with higher rates achieved in Child class A compared with Child class B patients. Similarly, the study of Burrel [65] with chemoembolization with doxorubicin-eluting beads for hepatocellular carcinoma, shows overall survival rates of 90, 66 and 38% at 1, 3 and 5 years after treatment.
In summary, the aim of the treatment is to improve survival of patients while maintaining the most preserved quality of life. This apparently simple statement is of paramount importance as quite frequently the debate of treatment indication is established around what can be done, rather than around what is worth being done. We are not discussing the real possibility to operate on patients with portal hypertension, multinodular lesions or large tumors. It is evident that in some cases, very skilled surgeons allow completion of liver resection with low mortality and low blood transfusion, but despite this, the survival is not optimal.
Feasibility should not be the main factor dictating the choice of treatment. Instead, the potential impact of the various options on chance of survival should guide decisions.
Conclusion
The endpoint of treatment is to provide the longest survival with the less impaired quality of life. Treatment indication should be changed from what can be done to what is worth doing.
Future perspective
Hepatocarcinoma continues to be the leading cause of death in patients with cirrhosis. Regular surveillance of patients at risk is essential to promote early diagnosis and the chance to apply the most appropriate treatments for the stage of disease, depending on team experience and the availability of donor organs. Development of effective preventive agents to prevent recurrence after liver resection are needed. Molecular cell biology will help identify new therapeutic strategies and novel targets for advanced stage hepatocellular carcinoma.
Footnotes
Financial & competing interests disclosure
J Fuster has been funded by an investigation grant from Association Llavaneres contra el Cáncer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann. Surg. 1993;2:145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llovet JM, Bruix J, Gores G. Surgical resection vs transplantation for early hepatocellular carcinoma: clues for the best strategy. Hepatology. 2000;31:1919–1921. doi: 10.1053/he.2000.6959. [DOI] [PubMed] [Google Scholar]
- 3.Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver. Transplant. 2004;(Suppl. 1):S39–S45. doi: 10.1002/lt.20040. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:519–524. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]; •• A practice guidelines on the management of hepatocellular carcinoma published by the American Association for the Study of Liver Diseases (AASLD).
- 5.Llovet JM, Fuster J, Bruix J. Intention to treat analysis of surgical treatment for early hepatocellular carcinoma: resection vs transplantation. Hepatology. 1999;39:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]; •• After listing for orthotopic liver transplantation, patients have to wait for a variable period of time during which the tumor may progress and preclude the operation. This adverse event worsens the outcomes when an intention-to-treat analysis of orthotopic liver transplantation results is performed.
- 6.Sala M, Fuster J, Llovet JM, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transplant. 2004;10:1294–1300. doi: 10.1002/lt.20202. [DOI] [PubMed] [Google Scholar]
- 7.Bedossa P, Poitou C, Veyre N, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidity obese patients. Hepatology. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 8.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 9.Ge PL, Du SD, Mao YL. Advances in preoperative assessment of liver function. Hepatobiliary Pancreat. Dis. Int. 2014;13:361–370. doi: 10.1016/s1499-3872(14)60267-8. [DOI] [PubMed] [Google Scholar]
- 10.Wong JSW, Wong GLH, Chan AWH, et al. Liver stiffness measurement by transient elastography as a predictor on posthepatectomy outcomes. Ann. Surg. 2013;257:922–928. doi: 10.1097/SLA.0b013e318269d2ec. [DOI] [PubMed] [Google Scholar]
- 11.Fung J, Poon RT, Yu WC, et al. Use of liver stiffness measurement for liver resection surgery: with indocyanine green clearance correlation testing and post-operative outcome. PLoS ONE . 2013;8(8):e72306. doi: 10.1371/journal.pone.0072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berzigotti A, Seijo S, Arena U, et al. Elastography, spleen size, platelet count and identify identity portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102–111. doi: 10.1053/j.gastro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Llop E, Berzigotti A, Reig M, et al. Assessment of portal hypertension by transient elastography in patients with compensated cirrhosis and potentially resectable liver tumors. J. Hepatol. 2012;56:103–108. doi: 10.1016/j.jhep.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin. Surg. Oncol. Liver Cancers. 1993;9:298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- 15.Lam CM, Fan ST, Lo CM, Wong J. Major hepatectomy for hepatocellularcarcinoma in patients with an unsatisfacory indocyanine green clearance test. J. Surg. Br. 1999;86:1012–1017. doi: 10.1046/j.1365-2168.1999.01204.x. [DOI] [PubMed] [Google Scholar]
- 16.Schneider PD. Preoperative assesment of liver function. Surg. Clin. North Am. 2004;84:355–373. doi: 10.1016/S0039-6109(03)00224-X. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Kwon CHD, Joh JW, et al. Can the model for end-stage liver disease score replace the indocyanine green clearance test in the selection of right hemihepatectomy in Child-Pugh class A? Ann. Surg. Treat. Res. 2014;86:122–129. doi: 10.4174/astr.2014.86.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 19.Cucchetti A, Ercolani G, Vivarelli M, et al. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12:966–971. doi: 10.1002/lt.20761. [DOI] [PubMed] [Google Scholar]
- 20.Delis SG, Bakoyiannis A, Biliatis I, Athanassiou K, Tassopoulos N, Dervenis C. Model for end-stage liver disease (MELD) score, as a prognostic factor for post-operative morbidity and mortality in cirrhotic patients, hepatectomy for hepatocellular carcinoma Undergoing. HPB (Oxford) 2009;11:351–357. doi: 10.1111/j.1477-2574.2009.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HBP. 2005;7:35–41. doi: 10.1080/13651820410024058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 1999;19:329–328. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]; • The Barcelona Clinic Liver Cancer staging and treatment strategy has gained wide acceptance and endorsement because of its stratification capacity and its linkage of staging with treatment indication.
- 23.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging score (JIS score) J. Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 24.Chevret S, Trinchet JC, Mathieu D, et al. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire. J. Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 25.Daniele B, Annunciata M, Barletta E, et al. Cancer of the Liver Italian Program (CLIP) score for staging hepatocellular carcinoma. Hepatol. Res. 2007;37(Suppl. 2):S206–S209. doi: 10.1111/j.1872-034X.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 26.Vauthey JN, Klimstra D, Blumgart LH. A simplified staging system for hepatocellular carcinomas. Gastroenterology. 1995;108:617–618. doi: 10.1016/0016-5085(95)90109-4. [DOI] [PubMed] [Google Scholar]
- 27.Ikai I, Takayasu K, Omata M, et al. Liver Cancer Study Group of Japan. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J. Gastroenterol. 2006;41:884–892. doi: 10.1007/s00535-006-1878-y. [DOI] [PubMed] [Google Scholar]
- 28.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 29.Fuster J, García-Valdecasas JC, Grande L, et al. Hepatocellular carcinoma and cirrhosis. Results of surgical treatment in a European series. Ann. Surg. 1996;223:297–302. doi: 10.1097/00000658-199603000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]; •• Absence of clinically significant portal hypertension measured by hepatic vein catheterization (hepatic vein pressure gradient <10 mmHg) is the best predictor of excellent outcome after surgery, with almost no risk for postoperative liver failure.
- 31.Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J. Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 33.Grieco A, Pompili M, Caminiti G, et al. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy:comparison of Okuda, CLIP, and BCLC staging system in a single Italian centre. Gut. 2005;54:411–418. doi: 10.1136/gut.2004.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]; • Analyzes the natural history and prognostic factors of patients with nonsurgical hepatocellular carcinoma (HCC). Twenty variables from 102 cirrhotic patients with HCC who were not treated within prospective randomized controlled trials (RCT) were investigated through uni- and multi-variate analyses. The outcome of nonsurgical HCC is not homogeneously grim and may be predicted by assessing the presence of symptoms and of an invasive tumoral pattern. Therapeutic trials should be designed and evaluated considering these characteristics.
- 35.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez de Lope C, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J. Hepatol. 2012:S75–S87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 37.Yau T, Tang WYF, Yao TJ, Fan ST, Lo CM, Poon RTP. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691–1700. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 38.Shermann M. Staging for hepatocellular carcinoma: complex and confusing. Gastroenterology. 2014;146:1599–1602. doi: 10.1053/j.gastro.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Bosch J. Vascular deterioration in cirrhosis: the big picture. J. Clin. Gastroenterol. 2007;41:S247–S253. doi: 10.1097/MCG.0b013e3181572357. [DOI] [PubMed] [Google Scholar]
- 40.García–Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N. Engl. Med. 2010;362:823–832. doi: 10.1056/NEJMra0901512. [DOI] [PubMed] [Google Scholar]
- 41.D'Amico G, García-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J. Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Reig M, Berzigotti A, Bruix J. If portal hypertension predicts outcome in cirrhosis, why should this not be the case after surgical resection? Liver Int. 2013;33:1454–1456. doi: 10.1111/liv.12273. [DOI] [PubMed] [Google Scholar]
- 43.Cappussotti L, Ferrero A, Vigano L, Muratore A, Polastri R, Bouzari H. Portal hipertensión: contraindication to liver surgery? World J. Surg. 2006;30:992–999. doi: 10.1007/s00268-005-0524-9. [DOI] [PubMed] [Google Scholar]
- 44.Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]; •• Concludes than neither the presence of multiple tumors nor portal hypertension is a contraindication to liver resection. However, their 5-year survival rate was poorer in their group with portal hypertension than in their group with no portal hypertension, either in Child A or Child B patients.
- 45.Cucchetti A, Ercolani G, Vivarelli M, et al. Is portal hypertension a contraindication to hepatic resection? Ann. Surg. 2009;250:922–928. doi: 10.1097/SLA.0b013e3181b977a5. [DOI] [PubMed] [Google Scholar]
- 46.Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal hypertension on the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology. 2014 doi: 10.1002/hep.27431. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.EASL-EORT Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East-West Study Group. Ann. Surg. 2013;257:929–937. doi: 10.1097/SLA.0b013e31828329b8. [DOI] [PubMed] [Google Scholar]
- 49.Bruix J, Fuster J. Letter to Editor. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? an observational study of the HCC East-West Study Group. Ann. Surg. Ann. Surg. 2015;262(1):e30. doi: 10.1097/SLA.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 50.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinoma in patients with cirrhosis. N. Engl. J. Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 51.Mazzaferro V, Roayaie S, Poon RT, Majno P. Letter to Editor. Dissecting EASL/AASLD. Recommendations with a more careful knife: a comment on ‘Surgical misinterpretation’ of the BCLC Staging System. Ann. Surg. 2015;262(1):e17–18. doi: 10.1097/SLA.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 52.Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br. J. Surg. 2007;94:320–326. doi: 10.1002/bjs.5622. [DOI] [PubMed] [Google Scholar]
- 53.Zhou WP, Lai EC, Li AJ, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann. Surg. 2009;249:195–202. doi: 10.1097/SLA.0b013e3181961c16. [DOI] [PubMed] [Google Scholar]
- 54.Yang T, Sun YF, Zhang J, et al. Partial hepatectomy for ruptured hepatocellular carcinoma. Br. J. Surg. 2013;100:1071–1079. doi: 10.1002/bjs.9167. [DOI] [PubMed] [Google Scholar]
- 55.Cheng LT, Chen MF, Li LA, et al. Long-term results of a randomized, observation-controlled, Phase III trial of adjuvant interferon alfa -2b in hepatocellular carcinoma after curative resection. Ann. Surg. 2012;255:8–17. doi: 10.1097/SLA.0b013e3182363ff9. [DOI] [PubMed] [Google Scholar]
- 56.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann. Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The survival results of patients with concomitant two or three nodules and cirrhosis were particularly poor compared with other subgroup of patients, and suggest that primary transplantation may be a more appropriate option for this subset of patients.
- 57.Nojiri K, Tanaka K, Takeda K, et al. The efficacy of liver resection for multinodular hepatocellular carcinoma. Anticancer Res. 2014;34:2421–2426. [PubMed] [Google Scholar]
- 58.Choi SH, Choi GH, Kim SU, et al. Role of surgical resection for multiple hepatocellular carcinoma. World. J. Gastroenterol. 2013;19:366–374. doi: 10.3748/wjg.v19.i3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim PT, Jang JH, Atenafu AG, et al. Outcomes after hepatic resection and subsequent multimodal treatment of recurrence for multifocal hepatocellular carcinoma. Br. J. Surg. 2013;100:1516–1522. doi: 10.1002/bjs.9263. [DOI] [PubMed] [Google Scholar]
- 60.Yin L, Li H, Li AJ, et al. Partial hepatectomy vs transcatheter arterial chemoembolization for resectable multiple hepatocarcinoma beyond Milan criteria: a RCT. J. Hepatol. 2014;61:82–88. doi: 10.1016/j.jhep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin. Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 62.Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver. Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 63.Takayasu K, Arii S, Kudo M, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J. Hepatol. 2012;56:886–892. doi: 10.1016/j.jhep.2011.10.021. [DOI] [PubMed] [Google Scholar]; •• Demonstrates that the 3- and 5-year survival rates of TACE were 55 and 34%. The outcome may not differ from that to be obtained by liver resection for those patients with multifocal HCC within the Milan criteria.
- 64.Malagari K, Pomoni M, Moschouris H, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc. Intervent. Radiol. 2012;35:1119–1128. doi: 10.1007/s00270-012-0394-0. [DOI] [PubMed] [Google Scholar]
- 65.Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolization (TACE) using drug eluting beads. Implications for clinical practice and trial design. J. Hepatol. 2012;56(6):1330–1335. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]