Abstract
Hepatocellular carcinoma is one of the most common malignancies and represents a unique challenge for physicians and patients. Treatment patterns are not uniform between areas despite efforts to promote a common protocol. Even if most hepatologists worldwide adopt the Barcelona Clinic Liver Cancer staging system, Asian and North American physicians are also independently making an effort to expand the indications of each treatment, combining therapies for better outcomes. Also, new therapeutic techniques have emerged and an increasing number of studies are trying to include these paradigm shifts into newer treatment guidelines. Controversial and diverging points in the current international guidelines are emphasized and discussed. Unanswered questions are also analyzed to identify the most needed and promising future perspectives.
KEYWORDS : chemoembolization, hepatocellular carcinoma, thermal ablation
Practice points.
Different guidelines regarding the therapeutic management of hepatocellular carcinoma (HCC) have been proposed and adopted in North America, Europe and Asia-Pacific region. Despite clearly visible differences in general concept, actual differences in the proposed treatments are limited.
The most relevant differences relate to liver resection, as American Association for the Study of Liver Diseases/European Association for Study of Liver guidelines recommend the presence of a single nodule and a Child-Pugh A functional status, whereas other guidelines accept multinodularity and a Child-Pugh B status, in selected cases.
Special therapeutic strategies have been included in particular guidelines, local ablation in hypovascular early HCC in the Japan Society of Hepatology guidelines and external-beam radiotherapy in National Comprehensive Cancer Network guidelines, respectively.
Currently, open issues include: role of the length of the HCC medical history in the prognosis; subclassification of the Barcelona Clinic for Liver Cancer B stage; possibility of combined treatments. Clinical adherence to the guidelines represents another challenging issue.
Liver cancer is the fifth most frequently diagnosed cancer worldwide, representing the second leading cause of cancer-related death in the world [1,2]. Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer and is still encumbered by an overall poor prognosis, resulting in 250,000–1,000,000 deaths globally per annum [3,4]. The incidence of HCC varies widely according to geographic location, as different etiologic factors are involved in the carcinogenic process.
According to the Globocan epidemiologic data, HCC is largely a problem of the less developed regions, where 83% (50% in China alone) of the estimated 782,000 new cancer cases worldwide occurred in 2012 [5]. The disease burden is highest in areas with endemic HBV infection, such as in sub-Saharan Africa and Eastern Asia, with incidence rates of over 20 per 100,000 individuals. Mediterranean countries, including Spain, Italy and Greece show less pronounced incidence rates (10–20 per 100,000 individuals), while the whole American continent still has a lower incidence (<5 per 100,000 individuals) [5]. However, incidence of HCC is on the rise in many western countries and a further increase of cases is expected in Central Europe and in the USA in the next years [5]. This trend is not supported by a higher prevalence of viral infections (for which more and more therapeutic options are becoming available) but rather by a parallel increase in prevalence of nonalcoholic fatty liver disease (NAFLD) and of its most aggressive form, nonalcoholic steatohepatitis (NASH) [6]. The number of deaths per year is virtually identical to the incidence (overall ratio of mortality to incidence of 0.95), underlining the high fatality rate of HCC. As such, the geographical patterns in incidence and mortality are very similar [5]. HCC usually develops on an underlying liver cirrhosis (80–90%), whatever the etiology of the disease [7]. Cirrhosis related to HBV and HCV infections, alcohol abuse and metabolic disorders (NAFLD-NASH) show a higher incidence of HCC in comparison to autoimmune hepatitis and cholestatic diseases [8]. Available therapeutic options for HCC are, therefore, dictated by the complex interplay of tumor stage and the extent of underlying liver disease.
Prognosis & staging
Four main factors affect prognosis in patients with HCC: stage, aggressiveness and growth rate of the tumor; general health of the patient; liver function of the patient; and HCC treatments administered [7].
HCC usually arises in cirrhotic livers, with different degrees of functional impairment [9]. Thus, the prognosis of HCC depends both on the degree of neoplastic spread and on the residual hepatic function.
An accurate evaluation of the hepatic function is of paramount importance for a correct prognosis and to avoid aggressive treatments potentially jeopardizing the patient’s safety.
In fact, treatments targeting a large portion of a cirrhotic liver may compromise the overall advantages of antineoplastic action or even reduce life expectancy.
Another critical factor for the identification of the most appropriate treatment is represented by the tumor staging.
The tumor nodes metastasis (TNM) staging system, according to the American Joint Committee on Cancer (AJCC) represented a first pathologic score to evaluate the prognosis of HCC patients [10].
Differently from what normally happens for the majority of solid tumors, however, many difficulties arise in evaluating histology parameters such as microscopic nodal vascular invasion and microscopic porta hepatis lymphnodes invasion.
In fact, only a small portion of HCC patients can be proposed for liver resection and lymphoadenectomy is not routinely performed due the high risk of postoperative ascites. Consequently, morphological staging is usually made on a radiologic base alone.
During the last decades different clinical staging systems have been proposed. The algorithms designed by the Barcelona Clinic for Liver Cancer (BCLC) [11] represent currently the most used staging system in western countries.
The BCLC staging system separates HCC patients in four groups including patients with less than three nodules smaller than 3 cm (BCLC-A, early stage), patients with multifocal or bigger nodules (BCLC B, intermediate stage), patients with macrovascular invasion and/or extrahepatic spread (BCLC C, advanced stage) and patients with terminal hepatic failure not amenable for liver transplantation (LT) and/or heavily compromised performance status, irrespective of the tumor spread (BCLC D, terminal stage).
The early stage is subsequently divided into five subgroups according to number of nodules, presence of portal hypertension and total bilirubin (ranging from A0, in other words, patients with single nodule <2 cm to A4, in other words, patients with up to three nodules possibly with portal hypertension and mild hyperbilirubinemia).
The importance of BCLC staging system relies on its ability to predict different median survival across the various groups, with an overall median survival of 43 months for BCLC A1 patients, 22 months for BCLC A4 patients, 18 months for BCLC B patients and 11 months for BCLC C patients [12].
Still, there is no universal consensus as to which staging system is best in predicting the survival of patients with HCC. In general, pathologic staging systems, such as the AJCC TNM staging system, predict prognosis better than do clinical systems when assessing the outcomes of resection in patients with well-preserved liver function, whereas BCLC and other clinical scores are more useful for predicting outcomes in patients undergoing nonsurgical therapy and/or with impaired liver function [13].
As per these evidences, ‘different systems for different patients’ was one of the bullet points in the consensus of the American Hepato Pancreato Biliary Association. Their consensus statement (updated in 2010) recommended the use of the TNM system to predict outcome following resection or LT and the BCLC scheme for patients with advanced HCC who are not candidates for surgery [14].
General overview of current guidelines
Until the 1990s the classifications of HCC were based on prognostic factors obtained from studies performed when most tumors were diagnosed at advanced stages and the survival rates were substantially poor.
Progresses in ultrasound technologies (which came with the possibility of an earlier diagnosis in the setting of screening programs), as well as in surgical and nonsurgical techniques, gradually offered most therapeutic options for a condition, which had been considered being an almost universal death sentence [15].
Reflecting this shift, in 1999 the first BCLC classification tried to match each different stage of the HCC with different therapeutic procedures [11]. The rational was to offer to the patients with early-stage disease (BCLC A) potentially curative options (such as surgical resection, radiofrequency ablation, percutaneous ethanol injection) as their liver function are more likely to recover from these radical procedures. On the other hand, it was stated that patients with multifocal disease and/or mildly compromised liver function should undergo palliative but better-tolerated procedures, such as transarterial chemoembolization [11].
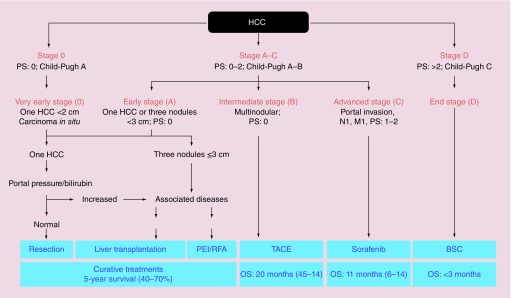
In these terms the BCLC represented the first staging system also providing evidence-based therapeutic recommendations. BCLC recommendation were updated in 2010 on the basis of new therapeutic evidences (Figure 1) [15], including but not limited to the SHARP clinical trial [16], that demonstrated the efficacy of sorafenib for the treatment of advanced HCC (BCLC C). As such, the BCLC recommendations are endorsed by the American Association for the Study of Liver Diseases (AASLD) in their guidelines for the treatment of HCC [15]. and also by the European Association for Study of Liver (EASL) and by the European Organization for Research and Treatment of Cancer (EORTC) [17].
Figure 1. . The 2010 Barcelona Clinic for Liver Cancer algorithm for the treatment of hepatocellular carcinoma.
BSC: Best supportive care; HCC: Hepatocellular carcinoma; OS: Overall survival; PEI: Percutaneous ethanol injection; PS: Performance status; RFA: Radiofrequency ablation; TACE: Transarterial chemoembolization.
Reproduced with permission from [15].
The BCLC staging system has come to be widely accepted in clinical practice and is also being used for many clinical trials of new drugs to treat HCC. Although widely used in many western countries, this system has been criticized because of its rigid algorithmic, rather than patient-centered approach. Furthermore, criticism arose as intermediate stage (BCLC B), the most frequent onset presentation, include a rather heterogeneous group of patients ranging from single large nodules to miliariform HCC for whom TACE was the only proposed therapeutic option [15].
Consequently, worldwide scientific medical societies have proposed different therapeutics algorithms, leading to the birth of new guidelines. The Japan Society of Hepatology (JSH) suggests a stratification of HCC patients according to five staging aspects: presence/absence of extrahepatic spread, residual liver function, presence/absence of vascular invasion, number and size of lesions [18]. According to the overall clinical picture each patient is proposed for a first-line treatment with specification of possible therapeutic alternatives, allowing a certain degree of flexibility for the clinician. In the USA, the National Comprehensive Cancer Network (NCCN) guidelines create three main groups of patients: resectable, unresectable and inoperable [19]. Each group is further divided into different subgroups according to specific stratification factors [19]. Despite clearly visible differences in general concept, actual differences in the proposed treatments are limited. Differences are usually dictated by the different clinical trials that have been considered in the creation of the guidelines (in particular in terms of perioperative mortality, disease recurrence and overall survival after each specific therapeutic procedure (Table 1).
Table 1. . Synopsis of the main parameters considered in the creation of the various guidelines.
| Procedure | Parameters | AASLD (%) | APASL (%) | NCCN (%) | JSH (%) |
|---|---|---|---|---|---|
| Resection | Mortality: | 2–3 | <5 | <5 | 0.8 |
| OS (follow-up): | 60 (5 years) | 35–50 (5 years) | 50–70 (5 years) | 53.4 (5 years) | |
| Transplantation | Mortality: | 3 | <5 | NR | NR |
| OS (follow-up): | 70 (5 years) | 60–75 (5 years) | 38–93 (5 years) | NR | |
| Percutaneous treatments (RFA) | Morbidity: | 4 | 0.7–7.9 | 4.8 | NR |
| Recurrence: | 2–18 (2 years) | NR | 50 (4 years) | NR | |
| OS (follow-up): | 70 (5 years) | 39.9–68.5 (5 years) | NR | NR | |
| Transarterial therapies | Mortality: | 2 | <5 | <5 | NR |
| PR rate: | 15–55 | 16–60 | 30 | NR | |
| OS (follow-up): | 50 (20 months) | 47 (3 years) | 51 (3 years) | NR | |
AASLD: American Association for the Study of Liver Diseases; APASL: Asian-Pacific Association for the Study of the Liver; JSH: Japanese Society of Heptology; NCCN: National Comprehensive Cancer Network; NR: Not reported; OS: Overall survival; PR: Partial response; RFA: Radiofrequency ablation.
In the following paragraphs, we will examine the indications and the contraindications for each therapeutic options according to the different international guidelines.
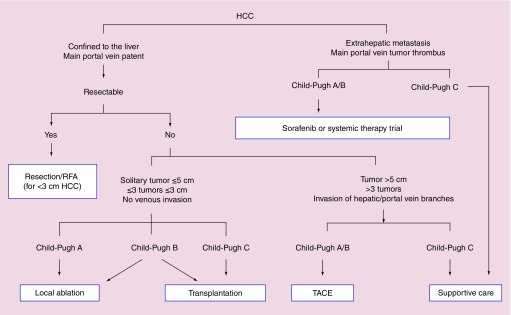
In particular, this review will focus on the following guidelines and recommendations: AASLD, EASL-EORTC [15], JSH [18], NCCN [19], Asian-Pacific Association for the Study of the Liver (APASL) [20] (Figure 2) and Italian Association for the Study of the Liver (AISF) [21].
Figure 2. . The 2010 Asian-Pacific Association for the Study of the Liver guidelines for the treatment of hepatocellular carcinoma.
HCC: Hepatocellular carcinoma; RFA: Radio-frequency ablation; TACE: transarterial chemoembolisation.
Reproduced with permission from [20].
Surgical resection
Surgical resection represents the best treatment, with curative intent, for early-stage HCC in patients with preserved liver function. This technique can now be performed with a low perioperative mortality (2–3%), blood transfusion requirement of less than 10% and with an expected 5-year survival rate of 60% in cirrhotic patients [22]. These outcomes have been obtained as a result of accurate selection of candidates, improved surgical techniques and optimal immediate postoperative management.
However, two main determinants of overall survival in patients undergoing this procedure are underlying liver function and tumor recurrence. Therefore, different international guidelines have, as common aim, the most appropriate selection criteria of the candidates.
The principal discrepancies between the different guidelines, in terms of tumor extension, portal hypertension, liver functional status and adjuvant/neoadjuvant therapies are reported in Table 2.
Table 2. . Synopsis of principal guidelines recommendations and contraindications for liver resection in patients with hepatocellular carcinoma.
| Organization | Extrahepatic metastasis | Vascular invasion | Liver function | Tumor extension | Adjuvant therapies |
|---|---|---|---|---|---|
| EASL-EORTC | Contraindication | Contraindication | Child-Pugh A, normal bilirubin with either hepatic venous pressure gradient 10 mmHg or platelet count 100,000/mmc | Solitary tumor | Not recommended. Further evidence required to be adopted |
| NCCN | Contraindication | Contraindication, but major vascular invasion can be considered | Child-Pugh A (B in highly selected cases) Adequate FLR |
Solitary tumor. Multinodular is controversial but can be considered | Not recommended |
| APASL | Contraindication | Not main portal vein, but major intrahepatic venous branches can be considered | ‘Satisfactory liver function reserve’ | Solitary or multifocal tumor | Not recommended |
| JSH | Contraindication | Third or fourth branch of portal venous invasion is sometimes admitted | Child-Pugh A or B | First choice for patients with up to three nodules. Can be considered for patients with more nodules | Not recommended |
| AISF | Contraindication | Contraindication. Peripheral portal invasion requires comparative clinical studies | Similar to EASL, but Child-Pugh B, hyperbilirubinemia, portal hypertension can be evaluated by a multidisciplinary team with great expertise | Solitary tumor. Multinodular can be evaluated by a multidisciplinary team with great expertise | Not recommended. Should be tested in prospective studies |
AISF: Italian Association for the Study of the Liver; APASL: Asian-Pacific Association for the Study of the Liver; EASL-EORTC: European Association for Study of Liver-European Organization for Research and Treatment of Cancer; FLR: Future liver remnant; JSH: Japanese Society of Heptology; NCCN: National Comprehensive Cancer Network.
About this, all guidelines recommend, as essential conditions, the presence of a preserved liver function, represented in general by Child-Pugh class A and absence of significant portal hypertension. Only JSH consider resection as mainstay therapy also for Child-Pugh class B patients. Furthermore, extrahepatic metastasis and gross vascular thrombosis are always recognized as contraindications.
In particular, both NCCN and EASL-EORTC recommend hepatectomy in patients with solitary tumor with adequate liver function, mild or moderate portal hypertension and without vascular invasion and extrahepatic metastasis. In addition, NCCN establish that the postoperative future liver remnant (FLR) volume should be at least 20% in noncirrhotic patients and approximately 30–40% in Child-Pugh A cirrhosis [23].
On the other hand, APASL practice guidelines appear to be more liberal as they recommend resection (when it is technically feasible) in patients with a preserved liver function and solitary or multifocal HCC confined to liver, without mentioning any limit of size or number of nodules [20]. Indeed, although single nodule ≤5 cm represents the best candidate, several studies have shown reasonable long-term survival also for those with a larger tumor [24,25]. In effect, there are solid evidences that resection can be successfully performed also in patients with portal hypertension and multinodular tumor provided that they are adequately selected [26,27].
Hence, NCCN suggests that hepatic resection is controversial, but could be considered, in patients with multifocal disease and major vascular invasion, in selected cases [19]. Instead, according to EASL-EORTC, is under investigation but not yet recommended resection in multifocal tutors (three nodules 3 cm) or in patients with mild hypertension not suitable for LT [23].
On the other hand, JSH Guidelines seems to be less restrictive about resection. Effectively, it is considered the mainstay therapy in patients with three or less HCC without extrahepatic lesions or vascular invasion in which liver function is good (if ≤3 cm local ablation can be performed with the same favorable prognosis; if >3 cm TACE can be performed as second-line treatment, or adding local ablation to previous TACE as third-line treatment) [18]. In the presence of vascular invasion resection is performed for patients with third- or fourth-branch of portal venous invasion, if possible. Furthermore, it can be a third treatment choice in four or more lesions (in this case TACE is the first choice) [18].
AISF position is similar to EASL-EORTC guidelines but has some adjustments in candidate selection. Indeed, patients with preserved liver function and single tumor >5 cm have to be multidisciplinary evaluated for hepatectomy, as well as those with hyperbilirubinemia and Model for End-Stage Liver Disease (MELD) score 8–10 [21]. Furthermore, they suggest, as example of potential candidates selection protocol, the use of Bologna Liver Oncology Group (BLOG) algorithm: it is based on MELD values (≤10), serum sodium level (≤140) and the size of hepatic resection [26].
In patients with borderline or inadequate FLR volume, some groups apply preoperative portal vein embolization of the branches supplying the portion of the liver to be resected, in order to increase the residual liver volume [28,29]. However, the efficacy of portal vein embolization in the frame of HCC in cirrhosis has not been yet properly tested in large controlled studies. For this reason, currently none of the guidelines incorporate that approach in its recommendations, even if NCCN allow that it can be considered for major resection.
About neoadjuvant or adjuvant therapies, all guidelines agree that there are no proven to improve outcome of patients treated with resection or local ablation [30], hence they are not recommended.
Finally, many data have been collected on laparoscopic liver resection, that is now considered an alternative noninvasive technique aimed to prevent liver deterioration [31]. The recent advances in this approach have led to a rising use of video-laparoscopic resection, but all agree that it needs prospective comparison with traditional laparotomic resection before any change in current practice is made.
LT
LT is an efficient, potentially curative therapeutic option for patients who meet Milan criteria (solitary tumor not more than 5 cm or up to three nodules, each one not more than 3cm and no evidence of macrovascular involvement or extrahepatic disease) [32].
All international guidelines recognize Milan criteria as valid method of candidates selection. Following strictly these criteria for LT, transplanted patients with cirrhosis and HCC, present, today, a 4-year overall survival and a disease-free survival of 85 and 92%, respectively [32]. Furthermore perioperative, 1- and 5-year mortality is 3, <10 and <30%, respectively [32]. In general, liver transplantation by cadaveric donor represents the curative treatment of choice for patients with early-stage HCC and compromised liver function (Child-Pugh class B or C) and who are eligible for this procedure. Currently, there are not studies that compare transplantation to surgical resection, but at the moment the general recommendation is to perform the first when liver function is compromised and the second when liver function is preserved [33].
EASL/EORTC guidelines recommend LT for patients with Child-Pugh A, B or C liver function who meet Milan criteria and are not suitable for resection [16]. NCCN are similar since they adopt United Network for Organ Sharing (UNOS) criteria (that are analog to Milan criteria) and recommend LT for patients with Child-Pugh B or C liver function, including Child-Pugh A only when resection is not applicable [19]. APASL guidelines consider LT the first therapeutic option for HCC meeting Milan criteria in Child-Pugh B or C liver function [20], even if MELD score ≥20 and AFP ≥455 ng/ml are recognized as poor prognostic markers [34].
Otherwise, JSH recommends LT as first-choice treatment to patients aged 65 years or younger, with an unfavorable liver function and Child-Pugh class C in the absence of vascular invasion. However, even when liver function is good (Child-Pugh A/B), transplantation is sometimes considered for frequently recurring HCC patients.
A controversial topic is the possibility of expanding Milan criteria. There is evidence that the transplant can provide good results in patients who exceed the limits set by the Milan criteria, provided they meet other criteria, such as the University of California at San Francisco (UCSF) criteria [35] and the methods defined ‘up to seven’ (the sum of the size – in centimeters – the number of nodules should be ≤seven) [36] or the criterion based on total tumor volume [37].
Although the expanded criteria have been supported by some other studies [38], there are inadequate data in the literature to validate the long-term survival results using expanded criteria. Moreover, expanding Milan criteria involves a greater liver demand resulting in a lengthening of the waiting list, that is another important topic. About that, AISF recommendations suggest that live donor liver transplantation represents the ideal procedure to test, through controlled clinical trials, the use of ‘expanded criteria’ application. In fact, the use of an organ obtained outside of donations from cadaver does not involve any disadvantage for patients with or without HCC within the Milan criteria [21].
The long waiting list can bring patients out from selection criteria. Almost all guidelines suggest, as a possible solution, bridge treatments and live donor LT. In fact, several studies have investigated the role of loco-regional treatment of HCC as a bridge to LT [39,40] and, according to EASL-EORTC, APASL and AISF, if the wait is more than 6 months, bridge therapies including resection, percutaneous ablation and TACE can be adopted before liver graft.
An additional choice to reduce significantly the waiting list is live donor LT that, if realized in centers with significant experience of hepatic resection and partial-liver transplantation, can lead to a potential advantage [41,42].
Finally, in all guideline there are no formal recommendation about downstaging therapy despite promising results have been obtained by some transplant centers adopting this technique [43–45].
Locoregional therapies
All HCC patients should be evaluated for potential curative treatments, such as resection and transplantation. Those patients not candidates for these therapies may be treated with locoregional approaches. They mainly consist of percutaneous ablation, which is still considered a curative treatment, and transarterial therapies.
Percutaneous ablation
Ablation is a locoregional therapy that represents primary treatment for small HCCs not candidate to resection or transplantation, but also in some patients with resectable HCC, such as those with tumors <2 cm, and as bridge therapy [40,46]. It can be performed with chemical substances, such as ethanol (percutaneous ethanol injection [PEI]) and acetic acid, or using physical methods such as radiofrequency ablation (RFA), microwave or cryoablation, in order to induce cellular death and tumor necrosis. Ablation is usually performed using percutaneous approach, under ultrasound guide, but it can be implemented also with open or laparoscopic surgery. Obviously, as long as this technique is successful, it should be done with caution when tumor is subcapsular, near major vessels or gross bile ducts [19].
Actually, the most widely used are RFA and PEI [18], but RFA proved to be more effective in terms of local recurrence, treatment response and overall survival [47,48]. The largest study comparing RFA to PEI involved 232 patients with HCC and demonstrated that RFA is superior to ethanol injection with respect to the local tumor progression rate (1.7 vs 11% at 4 years), the local recurrence rate (70 vs 85% at 4 years), overall survival (74 vs 57% at 4 years) and the number of treatment sessions required (2.1 vs 6.4 sessions) with equal morbidity [49]. Meta-analysis of RCTs comparing PEI and RFA, confirm these figures [47,48]. For this reason all guidelines recommend the use of RFA rather than PEI, that can be performed when RFA is not technically feasible or when there are contraindications (for risk of complications or ineffectiveness) to the use of RFA (that are more common [49–51]).
In general, all guidelines agree that RFA is the treatment of choice for patients with early-stage HCC, unresectable and not transplantable, meeting Milan criteria (≤ three nodules, each ≤3 cm), without vascular invasion and with good liver function (Child-Pugh A or B) (Table 3).
Table 3. . Synopsis of principal guidelines recommendations and contraindications for ablative therapies in patients with hepatocellular carcinoma.
| Organization | Extrahepatic metastasis/vascular invasion | Tumor extension | Liver function | Technique | Combined treatments |
|---|---|---|---|---|---|
| EASL-EORTC | Contraindication | Single nodule or three or fewer nodules each ≤3 cm when resection is not possible | Child-Pugh A or B | RFA better than PEI in nodules <5 cm RFA = PEI in nodules <2 cm | Not considered |
| NCCN | Contraindication | First choice (as resection) in patients with three or fewer nodules of ≤3 cm | Child-Pugh A or B | No clear recommendations | TACE + ablation in nodules between 3 and 5 cm |
| APASL | Contraindication | Small HCC (<3 cm) with three or fewer nodules when resection is not possible Acceptable alternative to resection for small HCC | Child-Pugh A or B | No clear recommendations | No clear recommendations |
| JSH | Contraindication | First choice (as resection) in patients with three or fewer nodules of ≤3 cm (NCCN) Only guidelines that consider local ablation in hypovascular early HCC |
Child-Pugh A or B. Child-Pugh C is not an absolute contraindication (experimental treatment in patients meeting Milan criteria when hepatic encephalopathy and intractable ascites are absent and bilirubin is less than 3 mg/dl) | No clear recommendations | TACE + ablation in nodules >3 cm, when resection is not indicated or TACE + ablation in >four nodules |
| AISF | Contraindication | EASL but it is the first choice (as resection) in nodules ≤2 cm | Child-Pugh A or B | RFA better than PEI in nodules <2 cm | TACE + ablation in unresectable nodules >3 cm (<5?) |
AISF: Italian Association for the Study of the Liver; APASL: Asian-Pacific Association for the Study of the Liver; EASL: European Association for Study of Liver; EORTC: European Organization for Research and Treatment of Cancer; HCC: Hepatocellular carcinoma; JSH: Japanese Society of Heptology; NCCN: National Comprehensive Cancer Network; PEI: Percutaneous ethanol injection; RFA: Radiofrequency ablation; TACE: Transarterial chemoembolization.
However, an important topic is whether RFA could replace or be a valuable alternative to surgery.
Four randomized trials have not documented the superiority of resection compared with percutaneous ablation, in terms of survival and disease-free survival [52–55]. However, all these studies suffer from important methodological biases that do not allow to achieve incontrovertible evidence.
A fifth randomized study, involving 230 patients with HCC by the Milan criteria, indicates superiority of resection (with no perioperative mortality) than the RF, regardless of the size and number of HCC [56]. Finally, a comparative study between resection and RFA showed no difference in overall survival for patients with very early or early HCC, based on BCLC staging, after adjustment for confounding factors. The benefit observed with resection in terms of relapse-free survival was probably offset by greater repeatability ablation reported in the group receiving RF [57].
Finally, compared with resection, ablation is burdened with lower rates of morbidity and mortality, length of hospitalization and healthcare costs [53,56]. All these data refer to HCC ≤3 cm, because large retrospective studies and meta-analysis have further reaffirmed the superiority survival outcomes from surgical resection of HCCs measuring >3 cm [58,59].
Therefore, APASL [20] and JSH [18] recommend RFA as first choice, such as resection, for three or fewer than 3 cm or smaller nodules with no extrahepatic lesion, good liver function and no vascular invasion. Similarly, AISF guidelines affirm that RFA can be considered, in a multidisciplinary context and evaluated the localization of the lesion, the first-line treatment for single nodule up to 2 cm as, compared with resection, it is burdened with lower rates of morbidity and mortality, length of hospitalization and lower healthcare costs [21].
As already mentioned, when the tumor size exceeds 3 cm, hepatectomy is preferred. Alternatively, in unresectable patients, it is reasonable, in the actual clinical practice to consider sequential or combined treatments (additional local ablation following transarterial treatment), because it may increase curability [60–62]. However, combined treatments are still not considered by western (AASLD and EASL) guidelines and, actually, only JSH and NCCN include this option among their recommendations, NCCN specifying that this is the only application in tumor between 3 and 5 cm [18,19]. Ultimately JSH consider combined (ablation + TACE) treatments even when the lesions are more than 4 cm, but not as first therapeutic choice.
Noteworthy, the JSH guideline’s distinctive feature is the addition in their treatment algorithm of hypovascular lesions considered as early HCC. Indeed, hypovascular nodules diagnosed as probably malignant by biopsy, CT hepatic arteriography/CT arterial portography or SPIO-MRI may be subjected not only to intensive follow-up, but also to local ablation in clinical setting [18].
Concerning the assessment of ablation response, EASL-EORTC, NCCN recommend the use of dynamic CT or MRI (1 month and, thereafter, every 3–4 months after the procedure according to EASL-EORTC, 3 months after according to NCCN). Otherwise, AISF retains that contrast-enhanced ultrasound (CEUS) can provide documentary evidence of response/persistence of lesion’s vitality underwent to local ablation [63,64]; hence AISF accounts CEUS for response evaluation, suggesting however to repeat CT or MRI every 6-8 months [21]. JSH and APASL do not provide indication about this topic.
It is important to note that imaging findings after tumor ablation differ based both on ablation modality and imaging modality; furthermore, the accuracy is limited by both spatial and contrast resolution to approximately 2–3 mm depending on the imaging modality employed. Therefore, in order for the ablation to be considered successful, the target tumor should be completely covered by the ablation zone that includes at least a 5–10 mm margin all around the expected tumor margin [65].
Transarterial therapies
HCC exhibits intense neoangiogenic activity during its progression [66], and even well-differentiated HCC is mostly dependent on hepatic artery for blood supply. This characteristic provides the rationale to support that arterial obstruction with or without intra-arterial administration of chemotherapeutic agents induces ischemic tumor necrosis with a high-rate objective response [15]. Indeed, a meta-analysis including seven randomized controlled trials (RCT), with a total of 516 patients, showed a beneficial survival effect of embolization/chemoembolization in comparison to the control group [2].
Transarterial therapies can be performed with transcatheter embolization (TAE) where no chemotherapeutic agent is delivered, infusing intra-arterial chemotherapy where no embolization is performed (TAI/HAIC) or through intra-arterial infusion of a cytotoxic agent, usually mixed with lipiodol, followed by embolization of the tumor-feeding blood vessels usually with gelfoam (TACE).
The principal discrepancies between the different guidelines, in terms of tumor extension, liver functional status and presence of macrovascular neoplastic invasion are resumed in Table 4.
Table 4. . Synopsis of principal guidelines recommendations and contraindications for transarterial chemoembolization in patients with hepatocellular carcinoma.
| Organization | Extrahepatic metastasis | Vascular invasion | Liver function | Tumor extension |
|---|---|---|---|---|
| EASL-EORTC | Contraindication | Contraindication | Child-Pugh A or B7 without ascites. Performance status: 0 | Multinodular (BCLC B stage) |
| NCCN | Contraindication | Contraindicated in case of main portal vein thrombosis | Child-Pugh A or B Bilirubin <3 mg |
No limit (unresectable or nontransplantable patients) |
| APASL | Contraindication | TACE is admitted is case of invasion of hepatic/portal vein branches | Child-Pugh A or B | Tumor >5 cm or >three tumors that are unresectable Early stage in whom RFA cannot be performed |
| JSH | Contraindication | TACE is admitted in case of minor portal vein thrombosis. HAIC also in case of main portal branch invasion | Child-Pugh A or B Child-Pugh C is not an absolute contraindication (experimental treatment in patients meeting Milan criteria when hepatic encephalopathy and intractable ascites are absent and bilirubin is less than 3 mg/dl) |
First choice in patients with up to three nodules In ≤three nodules >3cm is a resection’s alternative First-line therapy for downstaging tumors that exceed the criteria for transplantation |
| AISF | Contraindication | Peripheral, segmental portal invasion is not an absolute contraindication | Child-Pugh A or B7 Performance status: 0 or 1 |
EASL and also early-stage HCC, if surgical or ablative techniques are not applicable |
AISF: Italian Association for the Study of the Liver; APASL: Asian-Pacific Association for the Study of the Liver; BCLC: Barcelona Clinic for Liver Cancer; EASL: European Association for Study of Liver; EORTC: European Organization for Research and Treatment of Cancer; HAIC: Hepatic arterial infusion chemotherapy; HCC: Hepatocellular carcinoma; JSH: Japanese Society of Heptology; NCCN: National Comprehensive Cancer Network; RFA: Radiofrequency ablation; TACE: Transarterial chemoembolization.
Conventional chemoembolization (cTACE) is the most widely used primary treatment for unresectable HCC [67], yet there is not convincing evidence in favor of TACE over TAE in terms of patient survival [68,69]. As a result, all guidelines recommend TACE as first-line treatment in unresectable large or multinodular disease that is not amenable to ablation therapy only, and absence of macrovascular invasion and extrahepatic spread [17,19–20].
Also, JSH guidelines agree with these recommendations but add more information: TACE is selected when the number of nodules are three or fewer and the tumor size exceeds 3 cm (as resection’s alternative), and when there are more than four nodules (TACE or hepatic arterial infusion chemotherapy) [18]. Besides it is also the first-line treatment for downstaging tumor that exceeds the criteria for transplantation [12,45].
As for exclusion criteria, all guidelines identify: main portal vein invasion and extrahepatic metastases. However, each one specifies some more contraindications. NCCN guidelines specify that TACE is contraindicated in Child-Pugh C patients and in subjects with total bilirubin >3 mg/dl [19].
According to EASL-EORTC, patients with Child-Pugh class B and C are not good candidates for TACE because of the elevated hepatic failure and mortality risk [70]. Instead, JSH Guidelines permit TACE also in Child-Pugh B patients: supporting that, a recent polycentric Japanese cohort study, which reflected the experience gained in 1296 patients belonging to this class, reports survivals of 82, 43 and 22% at 1, 3 and 5 years, respectively, and a mortality periprocedural of 0.62% [71]. Furthermore, always according to JSH, Child-Pugh class C is not an absolute contraindication because it is admitted as experimental treatment, in patients meeting Milan criteria when hepatic encephalopathy and intractable ascites are absent and serum bilirubin level is less than 3 mg/dl [18]. In addition, TACE can be performed in many cases of mild portal invasion (portal invasion at the third or more peripheral portal branch at the second portal branch) [19], and in main trunk or first branch portal vein invasion hepatic arterial infusion chemotherapy (HAIC) is a choice of treatment [18]. AISF position is close to JSH because it defines as best candidates for TACE asymptomatic Child-Pugh class A patients, although those with a Child-Pugh score of B7 or Eastern Cooperative Oncology Group – Performance Status (ECOG PS 1) can also be considered [21].
Furthermore, they are similar because the presence of peripheral, segmental portal invasion is not considered by AISF an absolute contraindication to TACE, that can be associated with systemic treatment in the frame of controlled clinical studies [21]. Consequently, TACE in mild macrovascular invasion is formally considered by Asian and AISF guidelines but not by EASL and NCCN.
There is a new strategy of chemoembolization using drug-eluting beads (DEB-TACE), that has been shown to grow the local drug concentration with less systemic toxicity [72]. DEB-TACE has also shown similar response rates than gelfoam-lipiodol and in the multicentric randomized PRECISION V study it proved to be more effective than cTACE in some subgroups of patients (Child-Pugh B, ECOG PS1, bilobar or relapsing HCC) [73]. On the other hand, a randomized controlled trial conduct by Golfieri et al. [74] did not demonstrate any difference in local response and overall survival between cTACE and DEB-TACE. For these inconclusive results, all guidelines agree that are necessary further confirmations to propose preferential use of DEB TACE in clinical practice. A complete response to TACE is uncommon [69] and it frequently entails the need to retreatment [75]. No guidelines lean toward the use of repeated TACE at regular, short intervals or ‘a la demande,’ because there are no solid evidences of the higher survival rates of one rather than the other strategy [76]. Only AISF recommend the retreatment ‘a la demande’ in the absence of radiologic evidence of disease persistence (complete response), due to its risks, costs and impact on the patient’s quality of life [69,73].
The definition of TACE failure is an important topic that, interestingly, is insufficiently discussed in most guidelines. Indeed, the only ones that provide a precise definition are JSH and AISF. JSH Guidelines define TACE failure or refractory when more than two consecutive incomplete necrosis (depositions [<50%] of lipiodol) or appearances of a new lesion (recurrence) are seen by response evaluation CT within the treated tumors 4 weeks after adequately performed TACE; when vascular invasion or extrahepatic spread appear or when there is continuous elevation of tumor markers even though right after TACE [18]. Similarly, the AISF expert panel considers failure of TACE the lack of objective response of the treated lesions after two procedures [21]. Nonetheless, considering bilobar distribution, number of lesions and patient tolerability, the number of sessions to define the failure should be established case-by-case in multidisciplinary decisional setting, and may greatly vary on an individual basis [21].
But how should response to TACE be evaluated? Generally it is defined by the intratumoral necrosis and the tumor mass reduction and it is assessed with dynamic CT or MRI. However, various imaging modalities have been used to evaluate the vascularity of HCCs treated with TACE [77,78] and many studies have shown the effectiveness of contrast-enhanced sonography in evaluating the therapeutic response of HCC treated with TACE [79,80]. Formally no guidelines recommend the use of CEUS to assess TACE response. Only AISF recommendations admit that CEUS can be used to ascertain disease persistence in patients in which the targets are one or two lesions [21].
Regardless of the imaging techniques, it should be stresses that a mere measurement of the overall diameter of the treated lesions can be misleading because the necrosis induced by the treatment may cause an initial increase in size. For this reason, methods which evaluate only the residual viable portion of the tumor (for instance the modified Response Evaluation Criteria in Solid Tumors – mRECIST) should be used to evaluate treatment response [81].
Miscellaneous
There are other therapeutic strategies that are sometimes used in nonresectable HCC, in clinical practice, but are not recommended by guidelines because of the current lack of strong efficacy or safety evidences.
One of these, transarterial radioembolization (TARE) with lipiodol-I 131 or y90 microspheres, has raised a new interest in the treatment of HCC. It differs from TACE because it does not base its effect in arterial obstruction but rather in the local action of β-radiation through the lodging of yttrium-loaded glass or resin spheres in vessels feeding the tumor [82,83]. Two cohort studies have documented the therapeutic equivalence between cTACE and TARE in terms of overall survival and toxicity in patients with unresectable HCC [84,85].
A recent study of large clinical record has shown minor toxic systemic effects and a time to tumor progression (TTP) with TARE better than the TACE [86].
TARE has shown good results particularly in the setting of portal vein thrombosis [87,88]. In fact, in patients undergoing TARE, thrombosis of intrahepatic portal branch, that in many guidelines is considered a TACE contraindication [17,19], does not seem to be a negative predictor [88,89]. Therefore, TARE may be indicated in patients with large masses and/or portal thrombosis/invasion, but it should be utilized in the context of prospective studies aimed at ascertaining its cost–effectiveness profile [21].
External-beam radiotherapy (3D conformal or stereotactic) is another possibility, which is not widely used, but can be performed to treat lesions not amenable to surgery or ablation, such as those adjacent to the central biliary system or central portal area [90–92]. NCCN guidelines version 2.2014 have incorporated external beam radiation therapy to the therapeutic algorithm for HCC, listing as an option for patients with unresectable disease characterized as extensive or otherwise not suitable for LT, and those with local disease only who are not operable because of performance status or comorbidity [19]. Actually there are no many evidences about efficacy and gain in term of survival compared with other locoregional therapies, and it is not mentioned by the other guidelines.
Systemic treatment
No systemic therapy had been proven effective in patients with advanced HCC until 2007, when two large randomized trials with sorafenib demonstrated a clinically relevant prolongation of survival in the setting of a well-compensated liver cirrhosis [16,93].
As such, all of the current guidelines (EASL-AASLD, APASL, JSH, NCCN, AISF) unanimously recognize sorafenib as the standard treatment for Child-Pugh A patients who have been diagnosed with advanced HCC.
Conversely, there is substantial agreement that advanced HCC patient with end-stage liver disease (Child-Pugh C) not amenable for liver transplantation should not receive any pharmacologic treatment apart the best supportive care.
Instead, differences arise in the evaluation of the appropriate therapy for Child-Pugh B patients. The JSH guidelines consider these subjects as compromised patients for whom palliative care is recommended [18], whereas APASL, NCCN and EASL-AASLD guidelines are more permissive and suggest a possible use of sorafenib [17,19–20]. Discrepancies are mainly due to lack of data in this clinical subset. Both the SHARP and the Asia-Pacific Phase III trials included an overwhelming majority of well-compensated liver cirrhosis (Child-Pugh A), in order to minimize the confounding effect of noncancer related deaths due to progressive hepatic failure [16,93]. Benefits of sorafenib on more compromised patients (Child-Pugh B) are more debated. Different studies seem to confirm that sorafenib is safe even in Child-Pugh B patients, with a rate of adverse effect, which is similar to that observed in more compensated patients [94–98]. There is, however, a narrow benefit in terms of overall survival that questions the overall risk/benefits balance [97,98].
This topic is currently being addresses by an ad hoc clinical trial comparing sorafenib + best supportive care (BSC) versus BSC alone in Child-Pugh B patients with advanced HCC [99].
Until specific results will be available, systemic treatment of Child-Pugh B will remain a controversial issue in the different guidelines. This uncertainty is also reflected by different rules adopted by different countries in the reimbursement of sorafenib for the treatment of advanced HCC, with some admitting also Child B and some limiting the reimbursement only to Child A. As such, a comprehensive clinical evaluation of single patients with a careful assessment of the parameters that entail the Child-Pugh B status may represent the best current approach.
Open issues & unanswered questions
As clinical guidelines strongly assist the modern hepatologist, suggesting the best therapeutic options, there is still room for some open questions.
For instance, we know that the BCLC classification holds an important prognostic value, as different stages entail different prognosis and treatment. We still do not know whether the disease duration also bears prognostic implications. There is a clear evidence that many hepatic and nonhepatic conditions have a different natural history in different patients, with a slow-evolving, indolent course and a fulminant presentation in other subjects.
Currently, we do not know whether patients in the intermediate stage since their first diagnosis of HCC share the same prognosis of patients that migrate from early to intermediate stage after a long history of potentially curative interventions.
Furthermore, patients with BCLC-B HCC present highly heterogenic features, and, therefore, the behavior of intermediate-stage HCC patients is difficult to anticipate [100]. In the setting of this complex situation, TACE is the only recommended treatment according to the EASL-AASLD guidelines, putting a challenge to the development of tailored treatment strategies [100].
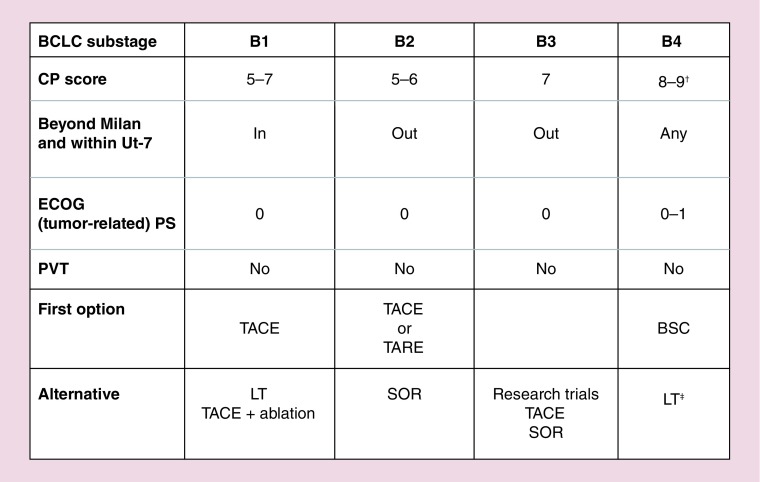
A subclassification of BCLC-B patients has been recently proposed in order to address these problems [101]. In this classification patients are divided according to their Child-Pugh score and performance status, presence/absence of segmental or subsegmental portal vein thrombosis, tumor burden evaluated according the ‘up-to-seven’ criterion. The criterion was chosen as it demonstrated a a good reliability in predicting survival after LT in patients with HCC beyond the Milan criteria and make provision for patients whose sum of number of nodules and diameter of biggest tumor is equal to or less than seven, in other words, a single nodule up to 6 cm, two nodules with a maximum diameter of 5 cm, three tumors with a maximum diameter of 4 cm, etc). Four different BLCL-B subclasses have been proposed (Figure 3), each one with one or more therapeutic option (conjugating classificative requirements and flexibility) [101]. A fifth subclass, named ‘Quasi-C,’ include patients with segmental or subsegmental portal vein thrombosis and with preserved liver function.
Figure 3. . Proposed substaging and treatment indications for patients at first observation with intermediate hepatocellular carcinoma.
†With severe/refractory ascites and/or jaundice.
‡Only if up to 7 IN and PS 0.
BCLC: Barcelona Clinic for Liver Cancer; BSC: Best supportive care; CP: Child-Pugh; ECOG PS: Eastern Cooperative Oncology Group; LT: Liver transplantation; PS: Performance status; PVT: Portal vein thrombosis; SOR: Sorafenib; TACE: Transarterial chemoembolization; TARE: Transarterial radioembolization.
Reproduced with permission from [101].
Proposed treatments range from OLT after a downstaging strategy for patients within the up-to-seven criterion (B1 subclass), to conventional TACE or TARE for well-compensated patients not amenable for OLT (B2 subclass) and from BSC for patients with significant liver function impairment (B4 subclass) to sorafenib for patients with segmental portal vein thrombosis (Quasi-C) or slightly compromised liver function (B3 subclass) or as a second-line option in patients with unsatisfactory response to TACE [101].
The possibility of combined therapies is another hot topic in hepatic oncology that has not yet received a full answer by the current guidelines. The JSH guidelines suggest to consider a multidisciplinary approach for intermediate-stage patients with bilobar extension of disease and nodules >3 cm, as they could benefit from a combined RF + TACE approach [18]. AISF recommendations transpose this statement outlining that a combination of different locoregional treatments offers the maximal flexibility in HCC treatment, allowing a tailored approach to each nodule in each patient [21]. The authors recommends that a combined/sequential strategy (based on RF ablation, PEI and/or TACE) should be evaluated in each patient with multinodular disease after a multidisciplinary assessment [21]. However, there is still no universal consensus on this topic, as the remaining international guidelines do not provide specific recommendations.
Also, a combination of locoregional and systemic treatments (TACE + sorafenib) in patients with intermediate-stage HCC has been largely investigated in recent years. Overall, the results of suggest that this combination could result in some clinical benefits, showing longer time to progression but failing to demonstrate a clear impact on overall survival.
However, it should be noted that many studies conducted so far included rather heterogeneous populations with a remarkable proportion of patients with advanced HCC. Therefore, there is still need of more accurate studies on this topic before recommendation can be made.
Conclusion
The ever progressing advances in surgical and nonsurgical techniques offers more and more possibilities for HCC patients, even with the potential to radically change the natural history of this disease that is still burdened by an overall grim prognosis.
Currently, many different guidelines seek to channel this continuous flow of knowledge into those therapies that experimental evidence identifies as being the most effective and safe, trying to balance these two aspects. Today, challenges include a more accurate classification of intermediate-advanced HCC and finding an appropriate allocation of new therapeutic resources.
Besides, there is a limit to the ability of each single guideline to foresee all of the possible clinical variable, therefore it may not be surprising that the overall strict implementation of AASLD/EASL-EORTC guidelines ranges from 51 to 66% in the real-life clinical practice [102,103].
An even bigger challenge awaits, therefore, the modern liver oncology clinician: finding the balance between the scientific rigor that stems from the guidelines with the ever increasing need for a tailored approach to optimize the clinical outcomes in each single patient.
Future perspective
The management of the guidelines is in a perpetual balance between two different needs: to contemplate the many possibilities that arise in real-life clinic practice and to maintain a simple structure.
It can be expected that the need for individualized therapies on one hand and the availability of new therapeutic techniques on the other hand will result in a greater structural complexity of most of the current guidelines.
In detail, the more relevant novelty will be represented by the introduction of TARE as a new therapeutic tool. In this case, it will be challenging to identify which subsets of patients will benefit the most from this therapy.
At the same time, it is expected that the combination therapies will gain increasing attention and, therefore, more and more space in the future guidelines. Overall, the future holds more articulated guidelines, allowing more individualized therapies and a greater application in real-life clinical practice.
Footnotes
Financial & competing interests disclosure
L Bolondi in the last 2 years has received honoraria for lectures and/or participation to advisory boards by the following companies: Bayer, Bristol Myers Squibb, Syrtex, MSD. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J. Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Bruix J. Sistematic review of randomized trials for unresectable hepatocellular carcinoma: chemioembolization improves survival. Hepatology. 2003;37(2):429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Munoz N. Hepatocellular carcinoma in the world: epidemiologic questions. In: Tabor E, DiBisceglie AM, Purcell RH, editors. Etiology, Pathology and Treatment of Hepatocellular Carcinoma in America. Advances in Applied Technology Series. Gulf, Houston, TX, USA: 1991. [Google Scholar]
- 4.Okuda K. Epidemiology of primary liver cancer. In: Tobe T, editor. Primary Liver Cancer in Japan. Springer-Verlag; Tokyo, Japan: 1992. [Google Scholar]
- 5.Ferlay J, Soerjomataram I, Ervik M, et al. Globocan 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11. http://globocan.iarc.fr [Google Scholar]
- 6.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J. Hepatol. 2012;56(6):1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J. Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 8.Colombo M, de Franchis R, Del Ninno E, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N. Engl. J. Med. 1991;325(10):675–680. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 9.Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract. Res. Clin. Gastroenterol. 2014;28(5):753–770. doi: 10.1016/j.bpg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Sobin LH, Wittekind CH. TNM Classification of Malignant Tumors (6th Edition) Wiley; New York, NY, USA: 2002. [Google Scholar]
- 11.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 12.Grieco A, Pompili M, Caminiti G, et al. Liver prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005;54(3):411–418. doi: 10.1136/gut.2004.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho YK, Chung JW, Kim JK, et al. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer. 2008;112(2):352–361. doi: 10.1002/cncr.23185. [DOI] [PubMed] [Google Scholar]
- 14.Vauthey JN, Dixon E, Abdalla EK, et al. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 2010;12(5):289–299. doi: 10.1111/j.1477-2574.2010.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig. Dis. 2011;29(3):339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 19.Benson AB, 3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J. Natl Compr. Canc. Netw. 2015;7(4):350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol. Int. 2010;4(2):439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolondi L, Cillo U, Colombo M, et al. Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig. Liver Dis. 2013;45(9):712–723. doi: 10.1016/j.dld.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J. Hepatol. 2008;48(S1):S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Shoup M, Gonen M, D’Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J. Gastrointest. Surg. 2003;7(3):325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 24.Regimbeau JM, Farges O, Shen BY, et al. Is surgery for large hepatocellular carcinoma justified? J. Hepatol. 1999;31(6):1062–1068. doi: 10.1016/s0168-8278(99)80319-5. [DOI] [PubMed] [Google Scholar]
- 25.Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J. Am. Coll. Surg. 2002;194(5):592–602. doi: 10.1016/s1072-7515(02)01163-8. [DOI] [PubMed] [Google Scholar]
- 26.Cescon M, Cucchetti A, Grazi GL. Indication of the extent of hepatectomy for hepatocellular carcinoma on cirrhosis by a simple algorithm based on preoperative variables. Arch. Surg. 2009;144(1):57–63. doi: 10.1001/archsurg.2008.522. [DOI] [PubMed] [Google Scholar]; • Illustrates prognostic factors for liver resection.
- 27.Ishizawa T, Hasegawa K, Aoki T. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134(7):1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 28.Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann. Surg. 2003;237(2):208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemming AW, Reed AI, Howard RJ, et al. Preoperative portal vein embolization for extended hepatectomy. Ann. Surg. 2003;237(5):686–691. doi: 10.1097/01.SLA.0000065265.16728.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuel M, Chow PK, Chan Shih-Yen E, et al. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst. Rev. 2009;21(1):CD001199. doi: 10.1002/14651858.CD001199.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Croome KP, Yamashita MH. Laparoscopic vs open hepatic resection for benign and malignant tumors: an updated meta-analysis. Arch. Surg. 2010;145(11):1109–1118. doi: 10.1001/archsurg.2010.227. [DOI] [PubMed] [Google Scholar]
- 32.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 33.Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia. Cancer. 2014;120(18):2824–2838. doi: 10.1002/cncr.28730. [DOI] [PubMed] [Google Scholar]
- 34.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134(5):1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am. J. Transplant. 2007;7(11):2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]; • Illustrates the expanded criteria for liver transplantation beyond Milan criteria.
- 36.Mazzaferro V, Romito R, Schiavo M, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44(6):1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 37.Toso C, Trotter J, Wei A, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14(8):1107–1115. doi: 10.1002/lt.21484. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez JA, Robles R, Marin C, et al. Can we expand the indications for liver transplantation among hepatocellular carcinoma patients with increased tumor size? Transplant Proc. 2003;35(5):1818–1820. doi: 10.1016/s0041-1345(03)00723-1. [DOI] [PubMed] [Google Scholar]
- 39.Pompili M, Mirante VG, Rondinara G, et al. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11(9):1117–1126. doi: 10.1002/lt.20469. [DOI] [PubMed] [Google Scholar]
- 40.Mazzaferro V, Battiston C, Perrone S, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann. Surg. 2004;240(5):900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berg CL, Merion RM, Shearon TH, et al. Liver transplant recipient survival benefit with living donation in the model for endstage liver disease allocation era. Hepatology. 2011;54(4):1313–1321. doi: 10.1002/hep.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14(7):935–945. doi: 10.1002/lt.21445. [DOI] [PubMed] [Google Scholar]
- 43.Chapman WC, Majella Doyle MB, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann. Surg. 2008;248(4):617–625. doi: 10.1097/SLA.0b013e31818a07d4. [DOI] [PubMed] [Google Scholar]
- 44.Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am. J. Transplant. 2008;8(12):2547–2557. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- 45.Yao FY, Kerlan RK, Jr, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48(3):819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41(5):1130–1137. doi: 10.1002/hep.20688. [DOI] [PubMed] [Google Scholar]
- 47.Cho YK, Kim JK, Kim MY. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49(2):453–459. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 48.Germani G, Pleguezuelo M, Gurusamy K, et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J. Hepatol. 2010;52(3):380–388. doi: 10.1016/j.jhep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129(1):122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Lin SM, Lin CJ, Lin CC, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma B4 cm. Gastroenterology. 2004;127(6):1714–1723. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Lin SM, Lin CJ, Lin CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54(8):1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen MS, Li JQ, Zheng Y. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann. Surg. 2006;243(3):321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho YK, Kim JK, Kim WT, et al. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51(4):1284–1290. doi: 10.1002/hep.23466. [DOI] [PubMed] [Google Scholar]; • Interesting comparison between liver resection and radiofrequency ablation outcomes.
- 54.Huang GT, Lee PH, Tsang YM, et al. Percutaneous ethanol injection versus surgical resection for the treatment of small hepatocellular carcinoma: a prospective study. Ann. Surg. 2005;242(1):36–42. doi: 10.1097/01.sla.0000167925.90380.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lü MD, Kuang M, Liang LJ, et al. Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yi Xue Za Zhi. 2006;86(12):801–805. [PubMed] [Google Scholar]
- 56.Huang J, Yan L, Cheng Z. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 2010;252(6):903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 57.Wang JH, Wang CC, Hung CH, et al. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J. Hepatol. 2012;56(2):412–418. doi: 10.1016/j.jhep.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 58.Huang J, Hernandez-Alejandro R, Croome KP, et al. Radiofrequency ablation versus surgical resection for hepatocellular carcinoma in Childs A cirrhotics – a retrospective study of 1,061 cases. J. Gastrointest. Surg. 2011;15(2):311–320. doi: 10.1007/s11605-010-1372-y. [DOI] [PubMed] [Google Scholar]
- 59.Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma (serial online) BMC Gastroenterol. 2010;10:78. doi: 10.1186/1471-230X-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng BQ, Jia CQ, Liu CT, et al. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299(14):1669–1677. doi: 10.1001/jama.299.14.1669. [DOI] [PubMed] [Google Scholar]
- 61.Yamakado K, Nakatsuka A, Takaki H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008;247(1):260–266. doi: 10.1148/radiol.2471070818. [DOI] [PubMed] [Google Scholar]
- 62.Maluccio M, Covey AM, Gandhi R, et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J. Vasc. Interv. Radiol. 2005;16(7):955–961. doi: 10.1097/01.RVI.0000161377.33557.20. [DOI] [PubMed] [Google Scholar]
- 63.Ricci P, Cantisani V, Drudi F, et al. Is contrast-enhanced US alternative to spiral CT in the assessment of treatment outcome of radiofrequency ablation in hepatocellular carcinoma? Ultraschall. Med. 2009;30(3):252–258. doi: 10.1055/s-2008-1027727. [DOI] [PubMed] [Google Scholar]
- 64.Shiozawa K, Watanabe M, Takayama R, et al. Evaluation of local recurrence after treatment for hepatocellular carcinoma by contrast-enhanced ultrasonography using Sonazoid: comparison with dynamic computed tomography. J. Clin. Ultrasound. 2010;38(4):182–189. doi: 10.1002/jcu.20685. [DOI] [PubMed] [Google Scholar]
- 65.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10 year update. J. Vasc. Interv. Radiol. 2014;25(11):1691–1705. doi: 10.1016/j.jvir.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giardina MG, Matarazzo M, Morante R, et al. Serum alpha-L-fucosidase activity and early detection of hepatocellular carcinoma: a prospective study of patients with cirrhosis. Cancer. 1998;83(12):2468–2474. [PubMed] [Google Scholar]
- 67.Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131(2):461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 68.Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224(1):47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 69.Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc. Intervent. Radiol. 2007;30(1):6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 70.Group d’Etude et de Traitment du Carcinome Hépatocellulaire. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N. Engl. J. Med. 1995;332(19):1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 71.Takayasu K, Arii S, Kudo M, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J. Hepatol. 2012;56(4):886–892. doi: 10.1016/j.jhep.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 72.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J. Hepatol. 2007;46(3):474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 73.Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc. Intervent. Radiol. 2010;33(1):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br. J. Cancer. 2014;111(2):255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Terzi E, Golfieri R, Piscaglia F, et al. Response rate and clinical outcome of HCC after first and repeated cTACE performed ‘on demand’. J. Hepatol. 2012;57(6):1258–1267. doi: 10.1016/j.jhep.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 76.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127(S1):S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 77.Kubota K, Hisa N, Nishikawa T, et al. Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of Lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdom. Imaging. 2001;26(2):184–190. doi: 10.1007/s002610000139. [DOI] [PubMed] [Google Scholar]
- 78.Shirato K, Numata K, Mitsui K, et al. Color Doppler sonography for evaluating response to transcatheter arterial embolization and percutaneous ethanol injection therapy and for detecting recurrence of hepatocellular carcinoma. J. Ultrasound Med. 2000;19(2):807–814. doi: 10.7863/jum.2000.19.12.807. [DOI] [PubMed] [Google Scholar]
- 79.Kim HJ, Kim TK, Kim PN, et al. Assessment of the therapeutic response of hepatocellular carcinoma treated with transcatheter arterial chemoembolization: comparison of contrast-enhanced sonography and 3-phase computed tomography. J. Ultrasound Med. 2006;25(4):477–486. doi: 10.7863/jum.2006.25.4.477. [DOI] [PubMed] [Google Scholar]
- 80.Cioni D, Lencioni R, Bartolozzi C. Therapeutic effect of transcatheter arterial chemoembolization on hepatocellular carcinoma: evaluation with contrast-enhanced harmonic power Doppler ultrasound. Eur. Radiol. 2000;10(10):1570–1576. doi: 10.1007/s003300000496. [DOI] [PubMed] [Google Scholar]
- 81.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010;30(1):52–56. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the currently accepted assessment of response to TACE and systemic therapies.
- 82.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology. 2013;58(6):2188–97. doi: 10.1002/hep.26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carr BI, Kondragunta V, Buch SC. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116(5):1305–1314. doi: 10.1002/cncr.24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kooby DA, Egnatashvili V, Srinivasan S. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2010;21(2):224–230. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 86.Salem R, Lewandowski RJ, Kulik L. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497–507.e2. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 88.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54(3):868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 89.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47(1):71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 90.Kwon JH, Bae SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. doi: 10.1186/1471-2407-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seo YS, Kim MS, Yoo SY, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J. Surg. Oncol. 2010;102(3):209–214. doi: 10.1002/jso.21593. [DOI] [PubMed] [Google Scholar]
- 92.Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(2):355–361. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 93.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 94.Lencioni R, Kudo M, Ye SL, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int. J. Clin. Pract. 2014;68(5):609–617. doi: 10.1111/ijcp.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Clearly reports the most frequent challenges in the management of sorafenib therapy.
- 95.DA Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol. Clin. Oncol. 2015;3(4):793–796. doi: 10.3892/mco.2015.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Federico A, Orditura M, Cotticelli G, et al. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma and Child-Pugh A or B cirrhosis. Oncol. Lett. 2015;9(4):1628–1632. doi: 10.3892/ol.2015.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pressiani T, Boni C, Rimassa L, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann. Oncol. 2013;24(2):406–411. doi: 10.1093/annonc/mds343. [DOI] [PubMed] [Google Scholar]
- 98.Zugazagoitia J, Manzano A, Sastre J, et al. Sorafenib for non-selected patient population with advanced hepatocellularcarcinoma: efficacy and safety data according to liver function. Clin. Transl. Oncol. 2013;15(2):146–153. doi: 10.1007/s12094-012-0902-3. [DOI] [PubMed] [Google Scholar]
- 99.Daniele B, di Maio M, Gallo C, et al. A randomized Phase III trial comparing sorafenib plus best supportive care (BSC) versus BSC alone in Child-Pugh B patients (pts) with advanced hepatocellular carcinoma (HCC): the BOOST study. J. Clin. Oncol. 2012;30 abstract TPS4151. [Google Scholar]
- 100.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 101.Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 2012;32(4):348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]; • Suggests different approaches for patients equally classified as intermediate stage HCC but with different clinical features.
- 102.Borzio M, Fornari F, De Sio I, et al. Adherence to American Association for the Study of Liver Diseases guidelines for the management of hepatocellular carcinoma: results of an Italian field practice multicenter study. Future Oncol. 2013;9(2):283–294. doi: 10.2217/fon.12.183. [DOI] [PubMed] [Google Scholar]
- 103.Leoni S, Piscaglia F, Serio I, et al. Adherence to AASLD guidelines for the treatment of hepatocellular carcinoma in clinical practice: experience of the Bologna Liver Oncology Group. Dig. Liver Dis. 2014;46(6):549–555. doi: 10.1016/j.dld.2014.02.012. [DOI] [PubMed] [Google Scholar]; • Clearly illustrates discrepancies between guidelines prescriptions and real-life clinical practice.