Abstract
This study was conducted to investigate whether the apparent ileal digestibility (AID) of GE or nitrogen (N) was influenced by inclusion level and type of digestibility index marker (DIM) and inclusion level of oat bran (OB) and whether the apparent total tract digestibility (ATTD) of GE or DIM recovery was influenced by the 3 aforementioned factors and duration of feces collection. Six diets were formulated as a 2 × 3 factorial arrangement with 2 levels of OB (0 or 100 g/kg) and 3 levels of DIM (2.5, 5.0, or 7.5 g/kg). Chromic oxide and titanium dioxide were used as DIM. Both chromic oxide and titanium dioxide were added to the same diet, and their inclusion levels were consistent in each experimental diet. In Exp. 1, 18 barrows fitted with T-cannulas at the distal ileum were used in a triplicate 6 × 2 incomplete Latin Square design with 6 dietary treatments and 2 periods. The ileal digesta were collected for 3 d after 5-d adaptation, and the AID of GE and N were determined by measuring Cr or Ti in diets and ileal digesta samples. In Exp. 2, a total of 72 barrows were used in a randomized complete block design, and the feces were collected for either 3 or 5 d after a 7-d adaptation according to the assignment. Experimental diets were same as Exp. 1. The ATTD of GE was determined by both total collection and index methods, and DIM recovery was determined by measuring Cr or Ti. In Exp. 1, there was no interaction among OB level, DIM level, and DIM type for AID of GE and N. The AID of GE and N determined by Ti were greater (P < 0.05) than Cr regardless of the OB level or DIM level. Neither the OB level nor the DIM level affected the AID of GE or N. In Exp. 2, no interaction among OB level, DIM level, duration of feces collection, and DIM type for ATTD of GE and DIM recovery was observed. The DIM level and duration of feces collection had no effect on ATTD of GE and DIM recovery. The total collection method resulted in greater (P < 0.05) ATTD of GE than Ti, which was greater (P < 0.05) than that determined by Cr. Similarly, the recovery of Ti was greater (P = 0.007) than Cr. Inclusion of 100 g/kg OB decreased (P = 0.003) ATTD of GE but did not affect the recovery of DIM. In conclusion, the AID of GE and N, the ATTD of GE, and the recovery of Cr or Ti were affected by DIM type, but not DIM level; the inclusion of OB had no effect on AID of GE and N, and DIM recovery; and the duration of feces collection had no effect on ATTD of GE and DIM recovery.
Keywords: chromic oxide, digestibility, digestibility index marker, oat bran, swine, titanium dioxide
INTRODUCTION
Apparent ileal digestibility (AID) and apparent total tract digestibility (ATTD) are 2 of the important indicators to evaluate the nutritional value or energy in feed ingredients and diets. The addition of digestibility index marker (DIM) as an index to determine digestibility avoids the need for quantitative records of feed intake and feces output (Adeola, 2001), and therefore, this index method has been widely used in digestibility studies in nonruminant animals. Theoretically, the nutrient digestibility values determined by different levels and types of DIM should be accordant. However, various DIM levels, which mostly range from 1 to 5 g/kg, and different DIM types, which mainly includes chromic oxide (Cr2O3) and titanium dioxide (TiO2), have been used, and inconsistent results about the effect of DIM level or type have been reported (Yin et al., 2000; Kavanagh et al., 2001; Olukosi et al., 2012). To the best of our knowledge, the verification of this theory using 3 equally spaced levels of DIM has not been investigated in pigs.
The previous study in our group indicated that the addition of 100 g/kg oat bran (OB) in diet decreased Ti recovery to 78.5% compared with 88.2% in diet without OB, which may be due to the relatively short duration of feces collection (Wang et al., 2017). Therefore, the objective of this study was to investigate the effect of DIM level and type on AID of GE and N, and the effect of DIM level and type, and duration of feces collection on ATTD of GE and DIM recovery of diets with or without OB in diets fed to growing pigs.
MATERIALS AND METHODS
All animal procedures used in this study were approved by the Purdue University Animal Care and Use Committee.
Dietary Treatments
To be consistent with previous study (Wang et al., 2017), the OB used in this study was purchased from the same local supplier. Two levels of OB (0 or 100 g/kg) and 3 levels of DIM (2.5, 5.0, or 7.5 g/kg) were used to formulate 6 corn–soybean meal-based diets in a 2 × 3 factorial arrangement (Table 1). All diets were formulated to meet or exceed the vitamin and mineral requirement estimates for pigs (NRC, 2012). Both Cr2O3 and TiO2 were added to the same diet as DIM, and their inclusion levels were consistent in each experimental diet.
Table 1.
Ingredient composition and analyzed nutrient concentration of experimental diets, g/kg as-fed basis1
| 0 g/kg oat bran | 100 g/kg oat bran | |||||
|---|---|---|---|---|---|---|
| DIM, g/kg | ||||||
| Item | 2.5 | 5.0 | 7.5 | 2.5 | 5.0 | 7.5 |
| Ingredient | ||||||
| Ground corn | 620.0 | 595.0 | 570.0 | 620.0 | 595.0 | 570.0 |
| Soybean meal, 48% CP | 203.5 | 203.5 | 203.5 | 203.5 | 203.5 | 203.5 |
| Oat bran | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | 100.0 |
| Cornstarch | 100.0 | 100.0 | 100.0 | 0.0 | 0.0 | 0.0 |
| Titanium dioxide premix2 | 12.5 | 25.0 | 37.5 | 12.5 | 25.0 | 37.5 |
| Chromic oxide premix3 | 12.5 | 25.0 | 37.5 | 12.5 | 25.0 | 37.5 |
| Soybean oil | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Limestone | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Monocalcium phosphate | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Salt | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Vitamin premix4 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Mineral premix5 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Selenium premix6 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Magnesium oxide | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Total | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 |
| Analyzed nutrients | ||||||
| DM | 89.1 | 88.5 | 88.6 | 88.5 | 88.9 | 88.7 |
| GE (kcal/g) | 3.86 | 3.95 | 3.83 | 3.91 | 3.91 | 3.83 |
| N | 2.46 | 2.47 | 2.42 | 2.75 | 2.66 | 2.63 |
| Cr | 1.64 | 3.09 | 4.65 | 1.66 | 3.18 | 4.70 |
| Ti | 1.49 | 2.99 | 4.41 | 1.52 | 3.04 | 4.48 |
1DIM = digestibility index marker.
2A 0.5 g titanium dioxide was added to 2.0 g corn.
3A 0.5 g chromic oxide was added to 2.0 g corn.
4Vitamin premix provided the following quantities per kilogram of diet: vitamin A, 3,960 IU; vitamin D3, 396 IU; vitamin E, 26.4 IU; menadione, 1.30 mg; riboflavin, 5.30 mg; d-pantothenic acid, 13.2 mg; niacin, 19.8 mg; and vitamin B12, 0.02 mg.
5Mineral premix provided the following quantities per kilogram of diet: Cu (as copper chloride), 9 mg; I (as calcium iodate), 0.36 mg; Fe (as ferrous carbonate), 194 mg; Mn (as manganese oxide), 17 mg; and Zn (as zinc oxide), 149 mg.
6Supplied 0.3 mg Se/kg of diet.
Experiment 1: Apparent Ileal Digestibility of GE and Nitrogen
Eighteen barrows were surgically fitted with T-cannulas at the distal ileum following the description of Dilger et al. (2004). All pigs were individually housed in stainless steel crates (1.22 × 1.22 m) equipped with a feeder and a nipple drinker. In the first period, 18 pigs (initial BW = 25.8 ± 0.4 kg) were grouped into 3 blocks based on BW, and 6 diets were randomly assigned to pigs within each block. In the second period, the same 18 pigs (initial BW = 27.4 ± 0.5 kg) went through the same procedure except that pigs consumed diets which were different from the first period. Daily feed allowance was set as 4% of the initial smallest BW in each period, and pigs were fed at 0800 and 1700 h in 2 equally divided meals. Water was available at all time during the experiment. Each experimental period consisted of 5 d of adaptation followed by 3 d of ileal digesta collection period. Plastic bag containing 10 mL of 10% formic acid was attached to the T-cannula with a rubber O-ring to collect ileal digesta from 0800 to 1700 h on days 6, 7, and 8. The attached bags were inspected every 30 min, and the filled bags were changed and stored at −20 °C until further processing.
Experiment 2: Apparent Total Tract Digestibility of GE and Digestibility Index Marker Recovery
A total of 72 barrows (initial BW = 21.1 ± 0.2 kg) were used in 3 periods and individually housed in the same crates and environmental condition with Exp. 1. Pigs were fed one of the 6 experimental diets used in Exp. 1 with a 7-d adaptation period followed by a 3- or 5-d total collection of feces period, which formulated 12 treatments with a 2 × 3 × 2 factorial arrangement. Pigs were allotted to 6 blocks based on BW within periods and assigned to a randomized complete block design with 12 treatments and 6 replicate pigs per treatment. Daily feed allowance was set as 4% of the initial mean BW, and pigs were fed at 0700 and 1700 h in 2 equally divided meals. The feces samples were collected according to the marker-to-marker procedure described by Akinmusire and Adeola (2009) except indigo carmine instead of ferric oxide was used to mark the initiation and end of feces collection. Feces collection period lasted 3 or 5 d according to treatment and was initiated and ended with the appearance of indigo carmine-marked feces. Feces were collected twice daily, and the collected feces were stored at −20 °C until further processing.
Chemical Analysis and Calculation
The ileal digesta in Exp. 1 and feces samples in Exp. 2 from each pig during each period were pooled, subsampled, and forced-air dried at 55 °C to constant weight. Diet, ileal digesta, and feces samples were ground through a 0.5-mm screen in a centrifugal grinder (Retsch ZM 200; Retsch GmbH, Haan, Germany). The DM contents of diets, ileal digesta, and feces samples were determined by drying in a forced-air oven at 105 °C for 24 h (Precision Scientific Co., Chicago, IL; method 934.01; AOAC, 2006). Chromium content was analyzed as described by Saha and Gilbreath (1991) with modifications of heating temperature at 400 °C during perchloric acid digestion and standing overnight after dilution with distilled water to 100 mL and determined by spectrophotometry at 450 nm (Spark 10 M; Tecan Group Ltd., Männedorf, Switzerland). Titanium content was analyzed with the method described by Myers et al. (2004) with a modification of heating time. In short, as-fed basis samples were digested in Kjeldahl digestion tubes with the presence of 13-mL concentrated sulfuric acid and catalyst at 420 °C for 4 h. Then, a 10-mL 30% hydrogen peroxide was added to the cooled digestion solution, and the total liquid weight was brought up to 100 g, which stood overnight. The Ti content was determined by spectrophotometry at 410 nm. Nitrogen content was determined by the combustion method (TruMac N; LECO Corp., St. Joseph, MI; method 990.03; AOAC, 2000). Gross energy content was analyzed by isoperibol bomb calorimeter (Model 6200; Parr Instrument Co., Moline, IL).
The AID and ATTD of GE (Adedokun and Adeola, 2005) and DIM recovery were calculated using the following equations:
where DIMdiet, DIMdigesta, and DIMfeces are the DIM concentration of the diet, ileal digesta, and feces, respectively (mg/kg DM); GEdiet, GEdigesta, and GEfeces are the GE concentration of the diet, ileal digesta, and feces, respectively (kcal/kg DM); and Wdiet and Wfeces are the total weight of diet consumed and output of feces during collection period, respectively (kg DM). The AID of N is calculated with GE replaced by N (mg/kg DM).
Statistical Analysis
In Exp. 1, the data on AID were analyzed as a split-plot arrangement, with the dietary treatments as whole-plot factor and the DIM type as split-plot factor. There was no interaction among factors. The model included OB level, DIM level, and DIM type as fixed effects and block, the interaction of pig and period, and the interaction of block and DIM type as random effects.
In Exp. 2, the ATTD of GE data were analyzed as a split-plot arrangement, where 2 × 3 × 2 factorial arrangement with 2 levels of OB inclusion, 3 levels of DIM inclusion, and 2 levels of duration of feces collection was whole-plot factors; the split-plot factor was the method, which refers to a generic term including total collection, Cr2O3, and TiO2. Reduced model was used by excluding the interactions among factors due to the absence of statistical significance. Finally, the fixed effects in the model were OB level, DIM level, duration of feces collection, and method, and the random effects were block, 2 way interactions of block and either one of OB level, DIM level, duration of feces collection, and method, and 3 way interaction of block, OB level, and DIM level.
In Exp. 2, the data on recovery of Cr and Ti were analyzed as a split-plot arrangement, where the whole-plot factor was same with the analysis of ATTD of GE and the split-plot factor was DIM type including Cr and Ti . Similarly, the interactions among factors were not statistically significant, and the reduced model was used. Finally, the model included OB level, DIM level, duration of feces collection, and DIM type as fixed effects, and the random effects were same with the model of ATTD of GE except replacing method with DIM type.
The analyzed and calculated concentration of DIM was analyzed using REG procedure of SAS (SAS Inst., Inc., Cary, NC). The rest of the data were analyzed using the MIXED procedure of SAS. Least squares means were separated by PDIFF option with the Tukey’s adjustment. The experimental unit was pig in all the analyses, and the statistical significance was declared at P < 0.05.
RESULTS
During the whole experimental process, 1 pig in Exp. 2 (with the treatment of OB level as 0, DIM level as 7.5 g/kg, and duration of feces collection as 5 d) had diarrhea and was removed from experiment.
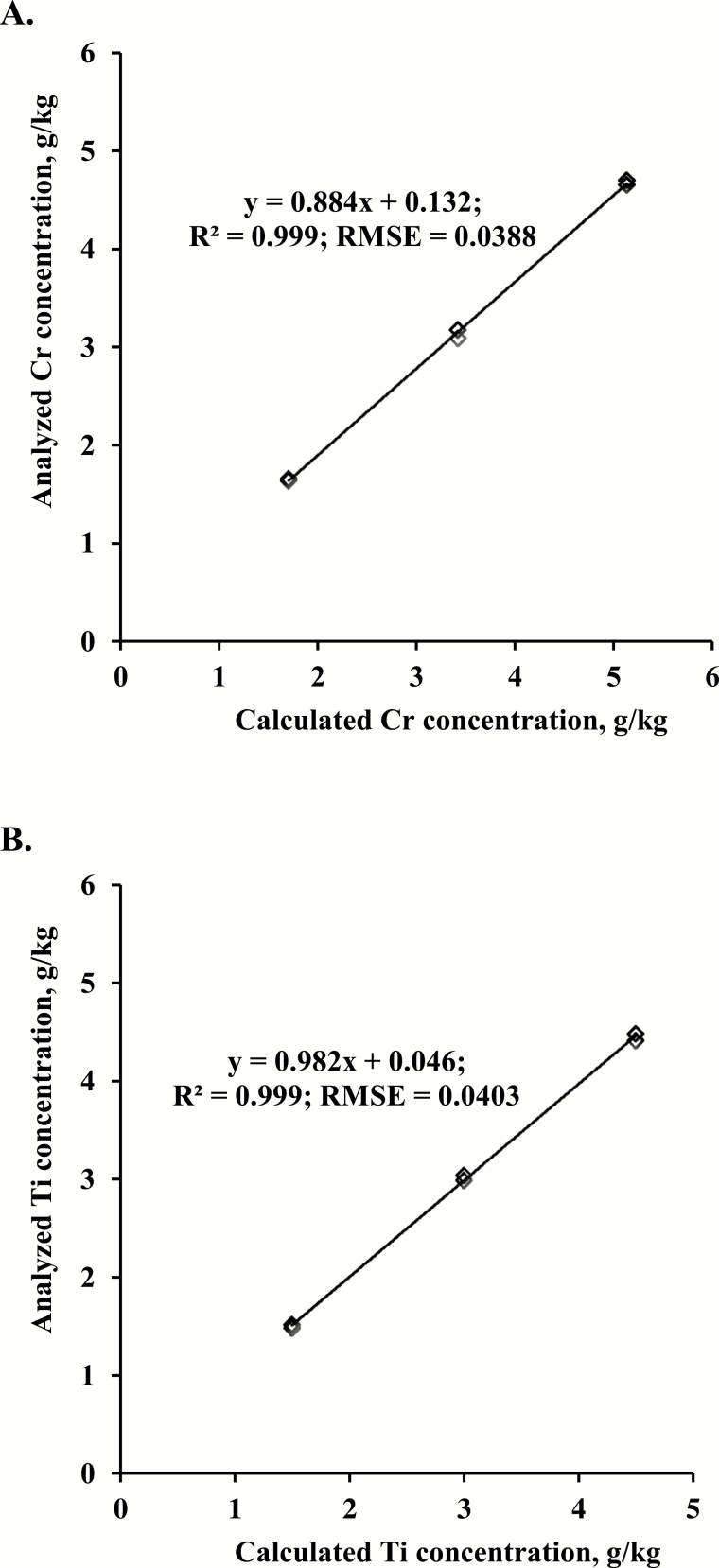
The analyzed Cr or Ti concentration in experimental diets was in agreement with the calculated value of Cr or Ti (Fig. 1), respectively. The slope of the regression line of dietary Cr concentration was 0.884, and the R2 and root of mean square error of the model were 0.999 and 0.0388, respectively. For dietary Ti concentration, the slope of the regression line was 0.982, and the R2 and root of mean square error of the model were 0.999 and 0.0403, respectively.
Figure 1.
The regression analysis between analyzed and calculated Cr (A) and Ti concentrations (B) in experimental diets. Each point represents the mean value of duplicated measurements. The chromic oxide and titanium dioxide were included at 2.5, 5.0, and 7.5 g/kg in experimental diets. RMSE = root of mean square error.
The results of AID of GE and N in Exp. 1 are presented in Table 2. No interaction among OB level, DIM level, and DIM type in the model of AID of GE or N was observed. The AID of GE and N were unaffected by either OB level or DIM level among the 6 experimental diets. However, the choice of DIM type affected the results of AID of GE (74.1% and 73.1% for Ti and Cr, respectively; P = 0.049) and N (72.7% and 71.7% for Ti and Cr, respectively; P = 0.042), where Ti determined greater values than Cr.
Table 2.
Influence of oat bran level, digestibility index marker level, and type on apparent ileal digestibility of GE and nitrogen in experimental diets fed to pigs in Exp. 11
| AID, % | ||||||
|---|---|---|---|---|---|---|
| Item | OB level, g/kg | DIM level, g/kg | DIM type | n | GE | Nitrogen |
| 0 | 2.5 | Cr2O3 | 6 | 73.2 | 73.1 | |
| 0 | 2.5 | TiO2 | 6 | 73.8 | 73.6 | |
| 0 | 5.0 | Cr2O3 | 6 | 74.1 | 72.5 | |
| 0 | 5.0 | TiO2 | 6 | 75.4 | 73.8 | |
| 0 | 7.5 | Cr2O3 | 6 | 72.1 | 67.7 | |
| 0 | 7.5 | TiO2 | 6 | 73.0 | 68.8 | |
| 100 | 2.5 | Cr2O3 | 6 | 72.8 | 71.7 | |
| 100 | 2.5 | TiO2 | 6 | 73.7 | 72.6 | |
| 100 | 5.0 | Cr2O3 | 6 | 74.1 | 73.3 | |
| 100 | 5.0 | TiO2 | 6 | 75.4 | 74.6 | |
| 100 | 7.5 | Cr2O3 | 6 | 72.1 | 71.9 | |
| 100 | 7.5 | TiO2 | 6 | 73.1 | 72.9 | |
| Cr2O3 | 36 | 73.1 | 71.7 | |||
| TiO2 | 36 | 74.1 | 72.7 | |||
| SED | ||||||
| OB level | 1.11 | 1.73 | ||||
| DIM level | 1.36 | 2.12 | ||||
| DIM type | 0.39 | 0.40 | ||||
| P value2 | ||||||
| OB level | 0.962 | 0.472 | ||||
| DIM level | 0.290 | 0.299 | ||||
| DIM type | 0.049 | 0.042 | ||||
1AID = apparent ileal digestibility; OB = oat bran; DIM = digestibility index marker; Cr2O3 = chromic oxide; TiO2 = titanium dioxide; n = number of observations.
2The P values for interactions including OB level × DIM level, OB level × DIM type, DIM level × DIM type, and OB level × DIM level × DIM type are all >0.10, and the P values are not presented.
The ATTD of GE in Exp. 2 is shown in Table 3, and there was no interaction among OB level, DIM level, duration of feces collection, and method. The result of DIM recovery is presented in Table 4, and no interaction among OB level, DIM level, duration of feces collection, and DIM type was observed. Digestibility index marker level and duration of feces collection did not affect the ATTD of GE and DIM recovery. The Ti determined greater (P < 0.05) ATTD of GE than Cr, whereas the greatest ATTD of GE was determined by the total collection method (P < 0.05). Including 100 g/kg OB in diet decreased (P = 0.003) the ATTD of GE, but did not affect the DIM recovery. Furthermore, the DIM recovery of Ti at 83.4% was greater (P = 0.007) than 78.9% for Cr.
Table 3.
Influence of oat bran level, digestibility index marker level, method, and duration of feces collection on apparent total tract digestibility of GE in experimental diets fed to pigs in Exp. 21
| Item | OB level, g/kg | DIM level, g/kg | Method | n 2 | ATTD of GE, % |
|---|---|---|---|---|---|
| 0 | 2.5 | Cr2O3 | 12 | 84.6 | |
| 0 | 2.5 | TiO2 | 12 | 86.0 | |
| 0 | 2.5 | TC | 12 | 87.9 | |
| 0 | 5.0 | Cr2O3 | 12 | 84.0 | |
| 0 | 5.0 | TiO2 | 12 | 84.6 | |
| 0 | 5.0 | TC | 12 | 87.7 | |
| 0 | 7.5 | Cr2O3 | 11 | 84.7 | |
| 0 | 7.5 | TiO2 | 11 | 85.1 | |
| 0 | 7.5 | TC | 11 | 87.7 | |
| 100 | 2.5 | Cr2O3 | 12 | 83.3 | |
| 100 | 2.5 | TiO2 | 12 | 84.9 | |
| 100 | 2.5 | TC | 12 | 86.9 | |
| 100 | 5.0 | Cr2O3 | 12 | 83.1 | |
| 100 | 5.0 | TiO2 | 12 | 84.0 | |
| 100 | 5.0 | TC | 12 | 86.9 | |
| 100 | 7.5 | Cr2O3 | 12 | 82.6 | |
| 100 | 7.5 | TiO2 | 12 | 83.1 | |
| 100 | 7.5 | TC | 12 | 86.0 | |
| 0 | 105 | 85.8 | |||
| 100 | 108 | 84.5 | |||
| Cr2O3 | 71 | 83.7z | |||
| TiO2 | 71 | 84.6y | |||
| TC | 71 | 87.2x | |||
| SED3 | |||||
| OB level | 0.24 | ||||
| DIM level | 0.39 | ||||
| Duration of feces collection | 0.34 | ||||
| Method | 0.18 | ||||
| P value4 | |||||
| OB level | 0.003 | ||||
| DIM level | 0.231 | ||||
| Duration of feces collection | 0.109 | ||||
| Method | <0.001 |
x,y,zMain effect means for method within a column without a common superscript letter differ (P < 0.05).
1ATTD = apparent total tract digestibility; OB = oat bran; DIM = digestibility index marker; Cr2O3 = chromic oxide; TiO2 = titanium dioxide; TC = total collection.
2 n = number of observations; it includes 3- and 5-d duration of feces collection.
3Duration of feces collection refers total collection of feces for either 3 or 5 d after a 7-d adaptation. Method refers the use of total collection, Cr2O3, or TiO2 to determine digestibility.
4The P values for all the interactions including OB level, DIM level, duration of feces collection, or method are all >0.10, and the P values are not presented.
Table 4.
Influence of oat bran level, digestibility index marker level and type, and duration of feces collection on recovery of digestibility index markers of pigs fed experimental diets in Exp. 21
| Item | OB level, g/kg | DIM level, g/kg | DIM type | n 2 | Recovery, % |
|---|---|---|---|---|---|
| 0 | 2.5 | Cr2O3 | 12 | 78.9 | |
| 0 | 2.5 | TiO2 | 12 | 86.1 | |
| 0 | 5.0 | Cr2O3 | 12 | 77.2 | |
| 0 | 5.0 | TiO2 | 12 | 80.1 | |
| 0 | 7.5 | Cr2O3 | 11 | 80.7 | |
| 0 | 7.5 | TiO2 | 11 | 82.6 | |
| 100 | 2.5 | Cr2O3 | 12 | 78.9 | |
| 100 | 2.5 | TiO2 | 12 | 87.2 | |
| 100 | 5.0 | Cr2O3 | 12 | 77.4 | |
| 100 | 5.0 | TiO2 | 12 | 81.9 | |
| 100 | 7.5 | Cr2O3 | 12 | 80.5 | |
| 100 | 7.5 | TiO2 | 12 | 82.6 | |
| Cr2O3 | 71 | 78.9 | |||
| TiO2 | 71 | 83.4 | |||
| SED3 | |||||
| OB level | 1.56 | ||||
| DIM level | 1.66 | ||||
| Duration of feces collection | 1.03 | ||||
| DIM type | 1.03 | ||||
| P value4 | |||||
| OB level | 0.782 | ||||
| DIM level | 0.129 | ||||
| Duration of feces collection | 0.322 | ||||
| DIM type | 0.007 |
1OB = oat bran; DIM = digestibility index marker; Cr2O3 = chromic oxide; TiO2 = titanium dioxide; DIM recovery is the ratio of digestibility index marker amount in feces and feed during the total collection of feces period.
2 n = number of observations; it includes 3- and 5-d duration of feces collection.
3Duration of feces collection refers total collection of feces for either 3 or 5 d after a 7-d adaptation.
4The P values for all the interactions including OB level, DIM level, duration of feces collection, or DIM type are all >0.10, and the P values are not presented.
DISCUSSION
The effect of inclusion level of DIM in diet on nutrients digestibility has been investigated in swine and poultry (Jagger et al., 1992; Olukosi et al., 2012). Based on the assumption about DIM that DIM should be nonabsorbable, indigestible, and completely voided in the feces (Adeola, 2001), the results determined by adding different inclusion levels of DIM should not be different. However, the validation of this theory might be related to animal species because of difference in gastrointestinal tract. Olukosi et al. (2012) reported that the AID of His, Trp, Cys, and Pro in corn–soybean meal-based diets were greater at 3 g/kg DIM concentration than 5 g/kg in broilers, either using Cr2O3 or TiO2. In addition, increased digestibility with decreasing levels of Cr2O3 from 20 to 5 g/kg in tilapia was observed (Shiau and Liang, 1995). But similar results of AID of N and AA between 3 and 5 g/kg DIM concentration were observed by Olukosi et al. (2012) in barrows. This is consistent with the results in the present study, where the AID of GE or N, ATTD of GE, and DIM recovery were not affected by 3 dietary DIM levels in barrows.
In the present study, the DIM recovery results were consistent with the results of ATTD of GE, which indicated that the lower recovery of Cr might be a reason for lower ATTD of GE determined by Cr than that determined by Ti. The equation used to calculate the recovery of DIM in this study is as follows: recovery of Cr, % = (Crfeces × Wfeces)/(Crdiet × Wdiet) × 100; recovery of Ti, % = (Tifeces × Wfeces)/(Tidiet × Wdiet) × 100. In the equation for calculating the recovery of Cr and Ti, the weight of feces and feed intake were same for both Cr and Ti, but the recovery of Ti was greater than that of Cr. This indicated that Tifeces/Tidiet was greater than Crfeces/Crdiet. The equation used to calculate ATTD of GE in this study is as follows: ATTD of GE, % = [1 − (Crdiet/Crfeces) × (GEfeces/GEdiet)] × 100 for Cr as DIM; ATTD of GE, % = [1 − (Tidiet/Tifeces) × (GEfeces/GEdiet)] × 100 for Ti as DIM. In the equation for calculating the ATTD using Cr or Ti, the GE of feces and feed intake were same, but the ATTD determined by Ti was greater than that by Cr. This indicated that Tidiet/Tifeces was less than Crdiet/Crfeces, which is consistent with the result of marker recovery. The lower recovery of Cr compared with Ti is consistent with several previous studies (Jagger et al., 1992; Yin et al., 2000; Olukosi et al., 2012), but is inconsistent with Köhler et al. (1990), where higher recovery of Cr than Ti was observed. One of the reasons for the difference in recovery of Ti and Cr is the accuracy of chemical analysis. First, the method used in quantification of both Cr and Ti in the present study is colorimetry, but the stability of the color-forming compounds in the chemical analysis of Cr and Ti is different. The intense orange or yellow color of peroxytitanic acid is formed when TiO2 reacts with hydrogen peroxide, which stays stable for at least 9 wk (Short et al., 1996; Myers et al., 2004). However, the chromic acid is formed when Cr2O3 reacts with perchloric acid (Fenton and Fenton, 1979), and it readily polymerizes by loss of water to form dichromic acid and higher polymers (Udy, 1956), which indicates the stability of the reaction of Cr is relatively low compared with that of Ti. Furthermore, the loss of Cr during digestion with the formation of volatile chlorine compound (Gorsuch, 1959) and the fusion or bonding of Cr to glassware (Cary and Allaway, 1971) could also contribute to the low recovery of Cr. Moreover, Jagger et al. (1992) indicated that the SE for AID values associated with Ti was lower than that of Cr.
The present study found that more accurate analysis of Ti was achieved than Cr, where the analyzed concentrations of Ti in diets were in better match with the expected values than Cr. Williams et al. (1962) found that silicate, Al, Ca, and Mg in aqueous solution interfered in the determination of Cr with atomic absorption spectroscopy, and later, Saha and Gilbreath (1991) indicated that this type of interference conceivably could happen in colorimetric determination, which was used in this study. It is worth noting that Ti was not digested during the chemical digestion process of Cr, but the undissolved Ti might interfere the absorbance of Cr solution with spectrophotometer. Both Olukosi et al. (2012) and Myers et al. (2004) indicated that the chemical analysis of Ti determined by colorimetry in diet or bovine fecal samples was not affected when Cr was present, but whether the determination of Cr was affected in the presence of Ti is still unknown.
The total collection method determined greater ATTD of GE than DIM method, which is in agreement with the previous studies (McCarthy et al., 1974; Adeola et al., 1986; Jagger et al., 1992), and the lower recovery of DIM might be the reason. In the present study, the DIM recovery ranged from 77.2% to 80.7% for Cr and 80.1% to 87.2% for Ti, which were within the range of literature values (Ishikawa and Sugimura, 1973; Köhler et al., 1990; Jagger et al., 1992; McClean, 1993; Wang et al., 2017). The factors that might contribute to the low DIM recovery have been investigated for decades, but there is still no accordant conclusion. Compared with the content of other components, the relatively small proportion of DIM in experimental diets, which ranged from 2.5 to 7.5 g/kg in the present study, might partly explain the low DIM recovery (Köhler et al., 1990). On the other hand, perfectly accurate record of intake of experimental diets is difficult in practice, especially when the diet is fed in mash form. Relatively higher Ti recovery was reported by Peddie et al. (1982), where pelleted diet was fed to mature laying hens. Except the 2 externally practical factors, the physical or biochemical interaction between markers and pigs could also explain the low marker recovery. The fineness of DIM and the tortuous intestine structure of nonruminant animals may cause the retention of DIM in intestine (McClean, 1993; Hill et al., 1996; Mroz et al., 1996). The possibility of biochemical reaction of Cr or Ti in animal body, as discussed by Olukosi et al. (2012), indicated the absorption of DIM and participation of reaction.
In the present study, the effect of the duration of feces collection on ATTD of GE and DIM recovery was investigated because the relatively short duration of feces collection might be the reason of the low recovery of DIM in the previous study (Wang et al., 2017). And at least of 4 d of total collection of feces was recommended by Adeola (2001). But the results obtained from this study indicated that there was no difference between 3 and 5 d of feces collection, and it was concluded that the duration of feces collection may not be one of the reasons of low marker recovery.
In conclusion, the choice of DIM type, but not marker level affected the AID of GE and N, the ATTD of GE, and the marker recovery. The inclusion of 100 g/kg OB did not affect the AID of GE and N and DIM recovery. The duration of feces collection had no effect on ATTD of GE and DIM recovery.
Footnotes
The authors gratefully acknowledge Dr. Darryl Ragland and Pat Jaynes (Purdue University, West Lafayette, IN) for their considerable contribution to this study.
LITERATURE CITED
- Adedokun S. A., and Adeola O.. 2005. Apparent metabolizable energy value of meat and bone meal for white pekin ducks. Poult. Sci. 84:1539–1546. doi:10.1093/ps/84.10.1539 [DOI] [PubMed] [Google Scholar]
- Adeola O. 2001. Digestion and balance techniques in pigs. In: A. J. Lewis and L. L. Southern, editors, Swine nutrition. 2nd ed CRC Press, Washington, DC: p. 903–916. [Google Scholar]
- Adeola O., Young L. G., McMillan E. G., and Moran E. T.. 1986. Comparative protein and energy value of OAC wintri triticale and corn for pigs. J. Anim. Sci. 63:1854–1861. doi:10.2527/jas1986.6361854x [Google Scholar]
- Akinmusire A. S., and Adeola O.. 2009. True digestibility of phosphorus in canola and soybean meals for growing pigs: Influence of microbial phytase. J. Anim. Sci. 87:977–983. doi:10.2527/jas.2007-0778 [DOI] [PubMed] [Google Scholar]
- AOAC 2000. Official methods of analysis. 17th ed Assoc. Off. Anal. Chem, Arlington, VA. [Google Scholar]
- AOAC 2006. Official methods of analysis. 18th ed Assoc. Off. Anal. Chem, Arlington, VA. [Google Scholar]
- Cary E. E., and Allaway W. H.. 1971. Determination of chromium in plants and other biological materials. J. Agric. Food Chem. 19:1159–1161. doi:10.1021/jf60178a009 [DOI] [PubMed] [Google Scholar]
- Dilger R. N., Sands J. S., Ragland D., and Adeola O.. 2004. Digestibility of nitrogen and amino acids in soybean meal with added soyhulls. J. Anim. Sci. 82:715–724. doi:10.2527/2004.823715x [DOI] [PubMed] [Google Scholar]
- Fenton T. W., and Fenton M.. 1979. An improved procedure for the determination of chromic oxide in feed and feces. Can. J. Anim. Sci. 59:631–634. doi:10.4141/cjas79-081 [Google Scholar]
- Gorsuch T. T. 1959. Radiochemical investigations on the recovery for analysis of trace elements in organic and biological materials. Analyst 84:135–173. doi:10.1039/AN9598400135 [Google Scholar]
- Hill R. C., Burrows C. F., Ellison G. W., and Bauer J. E.. 1996. The use of chromic oxide as a marker for measuring small intestinal digestibility in cannulated dogs. J. Anim. Sci. 74:1629–1634. doi:10.2527/1996.7471629x [DOI] [PubMed] [Google Scholar]
- Ishikawa S., and Sugimura K.. 1973. Movement of polyvinylalcohol through the digestive tract as a digestion indicator with swine. Agric. Biol. Chem. 37:203–206. doi:10.1080/00021369.1973.10860675 [Google Scholar]
- Jagger S., Wiseman J., Cole D. J., and Craigon J.. 1992. Evaluation of inert markers for the determination of ileal and faecal apparent digestibility values in the pig. Br. J. Nutr. 68:729–739. doi:10.1079/BJN19920129 [DOI] [PubMed] [Google Scholar]
- Kavanagh S., Lynch P. B., O’Mara F., and Caffrey P. J.. 2001. A comparison of total collection and marker technique for the measurement of apparent digestibility of diets for growing pigs. Anim. Feed Sci. Technol. 89:49–58. doi:10.1016/S0377-8401(00)00237-6 [Google Scholar]
- Köhler T., Huisman J., Den Hartog L. A., and Mosenthin R.. 1990. Comparison of different digesta collection methods to determine the apparent digestibilities of the nutrients at the terminal ileum in pigs. J. Sci. Food Agric. 53:465–475. doi:10.1002/jsfa.2740530405 [Google Scholar]
- McCarthy J. F., Aherne F. X., and Okai D. B.. 1974. Use of HCl insoluble ash as an index material for determining apparent digestibility with pigs. Can. J. Anim. Sci. 54:107–109. doi:10.4141/cjas74-016 [Google Scholar]
- McClean D. 1993. Effects of processing of raw materials on digestibility of diets for weaned pigs. PhD Diss. Queen’s Univ. Belfast, Northern Ireland. [Google Scholar]
- Mroz Z., Bakker G. C., Jongbloed A. W., Dekker R. A., Jongbloed R., and van Beers A.. 1996. Apparent digestibility of nutrients in diets with different energy density, as estimated by direct and marker methods for pigs with or without ileo-cecal cannulas. J. Anim. Sci. 74:403–412. [DOI] [PubMed] [Google Scholar]
- Myers W. D., Ludden P. A., Nayigihugu V., and Hess B. W.. 2004. Technical note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 82:179–183. [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Olukosi O. A., Bolarinwa O. A., Cowieson A. J., and Adeola O.. 2012. Marker type but not concentration influenced apparent ileal amino acid digestibility in phytase-supplemented diets for broiler chickens and pigs. J. Anim. Sci. 90:4414–4420. doi:10.2527/jas.2011-4801 [DOI] [PubMed] [Google Scholar]
- Peddie J., Dewar W. A., Gilbert A. B., and Waddington D.. 1982. The use of titanium dioxide for determining apparent digestibility in mature domestic fowls (Gallus domesticus). J. Agric. Sci. 99:233–236. doi:10.1017/S002185960005526X [Google Scholar]
- Saha D. C., and Gilbreath R. L.. 1991. Analytical recovery of chromium from diet and faeces determined by colorimetry and atomic absorption spectrophotometry. J. Sci. Food Agric. 55:433–446. doi:10.1002/jsfa.2740550311 [Google Scholar]
- Shiau S. Y., and Liang H. S.. 1995. Carbohydrate utilization and digestibility by tilapia, Oreochromis niloticus × O. aureus, are affected by chromic oxide inclusion in the diet. J. Nutr. 125:976–982. doi:10.1093/jn/125.4.976 [DOI] [PubMed] [Google Scholar]
- Short F. J., Gorton P., Wiseman J., and Boorman K. N.. 1996. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Tech. 59:215–221. doi:10.1016/0377-8401(95)00916-7 [Google Scholar]
- Udy M. J. 1956. The physical and chemical properties of compounds of chromium. In: M. J., Udy, editor, Chromium. Reinhold Publishing Corp, New York, NY: p. 113–250. [Google Scholar]
- Wang T., Ragland D., and Adeola O.. 2017. Combination of digestibility marker and fiber affect energy and nitrogen digestibility in growing pigs. Anim. Feed Sci. Technol. 230:23–29. doi:10.1016/j.anifeedsci.2017.05.012 [Google Scholar]
- Williams C. H., David D. J., and Iismaa O.. 1962. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J. Agric. Sci. 59:381–385. doi:10.1017/S002185960001546X [Google Scholar]
- Yin Y.-L., McEvoy J. D. G., Schulze H., Hennig U., Souffrant W.-B., and McCracken K. J.. 2000. Apparent digestibility (ileal and overall) of nutrients as evaluated with PVTC-cannulated or ileo-rectal anastomised pigs fed diets containing two indigestible markers. Livest. Prod. Sci. 62:133–141. doi:10.1016/S0301-6226(99)00130-X [Google Scholar]