SUMMARY
Transarterial radioembolization with yttrium-90 resin microspheres (SIR-Spheres; Sirtex Medical Limited, Sydney, Australia) is a liver-directed therapy that is gaining recognition as a treatment option for liver-dominant primary and metastatic cancers. The incidence of complications is low and can be further reduced by patient selection and rigorous pretreatment assessment. Ideal candidates for radioembolization have preserved liver function without ascites or encephalopathy, Child-Pugh score <7 and limited lung shunting. Phase III randomized controlled trials (RCTs) against other liver-directed therapies are lacking for intermediate-stage hepatocellular carcinoma. However, preliminary data from a recent RCT has suggested that radioembolization has a similar time-to-progression and comparable toxicity to selective chemoembolization. Phase II/III RCTs are now ongoing to evaluate the combination of radioembolization with systemic therapies in advanced-stage hepatocellular carcinoma and metastatic liver-dominant colorectal cancer in order to expand the treatment opportunities for patients with cancers in the liver.
KEYWORDS : colorectal cancer, hepatocellular carcinoma, liver metastases, neuroendocrine tumors, radioembolization, yttrium-90
Practice points.
An important initial step in the management of hepatocellular carcinoma (HCC) is the application of an appropriate and accurate staging system to stratify patients for either liver-directed (e.g., radiofrequency ablation, embolization or transarterial embolization [TACE]) or systemic treatment. One of the most commonly used standard classifications is the Barcelona Clinic Liver Cancer system, which has been validated in many studies and is endorsed by the American Association for the Study of Liver Diseases and European Association for the Study of the Liver.
Surgery (either R0 resection or transplantation [for HCC]) can provide potentially curative options for a minority of patients with liver-only primary and secondary liver tumors.
For the most part, patients with unresectable secondary liver metastases or advanced HCC receive palliative treatment with systemic chemotherapy and/or molecularly targeted approach (e.g., sorafenib for HCC).
However, in patients with unresectable liver-dominant disease (or whenever limited extrahepatic disease has an indolent clinical course), liver-directed locoregional therapies strategies may afford substantial clinical benefit in selected patients.
Among the novel liver-directed locoregional therapies currently under investigation for the treatment of unresectable liver-dominant or liver-only primary and secondary cancers is radioembolization with yttrium-90 (90Y) microspheres.
Phase III randomized controlled trials (RCTs) against other liver-directed therapies are lacking for intermediate-stage HCC. However, preliminary data from a recent RCT has suggested that radioembolization has a similar time-to-progression and comparable toxicity to selective TACE.
Phase II/III RCTs are now ongoing to evaluate the combination of radioembolization with systemic therapies in advanced-stage HCC and metastatic liver-dominant colorectal cancer in order to expand the treatment opportunities for patients with cancers in the liver.
Novel applications of radioembolization in HCC that deserve further research include: the application of high radiation doses to small sectors of liver tissue in 'radiation segmentectomy'; right-lobar radioembolization to induce significant contralateral hypertrophy that may enable anatomic liver resections otherwise contraindicated because of a small future liver remnant; and the application of radioembolization for enabling downsizing to liver transplantation or percutaneous ablation.
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and the third most common cause of cancer-related mortality [1,2]. Metastatic disease to the liver is the most common form of hepatic malignancy [3]. Common tumors that metastasize to the liver include colorectal (mCRC), breast cancers and neuroendocrine tumors (mNETs). Surgical interventions (resection/liver transplantation) provide potentially curative options for selected patients, but many patients are precluded from these treatments [4,5].
Among the novel liver-directed locoregional therapies for the treatment of unresectable liver-dominant or liver-only primary and secondary cancers, radioembolization with yttrium-90 (90Y)-microspheres has recently been considered as one of the most promising therapies currently under investigation. In HCC, transarterial chemoembolization (TACE), usually lobar, is the standard of care at many centers for the management of intermediate (Barcelona Clinic Liver Cancer [BCLC] stage B) HCC [6]. Both radioembolization and TACE are similar in delivery, since both require catheterization of the hepatic artery; however, TACE requires selective catheterization of the tumor-feeding vessel, while 90Y-radioembolization can be delivered using either a whole liver, lobar or segmental or subsegmental approach, depending on the nature and distribution of the hepatic tumors. TACE and 90Y-microspheres also differ in their mechanisms of action. 90Y-microspheres (∼30 µm diameter) achieve tumor necrosis through the localized effects of β-radiation with little or no embolic effect on the vessel [7]. By contrast, the larger TACE or bland embolization particles (100–500 µm diameter) have been designed to occlude medium-to-large-size arteries, so that ischemia drives the antitumor effect, with drug delivery (carried in lipiodol or drug-eluting beads) potentially enhancing tumor cell killing. Both TACE and 90Y-radioembolization delay disease progression and are used in a number of different settings to: downsize tumors for resection; as a bridge to liver transplantation (in HCC); or as palliative therapies in liver metastases [8]. In HCC, a number of randomized controlled trials (RCTs) comparing TACE or sorafenib and 90Y-radioembolization are underway (Table 1). For patients with unresectable mCRC of the liver, clinical trials with radioembolization and concomitant radiosensitizing chemotherapy have shown promising results (Table 2), rendering a significant proportion of patients amenable to potentially curative surgery or ablation. As a result, Phase III RCTs in this setting are now ongoing with 90Y-radioembolization.
Table 1. . Summary of published experience with radioembolization in hepatocellular carcinoma (by disease stage) in comparison with conventional transarterial embolization and sorafenib.
| Study (year) | Study design | Treatment | Child-Pugh class | Early | Intermediate | Advanced | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Patent portal vein | Portal vein occlusion | ||||||||||||
| n | Median OS (95% CI) mo | n | Median OS (95% CI), months | n | Median OS (95% CI), months | n | Median OS (95% CI), months | n | Median OS (95% CI), months | |||||
| Salem et al. (2011) | Retrospective case series | Conventional TACE | Overall A (n = 67) B (n = 53) | 47 | 45.4 (15.1–46.1) | 61 | 17.5 (14.8–18.7) | 12 | 9.3 (6.2–11.5) | – | – | – | – | [34] |
| Hilgard et al. (2010) | Retrospective case series | Radioembolization | Overall | – | – | 47 | 16.4 (12.1–NC) | 51 | NR | 75 | 16.4 (12.1–NC) | 33 | 10.0 (6.0–NC) | [35] |
| TheraSphere | A (n = 84) | 17.2 (12.1–NC) | ||||||||||||
| B (n = 24) | 6.0 (4.2–NC) | |||||||||||||
| Salem et al. (2010) | Prospective case series | Radioembolization | Overall | 48 | 26.9 (17–30.2) | 83 | 17.2 (13.5–29.6) | 107 | 7.3 (6.5–10.1)† | – | NR | – | NR | [36] |
| TheraSphere | A | 27 | 20.5 (15–27.4) | 48 | 17.3 (13.7–32.5) | 41 | 13.8 (8.8–17.7)† | 6 | 47.4 (NR)† | 35 | 10.4 (7.2–16.6)† | |||
| B | 21 | 29.1 (17–NC) | 35 | 13.5 (6.4–25.4) | 66 | 6.4 (4.9–7.7)† | 9 | 11.8 (NC–34)† | 57 | 5.6 (4.5–6.7)† | ||||
| Sangro et al. (2011) | Retrospective case series | Radioembolization | Overall | 52 | 24.4 (18.6–38.1) | 87 | 16.9 (12.8–22.8) | 183 | 10.0 (7.7–10.0) | 110 | 9.3 (7.4–11.4) | 73 | 10.2 (7.7–11.8) | [37] |
| SIR-Spheres | A | 47 | 30.9 (18.6–45.9) | 82 | 18.4 (13.6–23.2) | 137 | 9.7 (7.6–10.9) | |||||||
| B | 5 | 19.4 (6.5–27.4) | 5 | 3.6 (2.4–10.8) | 46 | 10.0 (6.1–14.5) | ||||||||
| Cheng et al. (2009) | Prospective RCT | Sorafenib | A | – | – | – | – | 150 | 6.5 (5.6–7.6) | 96 | NR | 54 | NR | [38] |
| Llovet et al. (2008) | Prospective RCT | Sorafenib | Overall A (n = 284) B (n = 14) | – | – | 54 | 14.5 (NR) | 244 | 9.7 (NR) | 191 | NR | 108 | NR | [39,40] |
†Without extrahepatic disease.
NC: Not calculated; NR: Not reported; OS: Overall survival; RCT: Randomized controlled trial; TACE: Transarterial chemoembolization.
Table 2. . Summary of published experience with radioembolization in liver metastases from neuroendocrine tumors, breast cancer and uveal melanoma.
| Study (year) | Study design | Microsphere type | Treatment setting | n/n with response | Response (%) | Median survival (months) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete | Partial | Stable disease | Any | Progressive disease | |||||||
| NET | |||||||||||
| Rhee et al. (2008) | Prospective | SIR-Spheres, TheraSphere | Salvage | 42/29 | 0 | 52 | 41 | 93 | 7 | 25.0 | [79] |
| Kennedy et al. (2008) | Retrospective | SIR-Spheres | NA | 168/148 | 3 | 67 | 25 | 95 | 5 | 70.0 | [80] |
| King et al. (2008) | Prospective | SIR-Spheres | Salvage | 34/34 | 15 | 35 | 15 | 65 | 35 | 27.6 | [81] |
| Saxena et al. (2010) | Retrospective | SIR-Spheres | Salvage | 48/48 | 15 | 40 | 23 | 78 | 22 | 35.0 | [82] |
| Cao et al. (2010) | Retrospective | SIR-Spheres | Mixed | 58/51 | 12 | 27.5 | 27.5 | 67 | 33 | 36.0 | [83] |
| Breast cancer | |||||||||||
| Cianni et al. (2013) | Retrospective | SIR-Spheres | Salvage | 52/52 | 0 | 56 | 33 | 89 | 10 | 11.5 | [84] |
| Jakobs et al. (2008) | Retrospective | SIR-Spheres | Salvage | 30/23 | 0 | 61 | 35 | 96 | 4 | 11.7 | [85] |
| Coldwell et al. (2007) | Retrospective | SIR-Spheres | Salvage | 44/36 | 0 | 47 | 47 | 94 | 6 | NA | [86] |
| Bangash et al. (2007) | Prospective | TheraSphere | Salvage | 27/23 | NA | 39 | 52 | 91 | 9 | NA | [87] |
| Uveal melanoma | |||||||||||
| Gonsalves et al. (2011) | Retrospective | SIR-Spheres | Salvage | 32/32 | 3 | 3 | 56 | 62 | 38 | 10.0 | [88] |
Studies in this table included more than 25 patients.
NA: Not available.
Rationale for 90Y-radioembolization
The application of external beam radiation therapy in patients with liver tumors is limited by the low tolerance of liver tissue to radiation beyond 30 Gy [9] compared with the doses required for a tumoricidal effect [10], which exceed 70 Gy [11]. Radioembolization exploits the well-characterized dual vasculature of the liver to selectively target tumors that are almost exclusively supplied by blood from the hepatic arterial branches. Owing to their size, the microspheres preferentially lodge within the tumor's microvascular plexus [12]. 90Y, a pure β-emitter with a half-life of 64.1 h, is the most commonly used radionuclide for the treatment of both primary and secondary liver malignancies. The decay of 90Y to stable zirconium-90 releases an average energy of 0.9367 MeV over a limited range (mean penetration into tissues: 2.4 mm), so that the radiation exposure is predominantly limited to tumor tissue, while normal liver tissue is spared [13,14]. Radioembolization is delivered using either 90Y-resin microspheres (SIR-Spheres; Sirtex Medical Limited, Sydney, Australia) or 90Y-glass microspheres (Therasphere; BTG International Canada Inc., Ontario, Canada), each with different physical characteristics (Table 3). The properties of the microspheres are similar; however, in contrast to the heavier 90Y-glass microspheres, 90Y-resin microspheres have a specific gravity similar to plasma, and more 90Y-resin microspheres than 90Y-glass microspheres are delivered in the typical radioembolization procedure. Consequently, 90Y-resin microspheres may theorectically achieve a more homogeneous coverage of the tumor tissue. One standard 3 GBq vial of 90Y-resin microspheres contains 40–80 million microspheres ranging in size from 20 to 60 μm. The average activity per resin microsphere is 50 Bq at the time of calibration. Each milligram of 90Y-glass microspheres contains between 22,000 and 73,000 microspheres, ranging in size from 20 to 30 μm, which are available in three activities (5, 10 and 20 Bq). Beyond the differences in the materials used for each type of microsphere, these devices differ in the amount of radioactive isotope loaded in each microsphere (greater for glass microspheres), which in turn determines the number of microspheres injected in a typical radioembolization procedure (lower for glass microspheres) (Table 3). Current evidence suggests that the primary method of action of both resin and glass microspheres is the same and is due to a localized radiotherapeutic effect (brachytherapy) rather than microvascular embolization and tumor ischemia [7,15,16].
Table 3. . Characteristics of commercially available yttrium-90 microspheres for radioembolization.
| SIR-Spheres† | TheraSphere‡ | |
|---|---|---|
| Isotope | 90Y | 90Y |
| Half-life (h) | 64.1 | 64.1 |
| Microsphere material | Resin | Glass |
| Microsphere diameter (μm) | 20–60 | 20–30 |
| Approximate activity per microsphere (Bq) | 50 | 2500 |
| Number of microspheres per 3 GBq | 40–80 × 106 | 1.2 × 106 |
| Specific gravity (g/ml) | 1.6 | 3.2 |
| Embolic effect | Moderate | Mild |
| Contrast agent injection | During infusion | None |
| Indication | USA (FDA PMA): colorectal liver metastases | USA (FDA HDE): hepatocellular carcinoma |
†Sirtex Medical, North Sydney, Australia.
‡BTG International Canada Inc., Ontario, Canada.
HDE: Humanitarian device exemption; PMA: Premarket approval.
Technical aspects of radioembolization
• Pretreatment evaluation
A multidisciplinary team consisting of professionals from interventional radiology, hepatology, medical, surgical and radiation oncology, transplant surgery and nuclear medicine is involved in selecting suitable candidates for radioembolization.
Patients are selected according to the following criteria:
Confirmed diagnosis of surgically unresectable HCC, intrahepatic cholangiocarcinoma (ICC) or liver-dominant metastases;
Age >18 years;
Eastern Cooperative Oncology Group (ECOG) performance status ≤2;
Adequate hematologic parameters (granulocyte count <1.5 × 109/l, platelet count >60 × 109/l), renal function (serum creatinine level <2.0 mg/dl) and liver function (serum total bilirubin level <2.0 mg/dl);
The ability to undergo angiography and selective visceral catheterization.
Most patients have a Child-Pugh score ≤7; although a Child-Pugh score >7 is not an absolute contraindication. The exclusion criteria are as follows:
Any other liver-directed therapy planned for cancer treatment;
Uncorrectable flow to the GI tract;
Lung shunting >20% (resin microspheres) or estimated radiation doses to the lungs >30 Gy (with single administration) or 50 Gy (with multiple administrations);
Significant extrahepatic disease representing imminent life-threatening outcome.
For patients with HCC and abnormal liver function (total bilirubin: 1.3–2.0 mg/dl), the tumor volume should not exceed 50% of the total liver volume; tumor volumes >70% or infiltrating disease (even in patients with normal liver function) are a relative contraindication for radioembolization. In patients with metastatic disease without cirrhosis, tumor volume should not exceed 50% with normal liver function tests (LFTs).
• Pretreatment angiography
In candidates for radioembolization, pretreatment angiography is performed (Figure 1) to detect and occlude aberrant vessels arising from hepatic arteries [17] that may feed nontarget tissue. Alternatively, the infusion catheter is placed distal to all vessels with hepatofugal flow [18–20].
Figure 1. . 90Y pretreatment planning.
(A) Arterial phase and (B) equilibrium phase computed tomography of residual hepatocellular carcinoma in VIII segment after a previous drug-eluting bead-based transarterial chemoembolization; (C & D) superselective angiography showing the positioning of the microcatheter for 90Y-radioembolization treatment.
• Pulmonary shunting
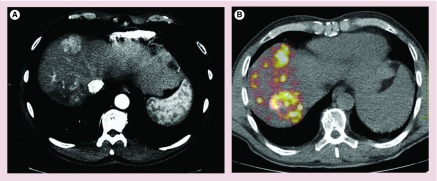
Another feature of hepatic tumors, particularly HCC, is the arteriovenous shunting of 90Y-microspheres to the lungs [21], thereby increasing the risk of radiation pneumonitis [22]. The fraction of shunting of microspheres from liver to the lung is assessed prior to radioembolization using technetium-99m macroaggregated albumin (99mTc-MAA), which closely mimic the distribution of the 90Y-microspheres. Using these data, the activity of 90Y delivered is modified so that the radiation dose delivered to the lung remains within tolerable limits. Correlation of 99mTc-MAA distribution through scintigram, or single-photon emission computed tomography (CT) or single-photon emission CT/CT with angiographic findings is also helpful in identifying potential accumulations at other extrahepatic sites (Figure 2) [23].
Figure 2. . 90Y-pretreatment planning.
(A) Technetium-99m macroaggregated albumin planar scintigram for calculation of the degree of hepatopulmonary shunting: lung/liver ratio: 11.48%; (B) technetium-99m macroaggregated albumin single-photon emission computed tomography/computed tomography without any obvious collateral vessels and good differential distribution of particles between tumor and normal liver tissue (tumor to normal ratio).
• Dose calculation for 90Y-microspheres
The most widely used dosimetry for 90Y-resin microspheres is the body surface area (BSA) method. It is calculated as follows:
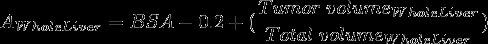 |
where AWholeLiver is the activity in GBq for a whole liver treatment and BSA is in m2. The calculated activity is then modified, as required, to take into account any lung shunting and the tumor burden [20].
The BSA equation may be adapted for lobar treatments as follows:
 |
For 90Y-glass microspheres, the dose absorbed to organs is estimated by the Medical Internal Radiation Dose schema, based on the assumption of uniformly distributed radioactive sources. Thus, the tumor and normal liver are assumed to share the same estimated absorbed dose [24]. Some groups proposed using the partition model to estimate the absorbed dose to tumor and normal liver parenchyma based on the uptake ratio of tumor to normal tissue of 99mTc-MAA imaging as a surrogate for 90Y-microsphere distribution, which provides a more realistic picture [25].
• 90Y-radioembolization procedure
The procedure is performed according to previously published guidelines [20,26]. The device for administering 90Y-resin microspheres is designed to minimize radiation exposure to the clinical team and optimize the flexibility and control of administration. The tumor is approached under fluoroscopic guidance and the predefined activity of 90Y is slowly injected into the tumor-bearing segments (i.e., one or more lobes/segments, as required). A medical physicist is present throughout the procedure to ensure that proper protocols are followed to minimize accidental radiation exposure. After infusion of the 90Y-microspheres, patients undergo a second nuclear medicine scan (i.e., Bremsstrahlung) to validate the distribution achieved by radioembolization within the liver and to confirm the absence of nontarget deposition of microspheres (Figure 3).
Figure 3. . Imaging evaluation of 90Y microsphere distribution.
(A) Pretreatment computed tomography and (B) PET/computed tomography of metastatic colorectal cancer of the liver performed immediately after radioembolization to check the distribution of 90Y-radioembolization within the liver and to exclude any nontarget deposition of microspheres to other organs. Notice that the deposition of 90Y-loaded microspheres corresponds with the sites of two large hepatocellular carcinoma lesions.
• Post-treatment assessment
Clinical, laboratory and radiologic follow-up is crucial to monitor response to treatment and to identify any toxicity. Cross-sectional imaging is performed at 1 month then every 3 months to assess the response.
• Imaging after radioembolization
Both the appearance of the tumor and surrounding liver can vary after radioembolization. Early scans may not be representative of the final extent of necrosis, since radiation effects can take time to manifest radiographically. Rim enhancement (Figure 4) around the lesion is a common early finding, representative of a fibrotic capsule and not residual tumor [27]. A total of 8–12 weeks after radioembolization, there is noticeable tumor shrinkage, which can be measured using CT or MRI to assess tumor response in the index lesions using either Response Evaluation Criteria in Solid Tumors 1.0 or 1.1. Alternatively, the European Association for the Study of the Liver guidelines measure change in the amount of enhancing (i.e., viable) tumor only. However, these anatomic changes often lag behind functional changes. The development of functional imaging techniques including: diffusion-weighted MRI for HCC and mCRC, gadolinium-ethoxybenzyl-diethylenetriaminepentaacetic acid in HCC and mCRC, and PET for liver metastases have allowed for the earlier (between 6 and 8 weeks postprocedure) and/or more sensitive assessment of treatment response compared with CT using Response Evaluation Criteria in Solid Tumors. However, further validation of these functional imaging techniques is still needed before they are adopted in clinical practice [28,29]. Accompanying tumor shrinkage observed at 8–12 weeks is atrophy of the parenchyma with hepatic fibrosis and capsular retraction of the treated lobe (Figure 5), especially if the treatment is lobar, rather than segmental or subsegmental. Atrophy also has the effect of stimulating hypertrophy in the untreated contralateral lobe, similar to that observed after hepatic lobe resection (Figure 6) [30–32]. Transient perfusion abnormalities in the region of treatment may also be observed, which are distinct from residual or recurrent tumor. Transient perivascular edema with accompanying hypodensity adjacent to the hepatic and portal veins can also be observed on CT.
Figure 4. . Computed tomography showing perilesional rim enhancement at 1 month after a successful 90Y-radioembolization.
(A) Arterial phase and (B) portal venous phase.
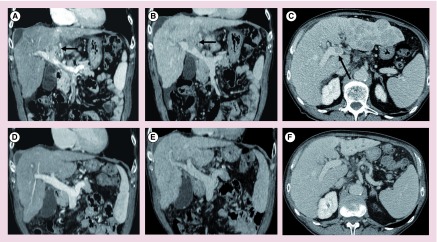
Figure 5. . Imaging pre- and post-treatment of a male (58 years of age) with hepatits C virus-related cirrhosis.
Pretreatment computed tomography demonstrates three hepatocellular carcinoma (HCC) lesions in segments: (A) VIII, (B) IV and (C) V (white arrows). (D) Dual super selective radioembolization was performed of the two lesions in segments VIII and V, with no treatment to the small HCC lesion in segment IV. Contrast-enhanced MRI study (gadolinium-ethoxybenzyl-diethylenetriaminepentaacetic acid) at 3 months showing the hepatic fibrosis and capsular retraction of the treated segments VIII and V, appearing as wedge-shaped areas, hypervascular in the (E) arterial phase, (F) isointense in portal phase, with low signal intensity in the (G & H) hepatobiliary phase and the untreated small HCC lesions (see red arrows in E–H).
Figure 6. . Contralateral lobe hypertrophy.
Imaging (A) pre- and (B) 3 months post-radioembolization of a male (58 years of age) with a single metastatic colorectal cancer nodule in the right lobe observed after multiple lines of chemotherapy (FOLFOX and FOLFIRI) combined with wedge resections; (B) computed tomography 3 months after the treatment showed marked contralateral left lobe hypertrophy (similar to that achieved with right portal vein embolization), thereby predisposing to the safe hepatectomy of the right lobe; (C) computed tomography volumetric evaluation confirmed a 102% volume increase of the left lobe, from 750 cm3 at baseline to 1518 cm3.
Progression is often the result of new lesions (intra/extra-hepatic) beyond the treated area, since radioembolization will have a limited effect on hepatic micronodules, which are poorly arterialized. Identifying early progressors is important, since the role of systemic agents (e.g., sorafenib in HCC and FOLFOX in secondary liver lesions [even the refractory setting]) is likely to be key component in improving long-term outcomes [33].
Clinical indications & outcomes
• Hepatocellular carcinoma
The use of 90Y-radioembolization in HCC is mainly supported by data from retrospective series and uncontrolled prospective studies (levels of evidence II-2 and II-3) (Table 1) [34–40]. However, the provisional evidence from the prospective SIRTACE study recently showed equivalence between radioembolization and TACE for intermediate-stage HCC [41]. Further Phase III RCTs are now ongoing evaluating the systemic therapy (i.e., sorafenib with or without the addition of 90Y-radioembolization in intermediate–advanced HCC).
Furthermore, the publication of three large series including over 700 patients has provided important insights into the overall survival (OS) according to BCLC stage (Table 1), and safety/tolerability profile of 90Y-radioembolization in the real-world clinical setting [34–37,42]. Many patients included in these series (at different stages of HCC) had either progressed or relapsed after TACE or were considered poor candidates for TACE due to the presence of portal vein invasion in advanced HCC [6] or bulky tumors [43]. (NB: patients with segmentary or subsegmentary portal vein invasion may be considered for TACE [44], while tumors ≥10 cm in diameter should be considered a relative contraindication to TACE [43]). The use of radioembolization in these cohorts slowed disease progression and also provided a bridge to transplantation for some patients by extending the time patients could remain eligible for donor organs [45]. In a retrospective analysis in patients with HCC beyond Milan criteria, Lewandowski et al. [46] compared radioembolization to TACE showing that radioembolization was a better tool than TACE for downstaging the disease to within transplants criteria. Malignant portal invasion in patients with advanced HCC is an exclusion criterion for transplantation and is associated with a poor prognosis, regardless of the treatment modality. TACE is also contraindicated in patients with advanced HCC and portal vein invasion (especially main) [6] because of the embolic nature of this therapy, which may lead to further deterioration of blood supply in patients with an already compromised liver parenchyma. By contrast, studies with 90Y-radioembolization have shown no significant difference in survival between patients with and without branch or main portal vein invasion (Figure 7) [37,42,47,48]. When compared with transarterial embolization and sorafenib, radioembolization consistently provides similar survival rates across tumor stages: for intermediate BCLC stage B (without portal vein occlusion and/or extrahepatic metastases) [26] and advanced BCLC stage C (Table 1) [49,50].
Figure 7. . Hepatocellular carcinoma and portal vein thrombosis.
(A–C) Pretreatment CT scan of left lobe showing infiltrating hepatocellular carcinoma and left portal branch thrombosis (arrows) adjoining the portal confluence; (D–F) CT scan 3 months after 90Y-radioembolization showing complete retraction of portal thrombus, marked left lobe shrinkage and almost undetectable hepatocellular carcinoma nodules.
Potential indications for 90Y-radioembolization [42] include the treatment of patients with:
Intermediate stage HCC who are poor candidates for TACE because of numerous or large tumors [42];
Advanced stage HCC with solitary tumors invading a segmental or lobar branch of the portal vein;
The option of downstaging, thereby opening the door to a radical approach;
Disease progression requiring TACE or sorafenib.
The National Comprehensive Cancer Network and European Society of Medical Oncology guidelines recommend radioembolization as either a ‘bridging’ option before other treatment modalities (partial hepatectomy or liver transplantation) or as a main therapy for patients with diffuse intrahepatic tumor spread, or as an alternative to TACE in selected patients with contraindications for TACE [51]. Large clinical trials are now underway to establish the precise roles of radioembolization for the treatment of HCC relative to the tyrosine kinase inhibitor, sorafenib, which is the recommended treatment of choice for advanced HCC following the SHARP trial. In particular, a number of multicenter RCTs are ongoing in advanced HCC (stratifying patients with and without portal vein invasion) either combining radioembolization with sorafenib or comparing radioembolization versus sorafenib.
• Intrahepatic cholangiocarcinoma
To date, four small series have examined 90Y-radioembolization as a potential treatment for unresectable ICC with median OS of 9.3, 11.5, 22.0 and 14.9 months, respectively, in patients who had received prior systemic chemotherapy [52–55]. In another cohort of 24 patients, survival following 90Y-radioembolization varied based on presence of multifocal (5.7 vs 14.6 months), infiltrative (6.1 vs 15.6 months) and bilobar disease (10.9 vs 11.7 months); disease was converted to resectable status in five (20.8%), who successfully underwent R0 resection [56]. Although the evidence is limited, early data suggest that the survival in ICC does not vary significantly regardless of the type of intra-arterial therapy (coventional TACE, drug-eluting beads TACE or radioembolization) [57].
• Liver metastases
Most published data on 90Y-radioembolization for the treatment of unresectable liver-dominant mCRC (including some Phase II and III RCTs) are based on the experience with on 90Y-resin microspheres rather than 90Y-glass microspheres (Table 4) [3,58–78]. In addition, small case series have been published on 90Y-radioembolization for the treatment of liver metastases from neuroendocrine tumors, breast cancer and anduveal melanoma (Table 2) [79–88]. Therapeutic benefits appear to be greatest when radioembolization is used as an earlier line of therapy or is combined with chemotherapy [58–61,89].
Table 4. . Summary of published experience with radioembolization in liver metastases from colorectal cancer.
| Study (year) | Study design | Microsphere type | Combination (per protocol) | Liver only or liver-dominant | n/n with response | Response (%) | Median survival (months) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete | Partial | Stable disease | Any | Progressive disease | ||||||||
| First-line | ||||||||||||
| Gray et al. (2001) | Prospective | SIR-Spheres | SIRT + FUDR-HAC | LO | 36/32 | 6 | 44 | 41 | 91 | 9 | 39% at 2 years | [58] |
| RCT | FUDR-HAC | LO | 34/27 | 0 | 22 | 48 | 70 | 30 | 29% at 2 years | |||
| van Hazel et al., (2004) | Prospective | SIR-Spheres | SIRT + 5FU/LV | LD | 11/11 | 0 | 73 | 27 | 100 | 0 | 29.4 | [59] |
| RCT | 5FU/LV | LD | 10/10 | 0 | 0 | 60 | 60 | 40 | 12.8 | |||
| Sharma et al. (2007) | Prospective | SIR-Spheres | SIRT + FOLFOX | LD | 22/20 | 0 | 90 | 10 | 100 | 0 | NA | [60] |
| Kosmider et al. (2011) | Retrospective | SIR-Spheres | SIRT + FOLFOX4 or 5FU/LV | LD | 19/19 | 11 | 74 | 5 | 90 | 10 | 29.4 | [61] |
| LO | 19/19 | 11 | 79 | 5 | 95 | 5 | 37.8 | |||||
| Tie et al. (2010) | Retrospective | SIR-Spheres | SIRT + FOLFOX4 or 5FU/LV | LD | 21/21 | 10 | 71 | 5 | 86 | 14 | 17.7 | [62] |
| LO | 21/21 | 14 | 71 | 5 | 90 | 10 | 29.0 | |||||
| Second line or third line | ||||||||||||
| van Hazel et al. (2009) | Prospective | SIR-Spheres | Irinotecan | LD | 25/23 | 0 | 48 | 39 | 87 | 13 | 12.2 | [63] |
| Lim et al. (2005) | Retrospective | SIR-Spheres | SIRT + 5FU in 70% of patients | LD | 32/32 | 0 | 31 | 28 | 59 | 41 | NA | [64] |
| Lewandowski et al. (2005) | Prospective | TheraSphere | None | LD | 27/26 | 0 | 35 | 52 | 87 | 13 | 9.3 | [3] |
| Evans et al. (2010) | Retrospective | SIR-Spheres | None | LD | 140/NA | NA | NA | NA | NA | NA | 7.9 | [65] |
| Salvage | ||||||||||||
| Seidensticker et al. (2012) | Prospective | SIR-Spheres | SIRT + BSC | LD | 29/28 | 0 | 43 | 18 | 72 | 28 | 8.3 | [66] |
| BSC (matched-pair controls) | LD | 29/NA | NA | NA | NA | NA | NA | 3.5 | ||||
| Hendlisz et al. (2010) | Prospective | SIR-Spheres | SIRT + 5FU | LO | 21/20 | 0 | 10 | 80 | 90 | 10 | 10.0 | [67] |
| RCT | 5FU (+ SIRT upon progression) | LO | 23/22 | 0 | 0 | 36 | 36 | 64 | 7.3 | |||
| Bester et al. (2013) | Retrospective | SIR-Spheres | None | LD | 224/NA | NA | NA | NA | NA | NA | 11.9 | [69] |
| BSC or conventional therapy | LD | 29/NA | NA | NA | NA | NA | NA | 6.6 | ||||
| Cosimelli et al. (2010) | Prospective | SIR-Spheres | None | LD | 50/46 | 2 | 24 | 26 | 52 | 48 | 12.6 | [68] |
| Mulcahy et al. (2009) | Prospective | TheraSphere | None | LD | 72/72 | 0 | 40 | 45 | 85 | 15 | 14.5 | [70] |
| Mancini et al. (2006) | Prospective | SIR-Spheres | None | LD | 35/35 | 0 | 12 | 76 | 88 | 13 | NA | [71] |
| Chua et al. (2011) | Retrospective | SIR-Spheres | None | LD | 140/140 | 1 | 31 | 31 | 63 | 37 | 9.0 | [72] |
| Stubbs et al. (2006) | Retrospective | SIR-Spheres | HAIC with 5FU | LD | 100/80 | 1 | 73 | 20 | 94 | 6 | 11.0 | [73] |
| Jakobs et al. (2008) | Retrospective | SIR-Spheres | None | LD | 41/36 | 0 | 19 | 70 | 89 | 11 | 10.5 | [74] |
| Cianni et al. (2009) | Retrospective | SIR-Spheres | None | LD | 41/41 | 5 | 40 | 36 | 81 | 19 | 11.9 | [75] |
| Kennedy et al. (2006) | Retrospective | SIR-Spheres | None | LD | 208/208 | 0 | 36 | 55 | 91 | 10 | 10.5 responders, 4.5 nonresponders | [77] |
| Benson et al. (2013) | Prospective | TheraSphere | None | NA | 61/61 | 0 | – | – | 59 | 31 | 8.8 | [78] |
5FU: 5-fluorouracil; BSC: Best supportive care; FOLFOX: Folinic acid, 5-fluorouracil plus oxalipaltin; FOLFOX4: 5-fluorouracil/leucovorin plus oxaliplatin; FUDR-HAC: Floxuridine-hepatic arterial chemotherapy; HAIC: Hepatic arterial infusion chemotherapy; LD: Liver dominant; LO: Liver only; LV: Leucovorin; NA: Not available; RCT: Randomized controlled trial; SIRT: Selective internal radiation therapy.
Liver metastases from mCRC
In 2001, a Phase III RCT compared 90Y-resin microspheres plus hepatic artery chemotherapy versus hepatic artery chemotherapy alone in 74 patients with mCRC confined to the liver. The combined modality treatment had a significantly better tumor response (44 vs 17.6%; p = 0.01), a longer time-to-progression (TTP; 15.9 vs 9.7; p = 0.001), similar OS at 1, 2, 3 and 5 years (72, 39, 17 and 3.5% vs 68, 29, 6.5 and 0%, respectively) and an acceptable safety profile [58]. In 2004, the same investigators [59] reported favorable data from a small RCT on the use of systemic 5-fluorouracil (5FU)/leucovorin chemotherapy with or without an additional single administration of 90Y-resin microspheres in the first-line treatment of patients with liver-dominant mCRC. In this study of 21 patients, both TTP (18.6 vs 3.6 months) and median survival (29.4 vs 12.8 months) were significantly longer for patients receiving combined treatment. Sharma et al. [60] conducted a Phase I study evaluating radioembolization combined with modified FOLFOX4 (5FU/leucovorin plus oxaliplatin) first-line systemic chemotherapy in patients with unresectable mCRC in the liver. The objective response rate in this study was 90%. Median progression-free survival and TTP in the liver were 9.3 and 12.3 months, respectively. Based on the results of this study, further investigation of first-line 90Y-resin microspheres combined with FOLFOX-based regimens (with or without bevacizumab) in two Phase III trials are now ongoing, with the first results expected in 2015. A recent meta-analysis of the effects of radioembolization on liver metastases showed high response rates for 90Y-radioembolization, particularly if used as neoadjuvant to chemotherapy [90]. Unfortunately, the quality of the data (at present) does not permit reliable analysis of survival in this setting.
In the chemotherapy-refractory setting, a multicenter Phase III RCT by Hendlisz et al. [67] compared radioembolization combined with 5FU versus protracted intravenous infusion of 5FU alone in 46 patients with liver-limited mCRC. TTP (overall) and TTP in the liver were significantly in favor of the combination treatment arm: 4.5 and 5.5 months versus 2.1 and 2.1 months, respectively (p = 0.003), and was associated with a lower incidence of Grade 3 or 4 toxicities (six vs one patient; p = 0.10).
Studies have shown a better response as the dose of 90Y in the tissue increases: with mortality reduced by 50% and the odds of a tumor response 3.1-times greater with median doses >95 Gy compared with median doses ≤95 Gy [91]. More recently, Bester et al. [69] compared survival following 90Y-resin microspheres versus best supportive care (BSC) in patients with chemotherapy-refractory liver metastases in the salvage setting. Median OS was significantly extended with the addition of 90Y-resin microspheres to BSC versus BSC alone: 11.9 (95% CI: 10.1–14.9 months) versus 6.6 months (log-rank test, p < 0.001); these survival figures have also been corroborated by the multicenter prospective study by Cosimelli et al. [68] and by the matched-pair analysis by Seidensticker et al. [66].
Liver metastases from neuroendocrine tumors
Radioembolization was recognized by the National Comprehensive Cancer Network as a treatment option for mNETs [92]. Unresectable mNETs treated with radioembolization demonstrated limited toxicity and prolonged responses. Kennedy et al. [80], in a 148 patient analysis, reported response rates of 63.2% with a survival of 70 months. Rhee et al. [79] in a multicenter Phase II study with 42 patients with hepatic mNETs observed that 92 and 94% of patients treated with glass and resin microspheres, respectively, showed either partial response or stable disease at 6 months, and the median survival was 22 and 28 months, respectively. Grade 3 toxicities were recorded in only six patients. They concluded that 90Y-radioembolization is a viable therapy with acceptable toxicity for hepatic mNETs.
Other liver metastases
90Y-radioembolization has been used for the treatment of patients with metastases other than mCRC with variable results (Table 2) [79–88] and a survival benefit has not been firmly established in prospective comparative studies. Bangash et al. [87] investigated 90Y-radioembolization in 27 patients with progressing liver metastases of breast cancer on standard polychemotherapy. The response rate was 39.1%; stable and progressive disease was observed in 52.1 and 8.8%, respectively. A response on fluorodeoxyglucose PET was noted in 63%. The median survival was 6.8 months in patients with an ECOG performance status of 0 compared with 2.6 months (ECOG 1, 2 and 3). They concluded that 90Y-radioembolization might be a viable option for the management of patients with liver metastases from breast cancer that have progressed on standard polychemotherapy. Coldwell et al. [86] investigated the use of 90Y-microspheres in the treatment of chemorefractory liver metastases from breast cancer in 44 patients in a multi-institutional study. No treatment-related procedure deaths or liver toxicity were noted. Partial responses were recorded in 47% of patients (by CT) and in 95% of patients (by PET). Survival was longer for responders and patients with slowly progressing disease (median OS not reached after 14 months follow-up) compared with nonresponders (median OS: 3.6 months). These findings were corroborated in a prospective evaluation by Jakobs et al. [85] in 30 patients with chemorefractory disease, which also showed a longer survival among responders compared with nonresponders (23.6 vs 5.7 months; p = 0.005) and in patients with liver-only disease compared with those with extrahepatic disease (16 vs 9.6 months; p = 0.077).
Because benefits have not been definitely established in these tumor types, 90Y-radioembolization should be limited to patients either unsuitable for, or refractory to, standard systemic therapies [93].
Safety & toxicity
Radioembolization is a relatively safe procedure with a lower toxicity compared with TACE [94] as a consequence of its small particle size and microembolic effect [77,95]. The most common side effect is the postradioembolization syndrome, which occurs in approximately 50% of patients. Similar to postembolization syndrome observed with TACE but less severe, the main clinical symptoms of postradioembolization syndrome are transient in nature: mild-to-moderate abdominal discomfort, nausea, vomiting, fever, anorexia and fatigue over the first 2 weeks post-treatment. Transient elevations in LFTs, specifically increases in alkaline phosphatase, bilirubin, and alanine transferase levels, are common and to be expected after treatment [96,97]. In addition, there are several specific complications associated with the nontargeted distribution of 90Y-radioembolization, including: radiation-induced gastroduodenal ulcerations and pancreatitis, radioembolization-induced liver disease (REILD), portal hypertension, radiation cholecystitis and bile duct injuries [98]. Radiation-induced pneumonitis is an exceedingly rare event due to the mandatory quantification of liver-to-lung shunting prior to 90Y-radioembolization [96,99]. Radiation-induced gastroduodenal ulcerations and pancreatitis, due to the inadvertent deposition of microspheres, occur in less than 5% of patients and less than 1% of patients (for pancreatitis) if meticulous patient preparation and proper techniques are used (i.e., slow and controlled injection of microspheres and prophylactic coil embolization of vessels to prevent nontarget deposition of microspheres) [96,100,101].
REILD usually occurs with 8 weeks of radioembolization; its incidence varies from 0 to 4%and is more common in patients with pre-existing liver dysfunction [102] and in patients with metastatic liver disease who have undergone extensive prior chemotherapy [96]. REILD is commonly defined by the occurrence of jaundice and nonmalignant ascites in the absence of tumor progression or bile duct obstruction. Bilirubin and alkaline phosphatase are usually markedly elevated; however, there may be no change in transaminase levels. Changes in LFTs after radioembolization have ranged between 15 and 20% [96,97]. The presence of factors, such as abnormal hepatic function at baseline, increased age, and activity delivered may also increase the risk of REILD and changes in LFTs.
The incidence of biliary sequelae after radioembolization is reported to be <10%. According to Atassi et al. [103], less than 2% of patients required intervention for the biliary toxicity induced by radioembolization (including drainage of bilomas, treatment of abscesses and cholecystectomies). Radiation-induced cholangitis is also rarely reported.
In the long-term, radioembolization has been shown to cause liver fibrosis in the treated portions, resulting in the contraction of the treated hepatic parenchyma and portal hypertension (based on radiological findings). This finding is more common with bilobar treatment, and its incidence increases in patients who have chemotherapy-associated steatohepatitis or pre-existing cirrhosis. However, portal hypertension has little clinical significance [104] because clinically relevant manifestations, such as reduced platelet counts (<100,000/dl) or variceal bleeding [31,104], are rare.
Conclusion
90Y-radioembolization represents a promising option, which challenges the current treatment paradigm due to its high antitumoral effect and survival equivalence to TACE in HCC. Its clinical application requires further testing for the treatment of advanced HCC complicated by portal vein occlusion, in downstaging to transplantation as an alternative to TACE, and in the conversion of surgically inoperable patients (due to small liver remnant) to potential cure with resection.
In liver metastases, a clinically relevant survival benefit for 90Y-therapy has been demonstrated in patients not responding to chemotherapy, including those heavily pretreated who would otherwise have few treatment options and a poor prognosis [105,106]. Future research will recognize the precise role of 90Y-radioembolization in clinical practice as a first- or second-line treatment modality in combination with modern chemotherapy.
Footnotes
Financial & competing interests disclosure
R Golfieri has previously received speaker fees form Bayer, Boston Scientific and Sirtex. In addition to the peer-review process, with the author's consent, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made at the discretion of the author and based on scientific or editorial merit only. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Diaz M, et al. Primary liver cancer, worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Lewandowski RJ, Thurston KG, Goin JE, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135–150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J. Vasc. Interv. Radiol. 2005;16:1641–1651. doi: 10.1097/01.RVI.0000179815.44868.66. [DOI] [PubMed] [Google Scholar]
- 4.Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma, implications for the current organ allocation policy. Liver Transpl. 2003;9:684–692. doi: 10.1053/jlts.2003.50147. [DOI] [PubMed] [Google Scholar]
- 5.Maddala YK, Stadheim L, Andrews JC, et al. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation, outcome with chemoembolization. Liver Transpl. 2004;10:449–455. doi: 10.1002/lt.20099. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. Clinical practice guidelines, management of hepatocellular carcinoma. J. Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Bilbao JI, de Martino A, de Luis E, et al. Biocompatibility, inflammatory response, and recannalization characteristics of nonradioactive resin microspheres: histological findings. Cardiovasc. Intervent. Radiol. 2009;32:727–736. doi: 10.1007/s00270-009-9592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 9.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991;21(1):109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 10.Dawson LA, McGinn CJ, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J. Clin. Oncol. 2000;18(11):2210–2218. doi: 10.1200/JCO.2000.18.11.2210. [DOI] [PubMed] [Google Scholar]
- 11.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int. J. Radiat. Oncol. Biol. Phys. 2002;53(4):810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 12.Wang LM, Jani AR, Hill EJ, et al. Anatomical basis and histopathological changes resulting from selective internal radiotherapy for liver metastases. J. Clin. Pathol. 2013;66:205–211. doi: 10.1136/jclinpath-2012-201231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell AM, Bailey IH, Burton MA. Tumour dosimetry in human liver following hepatic yttrium-90 microsphere therapy. Phys. Med. Biol. 2001;46(2):487–498. doi: 10.1088/0031-9155/46/2/315. [DOI] [PubMed] [Google Scholar]
- 14.Sarfaraz M, Kennedy AS, Lodge MA, Li XA, Wu X, Yu CX. Radiation absorbed dose distribution in a patient treated with yttrium-90 microspheres for hepatocellular carcinoma. Med. Phys. 2004;31(9):2449–2453. doi: 10.1118/1.1781332. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc. Intervent. Radiol. 2006;29:522–229. doi: 10.1007/s00270-005-0171-4. [DOI] [PubMed] [Google Scholar]
- 16.MacKie S, de Silva S, Aslan P, et al. Super selective radio embolization of the porcine kidney with 90yttrium resin microspheres: a feasibility, safety and dose ranging study. J. Urol. 2011;185:285–290. doi: 10.1016/j.juro.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Covey AM, Brody LA, Maluccio MA, et al. Variant hepatic arterial anatomy revisited, digital subtraction angiography performed in 600 patients. Radiology. 2002;224:542–547. doi: 10.1148/radiol.2242011283. [DOI] [PubMed] [Google Scholar]
- 18.Liu DM, Salem R, Bui JT, et al. Angiographic considerations in patients undergoing liver-directed therapy. J. Vasc. Interv. Radiol. 2005;16(7):911–935. doi: 10.1097/01.RVI.0000164324.79242.B2. [DOI] [PubMed] [Google Scholar]
- 19.Murthy R, Nunez R, Szklaruk J, et al. Yttrium-90 microsphere therapy for hepatic malignancy, devices, indications, technical considerations, and potential complications. Radiographics. 2005;25(Suppl. 1):S41–S55. doi: 10.1148/rg.25si055515. [DOI] [PubMed] [Google Scholar]
- 20.Salem R, Thurston KG. Radioembolization with 90-yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies (part 1: technical and methodologic considerations) J. Vasc. Interv. Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 21.Chen JH, Chai JW, Huang CL, et al. Proximal arterioportal shunting associated with hepatocellular carcinoma, features revealed by dynamic helical CT. Am. J. Roentgenol. 1999;172:403–407. doi: 10.2214/ajr.172.2.9930792. [DOI] [PubMed] [Google Scholar]
- 22.Ho S, Lau WY, Leung TW, et al. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur. J. Nucl. Med. 1996;23:947–952. doi: 10.1007/BF01084369. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadzadehfar H, Sabet A, Biermann K, et al. The significance of 99mTc-MAA SPECT/CT liver perfusion imaging in treatment planning for 90Y-microsphere selective internal radiation treatment. J. Nucl. Med. 2010;51(8):1206–1212. doi: 10.2967/jnumed.109.074559. [DOI] [PubMed] [Google Scholar]
- 24.Loevinger R, Budinger TF, Watson EE. MIRD Primer for Absorbed Dose Calculations Revised. Society of Nuclear Medicine; NY, USA: 1991. [Google Scholar]
- 25.Ho S, Lau WY, Leung WT, et al. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur. J. Nucl. Med. 1996;23:947–952. doi: 10.1007/BF01084369. [DOI] [PubMed] [Google Scholar]
- 26.Salem R, Lewandowski RJ, Gates VL, et al. Research reporting standards for radioembolization of hepatic malignancies. J. Vasc. Interv. Radiol. 2011;22:265–278. doi: 10.1016/j.jvir.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riaz A, Kulik L, Lewandowski RJ, et al. Radiologic–pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium–90 microspheres. J. Hepatol. 2009;49:1185–1193. doi: 10.1002/hep.22747. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y, Yaghmai V, Salem R, et al. Imaging tumor response following liver-directed intra-arterial therapy. Abdom. Imaging. 2013;38:1286–1299. doi: 10.1007/s00261-013-0017-5. [DOI] [PubMed] [Google Scholar]
- 29.Salem R, Lewandowski RJ, Gates VL, et al. Research reporting standards for radioembolization of hepatic malignancies. J. Vasc. Interv. Radiol. 2011;22:265–278. doi: 10.1016/j.jvir.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atassi B, Bangash AK, Bahrani A, et al. Multimodality imaging following 90Y-radioembolization, a comprehensive review and pictorial essay. Radiographics. 2008;28:81–99. doi: 10.1148/rg.281065721. [DOI] [PubMed] [Google Scholar]; •• Complete and exhaustive review of imaging patterns following yttrium-90 (90Y)-radioembolization.
- 31.Gaba RC, Lewandowski RJ, Kulik LM, et al. Radiation lobectomy, preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann. Surg. Oncol. 2009;16:1587–1596. doi: 10.1245/s10434-009-0454-0. [DOI] [PubMed] [Google Scholar]
- 32.Sato KT. Yttrium-90 radioembolization for the treatment of primary and metastatic liver tumors. Semin. Roentgenol. 2011;46(2):159–165. doi: 10.1053/j.ro.2010.08.004. [DOI] [PubMed] [Google Scholar]; •• Comprehensive review of clinical indications of 90Y-radioembolization.
- 33.Weintraub JL, Salem R. Treatment of hepatocellular carcinoma combining sorafenib and transarteriallocoregional therapy: state of the science. J. Vasc. Interv. Radiol. 2013;24:1123–1134. doi: 10.1016/j.jvir.2013.01.494. [DOI] [PubMed] [Google Scholar]
- 34.Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicitycompared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497–507. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma, European experience on safety and long–term survival. Hepatology. 2010;52:1741–1749. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 36.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres, a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]; • Key evidence on the impact of 90Y-glass microspheres on overall survival in hepatocellular carcinoma by Barcelona Clinic Liver Cancer stage.
- 37.Sangro B, Carpanese L, Cianni R, et al. Survival after 90Y resin microsphere radioembolization of hepatocellularcarcinoma across BCLC stages, a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]; • Key evidence on the impact of 90Y-resin microspheres on overall survival in hepatocellular carcinoma by Barcelona Clinic Liver Cancer stage.
- 38.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia–Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 39.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 40.Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with hepatocellular carcinoma (HCC): subanalysis of SHARP trial based on Barcelona Clinic Liver Cancer (BCLC) stage. J. Hepatol. 2009;50(Suppl.):S28–S29. [Google Scholar]
- 41.Kolligs FT, Bilbao JI, Jakobs T, et al. International Liver Cancer Association Seventh Annual Conference. Washington, DC, USA: 13–15 September 2013. SIRTACE: a randomised multicentre pilot trial of selective internal radiation therapy (SIRT) with yttrium-90 (90Y) resin microspheres versus transarterial chemo-embolisation (TACE) in patients with unresectable hepatocellular carcinoma (HCC). Presented at. Abstract P-136. [Google Scholar]
- 42.Sangro B, Iñarrairaegui M, Bilbao J, et al. Radioembolization for hepatocellular carcinoma. J. Hepatol. 2012;56:464–473. doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]; •• Comprehensive review of clinical indications for 90Y-radioembolization in hepatocellular carcinoma.
- 43.Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat. Rev. 2011;37:212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]; •• New proposal recognizing the heterogeneity of patients with intermediate hepatocellular carcinoma.
- 45.Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere®) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J. Surg. Oncol. 2006;94:572–586. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 46.Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterialdownstaging for hepatocellular carcinoma, chemoembolization versus radioembolization. Am. J. Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 47.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2007;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 48.Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a Phase 2 study. Hepatology. 2013;57:1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 49.Kooby DA, Egnatashvili V, Srinivasan S, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2010;21:224–230. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Carr BI, Kondragunta V, Buch SC, et al. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two cohort study. Cancer. 2010;116:1305–1314. doi: 10.1002/cncr.24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jelic S, Sotiropoulos GC. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow–up. Ann. Oncol. 2010;21(Suppl. 5):v59–v64. doi: 10.1093/annonc/mdq166. [DOI] [PubMed] [Google Scholar]
- 52.Saxena A, Bester L, Chua TC, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann. Surg. Oncol. 2010;17:484–491. doi: 10.1245/s10434-009-0777-x. [DOI] [PubMed] [Google Scholar]
- 53.Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc. Intervent. Radiol. 2013;36:440–448. doi: 10.1007/s00270-012-0463-4. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann RT, Paprottka PM, Schön A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc. Intervent. Radiol. 2012;35:105–116. doi: 10.1007/s00270-011-0142-x. [DOI] [PubMed] [Google Scholar]
- 55.Ibrahim SM, Mulcahy MF, Lewandowski RJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres, results from a pilot study. Cancer. 2008;113:2119–2128. doi: 10.1002/cncr.23818. [DOI] [PubMed] [Google Scholar]
- 56.Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J. Vasc. Interv. Radiol. 2013;24:1227–1234. doi: 10.1016/j.jvir.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann. Surg. Oncol. 2013;20:3779–3786. doi: 10.1245/s10434-013-3127-y. [DOI] [PubMed] [Google Scholar]
- 58.Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann. Oncol. 2001;12(12):1711–1720. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- 59.Van Hazel G, Blackwell A, Anderson J, et al. Randomised Phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J. Surg. Oncol. 2004;88(2):78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 60.Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J. Clin. Oncol. 2007;25:1099–1106. doi: 10.1200/JCO.2006.08.7916. [DOI] [PubMed] [Google Scholar]
- 61.Kosmider S, Tan TH, Yip D, Dowling R, Lichtenstein M, Gibbs P. Radioembolization in combination with systemic chemotherapy as first-line therapy for liver metastases from colorectal cancer. J. Vasc. Interv. Radiol. 2011;22(6):780–786. doi: 10.1016/j.jvir.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 62.Tie J, Yip D, Dowling R, et al. Radioembolization and systemic chemotherapy in patients with hepatic metastases from primary colorectal cancer. Ann. Oncol. 2010;21(Suppl. 8) Abstract 698. [Google Scholar]
- 63.van Hazel GA, Pavlakis N, Goldstein D, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J. Clin. Oncol. 2009;27(25):4089–4095. doi: 10.1200/JCO.2008.20.8116. [DOI] [PubMed] [Google Scholar]
- 64.Lim L, Gibbs P, Yip D, et al. A prospective evaluation of treatment with selective internal radiation therapy (SIR–spheres) in patients with unresectable liver metastases from colorectal cancer previously treated with 5-FU based chemotherapy. BMC Cancer. 2005;5:132. doi: 10.1186/1471-2407-5-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans KA, Richardson MG, Pavlakis N, Morris DL, Liauw W, Bester L. Survival outcomes of a salvage patient population after radioembolization of hepatic metastases with yttrium-90 microspheres. J. Vasc. Interv. Radiol. 2010;21(10):1521–1526. doi: 10.1016/j.jvir.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 66.Seidensticker R, Denecke T, Kraus P, et al. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc. Intervent. Radiol. 2012;35:1066–1073. doi: 10.1007/s00270-011-0234-7. [DOI] [PubMed] [Google Scholar]
- 67.Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J. Clin. Oncol. 2010;28(23):3687–3694. doi: 10.1200/JCO.2010.28.5643. [DOI] [PubMed] [Google Scholar]
- 68.Cosimelli M, Golfieri R, Cagol PP, et al. Multicentre Phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br. J. Cancer. 2010;103:324–331. doi: 10.1038/sj.bjc.6605770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bester L, Meteling B, Pocock N, et al. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J. Vasc. Interv. Radiol. 2012;23:96–105. doi: 10.1016/j.jvir.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 70.Mulcahy MF, Lewandowski RJ, Ibrahim SM, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer. 2009;115:1849–1858. doi: 10.1002/cncr.24224. [DOI] [PubMed] [Google Scholar]
- 71.Mancini R, Carpanese L, Sciuto R, et al. A multicentric Phase II clinical trial on intraarterial hepatic radiotherapy with 90-yttrium SIR-Spheres in unresectable, colorectal liver metastases refractory to i.v. chemotherapy, preliminary results on toxicity and response rates. In Vivo. 2006;20(6A):711–714. [PubMed] [Google Scholar]
- 72.Chua TC, Bester L, Saxena A, Morris DL. Radioembolization and systemic chemotherapy improves response and survival for unresectable colorectal liver metastases. J. Cancer Res. Clin. Oncol. 2011;137(5):865–873. doi: 10.1007/s00432-010-0948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stubbs RS, O’Brien I, Correia MM. Selective internal radiation therapy with 90Y microspheres for colorectal liver metastases, single-centre experience with 100 patients. ANZ J. Surg. 2006;76(8):696–703. doi: 10.1111/j.1445-2197.2006.03834.x. [DOI] [PubMed] [Google Scholar]
- 74.Jakobs TF, Hoffmann RT, Dehm K, et al. Hepatic yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. J. Vasc. Interv. Radiol. 2008;19(8):1187–1195. doi: 10.1016/j.jvir.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Cianni R, Urigo C, Notarianni E, et al. Selective internal radiation therapy with SIR-Spheres for the treatment of unresectable colorectal hepatic metastases. Cardiovasc. Intervent. Radiol. 2009;32(6):1179–1186. doi: 10.1007/s00270-009-9658-8. [DOI] [PubMed] [Google Scholar]
- 76.Mulcahy MF, Lewandowski RJ, Ibrahim SM, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer. 2009;115(9):1849–1858. doi: 10.1002/cncr.24224. [DOI] [PubMed] [Google Scholar]
- 77.Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases, modern USA experience. Int. J. Radiat. Oncol. Biol. Phys. 2006;65(2):412–425. doi: 10.1016/j.ijrobp.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 78.Benson AB, 3rd, Geschwind JF, Mulcahy MF, et al. Radioembolisation for liver metastases: results from a prospective 151 patient multi-institutional Phase II study. Eur. J. Cancer. 2013;49:3122–3130. doi: 10.1016/j.ejca.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 79.Rhee TK, Lewandowski RJ, Liu DM, et al. 90Y radioembolization for metastatic neuroendocrine liver tumors, preliminary results from a multi-institutional experience. Ann. Surg. 2008;247:1029–1035. doi: 10.1097/SLA.0b013e3181728a45. [DOI] [PubMed] [Google Scholar]
- 80.Kennedy AS, Dezarn WA, McNeillie P, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am. J. Clin. Oncol. 2008;31:271–279. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 81.King J, Quinn R, Glenn DM, et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer. 2008;113(5):921–929. doi: 10.1002/cncr.23685. [DOI] [PubMed] [Google Scholar]
- 82.Saxena A, Chua TC, Bester L, Kokandi A, Morris DL. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases, a critical appraisal of 48 cases. Ann. Surg. 2010;251(5):910–916. doi: 10.1097/SLA.0b013e3181d3d24a. [DOI] [PubMed] [Google Scholar]
- 83.Cao CQ, Yan TD, Bester L, Liauw W, Morris DL. Radioembolization with yttrium microspheres for neuroendocrine tumour liver metastases. Br. J. Surg. 2010;97(4):537–543. doi: 10.1002/bjs.6931. [DOI] [PubMed] [Google Scholar]
- 84.Cianni R, Pelle G, Notarianni E, et al. Radioembolisation with 90Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer. Eur. Radiol. 2013;23:182–189. doi: 10.1007/s00330-012-2556-5. [DOI] [PubMed] [Google Scholar]
- 85.Jakobs TF, Hoffmann RT, Fischer T, et al. Radioembolization in patients with hepatic metastases from breast cancer. J. Vasc. Interv. Radiol. 2008;19(5):683–690. doi: 10.1016/j.jvir.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 86.Coldwell DM, Kennedy AS, Nutting CW. Use of yttrium-90 microspheres in the treatment of unresectable hepatic metastases from breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:800–804. doi: 10.1016/j.ijrobp.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 87.Bangash AK, Atassi B, Kaklamani V, et al. 90Y radioembolization of metastatic breast cancer to the liver, toxicity, imaging response, survival. J. Vasc. Interv. Radiol. 2007;18:621–628. doi: 10.1016/j.jvir.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 88.Gonsalves CF, Eschelman DJ, Sullivan KL, Anne PR, Doyle L, Sato T. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma, a single-institution experience. Am. J. Roentgenol. 2011;196(2):468–473. doi: 10.2214/AJR.10.4881. [DOI] [PubMed] [Google Scholar]
- 89.Kennedy AS, Ball D, Cohen SJ, et al. Safety and efficacy of resin 90Y-microspheres in 548 patients with colorectal liver metastases progressing on systemic chemotherapy. J. Clin. Oncol. 2012;30(Suppl. 34) Abstract 264. [Google Scholar]
- 90.Vente MA, Wondergem M, van der Tweel I, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies, a structured meta-analysis. Eur. Radiol. 2009;19(4):951–959. doi: 10.1007/s00330-008-1211-7. [DOI] [PubMed] [Google Scholar]
- 91.Goin JE, Dancey JE, Hermann GA, et al. Treatment of unresectable metastatic colorectal carcinoma to the liver with intrahepatic Y90 microspheres: a dose-ranging study. World J. Nuc. Med. 2003;2:216–225. [Google Scholar]
- 92.Clark OH, Benson AB, 3rd, Berlin JD, et al. NCCN clinical practice guidelines in oncology: neuroendocrine tumors. J. Natl Compr. Canc. Netw. 2009;7:712–747. doi: 10.6004/jnccn.2009.0050. [DOI] [PubMed] [Google Scholar]
- 93.Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases, radioembolization with 90Y microsphere: safety, efficacy, and survival. Radiology. 2008;247:507–515. doi: 10.1148/radiol.2472062029. [DOI] [PubMed] [Google Scholar]
- 94.Salem R, Parikh P, Atassi B, et al. Incidence of radiation pneumonitis after hepatic intra–arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am. J. Clin. Oncol. 2008;31:431–438. doi: 10.1097/COC.0b013e318168ef65. [DOI] [PubMed] [Google Scholar]
- 95.Murthy R, Xiong H, Nunez R, et al. Yttrium-90 resin microspheres for the treatment of unresectable colorectal hepatic metastases after failure of multiple chemotherapy regimens: preliminary results. J. Vasc. Interv. Radiol. 2005;16:937–945. doi: 10.1097/01.RVI.0000161142.12822.66. [DOI] [PubMed] [Google Scholar]
- 96.Sangro B, Gil-Alzugaray B, Rodriguez J, et al. Liver disease induced by radioembolization of liver tumors, description and possible risk factors. Cancer. 2008;112:1538–1546. doi: 10.1002/cncr.23339. [DOI] [PubMed] [Google Scholar]
- 97.Young JY, Rhee TK, Atassi B, et al. Radiation dose limits and liver toxicities resulting from multiple yttrium-90 radioembolization treatments for hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2007;18:1375–1382. doi: 10.1016/j.jvir.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 98.Riaz A, Lewandowski RJ, Kulik LM, et al. Complications following radioembolization with yttrium-90 microspheres, a comprehensive literature review. J. Vasc. Interv. Radiol. 2009;20(9):1121–1130. doi: 10.1016/j.jvir.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 99.Leung TW, Lau WY, Ho SK, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int. J. Radiat. Oncol. Biol. Phys. 1995;33(4):919–924. doi: 10.1016/0360-3016(95)00039-3. [DOI] [PubMed] [Google Scholar]
- 100.Murthy R, Brown DB, Salem R, et al. Gastrointestinal complications associated with hepatic arterial yttrium-90 microsphere therapy. J. Vasc. Interv. Radiol. 2007;18:553–561. doi: 10.1016/j.jvir.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 101.Carretero C, Munoz–Navas M, Betes M, et al. Gastroduodenal injury after radioembolization of hepatic tumors. Am. J. Gastroenterol. 2007;102:1216–1220. doi: 10.1111/j.1572-0241.2007.01172.x. [DOI] [PubMed] [Google Scholar]
- 102.Kennedy AS, McNeillie P, Dezarn WA, et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int. J. Radiat. Oncol. Biol. Phys. 2009;74(5):1494–1500. doi: 10.1016/j.ijrobp.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 103.Atassi B, Bangash AK, Lewandowski RJ, et al. Biliary sequelae following radioembolization with yttrium-90 microspheres. J. Vasc. Interv. Radiol. 2008;19:691–697. doi: 10.1016/j.jvir.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Jakobs TF, Saleem S, Atassi B, et al. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with 90-yttrium microspheres. Dig. Dis. Sci. 2008;53:2556–2563. doi: 10.1007/s10620-007-0148-z. [DOI] [PubMed] [Google Scholar]
- 105.Memon K, Lewandowski RJ, Kulik L, et al. Radioembolization for primary and metastatic liver cancer. Semin. Radiat. Oncol. 2011;21:294–302. doi: 10.1016/j.semradonc.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kennedy A, Coldwell D, Sangro B, Wasan H, Salem R. Radioembolization for the treatment of liver tumors. General principles. Am. J. Clin. Oncol. 2012;35:91–99. doi: 10.1097/coc.0b013e3181f47583. [DOI] [PubMed] [Google Scholar]; •• Expert opinion on recommended radioembolization processes and procedures.