Abstract
When heated during a radiofrequency ablation (RFA) procedure to ≥40°C, lyso-thermosensitive liposomal doxorubicin (LTLD) produces high drug concentration in the surrounding margins of the ablation zone. The hypothesis that the RFA + LTLD combination can effectively treat hepatocellular carcinoma (HCC) was investigated in the HEAT study: adding LTLD did not improve the efficacy of normal practice RFA. However, among the 285 patients with a solitary lesion who received at least 45-min RFA dwell time, the hazard ratio for overall survival was 0.63 (95% CI: 0.41–0.96; p = 0.04). The OPTIMA study is currently ongoing to test the hypothesis that adding LTLD to a standardized RFA lasting ≥45 min increases survival compared with standardized RFA alone.
KEYWORDS : hepatocellular carcinoma, lyso-thermosensitive liposomal doxorubicin, radiofrequency ablation
Practice points.
Image-guided radiofrequency ablation (RFA) is established as the first-line therapy for patients with early-stage hepatocellular carcinoma when surgical options are precluded.
The rate of complete tumor eradication achieved by RFA is highly dependent on tumor size and is greatly reduced in tumors exceeding 3 cm in diameter.
Lyso-thermosensitive liposomal doxorubicin (LTLD), when heated to ≥40°C, produces a doxorubicin tumor concentration up to 25-times that of free (nonliposomal) doxorubicin.
In Phase I, LTLD showed a promising dose–response effect; however, the Phase III HEAT study conducted in patients with intermediate-size (3–7 cm) hepatocellular carcinoma lesions did not show any improvement in efficacy from adding LTLD to the normal practice of RFA.
HEAT study subgroup analysis supported with prospective experimental studies suggest that LTLD greatly enhances efficacy when RFA dwell time is standardized at ≥45 min.
The ongoing Phase III OPTIMA trial is aimed at confirming the hypothesis that the standardized RFA + LTLD combination increases survival compared with standardized RFA alone.
Background
Hepatocellular carcinoma is currently the second most common cause of cancer-related death worldwide [1]. Image-guided radiofrequency ablation (RFA) is established as the first-line therapy for nonsurgical patients with early-stage HCC [2–5]. Several reports have shown that the long-term survival of patients with compensated cirrhosis and small tumors who received RFA as the sole anticancer therapy is similar to that achieved with surgical resection [6]. Nevertheless, histological studies demonstrated that the rate of complete tumor eradication achieved by RFA is highly dependent on tumor size: the rate of complete necrosis was shown to be less than 50% in tumors exceeding 3 cm in diameter [7].
For tumors larger than 3 cm in longest diameter, multiple overlapping ablation cycles are typically required to ablate the target lesion plus a 360° 1-cm margin [8,9]. When multiple ablations are performed in large-volume tumors, RFA is more likely to leave viable tumor cells in the margins of overlapping ablation zones. This increases the possibility of recurrence at the original site as well as elsewhere, due to vascular spread. Several attempts have been made to increase the effectiveness of RFA in HCC treatment. Approaches that have been investigated include various combinations of locoregional interventions as well as the association of locoregional and systemic therapies [10–13].
Lyso-thermsensitive liposomal doxorubicin (LTLD; ThermoDox®, Celsion Corporation, Lawrenceville, NJ, USA) was designed for adjuvant use with RFA. It consists of the heat-enhanced cytotoxic doxorubicin within a heat-activated liposome. LTLD is administered by intravenous infusion. The liposomes selectively localize within and around tumor tissues because of their heightened permeability and retention properties (Figure 1) [14]. At normal body temperatures, doxorubicin remains encapsulated within the liposomes. When heated to ≥40°C, within seconds LTLD releases its doxorubicin, which quickly diffuses into the local tissue (Figure 2). Heated LTLD produces doxorubicin tumor concentrations up to 25-fold greater than free (nonliposomal) doxorubicin administered at the same doses [15]. Doxorubicin is heat-enhanced in that in vitro studies show an increase in cell killing when combined with hyperthermia compared with doxorubicin without hyperthermia [16–18].
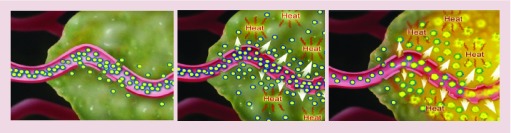
Figure 1. . Lyso-thermosensitive liposomal doxorubicin: mechanism of action.
Administered as a standard intravenous infusion, lyso-thermosensitive liposomal doxorubicin (LTLD) circulates through the bloodstream and into the tumor through the leaky tumor vasculature, concentrating at the tumor site (A). When an external heating device – such a radiofrequency ablation probe – heats the tissue, an increased amount of LTLD is carried into the tumor because of the heat-accentuated leakiness of the tumor vasculature (B). When tissue reaches a temperature of 40°C or greater, the heat-sensitive LTLD rapidly changes structure and the liposomal membrane selectively dissolves, creating openings that release the chemotherapeutic agent directly into the tumor and into the surrounding tissue (C).
Courtesy of Celsion Corporation.
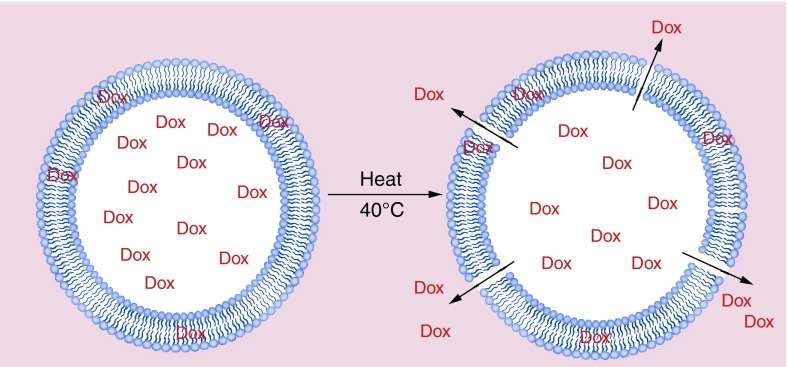
Figure 2. . Lyso-thermosensitive liposomal doxorubicin: effect of heating.
Lyso-thermosensitive liposomal doxorubicin is composed of lipid molecules that quickly change structure when heated to a specific temperature, creating channels in the liposome bilayer that allow encapsulated drug to rapidly disperse into the surrounding tissue. As a result, lyso-thermosensitive liposomal doxorubicin enables delivery of higher concentrations of chemotherapy drugs directly to the tumor, minimizing systemic toxicity.
Courtesy of Celsion Corporation.
Phase I study
The Phase I study was performed on 24 subjects, nine with HCC and 15 with metastatic liver tumors from nine other primary sites [19]. A total of 15 (62.5%) of the 24 subjects had tumors larger than 3 cm. The maximum tolerated dose of LTLD was found to be 50 mg/m2. Approximately 90% of the liposomal doxorubicin plasma area under the curve occurred during the first 3 h following infusion, establishing this period as optimal for RFA. Treatment failure was defined as radiologic disease progression and/or initiation of an alternative anticancer therapy. The study showed a statistically significant (p = 0.04) LTLD dose–response effect: median time to treatment failure for patients receiving the maximum tolerated dose of 50 mg/m2 was 374 days, while that for patients receiving less than 50 mg/m2 was 80 days. Time to treatment failure was significantly associated with LTLD dose but not with tumor size, tumor type or RFA approach. Research proceeded directly to Phase III [20,21].
Phase III trial: the HEAT study
The HEAT study was a double-blind, randomized controlled trial of RFA ± LTLD, registered with ClinicalTrials.gov (NCT00617981), in which 701 patients with intermediate-size (3–7 cm) HCC were recruited. The hypothesis tested in the HEAT study was that LTLD would produce a therapeutic doxorubicin tumor concentration when combined with the normal practice of RFA, thereby expanding the ‘treatment zone’ and targeting any micro-metastases outside the so-called ‘ablation zone’ (Figures 3 & 4). Patients had four or less unresectable HCC lesions, at least one of which had a longest diameter of 3 cm or more, with none exceeding 7 cm. They could be Child–Pugh A or B but were without vascular invasion or extrahepatic disease. Progression-free survival (PFS) was the primary end point and overall survival (OS) was a key secondary end point [22].
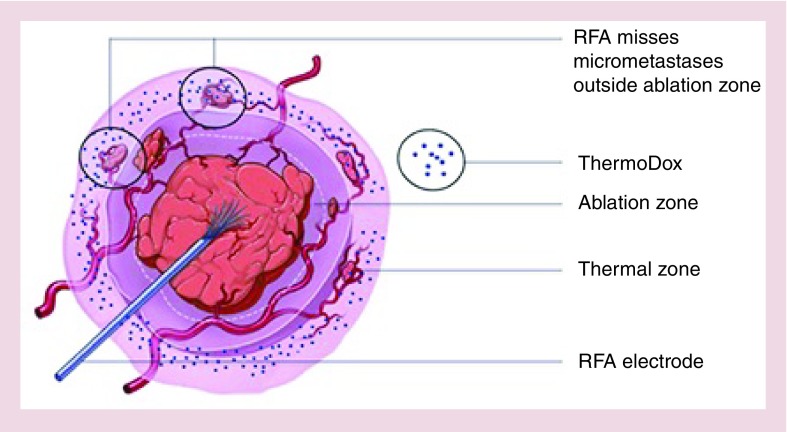
Figure 3. . Lyso-thermosensitive liposomal doxorubicin combined with radiofrequency ablation: effect on treatment zone.
Lyso-thermosensitive liposomal doxorubicin technology, when combined with RFA, can expand the ‘treatment zone’ for primary liver cancer, by targeting any micro-metastases outside the so-called ‘ablation zone’. Lyso-thermosensitive liposomal doxorubicin is infused 15 min prior to RFA administration. Ablation then releases doxorubicin in the ‘thermal zone’, where the drug concentrates while expanding the treatment area outward to the ablation zone.
RFA: Radiofrequency ablation.
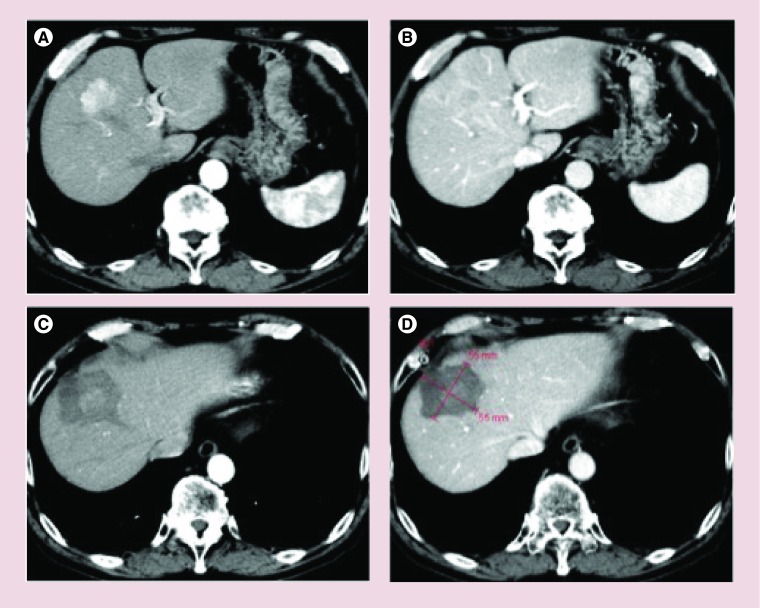
Figure 4. . Hepatocellular carcinoma tumor with complete response after treatment with radiofrequency ablation plus and lyso-thermosensitive liposomal doxorubicin.
Baseline arterial phase (A) and portal venous phase (B) CT scans. The lesion shows intratumoral arterial enhancement (A) with venous washout (B). After treatment with radiofrequency ablation plus lyso-thermosensitive liposomal doxorubicin, the treatment zone (calipers) is depicted as a low-attenuation area that fails to show any contrast enhancement in the arterial (C) and the portal venous (D) phase CT scans. The treatment zone appears to adequately cover the tumor with a 360° circumferential margin.
The HEAT study found that adding LTLD to RFA is safe, with reversible neutropenia similar to free doxorubicin but without congestive heart failure or hand–foot syndrome. The primary end point of PFS improvement from adding LTLD to RFA was not met. In intention-to-treatment (ITT) analysis, the PFS hazard ratio was 0.96 (95% CI: 0.79–1.18; p = 0.71) and the OS hazard ratio was 0.95 (95% CI: 0.76–1.20; p = 0.67). Median PFS was 13.9 months in both study arms. Median OS was 53.1 months in the RFA monotherapy arm and 55.2 months in the RFA + LTLD arm [22]. An important positive finding of the HEAT study is that RFA is an effective therapy for intermediate-size HCC, with median OS of about 4.5 years.
Computational models
While the HEAT study was still ongoing, a salient LTLD computational modeling study was published [23]. It investigated doxorubicin concentration in liver tumor tissue following combination therapy with LTLD and hyperthermia. This simulation combined a heat transfer model based on the bioheat equation with a drug delivery model. It simulated 5 h of 43°C hyperthermia beginning 15 min after completing a 15-min LTLD infusion. Maximum doxorubicin tumor tissue concentration was reached after 120 min of mild hyperthermia. This model found a direct correlation between duration of hyperthermia and doxorubicin concentration in tumor tissue, with 75% of the doxorubicin delivered in the first 45 min of heat and 25% of the doxorubicin delivery occurring in the next 75 min of applied heat [23].
The HEAT study: post hoc analysis
The criterion for RFA adequacy in the HEAT study was ablation of each target lesion plus a 360° 1-cm margin; there was no attempt to manage RFA approach or RFA dwell time. Dwell time is relevant for LTLD activity because the target and surrounding tissues remain at ≥40°C, even between ablation cycles. Manufacturers’ labeling of RFA devices do not instruct for tumor size related minimum heating times. In the HEAT study, RFA dwell times ranged from 12 to 230 min in the RFA alone arm (median 65 min) and from 12 to 180 min in the RFA + LTLD arm (median 60 min).
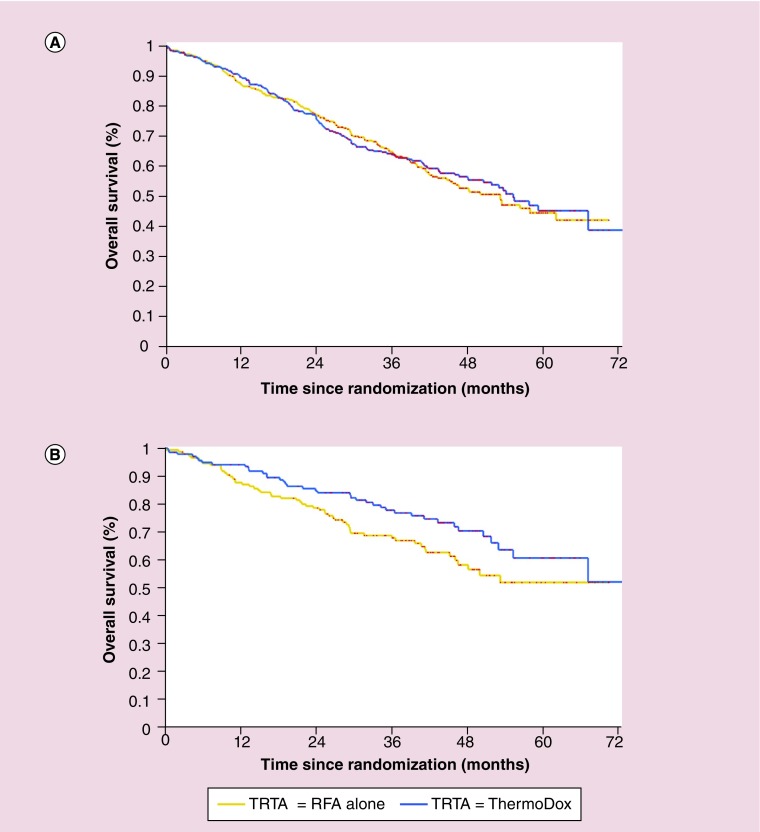
Because heat is essential to LTLD's mechanism of action, a post hoc subgroup analysis was performed [22]. A multivariate Cox regression model investigated the effect of eight potential prognostic factors: RFA dwell time (<45 vs ≥45 min, a breakpoint consistent with the computational modeling data), RFA approach, age, sex, geographical region, disease etiology, number of lesions (single vs multiple) and longest lesion diameter. Based on stepwise selection, the final two factors in the model were number of lesions (p < 0.001) and RFA dwell time (p < 0.05). In addition, both the investigation of the interaction of treatment (RFA alone vs RFA + LTLD) with number of lesions and the investigation of the three-way interaction (treatment × number of lesions × RFA dwell time) resulted in a p-value of less than 0.10, suggesting a potential efficacy advantage for patients with a solitary lesion who received both ≥45-min RFA dwell time and LTLD. In fact, patients treated with RFA for <45 min did not benefit from adding LTLD to RFA. However, among the 285 patients with a solitary lesion who received at least 45-min RFA dwell time, a subgroup that was balanced at baseline, the overall survival hazard ratio was 0.63 (95% CI: 0.41–0.96; p = 0.04). Figure 5 presents OS curves for all 701 subjects and also for the 285 patients with a solitary lesion who had ≥45-min RFA dwell time. Of course, post hoc subgroup analyses can only be used to generate hypotheses: they should not be regarded as definitive results, but rather as starting place for subsequent investigation.
Figure 5. . HEAT study.
Kaplan–Meier overall survival curves registered in the whole intention-to-treat 701-subject patient population (A) and in the subgroup analysis performed on 285 patients with solitary hepatocellular carcinoma and radiofrequency ablation (RFA) dwell time ≥45 min (B). In the subgroup analysis, the overall survival hazard ratio for the RFA + lyso-thermosensitive liposomal doxorubicin arm was 0.63 (95% CI: 0.41–0.96; p = 0.04). Treatment arms: RFA alone, yellow line; RFA + lyso-thermosensitive liposomal doxorubicin, blue line.
Experimental animal study
A study of RFA + LTLD in healthy pigs evaluated duration of RFA on doxorubicin target tissue concentration. Three heating times were tested: 15, 45 and 90 min. Overall, the data showed an increase in both the amount of doxorubicin deposited and the width of the tumor margin to which doxorubicin is delivered when heat time is increased from 15 to 45 min, with minimal further increase over the next 45 min. Fluorescence imaging showed that a high concentration of doxorubicin was found in the margin of the ablation zone and in areas where the ablations overlap [24].
Ongoing Phase III trial: the OPTIMA study
The computational modeling study, the animal trial and the HEAT study post hoc subgroup analysis are all consistent with each other and with LTLD's heat-based mechanism of action. The longer the target tissue is heated, the greater the doxorubicin tissue concentration. Therefore, it was hypothesized that the need for a minimum RFA dwell time for LTLD to be effective could explain the negative HEAT study results in the ITT population. Among all HEAT study subjects with a solitary lesion, 63.1% (285/452) had an RFA dwell time ≥45 min. Inadequate duration of heating may have resulted in subtherapeutic doxorubicin tumor concentrations for many subjects. Patients with more than one lesion may not have benefited due to insufficient time to heat all tumors for ≥45 min, resulting in subtherapeutic doxorubicin concentrations in one or more lesions. If so, such patients might be successfully treated one lesion at a time at 3-week intervals, the standard hematologic recovery period for doxorubicin.
To confirm this new hypothesis, the double-blind, randomized, controlled OPTIMA study (ClinicalTrials.gov NCT02112656) was initiated. The study will recruit 550 patients with a solitary 3–7 cm HCC. The OPTIMA study differs from the HEAT study in optimizing both RFA (by specifying ≥four overlapping ablation cycles) and doxorubicin tumor tissue concentration (by heating the target area ≥45 min to concentrate a therapeutic amount of doxorubicin in tumor tissue).
• Study design
Eligible subjects will be randomly assigned 1:1 to receive either standardized RFA (sRFA, i.e., RFA dwell time ≥45 min) plus LTLD at 50 mg/m2 or sRFA plus a dummy infusion. Randomization and analysis will be stratified by lesion longest diameter (3–5 cm vs >5–7 cm) and by RFA approach (laparoscopic, open surgical, percutaneous). All randomized patients will be evaluated for efficacy according to the ITT principle. OS will be the primary end point, with PFS and safety as secondary end points.
• Inclusion criteria
Males or females ≥18 years are eligible if they have a solitary unresectable HCC lesion ≥3 cm but ≤7.0 cm in longest diameter. Subjects are randomized without a biopsy if they meet American Association for the Study of Liver Disease (AASLD) criteria for the diagnosis of HCC [2]. Such patients are required to have a biopsy during the RFA procedure unless the biopsy is not possible or is contraindicated. Subjects not meeting AASLD criteria need a biopsy to confirm HCC prior to randomization. Subjects also have to be Child–Pugh Class A without current encephalopathy or ascites and to be ECOG performance status 0. Left ventricular ejection fraction (LVEF) has to be ≥50%. The position and accessibility of the target lesion must allow safe administration of multiple ablation cycles/deployments to achieve an RFA dwell time of ≥45 min.
• Exclusion criteria
Subjects are excluded if they are scheduled for liver transplantation, have any current or prior HCC treatment, prior exposure to any anthracycline, any contraindication to doxorubicin, extrahepatic metastasis, any concurrent malignancy (except treated squamous cell carcinoma of the skin or basal cell carcinoma of the skin), portal or hepatic vein tumor invasion/thrombosis, INR >1.5-times the upper normal limit, platelet count <75,000/mm3, neutrophil count <1500/mm3, hemoglobin <10.0 g/dl, serum creatinine ≥2.5 mg/dl, serum bilirubin >3.0 mg/dl, serum albumin <2.8 g/dl, body temperature >38.3°C (101°F), NYHA class III or IV functional classification for heart failure, evidence of hemochromatosis or any serious illness within the prior 6 months (e.g., congestive heart failure, myocardial infarction, life-threatening cardiac arrhythmia or cerebral vascular accident). The anticipated ablation volume can be no larger than removing three hepatic segments or 30% of total liver volume, whichever is less. Women who are pregnant or breastfeeding are excluded as are women of childbearing potential and men who are not practicing an acceptable form of birth control.
• Treatment & follow-up protocols
Blinded IV premedication is administered within 30 min before starting the study treatment infusion. The RFA + LTLD arm receives a steroid (e.g., 20 mg dexamethasone), H1 antihistamine (e.g., 50 mg diphenhydramine or 10 mg chlorpheniramine) and H2 antihistamine (e.g., 50 mg ranitidine or 20 mg famotidine). The control arm gets dummy IV premedication of sodium chloride 0.9 or 5% dextrose in water (D5W). Subjects then receive a blinded 30-min IV infusion of either 50 mg/m2 LTLD or D5W. RFA will be initiated at minute 15 following the start of study drug infusion, include at least 45-min dwell time and be completed within 3 h after starting the infusion. The goal is to ablate the tumor as well as a 360° 1.0-cm tumor-free margin surrounding the tumor. Only RFA devices approved by the US FDA will be used (Angiodynamics, Boston Scientific, Covidien).
The RFA procedure day will be day 0 and subjects will return to the clinic day 28 (±3 days). Subjects with a complete ablation by imaging will continue in the follow-up period described below. A subject who has an incomplete ablation is eligible for one retreatment procedure within 21 days after radiological imaging exam showing residual disease at day 28. Subjects will be retreated only once with the same RFA equipment and treatment assigned at randomization. Baseline safety evaluations must meet the eligibility parameters prior to a retreatment. Subjects with a complete ablation after retreatment will be followed for both OS PFS. If after two ablations the subject has local, distant intrahepatic or extrahepatic HCC, then the subject will be considered a treatment failure and will have met the PFS end point.
Following study treatment, subjects will undergo CT or MRI imaging scans (chest, abdomen and pelvis) at months 1, 5, 9, 13, 17, 21 and 25 (±2 weeks), then at 6-month intervals (±2 weeks) until radiological progression is seen. Investigator-determined radiological progression must be observed and recorded prior to beginning alternate treatments for HCC. Postprogression treatments will be reported and the subject will continue to be followed for OS until death, withdrawal of consent or the end of the study.
• Statistical assumptions
The OPTIMA study is designed to detect with 80% power a hazard ratio for OS of 0.67 (33% risk reduction) in the LTLD arm compared with the control arm with an overall 1-sided type 1 error of 0.025. A 3% per year loss to survival follow-up rate has been assumed and using a 1:1 treatment allocation (LTLD:control) of 550 subjects, a target of 197 events (deaths) will be required for the primary analysis. Two interim analyses, both for efficacy and futility, are planned for the study. The first is planned after 60% of the target events (118 deaths) and the second after 80% of the events (158 deaths) have occurred.
OS in the ITT population will be compared between the two treatment groups using the stratified log–rank test. The estimate of the hazard ratio and corresponding 95% CI will be provided using a Cox proportional hazards model including treatment and the stratification factors (lesion diameter, RFA approach) in the model. The survival curves will be estimated using Kaplan–Meier estimates. PFS will be analyzed using the same methodology.
Conclusion
It was hypothesized that LTLD would produce a therapeutic doxorubicin tumor concentration when combined with the normal practice of RFA and that this combination would offer a clinically meaningful benefit to patients with intermediate-size (3–7 cm) HCC. This hypothesis was falsified by the HEAT study. Preclinical studies, computational modeling and HEAT study subgroup analysis suggest that, if the practice of RFA is standardized to provide ≥45-min dwell time, LTLD efficacy is greatly enhanced. The alternative hypothesis that the sRFA + LTLD combination increases survival compared with sRFA alone is now being tested in the ongoing OPTIMA study.
Footnotes
Disclaimer
In addition to the peer-review process, with the author's consent, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made by the author at their discretion and based on scientific or editorial merit only. The author maintained full control over the manuscript, including content, wording and conclusions.
Financial & competing interests disclosure
R Lencioni is a research consultant for Celsion Corporation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
Celsion Corporation sponsored both the HEAT study and the OPTIMA study. Both protocols were approved by the institutional review board and/or independent ethics committee at each participating site. The HEAT study was and the OPTIMA study is being conducted according to Good Clinical Practice, the Declaration of Helsinki, and local laws. Both have an independent and unblinded Data Monitoring Committee (DMC) to review trial integrity and accumulating safety and efficacy data throughout the trial.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.International Agency for Research on Cancer - World Health Organization. Globocan 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr
- 2.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han KH, Kudo M, Ye SL, et al. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology. 2011;81(Suppl. 1):158–164. doi: 10.1159/000333280. [DOI] [PubMed] [Google Scholar]
- 4.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Verslype C, Rosmorduc O, Rougier P. ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012;23(Suppl. 7):vii41–vii48. doi: 10.1093/annonc/mds225. [DOI] [PubMed] [Google Scholar]
- 6.Breen DJ, Lencioni R. Image-guided ablation of primary liver and renal tumours. Nat. Rev. Clin. Oncol. 2015;12:175–186. doi: 10.1038/nrclinonc.2014.237. [DOI] [PubMed] [Google Scholar]
- 7.Lu DS, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954–960. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- 8.Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, Rajan DK. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J. Vasc. Interv. Radiol. 2009;20(7 Suppl.):S342–S347. doi: 10.1016/j.jvir.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc. Intervent. Radiol. 2010;33:11–17. doi: 10.1007/s00270-009-9736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lencioni R, Crocetti L, Petruzzi P, et al. Doxorubicin-eluting bead-enhanced radiofrequency ablation of hepatocellular carcinoma: a pilot clinical study. J. Hepatol. 2008;49:217–222. doi: 10.1016/j.jhep.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J. Clin. Oncol. 2013;31:426–432. doi: 10.1200/JCO.2012.42.9936. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a Phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Wang ZG, Fu SY, et al. Randomized clinical trial of chemoembolization plus radiofrequency ablation versus partial hepatectomy for hepatocellular carcinoma within the Milan criteria. Br. J. Surg. 2016;103:348–356. doi: 10.1002/bjs.10061. [DOI] [PubMed] [Google Scholar]
- 14.Zamboni WC. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist. 2008;13:248–260. doi: 10.1634/theoncologist.2007-0180. [DOI] [PubMed] [Google Scholar]
- 15.Kong G, Anyarambhatla G, Petros WP. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered release. Cancer Res. 2000;60:6950–6957. [PubMed] [Google Scholar]
- 16.Herman TS, Sweets CC, White DM, Gerner EW. Effect of heating on lethality due to hyperthermia and selected chemotherapeutic drugs. J. Natl Cancer Inst. 1982;68:487–491. [PubMed] [Google Scholar]
- 17.Herman TS. Temperature dependence of adriamycin, cis-diamminedichloroplatinum, bleomycin, and 1,3-bis(2-chloroethyl)-1-nitrosourea cytotoxicity in vitro . Cancer Res. 1983;43:517–520. [PubMed] [Google Scholar]
- 18.Bates DA, Mackillop WJ. Hyperthermia, adriamycin transport, and cytotoxicity in drug-sensitive and -resistant Chinese hamster ovary cells. Cancer Res. 1986;46:5477–5481. [PubMed] [Google Scholar]
- 19.Wood BJ, Poon RT, Locklin JK, et al. Phase I study of heat-deployed liposomal doxorubicin during radiofrequency ablation for hepatic malignancies. J. Vasc. Interv. Radiol. 2012;23:248–255. doi: 10.1016/j.jvir.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Report of the Phase I study of lyso-thermosensitive liposomal doxorubicin (LTLD).
- 20.Poon RTP, Borys N. Lyso-thermosensitive liposomal doxorubicin: a novel approach to enhance the efficacy of thermal ablation of liver cancer. Expert Opin. Pharmacother. 2009;10:333–343. doi: 10.1517/14656560802677874. [DOI] [PubMed] [Google Scholar]
- 21.Poon RT, Borys N. Lyso-thermosensitive liposomal doxorubicin: an adjuvant to increase the cure rate of radiofrequency ablation in liver cancer. Future Oncol. 2011;7:937–945. doi: 10.2217/fon.11.73. [DOI] [PubMed] [Google Scholar]; • Review of LTLD in the treatment of liver cancer.
- 22.Lencioni R, Tak WY, Chen MH, et al. Standardized radiofrequency ablation (sRFA) ≥ 45 minutes (m) plus lyso-thermosensitive liposomal doxorubicin (LTLD) for solitary hepatocellular carcinoma (HCC) lesions 3–7 cm: a retrospective analysis of Phase III HEAT study. J. Clin. Oncol. 2014;32(Suppl.) Abstract e15143. [Google Scholar]
- 23.Gasselhuber A, Dreher MR, Partanen A, et al. Targeted drug delivery by high intensity focused ultrasound mediated hyperthermia combined with temperature-sensitive liposomes: computational modelling and preliminary in vivo validation. Int. J. Hyperthermia. 2012;28:337–348. doi: 10.3109/02656736.2012.677930. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Report of the LTLD computational model.
- 24.Swenson CE, Haemmerich D, Maul DH, et al. Increased duration of heating boosts local drug deposition during radiofrequency ablation in combination with thermally sensitive liposomes (ThermoDox) in a porcine model. PLoS ONE. 2015;10(10):e0139752. doi: 10.1371/journal.pone.0139752. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Report of the experimental animal study of LTLD.