Abstract
Environmental conditions that impede heat dissipation and increase body temperature cause heat stress (HS). The study objective was to evaluate impacts of HS on the follicular phase of the estrous cycle. Postpubertal gilts (126.0 ± 21.6 kg) were orally administered altrenogest to synchronize estrus, and subjected to either 5 d of thermal-neutral (TN; 20.3 ± 0.5 °C; n = 6) or cyclical HS (25.4 − 31.9 °C; n = 6) conditions during the follicular phase preceding behavioral estrus. On d 5, blood samples were obtained, gilts were euthanized, and ovaries collected. Fluid from dominant follicles was aspirated and ovarian protein homogenates prepared for protein abundance analysis. HS decreased feed intake (22%; P = 0.03) and while plasma insulin levels did not differ, the insulin:feed intake ratio was increased 3-fold by HS (P = 0.02). Insulin receptor protein abundance was increased (29%; P < 0.01), but insulin receptor substrate 1, total and phosphorylated protein kinase B, superoxide dismutase 1, and acyloxyacyl hydrolase protein abundance were unaffected by HS (P > 0.05). Plasma and follicular fluid 17β-estradiol, progesterone, and lipopolysaccharide-binding protein concentrations as well as abundance of steroid acute regulatory protein, cytochrome P450 19A1, and multidrug resistance-associated protein 1 were not affected by HS (P > 0.05). HS increased estrogen sulfotransferase protein abundance (44%; P = 0.02), toll-like receptor 4 (36%; P = 0.05), and phosphorylated REL-associated protein (31%; P = 0.02). Regardless of treatment, toll-like receptor 4 protein was localized to mural granulosa cells in the porcine ovary. In conclusion, HS altered ovarian signaling in postpubertal gilts during their follicular phase in ways that likely contributes to seasonal infertility.
Keywords: environment, follicle development, ovary, porcine
INTRODUCTION
Seasonal infertility (SI) annually costs the American swine industry ~$450 million (Pollmann, 2010). Pigs are inherently susceptible to heat stress (HS), partly due to a lack of functional sweat glands and genetic selection for increased heat-producing traits have created a more thermal-sensitive pig (Brown-Brandl et al., 2004; Bloemhof et al., 2008; Ross et al., 2017). North American reproductive efficiency reaches an annual nadir in the summer, likely as a consequence of HS, and manifests as altered steroidogenesis (Nteeba et al., 2015), spontaneous abortion (Love, 1978), delayed puberty onset (Bertoldo et al., 2009), and fewer piglets/sow (Omtvedt et al., 1971).
During HS, blood flow is directed to the periphery for radiant heat dissipation and the splanchnic bed vasoconstricts, creating gastrointestinal tract hypoxia (Lambert et al., 2002). Intestinal barrier integrity is thus compromised (Hall et al., 1999), allowing luminal content translocation (including lipopolysaccharide [LPS]) into portal and systemic circulation (Hall et al., 2001; Turner, 2009; Pearce et al., 2013). LPS causes hyperinsulinemia; either directly as an insulin secretagogue or indirectly by increasing glucose-stimulated insulin secretion (Kvidera et al., 2017). Hyperinsulinemia is thought to reduce fecundity during insulin resistance (Nestler, 2000; Goodarzi et al., 2011). Negative reproductive impacts of LPS (Bidne et al., 2018) include reduced primordial follicle numbers (Bromfield and Sheldon, 2013), endocrine system alterations (Peter et al., 1990; Battaglia et al., 2000), and abortion (Harper and Skarnes, 1972; Giri et al., 1990; Newnham et al., 2005). Thus, both HS-induced hyperinsulinemia and endotoxemia could negatively influence reproductive performance. Our observations in prepubertal gilts (Nteeba et al., 2015), provided rationale to test the hypothesis that HS-induced hyperinsulinemia and circulating LPS affect ovarian signaling pathways in postpubertal gilts.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Iowa State University Institutional Animal Care and Use Committee and are thoroughly described elsewhere (Hale et al., 2017). Briefly, 12 postpubertal Duroc × PIC crossbred gilts (126.0 ± 21.6 kg) were synchronized for 14 d by orally administering altrenogest (15 mg/d) per manufacturer’s instructions (Matrix; Merck Animal Health; Summit, NJ) to ensure that gilts were heat-stressed during the entirety of the follicular phase of the estrous cycle. Immediately after altrenogest withdrawal, gilts were split in two cohorts (n = 6) and exposed to cyclical HS (25.4–31.9 °C) to imitate a diurnal heat load pattern or thermal-neutral (TN; 20.3 ± 0.5 °C) conditions for 5 d. Per industry standards, both groups were limit-fed 2.7 kg/d of a standard gestation diet and had ad libitum access to water. Feed intake was measured daily. Gilts were successfully heat-stressed as indicated by increased rectal temperatures (39.8 ± 0.2 °C) compared to TN (38.8 ± 0.2 °C) gilts (Hale et al., 2017).
Tissue Collection and Protein Isolation
Blood samples were collected prior to euthanasia into tubes containing sodium heparin and were centrifuged for 15 min at 1,500 × g at 4 °C. Plasma was removed and stored at −20°C for further analysis. At the end of the experimental period, gilts were euthanized by captive bolt penetration followed by exsanguination and ovaries were collected. One ovary was snap-frozen in liquid nitrogen and stored at −80 °C, and the contralateral ovary was fixed in 4% paraformaldehyde, transferred to 70% ethanol 24 h later, and stored at room temperature (RT) for histological staining. Briefly, tissue samples were placed in lysis buffer, homogenized, centrifuged (10 min, 10,621 × g, 4 °C), and supernatant was collected. Protein content was quantified by bicinchoninic acid assay (Pierce Protein Assay Kit; ThermoFisher Scientific 23227, Rockford, IL).
Western Blotting
Protein samples (50 µg) were separated at 50 volts (V) for 5 min followed by 90 V for 1 h on Mini-PROTEANTGX Gels (456–1096 BioRad Laboratories, Hercules, CA) with inclusion of a protein standard size marker (Precision Plus Protein Kaleidoscope BioRad Laboratories 161–0375, Hercules, CA). Protein was transferred onto a nitrocellulose membrane using the iBlot system (iBlot2 NC Regular Stacks IB23001, ThermoFisher Scientific), using the protocol; 20 V for 1 min, 23 V for 4 min, and 25 V for 2 min. Ponceau S (BP103-10 ThermoFisher Scientific) staining was performed to verify equal protein loading across wells. Membranes were blocked in 5% BSA (2153 Sigma Aldrich, St. Louis, MO) in PBS with 0.2% Tween 20 (PBST; PBS: BP665-1, Tween20: BP337-500, ThermoFisher Scientific) for a minimum of 1 h at RT. After blocking, membranes were incubated in primary antibodies (Table 1) directed against toll-like receptor 4 (TLR4; ThermoFisher Scientific); phosphorylated subunit Rel-A (pRELA pSer536; ThermoFisher Scientific); acyloxyacyl hydrolase (AOAH D-15; Santa Cruz); steroid acute regulatory protein (STAR; Cell Signaling Technology); cytochrome P450 family 19 subfamily A enzyme (CYP19A1; Cell Signaling Technology); protein kinase B (AKT; Cell Signaling Technology); phosphorylated AKT (pAKT T308; Cell Signaling Technology); superoxide dismutase (SOD1; Novus Biologicals, Littleton, CO); estrogen sulfotransferase (SULT1E1 H-40; Santa Cruz); multidrug resistant associated protein (ABCC1; Cell Signaling Technology); insulin receptor (INSR; Abbiotec, San Diego, CA) and insulin receptor substrate 1 (IRS1; Novus Biologicals) which were diluted in 5% BSA in PBST overnight at 4 °C. After primary incubation, membranes were washed three times in PBST. Species-specific secondary antibody was applied (Mouse anti-goat IgG-HRP; Santa Cruz; Anti-mouse IgG-HRP; Cell Signaling Technology; Anti-rabbit IgG-HRP; Cell Signaling) and membranes incubated for 1 h at RT, followed by three more washes in PBST. SignalFire enhanced chemiluminescent reagent (Cell Signaling Technology 6883) substrate was applied to the membrane for 3 min in the dark and the membrane imaged using a ChemiImager 5500 (Alpha Innotech, San Leandro, CA) with AlphaEaseFC software (v3.03 Alpha Innotech) or exposed to x-ray film. The sum of the gray values of all the pixels in the selection divided by the number of pixels, or mean gray value was quantified for each membrane using ImageJ software. Membranes were normalized to Ponceau S protein staining in which the entire lane of the transferred protein was quantified to account for loading variation. To ensure antibody specificity, negative control blots for each antibody used were performed in which the membranes were incubated with primary antibody only, secondary antibody only, or normal IgG in place of primary antibody with the inclusion of the appropriate secondary antibody. No protein bands were observed on these control blots indicating the specificity of the protein bands detected and analyzed.
Table 1.
List of antibodies used in this study
| Protein targeted | Vendor | Catalogue number | Dilution |
|---|---|---|---|
| TLR4 | ThermoFisher Scientific | HTA125 | 1:250 |
| pRELA pSer536 | ThermoFisher Scientific | PA5-6545 | 1:200 |
| AOAH D-15 | Santa Cruz Biotechnology | 163692 | 1:200 |
| STAR | Cell Signaling Technology | 8449S | 1:1000 |
| CYP19A1 | Cell Signaling Technology | 14528S | 1:500 |
| AKT | Cell Signaling Technology | 9272S | 1:1000 |
| pAKT T308 | Cell Signaling Technology | C31E5E | 1:500 |
| SOD1 | Novus Biologicals | NB1-31204 | 1:1000 |
| SULT1E1 H-40 | Santa Cruz Biotechnology | 292049 | 1:200 |
| ABCC1 | Cell Signaling Technology | 14685 | 1:100 |
| INSR | Abbiotec | 250721 | 1:1000 |
| IRS1 | Novus Biologicals | NB100-82001 | 1:1000 |
| Anti-goat IgG-HRP | Santa Cruz Biotechnology | 2354 | 1:1000 |
| Anti-mouse IgG-HRP | Cell Signaling Technology | 7076P2 | 1:1000 |
| Anti-rabbit IgG-HRP | Cell Signaling Technology | 7074P2 | 1:1000 |
ABCC1, multidrug resistance-associated protein; AKT, protein kinase B; pAKT, phosphorylated AKT; AOAH, acyloxyacyl hydrolase; CYP19A1, Cytochrome P450 isoform 19A1; INSR, insulin receptor; IRS1, insulin receptor substrate 1; pRELA, phosphorylated subunit Rel-A; SOD1, superoxide dismutase; STAR, steroidogenic acute regulatory protein; SULT1E1, estrogen sulfotransferase isoform 1E1; TLR4, toll-like receptor 4.
Immunofluorescence Staining
Ovarian tissues were paraffin embedded, sectioned 5 µm thick, and mounted onto microscope slides. Slides were deparaffinized using CitriSolv Hybrid Solvent (ThermoFisher Scientific 04-355-121), rehydrated by incubation for 3 min twice in 100% ethanol, 95% ethanol for 1 min, 80% ethanol for 1 min, followed by rinsing in distilled and deionized water. Antigen retrieval was performed by incubating slides in Tris-EDTA buffer (10 mM Trizma Base Sigma Aldrich T1503, 1 mM EDTA solution Sigma Aldrich E7889, 0.05% Tween 20, pH 9) at 95 °C for 30 min. Tissue sections were blocked in Image-iT FX signal enhancer (Novus Biologicals I36933) for 1 h at RT. Primary antibody against TLR4 (1:200; Novus Biologicals NB100-56566) was applied to each section and incubated overnight at 4 °C. Slides were washed thrice in PBS and incubated in fluorescent secondary antibody (1:1000; anti-mouse IgG (H + L) F(ab’)2 Fragment, AlexaFluor 488 conjugate; Cell Signaling Technology) for 1 h at RT. Slides were washed thrice in PBS and 4′,6-diamidino-2-phenylindole (DAPI; S36938, ThermoFisher Scientific) stain applied to each section and were stored in the dark at 4 °C to prevent signal fading. Images were captured on a Leica fluorescent microscope at 400× magnification. To ensure antibody binding specificity, negative controls omitted either the primary antibody, secondary antibody, or both. Mouse IgG was used in place of the primary antibody to control for antibody isotype nonspecific binding. No signal was obtained using these controls, thus the signal detected for the TLR4 protein is considered to be specific.
Quantification of Circulating Insulin
Mercodia Porcine Insulin ELISA kit (10-1200-01; Uppsala, Sweden) was used to quantify insulin in plasma samples collected from gilts on d 5 immediately before euthanasia. The intra-assay coefficient of variation was 4.6%.
Steroid Hormone Quantification From Plasma and Follicular Fluid
Concentrations of LPS-binding protein (LBP) were measured using a commercially available assay (Hycult Biotech; HK503; Uden, Netherlands). The intra-assay coefficient of variation was 4.4%. Plasma and follicular fluid 17β-estradiol and progesterone concentrations were measured using EIA (DRG Diagnostics; Marburg, Germany). The intra-assay coefficient of variation for plasma and follicular fluid 17β-estradiol were 10.7% and 3.3%, respectively, and for progesterone assays were 4.0% and 2.0%, respectively.
Statistical Analysis
Western blotting densitometric measurements were quantified using ImageJ and unpaired t-tests were analyzed using GraphPad Prism. Immunofluorescent staining was performed on ovaries from three animals per treatment with one histological slide per animal. A minimum of five images of different granulosa cell populations from tertiary follicles per slide were analyzed by densitometric analysis of TLR4 protein staining in granulosa cells surrounding the antrum using ImageJ and unpaired t-tests were analyzed using GraphPad Prism. Individual animal feed intake was analyzed using repeated measures with an autoregressive covariance structure. Day was the repeated effect. Effects of treatment, day, and treatment by day interaction were assessed using the mixed procedure of SAS (Cary, NC). The effect of treatment on blood and follicular fluid parameters was analyzed using mixed procedure of SAS. Data were reported as least square means and statistical significance was considered when the P value ≤ 0.05.
RESULTS
HS Reduced Feed Intake and Increased the Insulin:Feed Intake Ratio
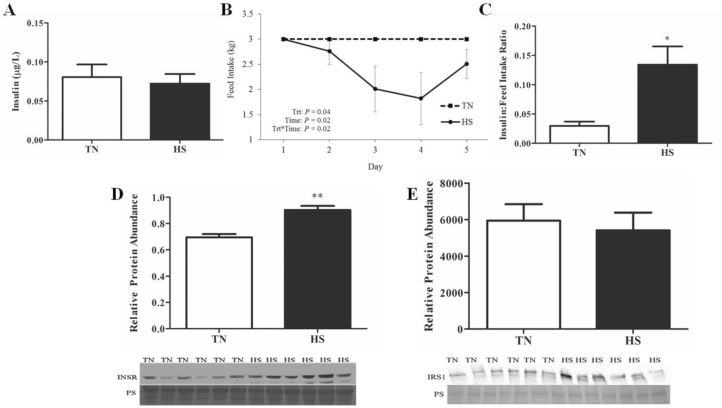
Environment did not affect circulating insulin (Figure 1A); however, feed intake decreased (22%; P = 0.03; Figure 1B) in HS gilts compared to TN controls. The insulin:feed intake ratio was increased 3-fold (P = 0.02; Figure 1C) in HS compared to TN gilts.
Figure 1.
Effect of heat stress on circulating insulin and ovarian insulin signaling proteins. Postpubertal cycling gilts were housed in either thermal-neutral (TN) or heat stress (HS) environment for 5 d during the follicular phase. Impact of HS on (A) plasma insulin concentration, (B) mean daily feed intake, and (C) the ratio of plasma insulin to feed intake on the morning of euthanasia; values represent mean ± SE. n = 6 animals per treatment. Graphs depict relative densitometric values of (D) INSR or (E) IRS1 protein in TN or HS porcine ovary; values represent mean ± SE. Equal protein loading was confirmed by Ponceau S (PS) staining of membranes. n = 6 animals per treatment; *P < 0.05; ** P < 0.01. INSR, insulin receptor; IRS1, insulin receptor substrate 1.
Insulin Signaling Was Unchanged by HS in Postpubertal Gilt Ovaries
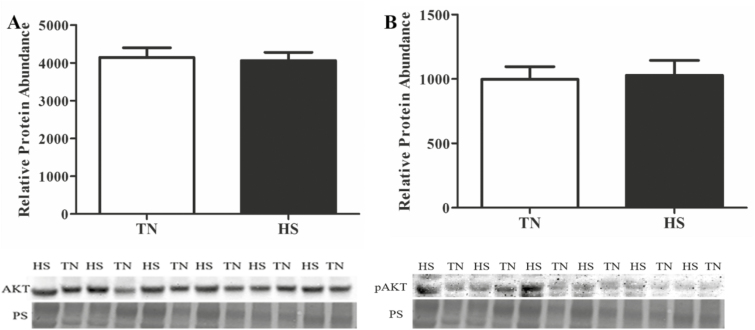
Increased INSR protein was observed in HS gilts (29%; P < 0.01; Figure 1D); however, IRS1 protein abundance was unchanged (Figure 1E) by HS. Additionally, the central mediator of phosphatidylinositol-3 kinase (PI3K) signaling, AKT, was not changed (Figure 2A) by HS, nor was AKT phosphorylation status impacted (Figure 2B) by environment.
Figure 2.
Influence of heat stress on total and pAKT protein abundance in whole ovarian lysate. Postpubertal cycling gilts were housed in either thermal-neutral (TN) or heat stress (HS) environment for 5 d during the follicular phase. The bar chart indicates relative densitometric values for (A) total and (B) pAKT protein in TN or HS porcine ovary; values represent mean ± SE. Equal protein loading was confirmed by Ponceau S (PS) staining of membranes. n = 6 animals per treatment. AKT, protein kinase B; pAKT, phosphorylated AKT.
HS Did Not Affect 17β-Estradiol and Progesterone Plasma or Follicular Fluid Concentrations, Steroidogenic Protein Abundance But Altered a Steroid Hormone Metabolism Protein
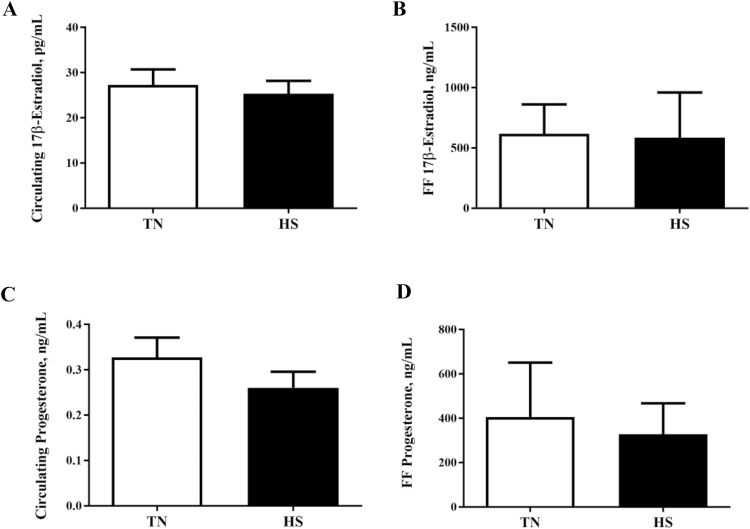
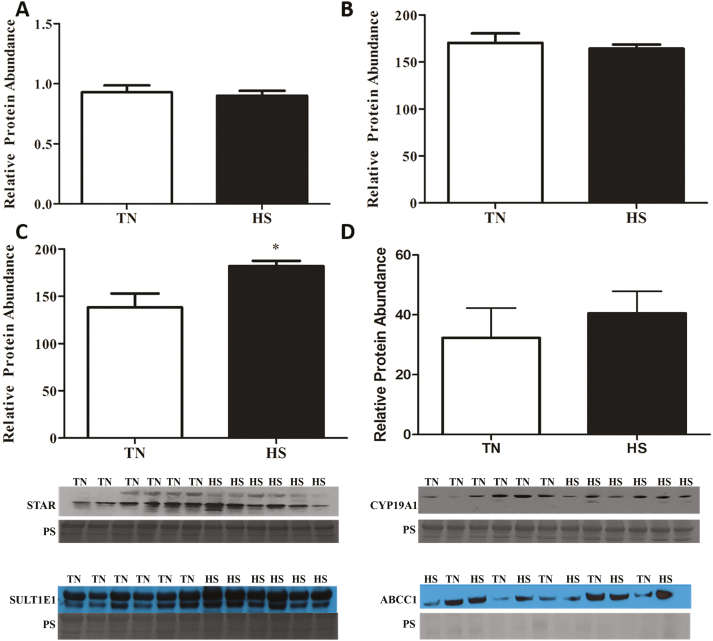
17β-Estradiol concentrations in follicular fluid or plasma were unaffected by environmental treatment (Figure 3A and B). Similarly, progesterone concentrations in either follicular fluid or plasma were not affected by treatment (Figure 3C and D). No impact of HS on protein abundance of STAR (Figure 4A) or CYP19A1 (Figure 4B) were observed; however, HS increased SULT1E1 (44%; P = 0.02; Figure 4C) but did not impact ABCC1 (Figure 4D) abundance.
Figure 3.
Heat stress effect on circulating and follicular fluid 17β-estradiol or progesterone concentration. Postpubertal cycling gilts were housed in either thermal-neutral (TN) or heat stress (HS) environment for 5 d during the follicular phase. The bar chart indicates (A) 17β-estradiol plasma concentration; (B) concentration of 17β-estradiol in follicular fluid; (C) progesterone plasma concentration; and (D) concentration of progesterone in follicular fluid; values represent mean ± SE, n = 6 animals per treatment.
Figure 4.
Consequence of heat stress on steroidogenic acute regulatory protein (STAR), cytochrome P450 isoform 19A1 (CYP19A1), estrogen sulfotransferase isoform 1E1 (SULT1E1), and multidrug resistance-associated protein (ABCC1) protein abundance in whole ovarian lysate. Postpubertal cycling gilts were housed in either thermal-neutral (TN) or heat stress (HS) environment for 5 d during the follicular phase. This bar chart indicates relative densitometric values of (A) STAR, (B) CYP19A1, (C) SULT1E1, and (D) ABCC1 proteins in TN or HS porcine ovary; values represent mean ± SE. Equal protein loading was confirmed by Ponceau S (PS) staining of membranes. n = 6 animals per treatment; *P < 0.05.
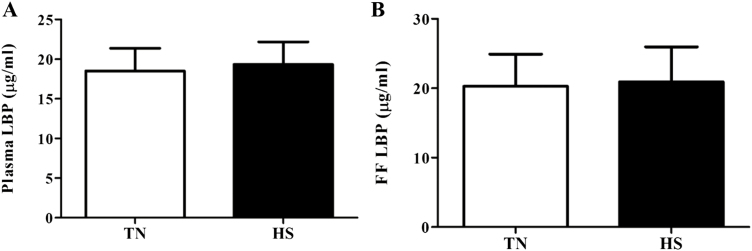
Circulating LBP Unaffected But LPS Induced Signaling Pathways Increased by HS
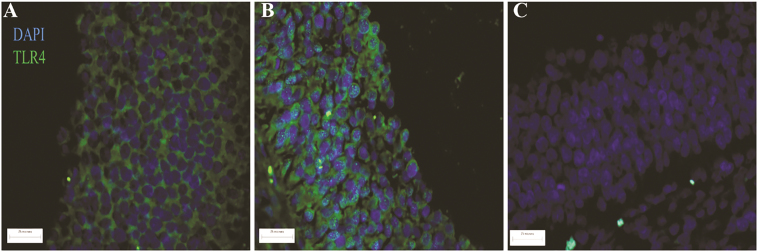
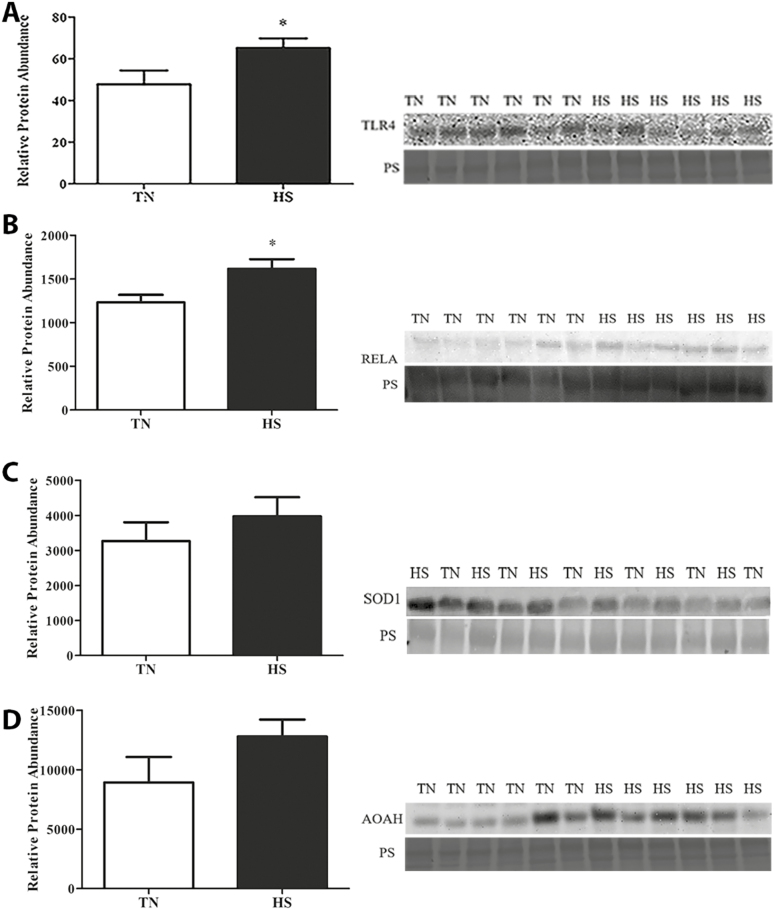
No difference in LBP concentration was observed (Figure 5) between TN and HS pigs in either follicular fluid or plasma. Localization of TLR4 protein to mural granulosa cells of antral follicles was observed (Figure 6A and B) and though no difference in granulosa cell TLR4 protein was observed, total ovarian TLR4 protein abundance was increased (36%; P = 0.05; Figure 7A) in HS pigs compared to TN controls. Additionally, downstream of TLR4, pRELA of transcription factor nuclear factor kappa B (NFкB) was also increased (31%; P = 0.02; Figure 7B) during HS.
Figure 5.
Effect of heat stress on circulating and follicular fluid lipopolysaccharide-binding protein (LBP) level. Postpubertal cycling gilts were housed in either thermal-neutral (TN) or heat stress (HS) environment for 5 d during the follicular phase. Impact of HS on (A) plasma concentration of LBP and (B) the concentration of LBP in follicular fluid; values represent mean ± SE. n = 6 animals per treatment. LBP, lipopolysaccharide-binding protein.
Figure 6.
Localization of TLR4 protein in the porcine ovary. Postpubertal cycling gilts were housed in either thermal-neutral (TN) or heat stress (HS) environment for 5 d during the follicular phase. TLR4 protein localization was assessed by immunofluorescence staining in (A) TN or (B) HS ovaries, (C) negative control shown an ovary that was not incubated in primary or secondary antibody. Blue color indicates DNA; green color represents TLR4 protein. TLR4, toll-like receptor 4.
Figure 7.
Impact of heat stress on TLR4, pRELA, SOD1, and AOAH protein abundance. Postpubertal cycling gilts were housed in either thermal-neutral (TN) or heat stress (HS) environment for 5 d during the follicular phase. The bar chart indicates relative densitometric values for (A) TLR4, (B) pRELA, (C) SOD1, and (D) AOAH proteins in TN or HS porcine ovary; values represent mean ± SE (n = 6 animals per treatment). Equal protein loading was confirmed by Ponceau S (PS) staining of membranes. Statistical significance is denoted by *P < 0.05. AOAH, acyloxyacyl hydrolase; pRELA, phosphorylated subunit Rel-A; SOD1, superoxide dismutase; TLR4, toll-like receptor 4.
Neither LPS Detoxification Nor Oxidative Stress Are Impacted in the Ovary of HS Gilts
Superoxide dismutase 1 (Figure 7C) and AOAH (Figure 7D) were detectable in ovarian tissue homogenates of postpubertal gilts but were unchanged in abundance due to HS.
DISCUSSION
Increased abortion (Love, 1978), delayed puberty (Bertoldo et al., 2009), longer wean-to-estrus interval (Prunier et al., 1994), and fewer piglets/gestation (Omtvedt et al., 1971) are phenotypic manifestations of HS and SI in pigs. Swine are inherently susceptible to HS and genetic selection for improved production variables has decreased their thermal tolerance (Brown-Brandl et al., 2004; Bloemhof et al., 2008; Renaudeau et al., 2012). Pearce et al., (2013) demonstrated compromised intestinal barrier integrity during HS, due to HS-induced redirecting blood flow to the periphery and vasoconstriction in the splanchnic bed (Hall et al., 1999; Lambert et al., 2002). An influx of LPS into circulation due to compromised intestinal integrity stimulates immunoactivation, characterized by an acute phase protein response and inflammation. Paradoxically, heat-stressed animals (despite being hypophagic) have increased basal and stimulated circulating insulin (Baumgard and Rhoads, 2013), a phenomenon likely mediated by LPS (Kvidera et al., 2017); thus, HS-induced endotoxemia and hyperinsulinemia are a consequence of compromised intestinal barrier integrity (Baumgard and Rhoads, 2013).
To characterize the effects of HS-induced endotoxemia and hyperinsulinemia on the ovary, postpubertal cycling gilts were exposed to either 5 d of TN or cyclical HS to encompass the entirety of the follicular phase of the estrous cycle and ovaries were collected immediately before ovulation. While there may be some small intra-animal variability in the proximity to ovulation, this experimental design was employed to capture the follicular fluid as well as ovarian tissue for analysis. The production and thermal phenotypes clearly indicated that pigs were experiencing a marked heat load, because they had increased rectal temperatures (Hale et al., 2017) and decreased feed intake. In addition, no impact of HS on dominant follicle number or size was observed; however, HS altered the abundance of ovarian proteins involved in autophagy-related pathways; thus, the ovary was clearly responding to thermal stress (Hale et al., 2017).
Heat-stressed gilts had increased insulin per unit of feed intake, which was not surprising considering that we have made similar observations in other studies (Sanz Fernandez et al., 2015). With regard to the impact on the ovary, insulin stimulates steroidogenesis (Barbieri et al., 1983), follicular development, and granulosa cell proliferation (Willis and Franks, 1995; Nestler et al., 1998; Poretsky et al., 1999). Once insulin binds to its cell surface receptor, it stimulates IRS proteins one to four auto-phosphorylation (Sweet et al., 1987; Backer et al., 1992; Cheatham and Kahn, 1995), which are upstream of the PI3K pathway (Akamine et al., 2010). We have previously discovered increased ovarian expression of both the INSR and IRS1 in prepubertal gilts exposed to 7 and 35 d of HS, demonstrating ovarian sensitivity to elevated insulin during HS (Nteeba et al., 2015). Additionally, increased phosphorylation of IRS1 protein at tyrosine 632 also occurs in prepubertal gilts (Nteeba et al., 2015) and this indicates ovarian insulin activation. In the current study, increased INSR was induced during HS, however, no impact on IRS1 protein abundance was detected. Thus, activation of insulin signaling in postpubertal gilts differed from those that had not entered puberty and in which the gonadotrophins and ovarian steroid hormones were absent.
Downstream of PI3K in the signaling cascade lies AKT (Kohn et al., 1996; Bandyopadhyay et al., 1997) and phosphorylating AKT causes its nuclear translocation where pAKT regulates a host of transcription factors. The PI3K/AKT signaling pathway is involved in many cell processes but proper PI3K signaling is integral for ovarian function, including regulation of primordial follicle recruitment and activation (Reddy et al., 2005; Liu et al., 2006; Reddy et al., 2008), oocyte viability (Brown et al., 2010), and steroidogenesis (Zeleznik et al., 2003; Chen et al., 2007). In the current experiment, no effects of HS on postpubertal gilt’s ovarian AKT or pAKT were observed, a surprising result considering that these impacts were evident in prepubertal gilts experiencing HS (Nteeba et al., 2015).
A major ovarian function is synthesis of 17β-estradiol and progesterone from the dominant follicles and corpora lutea. Ovarian hormone synthesis is initiated by cholesterol uptake in the mitochondria by the action of STAR. Progesterone is produced from both granulosa and theca cells while 17β-estradiol is synthesized only from granulosa cells (Falck, 1959). Granulosa cells produce the enzyme CYP19A1 which facilitates conversion of androgens to estrogens (Bjersing and Carstensen, 1964; Ryan and Short, 1965). Increased 17β-estradiol production from the dominant follicle(s) during the follicular phase precedes ovulation (Goding et al., 1969; Scaramuzzi et al., 1971; Diskin et al., 2003) and is integral to stimulating the LH surge required for ovulation. Altered 17β-estradiol production due to LPS exposure (Herath et al., 2007; Shimizu et al., 2012) and LPS-induced effects on CYP19A1 expression in granulosa cells have been demonstrated (Herath et al., 2007; Price et al., 2013). Dairy cows exposed to HS have decreased LH levels (Madan and Johnson, 1973), potentially due to decreased 17β-estradiol production. Granulosa cell proliferation, 17β-estradiol and progesterone secretion, as well as an abundance of genes involved in steroidogenesis, were decreased during HS in cultured murine granulosa cells (Luo et al., 2016). Thus, HS has the potential to alter ovarian steroid hormone production.
In prepubertal gilts, low-density lipoprotein receptor (LDLR), luteinizing hormone/choriogonadotropin receptor (LHCGR), and CYP19A1 mRNA abundance, as well as both STAR and CYP19A1 (responsible for the first and last steps, respectively, of 17β-estradiol production) were dramatically increased in abundance in whole ovarian tissue homogenates from heat-stressed gilts (Nteeba et al., 2015); thus, there was rationale for investigating potential steroidogenic impacts of HS in postpubertal gilts at the peak of 17β-estradiol production. Follicular fluid and serum 17β-estradiol and progesterone were quantified at the stage of Graafian follicle dominance where peak 17β-estradiol levels are expected. Both 17β-estradiol (Anderson, 2009) and progesterone (Babalola and Shapiro, 1988) were within the expected ranges at this stage of the estrous cycle in pigs. In spite of our previous observations of altered steroidogenic protein abundance in prepubertal gilts, HS had little to no effect on 17β-estradiol or progesterone concentrations in serum or follicular fluid in postpubertal gilts. Also, no environmental effects on STAR or CYP191A1 protein abundance were detected, in concurrence with lack of a detectable treatment effect on steroid hormone content. Thus, there may be differential ovarian endocrine disrupting effects of HS dependent on the stage of reproductive life at which a female is exposed.
Based upon our previous report in prepubertal gilts and despite lack of an observed effect of HS on circulating 17β-estradiol or progesterone, we hypothesized that HS might affect the abundance of SULT1E1 and ABCC1, thereby altering ovarian 17β-estradiol level through increased metabolism/turnover. Estrogen sulfonation is catalyzed by the cytosolic enzyme SULT1E1 (Li et al., 2013; Polei et al., 2014). In humans, SULT1E1 has a high affinity for catalyzing the sulfoconjugation of 17β-estradiol and estrone at the 3-hydroxyl terminal (Li et al., 2013). Sulfate conjugated substrates are transported by ABCC1, which is expressed in most tissues and localized in polarized cells of the basolateral membrane (Glavinas et al., 2004). Though not previously classified in the porcine ovary, we proposed that SULTE1 enzyme catalyzes sulfonation-mediated inactivation of 17β-estradiol, followed by transport from the ovary by the drug transporter protein ABCC1. While both ABCC1 and SULT1E1 are expressed in the ovary, HS did not influence ABCC1 although increased SULT1E1 abundance in gilt ovaries was observed. Additionally, it is also worth considering that the time of tissue collection represents a physiological time point at which steroid metabolism may be initiated, as indicated by increased SULT1E1. Alternatively, there is also potential for these enzymes to be altered at different stages of the estrous cycle during HS, dependent on the ovarian requirement for local steroid hormone metabolism.
As previously mentioned, due to compromised intestinal barrier function, circulating LPS is elevated during HS. In coordination with other proteins on the cell surface, TLR4 binds LPS and this initiates a signal cascade culminating in NFкB activation by phosphorylation of subunit RELA and nuclear translocation (Poltorak et al., 1998; Hoshino et al., 1999). Activated NFкB upregulates cytokine expression, such as IL-6 and IL-8 (Akira et al., 2006; Lu et al., 2008). Although cytokines are necessary for follicular development and ovulation (Machelon and Emilie, 1997; Bornstein et al., 2004; Gérard et al., 2004), excess cytokine synthesis can be detrimental for fertility (Adashi et al., 1989; Ghersevich et al., 2001; Herath et al., 2007). Demonstration of TLR4 localization in cultured granulosa cells has been documented (Bromfield and Sheldon, 2013; Price et al., 2013), and herein we also demonstrate TLR4 to be localized to granulosa cells in whole ovarian cross-sections. We discovered increased TLR4 protein abundance via western blotting in the ovary during HS suggesting that granulosa cells can respond to LPS via TLR4 signaling. Not surprisingly, protein abundance of pRELA was also increased in HS gilt ovaries suggesting ovarian TLR4 pathway activation during HS occurs, likely due to increased circulating LPS.
Detoxifying LPS is critical in preventing chronic endotoxemia. An acute phase protein, LBP, is produced and released from hepatocytes in response to immune system activation (Grube et al., 1994). Both proinflammatory and anti-inflammatory LBP properties have been observed (Hailman et al., 1994; Thompson et al., 2003), and LBP aides in transport of LPS to the TLR4 complex for signal cascade activation (Schumann, 2011) or in LPS detoxification (Thompson et al., 2003). Surprisingly, follicular fluid and serum levels of LBP were unchanged in this study due to HS. However, the infrequency of measuring LPS (one-time point collected 5 d post-HS initiation) may have limited the likelihood of detecting differences. Others have shown that LPS can infiltrate the follicular fluid (Herath et al., 2007; Magata et al., 2014) with detrimental impacts for ovarian function.
Another physiological route of LPS detoxification involves the action of AOAH (Hall and Munford, 1983). Produced primarily in macrophages, AOAH cleaves the lipid A portion of LPS, thereby tempering its potency (Munford and Hall, 1986). No change in ovarian AOAH protein abundance due to HS was observed, however, AOAH has not been previously demonstrated to be localized or functional in the ovary, specifically the porcine ovary; thus, the presence of AOAH in the ovary represents a novel finding. For both LBP and AOAH, it is important to note that this study did not characterize the activity or change in LBP and AOAH abundance over time, and it is possible that a temporal response to HS in abundance of both LBP and AOAH exists, but occurred outside the window of tissue collection (likely earlier). This remains a future investigation interest but was outside the financial and biological scope of the current investigation.
Increased oxidative stress has also been associated with HS in many species (Bernabucci et al., 2002; Altan et al., 2003; Montilla et al., 2014). Production of reactive oxygen species (ROS) during oxidative stress can have detrimental effects on fertility, including abortion (£agód et al., 2001) and elevated ROS due to HS can suppress embryonic development (Sakatani et al., 2004). A marker of oxidative stress, SOD1, is a cytosolic antioxidant, which works in coordination with mitochondrial SOD2 to bind ROS. We hypothesized that ROS and oxidative damage as a consequence of HS contributes to SI. However, no impact of HS on ovarian SOD1 protein abundance was observed.
Taken together, this study supports the hypothesis that hyperinsulinemia during HS may contribute to SI. Also, the data herein support that the ovary is responsive to HS-induced endotoxemia. As mentioned earlier, ovarian tissue collection occurred at a single time point before ovulation, thus, we cannot discount a temporal pattern of response to HS in the proteins investigated, nor can we alter the intrinsic interindividual variation in the exact timing of ovulation, which could have introduced variation and influenced our data. Endotoxemia has potential direct and indirect (through hyperinsulinemia) effects on the ovary during HS and our findings in the porcine ovary are original. Furthermore, potential differential effects of HS on the ovary of prepubertal and postpubertal swine are supported. Some considerations are that the previous prepubertal gilt study (Nteeba et al., 2015) had a longer length of exposure to HS (7 or 35 d), which was possible because the prepubertal ovary was not undergoing cyclicity, while the current study was constrained in the length of HS applied, because our aim was to subject gilts to HS during the entirety of the follicular phase of the estrous cycle (5 d in length). Also, the previous prepubertal study utilized a constant pattern of HS, compared to a cyclical pattern in this study, factors which may have impacted the observed differences between studies. A limitation to this study is that we relied on western blotting to determine alterations in protein quantification, whereas future studies including immunostaining will help determine localization of the proteins identified herein to determine specific ovarian cellular compartments directly affected by HS.
In summary, the ovary is a heterogeneous and dynamic organ in which folliculogenesis, steroidogenesis, and cellular atresia patterns change in a cyclical fashion with regulation by the hypothalamic–pituitary–gonadal axis and influence from environmental cues. Both hyperinsulinemia and endotoxemia are induced in the heat-stressed pig and HS disrupts ovarian signaling via increased INSR- and TLR4-pathway signaling, potentially being causative in the etiology of SI. Better understanding the mechanisms occurring in the ovary during times of HS can allow for the development of mitigation strategies and ultimately rescue the SI phenotype observed in swine.
ACKNOWLEDGEMENT
Thank you to Samantha Lei for analyzing lipopolysaccharide-binding protein content in plasma. We thank Dr. George Perry at South Dakota State University for RIA measurements. This work was funded by the National Pork Board.
LITERATURE CITED
- Adashi E. Y., C. E. Resnick C. S. Croft, and Payne D. W.. 1989. Tumor necrosis factor alpha inhibits gonadotropin hormonal action in nontransformed ovarian granulosa cells. A modulatory noncytotoxic property. J. Biol. Chem. 264:11591–11597. [PubMed] [Google Scholar]
- Akamine E. H., A. C. Marçal J. P. Camporez M. S. Hoshida L. C. Caperuto E. Bevilacqua, and Carvalho C. R.. 2010. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J. Endocrinol. 206:65–74. doi:10.1677/JOE-09-0461 [DOI] [PubMed] [Google Scholar]
- Akira S., S. Uematsu, and Takeuchi O.. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. doi:10.1016/j.cell.2006.02.015 [DOI] [PubMed] [Google Scholar]
- Altan Ö., Pabuçcuoğlu A., Altan A., Konyalioğlu S., and Bayraktar H.. 2003. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 44:545–550. doi:10.1080/00071660310001618334 [DOI] [PubMed] [Google Scholar]
- Anderson L. L. 2009. Reproductive Biology of Pigs. Animal Industry Report: AS 655, ASL R2443. [Google Scholar]
- Babalola G. O. and Shapiro B. H.. 1988. Correlation of follicular steroid hormone profiles with ovarian cyclicity in sows. J. Reprod. Fertil. 84:79–87. doi:10.1530/jrf.0.0840079 [DOI] [PubMed] [Google Scholar]
- Backer J. M., Myers M. G. Jr., Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., and Schlessinger J.. 1992. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. Embo J. 11:3469–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay G., M. L. Standaert L. Zhao B. Yu A. Avignon L. Galloway P. Karnam J. Moscat, and Farese R. V.. 1997. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J. Biol. Chem. 272:2551–2558. doi:10.1074/jbc.272.4.2551 [DOI] [PubMed] [Google Scholar]
- Barbieri R. L., A. Makris, and Ryan K. J.. 1983. Effects of insulin on steroidogenesis in cultured porcine ovarian theca. Fertil. Steril. 40:237–241. doi:10.1016/S0015-0282(16)47243-2 [PubMed] [Google Scholar]
- Battaglia D. F., H. B. Krasa V. Padmanabhan C. Viguié, and Karsch F. J.. 2000. Endocrine alterations that underlie endotoxin-induced disruption of the follicular phase in ewes. Biol. Reprod. 62:45–53. [DOI] [PubMed] [Google Scholar]
- Baumgard L. H. and Rhoads R. P. Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi:10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Bernabucci U., B. Ronchi N. Lacetera, and Nardone A.. 2002. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J. Dairy Sci. 85:2173–2179. doi:10.3168/jds.S0022-0302(02)74296-3 [DOI] [PubMed] [Google Scholar]
- Bertoldo M., C. G. Grupen P. C. Thomson G. Evans, and Holyoake P. K.. 2009. Identification of sow-specific risk factors for late pregnancy loss during the seasonal infertility period in pigs. Theriogenology 72:393–400. doi:10.1016/j.theriogenology.2009.03.008 [DOI] [PubMed] [Google Scholar]
- Bidne K. L., M. J. Dickson J. W. Ross L. H. Baumgard, and Keating A. F.. 2018. Disruption of female reproductive function by endotoxins. Reproduction 155:R169–R181. doi:10.1530/REP-17-0406 [DOI] [PubMed] [Google Scholar]
- Bjersing L. and Carstensen H.. 1964. The role of the granulosa cell in the biosynthesis of ovarian steroid hormones. Biochim. Biophys. Acta 86:639–640. [DOI] [PubMed] [Google Scholar]
- Bloemhof S., E. H. van der Waaij J. W. Merks, and Knol E. F.. 2008. Sow line differences in heat stress tolerance expressed in reproductive performance traits. J. Anim. Sci. 86:3330–3337. doi:10.2527/jas.2008-0862 [DOI] [PubMed] [Google Scholar]
- Bornstein S. R., H. Rutkowski, and Vrezas I.. 2004. Cytokines and steroidogenesis. Mol. Cell. Endocrinol. 215:135–141. doi:10.1016/j.mce.2003.11.022 [DOI] [PubMed] [Google Scholar]
- Bromfield J. J. and Sheldon I. M.. 2013. Lipopolysaccharide reduces the primordial follicle pool in the bovine ovarian cortex ex vivo and in the murine ovary in vivo. Biol. Reprod. 88:98. doi:10.1095/biolreprod.112.106914 [DOI] [PubMed] [Google Scholar]
- Brown C., J. LaRocca J. Pietruska M. Ota L. Anderson S. D. Smith P. Weston T. Rasoulpour, and Hixon M. L.. 2010. Subfertility caused by altered follicular development and oocyte growth in female mice lacking PKB alpha/akt1. Biol. Reprod. 82:246–256. doi:10.1095/biolreprod.109.077925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Brandl T. M., Nienaber J. A., Xin H., and Gates R. S.. 2004. A literature review of swine heat production. Trans. ASAE 47:259–270. doi:10.1016/S0301-6226(01)00184-1 [Google Scholar]
- Cheatham B. and Kahn C. R.. 1995. Insulin action and the insulin signaling network. Endocr. Rev. 16:117–142. doi:10.1210/edrv-16-2-117 [DOI] [PubMed] [Google Scholar]
- Chen Y. J., P. W. Hsiao M. T. Lee J. I. Mason F. C. Ke, and Hwang J. J.. 2007. Interplay of PI3K and cAMP/PKA signaling, and rapamycin-hypersensitivity in TGFbeta1 enhancement of FSH-stimulated steroidogenesis in rat ovarian granulosa cells. J. Endocrinol. 192:405–419. doi:10.1677/JOE-06-0076 [DOI] [PubMed] [Google Scholar]
- Diskin M. G., D. R. Mackey J. F. Roche, and Sreenan J. M.. 2003. Effects of nutrition and metabolic status on circulating hormones and ovarian follicle development in cattle. Anim. Reprod. Sci. 78:345–370. doi:10.1016/S0378-4320(03)00099-X [DOI] [PubMed] [Google Scholar]
- Falck B. 1959. Site of production of oestrogen in rat ovary as studied in micro-transplants. Acta Physiol. Scand. Suppl. 47:1–101. [DOI] [PubMed] [Google Scholar]
- Gérard N., M. Caillaud A. Martoriati G. Goudet, and Lalmanach A. C.. 2004. The interleukin-1 system and female reproduction. J. Endocrinol. 180:203–212. doi:10.1677/joe.0.1800203 [DOI] [PubMed] [Google Scholar]
- Ghersevich S., V. Isomaa, and Vihko P.. 2001. Cytokine regulation of the expression of estrogenic biosynthetic enzymes in cultured rat granulosa cells. Mol. Cell. Endocrinol. 172:21–30. doi:10.1016/S0303-7207(00)00396-8 [DOI] [PubMed] [Google Scholar]
- Giri S. N., P. Emau J. S. Cullor G. H. Stabenfeldt M. L. Bruss R. H. Bondurant, and Osburn B. I.. 1990. Effects of endotoxin infusion on circulating levels of eicosanoids, progesterone, cortisol, glucose and lactic acid, and abortion in pregnant cows. Vet. Microbiol. 21:211–231. doi:10.1016/0378-1135(90)90033-R [DOI] [PubMed] [Google Scholar]
- Glavinas H., P. Krajcsi J. Cserepes, and Sarkadi B.. 2004. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr. Drug Deliv. 1:27–42. doi:10.2174/1567201043480036 [DOI] [PubMed] [Google Scholar]
- Goding J. R., K. J. Catt J. M. Brown C. C. Kaltenbach I. A. Cumming, and Mole B. J.. 1969. Radioimmunoassay for ovine luteinizing hormone. Secretion of luteinizing hormone during estrus and following estrogen administration in sheep. Endocrinology 85:133–142. doi:10.1210/endo-85-1-133 [DOI] [PubMed] [Google Scholar]
- Goodarzi M. O., D. A. Dumesic G. Chazenbalk, and Azziz R.. 2011. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 7:219–231. doi:10.1038/nrendo.2010.217 [DOI] [PubMed] [Google Scholar]
- Grube B. J., C. G. Cochane R. D. Ye C. E. Green M. E. McPhail R. J. Ulevitch, and Tobias P. S.. 1994. Lipopolysaccharide binding protein expression in primary human hepatocytes and HepG2 hepatoma cells. J. Biol. Chem. 269:8477–8482. [PubMed] [Google Scholar]
- Hailman E., H. S. Lichenstein M. M. Wurfel D. S. Miller D. A. Johnson M. Kelley L. A. Busse M. M. Zukowski, and Wright S. D.. 1994. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J. Exp. Med. 179:269–277. doi:10.1084/jem.179.1.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale B. J., C. L. Hager J. T. Seibert J. T. Selsby L. H. Baumgard A. F. Keating, and Ross J. W.. 2017. Heat stress induces autophagy in pig ovaries during follicular development. Biol. Reprod. 97:426–437. doi:10.1093/biolre/iox097 [DOI] [PubMed] [Google Scholar]
- Hall D. M., K. R. Baumgardner T. D. Oberley, and Gisolfi C. V.. 1999. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am. J. Physiol. 276:G1195–G1203. [DOI] [PubMed] [Google Scholar]
- Hall D. M., G. R. Buettner L. W. Oberley L. Xu R. D. Matthes, and Gisolfi C. V.. 2001. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 280:H509–H521. doi:10.1152/ajpheart.2001.280.2.H509 [DOI] [PubMed] [Google Scholar]
- Hall C. L. and Munford R. S.. 1983. Enzymatic deacylation of the lipid A moiety of salmonella typhimurium lipopolysaccharides by human neutrophils. Proc. Natl. Acad. Sci. U. S. A. 80:6671–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. J. and Skarnes R. C.. 1972. Inhibition of abortion and fetal death produced by endotoxin or prostaglandin f2alpha. Prostaglandins 2:295–309. [DOI] [PubMed] [Google Scholar]
- Herath S., E. J. Williams S. T. Lilly R. O. Gilbert H. Dobson C. E. Bryant, and Sheldon I. M.. 2007. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction 134:683–693. doi:10.1530/REP-07-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K., O. Takeuchi T. Kawai H. Sanjo T. Ogawa Y. Takeda K. Takeda, and Akira S.. 1999. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749–3752. [PubMed] [Google Scholar]
- Kohn A. D., S. A. Summers M. J. Birnbaum, and Roth R. A.. 1996. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 271:31372–31378. doi:10.1074/jbc.271.49.31372 [DOI] [PubMed] [Google Scholar]
- Kvidera S. K., Horst E. A., Abuajamieh M., Mayorga E. J., Sanz Fernandez M. V., and Baumgard L. H.. 2017. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 100:2360–2374. doi:10.3168/jds.2016–12001 [DOI] [PubMed] [Google Scholar]
- Łagód L., T. Paszkowski R. Sikorski, and Rola R.. 2001. [The antioxidant-prooxidant balance in pregnancy complicated by spontaneous abortion]. Ginekol. Pol. 72:1073–1078. [PubMed] [Google Scholar]
- Lambert G. P., C. V. Gisolfi D. J. Berg P. L. Moseley L. W. Oberley, and Kregel K. C.. 2002. Selected contribution: hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J. Appl. Physiol. (1985). 92:1750–1761; discussion 1749. doi:10.1152/japplphysiol.00787.2001 [DOI] [PubMed] [Google Scholar]
- Li Y., Xu Y., Li X., Qin Y., and Hu R.. 2013. Effects of PPAR-α agonist and IGF-1 on estrogen sulfotransferase in human vascular endothelial and smooth muscle cells. Mol. Med. Rep. 8:133–139. doi:10.3892/mmr.2013.1483 [DOI] [PubMed] [Google Scholar]
- Liu K., S. Rajareddy L. Liu K. Jagarlamudi K. Boman G. Selstam, and Reddy P.. 2006. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev. Biol. 299:1–11. doi:10.1016/j.ydbio.2006.07.038 [DOI] [PubMed] [Google Scholar]
- Love R. J. 1978. Definition of a seasonal infertility problem in pigs. Vet. Rec. 103:443–446. [DOI] [PubMed] [Google Scholar]
- Lu Y. C., W. C. Yeh, and Ohashi P. S.. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42:145–151. doi:10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Luo M., L. Li C. Xiao Y. Sun, and Wang G. L.. 2016. Heat stress impairs mice granulosa cell function by diminishing steroids production and inducing apoptosis. Mol. Cell. Biochem. 412:81–90. doi:10.1007/s11010-015-2610-0 [DOI] [PubMed] [Google Scholar]
- Machelon V. and Emilie D.. 1997. Production of ovarian cytokines and their role in ovulation in the mammalian ovary. Eur. Cytokine Netw. 8:137–143. [PubMed] [Google Scholar]
- Madan M. L. and Johnson H. D.. 1973. Environmental heat effects on bovine luteinizing hormone. J. Dairy Sci. 56:1420–1423. doi:10.3168/jds.S0022-0302(73)85376-7 [DOI] [PubMed] [Google Scholar]
- Magata F., M. Horiuchi R. Echizenya R. Miura S. Chiba M. Matsui A. Miyamoto Y. Kobayashi, and Shimizu T.. 2014. Lipopolysaccharide in ovarian follicular fluid influences the steroid production in large follicles of dairy cows. Anim. Reprod. Sci. 144:6–13. doi:10.1016/j.anireprosci.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Montilla S. I., T. P. Johnson S. C. Pearce D. Gardan-Salmon N. K. Gabler J. W. Ross R. P. Rhoads L. H. Baumgard S. M. Lonergan, and Selsby J. T.. 2014. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature (Austin). 1:42–50. doi:10.4161/temp.28844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S. and Hall C. L.. 1986. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science 234:203–205. doi:10.1126/science.3529396 [DOI] [PubMed] [Google Scholar]
- Nestler J. E. 2000. Obesity, insulin, sex steroids and ovulation. Int. J. Obes. Relat. Metab. Disord. 24 (Suppl 2):S71–S73. [DOI] [PubMed] [Google Scholar]
- Nestler J. E., D. J. Jakubowicz A. F. de Vargas C. Brik N. Quintero, and Medina F.. 1998. Insulin stimulates testosterone biosynthesis by human Thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J. Clin. Endocrinol. Metab. 83:2001–2005. doi:10.1210/jcem.83.6.4886 [DOI] [PubMed] [Google Scholar]
- Newnham J. P., A. Shub A. H. Jobe P. S. Bird M. Ikegami I. Nitsos, and Moss T. J.. 2005. The effects of intra-amniotic injection of periodontopathic lipopolysaccharides in sheep. Am. J. Obstet. Gynecol. 193:313–321. doi:10.1016/j.ajog.2005.03.065 [DOI] [PubMed] [Google Scholar]
- Nteeba J., M. V. Sanz-Fernandez R. P. Rhoads L. H. Baumgard J. W. Ross, and Keating A. F.. 2015. Heat stress alters ovarian insulin-mediated phosphatidylinositol-3 kinase and steroidogenic signaling in gilt ovaries. Biol. Reprod. 92:148. doi:10.1095/biolreprod.114.126714 [DOI] [PubMed] [Google Scholar]
- Omtvedt I. T., R. E. Nelson R. L. Edwards D. F. Stephens, and Turman E. J.. 1971. Influence of heat stress during early, mid and late pregnancy of gilts. J. Anim. Sci. 32:312–317. [DOI] [PubMed] [Google Scholar]
- Pearce S. C., V. Mani T. E. Weber R. P. Rhoads J. F. Patience L. H. Baumgard, and Gabler N. K.. 2013. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 91:5183–5193. doi:10.2527/jas.2013-6759 [DOI] [PubMed] [Google Scholar]
- Peter A. T., J. E. Simon C. W. Luker, and Bosu W. T.. 1990. Site of action for endotoxin-induced cortisol release in the suppression of preovulatory luteinizing hormone surges. Theriogenology 33:637–643. [DOI] [PubMed] [Google Scholar]
- Polei M., T. Viergutz W. Tomek G. Schuler, and Fürbass R.. 2014. Estrogen-specific sulfotransferase (SULT1E1) in bovine placentomes: inverse levels of mRNA and protein in uninucleated trophoblast cells and trophoblast giant cells. Biol. Reprod. 91:48. doi:10.1095/biolreprod.114.118760 [DOI] [PubMed] [Google Scholar]
- Pollmann D. S. 2010. Seasonal effects on sow herds: industry experience and management strategies. In: N., Billy, editor, Day symposium. Midwest Amer. Soc. Anim. Sci, Des Moines, IA. [Google Scholar]
- Poltorak A., X., He I., Smirnova M. Y., Liu C., Van Huffel X., Du D., Birdwell E., Alejos M., Silva C., Galanos et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088. doi:10.1126/science.282.5396.2085 [DOI] [PubMed] [Google Scholar]
- Poretsky L., N. A. Cataldo Z. Rosenwaks, and Giudice L. C.. 1999. The insulin-related ovarian regulatory system in health and disease. Endocr. Rev. 20:535–582. doi:10.1210/edrv.20.4.0374 [DOI] [PubMed] [Google Scholar]
- Price J. C., J. J. Bromfield, and Sheldon I. M.. 2013. Pathogen-associated molecular patterns initiate inflammation and perturb the endocrine function of bovine granulosa cells from ovarian dominant follicles via TLR2 and TLR4 pathways. Endocrinology 154:3377–3386. doi:10.1210/en.2013-1102 [DOI] [PubMed] [Google Scholar]
- Prunier A., J. Y. Dourmad, and Etienne M.. 1994. Effect of light regimen under various ambient temperatures on sow and litter performance. J. Anim. Sci. 72:1461–1466. [DOI] [PubMed] [Google Scholar]
- Reddy P., L., Liu D., Adhikari K., Jagarlamudi S., Rajareddy Y., Shen C., Du W., Tang T., Hämäläinen S. L., Peng et al. 2008. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319:611–613. doi:10.1126/science.1152257 [DOI] [PubMed] [Google Scholar]
- Reddy P., L. Shen C. Ren K. Boman E. Lundin U. Ottander P. Lindgren Y. X. Liu Q. Y. Sun, and Liu K.. 2005. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev. Biol. 281:160–170. doi:10.1016/j.ydbio.2005.02.013 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., A. Collin S. Yahav V. de Basilio J. L. Gourdine, and Collier R. J.. 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi:10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Ross J. W., B. J. Hale J. T. Seibert M. R. Romoser M. K. Adur A. F. Keating, and Baumgard L. H.. 2017. Physiological mechanisms through which heat stress compromises reproduction in pigs. Mol. Reprod. Dev. 84:934–945. doi:10.1002/mrd.22859 [DOI] [PubMed] [Google Scholar]
- Ryan K. J. and Short R. V.. 1965. Formation of estradiol by granulosa and theca cells of the equine ovarian follicle. Endocrinology 76:108–114. doi:10.1210/endo-76-1-108 [DOI] [PubMed] [Google Scholar]
- Sakatani M., S. Kobayashi, and Takahashi M.. 2004. Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol. Reprod. Dev. 67:77–82. doi:10.1002/mrd.20014 [DOI] [PubMed] [Google Scholar]
- Sanz Fernandez M. V., Johnson J. S., Abuajamieh M., Stoakes S. K., Seibert J. T., Cox L., Kahl S., Elsasser T. H., Ross J. W., Isom S. C.. et al. 2015. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 3:e12315. doi:10.14814/phy2.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaramuzzi R. J., S. A. Tillson I. H. Thorneycroft, and Caldwell B. V.. 1971. Action of exogenous progesterone and estrogen on behavioral estrus and luteinizing hormone levels in the ovariectomized ewe. Endocrinology 88:1184–1189. doi:10.1210/endo-88-5-1184 [DOI] [PubMed] [Google Scholar]
- Schumann R. R. 2011. Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem. Soc. Trans. 39:989–993. doi:10.1042/BST0390989 [DOI] [PubMed] [Google Scholar]
- Shimizu T., K. Miyauchi K. Shirasuna H. Bollwein F. Magata C. Murayama, and Miyamoto A.. 2012. Effects of lipopolysaccharide (LPS) and peptidoglycan (PGN) on estradiol production in bovine granulosa cells from small and large follicles. Toxicol. in Vitro 26:1134–1142. doi:10.1016/j.tiv.2012.06.014 [DOI] [PubMed] [Google Scholar]
- Sweet L. J., B. D. Morrison, and Pessin J. E.. 1987. Isolation of functional alpha beta heterodimers from the purified human placental alpha 2 beta 2 heterotetrameric insulin receptor complex. A structural basis for insulin binding heterogeneity. J. Biol. Chem. 262:6939–6942. [PubMed] [Google Scholar]
- Thompson P. A., P. S. Tobias S. Viriyakosol T. N. Kirkland, and Kitchens R. L.. 2003. Lipopolysaccharide (LPS)-binding protein inhibits responses to cell-bound LPS. J. Biol. Chem. 278:28367–28371. doi:10.1074/jbc.M302921200 [DOI] [PubMed] [Google Scholar]
- Turner J. R. 2009. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9:799–809. doi:10.1038/nri2653 [DOI] [PubMed] [Google Scholar]
- Willis D. and Franks S.. 1995. Insulin action in human granulosa cells from normal and polycystic ovaries is mediated by the insulin receptor and not the type-I insulin-like growth factor receptor. J. Clin. Endocrinol. Metab. 80:3788–3790. doi:10.1210/jcem.80.12.8530637 [DOI] [PubMed] [Google Scholar]
- Zeleznik A. J., D. Saxena, and Little-Ihrig L.. 2003. Protein kinase B is obligatory for follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology 144:3985–3994. doi:10.1210/en.2003-0293 [DOI] [PubMed] [Google Scholar]