SUMMARY
Hepatocellular adenomas carry a risk of malignant transformation. However, little is known about the rate of this risk, the predisposing factors, the histological and/or immunohistochemical markers and the precursor lesions. Even the pathogenesis of malignant transformation itself is poorly understood. This review details the current knowledge on the subject.
KEYWORDS : β-catenin activation, hepatocellular adenoma, hepatocellular carcinoma, histopathology, immunohistochemistry, malignant transformation
Practice points.
The risk of malignant transformation of hepatocellular adenoma (HCA) approximates 5%.
This evaluation is biased by several parameters, mainly that not all patients with HCA are identified or undergo surgery and that the recognition of a pre-existing HCA behind a hepatocellular carcinoma developed in noncirrhotic liver is difficult to assess.
Male gender, β-catenin-activated HCA, lesion >5 cm, specific clinical backgrounds (glycogen storage disease, androgens, vascular diseases) are risk factors.
Obesity and the metabolic syndrome could represent a ‘fertile soil’.
At the microscope, the criteria to diagnose malignant transformation are still poorly defined and the terminology of the borderline lesions is still a matter of debate.
Some progress has been made in understanding the pathogenesis of malignant transformation of HCA: the identification of β-catenin mutation as an early event and the role of telomerase reverse transcriptase (TERT) promoter mutations.
Hepatocellular adenoma (HCA) is classically known as a benign liver tumor occurring in young woman taking oral contraception (OC) [1]. Actually, it represents a more complex heterogeneous entity, with four different subtypes recently identified (Table 1) [2–4]. HNF1A-inactivated HCA (H-HCA) occurs almost exclusively in women and represents approximately 35–40% of all HCAs. This subtype shows marked steatosis in a majority of cases. This specific histological feature, combined with an absence of immunostaining for liver fatty acid binding protein (LFABP) produced by a gene positively regulated by HNF1A, is characteristic of this HCA subtype. Inflammatory HCA (IHCA) is the largest subgroup, representing >50% of the cases. IHCA occurs mostly in women often in association with obesity and fatty liver disease. Inflammation, sinusoidal dilatation and ductular reaction are the main histological features. A strong immunohistochemical expression of inflammation-associated proteins such as serum amyloid A and C-reactive protein (CRP) helps in recognizing this subtype which is related to activation of the JAK/STAT pathway underlined by mutations in different genes. β-catenin mutations may coexist in 10% of IHCA (β-IHCA). β-catenin-activated HCA (β-HCA) is found in 10–15% of patients. This HCA subtype, underlined by an activating β-catenin mutation, is more often seen in men or associated with specific conditions such as glycogen storage disorders (GSD) or androgen administration. It has been reported to exhibit cellular atypia and pseudo-glandular growth pattern and to have an increased risk of malignant transformation. A strong expression of glutamine synthetase (GS) with or without aberrant cytoplasmic and nuclear expression of β-catenin by immunohistochemistry (IHC) is the clue to identify this type of HCA. The last group, still not fully understood, corresponds to all HCA devoid of specific histological and immunohistochemical features, and without known mutations. They represent <10% of all cases and were called, until now, unclassified adenomas (UHCA) (Table 1). This classification has been validated by several groups [5,6] and is included in the 2010 edition of the WHO classification of Tumors of the Digestive System [7].
Table 1. . Subtypes of hepatocellular adenomas.
| Subtype | Genotype | Phenotype (usual) | Immunohistochemistry | Frequency |
|---|---|---|---|---|
| H-HCA | HNF1A-inactivating mutations | Steatosis | LFABP negative | 30–40 |
| IHCA | IL6ST, STAT3, GNAS, FRK, Jak1-activating mutations (20% unknown) | Inflammation, sinusoidal dilatation, ductular reaction | SAA/CRP positive | 50 |
| β-HCA | β-catenin-activating mutations | Cellular atypia, rosettes (inconstant) | GS positivity, aberrant nuclear/cytoplasmic β-catenin expression | 10–15 |
| UHCA | Unknown | Unspecific | None | 10 |
CRP: C-reactive protein; GS: Glutamine synthetase; HCA: Hepatocellular adenoma; IHCA: inflammatory hepatocellular adenoma; LFABP: Liver fatty acid binding protein; SAA: Serum amyloid A; UHCA: Unclassified adenoma.
Surgical resection has been classically recommended in HCAs >5 cm because they were reported to carry a higher risk of bleeding and of transforming into hepatocellular carcinoma (HCC). However, recently, more precise algorithms have been developed to take into account the clinical context and the different subtypes of HCA before making a management decision, which is lately mainly based on the potential for malignant transformation [8–12].
The current review sought to better define the frequency of malignant transformation of HCA, the patients who are at risk to do so and, for the pathologists, the characteristics that can be observed at the microscope. In addition, recent data about the possible pathogenesis of malignant transformation of HCA will be discussed.
What is the frequency of malignant transformation?
The first case of malignancy in HCA was described in 1975 [13]. At that time, HCAs were known for their risk of bleeding, with a mortality as high as 60%, and the association with oral contraception was a recent discovery. In their case report, the authors suggested that if the risk of malignancy of these ‘pill tumors’ was similar to that found in cirrhosis, it could reach 10%. After that first case, several reports were published during the next decades establishing definitely a risk for malignant transformation in HCA, even if no detailed molecular studies were underwent to prove a direct progression from HCA to HCC [14–17]. In small series reported by specialized centers, malignant transformation was even shown to be quite high, from 13 to 17.6% of the patients [14,15]. However, today, almost 40 years after the first report of malignancy in HCA, malignant transformation in HCA is still a matter of debate, with difficulties to identify this complication histologically and immunohistochemically, to understand the pathogenesis and to identify the precursor lesions as well as to give them a name as we will see later [12]. Even the proportion of cases developing malignancy is difficult to establish [18]. Indeed, the series are biased, mainly because reporting only surgical cases, but also because not all patients undergo surgery (small lesions) or are identified (asymptomatic patients), and also because HCC developing in noncirrhotic liver, therefore potentially on a pre-existing HCA, are never taken into account.
Several recent publications aimed at collecting all published series of HCAs in order to better define the rate of malignant transformation. In 2010, Farges and Dokmak, looking at surgical series of more than 20 patients, reported a rate of malignancy stable across the last three decades: 5% in the 1980s, 4% in the 1990s and 6% from 2000 [18]. In 2011, Stoot et al. performed the largest review on this subject when collecting more than 1600 HCAs published between 1970 and 2010, coming from 157 series and 17 cases reports [19]. In their careful analysis, they showed that the risk of malignant transformation of HCA was 4.2% (Table 2), and 4.5% if taking into account only the surgical series. In this review, the authors also include more details about the three largest series of HCAs, all published in 2009. The series from Deneve et al. [20] reported malignant transformation in 4.2% of their 119 patients, none within HCA smaller than 8 cm; the series from Bioulac-Sage et al. [9] showed 4.7% of transformation into HCC in 128 patients, all in β-catenin-activated HCA (β-HCA and β-IHCA); and the series from Dokmak et al. [8] reported malignancy in ten of their 122 patients (8.1%), mostly in men and in larger lesions (>8 cm in 90%). Again, these numbers come from surgical series and are definitely biased by the recruitment of each center. Another systematic review, published in 2011, analyzed HCA publications between 1998 and 2008, and reported an overall rate of malignant transformation of 5.6%, with important geographical differences: 2.9% in Europe, 6.5% in North America, 6.7% in South-East Asia and 6.8% in China where 62% of HCA occur in men [21]. Very recently, reviewing 15 series published during the last 10 years, Nault et al. reported a rate of malignancy going from 0 to 18%, with malignant transformation occurring mainly in males, in lesions >5 cm and in β-HCAs [12].
Table 2. . Frequency of malignant transformation of hepatocellular adenoma and risk factors.
| Frequency | In largest surgical series: from 4.2 to 10.6% |
| Risk factors | Male gender, women older than 50 years or younger than 15 years? |
| Larger size | |
| Specific clinical background (androgens, GSD, vascular diseases) | |
| β-catenin activation: β-HCA and β-IHCA | |
GSD: Glycogen storage disorder; HCA: Hepatocellular adenoma; IHCA: Inflammatory hepatocellular adenoma.
Another way to look at the problem is the study performed by the Beaujon group in 2011, when they reviewed retrospectively 218 patients with HCA, screening them for the presence of malignant transformation [22]. The group study was mainly composed of women (84%) and the diagnosis of malignant transformation was based on the presence of abnormal trabecular pattern, decreased reticulin network and/or Glypican 3 expression by IHC. They found areas of HCC within HCA in 23 patients (10.6%, 16 men and seven women). Malignant transformation occurred mainly in men (47.1 vs 3.8%) and three lesions were <5 cm. Interestingly, 26.5% of malignant cases were associated with the presence of a metabolic syndrome and 64% occurred in β-catenin-activated HCA (β-HCA and β-IHCA).
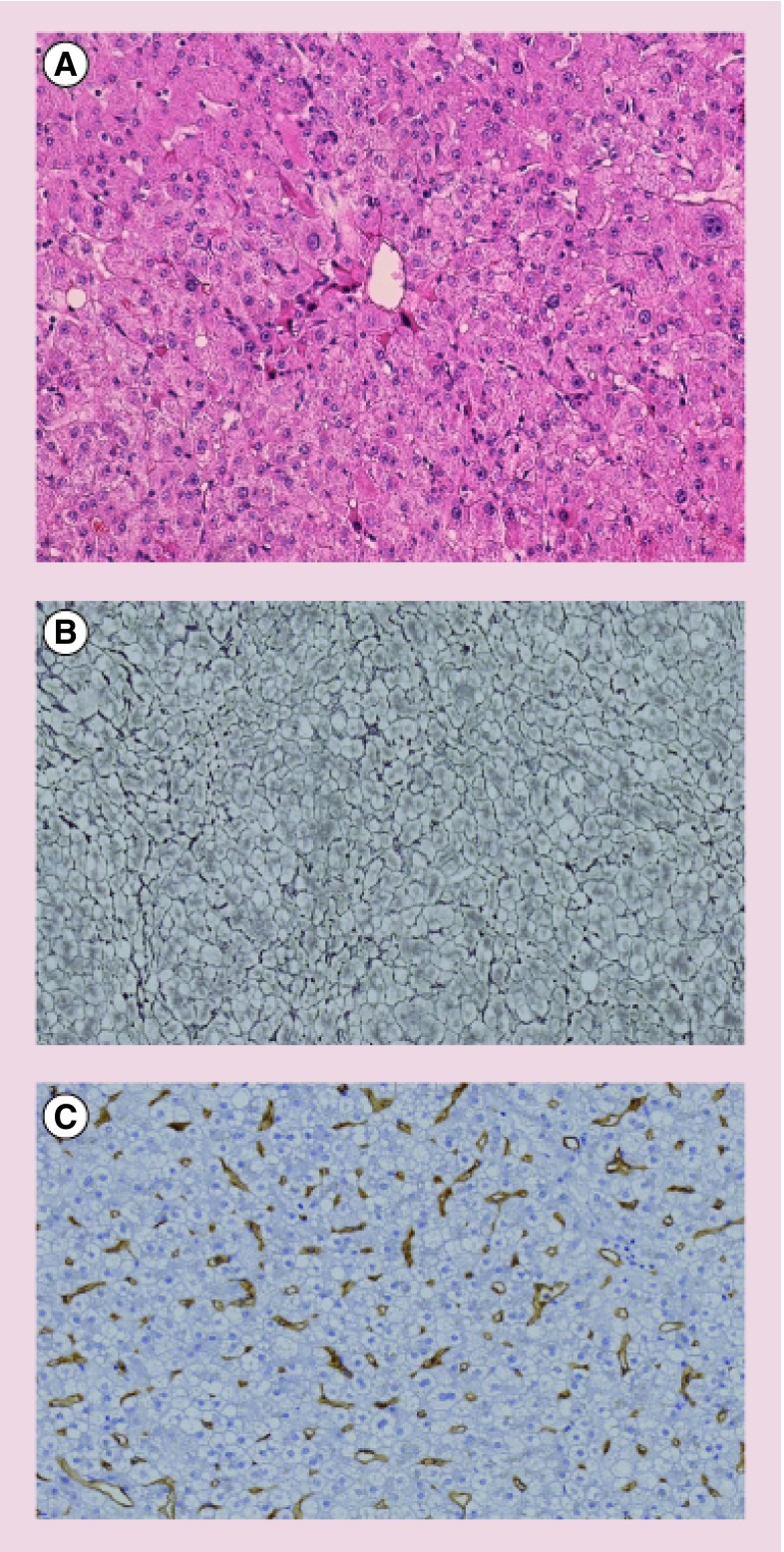
In children, HCA are very rare, mostly diagnosed during adolescence and in association with predisposing factors such as GSD Type 1, anabolic androgenic steroid treatments with or without Fanconi anemia, congenital or portosystemic shunts [23–28]. Exceptional cases of malignant transformation have been reported [29]. We experienced such a case of a 2-year-old boy presenting an 8 cm HCA developed in the background of portal vein agenesis (Abernethy malformation Type 1). The liver biopsy revealed a β-HCA (Figure 1A–C). Lost for follow-up, he came back 9 years later with a very large moderately differentiated HCC for which a liver transplantation was required (Figure 1D).
Figure 1. . Malignant transformation of hepatocellular adenoma in a young boy suffering from portal vein agenesis.
Liver biopsy at 2 years of age (A–C). Note the absence of atypia (A, original magnification ×10), the diffuse and intense staining for glutamine synthetase (B, original magnification ×5) and the very focal nuclear staining for β-catenin (C, original magnification ×20). Liver transplantation specimen at 11 years (D). Huge hepatocellular carcinoma in the right liver lobe.
Who is at risk?
Both clinical and histological features have been associated with a higher risk of malignant transformation of HCA. Scrutinizing the reported series already mentioned in the previous section, it appears that the higher risk is in men, in larger lesions, in some specific underlying clinical contexts and in β-catenin-activated HCA (β-HCA and β-IHCA) (Table 2).
Men are definitely more at risk than women to develop HCC within HCA. They are already known to exhibit a higher rate of HCC in general, maybe because of an increased exposure to risk factors such as viral hepatitis or alcohol consumption. In the context of HCA, men develop mainly β-catenin-activated HCA (β-HCA or β-IHCA) that is per se more at risk of malignant transformation (see below). Androgens have also been shown to increase HCA transformation which has been reported in bodybuilders for example, or in young patients treated for hematological disorders, as mentioned earlier [23,26]. In the study of Farges [22], the risk of malignant transformation in men was ten-times higher than the one observed in women, and they found an increased frequency of HCA in men during the last decade. According to the authors, this increased frequency could reflect the higher rate of metabolic syndrome, especially in men. They even proposed that HCC developing in this background could actually arise from a pre-existing HCA and not from a regenerative nodule, knowing that cirrhosis is not always present. This has been also suggested by other authors very recently [30] but is still a matter of debate [31]. Nevertheless, this observation let several authors to propose a different management of HCA in men, recommending surgical resection in all cases, independently from the size of the lesion [12].
The larger the lesion, the larger the risk of malignant progression, is a classical assumption. In the literature, the majority of HCC transformation of HCA is reported in lesions of more than 5 cm. However, they are two possible pitfalls, first the smaller lesions are not surgically resected and, second, small HCC in normal liver, without any sign of HCA, are never reported within series of HCA, although they could have risen from a pre-existing HCA. In the large review of Stoot [19], only three cases of malignant transformation were reported in lesions of <5 cm. One of these cases was the one reported by Micchelli et al. who reviewed their series of 17 HCA searching for malignancy [15]. They identified three cases (17.6%) of malignant transformation between 2003 and 2006, ranging from 4 to 8.5 cm. The background liver was steatotic and all cases occurred in women. In the series from Farges, 12% of malignant transformation was found in HCA <5 cm [22]. Two of the three cases were in men with specific underlying diseases. The recommendation is therefore again to surgically resect or destroy any lesion in men, even if small, and, in women, to treat lesions that increase in size or that do not regress after stopping OC, whatever their size and the background liver.
Some clinical contexts have been established not only as risk factors for HCA but also for malignant transformation. This has been shown for GSD [24,25] and in case of steroids consumption [26,32]. A large review published in 2004, reported on the association between androgens and liver tumors, showing the development of HCA, HCC and, in six patients, of both HCA and HCC, suggesting malignant transformation of HCA [26]. GSD is a classical background for developing HCA and cases of malignant transformation have been reported, mostly as the co-existence of HCC and HCA in the same diseased liver [24,25]. This, again, seems to be more frequent in men. In the USA, a rise of HCA has been reported [33], especially in men, and it has been attributed to obesity. Indeed, nonalcoholic fatty liver disease, the hepatic complication of the metabolic syndrome, has become an endemic disease in developed countries and has been worryingly associated with the development of HCC [34,35]. As it has been proposed for HCC, the metabolic syndrome could therefore also represent a ‘fertile soil’ for the development of HCA, later complicated by malignant transformation. Several studies have shown that the IHCA subtype of HCA was the most frequently observed subtype in case of obesity [36,37]. In their study on the nontumoral liver in case of IHCA, Han et al. [38] reported on the presence of small foci of IHCA scattered in a background of steatotic liver in patients with a metabolic syndrome. These minute foci, if left in place, could undergo further malignant transformation. This is concordant with the observation of Farges et al. [22] who reported a higher number of transformed HCA in men with a metabolic syndrome. However, not all researchers agree on that concept and in their series of 48 patients with 52 HCC on noncirrhotic liver, another group did not find any suggestive feature of a pre-existing HCA [31]. Obesity is a risk factor for HCA, particularly IHCA. Obesity and the metabolic syndrome are well known risk factors for cancer in general and HCC in particular. What remains to be studied is the identification of a prior HCA in the following situations: HCC developed in a patient with metabolic syndrome (and no other factors), HCC developed in a normal liver, in a steatotic liver and in NASH, with or without cirrhosis.
Eventually, β-catenin-activated HCA (β-HCA and β-IHCA) has been shown to represent a subgroup of HCAs at higher risk of malignant transformation [2,3,6,9,22,39,40]. Again this type of HCA is more frequent in men. Also, as already mentioned, this type of HCA is more frequently observed in specific clinical contexts such as steroids consumption and GSD. In their series, the Bordeaux group reported that 37.6% of β-catenin-activated HCA already showed HCC at the time of surgery [9]. In the study of Farges [22], 60% of malignant HCA were β-catenin-activated HCA. In our recent experience with 37 cases of HCA surgically ablated [6], the two HCAs showing malignant transformation were β-HCA, both occurring in men. So far, this subtype of HCA can only be recognized at the microscope, and/or by molecular analysis [11] as explained in the next section. Nevertheless, it is important to remind that no HCA subtype is devoid of risk of malignant transformation.
For women, an older age (50 years or older) or a younger age (15 years or less) has been suggested by several authors as a risk factor [41–43]. This clinical feature is taken into account to classify an HCA as atypical [41–43] or of uncertain malignant potential [44], by these authors but it remains to be firmly established.
What can the pathologist identify at the microscope?
The first important thing for the pathologist is to recognize and correctly identify β-catenin-activated HCA, either β-HCA or β-IHCA subtype. It has been shown that β-catenin-activated HCAs occur more frequently in men and are characterized by the presence of cellular atypia with frequent rosette formation [2,3]. However, later on, it was recognized that b-HCA could also be very bland, occurs in women and shows association with IHCA [4,6,9]. To make a diagnosis of β-catenin-activated HCA, the pathologist needs the help of IHC. Even if very specific, nuclear β-catenin immunostaining is of low sensitivity to detect accurately β-HCA and β-IHCA. Our recommendation is to perform GS staining on every single HCA, including IHCA to improve the detection of an activation of the β-catenin pathway. Indeed, GS is one of the target genes in case of β-catenin activation and it is usually diffusely and strongly expressed in β-catenin-activated HCA, either β-HCA or β-IHCA (Figure 1B). It is more reliable than β-catenin staining itself because it can be difficult to find a single β-catenin-positive nucleus (Figure 1C) even in front of a very strong GS staining (Figure 1B). GS staining can also be patchy or diffuse but less intense and still be related to β-catenin-activating mutations but in that situation a molecular analysis of the lesion has to be performed to confirm the suspected β-catenin activation.
In some patients, the specific clinical background will already alert the pathologist. Indeed, to find close to each other an HCA and an HCC, for example in the context of GSD or of vascular abnormalities, suggests a malignant transformation of a pre-existing HCA. In these patients, different subtypes of HCA can be present at the same time, mainly IHCA, β-HCA and β-IHCA [25]. Another possible situation is a past clinical history of HCA in a patient developing an HCC, suggesting again a malignant transformation. We experienced such a case in which the incomplete resection of a previous β-IHCA let the patient to develop an HCC 12 years later. Like the case of the young boy with agenesis of the portal vein, already described, this evolution suggests malignant transformation of an HCA, with a long delay that could represent an intrinsic characteristic of malignant transformation in HCA.
Cellular atypia can be present in HCA. It can either correspond to some area of small cells or, in contrast, to the presence of larger cells with bigger nuclei. A few rosettes or thicker liver cell plates, even some pseudogland formation can also focally be seen. The problem for the pathologist is to avoid an overdiagnosis in case of mild or focal cellular atypia. Indeed, lots of HCA bled before resection, got arterial embolization to stop the bleeding or portal embolization to increase the size of the remaining liver before large surgical resection. This can give rise to cellular atypia which therefore needs to be interpreted with caution when observed in this setting.
Some HCA can look much more worrisome to the pathologist. These HCAs are those showing architectural distortion, either in foci or more extensively, with a combination of thicker liver cell plates, extensive pseudoglands formation and decreased reticulin framework together with increased CD34 staining (Figure 2). These so-called atypical HCA [41–43] correspond to borderline lesions that have also recently be called well-differentiated hepatocellular neoplasms of uncertain malignant potential [44]. According to recent publications of the group of San Francisco [41–43], atypical HCA could represent a very well differentiated form of HCC because they share molecular abnormalities with HCC. Therefore, they have probably to be resected by surgery, even if their true potential of evolution and the delay of it are unknown. Evason et al. [42] also showed that HCA occurring in men or in older women have also to be considered as atypical HCA. In our experience, reticulin staining remains the most powerful tool to identify foci of definite malignant transformation, especially if combined with the presence of architectural distortion, cellular atypia and increased CD34 staining. Glypican 3 is very useful when it is positive, even focally; however, its negativity does not exclude malignancy, knowing that its expression increases in case of decreased differentiation [45]. Of note, CD34 has been shown to be increased in some HCA without malignant transformation [46]. Reviewing our recently published series of HCA [6] that we extended to the more recent cases, we identified three such atypical HCA/borderline lesions. Importantly, one of them was found in unclassified adenomas, which highlights the potential risk of malignancy in this so far poorly understood subgroup.
Figure 2. . Borderline hepatocellular adenoma.
Pathological features with cellular atypia and thickened liver cell plates on hematoxylin and eosin stain (A) with focally decreased reticulin network (B) and focal increased CD34 immunostaining (C). Original magnification ×20.
Eventually, the easiest situation for the pathologist will be the obvious presence of a focus or of several foci of HCC with a border or a rim of residual HCA (Figure 3). In this last histopathological category (Table 3), abnormal architectural and histological features are supported by total loss of reticulin network and diffuse increased CD34 expression. Glypican 3 can be present as well as an increased MIB1 staining. Some of these lesions have been studied at the molecular level (see next section) and they help in understanding the pathogenesis of malignant transformation in HCA.
Figure 3. . Hepatocellular carcinoma within an hepatocellular adenoma.
The two different parts are well identified on hematoxylin and eosin stain (A). The HCC part exhibits decreased reticulin network (B), diffuse increased CD34 immunostaining (C) and patchy/strong glutamine synthetase expression. The HCA part shows a preserved reticulin framework (B), no CD34 expression (C) and a strong, more diffuse glutamine synthetase immunostaining (D) underlying the β-catenin activation (β-HCA subtype). Original magnification ×10.
GS: Glutamine synthetase; HCA: Hepatocellular adenoma; HCC: Hepatocellular carcinoma.
Table 3. . Findings at the microscope.
| β-HCA/β-IHCA | Strong/diffuse (or patchy) GS-positive immunostaining and aberrant expression of β-catenin (can be very focal or absent) |
| Clinical background | HCC in a context strongly suggestive of HCA |
| Cellular and architectural atypia | Few foci of anisocaryosis or of smaller hepatocytes, thicker hepatocytic plates, decreased reticulin network, some pseudogland formations, cholestasis. Do not forget pitfall: vicinity of bleeding! |
| Borderline HCA | Well-differentiated hepatocellular neoplasm: several foci of either atypia, rosettes, loss of reticulin, increased CD34 … but insufficient for a straightforward diagnosis of HCC |
| HCC within HCA | One focus or several foci of HCC within an HCA, or rim of HCA surrounding HCC |
GS: Glutamine synthetase; HCA: Hepatocellular adenoma; HCC: Hepatocellular carcinoma; IHCA: Inflammatory hepatocellular adenoma.
To recognize worrisome features within an HCA is theoretically possible on a biopsy but the main problem will be the sampling error. Indeed in biopsy, you can miss the major point: the distribution of the abnormalities. Taking into account the parameters mentioned in Table 3, β-HCA will be recognized on biopsy by GS and β-catenin IHC staining and the clinical context has to be explained to the pathologist. Cellular and architectural atypia can be missed, as well as areas of borderline HCA. If a fully developed HCC is present, the diagnosis will be made, providing that the sampling is correct. In this particular situation if the focus is big enough, imaging techniques should be able to identify it.
In routine practice, the first step is to make a diagnosis of HCA and to do the subtyping and the second step is to establish a possible malignant transformation. For HCA identification and differential diagnosis with focal nodular hyperplasia, the pathologist needs a good hematoxylin and eosin section to search for specific characteristics such as steatosis or inflammation or sinusoidal dilatation for example. A Trichrome stain can be useful to identify pseudo-portal tracts or fibrotic bands. K7 is useful to detect the ductular reaction. Then the specific immunohistochemical stainings are performed. The order depends very much on the lab facilities and on the policy in terms of cost of the hospital/the social security system of the country. They can be done either at the same time or step by step, which is the most challenging. LFABP negativity will confirm an HNF1-α-inactivated HCA if diffuse steatosis; CRP positivity an inflammatory HCA if sinusoidal dilatation, inflammation, ductular reaction and pseudo-portal tracts are present. GS has to be performed in all cases to search for β-catenin activation. β-catenin immunostaining is only helpful if GS is abnormal but is less sensitive to identify β-catenin-activated HCA. Importantly, GS is also very helpful to diagnose focal nodular hyperplasia. All immunohistochemical markers have to give normal results (positivity of LFABP within and outside the HCA, negativity of CRP, GS and β-catenin within and outside the HCA) before to conclude to an unclassified subtype of HCA. Re-cuts are not really useful for surgical specimens, the best thing being to take enough samples, some of them at the junction with the nontumoral liver. On biopsies, it is preferable to prepare blank slides in advance and it is mandatory to have a biopsy of the nontumoral liver for comparison of the immunohistochemical results. To search for malignant transformation on surgical specimen, a careful macroscopic examination is mandatory with a good sampling of all different areas of the lesion. Some cytological or architectural variability is acceptable, especially in case of bleeding. When the pathologist suspects a malignant transformation or in case of β-catenin-activated HCA or specific clinical context, re-cuts, reticulin stain and CD34 will be performed. HSP70 can be useful. MIB1 can show an increased proliferation rate. GPC3 is often performed but usually negative. On a biopsy, the problem of sampling and the limited amount of material available for staining can represent a difficulty. There is so far no specific phenotype of HCC developed from HCA, except that in our experience these HCC are often pigmented or cholestatic. Not all HCC derived from HCA express GS, some can express CRP but we have little data concerning the expression of CRP or serum amyloid A during malignant transformation. LFABP expression is usually decreased in HCC. It is therefore not always easy to assess the HCA origin of a fully developed HCC on a nonfibrotic liver as it has recently been proposed [30,43].
What is the pathogenesis of malignant transformation?
The pathogenesis of malignant transformation of HCA is poorly understood. Very recently, some light was shed on the mechanisms by the group of Zucman-Rossi [47,48]. They demonstrated the importance of telomerase reverse transcriptase (TERT) promoter mutations in hepatocarcinogenesis. This was the most frequent abnormality observed in HCC, as well as in some dysplastic nodules, known to be preneoplastic lesions in cirrhotic livers. By contrast, in HCA, it was found to be a late event, not required for clonal benign proliferation, and always complicating β-catenin mutation, therefore probably cooperating to promote malignant transformation. In their analysis, comparing 60 benign HCAs to 16 transformed HCAs, 44% of malignant cases showed TERT promoter mutations whereas none of the benign HCAs presented this abnormality. This suggests that this molecular event is required for malignant transformation of HCA and could be a marker of it [47]. In addition, they showed that in the progression from HCA to HCC, β-catenin mutation is an early event and that genes classically mutated in HCA can be found in the HCC counterpart demonstrating a common origin [48]. All these β-catenin mutations involved in malignant transformation occur in exon 3 [48]. Interestingly enough is the discovery of other types of β-catenin mutations occurring in exon 7-8; these latter less activate β-catenin pathway and are probably not involved in malignant transformation [48]. An interesting field of investigation has been suggested a few years ago by Tretiakova et al. [49] who studied the expression of E-cadherin and of matrix metalloproteinases 7 and 9 in HCA and in HCC, showing specific features for HCA, some previously suggested [50]. The hypothesis is that the migration of the β-catenin into the nucleus leads to a decrease of E-cadherin therefore to a migratory phenotype. The proposed criteria on staining intensity score for E-cadherin and matrix metalloproteinases 7 and 9 remain to be studied in larger series, namely on β-HCA, β-IHCA and borderline HCA.
Conclusion & future perspective
Malignant transformation of HCA exists, even if we are still far from understanding its mechanism. Because of the weakness of the criteria to define malignant transformation of HCA, to quantify its risk, to reach a firm histological diagnosis, particularly on biopsy samples, it is difficult to propose an unequivocal approach to manage these lesions and to select patients who need to be surgically treated on the basis of specific event identified in HCA. According to the current knowledge, in case of HCA of less than 5 cm, a strict follow-up is recommended in classical clinical context (women on OC), including for β-HCA and β-IHCA, wherever HCA in men, HCA in specific clinical contexts and HCA with frank cytological or architectural abnormalities are recommended for destruction because they definitely carry a risk of malignant transformation (Box 1). Cellular atypia have to be interpreted with caution because it is frequently associated with prior bleeding, therefore representing a potential pitfall. TERT mutations and β-catenin activation are the first identified steps in understanding carcinogenesis. Well-differentiated HCC developed on noncirrhotic liver could represent the end of the spectrum, and pathologists have to look carefully for residual areas of HCA. The frequency of this particular type of lesions seems to increase in frequency maybe in relationship with the endemic evolution of the metabolic syndrome.
Box 1. . Suggested recommendations for resection/follow-up of borderline/atypical hepatocellular adenoma and hepatocellular carcinoma (for nodules <5 cm).
Borderline/atypical HCA with minor pathological abnormalities
Pathology (focal and minor degree)
Cytological abnormalities
Loss of reticulin
Pseudo-glands
Immunohistochemistry
Extension of CD34
GS diffuse and strong (with or without β-catenin nuclear staining)
MRI
Hypervascularization
In classical clinical conditions (women on oral contraception): strict follow-up (MRI)
In specific conditions (men, glycogen storage disorders, vascular diseases): destruction of the nodule
Borderline/atypical HCA with major pathological abnormalities or HCC
Destruction of the nodule
GS: Glutamine synthetase; HCA: Hepatocellular adenoma; HCC: Hepatocellular carcinoma.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Edmondson HA, Henderson B, Benton B. Liver-cell adenomas associated with use of oral contraceptives. N. Engl. J. Med. 1976;294:470–472. doi: 10.1056/NEJM197602262940904. [DOI] [PubMed] [Google Scholar]
- 2.Zucman-Rossi J, Jeannot E, Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 3.Bioulac-Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 4.Bioulac-Sage P, Cubel G, Balabaud C, Zucman-Rossi J. Revisiting the pathology of resected benign hepatocellular nodules using new immunohistochemical markers. Semin. Liver Dis. 2011;31:91–103. doi: 10.1055/s-0031-1272837. [DOI] [PubMed] [Google Scholar]
- 5.van Aalten SM, Verheij J, Terkivatan T, Dwarkasing RS, de Man RA, Jzermans JN. Validation of a liver adenoma classification system in a tertiary referral centre: implications for clinical practice. J. Hepatol. 2011;55:120–125. doi: 10.1016/j.jhep.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca S, Hoton D, Dardenne S, et al. Histological and immunohistochemical revision of hepatocellular adenomas: a learning experience. Int. J. Hepatol. 2013;2013:398308. doi: 10.1155/2013/398308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bioulac-Sage P, Balabaud C, Wanless I. Focal nodular hyperplasia and hepatocellular adenoma. In: Bosman F, Carneiro F, Hruban R, Theise ND, editors. Tumors of the Digestive Tract. World Health Organization, IARC; Lyon, France: 2010. pp. 198–204. [Google Scholar]
- 8.Dokmak S, Paradis V, Vilgrain V, et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698–1705. doi: 10.1053/j.gastro.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 9.Bioulac-Sage P, Laumonier H, Couchy G, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481–489. doi: 10.1002/hep.22995. [DOI] [PubMed] [Google Scholar]
- 10.van Aalten SM, Witjes CD, de Man RA, Ijzermans JN, Terkivatan T. Can a decision-making model be justified in the management of hepatocellular adenoma? Liver Int. 2012;32:28–37. doi: 10.1111/j.1478-3231.2011.02667.x. [DOI] [PubMed] [Google Scholar]
- 11.Sempoux C, Chang C, Gouw A, et al. Benign hepatocellular nodules: what have we learned using the patho-molecular classification. Clin. Res. Hepatol. Gastroenterol. 2013;37:322–327. doi: 10.1016/j.clinre.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology. 2013;144:888–902. doi: 10.1053/j.gastro.2013.02.032. [DOI] [PubMed] [Google Scholar]; •• A well-documented and up-to-date review by experts in the field.
- 13.Davis M, Portmann B, Searle M, Wright R, Williams R. Histological evidence of carcinoma in a hepatic tumour associated with oral contraceptives. Br. Med. J. 1975;29:496–498. doi: 10.1136/bmj.4.5995.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster JH, Berman MM. The malignant transformation of liver cell adenomas. Arch. Surg. 1994;129:712–717. doi: 10.1001/archsurg.1994.01420310044007. [DOI] [PubMed] [Google Scholar]
- 15.Micchelli ST, Vivekanandan P, Boitnott JK, Pawlik TM, Choti MA, Torbenson M. Malignant transformation of hepatic adenomas. Mod. Pathol. 2008;21:491–497. doi: 10.1038/modpathol.2008.8. [DOI] [PubMed] [Google Scholar]
- 16.Burri E, Steuerwald M, Cathomas G, et al. Hepatocellular carcinoma in a liver-cell adenoma within a non-cirrhotic liver. Eur. J. Gastroenterol. Hepatol. 2006;18:437–441. doi: 10.1097/00042737-200604000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Hechtman J, Raoufi M, Fiel I, et al. Hepatocellular carcinoma arising in a pigmented telangiectatic adenoma with nuclear-catenin and glutamine synthetase positivity: case report and review of the literature. Am. J. Surg. Pathol. 2011;35:927–932. doi: 10.1097/PAS.0b013e318218ca3f. [DOI] [PubMed] [Google Scholar]
- 18.Farges O, Dokmak S. Malignant transformation of liver adenoma: an analysis of the literature. Dig. Surg. 2010;27:32–38. doi: 10.1159/000268405. [DOI] [PubMed] [Google Scholar]
- 19.Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford) 2010;12:509–522. doi: 10.1111/j.1477-2574.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The largest review on the malignant transformation of hepatocellular adenoma (HCA).
- 20.Deneve JL, Pawlik TM, Cunningham S, et al. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann. Surg. Oncol. 2009;16:640–648. doi: 10.1245/s10434-008-0275-6. [DOI] [PubMed] [Google Scholar]
- 21.Lin H, van den Esschert J, Liu C, van Gulik TM. Systematic review of hepatocellular adenoma in China and other regions. J. Gastroenterol. Hepatol. 2011;26:28–35. doi: 10.1111/j.1440-1746.2010.06502.x. [DOI] [PubMed] [Google Scholar]
- 22.Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85–89. doi: 10.1136/gut.2010.222109. [DOI] [PubMed] [Google Scholar]; • Highlights the fact that men can develop HCA with a malignant potential.
- 23.Franchi-Abella S, Branchereau S. Benign hepatocellular tumors in children: focal nodular hyperplasia and hepatocellular adenoma. Int. J. Hepatol. 2013;2013:215064. doi: 10.1155/2013/215064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakellariou S, Al-Hussaini H, Scalori A, et al. Hepatocellular adenoma in glycogen storage disorder type I: a clinicopathological and molecular study. Histopathology. 2012;60(6B):E58–E65. doi: 10.1111/j.1365-2559.2011.04153.x. [DOI] [PubMed] [Google Scholar]
- 25.Calderaro J, Labrune P, Morcrette G, et al. Molecular characterization of hepatocellular adenomas developed in patients with glycogen storage disease type I. J. Hepatol. 2013;58:350–357. doi: 10.1016/j.jhep.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 26.Velazquez I, Alter BP. Androgens and liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am. J. Hematol. 2004;77:257–267. doi: 10.1002/ajh.20183. [DOI] [PubMed] [Google Scholar]
- 27.Franchi-Abella S, Branchereau S, Lambert V, et al. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J. Pediatr. Gastroenterol. Nutr. 2010;51(3):322–330. doi: 10.1097/MPG.0b013e3181d9cb92. [DOI] [PubMed] [Google Scholar]
- 28.Lisovsky M, Konstas A, Misdraji J. Congenital extrahepatic portosystemic shunts (Abernethy malformation): histopathologic evaluation. Am. J. Surg. Pathol. 2011;35:1381–1390. doi: 10.1097/PAS.0b013e3182230ce4. [DOI] [PubMed] [Google Scholar]
- 29.Morotti R, Killackey M, Shneider B, Repucci A, Emre S, Thung S. Hepatocellular carcinoma and congenital absence of the portal vein in a child receiving growth hormone therapy for Turner syndrome. Semin. Liver Dis. 2007;27:427–431. doi: 10.1055/s-2007-991518. [DOI] [PubMed] [Google Scholar]
- 30.Liu TC, Vachharajani N, Chapman WC, Brunt EM. Noncirrhotic hepatocellular carcinoma: derivation from hepatocellular adenoma? Clinicopathologic analysis. Mod. Pathol. 2013;27:420–432. doi: 10.1038/modpathol.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witjes CDM, Ten Kate FJW, van Aalten SM, et al. Hepatocellular adenoma as a risk factor for hepatocellular carcinoma in a non-cirrhotic liver: a plea against. Gut. 2012;61:1645–1646. doi: 10.1136/gutjnl-2012-302219. [DOI] [PubMed] [Google Scholar]
- 32.Giannitrapani L, Soresi M, La Spada E, Cervello M, D'Alessandro N, Montalto G. Sex hormones and risk of liver tumor. Ann. NY Acad. Sci. 2006;1089:228–236. doi: 10.1196/annals.1386.044. [DOI] [PubMed] [Google Scholar]
- 33.Chang CY, Hernandez-Prera JC, Roayaie S, Schwartz M, Thung SN. Changing epidemiology of hepatocellular adenoma in the United States: review of the literature. Int. J. Hepatol. 2013;2013:604860. doi: 10.1155/2013/604860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J. Hepatol. 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 35.Torres DM, Harrison SA. Nonalcoholic steatohepatitis and noncirrhotic hepatocellular carcinoma: fertile soil. Semin. Liver Dis. 2012;32:30–38. doi: 10.1055/s-0032-1306424. [DOI] [PubMed] [Google Scholar]
- 36.Bioulac-Sage P, Taouji S, Possenti L, Balabaud C. Hepatocellular adenoma subtypes: the impact of overweight and obesity. Liver Int. 2012;32:1217–1221. doi: 10.1111/j.1478-3231.2012.02786.x. [DOI] [PubMed] [Google Scholar]
- 37.Paradis V, Champault A, Ronot M, et al. Telangiectatic adenoma: an entity associated with increased body mass index and inflammation. Hepatology. 2007;46:140–146. doi: 10.1002/hep.21684. [DOI] [PubMed] [Google Scholar]
- 38.Han J, van den Heuvel MC, Kusano H, de Jong KP, Gouw AS. How normal is the liver in which the inflammatory type hepatocellular adenoma develops? Int. J. Hepatol. 2012;2012:805621. doi: 10.1155/2012/805621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monga SP. Hepatic adenomas: presumed innocent until proven to be beta-catenin mutated. Hepatology. 2006;43:401–404. doi: 10.1002/hep.21110. [DOI] [PubMed] [Google Scholar]
- 40.Van der Borght S, Libbrecht L, Katoonizadeh A, et al. Nuclear beta-catenin staining and absence of steatosis are indicators of hepatocellular adenomas with an increased risk of malignancy. Histopathology. 2007;51:855–856. doi: 10.1111/j.1365-2559.2007.02862.x. [DOI] [PubMed] [Google Scholar]
- 41.Kakar S, Chen X, Ho C, et al. Chromosomal abnormalities determined by comparative genomic hybridization are helpful in the diagnosis of atypical hepatocellular neoplasms. Histopathology. 2009;55:197–205. doi: 10.1111/j.1365-2559.2009.03343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evason KJ, Grenert JP, Ferrell LD, Kakar S. Atypical hepatocellular adenoma-like neoplasms with β-catenin activation show cytogenetic alterations similar to well-differentiated hepatocellular carcinomas. Hum. Pathol. 2013;44:750–758. doi: 10.1016/j.humpath.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An interesting basic research contribution to the understanding of malignant transformation of HCA.
- 43.Kakar S, Grenert JP, Paradis V, Pote N, Jakate S, Ferrell LD. Hepatocellular carcinoma arising in adenoma: similar immunohistochemical and cytogenetic features in adenoma and hepatocellular carcinoma portions of the tumor. Mod. Pathol. 2014 doi: 10.1038/modpathol.2014.50. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bedossa P, Burt AD, Brunt EM, et al. Well-differentiated hepatocellular neoplasm of uncertain malignant potential: proposal for a new diagnostic category. Hum. Pathol. 2014;45:658–660. doi: 10.1016/j.humpath.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of Glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod. Pathol. 2008;21:1011–1018. doi: 10.1038/modpathol.2008.85. [DOI] [PubMed] [Google Scholar]
- 46.Coston WM, Loera S, Lau SK, et al. Distinction of hepatocellular carcinoma from benign hepatic mimickers using Glypican-3 and CD34 immunohistochemistry. Am. J. Surg. Pathol. 2008;32:433–444. doi: 10.1097/PAS.0b013e318158142f. [DOI] [PubMed] [Google Scholar]
- 47.Nault JC, Mallet M, Pilati C, et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013;4:2218. doi: 10.1038/ncomms3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilati C, Letouze E, Nault JC, et al. Integrative genomic profiling of hepatocellular adenomas reveals recurrent FRK activating mutations and mutational processes of malignant transformation. Cancer Cell. 2014;25:428–441. doi: 10.1016/j.ccr.2014.03.005. [DOI] [PubMed] [Google Scholar]; •• The most recent review on the subject dealing with major findings.
- 49.Tretiakova MS, Hart J, Shabani-Rad MT, Zhang J, Gao Z. Distinction of hepatocellular adenoma from hepatocellular carcinoma with and without cirrhosis using E-cadherin and matrix metalloproteinase immunohistochemistry. Mod. Pathol. 2009;22:1113–1120. doi: 10.1038/modpathol.2009.75. [DOI] [PubMed] [Google Scholar]
- 50.Kozyraki R, Scoazec JY, Flejou JF, et al. Expression of cadherins and α-catenin in primary epithelial tumors of the liver. Gastroenterology. 1996;110:1137–1149. doi: 10.1053/gast.1996.v110.pm8613003. [DOI] [PubMed] [Google Scholar]