Abstract
Liver abscesses (LA) are a source of economic loss for feedlot cattle feedlots, and the 2017 veterinary feed directive has restricted further use of tylosin phosphate to prevention and control of LA. Our objective was to evaluate effects of intermittent tylosin phosphate feeding on incidence and severity of LA in feedlot cattle and presence of total antimicrobial-resistant Enterococcus spp. Steers (n = 312, 411.4 ± 6.71 kg) were blocked by initial BW and randomly assigned to a treatment group. Treatments included a negative control group (no tylosin phosphate throughout the finishing period), a positive control group (tylosin phosphate fed continuously throughout the finishing period), and a group that received tylosin phosphate off-label by feeding the drug on a repeated intermittent basis (1 wk on, 2 wk off). Steers were housed in 24 soil-surfaced pens with 13 steers per pen. Body weights of cattle were obtained every 28 d and at the end of 119 d the steers were weighed and harvested at a commercial abattoir. Fecal samples were collected on days 0, 21, and 118 to characterize antimicrobial-resistant Enterococcus spp. Total LA percentage was greater (P = 0.012) for the no tylosin phosphate treatment compared with the other treatments, but did not differ between the continuous tylosin phosphate treatment and the intermittently fed tylosin phosphate treatment (P = 0.716). No difference was observed among treatments for ADG (P = 0.21), DMI (P = 0.28), or G:F (P = 0.75). Marbling score was lower (P = 0.022) for tylosin phosphate treatment when compared with both intermittent treatment and continuous tylosin phosphate treatment. Enterococcus spp. bacterial counts did not differ by treatment group over time (P > 0.05); however, there was a strong period effect for macrolide resistance among all groups (P < 0.01), suggesting an important environmental component as cattle were first placed in pens and then progressed through the feeding period. We conclude that feeding tylosin phosphate intermittently during the finishing phase decreases the total percentage of LA and maintains feedlot performance and carcass characteristics to the same extent as feeding tylosin phosphate throughout the finishing phase; furthermore, we hypothesize that enteric antimicrobial resistance is a result of longer term antibiotic usage in a particular environment rather than a direct short-term result of the treatment during any given feeding period.
Keywords: beef cattle, feedlot, intermittent feeding, liver abscess, tylosin
INTRODUCTION
Liver abscesses (LA) are a source of economic loss in feedlot cattle. McKeith et al. (2012) reported that 5.4% of livers were condemned due to major abscesses and 8.3% of livers were condemned due to minor abscesses. Carcass value decreases due to the trim loss associated with LA (Brown & Lawrence, 2010; Rezac et al., 2014). Cattle with severe LA have an ADG that is 0.17 kg/d lower compared with animals that are healthy (Rezac et al., 2014), a significant decrease in G:F (Brink et al., 1990), and a decrease in feed intake (Brink et al., 1990).
Tylosin phosphate is a macrolide antibiotic that is used by U.S. feedlots (Berg & Scanlan, 1982; Nagaraja & Lechtenberg, 2007; Reinhardt & Hubbert, 2015). Various studies have shown that tylosin phosphate is effective in decreasing the incidence of LA (Tan et al., 1994; Vogel & Laudert, 1994; Meyer et al., 2013). In 2013, the FDA issued Guidance for Industry (GFI) no. 213, which established a time table for the reduction of medically important antimicrobials, such as macrolides, for performance enhancement in animal feeding operations. Guidance for Industry no. 213 also laid the framework for implementation of veterinary supervision when using these antimicrobials (FDA GFI no. 213, 2013).
It is therefore important to look at alternative approaches to control LA in feedlot cattle. Teague et al. (1966) observed that feeding chlortetracycline to growing-finishing pigs at a rate of 44 mg/kg DM every 4 wk or 88 mg/kg DM every 8 wk had a similar effect on growth performance as feeding chlortetracycline continuously at a rate of 11 mg/kg DM. We hypothesized that feeding tylosin phosphate intermittently will effectively control the incidence and severity of LA, while reducing total use. Our objectives for this experiment were to evaluate the effect of repeated intermittent feeding of tylosin phosphate on feedlot performance, carcass characteristics, incidence, and severity of LA in feedlot cattle, and the prevalence of antibiotic resistant Enterococcus spp.
MATERIALS AND METHODS
Procedures involving live animals were conducted and approved within the guidelines of the Kansas State University Institutional Animal Care and Use Committee.
Experimental Design
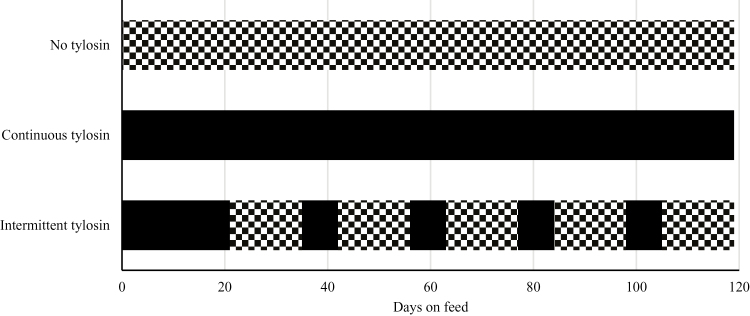
The trial was conducted from March 30, 2016 to July 26, 2016 and was designed as a randomized block design with 3 treatments using 312 crossbred steers (411.4 ± 6.71 kg). Steers were selected from a larger population and were selected based on their BW. Steers were then blocked based on their initial BW and were assigned within block to 1 of the 3 treatments. The treatments consisted of a positive control in which steers received a basal diet that contained tylosin phosphate throughout the feeding period, a negative control in which steers received a basal diet that contained no tylosin phosphate throughout the feeding period, and a treatment where steers received a basal diet with tylosin phosphate added to the diet during the step-up phase and then subsequently on a repeated intermittent basis (1 wk on, 2 wk off) (Figure 1). Tylosin phosphate was fed at a rate of 9.9 mg/kg DM.
Figure 1.
Treatment design to describe different tylosin phosphate feeding strategies. Diet containing no tylosin phosphate is represented by a checkered pattern, whereas a diet containing tylosin phosphate is represented by solid black. No tylosin treatment received no tylosin phosphate, tylosin treatment received tylosin phosphate continuously throughout the feeding period, and intermittent tylosin treatment received tylosin phosphate in a 1 wk on, 2 wk off pattern.
Animals were housed in 24 pens with each pen containing 13 animals/pen. Pens had a 10.1 × 30.5 m area with pipe fencing and soil surfaces. Each pen allowed approximately 20.44 m2 of space per animal. Automatic waterers were located approximately 6 to 9 m from feed bunks and shared by animals in adjacent pens. Each pen had concrete pads extending 3 m into the pen from the feed bunk and each feed bunk allowed for approximately 76 cm of bunk space per steer.
Cattle Processing
Three hundred eighty-five crossbred steers arrived from Cherokee (Oklahoma), Dodge City (Kansas), and Pratt (Kansas) at the Kansas State University Beef Cattle Research Centre (Manhattan, KS). It is unknown if the steers received any antibiotic treatments before they arrived at the research center. Steers were placed in holding pens and provided ad libitum access to ground alfalfa and water. Twenty-four hours after each load was received, steers were individually weighed, received ear tags for individual identification, were vaccinated with clostridial antigens (Ultrabac7 Somnubac, Zoetis Animal Health, Florham Park, NJ), a 5-way respiratory vaccine (Bovishield Gold5, Zoetis Animal Health), and received a pour-on treatment for parasites (Permectrin CDS, Bayer, Leverkusen, Germany). Fifty steers were given a tetanus toxoid vaccine (BarVac CD/T, Boehringer Ingelheim, Ingelheim am Rhein, Germany), but were supposed to have received a clostridial antigen vaccine (Ultrabac7 Somnubac, Zoetis). These steers were closely monitored and the incident was recorded as a deviation in protocol. Three hundred twelve steers (n = 312) were selected for use in the experiment. Steers were blocked according to BW and assigned to treatments. On day 1 of the study, the steers that were assigned to the experiment were individually weighed, implanted with Component TE-200 with Tylan (Elanco Animal Health, Greenfield, IN), and placed in their respective experimental pens.
Body weights were determined via pen weights collected at 28-d intervals and then at the end of the 119-d feeding period. Body weights were determined by taking the pen weight and dividing that number by the number of animals in the pen. Cattle in pens were weighed using a large platform pen scale (Central City Scale; Central City, NE) set on 4 electronic load cells; each load cell was calibrated with 1,021 kg of certified weights before each use. These values were used to determine ADG by subtracting the previous BW from the current BW and then dividing the value by days on feed (DOF). Gain:feed was determined by taking the ADG and dividing it by the DMI. Fecal samples were collected from 8 fresh fecal pats straight from the pen in each pen at the start of the experiment, after the 21-d step-up period, and one day before the end of the experiment. These samples were then prepared and sent to researchers in the Department of Veterinary Pathobiology at Texas A&M University for microbiological analyses of antimicrobial resistance.
Diet Preparation
Steers were transitioned to their finishing diets using 4 step-up diets over a 21-d period. Diets were formulated to gradually adapt steers to the finishing diet that contained a high concentration of grain. The composition of the diets is presented in Table 1. For the positive control diet, tylosin phosphate was added to the supplement, whereas no tylosin phosphate was added to the negative control diet supplement. For the intermittent treatment group, the diet contained tylosin phosphate during the 21-d transition period; thereafter, tylosin phosphate was fed in 1 wk on, 2 wk off cycle that repeated until the end of the trial. This pattern of tylosin phosphate feeding allowed for a 2-wk withdrawal period of tylosin phosphate imposed at the end of the 119-d feeding period. Feeding tylosin phosphate in this manner is off-label use of the drug. Monensin (Rumensin, Elanco Animal Health) was fed at a rate of 33 mg/kg DM, and ractopamine hydrochloride (Optaflexx, Elanco Animal Health) was provided at a level of 200 mg/steer for the last 29 d of the feeding period. Tylosin phosphate was fed at a rate of 9.9 mg/kg DM.
Table 1.
Diet composition of diets containing tylosin phosphate or containing no tylosin phosphate fed to finishing steers to assess the efficacy of intermittently feeding tylosin phosphate on liver abscess incidence
| Item | |
|---|---|
| Ingredient (%, DM) | |
| Steam-flaked corn | 57.68 |
| Sweetbran1 | 30.00 |
| Corn silage | 10.00 |
| Supplement2 | 2.32 |
| Nutrient composition (DM basis), calculated2 | |
| CP, % | 13.30 |
| NEm, MJ/kg | 2.14 |
| NEg, MJ/kg | 1.48 |
| NDF, % | 19.06 |
| Ca, % | 0.66 |
| P, % | 0.49 |
| Salt, % | 0.25 |
1Wet corn gluten feed, Cargill Starches & Sweeteners North America, Minneapolis, MN.
2Contains limestone, salt, urea, trace mineral/vitamin premix to provide (on a total diet DM basis) 0.15 mg/kg cobalt, 10 mg/kg copper, 0.50 mg/kg iodine, 20 mg/kg manganese, 0.10 mg/kg selenium, 30 mg/kg zinc, 2205 IU/kg vitamin A, 22 IU/kg vitamin E, and 33 mg/kg monensin (Rumensin, Elanco Animal Health). Tylosin phosphate (Tylan, Elanco Animal Health) was fed at 9.9 mg/kg.
2Calculated based on Nutrient Requirements of Beef Cattle (7th Revised Edition) values.
Diets were mixed once daily and fed to the steers at approximately 0800 h using a truck-mounted mixer. To avoid cross contamination, diets containing no tylosin phosphate were fed first, and after diets containing tylosin phosphate were fed, the truck was flushed with a diet containing no tylosin phosphate. Feed intakes were visually determined and adjusted daily to allow for ad libitum intake with minimal feed residues remaining in the feed bunk. Unconsumed feed was removed on days that the pens were weighed (to determine G:F) and after excessive precipitation events. To determine DM content, a subsample of the unconsumed feed was dried for 48 h in a 55 °C oven. Dry matter intake was determined for each 28-d interval and at the end of the experiment as follows: DMI = [(total feed accessible × %DM) – (unconsumed feed × %DM)]/(number of steers × day). Average tylosin phosphate consumption per steer was determined as follows: [pen intake × 0.0041 (percentage tylosin phosphate in the diet)]/animals in the pen. This equation was used to determine the tylosin phosphate consumption per steer per day; these values were then totaled.
Harvest
After 119 DOF, the steers were harvested. Pen weights were obtained before transporting steers to a commercial abattoir. Final BW was determined by taking the pen average and multiplying the value by 0.96 (pencil shrink). Liver abscess scores were assessed at the abattoir using the scoring system described by Brown et al. (1975): 0 = no abscesses, A− = 1 or 2 abscesses, A = 2 to 4 abscesses which are in average under 1 inch in diameter, and A+ = 1 or more large abscesses. Liver abscess scoring was done by a trained individual from KSU observing and scoring livers assessed by USDA officials. United States Department of Agriculture quality grades, yield grades, LM area, 12th subcutaneous rib fat thickness, marbling score, and incidence of dark cutters were obtained from the abattoir through camera images (VBG 2000; E+V Technology GmbH & Co. KG, Oranienburg, Germany).
Isolation of Fecal Enterococci and Estimation of Colony Forming Units
Fecal samples were collected from 8 randomly selected animals per pen, per sampling day (0, 20, and 118). Day 0 samples were collected before animals began their feeding trial and then were placed into the pens. One gram of thawed fecal sample and glycerol (50/50) (Fisher Chemical, Waltham, MA) mixture was added to 9 mL of sterile phosphate-buffered saline (PBS) (Gibco Lifetechnologies, Becton Dickinson, Sparks, MD). After thorough mixing, 50µL of this fecal suspension was spiral-plated onto each of plain selective m-Enterococcus (ME) agar (Difco, Becton Dickinson, Sparks, MD), erythromycin- (8µg/mL) (Sigma Aldrich, St. Louis, MO) infused ME agar, and tetracycline- (16µg/ml) (Sigma Aldrich, St. Louis, MO) infused ME agar using the Eddy Jet 2 instrument (Neutec Group Inc., NY). The plates were then incubated at 42 °C for a period of 48 h before estimating the number of typical Enterococcus spp. colonies formed per mL of the fecal sample suspension using the Flash & Go colony counter (Neutec Group Inc., NY). Further calculations based on all dilutions resulted in colony-forming units (cfu) estimates per gram of feces.
To initial assess the assay specificity, countable colonies—those with distinct Enterococcus spp. morphology—pink, mauve, and magenta—were randomly selected from plain ME agar plates and streaked to blood agar (BBL Stacker Becton Dickinson, Sparks, MD). The plates were incubated at 42 °C for 48 h. Isolates from each blood agar plate were inoculated into BBL Enterococcosel broth (Becton Dickinson, Sparks, MD) and incubated for 4 to 6 h at 37 °C. Approximately 95% of the isolates were positive on the Esculin hydrolysis test. Two Esculin positive isolates per sample were selected for cryo-bead preservation at −80 °C.
Furthermore, Enterococcus-presumptive isolates from 2 animals per pen/day were randomly selected from each treatment group for MALDI-TOF (Matrix-Assisted Laser Desorption Ionization-Time of Flight) analysis. For each selected animal, 2 distinct colonies were chosen from the plain, tetracycline-, and erythromycin- infused ME agar plates. Each colony was streaked onto individual blood agar plate and incubated for 48 h at 42 °C. Using sterilized wooden toothpicks, each isolate was smeared onto wells in the reusable 96-spot MALDI target plate (Bruker Daltonik GmbH., Billerica, MA). One microliter of bacteria test standard (BTS) solution (Bruker Daltonik GmbH., Billerica, MA) was applied to the first and second spots as positive controls. Once dry, 1 µL of 70% formic acid was added to the first member of each sample spot pair. After adequate drying, 1 µL of HCCA matrix solution (Bruker Daltonik GmbH., Billerica, MA) was then added to all sample-smeared spots, BTS spots, and at least 2 empty spots for negative control. The target plate was subsequently transferred to the MALDI-TOF benchtop microflex LT/SH mass spectrometer (Bruker Daltonik GmbH., Billerica, MA) for reading.
Statistical Analyses
Analyses for feedlot performance and carcass characteristics were performed using SAS version 9.4. PROC MIXED procedure was used for feedlot performance and noncategorical carcass data (continuous data), whereas PROC GLIMMIX procedure was used for categorical carcass data. The model contained treatment as a fixed effect, weight block as a random effect, and pen as the experimental unit. Treatment effects were declared significant at a level of P < 0.05. Least-squares means were compared between the treatment groups using the PDIFF function of SAS.
Antimicrobial resistance data were recorded and tabulated by pen (n = 24 divided into 3 treatments: 1) no tylosin phosphate fed, 2) tylosin phosphate fed continuously, or 3) tylosin phosphate fed intermittently, sample (n = 8 per pen) and day (0, 20, and 118), and these were further tabulated by selective medium (no antibiotics in ME agar or with erythromycin (ERY) at 8 µg/mL or tetracycline [TET] at 16 µg/mL). Log10 cfu were calculated with a single cfu added to each data point. The difference between Log10 cfu calculated for plain ME agar vs. ME plus ERY or vs. ME plus TET for each pen/sample/day was determined and used for multilevel mixed linear regression analysis (Stata 12.1, Stata Corp., College Station, TX). A full factorial model of treatment by day, adjusted for the clustering effects of pen, was built. Marginal means were estimated and plotted with 95% confidence intervals to examine the interactive and main effects of the pen-level treatments over time. Smaller differences signified a greater proportion of total enterococci that were resistant to either antibiotic; conversely, larger differences represented a smaller proportion of antibiotic-resistant bacteria.
RESULTS AND DISCUSSION
Two steers were removed from the positive control group. One steer was removed due to a back injury not related to treatment, and the other steer died due to a respiratory tract infection. One steer from the negative control group was euthanized due to an injury unrelated to the treatment.
Feedlot Performance
Overall feedlot performances for the 3 treatment groups are represented in Table 2. There were no treatment effects for ADG (P ≥ 0.207), DMI (P ≥ 0.278), and G:F (P ≥ 0.752). Our results are consistent with that of Brown et al. (1975), Heinemann et al. (1978), Potter et al. (1985), and Depenbusch et al. (2008). However, Meyer et al. (2009) observed improved performance when tylosin phosphate was added to the diet. Their reported improved performance may be due to the fact that they saw a greater percentage of severe LA in their control group (34% of total LA) compared with the amount of severe LA we observed (9.1% of total LA). The larger difference in the number of severe LA between the control group and tylosin phosphate treatment group in their study likely led to the significant difference they saw in performance between the treatments. Larger abscesses could lead to a portion of the liver losing function, or more severe predisposing factors such as ruminal acidosis, and thus reflect a greater decrease in growth performance. Sides et al. (2009) similarly observed no difference in feedlot performance when they performed a study with 4,000 heifers in a commercial feedlot setting. Sides et al. (2009) compared supplementation of a combination of monensin and tylosin phosphate throughout the feeding period to a treatment where they withdrew the combination of monensin and tylosin phosphate for 35 d preharvest. Sides et al. (2009) reported no difference for DMI, DOF, final BW, ADG, or G:F consistent with our results, where steers were fed tylosin phosphate intermittently. There was a 60% decrease in the amount of tylosin phosphate that was consumed by the intermittent treatment group, when compared with the tylosin phosphate treatment group (positive control).
Table 2.
Effect of feeding no tylosin phosphate, continuous tylosin phosphate, or feeding tylosin phosphate intermittently on finishing steer feedlot performance
| Item | Treatment1 | SEM | P-value | ||
|---|---|---|---|---|---|
| No tylosin | Tylosin | Intermittent tylosin | |||
| Initial BW, kg | 410 | 411 | 411 | 6.71 | 0.40 |
| Final BW, kg | 628 | 635 | 626 | 4.86 | 0.23 |
| ADG, kg/d | 1.83 | 1.87 | 1.80 | 0.036 | 0.21 |
| DMI, kg/d | 10.89 | 11.23 | 10.86 | 0.235 | 0.28 |
| G:F, kg/kg | 0.1678 | 0.1665 | 0.1657 | 0.0027 | 0.75 |
1No tylosin received no tylosin phosphate, Tylosin received tylosin phosphate continuously throughout the feeding period, and Intermittent tylosin received tylosin phosphate in a 1 week on, 2 weeks off pattern.
Carcass Characteristics
Carcass performance is represented in Table 3. No differences were observed between treatments for HCW (P = 0.512), dressed yield (P = 0.257), 12th rib fat thickness (P = 0.860), LM area (P = 0.921), quality grades (P ≥ 0.182), and yield grades (P ≥ 0.847). Brink et al. (1990) reported similar results for HCW and dressed yield. Previous studies have indicated that carcasses with mostly severe LA had lower 12th rib fat thickness and LM area compared with carcasses with normal livers (Brown & Lawrence, 2010; Rezac et al., 2014). This might explain why we did not see a difference in 12th rib fat thickness and LM area as only a few of the livers we saw had severe abscesses.
Table 3.
Effect of feeding no tylosin phosphate, continuous tylosin phosphate, or feeding tylosin phosphate intermittently on finishing steer feedlot performance
| Item | Treatment1 | SEM | P-value | ||
|---|---|---|---|---|---|
| No tylosin | Tylosin | Intermittent tylosin | |||
| Hot carcass weight, kg | 380 | 383 | 380 | 6.11 | 0.51 |
| Dressed yield, % | 65.4 | 64.9 | 65.8 | 0.21 | 0.26 |
| 12th rib fat thickness, cm | 1.25 | 1.27 | 1.25 | 0.030 | 0.86 |
| LM area, cm2 | 88.7 | 86.9 | 89.1 | 1.30 | 0.92 |
| Marbling score2 | 455a | 429b | 458a | 12 | 0.02 |
| USDA Prime, % | 1.0 | 0 | 1.9 | 1.38 | 0.38 |
| USDA Choice, % | 75.0 | 73.5 | 76.7 | 6.01 | 0.85 |
| USDA Select, % | 24.0 | 26.6 | 18.5 | 5.82 | 0.39 |
| Subselect3, % | 0.0a | 0.0a | 2.9b | 1.12 | 0.05 |
| USDA yield grade | 2.6 | 2.6 | 2.5 | 0.11 | 0.85 |
| Yield grade 1, % | 4.9 | 2.0 | 3.9 | 2.58 | 0.52 |
| Yield grade 2, % | 39.8 | 40.6 | 43.3 | 6.92 | 0.87 |
| Yield grade 3, % | 49.5 | 52.5 | 48.1 | 6.97 | 0.81 |
| Yield grade 4, % | 5.8 | 5.0 | 4.8 | 3.12 | 0.94 |
a,bMeans within a row and without a common superscript letter are different, P < 0.05.
1No tylosin received no tylosin phosphate, tylosin received tylosin phosphate continuously throughout the feeding period, and intermittent tylosin received tylosin phosphate in a 1k on, 2 wk off pattern.
2Marbling score determined by computer imaging system (VBG 2000, E+V Technology GmbH & Co. KG, Oranienburg, Germany). Small (400–499).
3Consists of carcasses grading USDA Standard and USDA Commercial carcasses.
There was a decrease in marbling score when the continuously fed tylosin phosphate treatment was compared with the other 2 treatments (P = 0.022). Our findings on marbling scores differed with what Sides et al. (2009) reported, in that they observed no differences observed in marbling score between cattle that had LA and those that did not have LA. We hypothesize that the decreased marbling score that we observed for the continuously fed tylosin phosphate treatment is due to the absence of carcasses that graded prime for the continuous tylosin phosphate treatment compared with the no tylosin phosphate and intermittent tylosin phosphate treatments that had a few carcasses that graded prime.
Liver Abscess Incidence and Severity
Liver abscess incidence and severity are represented in Table 4. There was an increase (P = 0.012) in the total number of LA when the no tylosin phosphate treatment was compared with the continuous tylosin phosphate and intermittent tylosin phosphate feeding treatments; however, no differences (P = 0.716) were observed between the two latter treatments. The no tylosin phosphate treatment group also had a greater (P = 0.026) number of moderate LA compared with the continuous tylosin phosphate feeding treatment. There were no further differences observed between treatments for mild LA or severe LA. Our overall results are consistent with what was found in various research projects in the late 1970s that looked at the LA mitigation properties of tylosin phosphate fed continuously (Brown et al., 1975; Heinemann et al, 1978; Pendlum et al., 1978; Vogel & Laudert, 1994); however, there are no published studies of intermittent feeding of tylosin phosphate upon which to base comparisons.
Table 4.
Effect of feeding no tylosin phosphate, continuous tylosin phosphate, or feeding tylosin phosphate intermittently on liver abscess incidence and severity
| Item | Treatment | SEM | P-value | ||
|---|---|---|---|---|---|
| No tylosin | Tylosin | Intermittent tylosin | |||
| Total liver abscesses, % | 21.36a | 7.84b | 9.62b | 4.655 | 0.01 |
| Liver abscess severity1, % | |||||
| Mild | 12.62 | 6.86 | 5.77 | 4.011 | 0.19 |
| Moderate | 6.88a | 0.98b | 2.88a,b | 2.589 | 0.07 |
| Severe | 1.94 | 0.00 | 0.96 | 1.378 | 0.37 |
1Liver abscess scores are assessed using the scoring system described by Brown et al. (1975): 0 = no abscesses (mild), A− = 1 or 2 abscesses (mild), A = 2 to 4 abscesses which are in average under 1 inch in diameter (moderate), and A+ = 1 or more large abscesses (severe).
a,bMeans within a row and without a common superscript letter are different, P < 0.05.
It is of considerable interest to note that there were no treatment effects for total LA between steers that were fed tylosin phosphate intermittently and steers that were fed tylosin phosphate throughout the feeding period. Sides et al. (2009) and Bohrer et al. (2016) also noted that simply withdrawing tylosin from the diet for the last 33 to 35 d on feed decreased the total amount of LA to the same extent as feeding tylosin for the entire time on feed. No explanation was provided by the authors; however, we hypothesize that it might be due to prolonged inhibition of regrowth of gram-negative bacteria after the initial exposure to the macrolide (Mathers et al., 2011). Our results indicate the possibility that tylosin phosphate has a prolonged effect in which LA can be decreased even after the removal of tylosin phosphate from the diet, possibly through the inhibition of gram-negative bacteria. Alternatively, the most important effects of tylosin phosphate in preventing LA might occur during the periods of greatest risk, that is, during “step up” periods of considerable dietary compositional change. During other constant feeding periods, the importance of the antibiotic may be diminished.
Antimicrobial-Resistant Enterococci
Based on MALDI-TOF characterization of 182 randomly selected presumptive isolates, we estimated that 81.8% of the colonies (95% CI: 75.1–87.0%) were Enterococcus spp. Among the enterococci, the dominant species included Enterococcus hirae (n = 76), Enterococcus faecium (n = 44), Enterococcus casseliflavus (n = 8), and Enterococcus gallinarum (n = 4), among others. Alternative bacterial genera and species that appeared like enterococci on ME agar plates included “No organism identification possible [by MALDI-TOF]” (n = 21), Streptococcus lutetiensis (n = 4), Paenibacillus barengoltzii (n = 2), Bacillus pumilus (n = 2), and Bacillus clausii (n = 1). Thus, the specificity of the cfu counts on 3 varieties of ME agar plates was estimated at 82%.
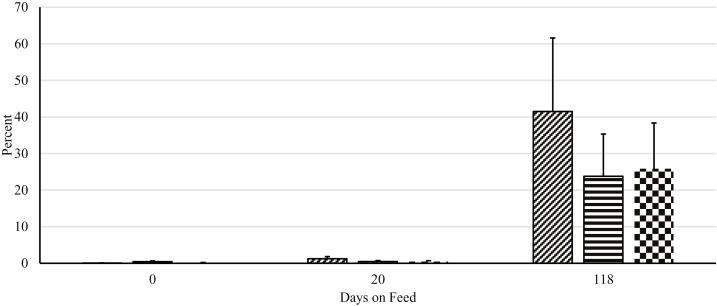
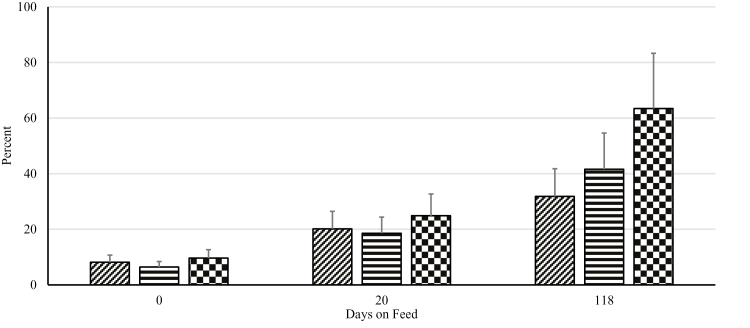
Overall, there were highly significant period (P < 0.01) effects when examining the difference between Log10 cfu of enterococci grown on plain ME agar vs. ME agar with 8 µg/mL of erythromycin (Figure 2). A large increase in the proportion of enterococci resistant to erythromycin is implied in the large reduction in differences in counts over the 118-d feeding trial. These period effects were much less pronounced with tetracycline, where levels of resistance were quite high initially (Figure 3); however, these differences did decrease over 118 d. The main effects of both erythromycin and tetracycline were nonsignificant (P > 0.05) at each time period, suggesting that time accrued in the feeding pen environment during the feeding trial was more important than the tylosin phosphate regimen being fed.
Figure 2.
Effect of different tylosin phosphate feeding strategies on percent of Enterococcus population resistant to erythromycin. Adapted from a multilevel mixed linear regression analysis. No tylosin treatment (diagonal lines) received no tylosin phosphate, tylosin treatment (horizontal lines) received tylosin phosphate throughout the feeding period, and intermittent tylosin treatment (checkered) received tylosin phosphate in a 1 wk on, 2 wk off pattern. Treatment × day, P = 0.35; Effect of sampling day, P < 0.001; Effect of treatment, P = 0.63.
Figure 3.
Effect of different tylosin phosphate feeding strategies on percent of Enterococcus population resistant to tetracycline. Adapted from a multilevel mixed linear regression analysis. No tylosin treatment (diagonal lines) received no tylosin phosphate, tylosin treatment (horizontal lines) received tylosin phosphate throughout the feeding period, and intermittent tylosin treatment (checkered) received tylosin phosphate in a 1 wk on, 2 wk off pattern. Treatment × day, P = 0.42; Effect of sampling day, P < 0.05; Effect of treatment, P = 0.71.
Generally speaking, there was little difference among treatment groups at each time point; notably, as the amount of time spent in the feeding pen increased, the total number of enterococci that were resistant to both erythromycin and tetracycline increased. This was further reflected in decreasing differences in the total cfu between plain ME agar and agar infused with the 2 antibiotics at breakpoint concentrations. This suggests that the major factor affecting the levels of resistance is not the use of the antibiotic; rather, it is likely the cumulative effect of continued exposure to an environment in which a macrolide such as tylosin phosphate has been fed for extended periods of time (e.g., years if not decades). Other authors have noted similar effects for other antibiotic-bacteria combinations (e.g., Agga et al., 2016). Unpublished work from members of our own group was notable for a similar dramatic step up of resistance upon placement in a feed yard, regardless of treatments (H.M. Scott, personal communication). Thus, it seems probable that beneficial effects of reduced use of tylosin phosphate will not manifest immediately, or at least not in the same environment in which the product or other antibiotics have been used in the recent past. Reductions in antibiotic resistance among enterococci are likely to accrue slowly in the absence of major environmental interventions.
IMPLICATIONS
In conclusion, feeding tylosin phosphate intermittently during the finishing phase decreased the incidence of LA and maintained feedlot performance and carcass characteristics in steers to the same extent as when tylosin phosphate was fed continuously throughout the finishing phase. Feeding tylosin phosphate intermittently is off-label use of the drug; therefore, feeding monensin and ractopamine hydrochloride with this treatment was also under off-label use. Furthermore, according to guidelines established by the VFD, if animals are fed intermittently, a new prescription from a licensed veterinarian will be required for every period that tylosin phosphate is fed (FDA CFR21, 2017). This warrants more research to determine when the optimum time to feed tylosin phosphate will be to reduce LA without having to feed tylosin phosphate multiple times.
Footnotes
Contribution no. 18-165-J from the Kansas Agricultural Experiment Station.
LITERATURE CITED
- Agga G. E., Schmidt J. W., and Arthur T. M.. 2016. Effect of in-feed chlortetracycline prophylaxis of beef cattle of animal health and antimicrobial-resistant Escherichia coli. Appl. Environ. Microbiol. 83:7197–7204. doi:10.1128/AEM.01928-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. N. and Scanlan C. M.. 1982. Studies of fusobacterium necrophorum from bovine hepatic abscesses: biotypes, quantitation, virulence, and antibiotic susceptibility. Am. J. Vet. Res. 43:1580–1586. [PubMed] [Google Scholar]
- Bohrer B. M., Galloway H. O., Meeuwse D. M., Beckett J. L., Edmonds M. D., Sharman E. D., Moseley W. M., Vanimisetti H. B., Schroeder A. L., Dilger A. C., and Boler D. D.. 2016. Effects of feeding generic ractopamine (Actogain) with or without the combination of monensin and tylosin phosphate on growth performance, carcass characteristics, and tenderness of finishing steers. Prof. Anim. Sci. 32:42–52. doi:10.15232/pas.2015-01414 [Google Scholar]
- Brink D. R., Lowry S. R., Stock R. A., and Parrott J. C.. 1990. Severity of liver abscesses and efficiency of feed utilization of feedlot cattle. J. Anim. Sci. 68:1201–1207. doi:10.2527/1990.6851201x [DOI] [PubMed] [Google Scholar]
- Brown H., Bing R. F., Grueter H. P., McAskill J. W., Cooley C. O., and Rathmacher R. P.. 1975. Tylosin and chloretetracycline for the prevention of liver abscesses, improved weight gains and feed efficiency in feedlot cattle. J. Anim. Sci. 40:207–213. doi:10.2527/jas1975.402207x [DOI] [PubMed] [Google Scholar]
- Brown T. R. and Lawrence T. E.. 2010. Association of liver abnormalities with carcass grading performance and value. J. Anim. Sci. 88:4037–4043. doi:10.2527/jas.2010-3219 [DOI] [PubMed] [Google Scholar]
- Depenbusch B. E., Drouillard J. S., Loe E. R., Higgins J. J., Corrigan M. E., and Quinn M. J.. 2008. Efficacy of monensin and tylosin in finishing diets based on steam-flaked corn with and without corn wet distillers grains with solubles. J. Anim. Sci. 86:2270–2276. doi:10.2527/jas.2007-0017 [DOI] [PubMed] [Google Scholar]
- Heinemann W. W., Hanks E. M., and Young D. C.. 1978. Monensin and tylosin in a high energy diet for finishing steers. J. Anim. Sci. 47:34–40. doi:10.2527/jas1978.47134x [Google Scholar]
- Mathers J. J., Flick S. C., and Cox L. A. Jr. 2011. Longer-duration uses of tetracyclines and penicillins in U.S. Food-producing animals: indications and microbiologic effects. Environ. Int. 37:991–1004. doi:10.1016/j.envint.2011.01.014 [DOI] [PubMed] [Google Scholar]
- McKeith R. O., Gray G. D., Hale D. S., Kerth C. R., Griffin D. B., Savell J. W., Raines C. R., Belk K. E., Woerner D. R., Tatum J. D., et al. 2012. National beef quality audit-2011: harvest-floor assessments of targeted characteristics that affect quality and value of cattle, carcasses, and byproducts. J. Anim. Sci. 90:5135–5142. doi:10.2527/jas.2012-5477 [DOI] [PubMed] [Google Scholar]
- Meyer N. F., Erickson G. E., Klopfenstein T. J., Benton J. R., Luebbe M. K., and Laudert S. B.. 2013. Effects of monensin and tylosin in finishing diets containing corn wet distillers grains with solubles with differing corn processing methods. J. Anim. Sci. 91:2219–2228. doi:10.2527/jas.2011-4168 [DOI] [PubMed] [Google Scholar]
- Meyer N. F., Erickson G. E., Klopfenstein T. J., Greenquist M. A., Luebbe M. K., Williams P., and Engstrom M. A.. 2009. Effect of essential oils, tylosin, and monensin on finishing steer performance, carcass characteristics, liver abscesses, ruminal fermentation, and digestibility. J. Anim. Sci. 87:2346–2354. doi:10.2527/jas.2008-1493 [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G. and Lechtenberg K. F.. 2007. Liver abscesses in feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 23:351–369. doi:10.1016/j.cvfa.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Pendlum L. C., Boling J. A., and Bradley N. W.. 1978. Levels of monensin with and without tylosin for growing-finishing steers. J. Anim. Sci. 47:1–5. doi:10.2527/jas1978.4711400773 [Google Scholar]
- Potter E. L., Wray M. I., Muller R. D., Grueter H. P., McAskill J., and Young D. C.. 1985. Effect of monensin and tylosin on average daily gain, feed efficiency and liver abscess incidence in feedlot cattle. J. Anim. Sci. 61:1058–1065. [DOI] [PubMed] [Google Scholar]
- Reinhardt C. D. and Hubbert M. E.. 2015. Control of liver abscesses in feedlot cattle: a review. Prof. Anim. Sci. 31:101–108. doi:10.15232/pas.2014-01364 [Google Scholar]
- Rezac D. J., Thomson D. U., Bartle S. J., Osterstock J. B., Prouty F. L., and Reinhardt C. D.. 2014. Prevalence, severity, and relationships of lung lesions, liver abnormalities, and rumen health scores measured at slaughter in beef cattle. J. Anim. Sci. 92:2595–2602. doi:10.2527/jas.2013-7222 [DOI] [PubMed] [Google Scholar]
- Röder B., Frühwirth K., Vogl C., Wagner M., and Rossmanith P.. 2010. Impact of long-term storage on stability of standard DNA for nucleic acid-based methods. J. Clin. Microbiol. 48:4260–4262. doi:10.1128/JCM.01230-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sides G. E., Swingle R. S., Vasconcelos J. T., Borg R. C., and Moseley W. M.. 2009. Effect of feeding melengestrol acetate, monensin and tylosin on performance, carcass measurements, and liver abscesses of feedlot heifers. Prof. Anim. Sci. 25:459–464. doi:10.15232/S1080-7446(15)30744-0 [Google Scholar]
- Tan Z. L., Lechtenberg K. F., Nagaraja T. G., Chengappa M. M., and Brandt R. T. Jr. 1994. Serum neutralizing antibodies against fusobacterium necrophorum leukotoxin in cattle with experimentally induced or naturally developed hepatic abscesses. J. Anim. Sci. 72:502–508. doi:10.2527/1994.722502x [DOI] [PubMed] [Google Scholar]
- Teague H. S., Grifo A. P. Jr, and Rutledge E. A.. 1966. Response of growing-finishing swine to different levels and methods of feeding chlortetracycline. J. Anim. Sci. 25:693–700.doi:10.2527/jas1966.253693x [DOI] [PubMed] [Google Scholar]
- Vogel G. J., and Laudert S. B.. 1994. The influence of Tylan on liver abscess control and animal performance -A 40 Trial Summary. J. Anim. Sci. 72(Suppl. 1):293. (Abstr). [Google Scholar]
- Wasfy M., Oyofo B., Elgindy A., and Churilla A.. 1995. Comparison of preservation media for storage of stool samples. J. Clin. Microbiol. 33:2176–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration 2013. Guidance for Industry# 213: new animal drugs and new animal drug combination products administered in or on medicated feed or drinking water of food-producing animals: recommendations for drug sponsors for voluntarily aligning product use conditions with GFI# 209https://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM299624 (Accessed 28 August 2017.)
- US Food and Drug Administration. 2017. Code of federal regulations. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=558.625 (Accessed 4 May 2018).