Abstract
Steers exposed to an endotoxin may require additional branched-chain AA (BCAA) to support an increase in synthesis of immune proteins. This study evaluated effects of bacterial lipopolysaccharide (LPS) and BCAA supplementation on blood metabolites and N balance of 20 ruminally-cannulated steers (177 ± 4.2 kg BW). The experiment was a randomized block design, with 14-d adaptation to metabolism stalls and diet (DM fed = 1.5% BW) and 6-d collection. Treatments were a 2 × 2 factorial of LPS (0 vs. 1.0 to 1.5 μg/kg BW; −LPS vs. +LPS) and BCAA (0 vs. 35 g/d; −BCAA vs. +BCAA). The LPS in 100 mL sterile saline was infused (1 mL/min via i.v. catheter) on day 15. The BCAA in an essential AA solution were abomasally infused (900 mL/d) three times daily in equal portions beginning on day 7. Blood, rumen fluid, and rectal temperature were collected on day 15 at h 0, 2, 4, 8, 12, and 24 after LPS infusion. Feces and urine were collected from day 16 to 20. Rectal temperatures were greater for +LPS vs. –LPS steers at 4 h and lower at 8 h after LPS infusion (LPS × h, P < 0.01). Serum cortisol and plasma urea N were greater for +LPS than −LPS steers at 2 (cortisol only), 4, 8, 12, and 24 h after LPS infusion (LPS × h, P < 0.01). Serum cortisol was greater for +BCAA than −BCAA steers at 12 h after LPS infusion (BCAA × h, P < 0.05). Serum glucose was greater for +LPS than −LPS steers at 2 h after LPS infusion (LPS × h, P < 0.01). Plasma Ile, Leu, and Val were lower, and plasma His was greater in +LPS than −LPS steers (LPS, P < 0.05). Plasma Lys, Met, Thr, and Trp of +LPS steers were lower than −LPS steers at 4 (Thr only), 8 (Lys and Trp only), 12, and 24 h after infusion (LPS × h, P < 0.05). Plasma Ile, Leu, and Val were greater (BCAA, P < 0.01), and Met, His, Phe, Thr, and Trp were lower for +BCAA than −BCAA steers at 0 and 24 h after LPS infusion (BCAA × h, P ≤ 0.05). Steers receiving +LPS had lower rumen pH at 8 h, greater total VFA at 8 h, and lower rumen NH3 at 24 h after LPS infusion compared with −LPS steers (LPS × h, P ≤ 0.04). Total tract passage rates, DM, OM, NDF, ADF, and N intake, fecal N, digested N, and retained N were lower (P < 0.05) for +LPS than −LPS steers. Total N supply (dietary plus infused) and fecal N were greater (P < 0.05) for +BCAA vs. −BCAA steers. The absence of LPS × BCAA interactions (P ≥ 0.20) for N balance indicated that post-ruminal supplementation of BCAA did not alleviate the negative effects of endotoxin on N utilization by growing steers.
Keywords: branched-chain amino acids, endotoxin, steers
INTRODUCTION
Morbidity due to exposure of newly received feedlot calves to infectious agents, such as bovine respiratory disease complex, has negative impacts on performance and gross income (Waggoner et al., 2007). Clinical and metabolic changes in morbid cattle alter protein requirements (Cullor, 1992; Waggoner et al., 2009b), possibly due, in part, to repartitioning of AA away from tissue protein synthesis and toward synthesis of immune system proteins (Le Floc’h et al., 2004).
Waggoner et al. (2009a,b) and Carter et al. (2010) demonstrated that steers exposed to bacterial lipopolysaccharide (LPS) had lower plasma branched-chain AA (BCAA), which is indicative of an increased demand to support an activated immune system (Reeds and Jahoor, 2001). Toyosawa et al. (2004) demonstrated that BCAA supplementation increases survival of LPS-challenged mice, and Garcia-de-Lorenzo et al. (1997) reported lower mortality of septic patients receiving supplemental BCAA. Furthermore, dietary BCAA restrictions decrease lymphocyte proliferation in humans (Nuwer et al., 1983) and rats (Tsukishiro et al., 2000), and supplemental BCAA alter exercise-induced production of cytokines (Bassit et al., 2002). Although BCAA are used for energy and as precursors for synthesis of other AA (e.g., Gln) important for immune function (Calder and Yaqoob, 2004), Calder (2006) concluded that BCAA use for energy or AA does not account for all the immunological benefits, and the immune system has absolute requirements for BCAA likely for immune cell protein synthesis.
In addition to the direct effects of BCAA on immune cells, post-ruminal BCAA supplementation increases N retention of growing steers (Löest et al., 2001a) and lambs (Nolte et al., 2008). Therefore, we hypothesized that post-ruminal supplementation of BCAA will support an activated immune system and improve N retention of endotoxin-challenged steers. The objective was to study the interactions of bacterial LPS exposure and supplemental BCAA on blood metabolites, rumen fermentation, and N balance of growing steers.
MATERIALS AND METHODS
Animals, Facilities, and Experimental Design
Procedures were approved by the New Mexico State University Institutional Animal Care and Use Committee. Twenty ruminally-cannulated Angus steers (177 ± 4.2 kg initial BW) were housed on rubber mats (2.54 cm thick) in individual tie-stalls of a metabolism barn with evaporative cooling (19 ± 0.6 °C), artificial lighting, and automatic water troughs (Nelson Manufacturing, Cedar Rapids, IA). Steers were fed a diet (Table 1) in two equal portions twice daily at 0700 and 1900. Daily DM offered was limited to 1.5% of BW to represent intake of newly received feedlot calves (NRC, 2000) and to minimize potential differences in DM intake among treatments.
Table 1.
Composition of the diet
| Item | DM basis |
|---|---|
| Ingredient, % | |
| Wheat grain, ground | 30.0 |
| Corn silage | 21.3 |
| Alfalfa hay | 20.0 |
| Soybean hulls | 20.0 |
| Molasses | 4.0 |
| Tallow | 2.5 |
| Minerals1 | 1.83 |
| Urea | 0.30 |
| Vitamins2 | 0.04 |
| Ruminsin-803 | 0.02 |
| Nutrient concentration | |
| NDF, % | 40.6 |
| ADF, % | 27.4 |
| CP, % | 14.3 |
| Ether extract, % | 4.94 |
| Ca4, % | 0.87 |
| P4, % | 0.39 |
| Fe4, mg/kg | 286 |
| Zn4, mg/kg | 67.8 |
| Cu4, mg/kg | 19.5 |
| Se4, mg/kg | 0.39 |
| ME5, Mcal/kg | 2.36 |
| NEm6, Mcal/kg | 1.48 |
| NEg7, Mcal/kg | 0.89 |
1Supplied (% of DM): limestone (0.50), dicalcium phosphate (0.50), sodium bicarbonate (0.50), salt (0.30), zinc sulfate (0.009), copper sulfate (0.004), and sodium selenite (0.000009).
2Supplied 1,500 IU of vitamin A and 100 IU of vitamin E per kg of diet DM.
3Supplied 33 mg monensin per kg of diet DM.
4Analyzed by SDK Laboratories, Huchinson, KS.
5ME, Mcal/kg = (4.103 − 0.0446 × %ADF) × 0.82 (Harlan et al., 1991).
6NEm, Mcal/kg = 1.37 × ME − 0.138 × ME2 + 0.0105 × ME3 − 1.12 (NRC, 2000).
7NEg, Mcal/kg = 1.42 × ME − 0.174 × ME2 + 0.0122 × ME3 − 1.65 (NRC, 2000).
The experiment was a randomized complete block design. The metabolism facility contained 12 tie-stalls, and steers were blocked by date of collection (8 steers in block 1, and 12 steers in block 2). Within each block, steers were allowed 14 d to adapt to the diet (Table 1) and metabolism facilities before the beginning of a 6-d collection period (day 15 to 20). The collection period consisted of 1 d for collection of blood and rumen fluid, and 5 d for collection of total feces and urine. On day 7, a flexible tube (3.2 mm i.d.; Tygon, Cole-Parmenter Instrument Co., Vernon Hills, IL) was inserted through the rumen cannula and reticulo-omasal orifice, and secured in the abomasum with a rubber flange (9-cm diameter) for daily infusion of AA treatments. On day 14, an indwelling jugular catheter (J-457A; Jorgenson Laboratories, Loveland, CO) was inserted for the infusion of LPS treatments and collection of blood samples.
Treatments
Treatments were arranged as a 2 × 2 factorial, and included two doses of LPS (0 vs. 1.0 to 1.5 μg/kg BW; −LPS vs. +LPS) and two amounts of BCAA (0 vs. 35 g/d; −BCAA vs. +BCAA). For the +LPS treatment, LPS (E. coli 055:B5; Sigma Chem. Co., St. Louis, MO) was dissolved in 100 mL of sterile saline and infused (1 mL/min via i.v. catheter) at 3 h after feeding on day 15. The dose of LPS was initially 1.5 µg/kg of BW for block 1, but after the death of a steer the LPS dose was lowered to 1.0 µg/kg of BW for block 2. For the −LPS treatment, steers were infused (1 mL/min via i.v. catheter) with 100 mL of sterile saline. For the −BCAA treatment, steers received abomasal infusions of an AA solution that supplied 5 g L-Arg, 5 g L-His, 10 g L-Lys, 5 g L-Met, 5 g L-Phe, 5 g L-Thr, and 2.5 g L-Trp daily. These amounts of each essential AA for the −BCAA infusions were 50% less than that used by Löest et al. (2001a), and were estimated to increase the metabolizable supply of each AA by at least 45%. The metabolizable AA supply from the basal diet was calculated using NRC (2000) estimates of the AA profiles for RUP of dietary ingredients (Table 1) and microbial protein synthesis (i.e., 13% of dietary TDN intake) when 177-kg steers were fed at 1.5% of BW (DM basis). For the +BCAA treatment, steers received abomasal infusions of 10 g L-Ile, 15 g L-Leu, and 10 g L-Val in addition to the −BCAA solution, which was estimated to increase the metabolizable supply of Ile, Leu, and Val by 80%, 70%, and 72%, respectively.
The AA solutions were prepared by dissolving L-Arg, L-His, L-Lys, L-Met, L-Phe, L-Thr, and L-Trp in 300 mL of deionized water. In a separate container, L-Ile, L-Leu, and L-Val were dissolved in 500 mL deionized water containing 18 mL of 6 M HCl. These two AA solutions were mixed together for the +BCAA treatment, whereas the BCAA solution was not added for the −BCAA treatment. Both the −BCAA and +BCAA solutions were adjusted to a pH of 5.0 with the addition of NaOH, and then brought to a final volume of 900 mL with deionized water. Both the −BCAA and +BCAA treatments were infused (900 mL/d) into the abomasum of steers in equal portions three times a day (0900, 1500, and 2100) from day 7 to 20 of the experiment.
Sample Collections
Samples of the diet were collected on day 15 through 19 and total feed refusals were obtained in correspondence with fecal and urine collections on day 16 through 20. For collection of urine, steers were individually fitted with rubber urine pouches (Itran-Tompkins, South Plainfield, NJ) that were connected with rubber vacuum tubing (12.7 mm i.d.; Fisher Scientific, Pittsburgh, PA) to 20-L vessels (C14907, Nasco, Modesto, CA). The urine collection vessels contained 600 mL of 3 M HCl to minimize volatilization of NH3, and were connected with rubber vacuum tubing (6.35 mm i.d.) to a vacuum pump (DOA-P704-AA, Gast, Benton Harbor, MI). For collection of feces, steers were fitted with canvas fecal collection bags (Awning & Canvas Specialty, El Paso, TX) that were secured using a harness around the girth and shoulders. Total feces and urine output was weighed, the weight recorded, and representative samples of feces (10%) and urine (1%) were stored at −20 °C for later analysis. To determine total gastrointestinal passage rates, 360 g of diet containing Cr-EDTA and 120 g of Yb-labeled diet were fed to steers at approximately 30 min before the 0700 feeding on day 15. Samples of the basal diet (Table 1) were used to prepare the Cr-EDTA and Yb-labeled feed as described by Waggoner et al. (2009a). Fecal samples were retrieved directly from the rectum at 24, 48, 72, 96, and 120 h after the diets with markers were fed, and then stored at −20 °C for later analysis. The weight of fecal samples retrieved directly from the rectum was included in the calculation of total fecal excretion.
On day 15, samples of blood and rumen fluid were collected 30 min before LPS infusion and 2, 4, 8, 12, and 24 h after LPS infusion. Blood samples were collected via the indwelling jugular catheter into blood vacuum tubes (Corvac Serum Separator and Monoject Sodium Heparin, Kendall, Ontario, CA). Blood samples for serum were allowed to coagulate at room temperature for 30 min, and samples for plasma were immediately chilled on ice. All blood samples were centrifuged (Sorvall RT600B, Thermo Electron Corp., Asheville, NC) at 1,500 × g for 20 min at 10 °C, and the supernatant was transferred to 6-mL sample vials (Cole-Parmer, Vernon Hills, IL) and stored at −20 °C. Rectal temperature of each steer was measured (Cooper TM99A digital thermometer, Cooper Atkins Corp., Middlefield, CT) at times similar to blood collection. Rumen fluid (approximately 100 mL) was collected via a suction strainer (Precision Machine Co., Inc, Lincoln, NE) that was passed through the rumen cannula (Lodge Ivey et al., 2009). The pH of the rumen fluid was immediately recorded using a portable pH meter (Accumet AP72, Fisher Scientific, Pittsburgh, PA), and an 8-mL sample of rumen fluid was added to 20 mL vials containing 2 mL of 25% (w/v) meta-phosphoric acid solution (Löest et al., 2001b) and frozen at −20 °C for later analysis.
Sample Analysis
Frozen samples of diet, feed refusals, and feces were allowed to thaw, and then composited for each steer before analysis. Samples were dried at 55 °C in a forced-air oven (Model #POM-326F Blue M Electric Company, Blue Island, IL) for 96 h, then allowed to air-equilibrate for 48 h, weighed to determine moisture loss, and then ground in a Wiley mill (Model 4, Thomas Scientific, Swedesboro, NJ) to pass a 2-mm screen. Ground samples were dried in a convection oven (Model 845, Precision Scientific Group, Chicago, IL) at 105 °C for 24 h and weighed to determine DM, and then placed in a muffle furnace (Model F-A1730, Thermolyne Corp., Dubuque, IA) at 550 °C for 8 h to determine ash. Ground samples of diet, orts, and feces were analyzed for NDF (ANKOM 200; ANKOM Technology Corp., Fairport, NY) with the use of heat-stable α-amylase, and without correction for residual ash. Also, ground samples of diet, orts, and feces, and urine samples were analyzed for N by total combustion and detection with a thermo-conductivity cell (LECO FP-528, LECO Corp., St. Joseph, MI).
Serum concentrations of cortisol (Kiyma et al., 2004) and insulin (Reimers et al., 1982) were analyzed by solid-phase RIA with intra- and inter-assay CV < 7.8%, and serum glucose concentrations were determined colorimetrically using a commercially available hexokinase reagent (Infinity TR15241, Thermo Scientific, Waltham, MA) as described by Waggoner et al. (2009b). Plasma AA concentrations were analyzed by gas chromatography (Varian CP-3800, Varian, Walnut Creek, CA) using a commercially available kit (EZ:FAAST; Phenomenex, Torrance, CA) as described by Waggoner et al. (2009a). Plasma AA assay CV were less than 15%. Ammonia concentrations were determined in rumen fluid using procedures described by Broderick and Kang (1980) modified for a plate reader (ELX 808 Ultra Microplate Reader, Bio-Tek Instruments Inc., Winooski, VT), and VFA concentrations were determined by capillary gas chromatography (Varian 3400; Varian Inc., Walnut Creek, CA) in accordance to the procedures by May and Galyean (1996). Concentrations of Cr and Yb were determined in fecal samples retrieved from the rectum by inductively coupled plasma spectrometry (Optima 4300; Perkin Elmer, Wellesley, MA) as described by Waggoner et al. (2009a). The slopes of the natural log of Yb (48 to 120 h) and Cr (24 to 96 h) concentrations regressed against hour were used to determine solid and liquid passage rates (%/h), respectively. Urinary Nτ-methylhistidine (NMH) was determined by gas chromatography mass spectrometry as described by Rathmacher et al. (1992). The internal standard (3-methyl-[methyl-2H3]-histidine) was added to urine, acidified, and absorbed onto cation exchange columns. It was then eluted from columns, dried, and derivatized with N-methyl-N-[tert-butyldimethylsilyl]trifluoroacetamide (Regis Technologies Inc., Morton Grove, IL) for gas chromatography mass spectrometry (Model 5890/5970B, Hewlett-Packard, Avondale, PA). The NMH and internal standard were monitored at 238.2 and 241.2 atomic mass units, respectively. To determine urea N in plasma and urine, 4 µL samples and 200 µL of urease reagent (Infinity TR12421, Thermo Scientific, Waltham, MA) were placed into 96-well plates. The 96-well plates were placed on a shaker for 15 s, incubated for 5 min at 37 °C, allowed to cool for 5 min at 4 °C, and then read on a microplate reader (Biotek ELx808, Biotek Instruments Inc., Winooski, VT) at 340 nm.
Statistical Analysis
The experiment was a randomized complete block design; the metabolism facility had only 12 tie-stalls; therefore, steers were blocked by date of sample collections (8 steers in block 1, and 12 steers in block 2). The minimum number of replicates per treatment required to detect (P < 0.05; 1 − β = 0.95) a 25% difference in N retention (the major response variable of interest) was determined by using error variance from results published by Waggoner et al. (2009b).
Data were analyzed using mixed models (SAS Inst. Inc., Cary, NC), and the experimental unit was steer. Data were missing for two experimental units (one in each block) because two steers receiving the +LPS and +BCAA treatment combination were removed from the experiment and later died due to severe respiratory reactions to the LPS infusions. Because the dose of LPS in block 2 differed from block 1, data were initially analyzed with block as a fixed effect in the model to determine if lowering the LPS dose from 1.5 µg/kg of BW in block 1 to 1.0 µg/kg of BW in block 2 affected responses to LPS. No block × LPS × BCAA interactions occurred (P ≥ 0.12), and therefore all data were analyzed with block as random. For all dietary measures, the statistical model included BCAA, LPS, and BCAA × LPS interaction as fixed effects. Rectal temperature, blood metabolites, and rumen fermentation measures were analyzed as repeated measures, and the model included BCAA, LPS, hour, and interactions for BCAA, LPS, and hour. The covariance structure was autoregressive order(1). Means were least squares, and significance was declared at P ≤ 0.05 and tendency at 0.05 < P < 0.10.
RESULTS
Effects of LPS × BCAA Interactions
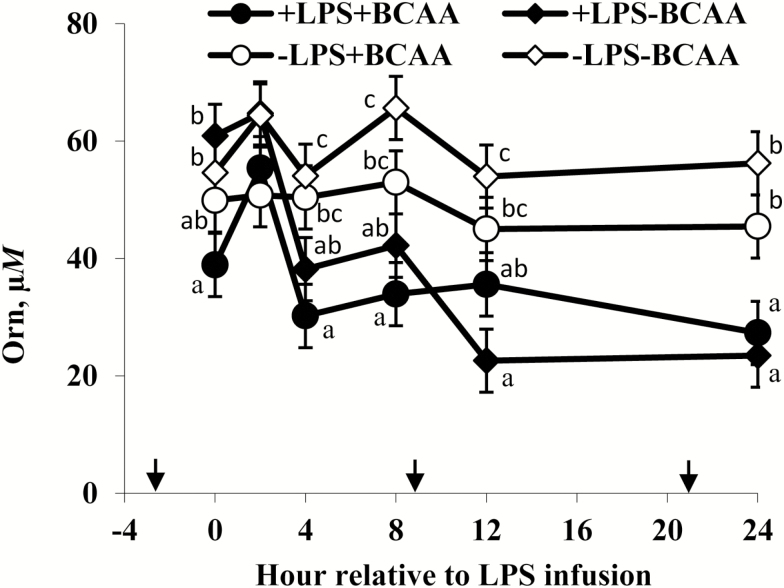
No LPS × BCAA × h interactions (P ≥ 0.10) and no LPS × BCAA interactions (P ≥ 0.10) were observed for rectal temperature, serum concentrations of cortisol, insulin, and glucose, plasma concentrations of essential and nonessential AA (except Orn and Ser), rumen concentrations of total VFA and NH3, and rumen pH. A LPS × BCAA × h interaction (P = 0.03) occurred for plasma Orn (Figure 1); plasma Orn tended to be lower for steers supplemented with +BCAA than –BCAA before LPS infusion, but after LPS infusion plasma Orn of +LPS steers decreased from 2 to 4 h and were lower than –LPS steers at 24 h. A tendency for an LPS × BCAA interaction (P = 0.06) occurred for plasma Ser; plasma Ser of −LPS steers tended to be lower for +BCAA (51.9 ± 4.4 µM) than –BCAA (70.7 ± 4.4 µM), whereas plasma Ser of +LPS steers were not different between +BCAA (38.9 ± 4.6 µM) and –BCAA (39.1 ± 4.4 µM; data not shown). Similarly, a tendency for an LPS × BCAA interaction (P = 0.08) occurred for plasma urea N; plasma urea N of −LPS steers were not different between +BCAA (6.01 ± 0.62 mg/dL) and −BCAA (6.89 ± 0.62 mg/dL), whereas urea N of +LPS steers tended to be greater for +BCAA (8.38 ± 0.64 mg/dL) than −BCAA (7.29 ± 0.62 mg/dL; data not shown). No LPS × BCAA interactions (P ≥ 0.20) were observed for intake and apparent digestibility of DM, OM, NDF, and ADF, N balance, urinary NMH, and total gastrointestinal tract passage of Cr and Yb.
Figure 1.
Plasma ornithine (Orn) concentrations of steers in response to lipopolysaccharide (LPS) and branched-chain AA (BCAA). Treatments were a 2 × 2 factorial arrangement of intravenous infusion (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusion of an essential AA solution that supplied either 0 or 35 g/d branched-chain AA (−BCAA vs. +BCAA). Effects for plasma Orn were LPS × BCAA × h (P = 0.03), LPS × h (P < 0.01), BCAA × h (P = 0.11), LPS × BCAA (P = 0.62), LPS (P < 0.01), and BCAA (P = 0.08). a,b,cMeans at specific hour with different letters differ (P < 0.05). Arrows on x-axis indicate time of feeding, and error bars are SEM.
Effects of LPS Infusion
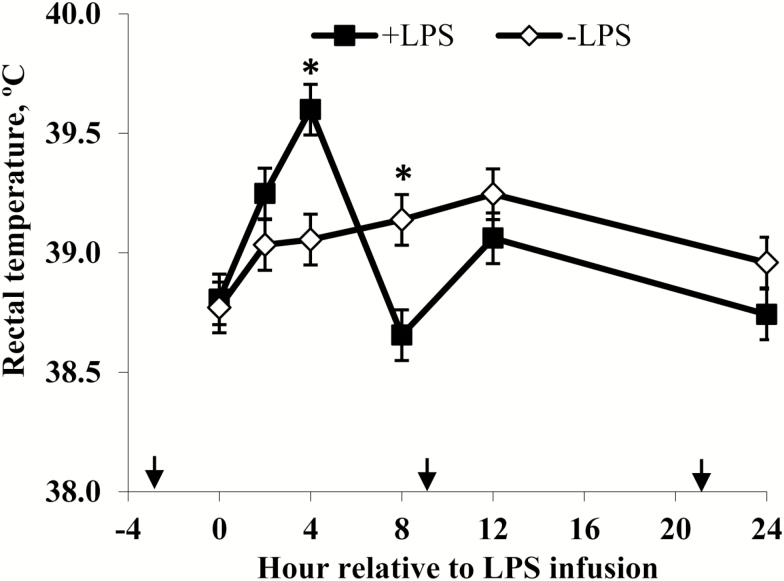
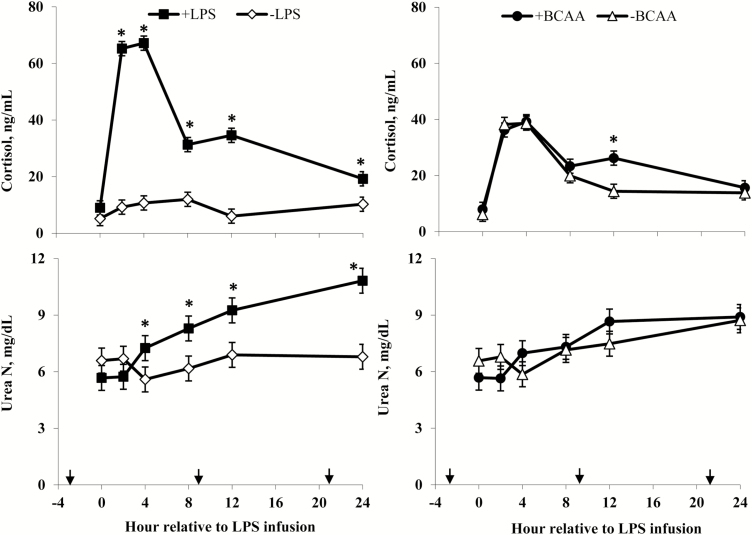
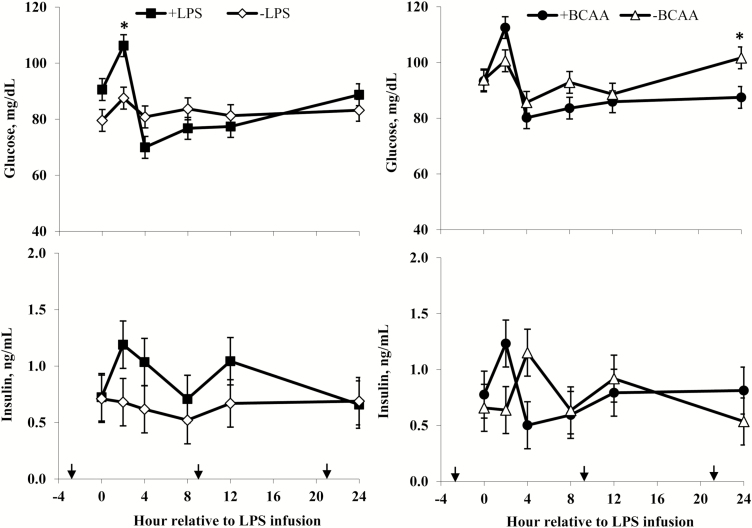
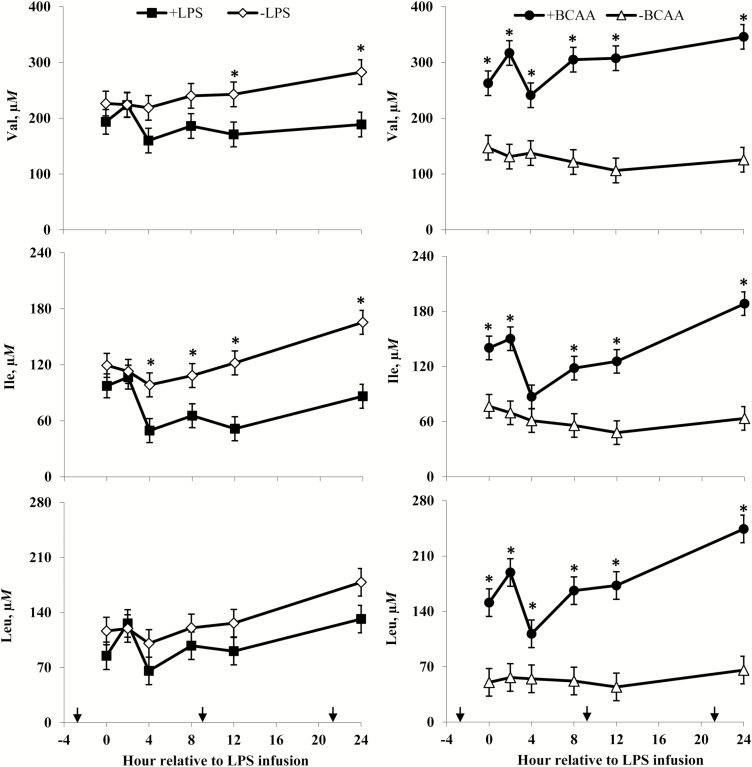
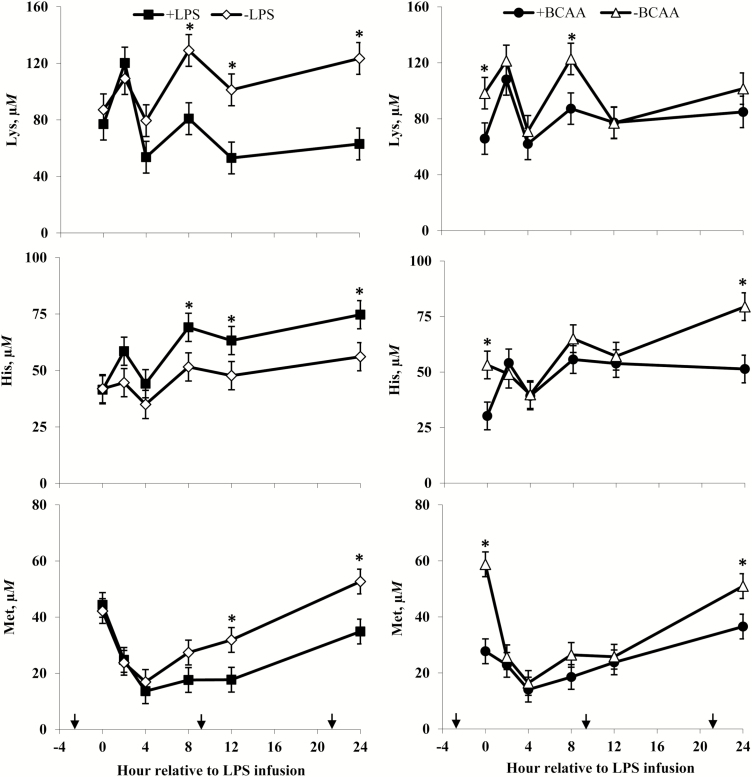
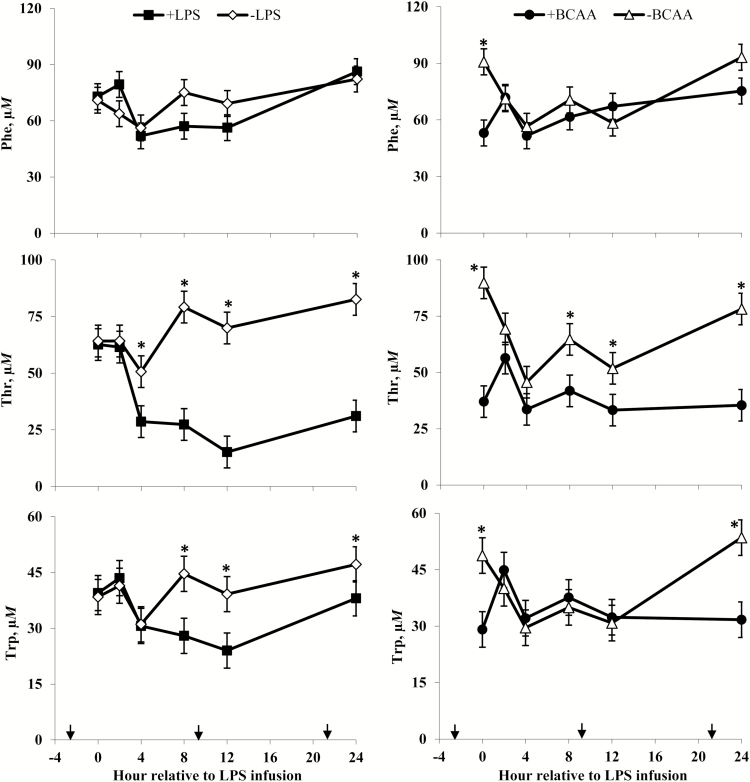
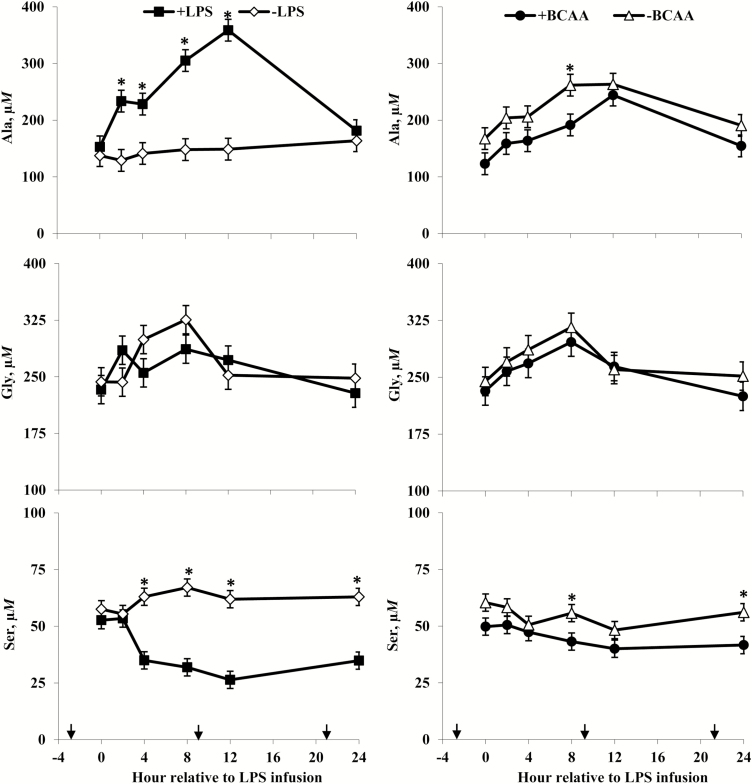
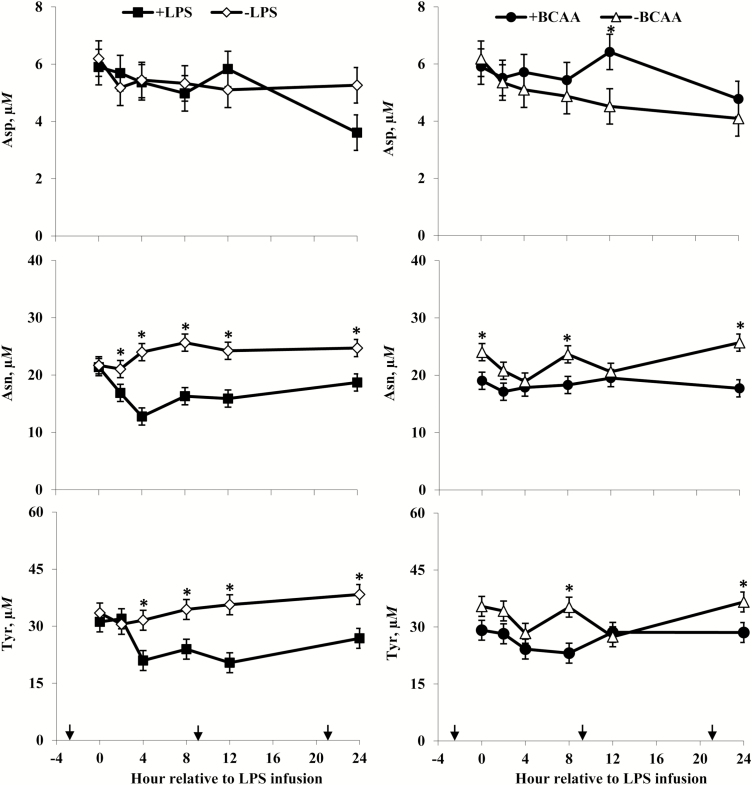
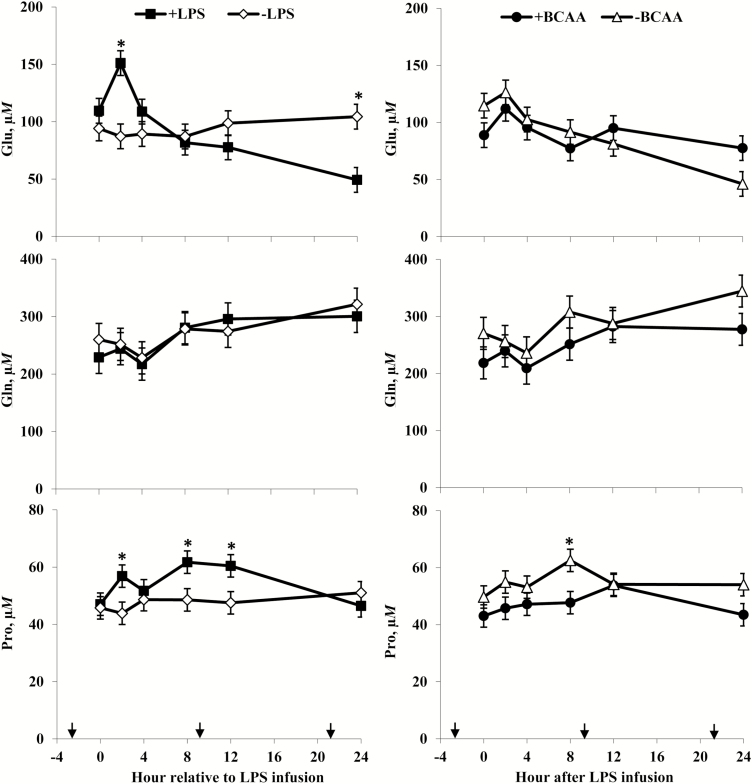
Rectal temperatures (Figure 2) of +LPS steers increased from 0 to 4 h and were greater than –LPS steers at 4 h after LPS infusion, then decreased 4 to 8 h and were lower than –LPS steers at 8 h; rectal temperatures were not different between +LPS and –LPS at 12 and 24 h after LPS infusion (LPS × h, P < 0.01). Serum cortisol concentrations (Figure 3) of +LPS steers increased from 0 to 2 h after LPS infusion, peaked at h 4, then decreased from 4 to 8 h but remained greater for +LPS than −LPS steers at 8, 12, and 24 h (LPS × h, P < 0.01). Plasma urea-N concentrations (Figure 3) were not different between +LPS and –LPS at 0 and 2 h, but increased in +LPS steers from 2 to 4 h and were greater than –LPS steers at 4, 8, 12, and 24 h after LPS infusion (LPS × h, P < 0.01). Serum glucose concentration (Figure 4) of +LPS steers increased from 0 to 2 h and were greater than –LPS steers at 2 h, but then decreased from 2 to 4 h and were not different between +LPS and –LPS steers at 4, 8, 12, and 24 h after LPS infusion (LPS × h, P < 0.01). Serum insulin concentrations (Figure 4) tended to be greater for +LPS than –LPS steers (LPS, P = 0.08). Plasma Val, Ile, and Leu concentrations (Figure 5) of +LPS steers were lower than −LPS steers (LPS, P < 0.05), and plasma His concentrations (Figure 6) were greater for +LPS than −LPS steers (LPS, P < 0.01). Plasma Lys and Met concentrations (Figure 6) were not different between +LPS and –LPS steers at 0, 2, and 4 h, but were lower for +LPS than –LPS steers at 8 (Lys only), 12, and 24 h after LPS infusion (LPS × h, P < 0.05). Plasma Thr and Trp concentrations (Figure 7) were not different between +LPS and –LPS steers at 0 and 2 h, but were lower for +LPS than –LPS steers at 4 (Thr only), 8, 12, and 24 h after LPS infusion (LPS × h, P < 0.05). Plasma Phe concentrations (Figure 7) tended to be lower at 8 h after infusion of LPS (LPS × h, P = 0.07). Plasma Ala concentrations (Figure 8) were not different at 0 h, but increased for +LPS steers from 0 to 2 h and were greater at 2, 4, 8, and 12 h (peak), then decreased and were not different than –LPS steers at 24 h after LPS infusion (LPS × h, P < 0.01). Plasma Gly concentrations (Figure 8) tended to be greater at 2 h, and lower at 4 and 8 h after LPS infusion for +LPS compared with –LPS steers (LPS × h, P < 0.01). Plasma Ser concentrations (Figure 8), as well as plasma Asn and Tyr concentrations (Figure 9) were not different between +LPS and –LPS steers at 0 h, and were lower for +LPS than −LPS steers at 2 (Asn only), 4, 8, 12, and 24 h after LPS infusion (LPS × h, P < 0.01). Infusion of LPS did not affect plasma Asp concentrations (LPS × h, P = 0.39). Plasma Glu (Figure 10) of +LPS steers increased from 0 to 2 h and was greater than –LPS steers at 2 h, then decreased and was not different at 4, 8, and 12 h, but lower for +LPS than –LPS steers at 24 h after LPS infusion (LPS × h, P < 0.01). Infusion of LPS did not affect (LPS × h, P = 0.41) plasma Gln concentrations (Figure 10). Plasma Pro concentrations (Figure 10) were not different at 0, 4, and 24 h, but were greater at 2, 8, and 12 h after LPS infusion for +LPS than –LPS steers.
Figure 2.
Rectal temperature of steers in response to lipopolysaccharide (LPS). Treatments were a 2 × 2 factorial arrangement of intravenous infusion (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusion of an essential AA solution that supplied either 0 or 35 g/d branched-chain AA (−BCAA vs. +BCAA). Effects for rectal temperature were LPS × BCAA × h (P = 0.59), LPS × h (P < 0.01), BCAA × h (P = 0.71), LPS × BCAA (P = 0.60), LPS (P = 0.88), and BCAA (P = 0.23). *P < 0.05 between treatments at specific hour. Arrows on x-axis indicate time of feeding, and error bars are SEM.
Figure 3.
Serum cortisol and plasma urea N of steers in response to lipopolysaccharide (LPS) and branched-chain AA (BCAA). Treatments were a 2 × 2 factorial arrangement of intravenous infusion (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusion of an essential AA solution that supplied either 0 or 35 g/d BCAA (−BCAA vs. +BCAA). Effects for serum cortisol were LPS × BCAA × h (P = 0.24), LPS × h (P < 0.01), BCAA × h (P = 0.05), LPS × BCAA (P = 0.42), LPS (P < 0.01), and BCAA (P = 0.23). Effects for plasma urea N were LPS × BCAA × h (P = 0.20), LPS × h (P < 0.01), BCAA × h (P = 0.07), LPS × BCAA (P = 0.08), LPS (P = 0.02), and BCAA (P = 0.85). *P < 0.05 between treatments at specific hour. Arrows on x-axis indicate time of feeding, and error bars are SEM.
Figure 4.
Serum glucose and insulin of steers in response to lipopolysaccharide (LPS) and branched-chain AA (BCAA). Treatments were a 2 × 2 factorial arrangement of intravenous infusion (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusion of an essential AA solution that supplied either 0 or 35 g/d BCAA (−BCAA vs. +BCAA). Effects for serum glucose were LPS × BCAA × h (P = 0.10), LPS × h (P < 0.01), BCAA × h (P = 0.05), LPS × BCAA (P = 0.65), LPS (P = 0.42), and BCAA (P = 0.28). Effects for serum insulin were LPS × BCAA × h (P = 0.21), LPS × h (P = 0.80), BCAA × h (P = 0.15), LPS × BCAA (P = 0.44), LPS (P = 0.08), and BCAA (P = 0.83). *P < 0.05 between treatments at specific hour. Arrows on x-axis indicate time of feeding, and error bars are SEM.
Figure 5.
Plasma valine (Val), isoleucine (Ile), and leucine (Leu) concentrations of steers in response to lipopolysaccharide (LPS) and branched-chain AA (BCAA). Treatments were a 2 × 2 factorial arrangement of intravenous infusion (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusion of an essential AA solution that supplied either 0 or 35 g/d BCAA (−BCAA vs. +BCAA). Effects for Val were LPS × BCAA × h (P = 0.86), LPS × h (P = 0.40), BCAA × h (P = 0.05), LPS × BCAA (P = 0.26), LPS (P < 0.01), and BCAA (P < 0.01). Effects for Ile were LPS × BCAA × h (P = 0.56), LPS × h (P = 0.06), BCAA × h (P = 0.02), LPS × BCAA (P = 0.13), LPS (P < 0.01), and BCAA (P < 0.01). Effects for Leu were LPS × BCAA × h (P = 0.66), LPS × h (P = 0.75), BCAA × h (P = 0.05), LPS × BCAA (P = 0.14), LPS (P = 0.02), and BCAA (P < 0.01). *P < 0.05 between treatments at specific hour. Arrows on x-axis indicate time of feeding, and error bars are SEM.
Figure 6.
Plasma lysine (Lys), histidine (His), and methionine (Met) concentrations of steers in response to lipopolysaccharide (LPS) and branched-chain AA (BCAA). Treatments were a 2 × 2 factorial arrangement of intravenous infusion (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusion of an essential AA solution that supplied either 0 or 35 g/d BCAA (−BCAA vs. +BCAA). Effects for Lys were LPS × BCAA × h (P = 0.79), LPS × h (P < 0.01), BCAA × h (P = 0.40), LPS × BCAA (P = 0.65), LPS (P < 0.01), and BCAA (P = 0.04). Effects for His were LPS × BCAA × h (P = 0.88), LPS × h (P = 0.20), BCAA × h (P < 0.01), LPS × BCAA (P = 0.30), LPS (P < 0.01), and BCAA (P < 0.01). Effects for Met were LPS × BCAA × h (P = 0.59), LPS × h (P = 0.04), BCAA × h (P < 0.01), LPS × BCAA (P = 0.12), LPS (P = 0.06), and BCAA (P = 0.01). *P < 0.05 between treatments at specific hour. Arrows on x-axis indicate time of feeding, and error bars are SEM.
Figure 7.
Plasma phenylalanine (Phe), threonine (Thr), and tryptophan (Trp) concentrations of steers in response to lipopolysaccharide (LPS) and branched-chain AA (BCAA). Treatments were a 2 × 2 factorial arrangement of intravenous infusion (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusion of an essential AA solution that supplied either 0 or 35 g/d BCAA (−BCAA vs. +BCAA). Effects for Phe were LPS × BCAA × h (P = 0.86), LPS × h (P = 0.07), BCAA × h (P < 0.01), LPS × BCAA (P = 0.78), LPS (P = 0.72), and BCAA (P = 0.13). Effects for Thr were LPS × BCAA × h (P = 0.25), LPS × h (P < 0.01), BCAA × h (P < 0.01), LPS × BCAA (P = 0.29), LPS (P < 0.01), and BCAA (P < 0.01). Effects for Trp were LPS × BCAA × h (P = 0.81), LPS × h (P = 0.01), BCAA × h (P < 0.01), LPS × BCAA (P = 0.64), LPS (P = 0.24), and BCAA (P = 0.35). *P < 0.05 between treatments at specific hour. Arrows on x-axis indicate time of feeding, and error bars are SEM.
Figure 8.
Plasma alanine (Ala), glycine (Gly), and serine (Ser) concentrations of steers in response to lipopolysaccharide (LPS) and branched-chain AA (BCAA). Treatments were a 2 × 2 factorial arrangement of intravenous infusion (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusion of an essential AA solution that supplied either 0 or 35 g/d BCAA (−BCAA vs. +BCAA). Effects for Ala were LPS × BCAA × h (P = 0.48), LPS × h (P < 0.01), BCAA × h (P = 0.69), LPS × BCAA (P = 0.99), LPS (P < 0.01), and BCAA (P = 0.05). Effects for Gly were LPS × BCAA × h (P = 0.85), LPS × h (P < 0.01), BCAA × h (P = 0.86), LPS × BCAA (P = 0.12), LPS (P = 0.71), and BCAA (P = 0.54). Effects for Ser were LPS × BCAA × h (P = 0.37), LPS × h (P < 0.01), BCAA × h (P = 0.26), LPS × BCAA (P = 0.06), LPS (P < 0.01), and BCAA (P = 0.05). *P < 0.05 between treatments at specific hour. Arrows on x-axis indicate time of feeding, and error bars are SEM.
Figure 9.
Plasma aspartate (Asp), asparagine (Asn), and tyrosine (Tyr) concentrations of steers in response to lipopolysaccharide (LPS) and branched-chain AA (BCAA). Treatments were a 2 × 2 factorial arrangement of intravenous infusion (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusion of an essential AA solution that supplied either 0 or 35 g/d BCAA (−BCAA vs. +BCAA). Effects for Asp were LPS × BCAA × h (P = 0.35), LPS × h (P = 0.39), BCAA × h (P = 0.51), LPS × BCAA (P = 0.79), LPS (P = 0.72), and BCAA (P = 0.27). Effects for Asn were LPS × BCAA × h (P = 0.74), LPS × h (P < 0.01), BCAA × h (P = 0.09), LPS × BCAA (P = 0.10), LPS (P < 0.01), and BCAA (P < 0.01). Effects for Tyr were LPS × BCAA × h (P = 0.73), LPS × h (P < 0.01), BCAA × h (P = 0.07), LPS × BCAA (P = 0.69), LPS (P < 0.01), and BCAA (P = 0.02). *P < 0.05 between treatments at specific hour. Arrows on x-axis indicate time of feeding, and error bars are SEM.
Figure 10.
Plasma glutamate (Glu), glutamine (Gln), and proline (Pro) concentrations of steers in response to lipopolysaccharide (LPS) and branched-chain AA (BCAA). Treatments were a 2 × 2 factorial arrangement of intravenous infusion (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusion of an essential AA solution that supplied either 0 or 35 g/d BCAA (−BCAA vs. +BCAA). Effects for Glu were LPS × BCAA × h (P = 0.14), LPS × h (P < 0.01), BCAA × h (P = 0.13), LPS × BCAA (P = 0.49), LPS (P = 0.72), and BCAA (P = 0.35). Effects for Gln were LPS × BCAA × h (P = 0.63), LPS × h (P = 0.41), BCAA × h (P = 0.12), LPS × BCAA (P = 0.16), LPS (P = 0.67), and BCAA (P = 0.05). Effects for Pro were LPS × BCAA × h (P = 0.50), LPS × h (P = 0.02), BCAA × h (P = 0.33), LPS × BCAA (P = 0.55), LPS (P = 0.12), and BCAA (P = 0.06). *P < 0.05 between treatments at specific hour. Arrows on x-axis indicate time of feeding, and error bars are SEM.
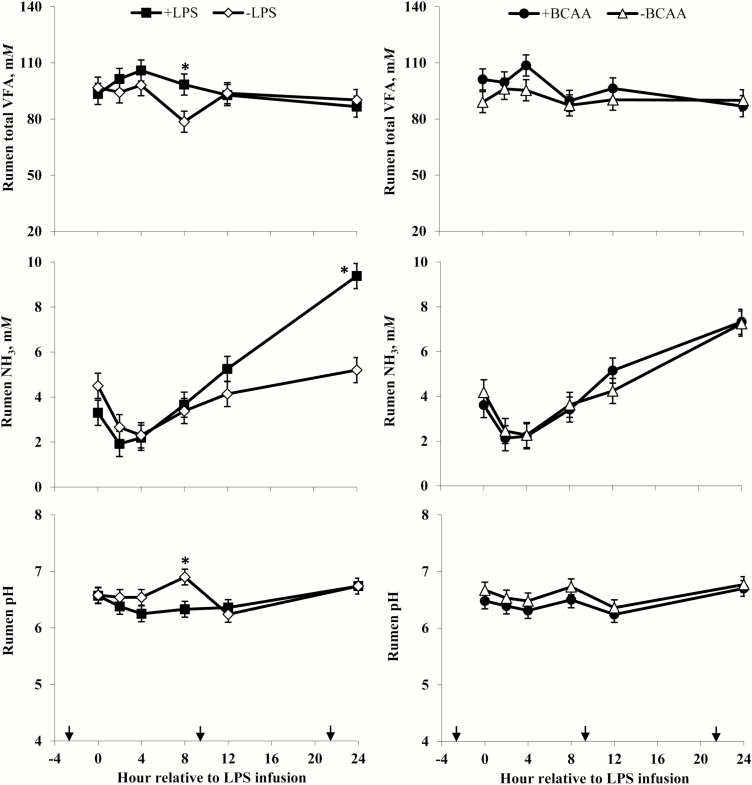
Ruminal pH (Figure 11) was not different between +LPS and –LPS steers at 0, 2, and 4 h, lower for +LPS than −LPS steers at 8 h, and not different between LPS treatments at 12 and 24 h after LPS infusion (LPS × h; P < 0.01). Ruminal total VFA concentrations (Figure 11) were not different between +LPS and –LPS steers at 0, 2, and 4 h, greater for +LPS than −LPS steers at 8 h, and not different between LPS treatments at 12 and 24 h after LPS infusion (LPS × h; P = 0.04). Ruminal NH3 concentrations (Figure 11) were not different between +LPS and –LPS steers at 0, 2, 4, 8, and 12 h, but increased and were greater for +LPS than –LPS steers at 24 h after LPS infusion (LPS × h; P < 0.01).
Figure 11.
Rumen total VFA, NH3, and pH of steers in response to lipopolysaccharide (LPS) and branched-chain AA (BCAA). Treatments were a 2 × 2 factorial arrangement of intravenous infusion (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusion of an essential AA solution that supplied either 0 or 35 g/d BCAA (−BCAA vs. +BCAA). Effects for VFA were LPS × BCAA × h (P = 0.90), LPS × h (P = 0.04), BCAA × h (P = 0.34), LPS × BCAA (P = 0.74), LPS (P = 0.28), and BCAA (P = 0.17). Effects for rumen NH3 were LPS × BCAA × h (P = 0.37), LPS × h (P < 0.01), BCAA × h (P = 0.79), LPS × BCAA (P = 0.34), LPS (P = 0.23), and BCAA (P = 0.95). Effects for rumen pH were LPS × BCAA × h (P = 0.56), LPS × h (P < 0.01), BCAA × h (P = 0.96), LPS × BCAA (P = 0.60), LPS (P = 0.10), and BCAA (P = 0.10). *P < 0.05 between treatments at specific hour. Arrows on x-axis indicate time of feeding, and error bars are SEM.
Steers infused with LPS had lower dietary intakes of DM, OM, NDF, and ADF (LPS, P ≤ 0.03) and lower total tract solid and liquid passage rates (LPS, P ≤ 0.02) compared with –LPS steers (Table 2). Apparent DM, OM, NDF, and ADF digestibility of steers was not altered by LPS infusion (LPS, P ≥ 0.11). Nitrogen intake, fecal N, digested N, and retained N were lower for +LPS vs. –LPS steers (LPS, P < 0.05). Urinary N excretion, apparent N digestibility (as a percentage of total N supply), and N retention (as a percentage of total N supply) were not different among LPS treatments (LPS, P ≥ 0.11). Urinary NMH excretion per unit of BW was greater for +LPS than –LPS steers (LPS, P = 0.05).
Table 2.
Dietary intake, apparent digestibility, N balance, and total tract passage rate of growing steers in response to i.v. infusion of LPS and abomasal infusion of BCAA
| Item | Treatment1 | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| −LPS | +LPS | |||||||
| −BCAA | +BCAA | −BCAA | +BCAA | LPS×BCAA | LPS | BCAA | ||
| n | 5 | 5 | 5 | 3 | ||||
| Intake, kg/d | ||||||||
| DM | 2.49 | 2.62 | 1.77 | 2.31 | 0.19 | 0.27 | 0.01 | 0.09 |
| OM | 2.30 | 2.41 | 1.63 | 2.13 | 0.18 | 0.27 | 0.01 | 0.09 |
| NDF | 1.01 | 1.06 | 0.70 | 0.95 | 0.08 | 0.20 | 0.01 | 0.06 |
| ADF | 0.69 | 0.72 | 0.51 | 0.64 | 0.07 | 0.34 | 0.03 | 0.12 |
| Apparent digestibility, % | ||||||||
| DM | 78.6 | 76.1 | 80.8 | 78.2 | 1.31 | 0.98 | 0.19 | 0.12 |
| OM | 80.3 | 78.2 | 82.7 | 79.8 | 1.23 | 0.78 | 0.19 | 0.11 |
| NDF | 72.4 | 68.8 | 76.9 | 72.9 | 2.14 | 0.95 | 0.11 | 0.16 |
| ADF | 72.4 | 68.8 | 76.2 | 72.5 | 1.92 | 0.98 | 0.13 | 0.13 |
| N balance | ||||||||
| Dietary N intake, g/d | 58.2 | 61.1 | 41.2 | 53.3 | 4.58 | 0.31 | 0.01 | 0.11 |
| Infused N, g/d | 6.3 | 10.2 | 6.3 | 10.2 | — | — | — | — |
| Total N supply, g/d | 64.4 | 71.2 | 47.4 | 63.5 | 4.58 | 0.31 | 0.01 | 0.02 |
| Fecal N, g/d | 15.7 | 18.0 | 10.2 | 14.5 | 1.50 | 0.49 | <0.01 | 0.03 |
| Urinary N, g/d | 29.7 | 29.0 | 30.4 | 33.5 | 1.31 | 0.25 | 0.11 | 0.45 |
| Apparent N digested | ||||||||
| g/d | 48.7 | 53.1 | 37.2 | 49.0 | 3.30 | 0.30 | 0.04 | 0.03 |
| % of total N supply | 76.1 | 74.8 | 78.3 | 77.2 | 1.23 | 0.95 | 0.13 | 0.43 |
| Retained N | ||||||||
| g/d | 18.9 | 24.0 | 6.5 | 15.6 | 3.56 | 0.65 | 0.02 | 0.11 |
| % of total N supply | 28.6 | 33.9 | 6.0 | 24.5 | 9.69 | 0.57 | 0.18 | 0.31 |
| Urinary NMH3 | ||||||||
| mmol/d | 3.60 | 3.51 | 4.04 | 4.76 | 0.52 | 0.49 | 0.15 | 0.59 |
| Daily µmol/kg of BW | 2.04 | 1.98 | 2.31 | 2.75 | 0.23 | 0.33 | 0.05 | 0.46 |
| Passage, %/h | ||||||||
| Cr | 4.17 | 4.43 | 1.95 | 2.27 | 0.40 | 0.92 | <0.01 | 0.37 |
| Yb | 3.07 | 3.55 | 1.96 | 2.03 | 0.50 | 0.67 | 0.02 | 0.56 |
1A 2 × 2 factorial arrangement of intravenous infusions (1 mL/min) of 100 mL sterile saline containing either 0 μg or 1.0 to 1.5 μg of LPS per kg of BW (−LPS vs. +LPS), and abomasal infusions of an essential AA solution that supplied either 0 or 35 g/d BCAA (−BCAA vs. +BCAA).
2n = steers per treatment; 2 steers receiving the +LPS and +BCAA treatment combination were removed from the experiment due to severe respiratory reactions to the LPS infusions. LPS = lipopolysaccharide; BCAA = branched-chain AA.
3Nτ-methylhistidine excreted in urine.
Effects of Post-ruminal BCAA Supplementation
Serum cortisol concentrations (Figure 3) were greater for +BCAA than −BCAA steers at 12 h, but not different at 0, 2, 4, 8, and 24 h after LPS infusion (BCAA × h, P < 0.01). Serum glucose concentrations (Figure 4) were not different between +BCAA and –BCAA steers at 0, 2, 4, 8, and 12 h, but were lower for +BCAA than –BCAA steers at 24 h after LPS infusion (BCAA × h, P = 0.05). Steers post-ruminally infused with +BCAA had greater plasma Val, Ile, and Leu concentrations (Figure 5) than –BCAA steers at 0, 2, 4 (except Ile), 8, 12, and 24 h after LPS infusion (BCAA × h, P ≤ 0.05). Plasma concentrations of Lys (Figure 6), Ala and Ser (Figure 8), Asn and Tyr (Figure 9), and Gln (Figure 10) were lower for +BCAA than –BCAA steers (BCAA, P ≤ 0.05). Similarly, plasma Pro concentrations (Figure 10) tended to be lower for +BCAA than –BCAA steers (BCAA, P = 0.06). Plasma concentrations of His and Met (Figure 6), and Phe and Trp (Figure 7), were lower for +BCAA than −BCAA steers at 0 and 24 h, but not different at 2, 4, 8, and 12 h after LPS infusion (BCAA × h, P < 0.01). Plasma Thr concentrations (Figure 7) were lower for +BCAA than −BCAA steers at 0, 8, 12, and 24 h, but not different at 2 and 4 h after LPS infusion (BCAA × h, P < 0.01). Post-ruminal infusion of BCAA did not affect (P ≥ 0.10) ruminal concentrations of total VFA and NH3, as well as ruminal pH (Figure 11).
Post-ruminal infusions of BCAA did not affect (BCAA, P ≥ 0.06) dietary DM, OM, NDF, ADF, and N intake, and apparent digestibility of DM, OM, NDF, and ADF (Table 2). Steers abomasally infused with +BCAA had greater (BCAA, P < 0.05) total N supply (dietary N plus infused N), fecal N excretion, and grams of apparent N digested than −BCAA steers (Table 2). However, apparent N digestibility as a percentage total N supply, urinary N excretion, and N retention were not different (BCAA, P ≥ 0.11) between +BCAA and –BCAA steers. Similarly, urinary excretion of NMH, and total tract solid and liquid passage rates were not different between +BCAA and –BCAA steers (BCAA, P ≥ 0.37).
DISCUSSION
Endotoxin Challenge
Observed increases in rectal temperatures and serum cortisol concentrations of steers exposed to LPS are indicative of stress and inflammation. Our previous research (Waggoner et al., 2009a; Carter et al., 2010) demonstrated that LPS-challenged steers had greater serum concentrations of cytokines (tumor necrosis factor-α and IL-6) that trigger inflammation and stimulate synthesis of immune system proteins (e.g., haptoglobin). A tendency for greater serum insulin concentrations in +LPS than –LPS steers are likely due to the observed initial increase in blood glucose concentrations in response to LPS. Steiger et al. (1999) attributed the initial increase in serum glucose concentrations to enhanced glycogenolysis. The observed hypoglycemia after the initial increase in serum glucose concentrations is a response that appears to occur often in ruminant animals exposed to LPS (Steiger et al., 1999; Waggoner et al., 2009b; Wang et al., 2015). As described by Waggoner et al. (2009b), hypoglycemia that follows the initial increase in serum glucose concentrations in LPS-challenged cattle is indicative of glucose utilization exceeding glucose synthesis. Steiger et al. (1999) suggested that LPS inhibits gluconeogenesis during the latter phase (after 3 h) of the metabolic responses to an endotoxin, and Caton et al. (2009) and Wang et al. (2015) demonstrated that the inhibitory effects of LPS on gluconeogenesis are linked to decreases in the expression of hepatic phosphoenolpyruvate carboxykinase, pyruvate carboxylase, and fructose-1,6-bisphosphatase.
Lower concentrations of seven essential AA (Ile, Leu, Lys, Met, Thr, Trp, Val) and five nonessential AA (Asn, Glu, Orn, Ser, Tyr) in plasma of steers exposed to LPS indicate altered AA metabolism, presumably because of an increase in the utilization of these AA as substrates for products generated from the inflammatory response (Reeds and Jahoor, 2001; Waggoner et al., 2009a,b). Li et al. (2007) describes that both the innate and adaptive immune systems are dependent on an adequate supply of AA for immune cell protein synthesis, and for synthesis of other immune supportive molecules, such as free radical scavengers, metabolic cofactors, and hormones. Hoskin et al. (2016) eloquently described that individual AA concentrations in the plasma pool are reflective of multiple factors that alter inflow (intestinal absorption, endogenous protein catabolism, de novo synthesis of nonessential AA) and outflow (extraction for protein synthesis, oxidation of AA), and that simple changes in plasma concentrations of an AA may not necessarily indicate a metabolic need specific for that AA. The metabolic demand of a different AA could cause an AA imbalance, thereby altering the plasma concentration of a specific AA indirectly (Hoskin et al., 2016). Nevertheless, the following are possible metabolic mechanisms that could explain some of the observed effects of LPS infusion on specific plasma AA concentrations in this study.
Decreases in the plasma BCAA of LPS-challenged steers are consistent with previous research (Waggoner et al., 2009a,b; Carter et al., 2010), perhaps due to increased uptake of BCAA by proliferating lymphocytes (Glassy and Furlong, 1981; Koch et al., 1990). Additionally, increased uptake of BCAA by skeletal muscle for synthesis and release of Gln and Ala into circulation (Holecek, 2002; Li et al., 2007) may have contributed to the observed decrease in plasma BCAA concentrations of LPS-challenged steers. Potential de novo synthesis of Ala could explain greater plasma Ala concentrations in steers exposed to LPS, which was also observed by Waggoner et al. (2009a,b). According to Wu (2009), Ala increases glucose production by inhibiting hepatocyte pyruvate kinase, which in addition to insulin insensitivity associated with stress (Wellen and Hotamisligil, 2005) could have contributed to the observed initial increase in serum glucose concentrations in +LPS steers. However, the inhibitory effects of LPS on key gluconeogenic enzymes (Wang et al., 2015) may have limited the use of Ala for glucose synthesis, which could also explain observed increases in plasma Ala concentrations. Although systemic inflammation enhances BCAA catabolism for synthesis and release of Gln into circulation (Holecek, 2002), plasma Gln concentrations did not increase in LPS-challenged steers, which is consistent with Waggoner et al. (2009a). Waggoner et al. (2009b) and Carter et al. (2010) observed decreases in plasma Gln concentrations for cattle exposed to LPS, perhaps because of increased utilization by cells of the immune system (Newsholme, 2001; Newsholme et al., 2003).
Plasma Asn concentrations decreased in LPS-challenged steers, which is a consistent response among studies with cattle (Waggoner et al., 2009a,b; Carter et al., 2010) and other species (Asai et al., 2008). Both Asn and Gln are activators of ornithine decarboxylase for polyamine synthesis in proliferating cells (Ray and Johnson, 2014), and Asn reduces intestinal damage of pigs after LPS exposure (Chen et al., 2016). Asparagine is also a precursor for Asp, Gln, and Glu, which serve as energy substrates for enterocytes and immune cells (Newsholme et al., 2003; Wang et al., 2015). Plasma Glu concentrations initially increased and then decreased after steers were exposed to LPS. Glutamate (together with Cys and Gly) is required for synthesis of glutathione, which is involved in antioxidant defense and other cellular functions such as cell proliferation and cytokine production (Newsholme et al., 2003; Wu et al., 2004). Although observed decreases in plasma concentrations of Glu, Met, and Ser may be partly due to increased glutathione production (because products of Met and Ser metabolism are Cys and Gly) in LPS-challenged steers, intracellular concentrations of glutathione in immune cells typically do not increase, but rather decrease in response to LPS exposure (Zhang et al., 2017; Zhang and White, 2017). Zhang et al. (2017) attributed observed decreases in intracellular glutathione concentrations to decreased glutamate cysteine ligase expression in macrophages exposed to LPS, but these authors also noted that decreased intracellular glutathione concentrations may be a result of glutathione consumption exceeding production during the inflammatory response.
The observed decrease in plasma Met concentrations of steers in response to LPS is consistent with previous research (Waggoner et al., 2009a,b; Carter et al., 2010). In addition to increased transulfuration of Met to Cys during inflammation (Malmezat et al., 2000), the role of S-adenosylmethionine in polyamine synthesis for replication of immune cells and production of cytokines (Grimble and Grimble, 1998) may have contributed to the observed decreases in plasma Met concentrations in LPS-challenged steers. Similarly, use of Orn for polyamine synthesis in immune cells could, in part, explain the observed decreases in plasma Orn concentrations in LPS-challenged steers, particularly during the later repair phase of the inflammatory response (Satriano, 2004). During the early phases of the inflammatory response (from 2 to 4 h after +LPS infusion), decreases in plasma Orn concentrations of +LPS steers perhaps indicate a shift toward the use of Arg for nitric oxide production due to stimulation of inducible nitric oxide synthase in macrophages and monocytes by bacterial endotoxin (Wu and Morris, 1998), thereby reducing substrate (i.e., Arg) availability for Orn production (Satriano, 2004). According to Wu and Meininger (2002), Arg uptake for synthesis of nitric oxide and(or) polyamines may be decreased by high extracellular Lys concentrations because Lys and Arg share the same transport system. In this study, plasma Lys concentrations decreased and were lower in +LPS than −LPS steers, which would imply that any potential antagonism of cellular Arg uptake by Lys was minimal. It is unfortunate that the GLC procedure used for plasma AA analysis in this study did not allow for the measurement of plasma Arg. Lower plasma Lys concentrations in steers exposed to LPS have been reported previously (Waggoner et al., 2009a,b; Carter et al., 2010), perhaps due to increased hepatic removal in response to LPS (McNeil et al., 2016).
The observed decreases in plasma concentrations of Phe (tendency only), Thr, Trp, and Tyr for steers exposed to LPS are consistent with Waggoner et al. (2009b). Lower plasma Thr, Trp, and Tyr (but not Phe) concentrations for endotoxin-challenged steers were also reported by Waggoner et al. (2009a) and Carter et al. (2010). These responses are perhaps not surprising considering the large concentration of aromatic AA and Thr found in acute-phase proteins (Reeds et al., 1994; Reeds and Jahoor, 2001). Decreases in plasma Trp concentrations may also be reflective of an increase in the catabolism of Trp via the kynurenine pathway during inflammation (Le Floc’h and Seve, 2007; Hoskin et al., 2016), and decreases in plasma Thr concentrations could be in response to the inflammatory demand for increased synthesis of mucosal proteins in addition to acute-phase proteins (Faure et al., 2006, 2007). However, Hoskin et al. (2016) also observed increased hepatic serine–threonine dehydratase activity in LPS-challenged lambs, which could be indicative of increased Thr catabolism. A decrease in plasma Tyr concentrations of steers in response to LPS infusion could also be related to the use of this AA as the immediate precursor for the synthesis of dopamine and the catecholamine hormones (epinephrine and norepinephrine), which increase in response to LPS in cattle (Li et al., 2007; Burdick et al., 2011).
Most of the essential AA decreased and were lower in the plasma of steers after LPS infusion, which suggest that AA oxidation and(or) extraction of these AA for production of components associated with the inflammatory response were perhaps greater than the essential AA supply from intestinal absorption or from endogenous protein degradation. However, plasma His concentrations increased after LPS infusion, perhaps indicating that the His supply from catabolism of mixed muscle proteins (Melchior et al., 2004) exceeded the His needed for histamine production during the inflammatory response (Tanaka and Ichikawa, 2006). Greater muscle protein breakdown by LPS-challenged steers was demonstrated by the greater daily urinary excretion of NMH (adjusted for BW), which is a post-translationally modified AA from the breakdown of contractile proteins and excreted in urine (Harris and Milne, 1981).
By design, daily DM offered to steers was limited to 1.5% of BW to represent low feed consumption that is typical for newly received feedlot calves (NRC, 2000), and to minimize anticipated differences in DM intake between endotoxin-challenged steers and control steers. Despite limiting the daily feed offered, the 5-day average DM intake of steers after LPS administration was approximately 1.0% of BW, which is somewhat lower than previous reports for steers infused with equivalent amounts of LPS (Waggoner et al., 2009a,b). This remarkable reduction in feed intake likely decreased the supply of intestinally absorbable AA after LPS exposure. However, the observed decreases in plasma AA concentrations of LPS-challenged steers are likely not reflective of decreased nutrient intake, because changes in plasma AA concentrations occurred within 4 h of LPS infusion, and LPS administration occurred 3 h after cattle were fed, at which time all steers had consumed all of their diet (i.e., there were no differences in DM intake during the blood collection period on day 15). Nevertheless, effects of LPS on gastrointestinal motility (Lohuis et al., 1988; Waggoner et al., 2009a,b) and intestinal nutrient absorption (Albin et al., 2007; Mani et al., 2012) may have contributed to some of the observed decreases in plasma AA concentrations for LPS-challenged steers.
The reduction in feed intake for immune-challenged animals is a result of several physiological factors, including the suppression of appetite via interactions between cytokines and the central nervous system (Johnson, 1998; Mani et al., 2012). Additionally, slower total tract liquid and solid passage rates for +LPS compared with −LPS steers are indicative of decreased gastrointestinal motility in response to the endotoxin (Lohuis et al., 1988), which likely contributed to the reduction in DM intake (Waggoner et al., 2009a,b; Lippolis et al., 2017). Reduced gastrointestinal motility may also explain observed tendencies for greater apparent fiber (NDF and ADF) digestibility because retention of digestive contents was potentially greater in the rumen of +LPS steers compared with −LPS steers. The observed increase in apparent fiber digestibility is consistent with Bernabucci et al. (1999) who reported that heat-stressed cows had greater NDF digestibility. However, our results are in contrast to Waggoner et al. (2009b), who reported no change in total tract NDF digestibility (expressed as a percentage of intake) in cattle exposed to endotoxin, even when digestive passage rates were slower. These contrasting observations between this study and Waggoner et al. (2009b) are perhaps explained by the differences in the magnitude of the decrease in passage rates (40% vs. 24% for solid passage rates) and DM intake (20% vs. 6%). Lopez et al. (2017) also reported no effects of LPS on ruminal in situ disappearance of NDF in cattle, whereas Lippolis et al. (2017) reported lower ruminal in situ disappearance of NDF when beef steers were administered with LPS.
Lower rumen pH coincided with greater total VFA concentrations in the rumen of +LPS compared with –LPS steers at 8 h after LPS infusion, which is indicative of either increased ruminal carbohydrate fermentation (demonstrated by tendencies for greater apparent NDF and ADF digestibility), or decreased absorption of VFA, or both. Galyean et al. (1981), who also observed greater rumen VFA concentrations in steers exposed to transportation stress, attributed the responses to decreased rumen motility and poor absorption of VFA. Similarly, increases in rumen NH3 in this study suggest reduced NH3 removal (absorption or passage) from the rumen or altered N utilization by rumen microorganisms. For example, there is evidence that mammalian stress hormones (i.e., cortisol and catecholamines) entering the rumen via the saliva of stressed animals could directly affect rumen microbial fermentation (Rath et al., 2016; Samuelson et al., 2016; Lippolis et al., 2017).
Steers exposed to LPS retained less N than control steers, in part because these +LPS steers consumed less dietary N and had less N digested, and in part because they tended to excrete more N in urine than –LPS steers. The tendency for greater urinary N excretion, even when the absorbable supply of N (intake and digestibility) was lower, is perhaps due to increased tissue protein catabolism for the mobilization of specific AA to support synthesis of immune cell protein. An increase in tissue protein catabolism for +LPS steers was supported by greater urinary NMH excretion, a marker used to measure muscle protein breakdown (Harris and Milne, 1981). The tendency for LPS to increase urinary N excretion in steers also suggests that not all the AA mobilized from tissue protein catabolism were used for synthesis of immune proteins, perhaps because of imbalances between tissue AA supply and immune system AA requirements (Reeds and Jahoor, 2001), or because of increased AA oxidation to support other metabolic functions, such as to supply additional energy for an activated immune system (Calder and Yaqoob, 2004; Calder, 2006). Greater oxidation of AA is supported by greater urea-N concentrations in the plasma of +LPS compared with –LPS steers, particularly at 4, 8, and 12 h after LPS infusion. Plasma urea-N concentrations of +LPS steers increased further at 24 h after LPS infusion, but this increase is possibly not only due to increased AA oxidation, but perhaps also due to a potential increase in NH3 absorption associated with the observed increase in ruminal NH3 concentrations at 24 h after LPS infusion.
Supply of BCAA
Decreases in plasma BCAA of steers immediately after LPS administration are consistent with previous research (Waggoner et al., 2009a,b; Carter et al., 2010), and may signify a demand for BCAA to support an activated immune system (Reeds and Jahoor, 2001) perhaps due to increased uptake of BCAA by proliferating lymphocytes (Glassy and Furlong, 1981; Koch et al., 1990) and(or) uptake by skeletal muscle for synthesis and release of Gln and Ala into circulation (Holecek, 2002; Li et al., 2007). Our hypothesis was that supplementation of LPS-challenged steers with a source of BCAA that escape ruminal degradation will increase the absorbable BCAA supply to support an activated immune system and thus potentially decrease the need for tissue protein catabolism to supply these AA for immune system support. To test this hypothesis, N retention was used as a measure of the animal’s performance response. However, an animal’s response (particularly protein accretion) to the supply of a single essential AA is not only dependent on whether an inadequate supply of that AA limits the response, but it is also dependent on whether inadequate supplies of other nutrients (e.g., other essential AA, energy, vitamins, etc.) also limit the ability of the animal to respond to the limiting AA (Titgemeyer and Löest, 2001; Titgemeyer, 2003). Even if the AA of interest is the first-limiting AA, the magnitude of the response to an increase in the supply of that AA may be limited by the second limiting or “co-limiting” AA (Merchen and Titgemeyer, 1992; Greenwood and Titgemeyer, 2000). Therefore, rather than abomasally infusing only BCAA to steers, we infused all 10 essential AA to steers receiving the +BCAA treatment, and removed only the BCAA from the essential AA solution for steers receiving the –BCAA treatment. This “deletion approach” minimized the potential for any of the essential AA besides the BCAA to limit protein accretion (i.e., N retention response). The necessity of using this approach was demonstrated by Greenwood and Titgemeyer (2000), who observed greater N retention responses in steers when the first-limiting AA (Met) was post-ruminally supplemented with other essential AA than when supplemented alone.
Typically, intestinal absorption of post-ruminally infused individual crystalline AA is efficient (i.e., near 100%; Nolte et al., 2008). However, in this study greater fecal excretion of N from steers receiving abomasal infusions of +BCAA compared with −BCAA, and no significant differences in dietary N intake, indicated that absorption of the abomasally infused BCAA (and potentially some of the other AA in the infusion) was less than 100%. This might be explained by the AA infusion technique. In this study, the BCAA treatments (both −BCAA and +BCAA) were infused into the abomasum of steers as a bolus three times a day, whereas our previous studies (see Nolte et al., 2008) utilized infusion pumps for continuous abomasal infusions of AA throughout a day. Nevertheless, an increase in the plasma Ile, Leu, and Val concentrations of steers receiving +BCAA compared with –BCAA suggests that a measurable quantity of the BCAA in the abomasal infusions was absorbed.
By design, post-ruminal infusions of BCAA were expected to have no effect on ruminal fermentation characteristics although an increase in the supply of absorbed AA could alter N recycling and thus potentially affect rumen microbial fermentation if the diet was deficient in RDP (Nolte et al., 2008). However, similar ruminal concentrations of VFA and NH3, ruminal pH, apparent digestibility of nutrients (as % of intake), and total tract passage rates for steers supplemented with +BCAA compared with −BCAA indicated that the abomasal infusion of BCAA did not significantly alter ruminal fermentation and total tract digestion characteristics of steers. Therefore, the observed metabolic responses to the abomasal infusion of BCAA are likely not an indirect effect of altered nutrient supply because of changes in ruminal digestion.
Greater plasma Ile, Leu, and Val concentrations for steers receiving +BCAA vs. –BCAA not only reflect an increase in the intestinal absorption of these BCAA, but also indicate that intestinal absorption exceeded the amounts extracted by tissues to meet requirements for synthesis of protein and(or) to support other metabolic functions (i.e., oxidation for energy and de novo synthesis of nonessential AA). Estimates of the metabolizable supplies of Leu, Ile, and Val for −LPS steers receiving −BCAA infusion were 98%, 134%, and 105% of requirements, respectively (AA requirements were calculated for the −LPS steers using O’Connor et al. (1993) equations with observed retained protein [grams of retained N × 6.25] and fecal DM output from Table 2). The supplies of all other essential AA, except Arg, were greater than 130% of the requirements based on the steers’ observed N retention (from Table 2); estimates of Arg supply were lower than the Arg requirements to support the N retention observed, which might suggest that calculations of the Arg supply and(or) requirements were not accurate. Because supplies of Leu and Val were approximately equal to the Leu and Val needed to support the observed N retained by −LPS steers receiving −BCAA infusions, it is plausible that these BCAA were the limiting AA for lean tissue accretion (assuming that energy was not first limiting).
Lower concentrations of His, Met, Phe, Thr, Trp, Ala, Asn, Ser, and Tyr and a tendency for a 56% increase in the grams of N retained by BCAA-supplemented steers suggests that the MP supply from the basal diet was limiting in at least one of the BCAA, and increasing the absorbable BCAA supply via abomasal infusions potentially increased the utilization of plasma AA for protein synthesis, regardless of whether the steers were or were not challenged with LPS. These results are similar to the findings in growing cattle and sheep reported by Löest et al. (2001a) and Nolte et al. (2008) where the infusion of BCAA decreased plasma AA concentration and increased N retention. Löest et al. (2001a) also noted decreases in N retention when Leu and Val were individually removed from the abomasal supply of 10 essential AA, and suggested these to be the most limiting of the BCAA. The results of Löest et al. (2001a) also support the NRC (2000) model predictions of the essential AA supply and demand for this study, where both Leu and Val were predicted to be near requirements based on the observed N retained by −LPS steers receiving −BCAA infusions. The tendency for greater N retention by steers abomasally infused with BCAA could also be due to the muscle protein synthesis stimulatory effect of Leu via activation of the mammalian target of rapamycin signaling pathway (Kimball and Jefferson, 2006).
The lack of BCAA × LPS interactions for all response variables measured (except plasma Orn) suggests that supplying additional metabolizable BCAA via abomasal infusions to LPS-challenged steers does not alleviate negative effects of the endotoxin on blood metabolites, diet digestibility, and N balance. However, despite no significant BCAA × LPS interaction, the magnitude by which N retention (as a % of N supply) decreased in response to the LPS challenge was 79% for steers receiving no supplemental BCAA and only 28% for steers supplemented with BCAA. Therefore, post-ruminal BCAA supplementation perhaps did lesson the negative impact of inflammation on N retention, possibly by reducing skeletal muscle breakdown, which was demonstrated by Wan et al. (2017) when LPS-challenged rats were supplemented with Leu.
Two steers receiving +BCAA developed severe respiratory reactions to the LPS infusions and later died despite efforts of intervention (these steers were removed from the study and given anti-inflammatory and analgesic medication), which is perhaps an indication that the abomasal infusion of supplemental BCAA may have affected immune responses that were not measured in this study. For example, Ananieva et al. (2016) described that hyper-activation of immune cells could result from an increase in the supply of Leu to the mammalian target of rapamycin signaling pathway. The severe respiratory reaction to the LPS, which resulted in the death of the two steers receiving +BCAA was surprising, because Toyosawa et al. (2004) and Lang et al. (2010) demonstrated that BCAA supplementation increased survival of LPS-challenged mice, and Garcia-de-Lorenzo et al. (1997) reported lower mortality of septic patients receiving supplemental BCAA.
CONCLUSIONS
The results of this study indicate that the MP supply in growing steers with dietary DM intake (1.5% of BW) equivalent to that of highly-stressed newly received feedlot calves could be limiting in at least one of the BCAA. The absence of interactions between BCAA supplementation and LPS infusion for diet intake, digestibility, and N balance suggests that increasing the metabolizable supply of BCAA via post-ruminal supplementation did not alleviate the negative effects of endotoxin on N utilization by growing steers.
Footnotes
This research is supported in part by the New Mexico Agric. Exp. Stn., Las Cruces, 88003. The authors acknowledge L. Chen (New Mexico State University) for analysis of plasma AA and rumen VFA, D. M. Hallford (New Mexico State University) for analysis of serum cortisol and insulin, and J. A. Rathmacher (Iowa State University) for analysis of urine Nτ-methylhistidine.
LITERATURE CITED
- Albin D. M., Wubben J. E., Rowlett J. M., Tappenden K. A., and Nowak R. A.. 2007. Changes in small intestinal nutrient transport and barrier function after lipopolysaccharide exposure in two pig breeds. J. Anim. Sci. 85:2517–2523. doi:10.2527/jas.2006-237 [DOI] [PubMed] [Google Scholar]
- Ananieva E. A., Powell J. D., and Hutson S. M.. 2016. Leucine metabolism in T cell activation: mTOR signaling and beyond. Adv. Nutr. 7:798S–805S. doi:10.3945/an.115.011221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai Y., Bajotto G., Yoshizato H., Hamada K., Higuchi T., and Shimomura Y.. 2008. The effects of endotoxin on plasma free amino acid concentrations in rats. J. Nutr. Sci. Vitaminol. (Tokyo). 54:460–466. doi:10.3177/jnsv.54.460 [DOI] [PubMed] [Google Scholar]
- Bassit R. A., Sawada L. A., Bacurau R. F., Navarro F., Martins E. Jr, Santos R. V., Caperuto E. C., Rogeri P., and Costa Rosa L. F.. 2002. Branched-chain amino acid supplementation and the immune response of long-distance athletes. Nutrition 18:376–379. doi:10.1016/S0899-9007(02)00753-0 [DOI] [PubMed] [Google Scholar]
- Bernabucci U., Bani P., Ronchi B., Lacetera N., and Nardone A.. 1999. Influence of short- and long-term exposure to a hot environment on rumen passage rate and diet digestibility by Friesian heifers. J. Dairy Sci. 82:967–973. doi:10.3168/jds.S0022-0302(99)75316-6 [DOI] [PubMed] [Google Scholar]
- Broderick G. A. and Kang J. H.. 1980. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63:64–75. doi:10.3168/jds.S0022-0302(80)82888-8 [DOI] [PubMed] [Google Scholar]
- Burdick N. C., Carroll J. A., Hulbert L. E., Dailey J. W., Ballou M. A., Randel R. D., Willard S. T., Vann R. C., and Welsh T. H. Jr. 2011. Temperament influences endotoxin-induced changes in rectal temperature, sickness behavior, and plasma epinephrine concentrations in bulls. Innate Immun. 17:355–364. doi:10.1177/1753425910379144 [DOI] [PubMed] [Google Scholar]
- Calder P. C. 2006. Branched-chain amino acids and immunity. J. Nutr. 136(1 Suppl):288S–293S. doi:10.1093/jn/136.1.288S [DOI] [PubMed] [Google Scholar]
- Calder P. C., and Yaqoob P.. 2004. Amino acids and immune function. In: Cynober L. A., editor, Metabolic and therapeutic aspects of amino acids in clinical nutrition. CRC Press, Boca Raton, FL: p. 305–319. [Google Scholar]
- Carter B. H., Löest C. A., Chen L., Gilliam G. G., Graham B. C., Carroll J. A., Collier C. T., and Hallford D. M.. 2010. Arginine supplementation does not alter nitrogen metabolism of beef steers during a lipopolysaccharide challenge. Proc. Wes. Sec. Am. Soc. Anim. Sci. 61:55–59. [Google Scholar]
- Caton P. W., Nayuni N. K., Murch O., and Corder R.. 2009. Endotoxin induced hyperlactatemia and hypoglycemia is linked to decreased mitochondrial phosphoenolpyruvate carboxykinase. Life Sci. 84:738–744. doi:10.1016/j.lfs.2009.02.024 [DOI] [PubMed] [Google Scholar]
- Chen S., Liu Y., Wang X., Wang H., Li S., Shi H., Zhu H., Zhang J., Pi D., Hu C. A., et al. 2016. Asparagine improves intestinal integrity, inhibits TLR4 and NOD signaling, and differently regulates p38 and ERK1/2 signaling in weanling piglets after LPS challenge. Innate Immun. 22:577–587. doi:10.1177/1753425916664124 [DOI] [PubMed] [Google Scholar]
- Choudry H. A., Pan M., Karinch A. M., and Souba W. W.. 2006. Branched-chain amino acid-enriched nutritional support in surgical and cancer patients. J. Nutr. 136(1 Suppl):314S–318S. doi:10.1093/jn/136.1.314S [DOI] [PubMed] [Google Scholar]
- Cole N. A. and Hutcheson D. P.. 1985. Influence of prefast feed intake on recovery from feed and water deprivation by beef steers. J. Anim. Sci. 60:772–780. doi:10.2527/jas1985.603772x [DOI] [PubMed] [Google Scholar]
- Cullor J. S. 1992. Shock attributable to bacteremia and endotoxemia in cattle: clinical and experimental findings. J. Am. Vet. Med. Assoc. 200:1894–1902. [PubMed] [Google Scholar]
- Faure M., Choné F., Mettraux C., Godin J. P., Béchereau F., Vuichoud J., Papet I., Breuillé D., and Obled C.. 2007. Threonine utilization for synthesis of acute phase proteins, intestinal proteins, and mucins is increased during sepsis in rats. J. Nutr. 137:1802–1807. doi:10.1093/jn/137.7.1802 [DOI] [PubMed] [Google Scholar]
- Faure M., Mettraux C., Moennoz D., Godin J. P., Vuichoud J., Rochat F., Breuillé D., Obled C., and Corthésy-Theulaz I.. 2006. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J. Nutr. 136:1558–1564. doi:10.1093/jn/136.6.1558 [DOI] [PubMed] [Google Scholar]
- Fluharty F. L., Loerch S. C., and Dehority B. A.. 1994. Ruminal characteristics, microbial populations, and digestive capabilities of newly weaned, stressed calves. J. Anim. Sci. 72:2969–2979. doi:10.2527/1994.72112969x [DOI] [PubMed] [Google Scholar]
- Galyean M. L., Lee R. W., and Hubbert M. E.. 1981. Influence of fasting and transit on ruminal and blood metabolites in beef steers. J. Anim. Sci. 53:7–18. doi:10.2527/jas1981.5317 [DOI] [PubMed] [Google Scholar]
- Galyean M. L., Perino L. J., and Duff G. C.. 1999. Interaction of cattle health/immunity and nutrition. J. Anim. Sci. 77:1120–1134. doi:10.2527/1999.7751120x [DOI] [PubMed] [Google Scholar]
- García-de-Lorenzo A., Ortíz-Leyba C., Planas M., Montejo J. C., Núñez R., Ordóñez F. J., Aragón C., and Jiménez F. J.. 1997. Parenteral administration of different amounts of branch-chain amino acids in septic patients: clinical and metabolic aspects. Crit. Care Med. 25:418–424. [DOI] [PubMed] [Google Scholar]
- Glassy M. C. and Furlong C. E.. 1981. Neutral amino acid transport during the cell cycle of cultured human lymphocytes. J. Cell. Physiol. 107:69–74. doi:10.1002/jcp.1041070109 [DOI] [PubMed] [Google Scholar]
- Greenwood R. H. and Titgemeyer E. C.. 2000. Limiting amino acids for growing Holstein steers limit-fed soybean hull-based diets. J. Anim. Sci. 78:1997–2004. doi:10.2527/2000.7871997x [DOI] [PubMed] [Google Scholar]
- Grimble R. F. and Grimble G. K.. 1998. Immunonutrition: role of sulfur amino acids, related amino acids, and polyamines. Nutrition 14:605–610. doi:10.1016/S0899-9007(98)80041-5 [DOI] [PubMed] [Google Scholar]
- Harlan D. W., Holter J. B., and Hayes H. H.. 1991. Detergent fiber traits to predict productive energy of forages fed free choice to nonlactating dairy cattle. J. Dairy Sci. 74:1337–1353. doi:10.3168/jds.S0022-0302(91)78289-1 [DOI] [PubMed] [Google Scholar]
- Harris C. I. and Milne G.. 1981. The urinary excretion of N tau-methyl histidine by cattle: validation as an index of muscle protein breakdown. Br. J. Nutr. 45:411–422. doi:10.1079/BJN19810116 [DOI] [PubMed] [Google Scholar]
- Holecek M. 2002. Relation between glutamine, branched-chain amino acids, and protein metabolism. Nutrition 18:130–133. doi:10.1016/S0899-9007(01)00767-5 [DOI] [PubMed] [Google Scholar]
- Hoskin S. O., Bremner D. M., Holtrop G., and Lobley G. E.. 2016. Responses in whole-body amino acid kinetics to an acute, sub-clinical endotoxin challenge in lambs. Br. J. Nutr. 115:576–584. doi:10.1017/S0007114515004894 [DOI] [PubMed] [Google Scholar]
- Johnson R. W. 1998. Immune and endocrine regulation of food intake in sick animals. Domest. Anim. Endocrinol. 15:309–319. doi:10.1016/S0739-7240(98)00031-9 [DOI] [PubMed] [Google Scholar]
- Kimball S. R. and Jefferson L. S.. 2006. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 136(1 Suppl):227S–231S. doi:10.1093/jn/136.1.227S [DOI] [PubMed] [Google Scholar]
- Kiyma Z., Alexander B. M., Van Kirk E. A., Murdoch W. J., Hallford D. M., and Moss G. E.. 2004. Effects of feed restriction on reproductive and metabolic hormones in ewes. J. Anim. Sci. 82:2548–2557. doi:10.2527/ 2004.8292548x [DOI] [PubMed] [Google Scholar]
- Koch B., Schröder M. T., Schäfer G., and Schauder P.. 1990. Comparison between transport and degradation of leucine and glutamine by peripheral human lymphocytes exposed to concanavalin A. J. Cell. Physiol. 143:94–99. doi:10.1002/jcp.1041430112 [DOI] [PubMed] [Google Scholar]
- Lang C. H., Lynch C. J., and Vary T. C.. 2010. BCATm deficiency ameliorates endotoxin-induced decrease in muscle protein synthesis and improves survival in septic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299:R935–R944. doi:10.1152/ajpregu.00297.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foc’h N., Melchior D., and Obled C. Modifications of protein and amino acid metabolism during inflammation and immune system activation. Livest. Prod. Sci. 87:37–45. doi:10.1016/j.livprodsci.2003.09.005 2004 [Google Scholar]
- Le Floc’h N., and Seve B.. 2007. Biological roles of tryptophan and its metabolism: potential implications for pig feeding. Livest. Sci. 112:23–32. doi:10.1016/j.livsci.2007.07.002 [Google Scholar]
- Li P., Yin Y. L., Li D., Kim S. W., and Wu G.. 2007. Amino acids and immune function. Br. J. Nutr. 98:237–252. doi:10.1017/S000711450769936X [DOI] [PubMed] [Google Scholar]
- Lippolis K. D., Cooke R. F., Schubach K. M., Marques R. S., and Bohnert D. W.. 2017. Effects of intravenous lipopolysaccharide administration on feed intake, ruminal forage degradability, and liquid parameters and physiological responses in beef cattle. J. Anim. Sci. 95:2859–2870. doi:10.2527/jas.2017.1502 [DOI] [PubMed] [Google Scholar]
- Lodge-Ivey S. L., Browne-Silva J., and Horvath M. B.. 2009. Technical note: bacterial diversity and fermentation end products in rumen fluid samples collected via oral lavage or rumen cannula. J. Anim. Sci. 87:2333–2337. doi:10.2527/jas.2008-1472 [DOI] [PubMed] [Google Scholar]
- Löest C. A., Titgemeyer E. C., Drouillard J. S., Lambert B. D., Trater A. M.. 2001b. Urea and biuret as a nonprotein nitrogen sources in cooked molasses blocks for steers fed prairie hay. Anim. Feed and Tech. 94:115–126. doi:10.1016/S0377-8401(01)00312-1 [Google Scholar]
- Löest C. A., Titgemeyer E. C., Lambert B. D., and Trater A. M.. 2001a. Branched-chain amino acids for growing cattle limit-fed soybean hull-based diets. J. Anim. Sci. 79:2747–2753. doi:10.2527/2001.79102747x [DOI] [PubMed] [Google Scholar]
- Löest C. A., Titgemeyer E. C., G. St-Jean, Van Metre D. C., and Smith J. S.. 2002. Methionine as a methyl group donor in growing cattle. J. Anim. Sci. 80:2197–2206. doi:10.1093/ansci/80.8.2197 [DOI] [PubMed] [Google Scholar]
- Lohuis J. A., Verheijden J. H., Burvenich C., and van Miert A. S.. 1988. Pathophysiological effects of endotoxins in ruminants. 1. Changes in body temperature and reticulo-rumen motility, and the effect of repeated administration. Vet. Q. 10:109–116. doi:10.1080/01652176.1988.9694157 [DOI] [PubMed] [Google Scholar]
- Lopez F. A., Samuelson K. L., Carey R. E., Pillmore S. L., Brooks J. M., Klump L. T., Oosthuysen E. R., and Löest C. A.. 2017. Supplemental glycerin alters rumen fermentation and in situ degradation in steers exposed to an endotoxin. Proc. West. Sec. Am. Soc. Anim. Sci. 68:310–315. doi:10.2527/asasws.2017.0062 [Google Scholar]
- Malmezat T., Breuillé D., Pouyet C., Buffière C., Denis P., Mirand P. P., and Obled C.. 2000. Methionine transsulfuration is increased during sepsis in rats. Am. J. Physiol. Endocrinol. Metab. 279:E1391–E1397. doi:10.1152/ajpendo.2000.279.6.E1391 [DOI] [PubMed] [Google Scholar]
- Maltby S. A., Reynolds C. K., Lomax M. A., and Beever D. E.. 2005. Splanchnic metabolism of nitrogenous compounds and urinary nitrogen excretion in steers fed alfalfa under conditions of increased absorption of ammonia and L-arginine supply across the portal-drained viscera. J. Anim. Sci. 83:1075–1087. doi:10.2527/2005.8351075x [DOI] [PubMed] [Google Scholar]
- Mani V., Weber T. E., Baumgard L. H., and Gabler N. K.. 2012. Growth and development symposium: endotoxin, inflammation, and intestinal function in livestock. J. Anim. Sci. 90:1452–1465. doi:10.2527/jas.2011-4627 [DOI] [PubMed] [Google Scholar]
- May T., and Galyean M.. 1996. Laboratory procedures in animal nutrition research. New Mexico State University, Las Cruces. [Google Scholar]
- McNeil C. J., Hoskin S. O., Bremner D. M., Holtrop G., and Lobley G. E.. 2016. Whole-body and splanchnic amino acid metabolism in sheep during an acute endotoxin challenge. Br. J. Nutr. 116:211–222. doi:10.1017/S0007114516001860 [DOI] [PubMed] [Google Scholar]
- Melchior D., Sève B., and Le Floc’h N.. 2004. Chronic lung inflammation affects plasma amino acid concentrations in pigs. J. Anim. Sci. 82:1091–1099. doi:10.2527/2004.8241091x [DOI] [PubMed] [Google Scholar]
- Merchen N. R. and Titgemeyer E. C.. 1992. Manipulation of amino acid supply to the growing ruminant. J. Anim. Sci. 70:3238–3247. doi:10.2527/1992.70103238x [DOI] [PubMed] [Google Scholar]
- Newsholme P. 2001. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection?J. Nutr. 131(9 Suppl):2515S–2522S; discussion 2523S. doi:10.1093/jn/131.9.2515S [DOI] [PubMed] [Google Scholar]
- Newsholme P., Lima M. M., Procopio J., Pithon-Curi T. C., Doi S. Q., Bazotte R. B., and Curi R.. 2003. Glutamine and glutamate as vital metabolites. Braz. J. Med. Biol. Res. 36:153–163. doi:10.1590/S0100-879X2003000200002 [DOI] [PubMed] [Google Scholar]
- Nolte J. van E., Löest C. A., Ferreira A. V., Waggoner J. W., and Mathis C. P.. 2008. Limiting amino acids for growing lambs fed a diet low in ruminally undegradable protein. J. Anim. Sci. 86:2627–2641. doi:10.2527/jas.2007-0771 [DOI] [PubMed] [Google Scholar]
- NRC 2000. Nutrient requirements of beef cattle. 7th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Nuwer N., Cerra F. B., Shronts E. P., Lysne J., Teasley K. M., and Konstantinides F. N.. 1983. Does modified amino acid total parenteral nutrition alter immune-response in high level surgical stress. JPEN. J. Parenter. Enteral Nutr. 7:521–524. doi:10.1177/0148607183007006521 [DOI] [PubMed] [Google Scholar]
- O’Connor J. D., Sniffen C. J., Fox D. G., and Chalupa W.. 1993. A net carbohydrate and protein system for evaluating cattle diets: IV. Predicting amino acid adequacy. J. Anim. Sci. 71:1298–1311. doi:10.2527/1993.7151298x [DOI] [PubMed] [Google Scholar]
- Rath L. L., Samuelson K. L., Salazar A. L., Lopez F. A., Scholljegerdes E. J., and Löest C. A.. 2016. Mammalian hormones associated with stress impact microbial fermentation of rumen fluid in vitro. Proc. West. Sec. Am. Soc. Anim. Sci. 67:190–193. doi:10.2527/jam2016-1647 [Google Scholar]
- Rathmacher J. A., Link G. A., Flakoll P. J., and Nissen S. L.. 1992. Gas chromatographic/mass spectrometric analysis of stable isotopes of 3-methylhistidine in biological fluids: application to plasma kinetics in vivo. Biol. Mass Spectrom. 21:560–566. doi:10.1002/bms.1200211107 [DOI] [PubMed] [Google Scholar]
- Ray R. M. and Johnson L. R.. 2014. Regulation of intestinal mucosal growth by amino acids. Amino Acids 46:565–573. doi:10.1007/s00726-013-1565-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeds P. J., Fjeld C. R., and Jahoor F.. 1994. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states?J. Nutr. 124:906–910. doi:10.1093/jn/124.6.906 [DOI] [PubMed] [Google Scholar]
- Reeds P. J., and Jahoor F.. 2001. The amino acid requirements of disease. Clin. Nutr. (Suppl. 1):15–22. doi:10.1054/clnu.2001.0402 [Google Scholar]
- Reimers T. J., Cowan R. G., McCann J. P., and Ross M. W.. 1982. Validation of a rapid solid-phase radioimmunoassay for canine, bovine, and equine insulin. Am. J. Vet. Res. 43:1274–1278. [PubMed] [Google Scholar]
- Samuelson K. L., Salazar A. L., Rath L. L., Alford J. B., Oosthyusen E. R., Lodge-Ivey S. L., Hallford D. M., and Löest C. A.. 2016. Salivary cortisol concentrations affect rumen microbial fermentation and nutrient digestibility in vitro. Proc. West. Sec. Am. Soc. Anim. Sci. 67:209–213. doi:10.2527/jam2016-1670 [Google Scholar]
- Satriano J. 2004. Arginine pathways and the inflammatory response: interregulation of nitric oxide and polyamines: review article. Amino Acids 26:321–329. doi:10.1007/s00726-004-0078-4 [DOI] [PubMed] [Google Scholar]
- Steiger M., Senn M., Altreuther G., Werling D., Sutter F., Kreuzer M., and Langhans W.. 1999. Effect of a prolonged low-dose lipopolysaccharide infusion on feed intake and metabolism in heifers. J. Anim. Sci. 77:2523–2532. doi:10.2527/1999.7792523x [DOI] [PubMed] [Google Scholar]
- Tanaka S. and Ichikawa A.. 2006. Recent advances in molecular pharmacology of the histamine systems: immune regulatory roles of histamine produced by leukocytes. J. Pharmacol. Sci. 101:19–23. doi:10.1254/jphs.FMJ06001X5 [DOI] [PubMed] [Google Scholar]
- Titgemeyer E. C. 2003. Amino acid utilization by growing and finishing ruminants. In: D’Mello J.P.F., editor, Amino acids in animal nutrition. CAB International, Cambridge, MA: p. 329–346. [Google Scholar]
- Titgemeyer E. C., and Löest C. A.. 2001. Amino acid nutrition: demand and supply in forage-fed ruminants. J. Anim. Sci. 79(E. Suppl):E180–E189. doi:10.2527/jas2001.79E-SupplE180x [Google Scholar]
- Toyosawa T., Suzuki M., Kodama K., and Araki S.. 2004. Potentiation by amino acid of the therapeutic effect of highly purified vitamin B2 in mice with lipopolysaccharide-induced shock. Eur. J. Pharmacol. 493:177–182. doi:10.1016/j.ejphar.2004.04.019 [DOI] [PubMed] [Google Scholar]
- Tsukishiro T., Shimizu Y., Higuchi K., and Watanabe A.. 2000. Effect of branched-chain amino acids on the composition and cytolytic activity of liver-associated lymphocytes in rats. J. Gastroenterol. Hepatol. 15:849–859. doi:10.1046/j.1440-1746.2000.02220.x [DOI] [PubMed] [Google Scholar]
- Waggoner J. W., Löest C. A., Mathis C. P., Hallford D. M., and Petersen M. K.. 2009. Effects of rumen-protected methionine supplementation and bacterial lipopolysaccharide infusion on nitrogen metabolism and hormonal responses of growing beef steers. J. Anim. Sci. 87:681–692. doi:10.2527/jas.2008-1068 [DOI] [PubMed] [Google Scholar]
- Waggoner J. W., Löest C. A., Turner J. L., Mathis C. P., and Hallford D. M.. 2009. Effects of dietary protein and bacterial lipopolysaccharide infusion on nitrogen metabolism and hormonal responses of growing beef steers. J. Anim. Sci. 87:3656–3668. doi:10.2527/jas.2009-2011 [DOI] [PubMed] [Google Scholar]
- Waggoner J. W., Mathis C. P., Löest C. A., Sawyer J. E., McCollum F. T., and Banta J. P.. 2007. Case study: impact of morbidity in finishing beef steers on feedlot average daily gain, carcass characteristics, and carcass value. Prof. Anim. Sci. 23:174–178. doi:10.15232/S1080-7446(15)30958-X [Google Scholar]
- Wan J., Chen D., Yu B., Luo Y., Mao X., Zheng P., Yu J., Luo J., and He J.. 2017. Leucine protects against skeletal muscle atrophy in lipopolysaccharide-challenged rats. J. Med. Food 20:93–101. doi:10.1089/jmf.2016.3759 [DOI] [PubMed] [Google Scholar]
- Wang L. F., Yang G. Q., Yang S., Yang G. Y., Li M., Zhu H. S., Wang Y. Y., Han L. Q., Liu R. Y., Jia S. D., et al. 2015. Alteration of factors associated with hepatic gluconeogenesis in response to acute lipopolysaccharide in dairy goat. J. Anim. Sci. 93:2767–2777. doi:10.2527/jas.2014-8718 [DOI] [PubMed] [Google Scholar]
- Wellen K. E. and Hotamisligil G. S.. 2005. Inflammation, stress, and diabetes. J. Clin. Invest. 115:1111–1119. doi:10.1172/JCI25102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. 2009. Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17. doi:10.1007/s00726-009-0269-0 [DOI] [PubMed] [Google Scholar]
- Wu G., Fang Y. Z., Yang S., Lupton J. R., and Turner N. D.. 2004. Glutathione metabolism and its implications for health. J. Nutr. 134:489–492. doi:10.1093/jn/134.3.489 [DOI] [PubMed] [Google Scholar]
- Wu G. and Meininger C. J.. 2002. Regulation of nitric oxide synthesis by dietary factors. Annu. Rev. Nutr. 22:61–86. doi:10.1146/annurev.nutr.22.110901.145329 [DOI] [PubMed] [Google Scholar]
- Wu G. and Morris S. M. Jr. 1998. Arginine metabolism: nitric oxide and beyond. Biochem. J. 336 (Pt 1):1–17. doi:10.1042/bj3360001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Liu H., Zhou L., Yuen J., and Forman H. J.. 2017. Temporal changes in glutathione biosynthesis during the lipopolysaccharide-induced inflammatory response of THP-1 macrophages. Free Radic. Biol. Med. 113:304–310. doi:10.1016/j.freeradbiomed.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. and White H. M.. 2017. Regulation of inflammation, antioxidant production, and methyl-carbon metabolism during methionine supplementation in lipopolysaccharide-challenged neonatal bovine hepatocytes. J. Dairy Sci. 100:8565–8577. doi:10.3168/jds.2017-12932 [DOI] [PubMed] [Google Scholar]