Abstract
Dendritic cells (DCs) are essential antigen presenting cells that sample the extra- and intracellular milieu to process antigens for instructing T cell responses. The mammalian target of rapamycin (mTOR) network senses environmental cues and is important for numerous cellular processes. This review discusses how DCs use mTOR complexes (mTORC1 and 2) to adapt their cellular metabolism, transcriptional responses and protein translation machinery to control DC development, antigen processing, cytokine production and T cell stimulation. We present a model suggesting that the mTOR network integrates pattern recognition and growth factor receptor activation with nutritional information from the cell and surrounding tissue to support T cell stimulation and tolerance. mTOR develops into a central player that regulates DC differentiation and immune functions.
mTOR: TORching and Chilling Immune Responses
Dendritic cells (DCs) are hematopoietic cells that have a unique ability to activate naïve T cells [1]. They are found in most peripheral tissues and lymph nodes as immature DCs (iDCs), which constantly sample the extracellular or intracellular environment to process antigen and present it on their surface by MHC-II or MHC-I complexes. They are able to distinguish between self and foreign antigens by co-sampling pathogen- or danger-associated molecular patterns (PAMPs, DAMPs) with pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors, retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), or C type lectins [2]. Such activated DCs mature and induce T cell mediated immunity when antigen is captured in the presence of PAMPs or DAMPS but may support peripheral T cell tolerance in the absence of these signals [3].
The mammalian target of rapamycin (mTOR) is an evolutionary conserved serine-threonine kinase that is present in at least two larger protein complexes: mTOR complex 1 (mTORC1) and mTORC2 (Box 1) [4]. Over the last years, it has become increasingly clear that mTORC1 and mTORC2 are part of a larger network, which integrates intra- and extracellular nutrient sensing with growth factor and PRR signaling [5]. This review discusses how the mTOR network uses this integrative information to control a wide array of basic cellular processes such as metabolism and protein synthesis that subsequently dictates and shapes inflammatory immune responses of DCs. Moreover, we present the current understanding of the roles of mTORC1 and mTORC2 in DCs, but also synthesize mTOR-dependent functions into a model that incorporates time and location within the life span of a DC.
Box 1. mTORC1, mTORC2, and their inhibitors.
The serine/threonine kinase mTOR is part of two multimeric proteins : mTOR complex 1 (mTORC1) and mTORC2 [4]. mTORC1 consists of mTOR, regulatory-associated protein of mTOR (Raptor), proline-rich AKT1 substrate of 40 kDa (Pras40), mLST8 (also known as GβL), and DEP domain-containing mTOR-interacting protein (Deptor). mTORC2 is composed of mTOR, mLST8, the adaptor proteins rapamycin-insensitive companion of mTOR (Rictor) and stress-activated MAP kinase-interacting protein 1 (Sin1). The prototypic mTOR inhibitor rapamycin inhibits mTORC1 by associating with FK506-binding protein 12 (FKBP12), which then directly binds to mTORC1 to inhibit substrate positioning to the catalytic cleft [15]. Rapamycin is more effective in blocking the phosphorylation of S6K1 than 4E-BP1. In addition, rapamycin can also variably inhibit mTORC2 at higher concentrations and at later time points in a cell-type specific manner. Novel ATP-competitive catalytic inhibitors, that block mTOR kinase activity, such as Torin1, PP242, or AZD8055, inhibit mTORC1 as well as mTORC2.
Dual Regulation of the mTOR Network by PRR Signals and Cellular Nutrients
After PRR-mediated activation, DCs start to change their morphology and rapidly produce early cytokines such as TNF-α or the gaseous signaling molecule nitric oxide (NO) [6]. Later they migrate to secondary lymphoid organs to stimulate adaptive T cell responses. The change from an endocytosing tissue-resident cell into an activated anabolic cell that secretes many immune modulators and stimulates T cells causes a drastic shift in metabolic and biosynthetic requirements [6]. Therefore, DCs need to sense the available nutrients to coordinate and adapt energy metabolism and cytokine molecule production. A main cellular regulator that organizes this adaptation is the mTOR network [4,7].
mTORC1 and mTORC2 are activated by PAMPs such as TLR ligands but also by the growth factors FMS-related tyrosine kinase 3 ligand (Flt3L) and granulocyte/macrophage colony-stimulating factor (GM-CSF) to support DC development from hematopoietic progenitors [8–11] (Figure 1 and Box 2). Full mTORC1 activation by growth factors or TLR ligands requires an intracellular sufficiency of nutrients - amino acids, glucose-6 phosphate and the lipid metabolite phosphatidic acid as well as oxygen and energy). mTORC1 and mTORC2 are therefore ideally suited to integrate many external and intracellular signals and control a wide array of basic cellular processes such as translation and protein synthesis, transcription, metabolism and anabolic processes that specifically shape the immune response of DCs (Figure 2).
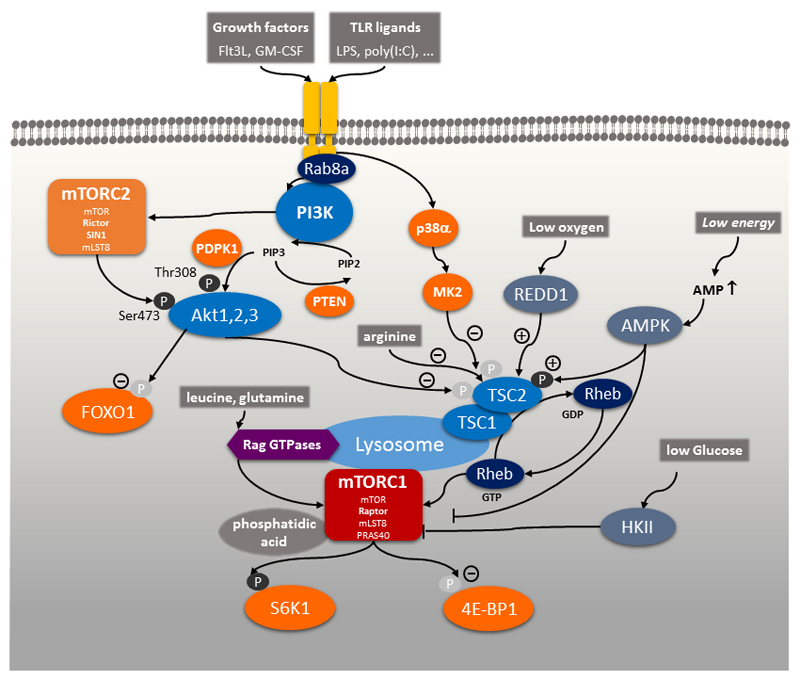
Figure 1. The mTORC1-mTORC2 Network.
In dendritic cells the mTOR network is activated either via the growth factors Flt3L or GM-CSF or via pattern recognition receptors such as Toll-like receptors via PI3K, which then triggers Akt1, Akt2, Akt3 to inactivate TSC2. This inactivation of TSC1/TSC2 promotes stimulation of mTORC1 via the small GTP binding protein Rheb. Full activation of mTORC1 by these signals requires a sufficiency of nutrients and energy in the cellular microenvironment such as amino acids, glucose, the phospholipid phosphatidic acid, oxygen, and energy in the form of ATP. mTORC2 is also stimulated by PI3K to support Akt activation. Signals that promote deactivation of TSC2 and hence, activate mTORC1 are indicated by (-), whereas signals that activate TSC2 and inhibit mTORC1 are indicated by (+). Abbreviations: mTOR, mammalian target of rapamycin; Flt3L, FMS-like tyrosine kinase 3 ligand; GM-CSF, granulocyte macrophage colony-stimulating factor; PI3K, phosphatidylinositol-3 kinase; TSC2, tuberous sclerosis complex 2; Rheb, RAS homologue enriched in brain; mTORC1, mTOR complex 1; mTORC2, mTOR complex 2.
Box 2. Molecular Mechanisms of mTORC1 and mTORC2 Activation.
Triggering of growth factor receptors or PRRs activates tyrosine kinase adaptor molecules including the small GTPase Rab8a at the cell membrane leading to the recruitment of the class I family of phosphatidylinositol-3 kinases (PI3K) to the receptor complex (Figure 1) [7,84]. Following receptor engagement, PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PtdInsP2) to generate phosphatidylinositol-3,4,5-trisphosphate (PtdInsP3) as a second messenger to recruit and activate the serine-threonine kinases Akt1, Akt2 and Akt3 [85] via phosphorylation on threonine 308 by phosphoinositide-dependent protein kinase 1 (PDPK1). The tumor suppressor phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is a lipid phosphatase and dephosphorylates PtdInsP3 to negatively regulate PI3K signaling [15]. mTORC2 is activated by PI3K through an uncharacterized mechanism and phosphorylates Akt on serine 473, which is important for full activation and substrate specificity of Akt. Two main targets of Akt are forkhead box O1 (FoxO1) and tuberous sclerosis 2 (TSC2) [4]. TSC2 forms a heterodimeric complex with TSC1 and inhibits mTORC1. Phosphorylation of TSC2 at threonine 1462 (Thr1462) by Akt inhibits its GTPase-activating protein (GAP) activity for the small GTPase RAS homologue enriched in brain (Rheb), which therefore remains in a GTP-bound active state and activates mTORC1 [86]. Thus, PI3K activation ultimately leads to mTORC1 activation through the inhibition of TSC2. Inhibition of TSC2 activity can also occur via a PI3K-independent pathway that involves p38 and MK2 in monocytes or macrophages [87].
In addition, mTORC1 senses amino acid sufficiency, especially leucine and arginine on the lysosome via Ras-related GTPases (Rag) and the ragulator complex [5]. In addition, arginine, independently of Rag family members, inhibits lysosomal localization of TSC2 to stimulate mTORC1 activity [88]. Low levels of glucose-6-phosphate inactivate mTORC1 by inducing binding to hexokinase 2 (HK2) [89,90]. Binding of the lipid metabolite phosphatidic acid (PA) to mTORC1 is also a prerequisite for mTORC1 activation [91]. Oxygen deprivation stimulates expression of REDD1 (regulated in development and DNA damage responses 1; also known as RTP801), which inhibits mTORC1 via TSC2 [92]. Thus, mTORC1 senses all main classes of nutrient and energy sources: amino acids, glucose, lipids, oxygen and ATP/AMP ratio via AMPK [18].The presence of these nutrients and energy is supposed to be required for full mTORC1 activation.
Figure 2. Role of mTOR for DC Function.
Triggering of mTORC1 and mTORC2 activates many basic cellular processes in DCs such as metabolism, protein synthesis and transcription (light green), but inhibit lysosome biogenesis and autophagy (dark green). These processes positively (light blue) or negatively (dark blue) control the expression of important cytokines and cell surface molecules that regulate many important DC functions (yellow). Abbreviations: DCs, dendritic cells; mTORC1, mammalian target of rapamycin complex 1; mTORC2, mammalian target of rapamycin complex 2.
Translational and Metabolic Reprogramming by mTORC1 in DCs
A massive increase in total protein synthesis occurs rapidly after LPS stimulation in DCs to support their maturation [12,13]. This increase in protein synthesis peaks after 4 hours and is vastly dependent on PI3K-mTORC1 [13,14]. The ribosomal protein S6 kinase 1 (S6K1) and S6K2 are two important targets of mTORC1-mediated phosphorylation, which in turn induce the phosphorylation and activation of S6 to stimulate protein translation. mTORC1 regulates cap-dependent and independent translation of mRNAs by phosphorylating the translation inhibitors 4E-BP1 and 4E-BP2, which release the initiation factor eIF4E [4]. mTORC1 is especially efficient in translation initiation of mRNAs containing a 5′ oligopyrimidine tract (termed a 5′ TOP) and pyrimidine-rich translational element (PRTE). Interestingly, these mRNAs encode many translation and metabolism-related genes [15,16]. Accordingly, adaptation and reprogramming of the metabolism after LPS stimulation mainly occurs through proteome remodeling at the translational level [17]. Thus, one main function of mTORC1 is to stimulate global protein synthesis after DC activation, which further induces long term reconfiguration of the cellular metabolism.
mTORC1 drives an increase in anabolism through hypoxia-inducible factor 1α (HIF1α), peroxisome proliferator-activated receptor-γ (PPARγ), sterol regulatory element-binding proteins (SREBPs), and Myc that together induce the synthesis of nucleic acids, proteins and lipids [4,18]. In addition, it drives processes such as glycolysis and mitochondrial respiration to provide both the cellular energy and building blocks for these responses. mTORC2 also participates in enhancing glycolytic metabolism by activating Akt and inactivating forkhead box protein O1 (FoxO1) and FoxO3, which turn on Myc transcription. Activation of mTORC1 by the conditional genetic deletion of tuberous sclerosis 1 (TSC1) promotes glycolysis as well as mitochondrial respiration and lipid synthesis in mouse DCs and hematopoietic stem cells [19–21]. These metabolic adaptions coordinated by the mTOR network regulate DC development, maturation, and other immune responses.
mTOR-Dependent DC Differentiation in the Steady and Inflammatory States
The PI3K-Akt-mTORC1 pathway is important for the development of normal numbers of mouse DCs in vivo, particularly the Flt3L-dependent CD8+ DC and plasmacytoid DC (pDC) subsets as well as Langerhans cells [9,22,23]. Activation of mTORC1 by deletion of PTEN causes an expansion of mouse CD8+ and CD103+ DCs [9]. Similarly, DC-specific loss of mTORC1 leads to a progressive decrease in Langerhans cells in the skin of mice [23]. mTORC1-deficient Langerhans cells have an increased tendency to leave the skin and show increased apoptosis, suggesting that mTORC1 is crucial for the preservation of the Langerhans cell network in mice. Mice with a deletion of the gene encoding late endosomal/lysosomal adaptor, MAPK and mTOR activator 2 (Lamtor2) in CD11c+ DCs display a virtually complete loss of Langerhans cells in the epidermis early after birth due to impaired proliferation and increased apoptosis [24]. Lamtor2 (also known as p14) is a component of the ragulator complex and essential for amino acid-dependent activation of mTORC1 on lysosomes [25]. Oddly, conventional DCs (cDCs) and pDCs are markedly expanded in ageing Lamtor2-deficient mice and demonstrate enhanced mTORC1 activation [26]. Lamtor2 also regulates endosomal biogenesis and therefore decreases Flt3 receptor turnover leading to increased levels of surface Flt3 and increased responsiveness to Flt3L in Lamtor2-deficient mouse DCs. This in turn indirectly activates mTORC1 and promotes the expansion of DCs [26]. However, constitutive activation of mTORC1 by deletion of TSC1 impairs DC development in vivo and in vitro, and is associated with spontaneous maturation, defective proliferation and cell survival [20]. Constitutive activation of mTORC1 in DCs may stimulate harmful generation of reactive oxygen species due to enhanced mitochondrial biogenesis [19]. Collectively, these results argue that a precisely controlled activation of mTORC1 is essential for steady-state myeloid cell development, however, constitutive activation of mTORC1 disturbs DC homeostasis and promotes spontaneous maturation.
Recently, a link between the organismal nutrient status and DC development has been reported. Energy restriction alters myelopoiesis to decrease cDCs and pDCs [27]. Interestingly, such an energy restriction diet increases the numbers of monocytes and macrophages suggesting a reciprocal relation between DCs and macrophages. Whether mTORC1 senses energy restriction to regulate DC and macrophage development is currently unknown.
Human DC development has been modelled in DC differentiation protocols in vitro that mostly rely on human monocytes that are cultured with GM-CSF and IL-4. Inhibition of mTORC1 by rapamycin for the complete differentiation period of about six days has a strong deleterious effect on these monocyte-derived DCs (moDCs) [28,8]. DC numbers are markedly reduced and rapamycin treatment reprograms these moDCs into tolerogenic cells that inhibit subsequent T cell stimulation. Transplant patients under mTOR inhibitor therapy do not have an alteration in cDC and pDC numbers in their blood compared to control patients suggesting that the amount of rapamycin used does not compromise the DC compartment in humans in vivo or that the in vitro models do not adequately reflect the in vivo situation [8,29]. In mice, monocytes can differentiate into DC-SIGN-positive moDCs under inflammatory conditions in vivo and act as classical DCs with potent antigen-presenting functions [30]. Thus, it remains to be determined whether the inhibitory function of mTOR inhibitors for human moDC development is also applicable to inflammatory mouse moDCs.
Spatiotemporal-Dependent Activation of mTOR Signaling in DCs
There are seemingly contrasting reports on immune functions of mTOR in DCs and therefore, mTOR inhibition is sometimes considered pro- as well as anti-inflammatory. Before we discuss these functions of mTOR in detail, we would like to reconcile those discrepancies by proposing a novel spatiotemporal model that considers the role of mTOR in DCs and the effects of mTOR inhibitors in a time and location-dependent manner to define a coherent function of mTOR for this peculiar cell type (Figure 3).
Figure 3. Spatiotemporal-Dependent Activation of mTORC1 Signaling in DCs.
Activation of the mTOR network is likely to be mediated in a context-dependent manner sensing nutrient levels of the cellular microenvironment. Initial activation by pathogen-associated molecular patterns (PAMPs) in tissues stimulates a glycolytic-dependent response in DCs that is mediated by a strong activation of mTORC1. Later in lymph nodes, DCs locally compete for nutrients with T cells, which likely reduces DC-specific mTORC1 signaling to support antigen presentation and T cell stimulation. Pharmacological inhibition of mTORC1 reduces inflammatory responses in the early phase, but enhances T cell responses in lymph nodes by exacerbating a physiological downregulation of mTORC1, which occurs in lymph nodes due to metabolic competition. Downregulation of mTOR activity may support stimulatory or regulatory responses dependent on whether the DCs were stimulated by PAMPs or not, respectively. Abbreviations: mTOR, mammalian target of rapamycin; mTORC1, mTOR complex 1; DCs, dendritic cells.
Many DCs are present in the periphery, where they constantly sense the environment by endocytosis. Once activated by PAMPs or DAMPs, they rapidly produce early proinflammatory signals such as NO, TNF-α or type I interferons to combat the infection or tissue danger. mTORC1 and mTORC2 are activated by TLR ligands and they usually support these responses. Therefore, inhibition of mTOR is considered anti-inflammatory at this time point. Later, these DCs upregulate CCR7 and migrate to lymph nodes to activate T cells. They shut down antigen uptake, optimize antigen presentation and increase the expression of the costimulatory molecule CD86, whereas inhibitory PD-L1 is also induced to prevent excessive T cell activation. Moreover, immunomodulatory cytokines such as IL-12 and IL-10 are maximally expressed at these late time points to guide T helper cell activation and differentiation. Inhibition of mTOR at this time point usually enhances antigen presentation, CCR7, CD86 and IL-12 production and blocks PD-L1 and IL-10 expression, which in total promotes T cell activation. So why do mTORC1 and mTORC2 stimulate the immediately-early response, whereas they limit the T cell stimulatory potential of DCs? We suggest that the nutrient and energy environment along with the oxygen content in individual tissues is key for this difference, which is sensed by the mTOR network to guide DC responses. For example, free amino acid, glucose and lactate as well as oxygen concentrations are profoundly different in interstitial fluid of individual organs, lymph, plasma, or also tumors [31–34]. Moreover, these concentrations are dramatically altered during inflammation [35]. In the periphery, DCs are mostly surrounded by aspirating tissues cells and we can speculate that a certain amount of glucose and other nutrients is present. mTORC1 senses this environment, and hence, is fully activated to promote a glycolytic response to support IFN and NO production. When the DCs then migrate to the lymph nodes, the local metabolic environment changes. Once the DCs activate T cells in the lymph nodes, these T cells become highly glycolytic within minutes and start to compete for glucose and other nutrients, a process that is known in tumors as metabolic competition [36]. Hence, it can be assumed that this limiting amount of nutrients downregulates mTOR in DCs, which may act as intrinsic signal to support their T cell stimulatory capacities. Pharmacological inhibition of mTOR thus exacerbates a response that is physiologically occurring in lymph nodes to support T cell activation. Moreover, the presence of metabolites that are produced by glycolytic T cells such as lactate may further shape T helper cell differentiation by modulating IL-12 and IL-10 expression in DCs [37].
iDCs that are not stimulated by PAMPs can also mature and migrate in a CCR7-dependent way to lymph nodes to generate tolerance by inducing peripheral Treg cells during homeostasis and under non-inflammatory conditions [38]. Inhibition of mTOR maintains CCR7 expression on non-TLR-activated DCs and therefore may also promote Treg induction under these conditions [28]. Alternatively, Treg cells rely on mitochondrial respiration and hence, one can envision that under these conditions metabolic competition in the lymph nodes will be lower and allows these tolerogenic DCs to maintain a stronger mTOR activity in DCs, which may also support Treg induction [39].
In the following paragraphs, we will detail the individual functions of mTORC1 and mTORC2 for DC biology that form the basis of this model and that show the critical role of the mTOR network for DC development, antigen presentation, cytokine production, and T cell stimulation.
mTOR Links Autophagy, Lysosome Function and Antigen Presentation
iDCs continuously sample their environment via macropinocytosis or via receptor-mediated endocytosis and phagocytosis [40]. Mouse bone marrow-derived DCs that are generated in the presence of rapamycin have reduced levels of macropinocytosis and mannose receptor-mediated endocytosis [28], whereas short-term inhibition of mTORC1 does not impact endocytosis in human myeloid DCs and moDCs [8]. In iDCs, internalized exogenous antigens are only slowly degraded and inefficiently used for peptide loading in MHC-II containing compartments, which are equivalent to late endosomes/lysosomes in other cell types [41,42]. Endogenous antigens are relatively well presented on MHC-II molecules by iDCs, because a basal level of autophagy allows cytosolic proteins to transit from the cytosol to endosomes and enter the MHC-II–restricted presentation pathway [43]. mTORC1 inhibits autophagy through its recruitment into the Atg1/ULK1–mAtg13–FIP200 autophagy initiation complex and subsequent Ser757 phosphorylation of ULK1 [44]. Upon TLR stimulation, mTORC1 activity is increased and likely reduces basal levels of autophagy in activated DCs to limit endogenous but enhance exogenous antigen presentation [45]. Inhibition of mTOR during activation could therefore enhance the presentation of endogenous self-antigens on MHC-II molecules. Indeed, rapamycin-treated patients have more alloreactive T cells [46]. In addition, a recent study showed that IL-4 induces autophagy with the consequence of augmenting the processing of endogenous antigens and their MHC-II–restricted presentation [47]. It is interesting to note that interferon (IFN)-γ also enhances autophagy by blocking mTORC1 activation [48] and is also known to enhance endogenous and exogenous antigen presentation [49].
Activated DCs then start to maximize exogenous antigen presentation and co-stimulation to T cells in a process called maturation [40]. Generally, DC maturation involves acidification of the lysosomal compartment by an mTOR-dependent assembly of the vacuolar proton pump V-ATPase and its activation to enhance antigen processing [50]. This facilitates the efficient formation of peptide–MHC-II complexes and their presentation on the plasma membrane for recognition by T cells [41]. In addition, DCs deliver peptide-loaded class II MHC molecules from endolysosomes to the cell surface via generation of long tubular structures, a process that is known as lysosome tubulation and positively supported by mTOR activation [51,52]. Some hours after activation DCs actively induce the expression of the transcription factor TFEB, which induces lysosomal biogenesis to promote exogenous antigen presentation on MHC-II [53]. Because TFEB is directly phosphorylated and inactivated by mTORC1 but also regulated by ERK2 (also known as MAPK1), protein kinase Cβ, GSK3 or lysosomal calcium signaling [54], further work is needed to investigate the functional relationship of mTORC1 and TFEB for antigen presentation in DCs.
There is another aspect of antigen presentation that is regulated by mTORC1: activation of mTORC1 reduces MHC-II mRNA transcription in mouse DCs [55]. iDCs constitutively express class II coactivator (CIITA) to produce high levels of MHC-II mRNA. However, iDCs maintain low levels of MHC-II protein at the cell surface because of ubiquitination and internalization of the MHC-II protein [56]. Upon DC maturation after TLR stimulation, MHC-II ubiquitination is rapidly decreased allowing translocation of MHC-II to the cell surface. Concurrently, TLR stimulation or TSC1 deletion activates mTORC1 and shuts off Ciita transcription [55]. Thus, further transcription of new MHC-II molecules is prevented to allow cell surface presentation of existing peptide-MHC-II complexes. This silencing of MHC-II transcription in mature DCs has been proposed to temporally “fix” the current peptide–MHC-II complexes expressed on the DC surface to promote antigen-specific T cell responses. Thus, activation of mTORC1 on the one hand reduces endogenous antigen presentation by blocking autophagy, stimulates lysosomal activation to augment exogenous antigen processing, and decreases transcription of new MHC-II molecules to adjust antigen presentation. Therefore, it becomes clear that mTORC1 organizes a serial sequence of events that is needed to optimize MHC-II mediated antigen presentation by DCs. Inhibition of mTOR at early time points may therefore reduce antigen uptake but may promote (preferentially endogenous) antigen presentation at later time points.
mTOR Orchestrates Immunomodulatory Cytokine Production in Activated DCs
Activated DCs not only raise antigen presentation, but start to sequentially express a number of inflammatory and immunomodulatory cytokines that shape subsequent immune responses and T cell polarization. Early response cytokines such as IL-6, IL-1 or TNF-α are usually positively or neutrally regulated by mTORC1 [10,8,57]. For example, mTORC1-mediated glycolysis supports inflammasome activation to promote maturation of pro-IL-1β [58], and hence, inhibition of mTOR at this early time point is therefore considered anti-inflammatory. Hours after activation, DCs start to maximally produce immunomodulatory cytokines such as IL-12 and IL-10. Deletion of Rictor and hence mTORC2 activity in mouse DCs boosts IL-12 production [59,60], while one report suggests that mTORC2 deletion in human moDCs decreases IL-12 expression [61]. Inhibition of mTORC1 with rapamycin or catalytic mTOR inhibitors (Box 1) augment expression of IL-12, IL-23, and IL-27 in primary human myeloid cDCs or monocytes [62,63,8,11]. Activation of mTORC1 by deletion of TSC1 or by the addition of excess amino acids reduces IL-12 production by mouse bone marrow-derived DCs [55,20,11]. Whereas inhibition of mTOR inhibits the expression of the IL-12 subunit IL-12p40 in LPS-treated moDCs, co-treatment with IFN-γ again enhances bioactive IL-12p70 production by inducing the second IL-12 subunit IL-12p35 [8]. Interestingly, IFN-γ inhibits mTORC1, which suppresses the translation of repressors of inflammation such as the transcription factor HES1 resulting in the augmentation of NF-κB-dependent inflammatory responses [48]. Therefore, activating mTORC1-dependent translation can bias the expression towards anti-inflammatory cytokines: low abundant mRNAs such as IL-10 are more strongly translated than high abundant pro-inflammatory cytokine mRNAs [14]. Hence, inhibition of mTORC1 by rapamycin decreases production of IL-10 in various cells including DCs [8,10,7]. mTORC1 also promotes IL-10 expression in CD11c+CD11b+ intestinal DCs, which prevents a severe inflammatory response to enteric bacteria following treatment with dextran sulfate sodium in vivo [64]. These modulating effects of mTORC1 inhibition of the late cytokines IL-12 and IL-10 can therefore be considered immunostimulatory. The mTORC1- and mTORC2-dependent molecular pathways that control IL-12 and IL-10 production have been recently reviewed [7].
Dual Control of Type I IFN Production
Type I IFN are important antiviral and immunomodulatory proteins that are most prominently produced by activated pDCs but also cDCs and other cells in response to ssRNA, dsRNA, or unmethylated CpG DNA [65]. Activation of mTORC1 after stimulation by these signals controls type I IFN expression in a dual way by regulating the translation and activation of the transcription factor IFN regulatory factor 7 (IRF7) [66,57,67,68]. Mouse and human pDCs show a constitutive expression of IRF7, which potently stimulates IFNα and IFNβ production in these cells after stimulation of their endosomal TLR7 or TLR8. Efficient translation of IRF7 is induced by mTORC1-dependent phosphorylation and inactivation of the translational inhibitors 4E-BPs [66]. Interestingly, mTORC1 is constitutively active in these cells, potentially supporting continuous IRF7 translation [57]. As second layer of mTORC1-dependent regulation, S6K1 and S6K2 phosphorylate and induce the nuclear translocation of IRF7, which then activates type I IFN transcription [57].
mTOR and the DC: T cell Interaction
The function of mTOR for antigen presentation and immunomodulatory cytokine production suggest that mTOR in DCs modulates activation and polarization of T cells. Moreover, inhibition of mTOR enhances surface expression of the T cell costimulatory molecule CD86, whereas the T cell inhibitory molecule PD-L1 is inhibited [69,64,11,70]. Interestingly, inhibition of mTORC1 blocks NO synthesis, which normally poisons the mitochondria, and thus enhances the life span of LPS-stimulated mouse DCs to potentially increase the time available for T cell activation [69,71]. Initials studies showed that treatment of human or mouse DCs during TLR stimulation with mTORC1 inhibitors augment the proliferation of CD4+ T helper 1 (TH1) and TH17 cells in vitro [11,70,10,69,63]. One study demonstrated that rapamycin can also enhance TH2 responses in vitro [72]. In addition, mTORC2 deficiency in mouse DCs by conditional deletion of the mTORC2 subunit Rictor in CD11c+ cells enhances allogeneic TH1 and TH17 cell responses in vitro and in vivo [60]. Intratumoral delivery of mTORC2-deficient mouse DCs inhibits B16 melanoma growth by promoting CD8+ effector T cell responses [73]. Mice immunized with rapamycin-treated DCs infected with the tuberculosis-vaccine strain BCG show enhanced TH1 cell-mediated protection against virulent Mycobacterium tuberculosis [74]. Therapeutic vaccination using DCs treated with TLR agonists plus rapamycin results in improved generation of CD8+ T cells and improved antitumour immunity in a mouse melanoma model [69]. Treatment of mice with an immunostimulatory agonistic CD40 antibody, together with AZD8055, an ATP-competitive mTOR inhibitor (Box 1), elicits synergistic antitumour responses in a metastatic renal cell carcinoma model [75]. Similarly, administration of rapamycin at the time of DC vaccination prolongs survival in a rat glioma tumor model, which was attributed to enhanced DC activation [76]. Blockade of mTORC1 with rapamycin efficiently boosts TLR-induced antigen-specific T and B cell responses to HBV and HCV vaccines [77]. Accordingly, constitutive activation of mTORC1 in DCs by deletion of Tsc1 inhibits T cell responses in vivo [55,20]. As example in humans, rapamycin-treated transplant patients show increased alloreactive and memory T cell frequencies compared with control patients [46,78]. This effect has been attributed to modulation of the innate immune system by activation of antigen presentation, IL-12 signaling, and increased NF-κB-dependent inflammatory immune responses [78,46]. However, some direct stimulatory effects of rapamycin on memory T cell activation cannot be excluded [79].
However, quite the opposite outcome is also possible: as noted above the differentiation of moDCs in vitro in the presence of rapamycin generates tolerogenic DCs that inhibit effector T cell activation but maintain the ability to stimulate regulatory T cells (Treg) [28]. Tolerogenic DCs generated in the presence of the catalytic mTOR inhibitor Torin 1 also promote the differentiation of Treg cells [80]. Injection of such tolerogenic rapamycin-conditioned DCs into mice can prolong graft survival in various transplantation models [81,82]. Along these lines, administration of antigen together with rapamycin but without a potent TLR stimulus promotes the deletion of conventional CD4+ T cells and induces Treg cells [83]. Interestingly, such an effect is enhanced by Flt3L, which together with rapamycin synergistically promote induction of antigen-specific Tregs via selective expansion of pDCs [83]. These studies in toto suggest, that mTORC1 inhibition in TLR-activated and matured DCs enhance T cell responses, whereas it generates tolerance in non-TLR-activated DCs.
Concluding Remarks
Interest in metabolic regulation of immune responses is growing rapidly. The mTORC1-mTORC2 network constitutes a main rheostat that receives nutrient and energy signals from the intra- and extracellular environment and connects them to growth factor receptor or PRR stimulation to organize many cellular processes including metabolic reprogramming, translation, autophagy and others. In this review, we proposed a novel spatiotemporal activation model of mTOR that might help to reconcile seemingly contrasting effects of mTOR inhibition in DCs. Hence, DCs may emerge as multi-faceted and hierarchical controllers for monitoring nutritional physiology to adjust subsequent immune responses. In that regard, we have to identify the functionally most important nutrients and metabolic pathways that are sensed and regulated by mTORC1 in DCs, respectively. Future research on DC function and metabolism should also include the assessment of the extracellular tissue environment in vivo and the cell culture supernatant in vitro when interpreting results (see Outstanding Questions). For example, cell density and cell culture medium composition profoundly influence immune responses of DCs in vitro [37]. Moreover, we need to develop novel tools that analyze the cellular metabolism of distinct untouched cell types in tissues in vivo without prior isolation. We can expect many novel findings in DC biology regarding metabolic regulation and the mTOR network is likely to take center stage.
Acknowledgements
This work is supported by grants from the Austrian Science Fund (FWF) grant FWF-P27701-B20, the Else-Kröner-Fresenius-Stiftung (P2013_A149), and the Herzfelder’sche Familienstiftung.
References
- 1.Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013;34:440–445. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Walsh KP, Mills KH. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34:521–530. doi: 10.1016/j.it.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Lewis KL, Reizis B. Dendritic cells: arbiters of immunity and immunological tolerance. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a007401. a007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jewell JL, et al. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce EJ, Everts B. Dendritic cell metabolism. Nat Rev Immunol. 2015;15:18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weichhart T, et al. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haidinger M, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 9.Sathaliyawala T, et al. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohtani M, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weichhart T, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Schott J, et al. Translational regulation of specific mRNAs controls feedback inhibition and survival during macrophage activation. PLoS Genet. 2014;10:e1004368. doi: 10.1371/journal.pgen.1004368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lelouard H, et al. Regulation of translation is required for dendritic cell function and survival during activation. J Cell Biol. 2007;179:1427–1439. doi: 10.1083/jcb.200707166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov SS, Roy CR. Pathogen signatures activate a ubiquitination pathway that modulates the function of the metabolic checkpoint kinase mTOR. Nat Immunol. 2013;14:1219–1228. doi: 10.1038/ni.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh AC, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jovanovic M, et al. Immunogenetics. Dynamic profiling of the protein life cycle in response to pathogens. Science. 2015;347 doi: 10.1126/science.1259038. 1259038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoki K, et al. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, et al. Tuberous sclerosis 1 (Tsc1)-dependent metabolic checkpoint controls development of dendritic cells. Proc Natl Acad Sci U S A. 2013;110:E4894–4903. doi: 10.1073/pnas.1308905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan H, et al. The role of tuberous sclerosis complex 1 in regulating innate immunity. J Immunol. 2012;188:3658–3666. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Laar L, et al. PI3K-PKB hyperactivation augments human plasmacytoid dendritic cell development and function. Blood. 2012;120:4982–4991. doi: 10.1182/blood-2012-02-413229. [DOI] [PubMed] [Google Scholar]
- 23.Kellersch B, Brocker T. Langerhans cell homeostasis in mice is dependent on mTORC1 but not mTORC2 function. Blood. 2013;121:298–307. doi: 10.1182/blood-2012-06-439786. [DOI] [PubMed] [Google Scholar]
- 24.Sparber F, et al. The late endosomal adaptor molecule p14 (LAMTOR2) represents a novel regulator of Langerhans cell homeostasis. Blood. 2014;123:217–227. doi: 10.1182/blood-2013-08-518555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sancak Y, et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheffler JM, et al. LAMTOR2 regulates dendritic cell homeostasis through FLT3-dependent mTOR signalling. Nat Commun. 2014;5:5138. doi: 10.1038/ncomms6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duriancik DM, Gardner EM. Energy restriction impairs dendritic cell development in C57BL/6J mice. Mech Ageing Dev. 2016;154:9–19. doi: 10.1016/j.mad.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Thomson AW, et al. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hackstein H, et al. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 30.Cheong C, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertolo RF, et al. Organ and plasma amino acid concentrations are profoundly different in piglets fed identical diets via gastric, central venous or portal venous routes. J Nutr. 2000;130:1261–1266. doi: 10.1093/jn/130.5.1261. [DOI] [PubMed] [Google Scholar]
- 32.Maggs DG, et al. Interstitial fluid concentrations of glycerol, glucose, and amino acids in human quadricep muscle and adipose tissue. Evidence for significant lipolysis in skeletal muscle. J Clin Invest. 1995;96:370–377. doi: 10.1172/JCI118043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gullino PM, et al. The Interstitial Fluid of Solid Tumors. Cancer Res. 1964;24:780–794. [PubMed] [Google Scholar]
- 34.Carreau A, et al. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerra-Romero L, et al. Amino acids in cerebrospinal and brain interstitial fluid in experimental pneumococcal meningitis. Pediatr Res. 1993;33:510–513. doi: 10.1203/00006450-199305000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Chang CH, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasi A, et al. Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol. 2013;191:3090–3099. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- 38.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira GB, et al. Vitamin D3 Induces Tolerance in Human Dendritic Cells by Activation of Intracellular Metabolic Pathways. Cell Rep. 2015;10:711–725. doi: 10.1016/j.celrep.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15:203–216. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trombetta ES, et al. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 42.Delamarre L, et al. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 43.Schmid D, et al. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deretic V. Autophagy as an innate immunity paradigm: expanding the scope and repertoire of pattern recognition receptors. Curr Opin Immunol. 2012;24:21–31. doi: 10.1016/j.coi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallon L, et al. Cellular and molecular immune profiles in renal transplant recipients after conversion from tacrolimus to sirolimus. Kidney Int. 2015;87:828–838. doi: 10.1038/ki.2014.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terawaki S, et al. RUN and FYVE domain-containing protein 4 enhances autophagy and lysosome tethering in response to Interleukin-4. J Cell Biol. 2015;210:1133–1152. doi: 10.1083/jcb.201501059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su X, et al. Interferon-gamma regulates cellular metabolism and mRNA translation to potentiate macrophage activation. Nat Immunol. 2015;16:838–849. doi: 10.1038/ni.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroder K, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 50.Liberman R, et al. Regulated assembly of vacuolar ATPase is increased during cluster disruption-induced maturation of dendritic cells through a phosphatidylinositol 3-kinase/mTOR-dependent pathway. J Biol Chem. 2014;289:1355–1363. doi: 10.1074/jbc.M113.524561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saric A, et al. mTOR controls lysosome tubulation and antigen presentation in macrophages and dendritic cells. Mol Biol Cell. 2016;27:321–333. doi: 10.1091/mbc.E15-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vyas JM, et al. Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells. J Immunol. 2007;178:7199–7210. doi: 10.4049/jimmunol.178.11.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samie M, Cresswell P. The transcription factor TFEB acts as a molecular switch that regulates exogenous antigen-presentation pathways. Nat Immunol. 2015;16:729–736. doi: 10.1038/ni.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Napolitano G, Ballabio A. TFEB at a glance. J Cell Sci. 2016;129:2475–2481. doi: 10.1242/jcs.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan H, et al. Critical role of the tumor suppressor tuberous sclerosis complex 1 in dendritic cell activation of CD4 T cells by promoting MHC class II expression via IRF4 and CIITA. J Immunol. 2013;191:699–707. doi: 10.4049/jimmunol.1201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin JS, et al. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 57.Cao W, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moon JS, et al. mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation. Cell Rep. 2015;12:102–115. doi: 10.1016/j.celrep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Brown J, et al. Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J Biol Chem. 2011;286:44295–44305. doi: 10.1074/jbc.M111.258053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raich-Regue D, et al. mTORC2 Deficiency in Myeloid Dendritic Cells Enhances Their Allogeneic Th1 and Th17 Stimulatory Ability after TLR4 Ligation In Vitro and In Vivo. J Immunol. 2015;194:4767–4776. doi: 10.4049/jimmunol.1402551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei WC, et al. Mammalian target of rapamycin complex 2 (mTORC2) regulates LPS-induced expression of IL-12 and IL-23 in human dendritic cells. J Leukoc Biol. 2015;97:1071–1080. doi: 10.1189/jlb.2A0414-206RR. [DOI] [PubMed] [Google Scholar]
- 62.Turnquist HR, et al. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood. 2010;115:4758–4769. doi: 10.1182/blood-2009-10-251488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macedo C, et al. Rapamycin augments human DC IL-12p70 and IL-27 secretion to promote allogeneic Type 1 polarization modulated by NK cells. Am J Transplant. 2013;13:2322–2333. doi: 10.1111/ajt.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohtani M, et al. Cutting Edge: mTORC1 in Intestinal CD11c+CD11b+ Dendritic Cells Regulates Intestinal Homeostasis by Promoting IL-10 Production. J Immunol. 2012;188:4736–4740. doi: 10.4049/jimmunol.1200069. [DOI] [PubMed] [Google Scholar]
- 65.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colina R, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 67.Fekete T, et al. The antiviral immune response in human conventional dendritic cells is controlled by the mammalian target of rapamycin. J Leukoc Biol. 2014;96:579–589. doi: 10.1189/jlb.2A0114-048RR. [DOI] [PubMed] [Google Scholar]
- 68.Boor PP, et al. Rapamycin has suppressive and stimulatory effects on human plasmacytoid dendritic cell functions. Clin Exp Immunol. 2013;174:389–401. doi: 10.1111/cei.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amiel E, et al. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J Immunol. 2012;189:2151–2158. doi: 10.4049/jimmunol.1103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weichhart T, et al. Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood. 2011;117:4273–4283. doi: 10.1182/blood-2010-09-310888. [DOI] [PubMed] [Google Scholar]
- 71.Amiel E, et al. Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function. J Immunol. 2014;193:2821–2830. doi: 10.4049/jimmunol.1302498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hussaarts L, et al. Rapamycin and omega-1: mTOR-dependent and -independent Th2 skewing by human dendritic cells. Immunol Cell Biol. 2013;91:486–489. doi: 10.1038/icb.2013.31. [DOI] [PubMed] [Google Scholar]
- 73.Raich-Regue D, et al. Intratumoral delivery of mTORC2-deficient dendritic cells inhibits B16 melanoma growth by promoting CD8(+) effector T cell responses. Oncoimmunology. 2016;5:e1146841. doi: 10.1080/2162402X.2016.1146841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jagannath C, et al. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 75.Jiang Q, et al. mTOR kinase inhibitor AZD8055 enhances the immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res. 2011;71:4074–4084. doi: 10.1158/0008-5472.CAN-10-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mineharu Y, et al. Blockade of mTOR signaling via rapamycin combined with immunotherapy augments antiglioma cytotoxic and memory T-cell functions. Mol Cancer Ther. 2014;13:3024–3036. doi: 10.1158/1535-7163.MCT-14-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He L, et al. mTOR regulates TLR-induced c-fos and Th1 responses to HBV and HCV vaccines. Virol Sin. 2015;30:174–189. doi: 10.1007/s12250-015-3606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brouard S, et al. Comparative transcriptional and phenotypic peripheral blood analysis of kidney recipients under cyclosporin A or sirolimus monotherapy. Am J Transplant. 2010;10:2604–2614. doi: 10.1111/j.1600-6143.2010.03302.x. [DOI] [PubMed] [Google Scholar]
- 79.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosborough BR, et al. Murine dendritic cell rapamycin-resistant and rictor-independent mTOR controls IL-10, B7-H1, and regulatory T-cell induction. Blood. 2013;121:3619–3630. doi: 10.1182/blood-2012-08-448290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taner T, et al. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5:228–236. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 82.Li X, et al. Tolerance induction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model. PLoS One. 2012;7:e44045. doi: 10.1371/journal.pone.0044045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biswas M, et al. Synergy between rapamycin and FLT3 ligand enhances plasmacytoid dendritic cell-dependent induction of CD4+CD25+FoxP3+ Treg. Blood. 2015;125:2937–2947. doi: 10.1182/blood-2014-09-599266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luo L, et al. Rab8a interacts directly with PI3Kgamma to modulate TLR4-driven PI3K and mTOR signalling. Nat Commun. 2014;5:4407. doi: 10.1038/ncomms5407. [DOI] [PubMed] [Google Scholar]
- 85.Brazil DP, et al. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 86.Cornu M, et al. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013;23:53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Katholnig K, et al. p38alpha senses environmental stress to control innate immune responses via mechanistic target of rapamycin. J Immunol. 2013;190:1519–1527. doi: 10.4049/jimmunol.1202683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carroll B, et al. Control of TSC2-Rheb signaling axis by arginine regulates mTORC1 activity. Elife. 2016;5:e11058. doi: 10.7554/eLife.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberts DJ, et al. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol Cell. 2014;53:521–533. doi: 10.1016/j.molcel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haller JF, et al. Glucose-6-phosphate isomerase deficiency results in mTOR activation, failed translocation of lipin 1alpha to the nucleus and hypersensitivity to glucose: Implications for the inherited glycolytic disease. Biochim Biophys Acta. 2011;1812:1393–1402. doi: 10.1016/j.bbadis.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Foster DA, et al. Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR) J Biol Chem. 2014;289:22583–22588. doi: 10.1074/jbc.R114.566091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15:555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]