Abstract
The innate immune system is central for the maintenance of tissue homeostasis and quickly responds to local or systemic perturbations by pathogenic or sterile insults. This rapid response must be metabolically supported to allow cell migration and proliferation and to enable efficient production of cytokines and lipid mediators. This Review focuses on the role of mammalian target of rapamycin (mTOR) for controlling and shaping the effector responses of innate immune cells. mTOR reconfigures cellular metabolism and regulates translation, cytokine responses, antigen presentation, macrophage polarization and cell migration. The mTOR network emerges as integrative rheostat that couples cellular activation to the environmental and intracellular nutritional status to dictate and optimize the inflammatory response. A detailed understanding of how mTOR metabolically coordinates effector responses by myeloid cells will provide important insights into immunity in health and disease.
Introduction
Rapamycin, the prototypical inhibitor of mammalian target of rapamycin (mTOR), was discovered in soil samples from Easter Island (locally known as Rapa Nui) in the 1970s, and found to have antifungal activity. Now, it is increasingly recognized that mTOR has manifold functions in mammalian cells and acts as a central regulator of cellular metabolism1,2. As such, the mTOR pathway is targeted as an immunosuppressive and anti-proliferative therapy for transplantation and cancer. Recent studies have established an important role for mTOR complex 1 (mTORC1) and mTORC2 in regulating the functions of innate immune cell populations. It is now clear that the mTOR signalling network functions as an integrative rheostat that orchestrates a broad network of cellular and metabolic activities that shape immune effector responses. Depending on the type of cell, its target tissue, and its extracellular and intracellular nutrient status, the response of an individual immune cell OK is tailored and optimized to its specific needs.
This Review describes the multiple roles of mTOR in monocytes, macrophages, dendritic cells (DCs), neutrophils, mast cells and innate-like natural killer (NK) cells. We discuss the stimuli and signals that activate the mTOR pathway in these cells and the specific effector functions that are controlled by mTORC1 and mTORC2. We review the recent evidence showing that the control of cellular energy metabolism by mTOR is central to directing innate effector responses.
Activation of mTOR in myeloid cells
Activated innate immune cells dramatically change their cell morphology, migrate to new tissue sites, and produce large amounts of cytokines, chemokines and lipid mediators, such as prostaglandins and leukotrienes. Many of these metabolically demanding processes are controlled and shaped by the mTORC1–mTORC2 network after cell activation. The mTORC1–mTORC2 network in innate immune cells is activated by various extracellular signals, including growth factors, Toll-like receptor (TLR) ligands and cytokines (Fig. 1). For example, the growth factors granulocyte/macrophage colony-stimulating factor (GM-CSF) and FMS-related tyrosine kinase 3 ligand (FLT3L) induce mTORC1 activation in DCs and neutrophils3–5; TLR ligands activate mTORC1 and mTORC2 in human and mouse monocytes, macrophages and DCs3,6–12, and mTORC1 in mouse neutrophils13; and the cytokine interleukin-4 (IL-4) promotes mTORC1 and mTORC2 activation in mouse macrophages14,15, whereas IL-15 induces mTOR activity in human and mouse NK cells16. Without these activating signals, the mTOR pathway is strikingly inactive in these cells in vitro, which is in contrast to resting fibroblasts and other primary cells. Activation of mTORC1 and mTORC2 controls a wide array of basic cellular processes such as translation and protein synthesis, cell growth, metabolism and anabolic processes. For example, mouse macrophages and DCs undergo a massive increase in protein synthesis, peaking after 4 hours of lipopolysaccharide (LPS) stimulation, that is largely dependent on the phosphatidylinositol 3-kinase (PI3K)-mTORC1 pathway17,18. Interestingly, this early increase in protein synthesis seems to be independent of glucose influx and glycolysis19. mTORC1 regulates cap-dependent and cap-independent translation of mRNAs by directly phosphorylating the translation inhibitors eIF4E-binding protein 1 (4E-BP1) and 4E-BP2, which then release eukaryotic translation initiation factor 4E (eIF4E). mTORC1 is particularly efficient in promoting translation initiation of mRNAs containing a 5′ terminal oligopyrimidine tract (5′ TOP) or a pyrimidine-rich translational element (PRTE), which encode many ribosomal and translation-related proteins but also metabolism-related genes2,20. In addition, ribosomal protein S6 kinase 1 (S6K1; also known as RPS6KB1) and S6K2 (also known as RPS6KB2) are two other well-characterized targets of mTORC1-mediated phosphorylation, which in turn induce the phosphorylation and activation of S6 to stimulate protein translation. Thus, one major function of mTORC1 is to stimulate global protein synthesis after innate cell activation, which further induces long term reconfiguration of cellular metabolism. In line with this, a recent report showed that the adaptation of metabolism after LPS stimulation in macrophages mainly occurs through proteome remodelling at the translational level21.
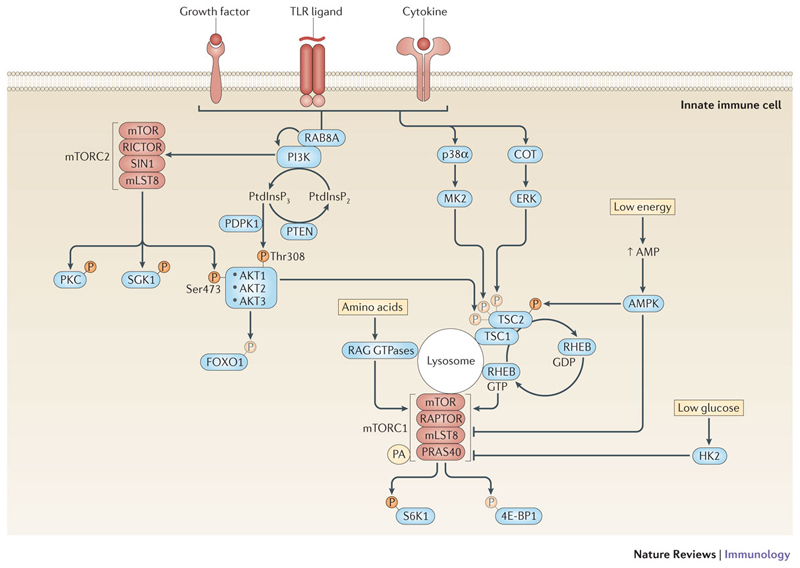
Figure 1. The mTOR pathway in innate immune cells.
In innate immune cells, the mammalian target of rapamycin (mTOR) network can be activated via multiple signals. Growth factors, Toll-like receptor (TLR) ligands or cytokines activate mTOR complex 1 (mTORC1) and mTORC2 through their cognate receptors. Receptor activation leads to the recruitment of class I phosphatidylinositol-3 kinases (PI3Ks) to the receptor complex by different adaptors including the small GTPase RAB8A in macrophages60. PI3Ks generate phosphatidylinositol-3,4,5-trisphosphate (PtdInsP3) as a second messenger to recruit and activate the serine-threonine kinases AKT1, AKT2 and AKT3 via phosphorylation on threonine 308 by phosphoinositide-dependent protein kinase 1 (PDPK1). This process is negatively regulated by phosphatase and tensin homologue (PTEN), which dephosphorylates PtdInsP32. mTORC2 phosphorylates AKT on serine 473, which seems to be required for full activation and substrate specificity of AKT. In addition, mTORC2 phosphorylates protein kinase C (PKC) and serum and glucocorticoid-regulated kinase 1 (SGK1) to regulate important cellular processes such as cytoskeleton reorganization. Two main effectors of AKT are forkhead box O1 (FOXO1) and tuberous sclerosis 2 (TSC2)1. TSC2 forms a heterodimeric complex with TSC1 and inhibits mTORC1. Phosphorylation of TSC2 at threonine 1462 (Thr1462) by AKT inhibits its GTPase-activating protein (GAP) activity for the small GTPase RAS homologue enriched in brain (RHEB), which therefore remains in a GTP-bound active state and activates mTORC1 on the lysosome. In parallel to the PI3K-AKT pathway, the mitogen-activated protein kinases (MAPKs) p38α163,164 and COT also activate mTORC1 via MK2- and ERK-mediated phosphorylation of TSC2, respectively72,165. AMP-activated protein kinase (AMPK) detects low cellular energy by sensing the AMP levels and inhibits mTORC1 by phosphorylating regulatory-associated protein of mTOR (RAPTOR) at serine 792 and TSC2 at serine 1387 that promotes the inhibitory function of the TSC1-TSC2 complex. mTORC1 senses amino acid sufficiency on the lysosome via RAG GTPases and the ragulator complex, which seems to be a prerequisite for mTORC1 activation by growth factors. Moreover, low levels of glucose-6-phosphate inactivate mTORC1 by inducing binding to hexokinase 2 (HK2). Binding of the lipid metabolite phosphatidic acid (PA) to mTORC1 is also a prerequisite for mTORC1 activation. mTORC1-dependent phosphorylation of a variety of downstream effectors, such as eIF4E-binding protein 1 (4E-BP1), 4E-BP2 and ribosomal protein S6 kinase 1 (S6K1), promotes protein synthesis.
mTOR, metabolism and innate immunity
It is now well known that T cells depend on proper metabolic reprogramming to mount effective responses22. Accumulating evidence suggests that innate immune cells also actively control metabolic processes to adapt and optimize their effector functions23. These various effector functions are supported by an adaptation of energy metabolism to cover their metabolic needs and link it to the availability of nutrients. The mTOR network is a central regulator of many core metabolic processes1,24. Activation of mTORC1 usually drives an anabolic response through hypoxia-inducible factor 1α (HIF1α), peroxisome proliferator-activated receptor-γ (PPARγ), sterol regulatory element-binding proteins (SREBPs), and MYC that induces the synthesis of nucleic acids, proteins and lipids1,25. In addition, it drives energy processes such as glycolysis and mitochondrial respiration to provide the cellular energy and building blocks for these responses (Fig. 2). mTORC2 also participates in enhancing glycolytic metabolism by activating AKT and promoting an inactivating phosphorylation of class IIa histone deacetylases24,26. This leads to the acetylation and inactivation of forkhead box protein O1 (FOXO1) and FOXO3, which in turn activates MYC transcription.
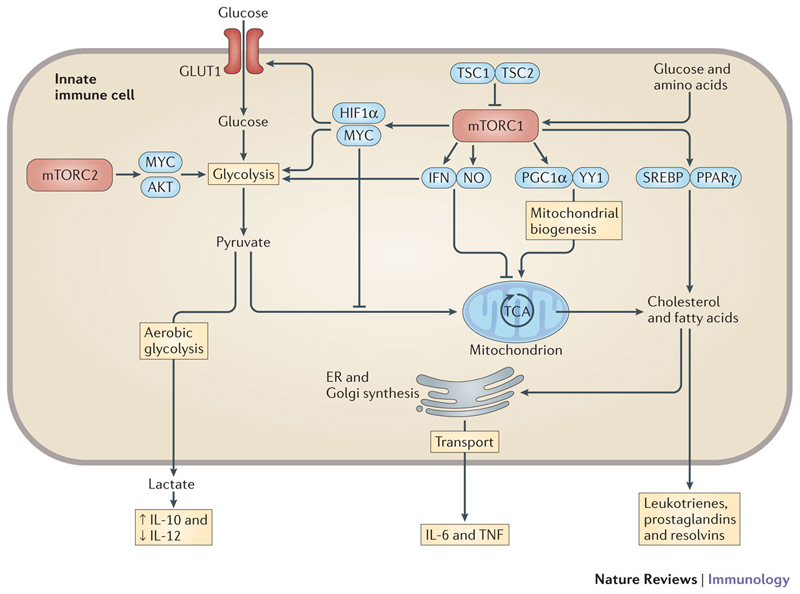
Figure 2. Metabolic control by mTOR in innate immunity.
The mammalian target of rapamycin complex 1 (mTORC1) promotes glycolysis through hypoxia-inducible factor 1α (HIF1α) and MYC, which enhances glucose import by increased expression and surface translocation of glucose transporter 1 (GLUT1) and increased expression of glycolytic genes. Activation of mTORC1 induces mitochondrial biogenesis through the transcription factors PPARγ coactivator 1α (PGC1α) and ying yang 1 (YY1), and promotes cholesterol and fatty acid synthesis from the tricarboxylic acid (TCA) cycle by sterol regulatory element-binding proteins (SREBPs) and peroxisome proliferator-activated receptor-γ (PPARγ). Fatty acids are further metabolized to lipid mediators such as leukotrienes, prostaglandins and resolvins. Cholesterol and fatty acids are also used as building blocks for endoplasmic reticulum (ER) and Golgi synthesis, which can promote the secretion of pro-inflammatory cytokines. This is especially important in lipopolysaccharide-activated dendritic cells, which promote ER and Golgi synthesis predominantly through AKT. mTORC1 can also have a negative effect on mitochondrial respiration by inducing the expression of interferon (IFN) and nitric oxide (NO), which subsequently promote aerobic glycolysis. mTORC2 promotes metabolic reprogramming by activating MYC and AKT, which enhance the expression of various glycolytic enzymes24. Lactate, the end product of aerobic glycolysis, can directly reprogramme macrophages and dendritic cells and reduce the expression of interleukin-12 (IL-12), while enhancing the production of IL-10.
One key organelle where mTORC1 integrates extracellular growth factor activation with nutrient and energy sensing is the lysosome27. Amino acid sufficiency is sensed on the lysosomal surface by the pentameric Ragulator complex and the vacuolar ATPase. This promotes the accumulation of active heterodimeric GTPases called RAGs, which recruit mTORC1 to the lysosomal membrane. Other organelles such as the Golgi or peroxisomes have also been implicated as places of mTORC1 activation28,29. In addition to amino acids, mTORC1 can sense and integrate the AMP/ATP ratio via AMPK25 and the availability of glucose via glucose-6 phosphate and binding of hexokinase II to mTORC130,31 (Fig. 1)30,31. Moreover, the binding of the lipid metabolite phosphatidic acid (PA) is required for mTORC1 activation on the lysosome32. Hence, mTORC1 can sense and integrate all of the main classes of nutrients and energy sources: amino acids, glucose, lipids and ATP. The current model proposes that mTORC1 activation by extracellular signals requires an intracellular sufficiency of nutrients and energy. In turn, mTORC1 stimulates further glucose uptake through the translation of HIF1α, enhances mitochondrial biogenesis through activation of PPARγ coactivator 1α (PGC1α) and ying yang 1 (YY1), and promotes lipid biosynthesis through activation of SREBPs and PPARγ33 (Fig. 2). Genetic activation of mTORC1 by deletion of tuberous sclerosis 1 (TSC1) promotes glycolysis, mitochondrial respiration and lipid synthesis in mouse DCs and haematopoietic stem cells34,35. This metabolic reprogramming in TSC1-deficient DCs occurs partly via mTORC1-mediated expression of MYC, although mTORC2 and AKT are inhibited in these cells35,36. Nevertheless, the specific functions of mTORC1-mediated metabolic reprogramming in innate immune cells are just beginning to be explored.
TLR stimulation of mouse bone marrow-derived DCs, but not primary human myeloid DCs, induces a rapid (after 1 hour) flux of glucose-derived carbon into the tricarboxylic acid (TCA) cycle and pentose phosphate pathway that is dependent on TANK-binding kinase 1 (TBK1), IκB kinase ε (IKKε) and AKT but independent of mTORC1 and HIF1α19,37. This initial increase in glycolysis supports the de novo synthesis of fatty acids that are required for the expansion of the endoplasmic reticulum and Golgi necessary to stimulate the production and secretion of pro-inflammatory molecules such as IL-12, tumour necrosis factor (TNF), IL-6 and CD8619. This is followed (hours later) by an mTOR- and HIF1α-dependent metabolic switch from oxidative phosphorylation to aerobic glycolysis in mouse DCs38–41. This switch is due to the induction of nitric oxide (NO) and type I interferon (IFN) production. NO poisons the mitochondrial respiratory chain necessitating an increase in aerobic glycolysis to promote DC survival39. In these inflammatory mouse DCs, inhibition of mTORC1 inhibits NO production, preserves mitochondrial respiration and extends cell survival40. As human DCs usually produce only low levels of NO, at least in vitro, it is currently unclear whether these results can be extrapolated to human DC biology. Autocrine IFN production by mouse splenic DCs activated with polyinosinic–polycytidylic acid also induces the switch from mitochondrial respiration to aerobic glycolysis via activation of HIF1α41. Because the synthesis of NO by inducible NO synthase (iNOS) is HIF1α dependent42, IFN and NO may both promote the switch to aerobic glycolysis via the activation of HIF1α. As mTORC1 positively controls IFN production (see below), mTORC1 regulates metabolic reprogramming in DCs at least via type I IFN and NO production.
mTORC1-mediated reprogramming from oxidative phosphorylation towards aerobic glycolysis represents the metabolic basis of an effect known as trained immunity. Trained immunity refers to the non-specific protection against secondary infections mediated by epigenetic reprogramming of myeloid immune cells to allow a faster and more pronounced secondary immune response43. Low doses of the cell wall component β-glucan of Candida albicans activate an AKT-mTORC1-HIF1α-dependent glycolytic response in human monocytes and this response is essential for the exacerbated IL-6 and TNF expression that occurs following restimulation of the monocytes after seven days with TLR ligands44. Deletion of HIF1α in myeloid cells in mice impairs the protective effect of β-glucan priming on survival following a lethal S. aureus infection44. In contrast, vitamin D3-induced tolerance in human DCs also requires activation of mTORC1 and aerobic glycolysis45. Interestingly, mTORC1-dependent induction of glycolysis is also important for activation of the NLRP3 inflammasome and maturation of pro-IL-1β in peritoneal macrophages46. Finally, mTORC1-mediated metabolic reprogramming via HIF1α is essential for the proliferation and cytotoxicity in mouse NK cells (see below)47. In conclusion, the current evidence suggests that mTORC1 primes metabolic reprogramming towards enhanced glycolysis via non-mutually exclusive mechanisms in innate immune cells. This can lead to seemingly opposing functional outputs: either enhanced restimulatory potential or tolerance induction; the reasons for these differences are currently unclear. It is important to note that constitutive activation of mTORC1 by deletion of TSC1 or TSC2 also enhance mitochondrial respiration and lipid synthesis35. Therefore, although an increase in mTORC1 activity promotes glycolysis, the generation of pyruvate through this pathway can be used for aerobic glycolysis as well as mitochondrial respiration. It will be important to further link the metabolic mechanisms controlled by the mTOR network to distinct effector responses.
mTOR in myeloid phagocytes
mTOR in innate immune cell development
Activation of the PI3K-AKT-mTORC1 pathway is required for the in vivo development of normal numbers of mouse and human DCs, particularly pDCs and conventional CD8+ DCs, in the presence of FLT3L4,48,49. Genetic activation of PI3K and mTORC1 by deletion of phosphatase and tensin homologue (Pten) in mouse DCs causes a rapamycin-dependent expansion of CD8+ DCs and CD103+ DCs4. However, renal transplant patients treated with the mTOR inhibitor rapamycin have similar numbers of myeloid DCs and pDCs in their blood compared to control patients, suggesting that the doses of rapamycin used in the patients do not compromise the DC compartment in humans in vivo3,49. Deletion of the gene encoding late endosomal/lysosomal adaptor, MAPK and MTOR activator 2 (LAMTOR2; also known as p14) in DCs activates mTORC1 and results in a marked expansion of conventional DCs and pDCs in ageing mice50. LAMTOR2 is a component of the ragulator complex and is thought to be essential for amino acid-dependent activation of mTORC1 on lysosomes. However, LAMTOR2 also regulates endosomal biogenesis and therefore decreases FLT3 turnover, leading to increased levels of surface FLT3 and increased responsiveness to FLT3L in LAMTOR2-deficient mouse DCs. This in turn indirectly activates mTORC1 and promotes the expansion of DCs. By contrast, deletion of TSC1 activates mTORC1 but impairs DC development in vivo and in vitro, and is associated with defective cell survival and proliferation35. Indeed, constitutive activation of mTORC1 might be detrimental as it promotes the generation of reactive oxygen species owing to enhanced mitochondrial biogenesis34. Moreover, deletion of TSC1 is associated with diminished phosphorylation of AKT that usually promotes the survival of various cell types15,34.
DC-specific loss of mTORC1, but not of mTORC2, leads to a progressive decrease in Langerhans cells in the skin of mice51. mTORC1-deficient Langerhans cells have an increased tendency to leave the skin and show increased apoptosis, suggesting that mTORC1 is crucial for the preservation of the Langerhans cell network in mice. Mice with a deletion of LAMTOR2 in CD11c+ DCs display a virtually complete loss of Langerhans cells in the epidermis early after birth due to impaired proliferation and increased apoptosis52. Overall, these results indicate that precisely controlled activation of mTORC1 is crucial for myeloid cell development, whereas constitutive activation of mTORC1 disturbs cell homeostasis.
mTOR-mediated regulation of cytokine production
Treatment of human monocytes or primary myeloid DCs with rapamycin or ATP-competitive mTOR inhibitors (Box 1) enhances their production of IL-12p40 and IL-12p70 after stimulation with TLR ligands3,8,9,11,53,54. Mycobacteria- or LPS-induced production of IL-23 by human monocyte-derived macrophages and monocytes is also enhanced by rapamycin8,55. An immunostimulatory effect of mTOR inhibitors is also seen in human transplant patients in vivo9,56,57. Two human studies showed activation of IL-12-induced signalling and increased inflammatory responses by blood leukocytes from rapamycin-treated transplant recipients56,57. Inhibition of the mTOR pathway with rapamycin can also have immunostimulatory effects in mouse cells and models after bacterial infections8,18,58. Inactivation of mTORC1 by genetic deletion of Rptor (which encodes regulatory-associated protein of mTOR (RAPTOR)) in mouse intestinal DCs induces a severe inflammatory response to enteric bacteria following treatment with dextran sulfate sodium (DSS)59. Genetic inactivation of PI3K and pharmacological inhibition of mTORC1 promotes IL-12 production by mouse immune cells6,8,58,60, although this seems to be more cell-type dependent than mTORC1-mediated enhancement of IL-12 expression by primary human immune cells. Accordingly, deletion of Tsc1 reduces IL-12 production by mouse bone marrow-derived DCs, but promotes IL-12 production by mouse macrophages via activation of Janus kinase 1 (JNK1) and/or JNK214,35,36,61. Interestingly, rapamycin treatment or genetic deletion of mTORC1 does not prevent and still increases IL-12 production by TSC1-deficient macrophages14, suggesting that some TSC1-mediated effects are independent of mTORC1 and that some of the effects of mTOR inhibitors are mediated by inhibition of mTORC2 (Box 1). Indeed, deletion of the mTORC2 component Rictor enhances IL-12 production by macrophages and DCs62–64, potentially through the inactivation of an AKT-dependent inflammatory feedback pathway. After LPS stimulation, mTORC2 directly phosphorylates AKT, which phosphorylates and limits the activation of the pro-inflammatory transcription factor FOXO162. Hence, deletion of mTORC2 activates FOXO1, which augments inflammatory gene expression and IL-12 production62,65. Inactivation of TSC1 and TSC2 could therefore promote inflammatory responses by inhibiting mTORC2, which in turn activates FOXO1.
Box 1. Mechanism of action and clinical use of mammalian target of rapamycin (mTOR) inhibitors.
The prototypic mTOR inhibitor rapamycin inhibits mTOR complex 1 (mTORC1) by associating with FK506-binding protein 12 (FKBP12), which then directly binds to mTORC12. mTORC1 recruits substrates through regulatory-associated protein of mTOR (RAPTOR) that are then further aligned to the catalytic cleft of mTOR. Modelling of the rapamycin-FKBP12 complex bound to mTOR suggests that rapamycin does not inhibit initial substrate recruitment, but blocks correct alignment to the catalytic cleft of some substrates2. This could explain why rapamycin is more effective in blocking the phosphorylation and activation of ribosomal protein S6 kinase 1 (S6K1) than eIF4E-binding protein 1 (4E-BP1). In addition, treatment of some cell types and mice with rapamycin can also variably inhibit mTORC2 through a currently unknown mechanism156.
Rapamycin was approved in 1999 by the Food and Drug Administration (FDA) for use in the prevention of kidney allograft rejection. Rapamycin and its derivative everolimus (RAD0001) are currently used as immunosuppressive therapies in solid organ transplantation157. Pro-inflammatory side effects and dyslipidaemia are frequently observed in these patients157. Rapamycin-eluting stents are used in patients with coronary artery lesions to prevent recurrence of stenosis after percutaneous coronary revascularization158. Clinical anticancer effects of mTORC1 inhibitors are mainly restricted to advanced renal carcinoma, advanced oestrogen receptor-positive or human epidermal growth factor receptor 2 (HER2)-negative breast carcinomas, giant cell astrocytoma and neuroendocrine pancreatic tumours159. The limited success of rapamycin in cancer therapy and the recognition that classical mTOR inhibitors do not inhibit some mTORC1 substrates and are largely ineffective against mTORC2 prompted the development of the ATP-competitive catalytic mTOR inhibitor Torin1 that inhibits mTORC1 and mTORC2. Novel catalytic mTORC1 and mTORC2 inhibitors, such as PP242 or AZD8055, have improved anticancer activity in vitro and in vivo and some candidates have entered clinical trials159. Their clinical efficacy remains to be determined.
The immunostimulatory effects of mTOR inhibition can be explained by enhanced activation of nuclear factor-κB (NF-κB) and inhibition of signal transducer and activator of transcription 3 (STAT3) and glycogen synthase kinase 3β (GSK3β)8,54,57 (Fig. 3a). At the same time, pharmacological or genetic inhibition of mTORC1 strongly reduces expression of the anti-inflammatory cytokine IL-10 by human and mouse monocytes and DCs3,7,8,11,53,59,66,67. This occurs through mTORC1-stimulated degradation of programmed cell death protein 4 (PDCD4), which releases twist-related protein 2 (TWIST2) that subsequently activates IL-10 expression through the transcription factor macrophage-activating factor (MAF)68 (Fig. 3a). Rapamycin prevents the LPS-induced degradation of PDCD4 and suppresses IL-10 production by mouse macrophages. Overall, these data show that inhibition of mTORC1 or mTORC2 generally has an immunostimulatory effect in TLR-stimulated primary myeloid immune cells.
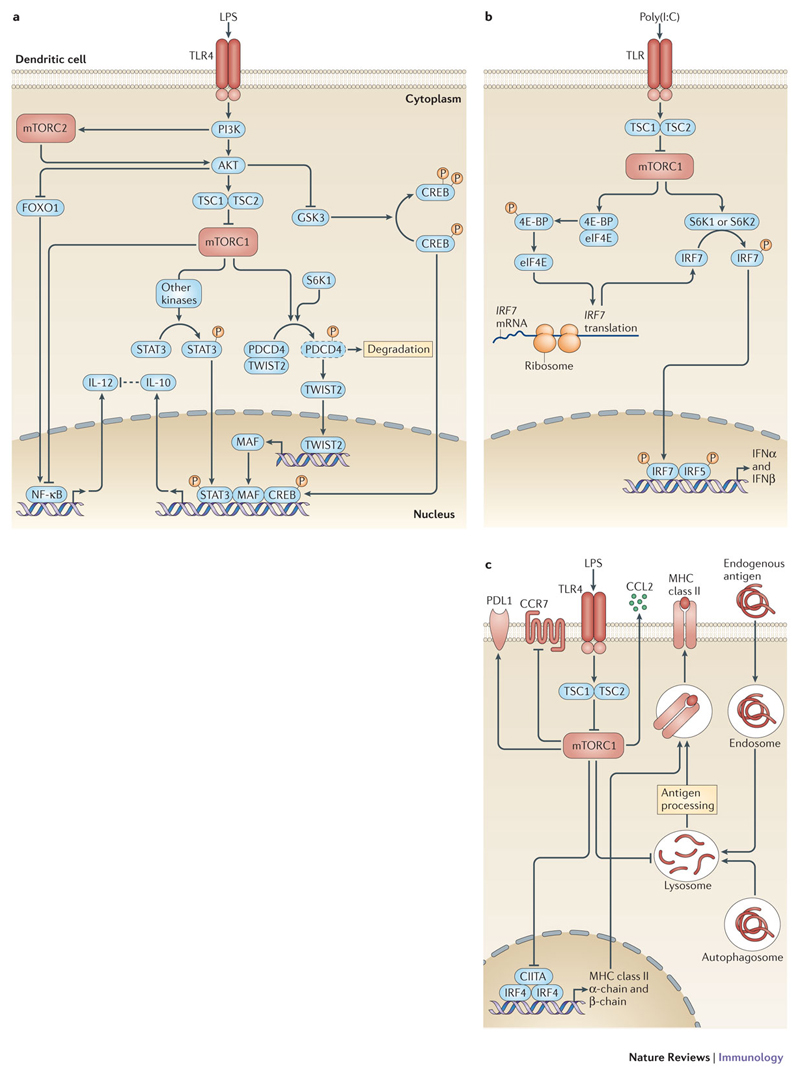
Figure 3. mTOR in dendritic cells (DCs) and its impact on T cell activation.
a| Toll-like receptor (TLR) stimulation regulates interleukin-10 (IL-10) and IL-12 production in a complex manner through mTOR complex 1 (mTORC1) and mTORC2. The AKT-mTORC1 network positively regulates IL-10 expression by at least three non-mutually exclusive mechanisms. mTORC1 induces the phosphorylation of signal transducer and activator of transcription 3 (STAT3) via currently unknown kinases. mTOR or ribosomal protein S6 kinase 1 (S6K1) phosphorylate programmed death cell protein 4 (PDCD4), which subsequently gets degraded by the proteasome. The transcription factor twist-related protein 2 (TWIST2) is released from PDCD4 and translocates to the nucleus to induce the expression of MAF. In addition, AKT phosphorylates and inhibits glycogen synthase kinase 3 (GSK3), which is then unable to phosphorylate and inhibit the transcriptional activity of cAMP response element-binding protein (CREB). Hence, mTORC1 promotes the expression of IL-10 via STAT3, MAF and CREB. mTORC1 decreases the activation of nuclear factor-κB (NF-κB) and the expression of IL-12. In addition, mTORC2 negatively regulates IL-12 production by inducing an AKT-dependent inactivation of forkhead box O1 (FOXO1), which usually promotes NF-κB activation. b| The expression of type I interferon production by DCs is regulated by S6Ks and eIF4E-binding proteins (4E-BPs) downstream of mTORC1. Phosphorylation of 4E-BP1 and 4E-BP2 by mTORC1 releases the eukaryotic translation initiation factor 4E (eIF4E), which promotes the translation of IFN regulatory factor 7 (IRF7) mRNA. In addition, S6K1 and S6K2 phosphorylate IRF7 to activate IFNα and IFNβ production, which is supported by IRF5. c| Lipopolysaccharide (LPS) activates mTORC1 and inhibits the transcription of new MHC class II mRNAs mediated by IRF4 and class II coactivator (CIITA). mTORC1 activation also limits antigen processing by decreasing autophagy, which contributes to the presentation of endogenous and exogenous antigen loading on MHC class I and class II molecules. mTORC1 promotes the expression of programmed cell death ligand 1 (PDL1), which limits T cell activation. mTORC1 positively regulates the production of CC-chemokine ligand 2 (CCL2) and negatively regulates signalling by CC-chemokine receptor 7 (CCR7), thereby affecting the migration of DCs to tissues and secondary lymphoid organs.
Inhibition of mTORC1 in in vitro-generated human monocyte-derived DCs (moDCs) during LPS stimulation usually has no immunostimulatory effect3. Rapamycin does not augment NF-κB signalling in these cells and instead inhibits IL-12p40, TNF and IL-6 expression. Interestingly, mTORC1 is constitutively active in moDCs in contrast to human myeloid DCs3,69; however, the molecular reasons for these discrepancies are currently unclear (Box 2). Moreover, in vitro differentiation of moDCs with GM-CSF and IL-4 in the continuous presence of rapamycin induces their conversion into tolerogenic DCs49,70,71. These tolerogenic DCs are characterized by an immature phenotype and low expression of pro-inflammatory cytokines and T cell stimulatory molecules70. In contrast to human DCs, rapamycin-conditioned mouse tolerogenic DCs are resistant to LPS- or cytokine-mediated maturation and T cell stimulation70, which has been harnessed for experimental cell-based tolerogenic therapies (see later).
Box 2. Pro-inflammatory versus anti-inflammatory effects of mammalian target of rapamycin (mTOR).
There are seemingly contrasting roles of mTOR in individual cells or models, which mirror contradictory data from the phosphatidylinositol 3-kinase (PI3K) pathway160. These observations might be a consequence of different experimental conditions, such as the analysis of primary versus in vitro generated cells or cell lines. Alternatively, species-specific differences between mice and humans concerning the importance of the arginine and nitric oxide system, which is regulated by PI3K-mTOR might explain these results. Importantly, the analysis of the investigated cytokines also influences the researcher’s perception. Inhibition of mTOR usually promotes interleukin-12 (IL-12) expression and blocks IL-10 expression, suggesting a pro-inflammatory (or immunostimulatory) role for mTOR inhibitors. However, tumour necrosis factor (TNF) and IL-6 are often blocked by rapamycin, leading to the conclusion that mTOR inhibitors are anti-inflammatory. The complexity of the pathway itself could also account for differential outputs. For example, inhibition of mTORC1 activates the PI3K-AKT pathway through a feedback loop via ribosomal protein S6 kinase 1 (S6K1). AKT acts upstream of mTORC1 and downstream of mTORC2, and individual AKT isoforms promote distinct macrophage polarization states and AKT phosphorylation status changes substrate specificity161. Tuberous sclerosis 1 (TSC1) and TSC2 have distinct and not completely overlapping functions that are not always mTOR dependent162. Constitutive activation of the mTOR network by TSC1 or TSC2 deletion may change manifold biological processes that differ from short-term activation that occurs after Toll-like receptor stimulation, for example. Deletion of TSC1 or TSC2 consistently reduces activation of mTORC2 and AKT (following serine 473 phosphorylation), which provide feedback to dampen forkhead box O1 (FOXO1)-induced innate immune activation. Incomplete inhibition of mTOR complex 1 (mTORC1) and mostly undefined inhibition of mTORC2 by classical mTOR inhibitors complicate the interpretation of results using inhibitors. Moreover, depending on the in vivo model used, the relative importance of the mTOR network for cell migration and chemotaxis versus cell-intrinsic immunomodulation will dictate the “inflammatory” outcome. These observations suggest that the PI3K-AKT-mTOR network is not a simple linear pathway that transmits a single signal when activated but integrates diverse intracellular and extracellular cues to regulate a diverse set of basic biological processes within the cell to adapt a given effector response to the environment.
Activating mTORC1-dependent translation can bias the expression towards anti-inflammatory cytokines in mouse macrophages: low abundant mRNAs such as IL-10 are more strongly expressed than high abundant pro-inflammatory cytokine mRNAs18. This is important in the defence against a virulent strain of Legionella pneumophila, which induces ubiquitylation and inactivation of mTOR and AKT in macrophages, stimulating a pro-inflammatory cytokine response and bacterial clearance18. Hence, blocking mTOR with rapamycin favours the translation of the more abundant pro-inflammatory cytokines. On the other hand, the proto-oncogene cancer Osaka thyroid oncogene (COT; also known as MAP3K8 and TPL2) positively controls cap-dependent mRNA translation of TNF and IL-6 through mTORC1 in mouse TLR-stimulated macrophages72. Nevertheless, translational control of mRNA expression can dampen the pro-inflammatory response73. Stimulation of mouse macrophages with LPS activates the translation of many feedback inhibitors such as NF-κB inhibitors and dual specificity protein phosphatase 1 (DUSP1) to limit an excessive inflammatory and self-destructive response73. A potential role of mTORC1 for regulating translation of these inhibitors has not been investigated but might explain some of the immunostimulatory effects of mTORC1 inhibition.
Translational control is also important for the regulation of type I IFN production by myeloid DCs and pDCs. Efficient translation of the transcription factor IFN regulatory factor 7 (IRF7), which stimulates IFNα and IFNβ production in pDCs, is induced by the mTORC1-dependent phosphorylation and inactivation of the translational inhibitors 4E-BPs74 (Fig. 3b). Of note, mouse and human pDCs show constitutive expression of IRF7 in contrast to other DCs, and this correlates with a constitutively active mTORC1 pathway in these cells75. The Leishmania major protease GP63 directly cleaves mTOR and maintains translational repression by 4E-BP1. This suppresses the translation of type I IFNs and microbicidal genes and confers an intracellular survival advantage for L. major76. Moreover, S6K1 and S6K2 phosphorylate and induce the nuclear translocation of IRF7, which together with IRF5, stimulates type I IFN production10,75,77 (Fig. 3b). Therefore, the mTORC1 downstream effectors 4E-BPs and S6Ks positively control the translation and activation of IRF7 and thus type I IFN expression.
mTOR-mediated control of macrophage polarization
Macrophage function depends on proper activation and polarization into distinct subtypes with individual effector functions, such as the M1 and M2 macrophage subsets (for a discussion see REF.78). Macrophage polarization is thought to be regulated not only by cytokines and growth factors but also by environmental cues, such as the presence of nutrients, fatty acids and other metabolites79,80. Therefore, it is not surprising that PI3K-AKT-mTOR-mediated sensing of environmental and metabolic cues influences macrophage polarization in a complex and incompletely understood manner (Table 1).
Table 1. Genetic deletion of mTOR network components in macrophages and their phenotypes.
| Target gene (protein encoded) | Genetic deletion | Number of M1 macrophages (relative to controls) | Number of M2 macrophages (relative to controls) | Polarization evidence | Biochemical features | In vivo phenotype | Refs. |
|---|---|---|---|---|---|---|---|
| Pten (PTEN) | LysM-Cre mediated | decreased | increased | in vivo | AKT enhanced; STAT6 and CEBPβ increased; polarization mediated by PI3K | Increased protection from ischaemia reperfusion injury and EAE | 82,83 |
| Inpp5d (SHIP) | Full gene knockout | no change | increased | in vitro | STAT6 increased; polarization mediated via PI3K | No obvious phenotype | 81 |
| Pdpk1 (PDPK1) | LysM-Cre mediated | increased | decreased | in vitro; in vivo | AKT reduced; enhanced migration via FOXO1 and CCR2 | Enhanced insulin resistance | 90 |
| Prkaa1 (AMPKα1) | Full gene knockout | increased | no change | in vitro | mTORC1, AKT, PI3K and SOCS3 reduced | No obvious phenotype | 91 |
| Prkab1 (AMPKβ1) | Deficient bone marrow transplanted into wild-type mice | increased | decreased | in vivo | AKT reduced; JNK enhanced | Enhanced insulin resistance and inflammation | 92 |
| Akt1 (AKT1) | Full gene knockout | increased | decreased | in vivo | STAT6 no significant change | Increased sensitivity to LPS-induced shock and DSS-induced colitis | 88 |
| Akt2 (AKT2) | Full gene knockout | decreased | increased | in vivo | CEBPβ increased; STAT6 no significant change | Increased resistance to LPS-induced shock and DSS-induced colitis | 88 |
| Akt2 (AKT2) | Deficient bone marrow transplanted into LDLR- mice | decreased | increased | in vivo | Impaired migration | Reduced atherosclerosis | 89 |
| Tsc1 (hamartin or TSC1) | LysM-Cre mediated | increased | decreased | in vitro, in vivo | mTORC1 enhanced, AKT reduced (causing decreased M2 polarization); STAT6 no significant change | reduced M2 polarization in response to IL-4 and chitin | 15 |
| Tsc1 | LysM-Cre mediated | increased | decreased | in vitro; in vivo | mTORC1 enhanced, AKT, reduced; enhanced M1 polarization due to RAS-ERK; reduced M2 polarization due to mTORC1-CEBPβ | Increased sensitivity to LPS; resistant to OVA-induced asthma | 14 |
| Tsc1 | LysM-Cre mediated | increased | increased | in vitro | Impaired migration and T cell activation | Spontaneous lymphoproliferative disorder | 86 |
| Rptor (RAPTOR) | LysM-Cre mediated | decreased | increased | in vivo | mTORC1 reduced; AKT enhanced; reduced JNK and NF-κB | Increased protection against insulin resistance and inflammation | 87 |
| Prr5 (Rictor 1) | LysM-Cre mediated | increased | decreased | in vitro | AKT reduced; MAP and NF-κB no significant change | Increased susceptibility to LPS-induced shock | 63 |
PTEN, phosphatase and tensin homologue; SHIP, SH2-domain containing inositol-5-phosphatase; PDPK1, phosphoinositide-dependent protein kinase-1; AMPK, AMP-activated protein kinase; TSC1, tuberous sclerosis 1; Raptor, regulatory associated protein of mTOR; Rictor, rapamycin-insensitive companion of mTOR; LysM, lysozyme 2; LDLR, low density lipoprotein receptor; STAT6, signal transducer and activator of transcription 6; Pi3K, phosphatidylinositol-3 kinases; Foxo1, forkhead box O1; CCR2, CC-chemokine receptor 2; SOCS3, suppressor of cytokine signaling 3; CEBPβ, CCAAT/enhancer-binding protein β; MAP, mitogen-activated kinase; EAE, experimental autoimmune encephalomyelitis; DSS, dextran sulfate sodium
Mouse macrophages with constitutively enhanced PI3K and mTOR signalling caused by myeloid cell-specific deletion of Pten or Inpp5d (which encodes SHIP) express higher levels of M2 markers and have enhanced activation of the STAT6 signalling pathway81–83. Inhibition of mTORC1 with rapamycin in human macrophages enhances M1 macrophage polarization67,84. These results indicate that activation of the PI3K-mTOR network promotes M2 macrophage polarization6,8,59,85. However, increased mTORC1 activity and reduced mTORC2 activity by ablation of Tsc1 promotes M1 macrophage polarization and reduces M2 macrophage polarization14,15. This seems to be mediated by different pathways that are independent of STAT6 and PPARγ, two well-known M2 macrophage-promoting proteins. Tsc1 deletion promotes M1 macrophage polarization by activating a RAS GTPase–RAF1–MEK–ERK pathway that is independent of mTORC114. In addition, deletion of Tsc1 reduces the expression of CEBPβ and M2 macrophage polarization by activation of mTORC114. In contrast, another group has found that deletion of Tsc1 in macrophages enhances M2 as well as M1 polarization86. Inhibition of mTORC1 by deletion of Rptor in myeloid cells protects against inflammation in an obesity model and seems to enhance M2 macrophage polarization87. Moreover, a positive signal of TSC1 to AKT can also drive M2 macrophage polarization15. However, the role of AKT is not straightforward, because the AKT isoforms differentially control macrophage polarization88. Ablation of AKT1 gives rise to M1 macrophages, and deletion of AKT2 promotes a M2 macrophage phenotype in in vivo models of colitis and atherosclerosis88,89. Akt2-knockout macrophages show enhanced expression of CEBPβ. Deletion of the kinase phosphoinositide-dependent protein kinase 1 (PDPK1) in macrophages that is crucial for AKT activation (Fig. 1) elevates M1 macrophage polarization and promotes insulin resistance90. In line with this, deletion of AMPK reduces AKT activation and promotes M1 macrophage polarization91,92. Finally, mice with a myeloid cell-specific mTORC2 deletion show stronger M1 macrophage polarization potential and reduced M2 macrophage polarization potential63. Overall, these results indicate that macrophage polarization is controlled by the PI3K-AKT-mTOR pathway, however, a complete understanding of the functional roles of the individual pathway members requires further research (Box 2).
There is initial evidence that macrophage polarization might also be regulated by metabolic reprogramming93. In that regard, activation of pyruvate dehydrogenase kinase 1 (PDK1), a positive regulatory enzyme in glucose metabolism, promotes mouse M1 macrophage polarization94. Surprisingly, MYC, a stimulator of aerobic glycolysis, is highly expressed in M2 macrophages and crucial for M2 macrophage polarization by inducing around half of the genes associated with alternative activation95. It is interesting that lactic acid — an end product of aerobic glycolysis — primes M2-like macrophage polarization in mouse macrophages and inhibits IL-12 production but enhances IL-10 production by human monocyte-derived DCs96,97. Hence, one can envision that strongly glycolytic M1 macrophages prime for subsequent M2 macrophage responses. Indeed, the presence of M1 macrophages is a prerequisite for the successive emergence of M2 macrophages and tissue homeostasis during wound healing or Listeria monocytogenes infections98,99. Moreover, these results, together with the notion that the AMPK-AKT-mTOR network is a global sensor of nutrients and energy, argue for caution with regard to cell density and cell culture composition in in vitro experiments when analysing macrophage polarization or innate immune cell activation. Whether the mTOR network mediates macrophage polarization via metabolic reprogramming is currently unclear, but warrants further investigation.
mTOR-mediated regulation of autophagy
The starvation-induced degradation of cytosolic components known as autophagy is crucial in providing substrates for energy production under catabolic conditions of limited nutrient supply. It is important to note that autophagy in macrophages is not a strictly catabolic process but is actively engaged after macrophage activation by a TLR-TRAF6-Beclin 1-dependent pathway to promote basic effector functions100. Autophagy supports the elimination of intracellular pathogens and phagocytosed materials, and facilitates the synthesis of secretory proteins, such as IL-6 and IL-8, by providing amino acids100–102. mTOR and AMPK control the Ser/Thr kinases ULK1 and ULK2, which are an additional system that controls autophagosomal formation and autophagic flux100. mTORC1 actively suppresses autophagy by phosphorylating ULK1 and, accordingly, inhibition of mTORC1 strongly induces autophagy100. However, termination of autophagy and reformation of lysosomes requires reactivation of mTORC1103. Autophagy regulation by mTORC1 in mouse macrophages is an important defence mechanism against Mycobacterium tuberculosis, Salmonella typhimurium and Pseudomonas aeruginosa and limits their intracellular growth104–107. Mycobacterial infection enhances the expression of miR-155, which inhibits the expression of the small GTPase RAS homologue enriched in brain (RHEB) by binding to the 3’-untranslated region of RHEB mRNA105. This leads to mTORC1 inhibition, activation of autophagy and enhanced intracellular killing of the mycobacteria in vitro and in vivo105. S. typhimurium encodes genes on the pathogenicity island 2 that activate focal adhesion kinase (FAK). This leads to enhanced AKT and mTOR signalling, which inhibits autophagy and promotes intracellular pathogen survival in mouse macrophages108. To evade immune-mediated control, HIV-1 induces a shutdown of autophagy after infection of human DCs by activating mTOR109.
Defects in macrophage autophagy are linked to the progression of atherosclerosis by regulating cholesterol homeostasis, oxidative stress and inflammation110. Autophagy promotes the degradation and clearance of ingested complex lipids such as low-density lipoprotein (LDL)-cholesterol. Accordingly, activation of mTOR enhances the accumulation of lipids and foam cell formation owing to inhibition of autophagy, whereas genetic or pharmacological inhibition of mTORC1 reduces atherosclerotic disease, macrophage plaque infiltration and systemic inflammation in various animal models111–114. These results suggest that mTORC1 inhibition could be an approach to treat atherosclerosis.
mTOR in antigen presentation and T cell stimulation
mTORC1 is intimately involved in antigen presentation by DCs and thereby able to modulate their T cell stimulatory capacity. As autophagy contributes to the presentation of endogenous and exogenous proteins on MHC class I and class II molecules115, it follows that autophagy induction by mTOR inhibitors enhances antigen presentation116 (Fig. 3c). However, endocytosis of mouse splenic DCs is decreased in rapamycin-treated mice in vivo117. Analysis of transplant patients shows that the antigen processing pathway is positively regulated in rapamycin-treated patients, predominantly by triggering antigen presentation56. Accordingly, chronic activation of mTORC1 by deletion of Tsc1 strongly reduces MHC class II expression by mouse DCs61. In resting wild-type DCs, mTORC1 activity is low, allowing expression of the class II coactivator (CIITA), which stimulates MHC class II mRNA transcription. LPS stimulation or Tsc1 deletion activates mTORC1 and rapidly shuts off Ciita transcription. Thereby, the further transcription of new MHC class II molecules is prevented allowing efficient cell surface expression of existing peptide-MHC class II complexes61. Inhibition of mTOR promotes the expression of the T cell costimulatory molecule CD86, whereas expression of the T cell inhibitory molecule PD-L1 is decreased3,8,37,59 (Fig. 3c). Moreover, inhibition of mTORC1 enhances the life span of LPS-stimulated mouse DCs, increasing the time available to drive T cell activation37,40.
This evidence, together with its IL-12 and IL-10 skewing potential, predicts that inhibition of mTORC1 in DCs promotes T cell activation. Indeed, inhibition of mTORC1 in human or mouse DCs during TLR stimulation augments the proliferation of CD4+ T helper 1 (TH1) and TH17 cells in vitro7–9,37,53. Similarly, mTORC2 deficiency in mouse DCs also enhances TH1 and TH17 cell responses64. Mice immunized with rapamycin-treated DCs and the tuberculosis-vaccine strain BCG show enhanced TH1 cell-mediated protection against virulent Mycobacterium tuberculosis116. Therapeutic vaccination using DCs treated with TLR agonists plus rapamycin results in improved generation of CD8+ T cells and improved antitumour immunity in a mouse melanoma model37. Treatment of mice with an immunostimulatory agonistic CD40 antibody, together with AZD8055, an ATP-competitive mTOR inhibitor, elicits synergistic antitumour responses in a metastatic renal cell carcinoma model12. Accordingly, constitutive activation of mTORC1 in DCs by deletion of Tsc1 inhibits T cell responses in vivo35,61. Finally, rapamycin-treated transplant patients show increased alloreactive T cell frequencies compared with control patients56. These potent immunostimulatory effects of rapamycin in DCs have to be considered in the context of the immunosuppressive and immunomodulatory effects on T cells on a systemic level118. Nevertheless, it might be possible to design novel human vaccination strategies, in which local administration of mTOR inhibitors together with an antigen-adjuvant complex or with antigen-loaded DCs might allow for an improved immune response. However, a seemingly different outcome with rapamycin can also be generated: as noted above the differentiation of moDCs in vitro in the presence of rapamycin generates tolerogenic DCs that inhibit effector T cell activation but maintain the ability to stimulate regulatory T cells71. Tolerogenic DCs generated with the catalytic mTOR inhibitor Torin 1 (Box 1) also promote the differentiation of regulatory T cells119. Injection of such tolerogenic rapamycin-conditioned DCs into mice can prolong graft survival in various transplantation models70,120.
mTOR in innate immune cell migration
mTOR signalling has contrasting effects on innate immune cell migration. On the one hand, mTORC1 inhibition enhances the expression of CC-chemokine receptor 7 (CCR7) by human monocytes and DCs67,121, as well as T cells122, and thereby promotes the migration of DCs and mouse Langerhans cells51,121 to secondary lymphoid nodes and the induction of adaptive immune responses (Fig. 3c). On the other hand, mTORC1 inhibition reduces CC-chemokine ligand 2 (CCL2) secretion by human and mouse monocytes, macrophages and DCs, and thereby reduces chemotactic activity of monocytes and basophils8,113,114,123. This effect of mTORC1 inhibition has been shown to be beneficial in mouse and rabbit models of atherosclerosis113,123. By contrast, AKT-dependent activation of mTORC1 induces a translational switch from the transcription factor isoform CEBPβ long (LAP) to the dominant-negative isoform CEBPβ liver inhibitory protein (LIP) after activation of the bile acid-responsive G protein-coupled receptor TGR5 in mouse macrophages. This diminishes the expression of the chemokines CCL2, CCL3 and CCL4 that have CEBPβ binding sites and the migratory properties of TGR5-activated macrophages124. Adherence of mouse peritoneal macrophages rapidly induces the mTORC1-dependent de novo translation of pre-existing mRNAs encoding Rho-associated kinase 1 (ROCK1), which promotes macrophage chemotaxis125. Therefore, the current evidence suggests that mTORC1 inhibition enhances the migration of DCs, whereas it usually inhibits the migratory properties of macrophages.
mTOR in granulocytes
Neutrophil granulocytes are a first line of defence during infections, inflammation and tissue injury. They are rapidly recruited by chemotaxis and migrate to the site of action to kill pathogens by phagocytosis, degranulation and neutrophilic extracellular trap (NET) formation126. Chemotactic migration is controlled by mTORC2 downstream of G-protein-coupled receptors in a dual manner127 (Fig. 4a). mTORC2 regulates actin cytoskeleton organization at the leading edge of migrating cells, and cells that lack the mTORC2 component Rictor exhibit defects in cell polarity and chemotaxis128,129. Small RHO GTPases and AKT2 serve as downstream effectors of mTORC2 to regulate actin assembly at the leading edge in human and mouse neutrophils128,130. In addition, mTORC2 is crucial for tail retraction in human neutrophils via the regulation of myosin II129. mTORC2 phosphorylates PKCβII, which activates adenylyl cyclase 9 to convert ATP into cAMP129,131. cAMP activates RHOA to further induce myosin II phosphorylation and tail retraction129,131. Interestingly, rapamycin inhibits human neutrophil migration very efficiently, indicating that mTORC1 might also have a direct role in cell migration132,133. Indeed, mTORC1 controls F-actin reorganization through S6K1 as well as focal adhesion formation and RHO GTPase activity in other cell types127.
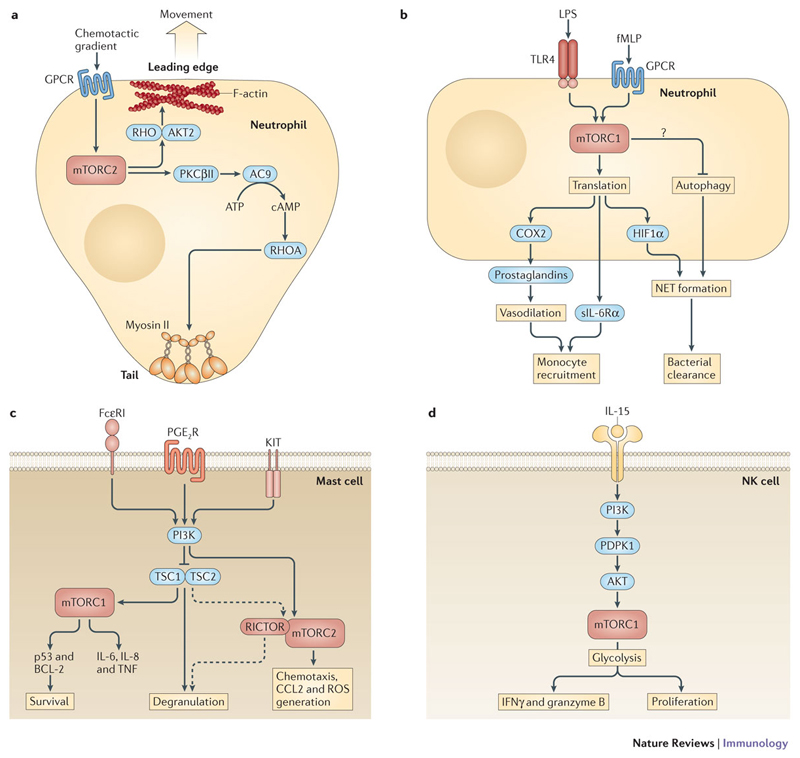
Figure 4. mTOR in neutrophils, mast cells and natural killer (NK) cells.
a| Chemotactic migration of neutrophils and mast cells is controlled by G-protein coupled receptor (GPCR)-mediated activation of mTOR complex 2 (mTORC2). mTORC2 activates RAS homologue (RHO) GTPases and AKT2 to regulate F-actin assembly at the leading edge. mTORC2 is also critical for tail retraction by phosphorylating protein kinase C βII (PKCβII). PKCβII then activates adenylyl cyclase 9 (AC9), which converts ATP into cAMP to activate RHOA. RHOA induces myosin II phosphorylation and tail retraction. b | mTORC1 controls the translation of preformed cyclooxygenase 2 (COX2) mRNA in LPS- or formyl-methionyl-leucyl-phenylalanine (fMLP)-activated neutrophils to induce the expression of prostaglandins that promote vasodilation. Soluble interleukin-6 receptor-α (sIL-6Rα) is synthesized via mTORC1. sIL-6Rα translocates to endothelial cells and induces a switch from neutrophil to mononuclear leukocyte recruitment to initiate the resolution of inflammation. mTORC1 promotes LPS-mediated neutrophil extracellular trap (NET) formation by translational control of hypoxia-inducible factor 1α (HIF1α) expression. Activation of mTORC1 can also negatively influence fMLP-stimulated NET formation by inhibiting autophagy. c | Activation of the high affinity receptor for IgE (FcεRI), prostaglandin E2 receptor (PGE2R) or the KIT receptor for stem cell factor (SCF) results in rapid phosphatidylinositol 3-kinase (PI3K)-dependent activation of mTORC1 and mTORC2 in mast cells. mTORC1 is required for mast cell survival and the expression of IL-8 and tumour necrosis factor (TNF) following FcεRI stimulation. However, constitutive activation of mTORC1 by loss of tuberous sclerosis 1 (TSC1) is detrimental and increases apoptosis by enhancing the expression of p53, which reduces the expression of B cell lymphoma 2 (BCL-2). mTORC2 promotes PGE2-mediated chemotaxis, CC-chemokine ligand 2 (CCL2) expression and reactive oxygen species (ROS) production. TSC1 promotes mast cell degranulation and histamine production independent of mTORC1. RICTOR contributes to FcεRI-induced degranulation independent of mTORC2. d | IL-15 activates mTORC1 through PI3K, phosphoinositide-dependent protein kinase 1 (PDPK1) and AKT in NK cells. mTORC1 promotes NK cell proliferation and the acquisition of cytotoxicity through the induction of interferon-γ (IFNγ) and granzyme B production. mTORC1-mediated cytotoxicity requires metabolic reprogramming and enhanced glycolysis.
The translation of constitutively present mRNAs by mTORC1 emerges as an important mechanism by which neutrophils rapidly react to inflammatory insults134–136 (Fig. 4b). For example, mTORC1 controls the translation of pre-existing cyclooxygenase 2 mRNA in human neutrophils to induce the rapid expression of prostaglandins that promote inflammation and vasodilation to attract further immune cells134. The soluble IL-6 receptor-α (sIL-6Rα) is synthesized in an mTORC1-dependent manner without the requirement of de novo transcription136. sIL-6Rα subsequently signals to endothelial cells and induces a switch from neutrophil recruitment to mononuclear leukocyte recruitment to initiate the resolution of acute inflammation137. Therefore, inhibition of mTORC1 in neutrophils is anti-inflammatory at early phases of inflammation, whereas it can prolong the acute inflammatory phase at later stages. Inhibition of mTOR by rapamycin can differentially regulate NET formation in primary human neutrophils depending on the stimulus138–140. Whereas rapamycin inhibits LPS-mediated NET formation by translational control of HIF1α expression, it enhances formyl-methionyl-leucyl-phenylalanine (fMLP)-stimulated NET formation138,139. The reason for these differences is currently unclear but may be due to differential activation of NADPH oxidases and autophagy by LPS and fMLP138,139,141. Finally, mTORC1 positively contributes to the expression of pro-inflammatory IL-8, IL-6 and TNF in human and mouse neutrophils via a transcriptional mechanism in vitro and in TLR4-induced lung injury in vivo13,142.
So far, relatively little is known about the role of mTOR in eosinophil granulocytes. Rapamycin inhibits the differentiation, proliferation and function of mouse eosinophils in vitro and in vivo143.
mTOR in mast cells
Mast cells contribute to host defence and also to allergic disorders and asthma144. They migrate into inflamed tissues and release pro-inflammatory mediators by degranulation upon activation of cell surface receptors such as the high affinity IgE receptor (FcεRI), the prostaglandin E2 (PGE2) receptor or the receptor for stem cell factor, KIT. In human and mouse mast cells, stimulation of these receptors results in a rapid PI3K-dependent activation of mTORC1 and mTORC2145–147 (Fig. 4c). Activation of mTORC1 is required for human and mouse mast cell survival and the expression of IL-8 and TNF after FcεRI stimulation145,148. mTORC2 activity also contributes to PGE2-mediated chemotaxis, CCL2 expression, and reactive oxygen species (ROS) production146. However, constitutive activation of mTORC1 by loss of Tsc1 is detrimental and increases mouse mast cell apoptosis by enhancing mitochondrial ROS production and expression of p53, which reduces the expression of the anti-apoptotic molecule B-cell lymphoma 2 (BCL-2)148. TSC1-deficient mast cells show defective degranulation and histamine production in vitro and in vivo148. Interestingly, rapamycin is incapable of restoring the impaired degranulation in TSC1-deficient cells suggesting an mTORC1-independent mechanism. Surprisingly, downregulation of Rictor increases FcεRI-induced degranulation independently of mTORC2149. Indeed, about 50% of Rictor is not localized to mTOR in human mast cells149.
mTOR in NK cells
NK cells are group 1 innate lymphoid cells (ILC1s) involved in immune surveillance of cancers and in the control of intracellular infections150. IL-15 is important for the homeostasis, activation and maturation of NK cells. High but not low concentrations of IL-15 activate mTORC1 via PDPK1 and AKT16,151,152 (Fig. 4d). This mTORC1 activation is important for efficient mouse NK cell proliferation and the acquisition of cytotoxicity through the production of IFNγ and granzyme B16,47,151. Rapamycin or genetic deletion of mTOR in NK cells blocks NK cell proliferation, IFNγ and granzyme B expression, and results in elevated viral burdens upon mouse cytomegalovirus infection16,151. Rapamycin inhibits NK cell proliferation and cytotoxicity in humans, and there is a significant decrease in NK cells in rapamycin-treated transplant recipients153. Upon activation, mouse NK cells undergo mTORC1-dependent metabolic reprogramming to upregulate glucose uptake and increase aerobic glycolysis47. Directly limiting the rate of glycolysis is sufficient to block cytotoxic activity by NK cells47, indicating that NK cells are exquisitely dependent on mTORC1-regulated glucose import and use. The finding that rapamycin inhibits IL-5 and IL-13 production by IL-33-stimulated ILC2s suggests a broader role of mTOR in the metabolic regulation of ILC functions154.
Summary and future directions
The past few years have provided us with a wealth of information about how the mTOR signalling pathway operates in innate immunity. A central paradigm emerges suggesting that activation of innate immune cells via pattern recognition or growth factor receptors triggers the pathway, which further integrates the environmental and intracellular nutritional and energy status to guide and shape the effector response. This occurs partially via the reconfiguration of cellular metabolism to provide energy and building blocks for the subsequent immune response of the cell. In addition, mTOR modulates central transcription pathways, such as NF-κB, STAT3, HIF1α and PPARγ pathways, in a cell-specific manner. While mTOR broadly supports many innate immune functions, a proper timed activation pattern of the mTOR network seems equally essential and might explain some of the unexpected observations in cells, in which mTORC1 is constitutively activated. Moreover, the realization that the environmental nutrient state is sensed by mTOR will have important implications in the design and interpretation of in vitro cell culture experiments. Our current knowledge is just the starting point for further studies that will shed new light on the impact of the mTOR signalling network on distinct effector responses. Which extracellular nutrients and metabolites are specifically sensed by mTOR in the context of inflammatory responses? How does mTOR regulate the metabolic system in the context of macrophage polarization and other effector responses? Does the mTOR network control macrophage proliferation as an important determinant for tissue homeostasis? Lysosomes seem to be central intracellular compartments in which mTOR signalling occurs. As lysosomes are essential for phagocytosing cells and the mTORC1-regulated lysosomal transcription factor TFEB is a regulator of host defence155, the intimidate relationship of mTORC1 and lysosome function for bacterial infections or atherosclerosis needs to be further explored. How important is the mTOR-mediated regulation of protein translation for the currently underappreciated role of total protein synthesis for immune effector responses? Answering these and other questions will provide exciting new insight into the regulation of innate immune responses by mTORC1 and mTORC2 and new opportunities for therapeutic approaches.
Glossary.
OKmTORC1
A complex consisting of: mammalian target of rapamycin (mTOR), which is a serine/threonine kinase; regulatory-associated protein of mTOR (RAPTOR); proline-rich AKT1 substrate of 40 kDa (PRAS40), which is an mTORC1 inhibitor; mLST8 (also known as GβL), which is of unknown function; and DEP domain-containing mTOR-interacting protein (DEPTOR), which is an mTOR inhibitor.
mTORC2
A complex composed of: mTOR, mLST8 and the adaptor proteins rapamycin-insensitive companion of mTOR (RICTOR) and stress-activated MAP kinase-interacting protein 1 (SIN1; also known as MAPKAP1).
Tuberous sclerosis 1
(TSC1). TSC1 forms a heterodimeric complex with TSC2. The TSC1-TSC2 complex actively inhibits mTORC1 in unstimulated cells. Stimulation promotes AKT-dependent phosphorylation and inactivation of the complex. TSC1-TSC2 seems to be a positive regulator of mTORC2.
Pyruvate
The end product of glycolysis, which can be further metabolized to lactate in a process known as aerobic glycolysis (also known as “Warburg effect”). Pyruvate can also oxidized in the mitochondria through the tricarboxylic acid (TCA) cycle followed by oxidative phosphorylation to generate ATP.
Langerhans cells
A specialized subset of dendritic cells that seed the epidermal layer of the skin.
M1 and M2 macrophage subsets
Macrophages display considerable plasticity and can change their function in response to local environmental stimuli. M1 (or classically activated) macrophages mediate defence against various pathogens and tumours and contribute to chronic inflammatory diseases and autoimmunity. M2 (or alternatively activated) macrophages are required for defence against parasitic infections, to promote the resolution of inflammation, and to initiate tissue repair, and they are also implicated in tumour promotion.
STAT6
A transcription factor that is important for M2 macrophage polarization after stimulation with interleukin-4 or interleukin-13. STAT6 regulates many M2-associated genes, such as arginase 1, CD206, resistin-like-α and chitinase-like protein 3 (also known as YM1).
Key points.
The mammalian target of rapamycin (mTOR) is an evolutionary conserved serine/threonine kinase that is present in two complexes, mTORC1 and mTORC2. mTORC1 is the main energy and nutrient sensor of the cell: it senses the presence of amino acids, glucose, lipids and ATP to allow efficient activation of the network in response to growth factors, Toll-like receptor ligands and cytokines.
Activation of the mTOR pathway usually promotes an anabolic response that induces the synthesis of nucleic acids, proteins and lipids. In addition, it stimulates glycolysis as well as mitochondrial respiration. Emerging data suggest that this metabolic reconfiguration is required for specific effector functions in myeloid cells.
Translational control of gene expression in myeloid immune cells emerges as one way in which mTORC1 controls cellular processes such as migration, interferon and pro- or anti-inflammatory cytokine expression as well as metabolic reprogramming.
Counterintuitively, inhibition of mTORC1 during TLR triggering generally promotes IL-12 production and inhibits IL-10 and type I interferon expression by dendritic cells (DCs) and augments their T cell stimulatory capacity. Inhibition of mTORC2 enhances a pro-inflammatory response and IL-12 production in DCs.
Inhibition of mTORC1 in macrophages promotes autophagy, which is important for intracellular pathogen killing and clearance of ingested complex lipids such as LDL cholesterol.
mTORC2 is especially important for cell polarity and chemotaxis in neutrophils and mast cells. mTORC2 controls the leading edge as well as tail retraction during chemotactic migration.
Activation of mTORC1 in NK cells by IL-15 triggers a glycolytic response, which is important for their proliferation and acquisition of cytotoxicity.
Acknowledgements
This work is supported by grants from the Austrian Science Fund (FWF) grant FWF-P27701-B20, the Else-Kröner-Fresenius-Stiftung (P2013_A149), and the Herzfelder’sche Familienstiftung. The authors apologize to those colleagues whose work has not been cited owing to space constraints.
Biographies
Author details
Thomas Weichhart is an associate professor at the Medical University of Vienna, Austria. He received a Ph.D. in 2008 from the University of Vienna, Austria, worked in the industry, and performed postdoctoral training at the Medical University of Vienna. His group is interested how environmental and metabolic signals influence innate immune cell activation and its consequences for tissue homeostasis and the adaptive immune response with a special focus on mTOR.
Markus Hengstschläger is a professor of medical genetics and head of the Institute of Medical Genetics at the Medical University of Vienna. He received a Ph.D from the University of Vienna, Austria and completed postdoctoral training at Yale University. His research focuses on mTOR, stem cells and human genetic diseases.
Monika Linke is currently a Ph.D. student in the Institute of Medical Genetics at the Medical University of Vienna. Her research interest in the laboratory of T. Weichhart involves the function of the mTOR pathway in macrophages for tissue homeostasis.
References
- 1.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–62. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 3.Haidinger M, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–31. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 4.Sathaliyawala T, et al. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehman JA, Calvo V, Gomez-Cambronero J. Mechanism of ribosomal p70S6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils: cooperation of a MEK-related, THR421/SER424 kinase and a rapamycin-sensitive, m-TOR-related THR389 kinase. J Biol Chem. 2003;278:28130–8. doi: 10.1074/jbc.M300376200. [DOI] [PubMed] [Google Scholar]
- 6.Fukao T, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–81. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 7.Ohtani M, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–43. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weichhart T, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–77. doi: 10.1016/j.immuni.2008.08.012. [References 7 and 8 were the first to describe immunostimulatory effects of rapamycin in DCs that lead to enhanced T cell activation.] [DOI] [PubMed] [Google Scholar]
- 9.Weichhart T, et al. Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood. 2011;117:4273–83. doi: 10.1182/blood-2010-09-310888. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz F, et al. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur J Immunol. 2008;38:2981–92. doi: 10.1002/eji.200838761. [DOI] [PubMed] [Google Scholar]
- 11.Turnquist HR, et al. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood. 2010;115:4758–69. doi: 10.1182/blood-2009-10-251488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Q, et al. mTOR kinase inhibitor AZD8055 enhances the immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res. 2011;71:4074–84. doi: 10.1158/0008-5472.CAN-10-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorne E, et al. Participation of mammalian target of rapamycin complex 1 in Toll-like receptor 2- and 4-induced neutrophil activation and acute lung injury. Am J Respir Cell Mol Biol. 2009;41:237–45. doi: 10.1165/rcmb.2008-0290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu L, et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat Commun. 2014;5 doi: 10.1038/ncomms5696. 4696. [DOI] [PubMed] [Google Scholar]
- 15.Byles V, et al. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4 doi: 10.1038/ncomms3834. 2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcais A, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. 2014;15:749–57. doi: 10.1038/ni.2936. [This study shows that proliferation and cytotoxicity of NK cells depend on activation of mTOR by high doses of IL-15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lelouard H, et al. Regulation of translation is required for dendritic cell function and survival during activation. J Cell Biol. 2007;179:1427–39. doi: 10.1083/jcb.200707166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov SS, Roy CR. Pathogen signatures activate a ubiquitination pathway that modulates the function of the metabolic checkpoint kinase mTOR. Nat Immunol. 2013;14:1219–28. doi: 10.1038/ni.2740. [This study shows that inhibition of mTOR in macrophages limits translation of low abundant anti-inflammatory cytokines and creates a bias towards pro-inflammatory responses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everts B, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15:323–32. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh AC, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jovanovic M, et al. Immunogenetics. Dynamic profiling of the protein life cycle in response to pathogens. Science. 2015;347 doi: 10.1126/science.1259038. 1259038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14:435–46. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–55. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 24.Masui K, Cavenee WK, Mischel PS. mTORC2 in the center of cancer metabolic reprogramming. Trends Endocrinol Metab. 2014;25:364–73. doi: 10.1016/j.tem.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 26.Masui K, et al. mTOR complex 2 controls glycolytic metabolism in glioblastoma through FoxO acetylation and upregulation of c-Myc. Cell Metab. 2013;18:726–39. doi: 10.1016/j.cmet.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–9. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, et al. A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS. Nat Cell Biol. 2013;15:1186–96. doi: 10.1038/ncb2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas JD, et al. Rab1A is an mTORC1 activator and a colorectal oncogene. Cancer Cell. 2014;26:754–69. doi: 10.1016/j.ccell.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts DJ, Tan-Sah VP, Ding EY, Smith JM, Miyamoto S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol Cell. 2014;53:521–33. doi: 10.1016/j.molcel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haller JF, Krawczyk SA, Gostilovitch L, Corkey BE, Zoeller RA. Glucose-6-phosphate isomerase deficiency results in mTOR activation, failed translocation of lipin 1alpha to the nucleus and hypersensitivity to glucose: Implications for the inherited glycolytic disease. Biochim Biophys Acta. 2011;1812:1393–402. doi: 10.1016/j.bbadis.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster DA, Salloum D, Menon D, Frias MA. Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR) J Biol Chem. 2014;289:22583–8. doi: 10.1074/jbc.R114.566091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–9. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, et al. Tuberous sclerosis 1 (Tsc1)-dependent metabolic checkpoint controls development of dendritic cells. Proc Natl Acad Sci U S A. 2013;110:E4894–903. doi: 10.1073/pnas.1308905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan H, O'Brien TF, Zhang P, Zhong XP. The role of tuberous sclerosis complex 1 in regulating innate immunity. J Immunol. 2012;188:3658–66. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amiel E, et al. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J Immunol. 2012;189:2151–8. doi: 10.4049/jimmunol.1103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krawczyk CM, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–9. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everts B, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–31. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amiel E, et al. Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function. J Immunol. 2014;193:2821–30. doi: 10.4049/jimmunol.1302498. [The authors demonstrate that the mTOR-induced production of NO poisons mitochondrial respiration and necessitates an increase in aerobic glycolysis for survival in mouse but not human DCs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pantel A, et al. Direct type I IFN but not MDA5/TLR3 activation of dendritic cells is required for maturation and metabolic shift to glycolysis after poly IC stimulation. PLoS Biol. 2014;12:e1001759. doi: 10.1371/journal.pbio.1001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–17. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quintin J, Cheng SC, van der Meer JW, Netea MG. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol. 2014;29:1–7. doi: 10.1016/j.coi.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Cheng SC, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira GB, et al. Vitamin D3 Induces Tolerance in Human Dendritic Cells by Activation of Intracellular Metabolic Pathways. Cell Rep. 2015;10:711–725. doi: 10.1016/j.celrep.2015.01.013. [References 44 and 45 demonstrate that mTOR-mediated glycolysis in myeloid immune cells triggers either tolerance or enhanced restimulatory potential depending on the context.] [DOI] [PubMed] [Google Scholar]
- 46.Moon JS, et al. mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Donnelly RP, et al. mTORC1-Dependent Metabolic Reprogramming Is a Prerequisite for NK Cell Effector Function. J Immunol. 2014;193:4477–84. doi: 10.4049/jimmunol.1401558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Laar L, et al. PI3K-PKB hyperactivation augments human plasmacytoid dendritic cell development and function. Blood. 2012;120:4982–91. doi: 10.1182/blood-2012-02-413229. [DOI] [PubMed] [Google Scholar]
- 49.Hackstein H, et al. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–63. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 50.Scheffler JM, et al. LAMTOR2 regulates dendritic cell homeostasis through FLT3-dependent mTOR signalling. Nat Commun. 2014;5:5138. doi: 10.1038/ncomms6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kellersch B, Brocker T. Langerhans cell homeostasis in mice is dependent on mTORC1 but not mTORC2 function. Blood. 2013;121:298–307. doi: 10.1182/blood-2012-06-439786. [DOI] [PubMed] [Google Scholar]
- 52.Sparber F, et al. The late endosomal adaptor molecule p14 (LAMTOR2) represents a novel regulator of Langerhans cell homeostasis. Blood. 2014;123:217–27. doi: 10.1182/blood-2013-08-518555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macedo C, et al. Rapamycin augments human DC IL-12p70 and IL-27 secretion to promote allogeneic Type 1 polarization modulated by NK cells. Am J Transplant. 2013;13:2322–33. doi: 10.1111/ajt.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, et al. Convergence of the mammalian target of rapamycin complex 1- and glycogen synthase kinase 3-beta-signaling pathways regulates the innate inflammatory response. J Immunol. 2011;186:5217–26. doi: 10.4049/jimmunol.1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang CS, et al. Intracellular network of phosphatidylinositol 3-kinase, mammalian target of the rapamycin/70 kDa ribosomal S6 kinase 1, and mitogen-activated protein kinases pathways for regulating mycobacteria-induced IL-23 expression in human macrophages. Cell Microbiol. 2006;8:1158–71. doi: 10.1111/j.1462-5822.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 56.Gallon L, et al. Cellular and molecular immune profiles in renal transplant recipients after conversion from tacrolimus to sirolimus. Kidney Int. 2014;87:828–38. doi: 10.1038/ki.2014.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brouard S, et al. Comparative transcriptional and phenotypic peripheral blood analysis of kidney recipients under cyclosporin A or sirolimus monotherapy. Am J Transplant. 2010;10:2604–14. doi: 10.1111/j.1600-6143.2010.03302.x. [DOI] [PubMed] [Google Scholar]
- 58.Foldenauer ME, McClellan SA, Berger EA, Hazlett LD. Mammalian target of rapamycin regulates IL-10 and resistance to Pseudomonas aeruginosa corneal infection. J Immunol. 2013;190:5649–58. doi: 10.4049/jimmunol.1203094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohtani M, et al. Cutting edge: mTORC1 in intestinal CD11c+ CD11b+ dendritic cells regulates intestinal homeostasis by promoting IL-10 production. J Immunol. 2012;188:4736–40. doi: 10.4049/jimmunol.1200069. [DOI] [PubMed] [Google Scholar]
- 60.Luo L, et al. Rab8a interacts directly with PI3Kgamma to modulate TLR4-driven PI3K and mTOR signalling. Nat Commun. 2014;5:4407. doi: 10.1038/ncomms5407. [DOI] [PubMed] [Google Scholar]
- 61.Pan H, et al. Critical role of the tumor suppressor tuberous sclerosis complex 1 in dendritic cell activation of CD4 T cells by promoting MHC class II expression via IRF4 and CIITA. J Immunol. 2013;191:699–707. doi: 10.4049/jimmunol.1201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown J, Wang H, Suttles J, Graves DT, Martin M. Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J Biol Chem. 2011;286:44295–305. doi: 10.1074/jbc.M111.258053. [The first demonstration that mTORC2 negatively regulates IL-12 production in DCs by inhibiting activation of FOXO1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Festuccia WT, Pouliot P, Bakan I, Sabatini DM, Laplante M. Myeloid-specific Rictor deletion induces M1 macrophage polarization and potentiates in vivo pro-inflammatory response to lipopolysaccharide. PLoS One. 2014;9:e95432. doi: 10.1371/journal.pone.0095432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raich-Regue D, et al. mTORC2 Deficiency in Myeloid Dendritic Cells Enhances Their Allogeneic Th1 and Th17 Stimulatory Ability after TLR4 Ligation In Vitro and In Vivo. J Immunol. 2015;194:4767–76. doi: 10.4049/jimmunol.1402551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan W, et al. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J. 2010;29:4223–36. doi: 10.1038/emboj.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jorgensen PF, et al. Sirolimus interferes with the innate response to bacterial products in human whole blood by attenuation of IL-10 production. Scand J Immunol. 2001;53:184–91. doi: 10.1046/j.1365-3083.2001.00862.x. [DOI] [PubMed] [Google Scholar]
- 67.Mercalli A, et al. Rapamycin unbalances the polarization of human macrophages to M1. Immunology. 2013;140:179–90. doi: 10.1111/imm.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van den Bosch MW, Palsson-Mcdermott E, Johnson DS, O'Neill LA. LPS induces the degradation of programmed cell death protein 4 (PDCD4) to release Twist2, activating c-Maf transcription to promote interleukin-10 production. J Biol Chem. 2014;289:22980–90. doi: 10.1074/jbc.M114.573089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang GY, et al. Rapamycin-treated mature dendritic cells have a unique cytokine secretion profile and impaired allostimulatory capacity. Transpl Int. 2009;22:1005–16. doi: 10.1111/j.1432-2277.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 70.Macedo C, Turquist H, Metes D, Thomson AW. Immunoregulatory properties of rapamycin-conditioned monocyte-derived dendritic cells and their role in transplantation. Transplant Res. 2012;1:16. doi: 10.1186/2047-1440-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–37. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lopez-Pelaez M, et al. Cot/tpl2-MKK1/2-Erk1/2 controls mTORC1-mediated mRNA translation in Toll-like receptor-activated macrophages. Mol Biol Cell. 2012;23:2982–92. doi: 10.1091/mbc.E12-02-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schott J, et al. Translational regulation of specific mRNAs controls feedback inhibition and survival during macrophage activation. PLoS Genet. 2014;10:e1004368. doi: 10.1371/journal.pgen.1004368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colina R, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–8. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 75.Cao W, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–64. doi: 10.1038/ni.1645. [Studies 74 and 75 showed for the first time that expression of type I IFNs is controlled by the mTOR pathway through activation and translation of IRF7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jaramillo M, et al. Leishmania repression of host translation through mTOR cleavage is required for parasite survival and infection. Cell Host Microbe. 2011;9:331–41. doi: 10.1016/j.chom.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Fekete T, et al. The antiviral immune response in human conventional dendritic cells is controlled by the mammalian target of rapamycin. J Leukoc Biol. 2014;96:579–89. doi: 10.1189/jlb.2A0114-048RR. [DOI] [PubMed] [Google Scholar]
- 78.Murray PJ, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–81. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–49. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weisser SB, et al. Alternative activation of macrophages by IL-4 requires SHIP degradation. Eur J Immunol. 2011;41:1742–53. doi: 10.1002/eji.201041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yue S, et al. Myeloid PTEN deficiency protects livers from ischemia reperfusion injury by facilitating M2 macrophage differentiation. J Immunol. 2014;192:5343–53. doi: 10.4049/jimmunol.1400280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sahin E, et al. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J Immunol. 2014;193:1717–27. doi: 10.4049/jimmunol.1302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen W, et al. Macrophage-induced tumor angiogenesis is regulated by the TSC2-mTOR pathway. Cancer Res. 2012;72:1363–72. doi: 10.1158/0008-5472.CAN-11-2684. [DOI] [PubMed] [Google Scholar]
- 85.Rocher C, Singla DK. SMAD-PI3K-Akt-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS One. 2013;8:e84009. doi: 10.1371/journal.pone.0084009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fang C, et al. Tsc1 is a Critical Regulator of Macrophage Survival and Function. Cell Physiol Biochem. 2015;36:1406–1418. doi: 10.1159/000430306. [DOI] [PubMed] [Google Scholar]
- 87.Jiang H, Westerterp M, Wang C, Zhu Y, Ai D. Macrophage mTORC1 disruption reduces inflammation and insulin resistance in obese mice. Diabetologia. 2014;57:2393–404. doi: 10.1007/s00125-014-3350-5. [DOI] [PubMed] [Google Scholar]
- 88.Arranz A, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A. 2012;109:9517–22. doi: 10.1073/pnas.1119038109. [The authors identify opposite functions of the isoforms AKT1 and AKT2 for macrophage polarization, highlighting the complexity of the PI3K-AKT-mTOR pathway.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Babaev VR, et al. Macrophage deficiency of Akt2 reduces atherosclerosis in Ldlr null mice. J Lipid Res. 2014;55:2296–308. doi: 10.1194/jlr.M050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kawano Y, et al. Loss of Pdk1-Foxo1 signaling in myeloid cells predisposes to adipose tissue inflammation and insulin resistance. Diabetes. 2012;61:1935–48. doi: 10.2337/db11-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu YP, Brown JR, Sag D, Zhang L, Suttles J. Adenosine 5'-Monophosphate-Activated Protein Kinase Regulates IL-10-Mediated Anti-Inflammatory Signaling Pathways in Macrophages. J Immunol. 2014 doi: 10.4049/jimmunol.1401024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Galic S, et al. Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011;121:4903–15. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galvan-Pena S, O'Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tan Z, et al. Pyruvate Dehydrogenase Kinase 1 Participates in Macrophage Polarization via Regulating Glucose Metabolism. J Immunol. 2015;194:6082–9. doi: 10.4049/jimmunol.1402469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pello OM, et al. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012;119:411–21. doi: 10.1182/blood-2011-02-339911. [DOI] [PubMed] [Google Scholar]
- 96.Nasi A, et al. Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol. 2013;191:3090–9. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- 97.Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63. doi: 10.1038/nature13490. [References 96 and 97 show that lactic acid, an end product of aerobic glycolysis, produced by DCs or tumour cells can directly influence innate immune responses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chazaud B. Macrophages: supportive cells for tissue repair and regeneration. Immunobiology. 2014;219:172–8. doi: 10.1016/j.imbio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 99.Bleriot C, et al. Liver-Resident Macrophage Necroptosis Orchestrates Type 1 Microbicidal Inflammation and Type-2-Mediated Tissue Repair during Bacterial Infection. Immunity. 2015;42:145–58. doi: 10.1016/j.immuni.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 100.Deretic V. Autophagy as an innate immunity paradigm: expanding the scope and repertoire of pattern recognition receptors. Curr Opin Immunol. 2012;24:21–31. doi: 10.1016/j.coi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Narita M, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–70. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang X, et al. CaMKIV-dependent preservation of mTOR expression is required for autophagy during lipopolysaccharide-induced inflammation and acute kidney injury. J Immunol. 2014;193:2405–15. doi: 10.4049/jimmunol.1302798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu L, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–6. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gutierrez MG, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–66. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 105.Wang J, et al. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 2013;9:e1003697. doi: 10.1371/journal.ppat.1003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281:11374–83. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- 107.Yuan K, et al. Autophagy plays an essential role in the clearance of Pseudomonas aeruginosa by alveolar macrophages. J Cell Sci. 2012;125:507–15. doi: 10.1242/jcs.094573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Owen KA, Meyer CB, Bouton AH, Casanova JE. Activation of focal adhesion kinase by Salmonella suppresses autophagy via an Akt/mTOR signaling pathway and promotes bacterial survival in macrophages. PLoS Pathog. 2014;10:e1004159. doi: 10.1371/journal.ppat.1004159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blanchet FP, et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–69. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sergin I, Razani B. Self-eating in the plaque: what macrophage autophagy reveals about atherosclerosis. Trends Endocrinol Metab. 2014;25:225–34. doi: 10.1016/j.tem.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Le Guezennec X, et al. Wip1-dependent regulation of autophagy, obesity, and atherosclerosis. Cell Metab. 2012;16:68–80. doi: 10.1016/j.cmet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 112.Zhai C, et al. Selective inhibition of PI3K/Akt/mTOR signaling pathway regulates autophagy of macrophage and vulnerability of atherosclerotic plaque. PLoS One. 2014;9:e90563. doi: 10.1371/journal.pone.0090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ai D, et al. Disruption of mammalian target of rapamycin complex 1 in macrophages decreases chemokine gene expression and atherosclerosis. Circ Res. 2014;114:1576–84. doi: 10.1161/CIRCRESAHA.114.302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen WQ, et al. Oral rapamycin attenuates inflammation and enhances stability of atherosclerotic plaques in rabbits independent of serum lipid levels. Br J Pharmacol. 2009;156:941–51. doi: 10.1111/j.1476-5381.2008.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mintern JD, Macri C, Villadangos JA. Modulation of antigen presentation by intracellular trafficking. Curr Opin Immunol. 2015;34C:16–21. doi: 10.1016/j.coi.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 116.Jagannath C, et al. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–76. doi: 10.1038/nm.1928. [This study is the first to demonstrate that mTOR inhibition enhances the efficacy of DCs in an autologous vaccination protocol.] [DOI] [PubMed] [Google Scholar]
- 117.Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood. 2002;100:1084–7. doi: 10.1182/blood.v100.3.1084. [DOI] [PubMed] [Google Scholar]
- 118.Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Curr Opin Cell Biol. 2011;23:707–15. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rosborough BR, et al. Murine dendritic cell rapamycin-resistant and rictor-independent mTOR controls IL-10, B7-H1, and regulatory T-cell induction. Blood. 2013;121:3619–30. doi: 10.1182/blood-2012-08-448290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5:228–36. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 121.Sordi V, et al. Differential effects of immunosuppressive drugs on chemokine receptor CCR7 in human monocyte-derived dendritic cells: selective upregulation by rapamycin. Transplantation. 2006;82:826–34. doi: 10.1097/01.tp.0000235433.03554.4f. [DOI] [PubMed] [Google Scholar]
- 122.Sinclair LV, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–21. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baetta R, et al. Everolimus inhibits monocyte/macrophage migration in vitro and their accumulation in carotid lesions of cholesterol-fed rabbits. J Pharmacol Exp Ther. 2009;328:419–25. doi: 10.1124/jpet.108.144147. [DOI] [PubMed] [Google Scholar]
- 124.Perino A, et al. TGR5 reduces macrophage migration through mTOR-induced C/EBPbeta differential translation. J Clin Invest. 2014 doi: 10.1172/JCI76289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fox R, et al. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J. 2007;26:505–15. doi: 10.1038/sj.emboj.7601522. [This study highlights the importance of translationally regulated gene expression for effector responses in macrophages.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 127.Liu L, Parent CA. Review series: TOR kinase complexes and cell migration. J Cell Biol. 2011;194:815–24. doi: 10.1083/jcb.201102090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He Y, et al. Mammalian target of rapamycin and Rictor control neutrophil chemotaxis by regulating Rac/Cdc42 activity and the actin cytoskeleton. Mol Biol Cell. 2013;24:3369–80. doi: 10.1091/mbc.E13-07-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu L, Das S, Losert W, Parent CA. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev Cell. 2010;19:845–57. doi: 10.1016/j.devcel.2010.11.004. [These authors uncover an important role of mTORC2 in neutrophil polarization and directed migration.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen J, Tang H, Hay N, Xu J, Ye RD. Akt isoforms differentially regulate neutrophil functions. Blood. 2010;115:4237–46. doi: 10.1182/blood-2009-11-255323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu L, Gritz D, Parent CA. PKCbetaII acts downstream of chemoattractant receptors and mTORC2 to regulate cAMP production and myosin II activity in neutrophils. Mol Biol Cell. 2014;25:1446–57. doi: 10.1091/mbc.E14-01-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gomez-Cambronero J. Rapamycin inhibits GM-CSF-induced neutrophil migration. FEBS Lett. 2003;550:94–100. doi: 10.1016/s0014-5793(03)00828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frondorf K, Henkels KM, Frohman MA, Gomez-Cambronero J. Phosphatidic acid is a leukocyte chemoattractant that acts through S6 kinase signaling. J Biol Chem. 2010;285:15837–47. doi: 10.1074/jbc.M109.070524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fernandez N, Gonzalez A, Valera I, Alonso S, Crespo MS. Mannan and peptidoglycan induce COX-2 protein in human PMN via the mammalian target of rapamycin. Eur J Immunol. 2007;37:2572–82. doi: 10.1002/eji.200737262. [DOI] [PubMed] [Google Scholar]
- 135.Wall M, et al. Translational control of c-MYC by rapamycin promotes terminal myeloid differentiation. Blood. 2008;112:2305–17. doi: 10.1182/blood-2007-09-111856. [DOI] [PubMed] [Google Scholar]
- 136.Lindemann SW, et al. Neutrophils alter the inflammatory milieu by signal-dependent translation of constitutive messenger RNAs. Proc Natl Acad Sci U S A. 2004;101:7076–81. doi: 10.1073/pnas.0401901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–9. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 138.McInturff AM, et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 alpha. Blood. 2012;120:3118–25. doi: 10.1182/blood-2012-01-405993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Itakura A, McCarty OJ. Pivotal role for the mTOR pathway in the formation of neutrophil extracellular traps via regulation of autophagy. Am J Physiol Cell Physiol. 2013;305:C348–54. doi: 10.1152/ajpcell.00108.2013. [References 138 and 139 demonstrate that mTOR regulates neutrophil extracellular trap formation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gupta AK, Giaglis S, Hasler P, Hahn S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One. 2014;9:e97088. doi: 10.1371/journal.pone.0097088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Remijsen Q, et al. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011;21:290–304. doi: 10.1038/cr.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vitiello D, Neagoe PE, Sirois MG, White M. Effect of everolimus on the immunomodulation of the human neutrophil inflammatory response and activation. Cell Mol Immunol. 2014 doi: 10.1038/cmi.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hua W, et al. Rapamycin inhibition of eosinophil differentiation attenuates allergic airway inflammation in mice. Respirology. 2015 doi: 10.1111/resp.12554. [DOI] [PubMed] [Google Scholar]
- 144.Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13:362–75. doi: 10.1038/nri3427. [DOI] [PubMed] [Google Scholar]
- 145.Kim MS, Kuehn HS, Metcalfe DD, Gilfillan AM. Activation and function of the mTORC1 pathway in mast cells. J Immunol. 2008;180:4586–95. doi: 10.4049/jimmunol.180.7.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kuehn HS, Jung MY, Beaven MA, Metcalfe DD, Gilfillan AM. Prostaglandin E2 activates and utilizes mTORC2 as a central signaling locus for the regulation of mast cell chemotaxis and mediator release. J Biol Chem. 2011;286:391–402. doi: 10.1074/jbc.M110.164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Smrz D, et al. mTORC1 and mTORC2 differentially regulate homeostasis of neoplastic and non-neoplastic human mast cells. Blood. 2011;118:6803–13. doi: 10.1182/blood-2011-06-359984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shin J, Pan H, Zhong XP. Regulation of mast cell survival and function by tuberous sclerosis complex 1. Blood. 2012;119:3306–14. doi: 10.1182/blood-2011-05-353342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smrz D, et al. Rictor Negatively Regulates High-Affinity Receptors for IgE-Induced Mast Cell Degranulation. J Immunol. 2014;193:5924–32. doi: 10.4049/jimmunol.1303495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11:658–71. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nandagopal N, Ali AK, Komal AK, Lee SH. The Critical Role of IL-15-PI3K-mTOR Pathway in Natural Killer Cell Effector Functions. Front Immunol. 2014;5:187. doi: 10.3389/fimmu.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang M, et al. PDK1 orchestrates early NK cell development through induction of E4BP4 expression and maintenance of IL-15 responsiveness. J Exp Med. 2015 doi: 10.1084/jem.20141703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wai LE, Fujiki M, Takeda S, Martinez OM, Krams SM. Rapamycin, but not cyclosporine or FK506, alters natural killer cell function. Transplantation. 2008;85:145–9. doi: 10.1097/01.tp.0000296817.28053.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Salmond RJ, et al. IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J Allergy Clin Immunol. 2012;130:1159–1166 e6. doi: 10.1016/j.jaci.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Visvikis O, et al. Innate host defense requires TFEB-mediated transcription of cytoprotective and antimicrobial genes. Immunity. 2014;40:896–909. doi: 10.1016/j.immuni.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lamming DW, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–43. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Touzot M, Soulillou JP, Dantal J. Mechanistic target of rapamycin inhibitors in solid organ transplantation: from benchside to clinical use. Curr Opin Organ Transplant. 2012;17:626–33. doi: 10.1097/MOT.0b013e32835a4be2. [DOI] [PubMed] [Google Scholar]
- 158.Toyota T, Shiomi H, Morimoto T, Kimura T. Meta-analysis of Long-Term Clinical Outcomes of Everolimus-Eluting Stents. Am J Cardiol. 2015 doi: 10.1016/j.amjcard.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 159.Chiarini F, Evangelisti C, McCubrey JA, Martelli AM. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci. 2015;36:124–35. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 160.Gunzl P, Schabbauer G. Recent advances in the genetic analysis of PTEN and PI3K innate immune properties. Immunobiology. 2008;213:759–65. doi: 10.1016/j.imbio.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 161.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–31. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 162.Neuman NA, Henske EP. Non-canonical functions of the tuberous sclerosis complex-Rheb signalling axis. EMBO Mol Med. 2011;3:189–200. doi: 10.1002/emmm.201100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Katholnig K, et al. p38alpha senses environmental stress to control innate immune responses via mechanistic target of rapamycin. J Immunol. 2013;190:1519–27. doi: 10.4049/jimmunol.1202683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McGuire VA, et al. Cross talk between the Akt and p38alpha pathways in macrophages downstream of Toll-like receptor signaling. Mol Cell Biol. 2013;33:4152–65. doi: 10.1128/MCB.01691-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]