Abstract
The nature of emotion deficits in schizophrenia and anhedonia is still unclear and understanding the nature of these deficits could help improve treatment of chronic symptoms and functional disability. An important mechanism in emotional functioning is attention to affective information. People with schizophrenia (n = 48) and a non-psychiatric comparison group (n = 28) completed an affective interference task, a task used to assess attention to affective information. Given that the affective interference task also involves prepotent response inhibition, participants also completed a very similar, but non-affective, cognitive interference task that involves prepotent inhibition but does not require attention to affective information. Results revealed a double dissociation in performance on these tasks in people with schizophrenia. Relative to controls, people with schizophrenia exhibited decreased affective interference but increased cognitive interference. In addition, decreased affective interference was associated with increased anhedonia and increased reports of wanting to ignore positive emotions. In contrast, increased cognitive interference was associated with increased communication disturbances and alogia. Overall, these results suggest that there is a decrease in attention to affective information in schizophrenia, and this deficit is related to anhedonia. At the same time, these results provide further evidence of cognitive control prepotent inhibition deficits in schizophrenia, which are related to communication disturbances and alogia.
Keywords: attention to emotion, affective interference, anhedonia, cognitive interference
There is evidence that schizophrenia is associated with impairments in emotion. For example, people with schizophrenia exhibit deficits on some tasks that involve emotional stimuli (Cohen & Minor, 2010; Kohler & Martin, 2006; Kring & Moran, 2008; Tremeau, 2006), with poor performance on these tasks associated with poor outcomes in the disorder (Kring & Moran, 2008; Kohler & Martin, 2006). At the same time, people with schizophrenia report some symptoms that seem to imply an underlying deficit in affective mechanisms. For example, people with schizophrenia report elevated anhedonia. Anhedonia refers to reports of diminished experience of positive emotion in response to social or physical stimuli not currently encountered (Horan, Green, Kring, & Nuechterlein, 2006; Strauss & Gold, 2012; Wolf, 2006). Anhedonia predicts future onset of the disorder (Gooding, Tallent, & Matts, 2005; Kwapil, 1998), and in people with the disorder, it is associated with poor outcomes and is currently not well treated (Blanchard, Mueser, & Bellack, 1998). Hence, understanding the nature of deficits in affective processing mechanisms in schizophrenia could help us potentially prevent the disorder as well as treat chronic symptoms and functional disability in people with the disorder. However, the nature of deficits in affective processing mechanisms in schizophrenia is still unclear (e.g., Kring & Moran, 2008). An important mechanism in affective processing is attention to affective information (Barrett, Mesquita, Ochsner, & Gross, 2007; Cunningham & Zelazo, 2007; Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007; Ochsner & Gross, 2005).
Attention to affect has been conceptualized as part of “meta-mood processing”, or processing that occurs after the onset of an emotion (Mayer & Gaschke, 1988). From this perspective, attention to affective information may be a first step to identify and regulate one’s affective experience (Mayer & Gaschke, 1988; Salovey, Mayer, Goldman, Turvey, & Palfai, 1995; Mayer & Salovey, 1997). Decreased attention to emotions may have multiple consequences for emotional functioning. People could be less aware of their emotions and hence might view themselves as less emotional than they really are. They could also be less likely to regulate emotional reactions as they are less likely to identify that an emotion has taken place.
The influence of attention to affect can be observed in tasks where affective information can be relevant or irrelevant (Bartholow, Riordan, Saults, & Lust, 2009; Gratton et al., 1992; Klauer, Rossnagel, & Musch, 1997). For example, on word priming tasks in which a cue word precedes a target word, attention to the cue word may facilitate or interfere with the response to the target word (Klauer & Musch, 2002). That is, on congruent trials, where the cue and target are matched on valence, attention to the cue word can produce facilitation (i.e. fewer errors and faster reaction times). In contrast, on incongruent trials, where the cue and target differ on valence, attention to the cue word can cause increased interference (i.e. more errors and slower reaction times).
Evidence for chronic inattention to affective information would presumably be most evident at the earliest time courses of processing (Bartholow et al., 2009; Cunningham & Zelazo, 2007; Klauer et al., 1997). This is because with more time participants might then have enough time to finally become aware of affective information. However, very few studies examining the processing of affective stimuli in schizophrenia have involved short enough time windows to be able to clearly examine attention to affect at the earliest time courses. For example, schizophrenia startle probe studies or facial affective priming tasks have involved at least 1,000 ms of affective stimulus processing before measuring affective responses (Curtis, Lebow, Lake, Katsanis, & Iacono, 1999; Hooker, Tully, Verosky, Fisher, Holland, Vinogradov, 2011; Kring, Germans Gard, & Gard, 2011; Schlenker, Cohen, & Hopmann, 1995; Yee et al., 2010). In addition, incidental learning tasks, which often do not use affective stimuli, tend to have longer exposure times as well (1000 – 3000 ms; e.g., Burch, Hemsley, Corr, & Gwyer, 2006; Danion, Meulemans, Kauffmann-Muller, & Vermaat, 2001; Dykes & McGhie, 1976; Horan, Green, Knowlton, Wynn, Mintz, & Nuechterlein, 2008; Payne, Hochberg, & Hawks, 1970).
Psychophysiology studies that have examined relatively early stimulus processing (i.e. stimulus processing durations ≤ 400 ms) have produced seemingly conflicting results, with only one of the studies finding diminished attention to affective information in schizophrenia (Horan, Foti, Hajcak, Wynn, & Green, 2012; Horan, Wynn, Kring, Simons, & Green 2010; Volz, Hamm, Kirsch, & Rey, 2003). It is possible that the apparent conflict between these results may be due to the particular component of emotion that was measured in each study: arousal vs. valence. All three of these studies have reported intact processing of affective arousal in schizophrenia (Horan et al., 2010, 2012; Volz et al., 2003). In contrast, the only study that examined affective valence processing reported diminished processing of affective valence in schizophrenia (Volz et al., 2003). One behavioral study that potentially investigated the early time course of attention to affective information did find that anhedonia in schizophrenia was associated with a reduced influence of affect (Suslow, Roestel, & Arolt, 2003). However, this study involved a non-speeded judgment task that might have introduced a greater influence of other cognitive processes. At the same time, there were only a small number of trials in that study before the introduction of a procedural variation that obscured the measurement of attention to affective information specifically. Thus, based on previous research, it is still unclear whether people with schizophrenia have an impairment of attention to affective information and whether it is associated with anhedonia.
A task that has often been used in previous non-schizophrenia research to examine the early time course of attention to affect is the affective interference task (Fazio, 2001; Klauer, Teige-Mocigemba, & Spruyt, 2009). Somewhat similar to the Stroop color-naming task, the affective interference task involves both (a) congruent, non-interference trials, and (b) incongruent, high-interference trials (Klauer & Musch, 2002). On this task (Fazio, 2001; Fazio, Sanbonmatsu, Powell, & Kardes, 1986) participants read a valenced cue word (e.g., ‘friendly’) and are asked to judge the valence of a target word (e.g., ‘birthday’). The affective interference effect is the extent to which people are slower and less accurate for incongruent trials than for congruent trials. Previous research has consistently found that inducement to increase or decrease attention to the affective cue word increases or decreases affective interference, respectively (Bartholow et al., 2009; Klauer et al., 1997). Decreased attention to affective information should result in less of an influence of the cue word’s valence when evaluating the target. Hence, if people with schizophrenia have decreased attention to affective information, then it is expected that they would exhibit decreased affective interference on this task, especially at the shortest stimulus onset asynchronies (SOAs), or the length of time between the onset of the cue and onset of the target.
In addition, if anhedonia in schizophrenia is related to decreased attention to affective information, then increased anhedonia should be correlated decreased affective interference. In addition to attention to affective information, the affective interference task also includes at least one other component, prepotent response inhibition. This is because on incongruent trials the cue valence can activate the incorrect response. Therefore, in responding to the target valence participants also need to overcome the prepotent response activated by the cue word. Consistent with this, a range of behavioral and brain imaging research has found evidence of prepotent response inhibition on the affective interference task (Bartholow et al., 2009; Hermans, De Houwer, & Eelen, 1994; Wentura, 1999; Herring, Taylor, White, & Crites, 2011). Previous research has also found that people with schizophrenia do exhibit deficits in prepotent response inhibition (e.g., Badcock, Michie, Johnson, & Combrinck, 2002; Clementz, 1998; Ford et al., 2004; Hahn et al., 2010; Hughes, Fulham, Johnston, & Michie, 2012). Importantly however, a deficit only in prepotent response inhibition would result in a different pattern of performance than a deficit in attention to affective information. Whereas decreased attention to affect should result in decreased affective interference, a deficit only in prepotent response inhibition should result in increased affective interference. This is because the cue should produce greater response interference on incongruent trials.
To help disentangle the role of both affective interference and prepotent inhibition on the affective interference task, we compared performance on the affective interference task with performance on a procedurally similar, non-affective cognitive interference task (Machado, Wyatt, Devine, & Knight, 2007). Importantly, both the affective interference task and the cognitive interference task involve the need for prepotent response inhibition because the occurrence of response conflict should slow down performance and increase error rates on incongruent trials compared to congruent trials. However, only the affective interference task involves affective stimuli. Hence, if people with schizophrenia have both decreased attention to affective information and increased prepotent inhibition deficits, then they should exhibit differential performance on these two tasks.
In the current study, we expected a double dissociation in performance on the affective interference and the cognitive interference tasks in people with schizophrenia. Specifically, we expected decreased affective interference (due to decreased attention to affective information) but increased cognitive interference (due to difficulties with prepotent response inhibition). In addition, we also expected that performance on these two interference tasks should be differentially correlated with schizophrenia symptoms. We expected that decreased affective interference should be correlated with increased anhedonia. In addition, given that affective interference is a behavioral result of one’s attention to emotion, we expected that it would be associated with self-reported attention to emotion. Based on previous factor analytic research and evidence of convergent and discriminant validity, focusing on vs. ignoring emotions are considered distinct aspects of attention to emotions (Gasper & Bramesfeld, 2006). Thus, we considered them separately for both positive and negative emotions. Also, previous research has found that prepotent response inhibition deficits in schizophrenia have been correlated with increased communication disturbances and alogia (Barch et al., 1999; Becker, Cicero, Cowan, & Kerns, 2012; Kerns & Berenbaum, 2002). Hence, we expected that increased cognitive interference in schizophrenia should be correlated with increased communication disturbances and alogia.
Method
Participants
The schizophrenia (SZP) group was comprised of 48 inpatients (not recent admissions and not in an acute state) with a wide range of functioning recruited from a long-term state psychiatric hospital (with a largely forensic population). Participants resided on units in which the average length of stay is approximately 8 years. All had a DSM-IV diagnosis of schizophrenia (n = 38) or schizoaffective disorder (n = 10) based on the Structured Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1998). None had a current comorbid substance use disorder. Control participants were 30 individuals recruited through community advertisements. General exclusionary criteria included diagnosis of a substance abuse disorder within the past 6 months, diagnosis of mental retardation, non-native English speakers, or a history of any neurologic event or disease (e.g., loss of consciousness for more than 10 minutes; stroke). In addition, control participants did not have any current Axis I (e.g., current major depressive disorder diagnosis) based on the SCID, and they denied having a first-degree relative with a psychotic disorder. Two people in the control group were excluded because they met criteria for current major depressive disorder, leaving the final control group with 28 participants. Table 1 contains demographic and clinical information. The groups did not differ in age, sex, or parental education, all ps > .25. Although the groups did differ in race/ethnicity,χ2 (2, n = 48) = 15.09 p < .001 (significantly more African-American participants in the SZP group than in the control group), race/ethnicity was not a significant predictor of performance on the affective or cognitive interference tasks, ps > .16. In addition, within the SZP group, performance on did not significantly differ between racial groups, ps > .21.
Table 1.
Demographic and Clinical Information
| SZP Group (n = 48) | Control Group (n = 28) | |
|---|---|---|
|
| ||
| Sex (% male) | 83.3 | 92.8 |
|
| ||
| Race/ethnicity (% Caucasian) | 58.3 | 96.4 |
|
| ||
| Age (years) | 40.78 (11.76) | 43.62 (9.49) |
| Education (years) | 11.57 (1.76) | 16.07 (1.83) |
|
| ||
| Parental Education (years) | 12.51 (2.12) | 12.55 (2.18) |
|
| ||
| Mini-Mental State Examination (out of 30) | 26.21 (2.6) | 29.23 (.81) |
|
| ||
| Medication | ||
| Chlorpromazine equivalents | 335.36 (355.8) | |
| Range | 0 – 1906.82 | |
| % taking antipsychotics | 97.91 | |
| % taking mood stabilizers | 43.75 | |
| % taking antidepressants | 56.25 | |
| % taking anticholinergics | 18.75 | |
| % taking anxiolytics | 29.17 | |
|
| ||
| SAPS Global Ratings M (SD) | ||
|
| ||
| Hallucinations | 1.88 (1.89) | |
|
| ||
| Delusions | 2.87 (1.41) | |
|
| ||
| Positive Formal Thought Disorder | 1.72 (1.5) | |
|
| ||
| Physical Anhedonia Scale | 16.92 (7.71) | 11.5 (6.91) |
|
| ||
| Social Anhedonia Scale | 15.78 (6.73) | 10.36 (7.69) |
Note: SAPS - Scale for the Assessment of Positive Symptoms
Materials
Anhedonia
To measure anhedonia, following previous research (Barch, Yodkovik, Sypher-Locke, & Hanewinkel, 2008: Heerey & Gold, 2007; Horan et al., 2010) participants completed two anhedonia instruments, the Revised Social Anhedonia Scale (SAS; Eckblad, Chapman, Chapman, & Mishlove, 1982) and the Revised Physical Anhedonia Scale (PAS; Chapman & Chapman, 1978). The SAS involves 40 true-false items and is designed to measure lack of relationships and lack of pleasure from relationships (e.g., “Having close friends is not as important as many people say.”). Reliability of the SAS in this study was α = .83. The PAS involves 61 true-false items and is designed to measure a lack of pleasure gained from physical stimuli, such as food or touch (e.g., “One food tastes as good as another to me.”). Reliability of the PAS in this study was α = .83. As expected, the SZP group reported significantly greater levels of anhedonia than the control group, t(75) = 3.53, p < .001, effect size r = .38.
In the current study, we report the results of a composite anhedonia score, rather than physical and social anhedonia scores separately, for several reasons. First, the use of a composite anhedonia score is consistent with schizophrenia research that uses clinical rating scales of anhedonia. For example, the Scale for the Assessment of Negative Symptoms (SANS, Andresen, 1984), the Clinical Assessment Interview for Negative Symptoms (CAINS; Blanchard et al., 2011; Horan et al., 2011), and the Brief Negative Symptom Scale (BNSS; Kirkpatrick et al., 2006) measure social and physical anhedonia on a single subscale. Also, the use of a composite anhedonia score is consistent with previous non-patient exploratory and confirmatory factor analytic research that has found that social and physical anhedonia scales load consistently on a common anhedonia factor (Kwapil et al., 2008; Mason et al., 1995). In addition, scores on the social and physical anhedonia are highly correlated in the current sample, r = .63, p < .001. This is consistent with previous research that has reported that physical and social anhedonia are correlated in both patient samples (e.g., r = .51; Blanchard, Bellack, & Mueser, 1998) and non-patient samples (Edell, 1995). Last, the results from the current study were similar when considering the composite anhedonia variable to the results when considering the scales individually.
Speech symptom ratings
To reliably assess the speech symptoms of communication disturbances and alogia, eight minutes of speech was collected from a structured interview in which people were asked about neutral memories (e.g., “Tell me about a time you were working.”). Communication impairments were measured with the Communication Disturbance Index (CDI; Docherty, DeRosa, & Andreasen, 1996; inter-rater reliability with four trained raters in current study = .92). The CDI rates the number of speech “unclarities,” or the number of times the speech lacks lucidity and impairs the overall meaning of the speech passage. Following previous research (Berenbaum, Kerns, Vernon, & Gomez, 2008; Kerns, 2007), the number of words spoken during the 8-minute period was used as a measure of alogia (which was then reversed, so that higher scores mean greater alogia).
Affect interference task
This task consisted of positively or negatively valenced cue and target words that appeared in succession on a computer screen (Klauer et al., 2009) through E-Prime software (2006). Participants were told to read the first word silently to themselves (i.e. pay attention to the cue word) and then to rate, or categorize, the second word as a “good” (or “positive”) word or a “bad” (or “negative”) word. Participants responded with a keyboard press, ‘1’ for good and ‘0’ for bad. Each trial began with a fixation cross for 500 ms, followed by a cue word for either a “short” (i.e., 85 ms), “intermediate” (i.e., 170 ms), or “long” (i.e., 270 ms) interval (i.e. SOA; note that the labels short, intermediate, and long are used relative to each other). Then the target word appeared until a participant made a response. Then the screen was blank for 2000 ms until the next trial. Participants were instructed to respond as quickly and accurately as possible. The proportion of cue and target pairs that had the same valence was 0.50. After completing 8 practice trials, participants completed 9 blocks of 24 trials, with three blocks of short, intermediate, and long SOA trials, respectively, with block order randomized across participants.
Trials with reaction times less than 200 ms or greater than 6000 ms were eliminated. Also, reaction times that were greater than 3.5 SD from each participant’s mean were eliminated. The percentage of trials eliminated for the SZP and control groups based on reaction times was mean = 7.8% (median = 4.1%) and mean = 2.8% (median = 1.2%), respectively. Following previous research (Klauer et al., 1997; Kerns, 2005), the affective interference effect was measured as the difference in reaction times and error rates between incongruent trials (i.e. where cue and target have different valences) versus congruent trials (i.e. where cue and target have the same valence). A single affective interference effect score was created by averaging standardized z-scores for reaction times and error rates, with higher scores reflecting poorer performance for incongruent than for congruent trials. Because we used the standard version of the affective interference task, which includes only positively and negatively valenced words but does not include neutral cue words (e.g., Bargh, Chaiken, Govender, & Pratto, 1992; Fazio et al., 1986; Klauer et al., 1997), we could not discriminate between the relative size of interference vs. facilitation effects.
In this task, we used a fixed word list for each SOA (i.e., word list 1 was used with short SOA trials, word list 2 was used with intermediate SOA trials, word list 3 was used with long SOA trials). We did this for two reasons. First, we wanted to use a fixed set of cue-target pairs because we wanted to insure that cue-target pairs were not semantically related (e.g., gift-birthday) and that affective interference effects could not be attributed to semantic relatedness. Second, it has been recommended that when examining associations with individual difference variables (e.g., diagnostic status or symptoms, as in the current study) that it is preferable for task parameters to be fixed to remove variation across participants due to order effects (Miyake et al., 2000; Miyake, Friedman, Rettinger, Shah, & Hegarty, 2001).
Previous research has reported that at shorter SOAs (e.g., < 420 ms), performance generally does not vary by SOA for healthy participants (Klauer, Teige-Mocigemba, & Spruyt, 2009). However, in the current study, the size of the affective interference effect did vary by SOA in control participants. Hence, we systematically varied both SOA and word list in a separate normative study with Introduction to Psychology students (n = 223) and did not find an effect of SOA but instead found an effect of word list. Thus, in the current study we do not interpret variations in control participants’ performance by SOA. Instead, we focus on between-group differences between people with schizophrenia and controls and whether this varied by SOA.
On this task, each cue and target word appeared only once (Klauer et al., 1997). Cue and target words (e.g., positive words: ‘kitten’, ‘angel’, ‘clothes’; negative words: ‘headache’, ‘funeral’, ‘lice’) were selected from previous published norms of affectively valenced words (Anderson, 1968; Bargh et al., 1992; Bellazza, Greenwald, & Banaji, 1986; Bradley & Lang, 1999; Brown & Ure, 1969; John, 1988; Rubin, 1980; Silverstein, & Dienstbier, 1968). Words in congruent word pairs (i.e. cue and target with the same valence) were matched to words in incongruent word pairs (i.e. cue and target with different valences) on word length, word frequency (Francis & Kučera, 1982), arousal level, and extremity of affective valence, all ps > .35.
To insure that participants did not evaluate words in an idiosyncratic manner, participants were given visual feedback when they responded incorrectly. In addition, following the affective interference task, participants were asked to rate all of the cue words from the task as “good” or “bad”. Trials from the affective interference task were excluded from analyses if the cue words were not correctly rated more than 70% of the time by patients (n = 13 cues; e.g., ‘acclaim’; ‘elated’). This was done to attempt to prevent the affective interference effect from being influenced by lack of knowledge about the cue words. Importantly, cue-rating accuracy was not related to the affective interference effect (at any SOA) and was not related to anhedonia, r = .11, p = .48. Thus, cue accuracy does not seem to be related to the affective interference effect or to anhedonia.
Cognitive interference task
Participants completed a cognitive interference task, matched (.040 vs. .043) to the affective interference task in true score variance (i.e. the product of reliability and variance; Melinder, Barch, Heydebrand, & Csernansky, 2005; Strauss, 2001). Just as on the affective interference task, on the cognitive interference task (Machado et al., 2007), participants first saw a central fixation cross, then a cue (a green or red color square above or below fixation), and then they evaluated a target (a green or red square that replaced the fixation cross). They were instructed to press “1” if the center square was green and “0” if the center square was red. Just as for the affective interference task, participants completed blocks with the same short, intermediate, and long SOAs, with 50% congruent trials and 50% incongruent trials. As with the affective interference task, trials with reaction times less than 200 ms or greater than 6000 ms were eliminated, and reaction times that were greater than 3.5 SD from each participant’s mean were eliminated. Just as for the affective interference effect, a cognitive interference effect was calculated as the difference in reaction times and error rates between incongruent trials versus congruent trials. A single cognitive interference effect score was created by averaging standardized z-scores for reaction times and error rates, with higher scores reflecting poorer performance for incongruent than for congruent trials.
Following Affective States Test (FAST; Gasper & Bramesfeld, 2006)
To measure self-reported attention to positive and negative emotions, participants completed the FAST. The FAST is comprised of 4 subscales: Focus on Positive Feelings, Ignore Positive Feelings, Focus on Negative Feelings, and Ignore Negative Feelings. Gasper and Bramesfeld (2006) reported that all four of the FAST subscales show convergent validity with a number of different published emotion trait measures. For example, the Focus on Positive Feeling subscale was positively associated with the Emotional Attention subscale of the Trait Meta Mood Scale (TMMS). In contrast, the Ignore Positive and Ignore Negative subscales were negatively associated with the Emotional Attention subscale of the TMMS (Salovey et al., 1995). In the current study, internal consistencies were comparable to those reported by Gasper and Bramesfeld (2006), ranging from α = .64–.68. To our knowledge, this is the first schizophrenia study examining attention to positive versus negative emotions and focusing on and ignoring of emotions.
Clinical symptom ratings
Positive schizophrenia symptoms were measured using the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984). In this study, all patients’ diagnostic interviews were video-recorded, and videos of 14 participants were randomly selected for reliability ratings. There was 100% agreement for diagnosis, and the interrater reliability, indexed by interclass correlations, for global symptom ratings for hallucinations, delusions, and positive thought disorder from the SAPS were all greater than .84.
Procedure
Participants underwent the semi-structured diagnostic interview and the structured speech interview. Then, they completed the affective and cognitive interference tasks in counterbalanced order, followed by questionnaire measures. The data presented here were part of a larger study in which additional, but unrelated tasks, were completed. One person from both the SZP and control groups did not complete the cognitive interference task because they were red-green color-blind.
Results
Variation in group differences by type of task
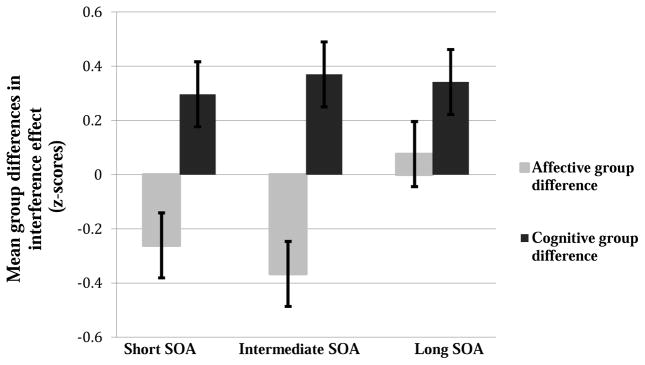
First, we investigated whether people with schizophrenia would exhibit a similar overall impairment on both types of tasks or whether the nature of group differences would vary by type of task. We conducted a task (affective interference task vs. cognitive interference task) by SOA (short vs. intermediate vs. long) by group (SZP vs. control) repeated measures ANOVA for the interference effects. Overall, there was a significant task X group interaction, F(1,71) = 8.67, p < .01,η2= .038, as between-group differences varied significantly by type of task. As can be seen in Figure 1 and in Tables 2 and 3, people with schizophrenia tended to exhibit a decreased affective interference effect compared to controls. In contrast, people with schizophrenia tended to exhibit an increased cognitive interference effect compared to controls. There were no other significant main effects or interactions. We next examined performance on each task separately.
Figure 1.
Mean group differences in the affective and cognitive interference effects by SOA.
Table 2.
Means (SD) of Trial Types on Affective Interference Task
| Schizophrenia Group | Control Group | |||||
|---|---|---|---|---|---|---|
| 75 SOA | 150 SOA | 250 SOA | 75 SOA | 150 SOA | 250 SOA | |
| Reaction Times | ||||||
| PP | 1634.97 (578.87) | 1607.53 (597.26) | 1512.77 (578.86) | 1002.88 (244.63) | 962.89 (223.33) | 922.44 (185.83) |
| PN | 1773.42 (737.51) | 1617.41 (632.13) | 1600.52 (612.97) | 1009.32 (232.07) | 984.87 (205.8) | 986.97 (212.16) |
| NP | 1534.38 (531.31) | 1548.68 (540.66) | 1433.62 (470.57) | 929.41 (182.91) | 1008.19 (240.55) | 936.66 (199.78) |
| NN | 1682.64 (626.27) | 1733.31 (682.09) | 1545.15 (529.28) | 1044.58 (250.4) | 986.58 (217.56) | 930.84 (196.67) |
| Error rates | ||||||
| PP | .13(.19) | .13 (.18) | .11 (.16) | .02 (.06) | .02 (.03) | .01 (.03) |
| PN | .13(.14) | .12 (.13) | .12 (.14) | .03 (.05) | .03 (.05) | .02 (.04) |
| NP | .10 (.17) | .10 (.13) | .10 (.15) | .02 (.03) | .02 (.04) | .03 (.03) |
| NN | .12 (.13) | .12 (.13) | .09 (.13) | .03 (.04) | .01 (.03) | .02 (.04) |
Note: PP = positive cue-positive target, PN = positive cue-negative target, NP = negative cue-positive target, NN = negative cue-negative target; The SZP group significantly differed from the control group on reaction times and error rates for all trial types, all ps <.05.
Table 3.
Means (SD) of Trial Types on Cognitive Interference Task
| Schizophrenia Group | Control Group | |||||
|---|---|---|---|---|---|---|
| 75 SOA | 150 SOA | 250 SOA | 75 SOA | 150 SOA | 250 SOA | |
| Reaction Times | ||||||
| RR | 789.96 (370.59)** | 777.41 (356.89)** | 723.79 (296.26)** | 574.9 (178.71) | 555.76 (162.49) | 551.34 (143.29) |
| RG | 864.87 (392.05)** | 893.89 (469.61)** | 833.78 (466.08)** | 610.16 (120.72) | 577.79 (144.86) | 566.12 (106.46) |
| GR | 879.39 (412.23)** | 920.57 (580.96)** | 811.56 (425.84)** | 622.87 (185.63) | 597.22 (197.53) | 577.93 (145.91) |
| GG | 795.05 (363.27)** | 797.4 (390.54)** | 734.02 (318.75)** | 546.36 (140.87) | 545.16 (186.29) | 535.07 (145.23) |
| Error rates | ||||||
| RR | .06 (.09)* | .08 (.12)* | .08 (.11) | .02 (.05) | .04 (.04) | .03 (.05) |
| RG | .13 (.18)* | .16 (.21)** | .10 (.17) | .04 (.08) | .04 (.06) | .04 (.07) |
| GR | .14 (.19)** | .17 (.22)** | .11 (.15)** | .03 (.05) | .04 (.05) | .02 (.04) |
| GG | .07 (.13) | .11 (.16)* | .07 (.11)* | .02 (.13) | .03 (.04) | .01 (.03) |
Note: RR = red cue-red target, RG = red cue-green target, GR = green cue-red target, GG = green cue-green target;
p< .05,
p < .01.
Affective interference effect
We conducted group (SZP vs. control) by SOA (short vs. intermediate vs. long) repeated measures ANOVA for the affective interference task. As can be seen in Figure 1, there was a trend for the SZP group to exhibit an overall smaller affective interference effect from the control group, F(1,74) = 3.32, p = .07, η2 = .05 . In addition, the SZP group only tended to exhibit a smaller affective interference effect at the shortest SOAs, but the group by SOA interaction was not significant, F(2, 148) = 2.17, p = .12, η2 =.028. People with SZP did exhibit a significantly smaller affective interference effect than control participants at the intermediate SOA, t(74) = 2.61, p =.01, r = .29. Also, there was a trend for the groups to differ at the short SOA, t(74) = 1.48, p = .14, r = .17. In contrast, the groups did not differ at the long SOA, t(74) = .44, p = .66, r = .05, with, if anything, people with schizophrenia exhibiting a numerically larger affective interference effect than controls. Thus, there is some evidence that people with SZP exhibit decreased affective interference at the shorter SOAs, which was significant at the intermediate SOA.
To investigate whether there were differences in responses to positive vs. negative cues or targets, we conducted a cue valence (positive vs. negative) by target valence (positive vs. negative) by SOA (short vs. intermediate vs. long) by group (SZP vs. control) repeated measures ANOVA for the affective interference effect. There was not a significant 4-way interaction, p = .83, nor any significant 3-way interactions (all ps < .43) or 2-way interactions (all ps < .59). There was a significant main effect of group, F(1, 74) = 44.93 p < .001. There were no main effects for cue valence, target valence, or SOA, all ps < .78. Thus, although the SZP group exhibited decreased affective interference compared to control participants, there were not differential effects of positive vs. negative cues or targets.
Cognitive interference effect
Next, we conducted a group (SZP vs. control) by SOA (short vs. intermediate vs. long) repeated measures ANOVA for the cognitive interference task. There was not a significant interaction between group and SOA, but there was a significant main effect of group, F(1,72) = 5.46, p < .05,η2 = .07. Overall, people with schizophrenia exhibited a significantly larger cognitive interference effect than controls. As can be seen in Figure 1, the SZP group exhibited a significantly larger cognitive interference effect than control participants at the intermediate SOA, t(72) = 2.34, p < .05, r = .27. Also, there were trends for differences between the groups at both the short SOA, t(72) = 1.78, p = .08, r = .21, and the long SOA, t(72) = 1.68, p = .097, r = .19. Thus, in contrast to the results involving the affective interference effect, people with SZP group show significantly increased cognitive interference compared to the control group.
Affective interference effect and anhedonia
Next, we examined whether affective interference was associated with anhedonia. In people with schizophrenia, there was a significant correlation between the affective interference effect at the two shortest SOAs and anhedonia, r = −.39, p < .01. Hence, higher levels of anhedonia were associated with decreased affective interference in schizophrenia. In contrast to the results for anhedonia, affective interference was not correlated with speech symptoms: communication disturbances, r = −.02; alogia, r = −.13; all ps > .37. In addition, the size of the correlation between anhedonia and affective interference was significantly more negative than the correlation between affective interference and communication disturbances, Z = −1.86, p < .05, and at trend level with alogia, Z = 1.33, p = .09 (Meng, Rosenthal, & Rubin, 1992).
Cognitive interference effect and speech symptoms
Next, we examined whether cognitive interference was associated with speech symptoms. In people with schizophrenia, there were significant correlations between speech symptoms and cognitive interference at the intermediate SOA: communication disturbances, r = .38, p < .01; alogia, r = .41, p < .01. Thus, it appears that symptoms previously associated with prepotent inhibition deficits were associated with increased cognitive interference. In contrast, the correlation between the cognitive interference effect and anhedonia was not significant, r = .19, p = .2. Furthermore, the correlation between anhedonia and cognitive interference was significantly different from the correlation between anhedonia and affective interference, Z = 1.74, p < .05. Thus, overall, there appeared to be some distinct associations between interference effects (affective vs. cognitive) and symptoms (anhedonia vs. speech symptoms).1
Group differences in self-reported attention to emotion
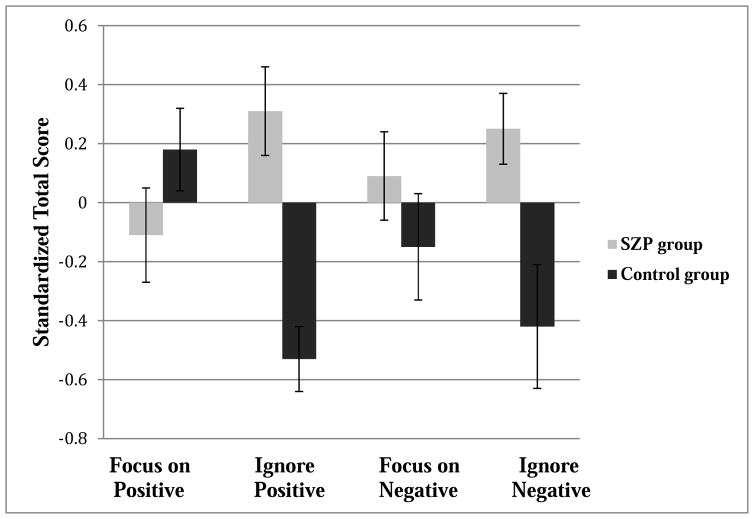
Next, we examined whether there were group differences on self-reported attention to emotions measured by the FAST. Overall, there was a significant valence (positive vs. negative) by attention (focus vs. ignore) interaction, F(1,73) = 430.05, p < .0001, as participants reported focusing on positive emotions and ignoring negative emotions more than focusing on negative emotions and ignoring positive emotions, all ps < .001. There was also a trend for group by valence interaction, F(1,73) = 3.28, p = .074, as individuals with schizophrenia tended to report relatively greater attention to negative than to positive emotions compared to controls. Most importantly, as can be seen in Figure 2, there was a significant group by attention (focus vs. ignore) interaction, F(1,73) = 15.08, p < . 001. Hence, we analyzed results separately for focusing on and ignoring of emotions. For focusing on emotions, there were no group differences, F(1, 73) = .023, p = .88. However, for ignoring emotions, people with schizophrenia reported increased ignoring of emotions, F(1, 73) = 18.03, p < .001. This was true for both ignoring positive emotions, t(73) = 3.84, p < .001, r = .41, and ignoring negative emotions, t(73) = 3.01, p < .01, r = .33. Overall, these results suggest that people with schizophrenia differ from controls in their self-reported desire to ignore both positive and negative emotions but not in their self-reported focusing on emotions.
Figure 2.
Standardized total subscale scores on the Following Affective States Test by group
Next, we examined whether self-reported attention to emotion was related to affective interference in the SZP group. Higher levels of ignoring positive emotions was significantly associated with decreased affective interference at the two shortest SOAs, r = −.37, p < .05. There were no other significant relationships between attention to emotion and affective interference, all ps > .1.
Last, we examined associations between anhedonia and reports of attention to emotion in the SZP group. Anhedonia was significantly correlated with increased ignoring of positive emotions, r =. 48. In addition, anhedonia was also significantly correlated with decreased focusing on positive emotions, r = −.36, and increased focusing on negative emotions, r = .40, but not with self-reported ignoring of negative emotions, r = .02, p = .9.
Discussion
In the current study, there were multiple pieces of evidence consistent with decreased attention to affective information in people with schizophrenia. First, we employed a well-validated behavioral measure of attention to affective information, the affective interference task (Fazio, 2001; Klauer & Musch, 2002). Hence, based on previous research with this task, the current findings of decreased affective interference in people with schizophrenia suggest decreased attention to affective information in this group. At the same time, attention to affective information is thought to be most evident on this task at the shortest SOAs (Klauer et al., 2009). Hence, if people with schizophrenia do have decreased attention to affective information, it would be expected to be most evident at the shortest SOAs. Consistent with this, we found decreased affective interference in schizophrenia at the shortest SOAs. It should be noted that the between-group effect size for the difference in performance on the affective interference task was not large and that power was somewhat limited our ability to detect some between-group differences.
Another piece of evidence that suggests decreased attention to affective information in schizophrenia is the indication of a double dissociation between the affective and cognitive interference tasks. Both the affective interference and cognitive interference tasks involve prepotent response inhibition, as incongruent trials involve response interference from the cue and require cognitive control to overcome the influence of the cue. On these tasks, a deficit only in prepotent inhibition would predict increased interference in schizophrenia. However, on the affective interference task, the occurrence of prepotent response interference also requires being sensitive to the affective valence of the cue word. Hence, decreased attention to affective information should prevent the occurrence of prepotent response interference and result in decreased affective interference. We found some evidence of this at the shortest SOAs. Thus, the indication of a possible double dissociation found between the affective and cognitive interference tasks further suggests a deficit in attention to affective information in schizophrenia. Also, the presence of prepotent inhibition deficits could account for why people with schizophrenia did not differ from controls at the longest SOA on the affective interference task. This is because with longer time to process the affective cue word, prepotent inhibition deficits in people with schizophrenia results in them rapidly catching up to controls in the amount of interference exhibited on the affective interference task. Hence, overall, the double dissociation in performance on the affective and cognitive interference tasks seems to further support a deficit in attention to affective information in schizophrenia.
The indication of a possible double dissociation between the affective and cognitive interference tasks not only provides evidence of decreased attention to affective information in schizophrenia, but it also suggests this deficit is a specific one and not reflective of a generalized deficit. Double dissociations (i.e. the experimental group performs worse on one task, while the control group performs worse on the other) have been called the “most powerful internal control” (MacDonald, Pogue-Geile, Johnson, & Carter, 2003, p. 58) and provide stronger evidence of a specific deficit than equating tasks on discriminating power based on the tasks’ true score variance. In fact, true score variance may actually be a fairly imperfect method for determining discriminating power (Kang & MacDonald, 2010). Thus, the combination of decreased affective interference in the face of increased cognitive interference in the schizophrenia group suggests that poor performance on the affective interference task in schizophrenia could be a specific deficit.
Another piece of evidence consistent with a deficit in attention to affective information in schizophrenia is the association between decreased affective interference and increased anhedonia. If decreased affective interference was due to affective deficits, then we would expect decreased affective interference to be associated with emotion-related symptoms such as anhedonia. At the same time, the association between decreased affective interference with both anhedonia and increased ignoring of positive emotion makes some other non-affective interpretations of the current results less likely. For instance, other possible interpretations of decreased affective interference in schizophrenia in the current study are a general problem with rapidly processing linguistic stimuli or a general decrease in semantic priming. However, it is not clear why anhedonia or ignoring of positive emotion would be associated with these general linguistic or semantic deficits. At the same time, previous schizophrenia research has not found evidence of decreased semantic priming at SOAs less than 200 ms (Pomarol-Clotet, Oh, Laws, & McKenna, 2008).
Hence, the association between anhedonia and decreased affective interference and previous evidence of intact short SOA semantic priming in schizophrenia further suggests that decreased affective interference in the current study reflects decreased attention to affective information in schizophrenia. Hence, overall, we interpret decreased affective interference in schizophrenia at the shortest SOAs as reflecting decreased attention to affective information. One possible explanation for decreased attention to affective information is that individuals with schizophrenia chronically do not attend to affective feelings and information. Consistent with this, we found that individuals with schizophrenia report wanting to ignore both their positive and negative emotions more than control participants. Hence, from this view, chronic inattention to affect makes them less likely to process affective valence and results in decreased affective interference. In addition, this chronic inattention to affect could contribute to anhedonia symptoms in schizophrenia. Chronic inattention to affect should result in people with schizophrenia being less likely to identify the presence of positive affect and should decreased self-reported trait levels of positive affect. It should also decrease the tendency to focus on and increase positive affect when it occurs (Garland et al., 2010), resulting in increased self-reported anhedonia. Therefore, we interpret the current evidence of decreased affective interference in schizophrenia and its relation to anhedonia as reflecting chronic inattention to affective feelings and valence information.
Previous research is also generally consistent with decreased chronic inattention to affective feelings and valence information in schizophrenia. First, our findings are consistent with Suslow et al. (2003), who reported anhedonia in schizophrenia was associated with aberrant attention to affective information on a task involving briefly presented picture cues and then evaluations of ideograph targets. In addition, people with schizophrenia have been found to report a general decrease in attention to emotions (Cedro, Kokoszka, Popiel, & Narkiewicz-Jodko, 2001; Maggini, Raballo, & Salvatore, 2002; Serper & Berenbaum, 2008; Stanghellini & Ricca, 1995). At the same time, decreased self-reported attention to emotions is also associated with anhedonia in people with schizophrenia (Becker, Cicero, & Kerns, 2007) and in people at risk for the development of a schizophrenia-spectrum disorder (Berenbaum et al., 2006; Kerns, 2006; Martin, Becker, Cicero, Docherty, & Kerns, 2011). Also, the current results are generally consistent with previous findings of decreased anticipatory pleasure in schizophrenia. If individuals with schizophrenia have chronic inattention to affective information, it is possible that they would be less likely to consider future pleasant events (Gard, Kring, Gard, Horan, & Green, 2007; Chan et al, 2010). Procedural differences between our study and other affective priming and incidental learning studies (e.g., length of SOA, stimuli type) might account for why some other studies have not reported possible evidence of decreased affective priming in schizophrenia. For example, with more time to process cue information, patients may show increased affective priming for negative emotional stimuli as was reported in Hooker et al. (2011).
In addition, previous non-schizophrenia research on affective attention retraining suggests that chronic inattention to affective stimuli should result in decreased neural responses to emotional stimuli (Eldar, Yankelevitch, Lamy, & Bar-Haim, 2010). Hence, if people with schizophrenia have chronic inattention to affective feelings and valence information, then it would be expected that schizophrenia would be associated with decreased activity in brain regions associated with processing of affective information. Consistent with this, previous research has reported that people with schizophrenia or people at-risk for schizophrenia do exhibit decreased ventral striatum and amygdala activity for emotional stimuli (e.g., Anticevic et al., 2012; Dowd & Barch, 2010; Gur et al., 2002; Gur et al., 2007; Juckel et al., 2006; Kirsch, Ronshausen, Mier, & Gallhofer, 2007; Lawrie & Abukmeil, 1998; Modinos, Ormel, & Aleman, 2010; Nelson, Saykin, Flashman, & Riordan, 1998; Schneider et al., 1998; Takahashi et al., 2004; Williams et al., 2004). In addition, anhedonia has also been associated with decreased striatum activity (Dowd & Barch, 2010; Gradin et al., 2011). Thus, overall, the current finding of decreased attention to affective information in schizophrenia is generally consistent with previous research suggesting inattention to affective valence and decreased emotion-related neural activity in schizophrenia and its relationship to anhedonia.
Future research on the hypothesis of inattention to affective valence in schizophrenia could employ a number of methodologies that could complement the affective interference task used in the current study. For example, the Dot Probe Task (Mathews & MacLeod, 2005), on which people with anxiety and other disorders shows negative attentional biases, could potentially be used to examine whether people with schizophrenia exhibit decreased attention to affective stimuli (Anticevic, Repovs, & Barch, 2011). Another value in using the Dot Probe Task is that attentional biases towards or away from affective information on this task can be modified in a single session (MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002), with evidence that attention bias modification can have important real world effects (MacLeod & Matthews, 2012). Hence, future research on the inattention hypothesis could use retraining on the Dot Probe Task to attempt to increase attention to emotion in people with schizophrenia. If chronic inattention to affective valence contributes to decreased affective interference, then it would be expected that after attention retraining, group differences between people with schizophrenia and controls on the affective interference task should be decreased or eliminated.
The current research also suggests that chronic inattention to affective valence contributes to anhedonia in schizophrenia. One implication of the current results is that potentially increased attention to emotion in schizophrenia could be used as a possible treatment for anhedonia. That is, attentional training may increase one’s ability to encode and subsequently retrieve memories of positive affect, which has recently been argued to be a core deficit in anhedonia (Strauss & Gold, 2012). Hence, one issue for future research might be to examine whether increasing attention to emotion in schizophrenia decreases anhedonia. At the same time, previous research on positive emotion regulation suggests that increased savoring of positive emotions increases the amount of positive affect experienced (Garland et al., 2010). In contrast, it has also been found that some people are motivated to attempt to decrease, or dampen, the amount of positive affect they experience (Wood, Heimpel, & Michela, 2003). This suggests that one possible implication of chronic inattention to affective valence in schizophrenia might be decreased savoring and possibly even increased dampening of positive emotions. Future research could examine whether anhedonia is associated with decreased savoring and increased dampening of positive affect. In addition, future research could examine whether attempts to increase savoring of positive affect decreases anhedonia in schizophrenia (Garland et al., 2010).
Another issue for future research would be to examine the effects of antipsychotic medication on decreased attention to affective information in people with schizophrenia. In the current study, all but one person with schizophrenia was taking anti-psychotic medication at the time of testing, with all participants having taken them previously. Given the possible relationship between anti-psychotic mediation usage and ventral striatum activity (e.g., Juckel et al., 2006; Kirsch et al., 2007; Schlagenhauf et al., 2008), it remains a possibility that decreased attention to affective information in schizophrenia could be a by-product of antipsychotic medication. Future research could examine whether unmedicated people with schizophrenia also exhibit decreased attention to affective information.
In addition, future research should examine whether the current results generalize to a more diverse patient population (e.g., non-forensic, outpatients, first episode patients, increased number of women) as the current study predominately consisted of chronically ill, male inpatient participants. In addition to finding decreased attention to affective information in schizophrenia and its association with anhedonia, the current study also provides further evidence of deficits in prepotent response inhibition in people with schizophrenia and of the association of these deficits with communication disturbances and alogia. As previously mentioned, the results for the cognitive interference task in people with schizophrenia were very different than the results for the affective interference task. Although the SZP group exhibited decreased interference on the affective interference task, they exhibited increased interference on the cognitive interference task. Also, performance on the cognitive interference task was not related to anhedonia but was related to increased communication disturbances and increased alogia. This is consistent with previous evidence of a relationship between increased prepotent response interference and verbal communication impairments (Barch et al., 1999; Becker et al., 2012; Kerns & Berenbaum, 2002). Overall, this suggests that future research on the nature of prepotent inhibition and cognitive control deficits in schizophrenia might in part help elucidate the nature of speech symptoms in the disorder.
Acknowledgments
This research was supported in part by a National Research Service Award, awarded to Elizabeth A. Martin (F31MH090669), and NIAAA (R21AA019492) and MU Research Board grants awarded to John G. Kerns.
Footnotes
Neither affective interference (all ps > .25) nor cognitive interference (all ps > .36) was associated with global ratings of hallucinations, delusions, or positive thought disorder on the SAPS.
References
- Anderson NH. Likeableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology. 1968;9:272–27. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa College of Medicine; Iowa City: 1984. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa College of Medicine; Iowa City: 1984. [Google Scholar]
- Anticevic A, Repovs G, Barch DM. Working Memory Encoding and Maintenance Deficits in Schizophrenia: Neural Evidence for Activation and Deactivation Abnormalities. Schizophrenia Bulletin. 2011 doi: 10.1093/schbul/sbr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala recruitment in schizophrenia in response to aversive emotional material: A meta-analysis of neuroimaging studies. Schizophrenia Bulletin. 2012;38:608–621. doi: 10.1093/schbul/sbq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badcock JC, Michie PT, Johnson L, Combrinck J. Acts of control in schizophrenia: Dissociating the components of inhibition. Psychological Medicine. 2002;32:287–97. doi: 10.1017/S0033291701005128. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Perlstein W, Baird J, Cohen JD, Schooler N. Increased Stroop facilitation effects in schizophrenia are not due to increased automatic spreading activation. Schizophrenia Research. 1999;39:51–64. doi: 10.1016/S0920-9964(99)00025-0. [DOI] [PubMed] [Google Scholar]
- Barch DM, Yodkovik N, Sypher-Locke H, Hanewinkel M. Intrinsic motivation in schizophrenia: Relationship to cognitive function, depression, anxiety, and personality. Journal of Abnormal Psychology. 2008;117:776–787. doi: 10.1037/a0013944. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Chaiken S, Govender R, Pratto F. The generality of the automatic attitude activation effect. Journal of Personality and Social Psychology. 1992;62:893–912. doi: 10.1037/0022-3514.62.6.893. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Riordan MA, Saults JS, Lust SA. Psychophysiological evidence of response conflict and strategic control of responses in affective priming. Journal of Experimental Social Psychology. 2009;45:655–666. doi: 10.1016/j.jesp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TM, Cicero DC, Kerns JG. Disorganized speech, cognitive control deficits, and emotion traits in schizophrenia. Poster presented at the annual meeting of the Society for Psychopathology Research; Iowa City, IA. 2007. Oct, [Google Scholar]
- Becker TM, Cicero DC, Martin EA, Docherty AR, Kerns JG. Increased cognitive control demands result in an increase in disorganized speech in people with schizophrenia. Poster presented at the annual meeting of the Society for Psychopathology Research; Boston, MA. 2011. Sep, [Google Scholar]
- Becker TM, Cicero DC, Cowan N, Kerns JG. Cognitive control components and speech symptoms in people with schizophrenia. Psychiatry Research. 2012;196:20–26. doi: 10.1016/j.psychres.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellazza FS, Greenwald AG, Banaji MR. Words high and low in pleasantness as rated by male and female college students. Behavior Research Methods, Instruments, & Computers. 1986;18:299–303. doi: 10.3758/BF03204403. [DOI] [Google Scholar]
- Berenbaum H, Boden MT, Baker JP, Dizen M, Thompson RJ, Abramowitz A. Emotional correlates of the different dimensions of schizotypal personality disorder. Journal of Abnormal Psychology. 2006;115:359–368. doi: 10.1037/0021-843X.115.2.359. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Kerns JG, Vernon LL, Gomez JJ. Cognitive correlates of schizophrenia signs and symptoms: I. Verbal communication disturbances. Psychiatry Research. 2008;159:147–56. doi: 10.1016/j.psychres.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophrenia Bulletin. 1998;24:413–424. doi: 10.1093/oxfordjournals.schbul.a033336. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophrenia Bulletin. 1998;24:413–424. doi: 10.1016/j.schres.2010.08.039. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Kring AM, Horan WP, Gur R. Towards the next generation of negative symptom assessments: The Collaboration to Advance Negative Symptom Assessment in Schizophrenia. Schizophrenia Bulletin. 2011;37:291–299. doi: 10.1093/schbul/sbq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): Instruction manual and affective ratings. (Technical Report C-1) 1999 Retrieved from University of Florida, Center for Research in Psychophysiology website: http://a.parsons.edu/~spani621/thesis/context/ANEW.pdf.
- Brown WP, Ure DM. Five rated characteristics of 650 word association stimuli. British Journal of Psychology. 1969;60:233–249. doi: 10.1111/j.2044-8295.1969.tb01196.x. [DOI] [PubMed] [Google Scholar]
- Burch GSJ, Hemsley DR, Corr PJ, Gwyer P. The relationship between incidental learning and multi-dimensional schizotypy as measured by the Oxford- Liverpool Inventory of Feelings and Experiences (O-LIFE) Personality and Individual Differences. 2006;40:385–394. doi: 10.1016/j.paid.2005.07.010. [DOI] [Google Scholar]
- Cedro A, Kokoszka A, Popiel A, Narkiewicz-Jodko W. Alexithymia in schizophrenia: An exploratory study. Psychological Reports. 2001;89:95–98. doi: 10.2466/pr0.2001.89.1.95. [DOI] [PubMed] [Google Scholar]
- Clementz BA. Psychophysiological measures of (dis)inhibition as liability indicators for schizophrenia. Psychophysiology. 1998;35:648–668. doi: 10.1111/1469-8986.3560648. [DOI] [PubMed] [Google Scholar]
- Clore GL, Ortony A. Appraisal theories: How cognition shapes affect into emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotions. 3. New York: Guilford Press; 2008. pp. 628–642. [Google Scholar]
- Chapman LJ, Chapman JP. Revised physical anhedonia scale. 1978 Unpublished manuscript. [Google Scholar]
- Chan RC, Wang Y, Huang J, Shi Y, Wang Y, Hong X, … Kring AM. Anticipatory and consummatory components of the experience of pleasure in schizophrenia: Cross-cultural validation and extension. Psychiatry Research. 2010;175:181–183. doi: 10.1016/j.psychres.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: Meta-analysis of laboratory studies. Schizophrenia Bulletin. 2008;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Zelazo PD. Attitudes and evaluations: A social cognitive neuroscience perspective. Trends in Cognitive Science. 2007;11:97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Lebow B, Lake DS, Katsanis J, Iacono WG. Acoustic startle reflex in schizophrenia patients and their first-degree relatives: Evidence of normal emotional modulation. Psychophysiology. 1999;36:469–475. doi: 10.1017/S0048577299980757. [DOI] [PubMed] [Google Scholar]
- Danion JM, Meulemans T, Kauffmann-Muller F, Vermaat H. Intact implicit learning in schizophrenia. American Journal of Psychiatry. 2001;158:944–948. doi: 10.1176/appi.ajp.158.6.944. [DOI] [PubMed] [Google Scholar]
- Docherty NM, DeRosa M, Andreasen NC. Communication disturbances in schizophrenia and mania. Archives of General Psychiatry. 1996;53:358–364. doi: 10.1001/archpsyc.1996.01830040094014. [DOI] [PubMed] [Google Scholar]
- Dowd E, Barch DM. Anhedonia and emotional experience in schizophrenia: Neural and behavioral markers. Biological Psychiatry. 2010;67:902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes M, McGhie A. A comparative study of attentional strategies of schizophrenic and highly creative normal subjects. British Journal of Psychiatry. 1976;128:50–56. doi: 10.1192/bjp.128.1.50. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ, Chapman JP, Mishlove M. The Revised Social Anhedonia Scale. 1982 Unpublished manuscript. [Google Scholar]
- Edell WS. The psychometric measurement of schizotypy using the Wisconsin Scales of Psychosis Proneness. In: Miller GA, editor. The Behavioral High-Risk Paradigm in Psychopathology. New York: Springer-Verlag; 1995. pp. 3–46. [Google Scholar]
- Eldar S, Yankelevitch R, Lamy D, Bar-Haim Y. Enhanced neural reactivity and selective attention to threat in anxiety. Biological Psychology. 2010;85:252–257. doi: 10.1016/j.biopsycho.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Eprime (Version 2) [Computer software] Sharpsburg, PA: Psychological Software Tools; [Google Scholar]
- Fazio RH. On the automatic activation of associated evaluations: An overview. Cognition and Emotion. 2001;15:115–141. doi: 10.1080/0269993004200024. [DOI] [Google Scholar]
- Fazio RH, Sanbonmatsu DM, Powell MC, Kardes FR. On the automatic activation of attitudes. Journal of Personality and Social Psychology. 1986;50:229–238. doi: 10.1037/0022-3514.50.2.229. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York State Psychiatric Institute; New York: 1998. [Google Scholar]
- Francis WN, Kučera H. Frequency analysis of English words. Boston, MA: Houghton Mifflin; 1982. [Google Scholar]
- Fredrickson BL. The role of positive emotions in positive psychology: The broaden-and-build theory of positive emotions. American Psychologist. 2001;56:218–226. doi: 10.1037/0003-066X.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Gray M, Whitfield S, Turken AU, Glover G, Faustman WO, Mathalon DH. Acquiring and inhibiting prepotent responses in schizophrenia. Archives of General Psychiatry. 2004;61:119–129. doi: 10.1001/archpsyc.61.2.119. [DOI] [PubMed] [Google Scholar]
- Garland EL, Fredrickson B, Kring AM, Johnson DP, Meyer PS, Penn DL. Upward spirals of positive emotions counter downward spirals of negativity: Insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical Psychology Review. 2010;30:849–864. doi: 10.1016/j.cpr.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Germans Gard M, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93:253–360. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasper K, Bramesfeld KD. Should I follow my feelings? How individual differences in following feelings influence affective well-being, experience, and responsiveness. Journal of Research in Personality. 2006;40:986–1014. doi: 10.1016/j.jrp.2005.10.001. [DOI] [Google Scholar]
- Gooding DC, Tallent KA, Matts CW. Clinical status of at-risk individuals 5 years later: Further validation of the psychometric high-risk strategy. Journal of Abnormal Psychology. 2005;114:170–175. doi: 10.1037/0021-843X.114.1.170. [DOI] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, … Steele JD. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–1764. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. Optimizing the use of information: Strategic control of activation and responses. Journal of Experimental Psychology: General. 1992;121:480–506. doi: 10.1037/0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, … Gur RC. An fMRI study of facial emotion processing in patients with schizophrenia. American Journal of Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, … Gur RC. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. American Journal of Psychiatry. 2007;64:1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, Harvey AN, Beck VM, Leonard CJ, … Gold JM. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biological Psychiatry. 2010;68:603–9. doi: 10.1016/j.biopsych.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. Journal of Abnormal Psychology. 2007;116:268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Herring DR, Taylor JH, White KR, Crites SL. Electrophysiological responses to evaluative priming: the LPP is sensitive to incongruity. Emotion. 2011;11:794–806. doi: 10.1037/a0022804. [DOI] [PubMed] [Google Scholar]
- Hermans D, De Houwer J, Eelen P. The affective priming effect: Automatic activation of evaluative information in memory. Cognition and Emotion. 1994;8:515–533. doi: 10.1080/02699939408408957. [DOI] [Google Scholar]
- Hooker CI, Tully LM, Verosky SC, Fisher M, Holland C, Vinogradov S. Can I trust you? Negative affective priming influences social judgments in schizophrenia. Journal of Abnormal Psychology. 2011;120:98–107. doi: 10.1037/a0020630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Green MF, Kring AM, Nuechterlein KH. Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? Journal of Abnormal Psychology. 2006;115:496–508. doi: 10.1093/schbul/sbj009. [DOI] [PubMed] [Google Scholar]
- Horan WP, Green MF, Knowlton BJ, Wynn JK, Mintz J, Nuechterlein KH. Impaired implicit learning in schizophrenia. Neuropsychology. 22:606–617. doi: 10.1037/a0012602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Wynn JK, Kring AM, Simons RF, Green MF. Electrophysiological correlates of emotional responding in schizophrenia. Journal of Abnormal Psychology. 2010;119:18–30. doi: 10.1037/a0017510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and Psychometric Validation of the Clinical Assessment Interview for Negative Symptoms (CAINS) Schizophrenia Research. 2011;132:140–145. doi: 10.1016/j.schres.2011.06.030. doi:0.1016/j.schres.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Foti D, Hajcak G, Wynn JK, Green MF. Schizophrenia Research. 2012;135:95–99. doi: 10.1016/j.schres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Fulham WR, Johnston PJ, Michie PT. Stop-signal response inhibition in schizophrenia: Behavioural, event-related potential and functional neuroimaging data. Biological Psychiatry. 2012;89:220–231. doi: 10.1016/j.biopsycho.2011.10.013. [DOI] [PubMed] [Google Scholar]
- John CH. Emotionality ratings and free-association norms of 240 emotional and non-emotional words. Cognition and Emotion. 1988;2:49–70. doi: 10.1080/02699938808415229. [DOI] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. doi:0.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wüstenberg T, Wustenberg T, … Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006;187:222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Kang SS, MacDonald AW., III Limitations of True Score Variance to Measure Discriminating Power: Psychometric Simulation Study. Journal of Abnormal Psychology. 2010;119:300–306. doi: 10.1037/a0018400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG. Positive schizotypy and emotion processing. Journal of Abnormal Psychology. 2005;114:392–401. doi: 10.1037/0021-843X.114.3.392. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Schizotypy facets, cognitive control, and emotion. Journal of Abnormal Psychology. 2006;15:418–427. doi: 10.1037/0021-843X.115.3.418. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Experimental manipulation of cognitive control processes causes an increase in communication disturbances in healthy volunteers. Psychological Medicine. 2007;137:995–1004. doi: 10.1017/S0033291706009718. [DOI] [PubMed] [Google Scholar]
- Kerns JK, Berenbaum H. Cognitive impairments associated with formal thought disorder in people with schizophrenia. Journal of Abnormal Psychology. 2002;111:211–224. doi: 10.1037/0021-843X.111.2.211. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BF, Daniel D, Cienfuegos A, Marder SR. The Brief Negative Symptom Scale: Psychometric properties. Schizophrenia Bulletin. 2011;37:300–305. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Ronshausen S, Mier D, Gallhofer B. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients. Pharmacopsychiatry. 2007;40:196–198. doi: 10.1055/s-2007-984463. [DOI] [PubMed] [Google Scholar]
- Klauer KC, Rossnagel C, Musch J. List-context effects in evaluative priming. Journal of Experimental Psychology Learning, Memory, and Cognition. 1997;23:246–255. doi: 10.1037/0278-7393.23.1.246. [DOI] [PubMed] [Google Scholar]
- Klauer KC, Musch J. Affective priming: Findings and theories. In: Musch J, Klauer KC, editors. The psychology of evaluation: Affective processes in cognition and emotion. Mahwah, NJ: Erlbaum; 2002. pp. 7–50. [Google Scholar]
- Klauer KC, Teige-Mocigemba S, Spruyt A. Contrast effects in spontaneous evaluations: A psychophysical account. Journal of Personality and Social Psychology. 2009;96:265–87. doi: 10.1037/a0013248. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Martin EA. Emotional processing in schizophrenia. Cognitive Neuropsychiatry. 2006;11:250–271. doi: 10.1080/13546800500188575. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: Insights from affective science. Schizophrenia Bulletin. 2008;35:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Germans Gard M, Gard DE. Emotion deficits in schizophrenia: Timing matters. Journal of Abnormal Psychology. 2011;120:79–87. doi: 10.1037/a0021402. [DOI] [PubMed] [Google Scholar]
- Kwapil TR. Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. Journal of Abnormal Psychology. 1998;107:558–565. doi: 10.1037/0021-843X.107.4.558. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Barrantes-Vidal N, Silvia P. The dimensional structure of the Wisconsin schizotypy scales: Factor identification and construct validity. Schizophrenia Bulletin. 2008;34:444–457. doi: 10.1093/schbul/sbm098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. British Journal of Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- Machado L, Wyatt N, Devine A, Knight B. Action planning in the presence of distracting stimuli: An investigation into the time course of distractor effects. Journal of Experimental Psychology Human Perception and Performance. 2007;33:1045–1061. doi: 10.1037/0096-1523.33.5.1045. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111:107–23. doi: 10.1037/0021-843X.111.1.107. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Pogue-Geile MF, Johnson MK, Carter CS. A specific deficit in context processing in the unaffected siblings of patients with schizophrenia. Archives of General Psychiatry. 2003;60:57–65. doi: 10.1093/schbul/sbp017. [DOI] [PubMed] [Google Scholar]
- Maggini C, Raballo A, Salvatore P. Depersonalization and basic symptoms in schizophrenia. Psychopathology. 2002;35:17–24. doi: 10.1159/000056211. [DOI] [PubMed] [Google Scholar]
- Martin EA, Becker TM, Cicero DC, Docherty AR, Kerns JG. Differential associations between schizotypy facets and emotion traits. Psychiatry Research. 2011;187:94–99. doi: 10.1016/j.psychres.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive Vulnerability to Emotional Disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mason O, Claridge G, Jackson M. New scales for the assessment of schizotypy. Personality and Individual Differences. 1995;18:7–13. doi: 10.1016/0191-8869(94)00132-C. [DOI] [Google Scholar]
- Mayer JD, Gaschke YN. The experience and meta-experience of mood. Journal of Personality and Social Psychology. 1988;55:102–111. doi: 10.1037/0022-3514.55.1.102. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P. What is emotional intelligence? In: Salovey P, Sluyter D, editors. Emotional development and emotional intelligence: Implications for educations. New York: Basic Books; 1997. pp. 3–31. [Google Scholar]
- Melinder MRD, Barch DM, Heydebrand G, Csernansky JG. Easier tasks can have better discriminating power: The case of verbal fluency. Journal of Abnormal Psychology. 2005;114:385–391. doi: 10.1037/0021-843X.114.3.383. [DOI] [PubMed] [Google Scholar]
- Meng X, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychological Bulletin. 1992;111:172–175. doi: 10.1037/0033-2909.111.1.172. [DOI] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager T. The unity and diversity of executive functions and their contributions to complex "frontal lobe" tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Rettinger DA, Shah P, Hegarty M. How are visuospatial working memory, executive functioning, and spatial abilities related? A latent variable analysis. Journal of Experimental Psychology: General. 2001;130:621–640. doi: 10.1037/0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Altered activation and functional connectivity of neural systems supporting cognitive control of emotion in psychosis proneness. Schizophrenia Research. 2010;118:88–97. doi: 10.1016/j.schres.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: A meta-analytic study. Archives of General Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Payne RW, Hochberg AC, Hawks D. Dichotic stimulation as a method of assessing disorder of attention in over-inclusive schizophrenic patients. Journal of Abnormal Psychology. 1970;76:185–193. doi: 10.1037/h0029886. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Oh TM, Laws KR, McKenna PJ. Semantic priming in schizophrenia: Systematic review and meta-analysis. British Journal of Psychiatry. 2008;192:92–97. doi: 10.1192/bjp.bp.106.032102. [DOI] [PubMed] [Google Scholar]
- Rubin DC. 51 properties of 125 words: A unit analysis of verbal behavior. Journal of Verbal Learning and Verbal Behavior. 1980;19:736–755. doi: 10.1016/s0022-5371(80)90415-6. [DOI] [Google Scholar]
- Salovey P, Mayer JD, Goldman S, Turvey C, Palfai T. Emotional attention, clarity and repair: Exploring emotional intelligence using the Trait Meta-Mood scale. In: Pennebaker JW, editor. Emotion, disclosure and health. Washington, DC: American Psychological Association; 1995. pp. 125–154. [Google Scholar]
- Schlagenhauf F, Juckel G, Koslowski M, Kahnt T, Knutson B, Dembler T, … Heinz A. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmocology (Berl ) 2008;196:673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- Schlenker R, Cohen R, Hopmann G. Affective modulation of the startle reflex in schizophrenic patients. European Archives of Psychiatry and Clinical Neuroscience. 1995;245:309–318. doi: 10.1007/BF02191873. [DOI] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, Salloum JB, Posse S, Grodd W, Müller-Gärtner HW. Differential amygdala activation in schizophrenia during sadness. Schizophrenia Research. 1998;34:133–42. doi: 10.1016/S0920-9964(98)00085-1. [DOI] [PubMed] [Google Scholar]
- Serper M, Berenbaum H. The relation between emotional awareness and hallucinations and delusions in acute psychiatric inpatients. Schizophrenia Research. 2008;101:195–200. doi: 10.1016/j.schres.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Stanghellini G, Ricca V. Alexithymia and schizophrenias. Psychopathology. 1995;28:263–272. doi: 10.1159/000284937. [DOI] [PubMed] [Google Scholar]
- Silverstein A, Dienstbier RA. Rated pleasantness and association value of 101 English nouns. Journal of Verbal Learning and Verbal Behavior. 1968;7:81–86. doi: 10.1016/S0022-5371(68)80168-9. [DOI] [Google Scholar]
- Smith NK, Cacioppo JT, Larsen JT, Chartrand TL. May I have your attention, please: Electrocortical responses to positive and negative stimuli. Neuropsychologia. 2003;41:171–183. doi: 10.1016/S0028-3932(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Smith NK, Larsen JT, Chartrand TL, Cacioppo JT, Katafiasz HA, Moran KE. Being bad isn’t always good: Affective context moderates the attention bias toward negative information. Journal of Personality and Social Psychology. 2006;90:210–220. doi: 10.1037/0022-3514.90.2.210. [DOI] [PubMed] [Google Scholar]
- Strauss ME. Demonstrating specific cognitive deficits: A psychometric perspective. Journal Abnormal Psychology. 2001;110:6–14. doi: 10.1037/0021-843X.110.1.6. [DOI] [PubMed] [Google Scholar]
- Strauss ME, Gold JM. A new perspective on anhedonia in schizophrenia. American Journal of Psychiatry. 2012;169:364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Roestel C, Arolt V. Affective priming in schizophrenia with and without affective negative systems. European Archives of Psychiatry and Clinical Neuroscience. 2003;253:292–300. doi: 10.1016/S0165-1781(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Koeda M, Oda K, Matsuda T, Matsushima E, Asai K, Okubo Y. An fMRI study of differential neural response to affective pictures in schizophrenia. NeuroImage. 2004;22:1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Tremeau F. A review of emotion deficits in schizophrenia. Dialogues in Clinical Neuroscience. 2006;8:59–70. doi: 10.31887/DCNS.2006.8.1/ftremeau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz M, Hamm AO, Kirsch P, Rey ER. Temporal course of emotional startle modulation in schizophrenia patients. International Journal of Psychophysiology. 2003;49:123–137. doi: 10.1016/S0167-8760Z03.00100-4. [DOI] [PubMed] [Google Scholar]
- Wentura D. Activation and inhibition of affective information: Evidence for negative priming in the evaluation task. Cognition and Emotion. 1999;13:65–91. doi: 10.1080/026999399379375. [DOI] [Google Scholar]
- Williams LM, Das P, Harris AWF, Liddell BB, Brammer MJ, Olivieri G, … Gordon E. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. American Journal of Psychiatry. 2004;161:480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- Wolf DH. Anhedonia in schizophrenia. Current Psychiatry Reports. 2006;8:322–328. doi: 10.1007/s11920-006-0069-0. [DOI] [PubMed] [Google Scholar]
- Wood JV, Heimpel SA, Michela JL. Savoring versus dampening: Self-esteem differences in regulating positive affect. Journal of Personality and Social Psychology. 2003;85:566–580. doi: 10.1037/0022-3514.85.3.566. [DOI] [PubMed] [Google Scholar]
- Yee CM, Mathis KI, Sun JC, Sholty GL, Lang PJ, Bachman P, … Nuechterlein KH. Integrity of emotional and motivational states during the prodromal, first-episode, and chronic phases of schizophrenia. Journal of Abnormal Psychology. 2010;119:71–82. doi: 10.1037/a0018475l. [DOI] [PMC free article] [PubMed] [Google Scholar]