Abstract
Objective
Most acute ischemic stroke (AIS) patients with unwitnessed symptom onset are ineligible for intravenous thrombolysis due to timing alone. Lesion evolution on FLAIR MRI correlates with stroke duration, and quantitative mismatch of diffusion-weighted MRI (DWI) with FLAIR (qDFM) might indicate stroke duration within guideline-recommended thrombolysis. We tested if intravenous thrombolysis ≤4.5 h from time of symptom discovery is safe in patients with qDFM in an open-label, phase 2a, prospective study (NCT01282242).
Methods
Patients aged 18–85 years with AIS of unwitnessed onset at 4.5–24 h since last known well, treatable within 4.5 h of symptom discovery with intravenous alteplase (0.9 mg/kg) and presenting with qDFM were screened across fourteen hospitals. The primary outcome was the risk of symptomatic intracranial hemorrhage (sICH) with pre-planned stopping rules. Secondary outcomes included symptomatic brain edema risk, and functional outcomes of 90-day modified Rankin Scale (mRS).
Results
Eighty subjects were enrolled between January 31, 2011 and October 4, 2015 and treated with alteplase at median 11.2 (9.5–13.3) h from last known well. There was one sICH (1.3%) and three cases of symptomatic edema (3.8%). At 90 days, 39% of subjects achieved mRS 0–1, as did 48% of subjects who had vessel imaging and were without large vessel occlusions.
Interpretation
Intravenous thrombolysis within 4.5 h of symptom discovery in patients with unwitnessed stroke selected by qDFM, who are beyond the recommended time windows, is safe. A randomized trial testing efficacy using qDFM appears feasible and is warranted in patients without large vessel occlusions.
INTRODUCTION
Intravenous (IV) thrombolysis of acute ischemic stroke (AIS) with recombinant tissue plasminogen activator (alteplase) is the only guideline-recommended IV AIS therapy, and it must be administered ≤4.5 h from when the patient was last known well. Despite two decades of availability, IV alteplase is given to <10% of patients worldwide.1 One reason for underuse is the strict time restriction. While time is easily measured, onset of symptoms is frequently not witnessed and difficult to establish in the emergency department setting; approximately 25–30% of AIS patients have stroke with unwitnessed symptom onset.2 Despite rapid presentation to the emergency department following symptom discovery, these patients do not qualify for guideline-recommended IV alteplase due to arrival >4.5 h from last known well, and most lack large vessel occlusions (LVO) required for endovascular thrombectomy. Even among stroke patients arriving at hospitals <6 h after last known well, less than 2% currently receive endovascular thrombectomy.3, 4 These numbers will likely increase due to several major trials showing profound benefit of endovascular thrombectomy.5 Recent clinical trial results6, 7 suggest that endovascular thrombectomy for patients with unwitnessed strokes and LVO is beneficial, and it is likely that this treatment approach that is now recommended will increasingly be adopted for unwitnessed LVO strokes. With increased screening of patients with unwitnessed strokes up to 24 h since last known well, there is urgent need to find effective new treatments for patients with unwitnessed strokes who lack LVO.
Retrospective studies of stroke patients with unwitnessed symptom onset treated with IV thrombolysis on a compassionate basis found that thrombolysis may be safely administered in a select subset with imaging patterns consistent with early stroke.8, 9 Small single-center studies using imaging-selection for prospectively treating stroke patients with unwitnessed symptom onset showed similar safety.10, 11 These studies suggested that an MRI diffusion-FLAIR mismatch, i.e., the presence of hyperintensity on diffusion-weighted MRI (DWI) with minimal or no evidence of hyperintensity on corresponding T2-weighted fluid attenuated inversion recovery (FLAIR) images, might identify a group of patients in whom the biological onset of symptom is more closely approximated by time of symptom discovery rather than time last known well. This supports FLAIR imaging being used as a “tissue clock” for ischemic injury, since its signal intensity increases over time after AIS, as has been demonstrated in experimental animal models.12 Therefore, diffusion-FLAIR mismatch might characterize stroke that is within 4.5 h of biological symptom onset.13 Diffusion-FLAIR mismatch has not yet been shown in a prospective multicenter study to be able to independently safely select patients for thrombolysis. In addition, simple qualitative diffusion-FLAIR mismatch has poor inter-rater agreement13 and hence might not be reproducible in a multi-center setting. We improved diffusion-FLAIR mismatch reproducibility14 by requiring that abnormal FLAIR be quantified; we hypothesized that a quantitative diffusion-FLAIR mismatch (qDFM) can be used in place of time from last known well to identify stroke patients with unwitnessed symptom onset who can safely be treated with thrombolytic therapy. The DWI-positive and FLAIR negative pattern proposed as a surrogate for short duration since symptom onset in our study is consistent with a pattern of restricted ADC with normal T2 signal that characterizes compromised tissue that may or may not recover with reperfusion as validated against histopathology in experimental stroke models.15, 16 Furthermore, the presence of hyperintense FLAIR lesions, in addition to being a possible indicator of late stage strokes, has also been linked to increased sICH risk even in patients treated within 3 h.17 Therefore, by excluding patients with substantial acute FLAIR lesions, we also excluded those patients with stroke deficits severe enough to have substantially delayed their initial presentation to medical assistance. Thus, qDFM is representative of not only short duration since symptom onset but also tissue with the greatest likelihood of recovery with reperfusion.
Prior to initiation of a randomized placebo-controlled trial (RCT) investigating the safety and efficacy of thrombolysis in imaging-selected stroke patients with unwitnessed symptom onset, a multicenter prospective phase 2a study was necessary to prove feasibility of acute MRI-based qDFM enrollment, of screening up to 24 h since last known well time, and to provide definitive evidence of safety of thrombolysis in this population. In stroke patients with unwitnessed symptom onset and qDFM, time of last known well could be replaced by time of discovery of symptoms and used in the treatment decision for thrombolysis. Therefore, the trial design contained both a time-based constraint (by requiring treatment within 4.5 h from first detection of symptoms), as well as a tissue-based constraint (qDFM). Both conditions needed to be met for enrollment in this trial. When the study was first formulated in 2009, MR WITNESS was designed based on the hypothesis that qDFM could be used to safely select patients with limited tissue injury due to brief stroke duration who might benefit from reperfusion, assuming that tissue infarction progresses at a relatively constant rate after symptom onset in most patients. As this was a safety study, it seemed prudent to constrain the upper limit of time since discovery of symptoms, to align with the available data on the known therapeutic time window of safety and efficacy of IV thrombolysis and the known risk of harm with delayed treatment. Our strategy was to enrich the study population with those subjects that likely still had brain at risk that can be salvaged by acute IV thrombolysis, as we believe that a substantial number of patients who awaken with symptoms did so shortly after the biological onset of their symptoms and patients with unwitnessed strokes might similarly have had symptom onset close to symptom discovery time. If our approach is successful, subsequent trials of IV thrombolysis could explore if time from last known well or symptom discovery could ultimately be abandoned, with treatment decisions based purely on brain imaging findings.
We therefore conducted the MR WITNESS trial using an imaging-based “MR witness” of infarct evolution rather than a “human witness” of chronologic time. We designed our subject selection criteria to select subjects who would be similar in stroke evolution to those treated in the ECASS3 trial, a trial that showed benefit from IV thrombolysis when given 3–4.5 h after symptom onset. We therefore excluded subjects with symptom discovery more than 4.5 h. Although there may exist patients who are more than 4.5 h since symptom discovery but in whom ischemia progresses much more slowly than expected and who still have a qDFM pattern that might benefit from thrombolysis, numerous independent studies have shown that such patients with diffusion-FLAIR mismatch beyond 6 h from stroke onset are extremely rare.18 Our study was not designed to assess if those who have greater stroke duration chronologically but who are slow progressors will respond to thrombolysis. Future studies that select subjects purely by imaging without regard to symptom discovery time will be needed to test this hypothesis.
SUBJECTS AND METHODS
Study design and participants
We conducted a multicenter, phase 2a, open-label safety trial of intravenous thrombolysis in stroke patients with unwitnessed symptom onset (ClinicalTrials.gov, NCT01282242). The study was conducted under an investigational new drug application with the Food and Drug Administration (FDA, IND 110075, 110088) and approved by the local institutional review board of each participating site. The trial launched with three sites and expanded in two waves to include a total of 14 US sites. The full study protocol including imaging details is available at http://www.mrwitness.org. Patients were recruited from the emergency department and inpatient areas. Each study site screened patients with unwitnessed symptom onset who were between 4.5 h to 24 h since last known well and could receive treatment within 4.5 h of symptom discovery.
At trial onset, subjects were excluded from the trial using criteria similar to the guideline-recommended exclusions in the United States for IV alteplase in the 3–4.5 h window, including severe stroke as assessed clinically (e.g., NIH Stroke Scale score >25) or by appropriate imaging techniques (DWI lesion volume > one-third of MCA by visual inspection or >100 cm3 using the ellipsoid estimation formula of ABC/2). After new data suggested safety of thrombolysis at 3–4.5 h in clinical practice, in 2012 the age limit was raised from 80 to 85 and the exclusion for diabetes plus stroke was removed. Patients with a history of recent dabigatran use but none in the past 24 h and with normal clotting studies were deemed eligible under specific circumstances. As this was a safety study, we did not exclude patients with pre-stroke disability (mRS>1). Additional MR-specific exclusion criteria were uninterpretable images, lack of DWI lesion, evidence of prior macroscopic intracranial hemorrhage (ICH), or microbleeds in a pattern suggestive of amyloid angiopathy. Full clinical and imaging inclusion and exclusion criteria can be found in the study protocol and in the Supplementary materials.
Prior to enrollment, all clinically eligible patients underwent MRI. Eligible subjects were 18–85 years of age, had a disabling neurological deficit at time of treatment lasting at least 30 min, a confirmed ischemic stroke on MRI, and a qDFM pattern, defined as minimal or no hyperintensity on FLAIR imaging in a region corresponding to that of restricted diffusion on DWI. The acceptable threshold for minimal FLAIR hyperintensity was specified a priori as a signal intensity ratio (SIR) of <1.15 when the mean signal intensity measured in a ROI involving the FLAIR hyperintensity was divided by a corresponding mean signal intensity in a ROI in normal appearing tissue in the contralesional hemisphere.14 Figure 1 shows the imaging-based selection algorithm.
Figure 1.
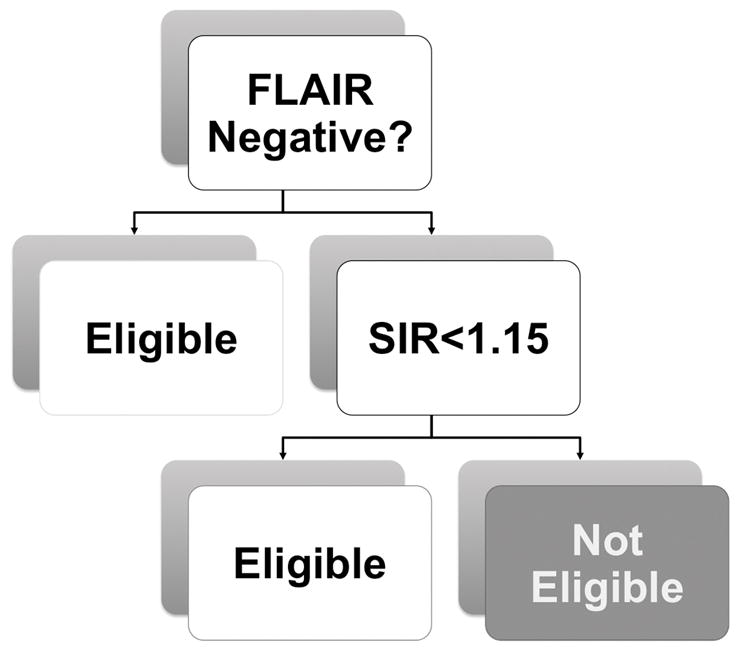
Flowchart demonstrating how to apply the Quantitative diffusion-FLAIR mismatch for enrollment
Study Procedures
The schedule of assessments for enrolled subjects is shown in Figure 2. Written informed consent was obtained prior to thrombolysis. Alteplase was administered at the usual dose and method of 0.9 mg/kg (maximum dose ≤90 mg) with 10% as a bolus over one min and the remainder by continuous infusion over one hour. The duration of the study was 90±14 d. Demographics, medical history, clinical and laboratory data were collected. Demographics consisted of age, sex, race and ethnicity. Medical history included atrial fibrillation, coronary artery disease, myocardial infarction, peripheral arterial disease, carotid stenosis, diabetes mellitus, dyslipidemia, congestive heart failure, hypertension, renal insufficiency, chronic obstructive pulmonary disease, prior stroke or transient ischemic attack, dementia and smoking. Laboratory factors included initial international normalized ratio (INR), and blood glucose. Systolic blood pressure (SBP) and diastolic blood pressure at admission were used. Neurological assessments consisted of NIH stroke scale (NIHSS), modified Rankin Scale (mRS) and Barthel Index (BI). NIHSS was required at all designated time-points except the 90-day visit, where a telephone interview was allowed. The last NIHSS score prior to treatment was entered as the initial NIHSS; if not repeated, arrival NIHSS was used. BI and mRS were performed at hospital discharge, 30-day and 90-day visits. Stroke subtype was performed using Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.19
Figure 2.
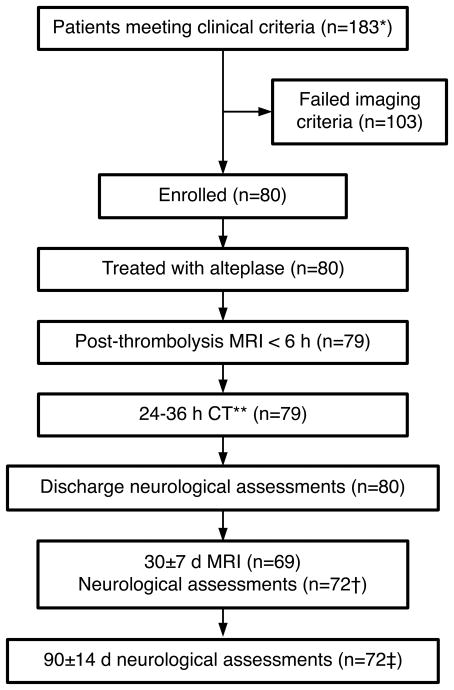
Trial Profile. *This number only includes patients deemed clinically eligible to undergo MRI screening. Other patients were not systematically tracked. The total does not include patients who refused study participation despite meeting MRI eligibility criteria (n=4). **At one site, the 24h imaging study was performed using MRI per site protocol. †Five subjects died by the 30±7 d assessment.
‡Seven subjects died by the 90±14 d assessment.
Image acquisition, triage and analysis
All clinically eligible patients underwent at minimum DWI, FLAIR, and gradient echo T2*-weighted sequences. Angiography and perfusion sequences were optional. An Imaging Protocol provided to all sites prior to site initiation contained recommended imaging acquisition parameters (See Supplementary Tables). All sites submitted a description of their protocol of standard stroke imaging to the Imaging Core and were provided feedback if the protocol parameters were outside of the suggested acquisition parameters. Feedback was sent to the site if images acquired during the trial deviated beyond the suggested parameters. Prior to receiving study drug, at least one reader at each site was required to pass training and certification by the Imaging Core. Each reader was provided an initiation packet containing 10 training cases and 20 test cases from patients with witnessed symptom onset as well as instructions for avoiding selecting chronic lesions. Correct classification results and sample ROIs were provided for the 10 training cases to each reader. Readers sent their classification results for the 20 test cases to the Imaging Core for grading. Before readers were considered trained and certified for performing imaging eligibility assessment for MR WITNESS, they had to obtain an intraclass correlation of at least 0.80 on signal intensity measurements and Fleiss’ kappa (κ≥0.80) on the qDFM classifications for the 20 test cases against the Imaging Core results. Prior to certification, readers were required to send a screenshot demonstrating their ability to perform real-time signal intensity ratio measurement to the Imaging Core.
Outcomes
The primary outcome was safety of IV alteplase, using the ECASS-2 symptomatic ICH (sICH) definition (i.e., any blood in the brain on CT and an NIHSS score that was higher by 4 points or more than the value at baseline or the lowest value in the first 7 days, or any hemorrhage leading to death).20 This sICH definition was chosen to avoid bias because this was an open-label safety trial. We assessed safety at pre-specified interim analyses that tested whether the sICH risk was larger than 5.3%, and at completion whether the sICH risk was significantly less than 5.3%. A secondary safety outcome was the risk of symptomatic brain edema, defined as brain edema with mass effect as the predominant cause of clinical deterioration, relative to the ECASS-3 risk of 6.9%.21 We chose to investigate symptomatic edema as a secondary safety outcome since it is a known potential risk of late reperfusion.22, 23
All images were screened for hemorrhagic transformation (HT) by the neuroimaging core, adjudicated by two board-certified neuroradiologists (AJY, MHL) who resolved discordant readings by consensus. HT was pre-specified to be classified using the European Cooperative Acute Stroke Study (ECASS)-1 criteria: hemorrhagic infarction (HI) type 1 (HI-1, non-confluent punctate foci within infarcted tissue), HI type 2 (HI-2, confluent foci or linear areas of signal loss within the infarcted area), parenchymal hemorrhage type 1 (PH-1, parenchymal hemorrhage <1/3 of the infarcted area), parenchymal hemorrhage type 2 (PH-2, Parenchymal hemorrhage >1/3 of the infarcted area).24 Hemorrhagic lesions distant from the infarcted area were all rated remote PH 1 (RPH-1) or 2 (RPH-2) according to the absence or presence of significant mass effect, respectively. Presence of intraventricular and subdural hemorrhages were also noted. The HT type was also categorized post-hoc according to the Heidelberg Bleeding Classification criteria to facilitate comparisons to future studies.5 In cases with more than one type of HT occurring simultaneously (Class 1 and Class 3), the more severe category was used (Class 3). Adverse events were classified per FDA criteria and are listed at ClinicalTrials.gov NCT01282242.
An independent medical monitor reviewed all cases of ICH and significant brain edema to classify them as symptomatic or asymptomatic, and reported the findings to an independent 3-person data safety monitoring board (DSMB). The DSMB reviewed every case of sICH or serious adverse event and applied a set of pre-specified stopping rules to determine if the trial should continue. Asymptomatic ICH (aICH) risk at 24 h was also evaluated. An additional secondary objective was assessment of good outcome, which was pre-specified as mRS 0–1 at 90 d. Last observation carried forward was used for mRS outcomes that were not available at 90 d.
Statistical Analyses
The primary outcome of sICH was based on the adjudicated sICH assessments. The stopping boundary for the trial was a hybrid of two conditions: (1) if the lower 95%CI of the hemorrhage risk observed in MR WITNESS was >5.3%, or (2) if the absolute number of sICH cases exceeded 6 during the trial. A sample size of 80 was calculated based on simulations (5000 repetitions) of hemorrhages as binary random variables and calculation of the exact 95% lower confidence bound, such that if the true hemorrhage risk were 5.3%, the study would detect a safety problem with probability 0.15, if the true risk was 8% it would detect a problem with probability 0.49, and if the true risk was 10% it would detect a problem with probability 0.68. At trial completion, we had 80% power using a one-sided, 0.2 significance level exact binomial test to detect a symptomatic edema risk of 12.5% or larger, assuming a symptomatic edema rate of 6.9% as seen in ECASS-3 treated patients.
Following successful trial completion, as secondary analyses, we performed one-sided, one-sample exact binomial tests to assess whether the risks of sICH and symptomatic edema were larger than those from ECASS-3. All other comparisons with ECASS-3 are based on two-sided Fisher’s exact tests, treating ECASS-3 as an independent group. Univariate comparisons between outcome groups and variables of interest were conducted using Fisher’s exact tests for categorical variables and exact Wilcoxon Rank-Sum tests (100,000 Monte Carlo repetitions) for continuous variables. Proportions are provided with 95% exact binomial confidence intervals [CIs]. Univariate logistic regressions were performed to estimate the associations between variables of interest and good outcomes (mRS of 0–1) at 90-d, aICH at 24 h and multiple logistic regression models were fitted with all variables with p<0.10 in univariate logistic regressions. Variables without cases in the predicted outcome group (i.e., with “complete separation”) were not considered as candidates for multiple regression. All odds ratio (OR) estimates are presented with 95% Wald confidence intervals. Relative risk (RR) was calculated when comparing MR WITNESS results with respect to ECASS-3 results. Stroke subtype was dichotomized into lacunar vs. non-lacunar. Subjects with documented medical conditions (e.g., hypertension), behaviors (e.g., current smoker) or wakeup strokes were classified as such; those without explicit documentation were assigned the absence of conditions.
To assess the impact of variables for which some subjects had missing values or were coded as “not documented,” we conducted sensitivity analyses in which all such cases were removed. We also conducted subgroup analyses for safety and efficacy among those subjects who underwent vessel imaging and who did not have an LVO of the internal carotid artery or M1 segment of the middle cerebral artery (ICA/M1). All analyses were conducted in SAS 9.4.
RESULTS
Between January 31, 2011 and October 4, 2015, 183 patients were screened to enroll 80 subjects (Figure 2). Figure 3 shows imaging examples of an enrolled (A) and an excluded (B) subject based on applying the qDFM imaging criteria. Baseline characteristics are shown in Table 1. Only medical history factors that differed between patients with good outcome (mRS≤1) and poor outcome (mRS≥2) with p-value≤0.10 are shown. The full table of results can be found in the Supplement. Among sites that recruited for > 1 year, median enrollment was 3.12 patients per year (IQR 1.78–4.47). Of the enrolled subjects, 40/80 (50.0% [38.6–61.4%]) were FLAIR positive. Vessel imaging was obtained at presentation in 70 subjects, of which 16 (22.9%) exhibited an ICA/M1-LVO potentially treatable with endovascular thrombectomy. There was one stroke mimic enrolled for a proportion of 1/80 (1.3% [0.0–6.8%]). Our median arrival-to-thrombolysis time was 1.78 h, and MRI-to-thrombolysis time was 0.9 h. Since subjects or their surrogates were consented for participation into the study after a routine MRI was acquired and screened for eligibility, comparing imaging to needle times between this study and clinical care is reasonable, but the door to needle times in our study include consent and thus are most appropriately compared to other thrombolysis trials that required consent. The primary outcome was safety, measured as sICH, which was observed in 1/80 cases, and was classified as a PH-2. (1.3% [0.0–6.8%]). This was not different than the ECASS-3 risk of 22/418 (5.30%; RR=0.24; p=0.07). Symptomatic edema occurred in 3/80 subjects (3.8% [0.8–10.6%]) and was not different than the ECASS-3 risk of 29/418 (6.9%; RR=0.54; p=0.19). AICH within 24 h occurred in 21/79 (26.6% [17.3–37.7%]) subjects without sICH, and was not different from ECASS-3 risk of 91/418 (21.8%; RR=1.22, p=0.38). Using ECASS 3 criteria, there were 22 ICH events: 7 HI-1, 5 HI-2, 1 PH-1, 1 HI-1+SAH, 1 HI-2+RPH-1, 1 HI-2+IVH, 1 HI-2+IVH+RPH, 1 HI-2+SAH+RPH-1, 1 PH-1+IVH, 2 SAH, and 1 PH-2. The equivalent Heidelberg Bleeding classification is: 13 Class 1, 1 Class 2 and 8 Class 3. There were 266 adverse events in total, with 46 bleeding events in 38 subjects of which nine were serious. Mortality at 90-day occurred in 7/80 (8.8%, [3.6–17.2%]) subjects, which was not different than the ECASS-3 risk of 32/418 (7.7%; RR=1.14; p=0.66).
Figure 3.
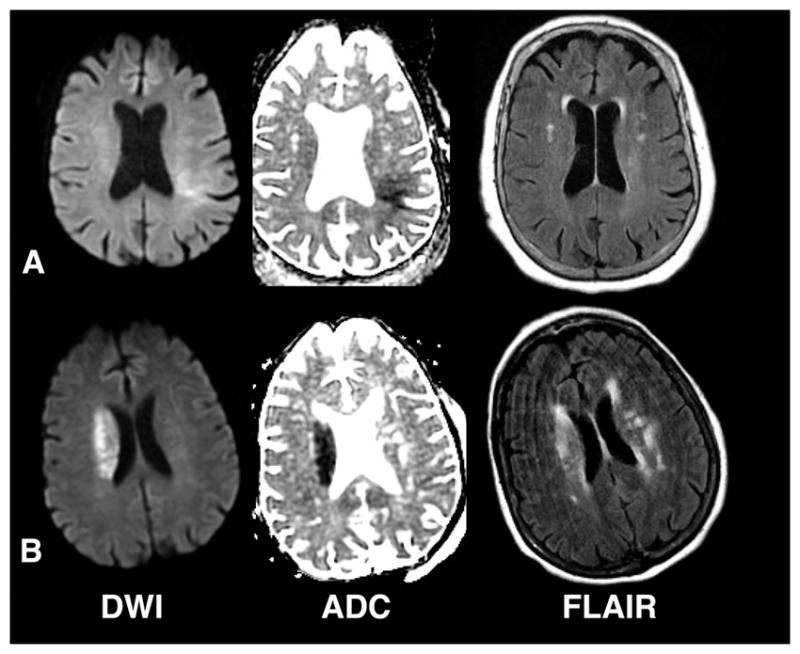
Examples of enrolled and non-enrolled subjects. (A) MRI from an enrolled subject obtained approximately 3 hours after symptom discovery in a 77 year old female presenting with unwitnessed symptom onset and right-sided weakness, numbness and aphasia. Her NIHSS score was 10. (B) MRI from a non-enrolled subject obtained approximately 1.25 hours after symptom discovery in a 78 year old female presenting with unwitnessed symptom onset and left-sided weakness, last known well the night before. Her NIHSS score was 15.
Table 1.
Baseline characteristics of the 80 enrolled subjects, comparing those who achieved a 90 d good outcome (mRS 0–1) compared to those who did not (mRS 2–6).
| All Subjects (n=80) | 90 d mRS 0–1 (n=31) | 90 d mRS 2–6 (n=49) | p-value | |
|---|---|---|---|---|
| Age, years | 67.5±13.5 | 67.2±15.2 | 67.6±12.5 | 0.73 |
| Male sex (%) | 43 (53.8%) | 19 (61.3%) | 24 (49.0%) | 0.36 |
| Race White (%) | 47 (58.8%) | 22 (71.0%) | 25 (51.0%) | 0.10 |
| Lacunar Subtype (vs non-lacunar)* | 21 (26.6%) | 4 (13.3%) | 17 (34.7%) | 0.06 |
| Pre-stroke mRS 0–1 | 69 (86.3%) | 30 (96.8%) | 39 (79.6%) | 0.04 |
| Medical History† (%) | ||||
| Current Smoker** | 18 (22.5%) | 3 (9.7%) | 15 (30.6%) | 0.03 |
| Dementia | 6 (7.5%) | 0 (0%) | 6 (12.2%) | 0.07 |
| Hypertension | 58 (72.5%) | 19 (61.3%) | 39 (79.6%) | 0.12 |
| Initial NIHSS | 7 (4–13.5) | 6 (4 – 9) | 10 (5 – 17) | 0.01 |
| Blood Glucose, mg/dL | 120 (103.5–172.5) | 122 (100 – 159) | 118 (105 – 188) | 0.25 |
| Systolic Blood Pressure, mm Hg | 155.5 (143.5–171) | 150 (136 – 168) | 162 (149 – 172) | 0.04 |
| Diastolic Blood Pressure, mm Hg | 83 (72.5–92) | 79 (75 – 90) | 85 (72 – 92) | 0.94 |
| Initial INR‡ | 1.00 (0.96–1.10) | 1.00 (0.90 – 1.10) | 1.00 (0.96 – 1.10) | 0.63 |
| FLAIR Signal Intensity Ratio | 1.08 (1.02–1.12) | 1.08 (1.02–1.12) | 1.08 (1.03–1.12) | 0.81 |
| FLAIR Negative (%) | 40 (50.0%) | 13 (41.9%) | 27 (55.1%) | 0.36 |
| Total alteplase dose, mg | 73·7±14·2 | 72·9±14·1 | 74·3±14·4 | 0.68 |
| Symptom Discovery to thrombolysis, h | 3·48 (2·90–4·01) | 3·68 (2·83 – 4·03) | 3·18 (2·90 – 3·93) | 0·52 |
| Last Known Well to thrombolysis, h | 11·24 (9·46–13·26) | 11·13 (8·85 – 14·40) | 11·25 (9·52– 12·93) | 0·90 |
| Arrival-to-thrombolysis, h§ | 1.78 (1.40–2.25) | 1.94 (1.33–2.40) | 1.77 (1.47–2.12) | 0.66 |
| Arrival-to-MRI, h§ | 0.85 (0.58–1.28) | 0.89 (0.60–1.67) | 0.80 (0.58–1.23) | 0.32 |
| MRI-to-thrombolysis, h | 0.89 (0.68–1.05) | 0.80 (0.52–0.95) | 0.97 (0.72–1.10) | 0.02 |
| Any ICH at 24h | 22 (27.5%) | 6 (19.4%) | 16 (32.7%) | 0.21 |
| Stroke upon awakening† | 57 (71.3) | 21 (67.7%) | 36 (73.5%) | 0.62 |
Data are mean±SD, n (%), or median (IQR). INR, international normalized ratio; NIHSS, NIH Stroke Scale.
The stroke mimic who had 90-day mRS<2 group was excluded since no stroke subtype could be assigned.
Subjects were assumed to not have the condition unless explicitly documented as present.
Current smokers are compared to everyone else, including past smokers, never smokers, and unknown status.
Two subjects were missing initial INR in the mRS>1 group.
Excluding one subject with symptom discovery after arrival.
At 90-day follow-up, the median BI was 95 (75–100). The median 90-day mRS was 2 (1–3), improving from 4 (2–4) at discharge and 3 (1–4) at 30-day (one subject had no 90-day visit, so the 30-day mRS score was carried forward). Among the 80 subjects enrolled, 31/80 (38.8% [28.1–50.3%] had a 90-day good outcome (Figure 2). On univariate testing, there was no difference in outcome between subjects with or without aICH at 24 h, or between those with wakeup vs non-wakeup stroke, and no patients with dementia had good outcome (Table 1). Only pre-existing disability prior to stroke, lacunar subtype, hypertension, current smoking, systolic blood pressure, blood glucose, MRI-to-thrombolysis time, arrival-to-MRI time and initial NIHSS were significant at p<0.10. When these covariates were included in multiple logistic regression, only absence of pre-stroke disability, non-lacunar subtype, non-smoking, and lower initial NIHSS remained significantly associated with good outcome (Table 2). Among the 69 subjects with pre-stroke mRS 0–1, 30/69 (43.5% [31.6–56.0%]) had 90-day good outcome (Figure 4) and univariate and multiple regression analyses produced similar results to the full 80 subject cohort (data not shown). In the subgroup of 70 subjects with vessel imaging, a larger proportion of subjects with excellent outcome occurred in the group without ICA/M1-LVO compared to the group with ICA/M1-LVO, (p=48.1% vs 18.8%, OR=4.02; p=0.045).
Table 2.
Unadjusted univariate and adjusted multivariable stepwise forward logistic regression model of odds of a good clinical outcome for all 80 subjects. Results were adjusted for baseline covariates available at the time of enrollment that were significant at the p<0.10 level in univariate logistic regression.
| Covariate | Unadjusted OR(95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Lacunar stroke | 0.29 (0.09, 0.97) | 0.04 | 0.06 (0.01, 0.51) | 0.01 |
| Pre-Stroke mRS >1 | 0.13 (0.02, 1.07) | 0.06 | 0.02 (0.001, 0.40) | 0.01 |
| Hypertension | 0.41 (0.15, 1.11) | 0.08 | 0.36 (0.07, 1.89) | 0.23 |
| Current Smoker | 0.24 (0.06, 0.92) | 0.04 | 0.03 (0.004, 0.27) | 0.002 |
| Initial NIHSS per point | 0.87 (0.79, 0.96) | 0.004 | 0.76(0.65, 0.89) | <0.001 |
| Blood Glucose, mg/dL | 0.99 (0.98, 1.00) | 0.098 | 0.99 (0.97, 1.004) | 0.16 |
| Systolic Blood Pressure, mm Hg | 0.98 (0.96, 1.00) | 0.094 | 1.0 (0.96, 1.03) | 0.87 |
| MRI-to-thrombolysis, per hour | 0.21 (0.04, 1.07) | 0.06 | 0.12 (0.01,1.54) | 0.10 |
| Arrival-to-MRI, per hour | 2.00 (0.89, 4.53) | 0.09 | 2.65 (0.53,13.12) | 0.23 |
OR, odds ratio; NIHSS, NIH stroke scale; mRS, modified Rankin Scale. Dementia was not included in multivariable analyses since there were no subjects with dementia who had good outcome, resulting in an unstable fit.
Figure 4.
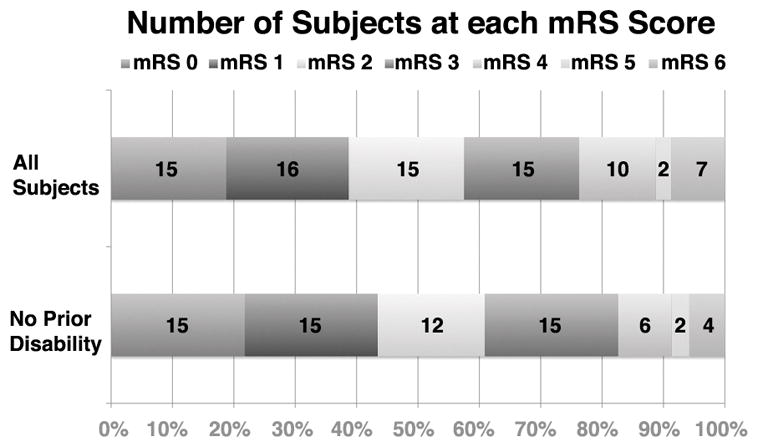
Modified Rankin Scale scores at day 90 in all treated subjects (n=80), and in the subset of subjects (n=69) who were without disability (mRS 0,1) prior to the index stroke.
Sensitivity analyses for 90-day good outcomes generated similar results. In univariate logistic regression, hypertension and systolic blood pressure were no longer significant at p<0.10, and dyslipidemia became significant (OR=0.44; p=0.095). Multiple regression produced similar results for variables with p-value of <0.05. Among the 69 subjects with pre-stroke mRS 0–1, hypertension was no longer significant in univariate logistic regression, but sensitivity analysis and multiple logistic regression provided similar results. Of the 54 subjects without ICA/M1-LVO (see Supplement), hypertension and blood glucose were no longer significant in univariate logistic regression, but race remained significant. However, multiple logistic regression produced similar results (see Supplement). Sensitivity analysis generated the same set of significant variables in both univariate and multivariable settings for 90-day good outcome. Among the 46 subjects with pre-stroke mRS 0–1, NIHSS and hypertension were no longer significant in the univariate setting. In the multivariable model including variables which were significant in univariate analyses (lacunar stroke, current smoker and systolic blood pressure), only current smoker was significant (OR=0.10; p=0.01).
On univariate testing for predictors of aICH at or before 24 h among the 79 subjects without sICH, symptom discovery to thrombolysis duration, history of atrial fibrillation, initial INR and initial NIHSS were significant at p<0.10 (Table 3, see Supplement for full results). When these covariates were included in multiple logistic regression, only initial NIHSS remained significantly associated with aICH (Table 4). Sensitivity analysis identified congestive heart failure (OR=5.16; p=0.09) and wake-up stroke (OR=0.37; p=0.08) in addition to the ones reported above. In multiple regression, as in the main analysis, only NIHSS remained significant at p-value of <0.05. Similar multivariable results were observed for the 53 subjects without ICA/M1-LVO or sICH (see Supplement) resulting in only NIHSS as a significant predictor of aICH at 24 h. Sensitivity analysis for multiple logistic regression resulted in no variable significant at the <0.05 level (NIHSS OR=1.42; p=0.06).
Table 3.
Baseline demographics and clinical characteristics of all enrolled subjects (comparing those with and without asymptomatic intracranial hemorrhage at or before the 24 h unenhanced head CT. The one subject with symptomatic ICH was excluded from this analysis.
| No ICH at 24 h (n=58) | Asymptomatic ICH at 24 h (n=21) | p value | |
|---|---|---|---|
| Age (±SD) | 68±13 | 66±15 | 0.56 |
| Male (%) | 33 (56.9%) | 9 (42.9%) | 0.31 |
| Race White (%) | 36 (62.1%) | 11 (52.4%) | 0.45 |
| Lacunar Subtype (vs. non-lacunar)* | 21 (36.8%) | 0 (0%) | <0.001 |
| Pre-stroke mRS 0–1 | 49 (84.5%) | 19 (90.5%) | 0.72 |
| Medical History† (%) | |||
| Atrial Fibrillation | 9 (15.5%) | 12 (57.1%) | <0.001 |
| Initial NIHSS | 5.5 (4–10) | 16 (10–19) | <0.001 |
| Blood Glucose, mg/dL | 119.5 (102–180) | 118 (104–160) | 0.65 |
| Systolic Blood Pressure, mm Hg | 158 (143–171) | 150 (148–171) | 0.66 |
| Diastolic Blood Pressure, mm Hg | 82.5 (72–89) | 84 (73–93) | 0.49 |
| Initial INR‡ | 1.00 (0.90–1.07) | 1.08 (1.00–1.10) | 0.04 |
| FLAIR Signal Intensity Ratio | 1.08 (1.02–1.11) | 1.10 (1.03–1.13) | 0.08 |
| FLAIR Negative (%) | 28 (48.3%) | 12 (57.1%) | 0.61 |
| Total alteplase dose, mg | 72.7±14.1 | 75.9±14.4 | 0.37 |
| Symptom Discovery to thrombolysis, h | 3.62 (3.02–4.03) | 3.00 (2.83–3.75) | 0.02 |
| Last Known Well to thrombolysis, h | 11.62 (10.23–13.42) | 10.53 (7.93–12.67) | 0.13 |
| Arrival-to-thrombolysis, h§ | 1.78 (1.40–2.23) | 1.76 (1.43–2.30) | 0.98 |
| Arrival-to-MRI, h§ | 0.89 (0.58–1.28) | 0.73 (0.57–1.06) | 0.55 |
| MRI-to-thrombolysis, h | 0.86 (0.62–1.02) | 0.90 (0.73–1.07) | 0.41 |
| Stroke upon awakening† | 44 (75.9%) | 12 (57.1%) | 0.16 |
Data are mean±SD, n (%), or median (IQR). ICH, intracranial hemorrhage, INR, international normalized ratio.
The stroke mimic who had no ICH was excluded since no stroke subtype.
Subjects were assumed to not have the condition unless explicitly documented in their medical record.
Current smokers are compared to everyone else, including past smokers, never smokers, and unknown status.
Two subjects were missing initial INR in the no-ICH group.
Excluding one subject with symptom discovery after arrival.
Table 4.
Unadjusted univariate and adjusted multivariable stepwise forward logistic regression model of the odds of an asymptomatic intracranial hemorrhage at or before the 24 h unenhanced head CT. Results were adjusted for baseline covariates available at the time of enrollment that were significant at the p<0.10 level in univariate logistic regression.
| Covariate | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Symptom Discovery to alteplase, h | 0.42 (0.21, 0.87) | 0.02 | 0.58 (0.24, 1.42) | 0.23 |
| History of Atrial Fibrillation | 7.26 (2.37, 22.23) | <0.001 | 3.20 (0.80, 12.77) | 0.099 |
| Initial INR | 711.17 (2.64, >999) | 0.02 | 4.56 (0.003, >999) | 0.68 |
| Initial NIHSS | 1.28 (1.14, 1.43) | <0.001 | 1.22 (1.08, 1.38) | 0.001 |
Time interval OR are per hour, NIHSS and INR are per point. OR, Odds Ratio; NIHSS, NIH stroke scale; mRS, modified Rankin Scale.
Lacunar subtype was not included in final multivariable analyses since there were no subjects with lacunar subtype who had AICH, resulting in an unstable fit.
DISCUSSION
We present the results of the first prospective study of the safety of IV thrombolysis for strokes with unwitnessed symptom onset selected using qDFM. We showed that in this population, IV alteplase administered within 4.5 h of symptom discovery did not increase the risks of sICH, symptomatic brain edema, aICH, or mortality when compared to the thrombolysis arm of the ECASS-3 trial. In addition, we showed that using qDFM can potentially double the enrollment rate over using qualitative diffusion-FLAIR mismatch without affecting rate of good outcomes and sICH. Our enrollment rate for sites enrolling over one year was similar to that of another prospective wake-up stroke study that used CT for inclusion.25 Furthermore, our median time from start of MRI to initiation of thrombolysis was less than one hour, including time for consent. This demonstrates the feasibility of using our qDFM approach expeditiously in clinical situations for which informed consent will not be required prior to initiation of treatment.
Our prospective study confirms the safety of IV alteplase that was suggested by prior retrospective, and small prospective, studies. Since our study launched in 2011, there have been several new trials involving subjects with unwitnessed symptom onset that have been initiated to investigate efficacy26, 27 or that have been completed. One of the recently completed studies used CT to select wakeup stroke patients in an open-label trial involving five centers (n=40) and found no cases of sICH.25 A single center study involving 20 wakeup stroke subjects (with ASPECTS >5, and angiographic or ultrasound evidence of arterial occlusion) showed similar results.28 Other prospective studies involving delayed thrombolysis (>4.5 h from last known well) of wakeup and non-wakeup unwitnessed stroke patients based on CT perfusion29, 30 or qualitative diffusion-FLAIR mismatch31 had no cases of sICH. However, none of these studies29–31 were designed to test for safety or efficacy of thrombolysis. One prospective multicenter study with 6 centers which treated 83 subjects with unwitnessed symptom onset within 6h of symptom discovery time reported an sICH risk of 6%.32 However, this study required presence of DWI and perfusion-MRI mismatch for patient enrollment, and absence of DWI-FLAIR mismatch was an exclusion criteria.
Our low rate of sICH might be related to our median admission NIHSS of 7, compared to the ECASS3 median score of 9,21 however 33/80 (41%) subjects had admission NIHSS ≥ 10. Furthermore, our study’s median NIHSS is comparable to another recently completed prospective study of wake-up strokes, with median NIHSS of 6.5.25 Like the CT-based study, our requirement for qDFM SIR<1.15 might have excluded patients with more severe strokes which could have improved the safety profile of alteplase in these patients. Other prospective studies involving subjects with unwitnessed symptom onset that required perfusion mismatch29, 30, 32 and/or arterial occlusion visible on vessel imaging28, 31 had more severe median NIHSS scores ranging from 12–18. We specifically designed the study to not a priori exclude milder strokes (NIHSS ≤4) since patients presenting with milder, but disabling, strokes may also benefit from thrombolysis.33, 34
Our design enriched the study population in 2 ways: by extending treatment up to 4.5 h from the time of symptom discovery, and by including both wakeup and non-wakeup unwitnessed strokes. The median time from last known well to treatment in our study was greater than 11 h, a time at which the pooled meta-analysis of IV thrombolysis trials35 suggests an unfavorable benefit to harm ratio. Though we have no concurrent controls, comparison to the alteplase arms of the major clinical alteplase trials and registries suggests comparable rates of benefit and harm. The presence of qDFM supports our hypothesis that in our enrolled subjects with stroke of unwitnessed symptom onset, the true symptom onset was likely close in time to the discovery of symptoms.
A substantial percentage of AIS patients present with uncertain time of symptom onset. Among all subjects with unwitnessed symptom onset, most are wakeup strokes. In our study, the ratio of wakeup to non-wakeup strokes was 2.5:1. Studies have found that 13% to 27% of all patients with stroke awaken with symptoms, making unwitnessed stroke a sizeable public health burden.36, 37 Since symptom onset is more frequent in the morning, and brain imaging in unwitnessed wakeup strokes is indistinguishable from that in patients with known symptom onset ≤3 h, waking up with symptoms may be a biomarker of symptom onset.38, 39 Many patients with wakeup stroke are likely to have a true symptom onset that is within the window in which IV alteplase has been proven effective. Thus, IV alteplase in wakeup stroke could benefit a large proportion of patients and significantly reduce long-term disability. Recent clinical trial data suggests a strong benefit from endovascular thrombectomy for subjects with unwitnessed symptom onset and documented ICA/M1-LVO,6 many of whom were wakeup strokes.
However, the proportion of patients with non-wakeup unwitnessed strokes may also be increasing (10% to 16% over 11-years) according to a single-center report.40 These non-wakeup unwitnessed stroke patients who did not receive IV alteplase had significantly higher baseline stroke severity, rates of in-hospital mortality and poorer functional outcome at 6 months compared to wakeup unwitnessed strokes.40 Non-wakeup unwitnessed stroke patients are therefore also in need of effective interventions and are important to include in randomized trials of unwitnessed strokes. These patients are also in need of an acute treatment if they do not have LVO or meet other criteria for endovascular thrombectomy.
We found that although aICH was identified frequently at 24 h, it was not significantly associated with outcome. This may have been due to competing effects of improvement and worsening, or simply to a lack of influence. Our sample size is too small to explore this further. We did investigate predictors of aICH and found that despite several univariate associations, only the NIHSS score remained significant in the multiple regression model, with an odds ratio of 1.22 for each one point increase in the score.
The proportion of good outcome in MR WITNESS was 38.8%. It is challenging to identify an appropriate comparison group for our trial in terms of good outcomes, due to the dramatically longer time window and our use of MRI to select subjects. The proportion of good clinical outcome following IV alteplase in the 3–4.5 h window among recent randomized trials or observational cohorts that report the proportion of subjects achieving a mRS of 0–1 at 90 d ranges from 35% in the pooled meta-analysis alteplase cohort (which included subjects up to age 85), to 41% in the Safe Implementation of Treatment in Stroke International Stroke Thrombolysis Register41 (open label, single arm) and 52% in the ECASS-3 trial. Our observed proportion of 39% lies within this range. We explored factors associated with clinical efficacy, and found that pre-stroke disability, lacunar subtype, current smoking and higher initial NIHSS score all reduced odds of a good outcome. When we excluded subjects with pre-stroke mRS>1 as has been the case in most trials of IV thrombolysis, the proportion of good outcome was 43.5%. We examined the covariates of presence of LVO or the time from symptom onset, measured either as time from last well or from symptom discovery, and their association with good outcome. Among subjects who had vessel imaging performed, those without LVO had better odds of achieving a good outcome (OR=4.02; p=0.045), consistent with data suggesting IV alteplase is more effective in distal than proximal occlusions. Duration from symptom onset was not associated with outcome, either because the true biological duration of the strokes was similar in those with a good vs. poor outcome, or that other factors are much more important such as initial NIHSS and the diagnosis of lacunar stroke.
While increasing NIHSS score has been shown to be the most powerful predictor of inpatient mortality and worse outcomes, the data on association between lacunar subtype or cigarette smoking and outcome are mixed.42 Two large European registry studies and IST3 did not find a difference in alteplase response comparing lacunar vs. non-lacunar strokes.43, 44 While it is possible that thrombi in patients with lacunar stroke are less susceptible to thrombolysis compared to other stroke subtypes, there is no clinical trial evidence to support this. It is possible that lacunar infarction in a subgroup of mild subjects is associated with a high proportion of excellent outcomes and that the absolute percent benefit with IV alteplase is so small that it could not be seen in our study. The recent results of the “Study of the Efficacy and Safety of Alteplase in Participants with Mild Stroke” (PRISMS) trial recently presented in abstract form showed no difference in the response rates among patients with non-disabling deficits, and suggests that IV alteplase is not beneficial in these patients. All subjects in MR WITNESS were required to have a deficit that the investigator felt would be disabling and thus these data reinforce the importance of excluding subjects with non-disabling deficits.
Our study has several important limitations. It was a phase 2a open-label single arm safety study and therefore we have no concurrent randomized controls for comparing safety or efficacy to a placebo arm. The small sample size was determined to assess safety, but not efficacy. Our open-label design presents a risk of unconscious bias at the site for assessment of adverse events and poor outcomes, despite the use of independent raters. Our use of an independent medical monitor to adjudicate all cases of ICH mitigates the risk of bias in the primary outcome. Our use of MRI in selecting subjects permitted exclusion of patients with large infarction and minimized enrollment of stroke mimics, but might limit generalizability. While we suspect that lack of FLAIR signal suggests potential reversibility of ischemia with reperfusion, we are unable to test this hypothesis. Most patients did not have both pre- and post-treatment vessel and perfusion imaging, limiting our ability to assess reperfusion or recanalization rates. Our study enrolled subjects at a rate of just over 3 patients per year, which might be viewed as slow given the estimates of the prevalence of this population among acute ischemic strokes. The rapid enrollment rates of the two endovascular trials of late window subjects suggests that with vigilant screening, there will be an abundant number of subjects who may be eligible for IV thrombolysis. Among those with vessel imaging in our study, only 22.9% exhibited an LVO potentially treatable with endovascular thrombectomy. This suggests that with large-scale, 24–7 screening at stroke centers, there may be greater than 4 treatable non-LVO subjects for every LVO subject identified. Lastly, our imaging assessment did not use perfusion imaging for patient selection and so we are limited in our ability to speculate on the influence of this method on safety or efficacy. While this method has been shown to select patients with LVO who benefit from thrombectomy in late windows, the nature and biology of stroke progression in these patients may be entirely different than in the non-LVO cohort, and it remains to be seen if it is a useful and reliable tool in selecting patients with more distal occlusions that can still benefit from IV thrombolysis. While the predominant effect of thrombolysis on outcome in LVO patients may be immediate reperfusion of a large region of reversible ischemia, in smaller infarcts thromboylysis may provide benefit in additional ways, such as preserving the microcirculation or preventing propagation of ischemia into areas that are not initially affected.
In conclusion, MR WITNESS confirms the safety of alteplase in qDFM selected patients with stroke of unwitnessed symptom onset, and our promising preliminary efficacy data warrant further exploration in a double-blinded RCT of IV alteplase in the broad population of both wakeup and non-wakeup strokes. We have shown that it is feasible to enroll subjects using qDFM, and others have shown similar success with CT-based strategies, laying the groundwork for randomized trials of the efficacy of thrombolysis in MRI or CT based selection of patients with stroke of unwitnessed symptom onset. These proposed IV thrombolysis trials would complement the recent successes in endovascular treatment of wakeup strokes with LVO,6, 7 since the majority of subjects who were enrolled in MR WITNESS would not have been eligible for these EVT trials since they lacked LVO. Non-LVO patients, although likely to have milder strokes, may still benefit from thrombolysis. A new day is dawning in AIS reperfusion therapy for patients with unwitnessed symptom onset who are beyond the traditional, guideline-recommended time windows. The current safety trial is an important step toward ensuring that the subjects with unwitnessed symptom onset and without ICA/M1-LVO are not excluded from potentially valuable treatment opportunities. Further research testing the efficacy of IV alteplase in subjects with unwitnessed symptom onset is warranted. CT-based selection is more widely available and simpler to execute, but CT is less sensitive to early infarction and so likely enrolls a greater proportion of mimics. MRI, while less readily available and harder to execute, is exquisitely sensitive and specific for infarction. The longer time to imaging with MRI may offset the greater accuracy, and so both methods should be investigated to determine if the benefit is equally present in both modalities. A prospective randomized trial of late window subjects selected by CT or MRI is warranted.
Supplementary Material
Acknowledgments
This work was funded in part by the National Institutes of Health NINDS Specialized Program of Transitional Research in Acute Stroke (SPOTRIAS) grant P50-NS051343 and NINDS Division of Intramural Research. This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. Additional support was provided to OW (NIH: R01NS059775, R01NS082285, R01NS086905), ALF (NIH: K23NS069807 R01 HL129241) and RB (Harvard NeuroDiscovery Center).
We would like to thank the members of the Data Safety and Monitoring Board: Howard Rowley (Chair), Eric Smith and Yuko Palesch and Independent Medical Monitor Barney Stern. We would also like to acknowledge Karen L. Furie, MD, and Steven M. Greenberg MD, Ph.D., who served as Program Directors of the MGH SPOTRIAS Program Project Grant during this study. We are grateful to the members of the teams at all of our enrolling sites: Washington Hospital Center, Washington DC, Suburban Hospital, Bethesda, MD; Washington University School of Medicine, St. Louis MO; Massachusetts General Hospital, Boston, MA; Cedars-Sinai Medical Center, Los Angeles, CA; Seton/UT Southwestern Medical Center Austin, TX; University of Tennessee Health Science Center, Memphis TN; Ronald Reagan UCLA Medical Center, Los Angeles, CA; Rush University Medical Center, Chicago IL; University of Iowa, Iowa City, IA; Intermountain Healthcare, Murray, Utah; University of Arizona, Tucson, AZ; Beth Israel Deaconess Medical Center, Boston, MA; University of Massachusetts, Worcester, MA.
Footnotes
Author Contributions:
LHS, OW, RB and SW contributed to the conception and design of this study; all authors contributed to acquisition and analysis of data, and to critical revisions of the manuscript; LHS and OW drafted the manuscript and prepared the figures.
Potential Conflicts of Interests
Genentech provided alteplase free of charge to the study for distribution to all sites except to the NINDS intramural branch, and starting in year 2 provided modest supplemental site payments to permit expansion to 14 sites. Genentech received details on the occurrence and nature of sICH and had the right to view the manuscript prior to submission, but had no control over design, data collection, analysis, interpretation, or publication decision. LHS has been a consultant for Lundbeck on their International Steering Committee for DIAS3 and DIAS4 trials which tested desmoteplase in late window acute ischemic stroke. MHL has been a consultant to General Electric Healthcare, which manufactures MRI devices. The other authors declare no relevant conflicts.
References
- 1.Rymer MM, Thrutchley DE. Organizing regional networks to increase acute stroke intervention. Neurol Res. 2005;27(Suppl 1):S9–16. doi: 10.1179/016164105X25315. [DOI] [PubMed] [Google Scholar]
- 2.Rimmele DL, Thomalla G. Wake-up stroke: clinical characteristics, imaging findings, and treatment option - an update. Front Neurol. 2014;5:35. doi: 10.3389/fneur.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeoye O, Kleindorfer D. Acute Stroke Reperfusion Treatment Rates at NIH StrokeNet Hospitals. Stroke. 2015;46:A56. [Google Scholar]
- 4.Menon BK, Saver JL, Goyal M, et al. Trends in endovascular therapy and clinical outcomes within the nationwide Get With The Guidelines-Stroke registry. Stroke. 2015 Apr;46(4):989–95. doi: 10.1161/STROKEAHA.114.007542. [DOI] [PubMed] [Google Scholar]
- 5.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg Bleeding Classification: Classification of Bleeding Events After Ischemic Stroke and Reperfusion Therapy. Stroke. 2015 Oct;46(10):2981–6. doi: 10.1161/STROKEAHA.115.010049. [DOI] [PubMed] [Google Scholar]
- 6.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2017 Nov 11; doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018 Jan 24; doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barreto AD, Martin-Schild S, Hallevi H, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009 Mar;40(3):827–32. doi: 10.1161/STROKEAHA.108.528034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho AH, Sohn SI, Han MK, et al. Safety and efficacy of MRI-based thrombolysis in unclear-onset stroke. A preliminary report. Cerebrovascular diseases (Basel, Switzerland) 2008;25(6):572–9. doi: 10.1159/000132204. [DOI] [PubMed] [Google Scholar]
- 10.Iosif C, Oppenheim C, Trystram D, Domigo V, Meder JF. MR imaging-based decision in thrombolytic therapy for stroke on awakening: report of 2 cases. AJNR American journal of neuroradiology. 2008 Aug;29(7):1314–6. doi: 10.3174/ajnr.A1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoki J, Kimura K, Iguchi Y, et al. Intravenous thrombolysis based on diffusion-weighted imaging and fluid-attenuated inversion recovery mismatch in acute stroke patients with unknown onset time. Cerebrovascular diseases (Basel, Switzerland) 2011;31(5):435–41. doi: 10.1159/000323850. [DOI] [PubMed] [Google Scholar]
- 12.Knight RA, Dereski MO, Helpern JA, Ordidge RJ, Chopp M. Magnetic resonance imaging assessment of evolving focal cerebral ischemia. Comparison with histopathology in rats. Stroke. 1994 Jun;25(6):1252–61. doi: 10.1161/01.str.25.6.1252. discussion 61–2. [DOI] [PubMed] [Google Scholar]
- 13.Thomalla G, Rossbach P, Rosenkranz M, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Annals of Neurology. 2009 Jun;65(6):724–32. doi: 10.1002/ana.21651. [DOI] [PubMed] [Google Scholar]
- 14.Song SS, Latour LL, Ritter CH, et al. A pragmatic approach using magnetic resonance imaging to treat ischemic strokes of unknown onset time in a thrombolytic trial. Stroke. 2012 Sep;43(9):2331–5. doi: 10.1161/STROKEAHA.111.630947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch KM, Windham J, Knight RA, et al. A model to predict the histopathology of human stroke using diffusion and T2-weighted magnetic resonance imaging. Stroke. 1995 Nov;26(11):1983–9. doi: 10.1161/01.str.26.11.1983. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Q, Chopp M, Zhang ZG, et al. The temporal evolution of MRI tissue signatures after transient middle cerebral artery occlusion in rat. J Neurol Sci. 1997 Jan;145(1):15–23. doi: 10.1016/s0022-510x(96)00286-9. [DOI] [PubMed] [Google Scholar]
- 17.Cho AH, Kim JS, Kim SJ, et al. Focal fluid-attenuated inversion recovery hyperintensity within acute diffusion-weighted imaging lesions is associated with symptomatic intracerebral hemorrhage after thrombolysis. Stroke. 2008 Dec;39(12):3424–6. doi: 10.1161/STROKEAHA.108.516740. [DOI] [PubMed] [Google Scholar]
- 18.Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011 Nov;10(11):978–86. doi: 10.1016/S1474-4422(11)70192-2. [DOI] [PubMed] [Google Scholar]
- 19.Adams HJ, Bedndixen B, Kappelle J, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998 Oct 17;352(9136):1245–51. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 21.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. New England Journal of Medicine. 2008 Sep 25;359(13):1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 22.Koudstaal PJ, Stibbe J, Vermeulen M. Fatal ischaemic brain oedema after early thrombolysis with tissue plasminogen activator in acute stroke. BMJ. 1988 Dec 17;297(6663):1571–4. doi: 10.1136/bmj.297.6663.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) [see comments] JAMA. 1995;274(13):1017–25. [PubMed] [Google Scholar]
- 24.Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct - Relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999 Nov;30(11):2280–4. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 25.Barreto AD, Fanale CV, Alexandrov AV, et al. Prospective, open-label safety study of intravenous recombinant tissue plasminogen activator in wake-up stroke. Ann Neurol. 2016 Aug;80(2):211–8. doi: 10.1002/ana.24700. [DOI] [PubMed] [Google Scholar]
- 26.Koga M, Toyoda K, Kimura K, et al. THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0.6 mg/kg (THAWS) Trial. International journal of stroke : official journal of the International Stroke Society. 2014 Dec;9(8):1117–24. doi: 10.1111/ijs.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomalla G, Fiebach JB, Ostergaard L, et al. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP) International journal of stroke : official journal of the International Stroke Society. 2014 Aug;9(6):829–36. doi: 10.1111/ijs.12011. [DOI] [PubMed] [Google Scholar]
- 28.Hill MD, Kenney C, Dzialowski I, et al. Tissue Window in Stroke Thrombolysis study (TWIST): a safety study. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2013 Jan;40(1):17–20. doi: 10.1017/s0317167100012890. [DOI] [PubMed] [Google Scholar]
- 29.Cortijo E, Garcia-Bermejo P, Calleja AI, et al. Intravenous thrombolysis in ischemic stroke with unknown onset using CT perfusion. Acta neurologica Scandinavica. 2014 Mar;129(3):178–83. doi: 10.1111/ane.12160. [DOI] [PubMed] [Google Scholar]
- 30.Michel P, Ntaios G, Reichhart M, et al. Perfusion-CT guided intravenous thrombolysis in patients with unknown-onset stroke: a randomized, double-blind, placebo-controlled, pilot feasibility trial. Neuroradiology. 2012 Jun;54(6):579–88. doi: 10.1007/s00234-011-0944-1. [DOI] [PubMed] [Google Scholar]
- 31.Aoki J, Kimura K, Shibazaki K, Sakamoto Y. Negative fluid-attenuated inversion recovery-based intravenous thrombolysis using recombinant tissue plasminogen activator in acute stroke patients with unknown onset time. Cerebrovascular diseases extra. 2013;3(1):35–45. doi: 10.1159/000348552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang DW, Sohn SI, Hong KS, et al. Reperfusion therapy in unclear-onset stroke based on MRI evaluation (RESTORE): a prospective multicenter study. Stroke. 2012 Dec;43(12):3278–83. doi: 10.1161/STROKEAHA.112.675926. [DOI] [PubMed] [Google Scholar]
- 33.Khatri P, Tayama D, Cohen G, et al. Effect of Intravenous Recombinant Tissue-Type Plasminogen Activator in Patients With Mild Stroke in the Third International Stroke Trial-3: Post Hoc Analysis. Stroke. 2015 Aug;46(8):2325–7. doi: 10.1161/STROKEAHA.115.009951. [DOI] [PubMed] [Google Scholar]
- 34.Smith EE, Fonarow GC, Reeves MJ, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from Get With The Guidelines-Stroke. Stroke. 2011 Nov;42(11):3110–5. doi: 10.1161/STROKEAHA.111.613208. [DOI] [PubMed] [Google Scholar]
- 35.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014 Nov 29;384(9958):1929–35. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fink JN, Kumar S, Horkan C, et al. The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke. 2002 Apr;33(4):988–93. doi: 10.1161/01.str.0000014585.17714.67. [DOI] [PubMed] [Google Scholar]
- 37.Nadeau JO, Fang J, Kapral MK, Silver FL, Hill MD. Outcome after stroke upon awakening. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2005 May;32(2):232–6. doi: 10.1017/s0317167100004029. [DOI] [PubMed] [Google Scholar]
- 38.Todo K, Moriwaki H, Saito K, Tanaka M, Oe H, Naritomi H. Early CT findings in unknown-onset and wake-up strokes. Cerebrovascular diseases (Basel, Switzerland) 2006;21(5–6):367–71. doi: 10.1159/000091545. [DOI] [PubMed] [Google Scholar]
- 39.Serena J, Davalos A, Segura T, Mostacero E, Castillo J. Stroke on awakening: looking for a more rational management. Cerebrovascular diseases (Basel, Switzerland) 2003;16(2):128–33. doi: 10.1159/000070592. [DOI] [PubMed] [Google Scholar]
- 40.Reid JM, Dai D, Cheripelli B, et al. Differences in wake-up and unknown onset stroke examined in a stroke registry. International journal of stroke : official journal of the International Stroke Society. 2015 Apr;10(3):331–5. doi: 10.1111/ijs.12388. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed N, Kellert L, Lees KR, et al. Results of intravenous thrombolysis within 4.5 to 6 hours and updated results within 3 to 4.5 hours of onset of acute ischemic stroke recorded in the Safe Implementation of Treatment in Stroke International Stroke Thrombolysis Register (SITS-ISTR): an observational study. JAMA Neurol. 2013 Jul;70(7):837–44. doi: 10.1001/jamaneurol.2013.406. [DOI] [PubMed] [Google Scholar]
- 42.Ali SF, Smith EE, Bhatt DL, Fonarow GC, Schwamm LH. Paradoxical association of smoking with in-hospital mortality among patients admitted with acute ischemic stroke. J Am Heart Assoc. 2013 Jun 19;2(3):e000171. doi: 10.1161/JAHA.113.000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindley RI, Wardlaw JM, Whiteley WN, et al. Alteplase for acute ischemic stroke: outcomes by clinically important subgroups in the Third International Stroke Trial. Stroke. 2015 Mar;46(3):746–56. doi: 10.1161/STROKEAHA.114.006573. [DOI] [PubMed] [Google Scholar]
- 44.Eggers CCJ, Bocksrucker C, Seyfang L Austrian Stroke Unit Registry C. The efficacy of thrombolysis in lacunar stroke - evidence from the Austrian Stroke Unit Registry. Eur J Neurol. 2017 Jun;24(6):780–7. doi: 10.1111/ene.13288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.