Abstract
The Restoring Insulin Secretion (RISE) study was initiated to evaluate interventions to slow or reverse the progression of β-cell failure in type 2 diabetes (T2D). To design RISE, we undertook an evaluation of methods for measurement of β-cell function and changes in β-cell function in response to interventions. Here we present a review of approaches for measurement of β-cell function, focusing on methodologic and feasibility considerations. Methodologic considerations included 1) the utility of each technique for evaluating key aspects of ß-cell function (first- and second-phase insulin secretion, maximal insulin secretion, glucose sensitivity, incretin effects); and 2) tactics for incorporating a measurement of insulin sensitivity in order to appropriately adjust insulin secretion measures for insulin sensitivity. Of particular concern were the capacity to accurately measure β-cell function in those with poor function, as is seen in established T2D, and the capacity of each method for demonstrating treatment-induced changes in β-cell function. Feasibility considerations included staff burden including time and required methodological expertise; participant burden including time and number of study visits; and the ease of standardizing methods across a multi-center consortium. Following this evaluation, we selected a 2-day measurement procedure, combining a 3-hour 75g OGTT and a 2-stage hyperglycemic clamp procedure augmented with arginine.
Introduction
The study of progressive metabolic dysfunction in prediabetes and type 2 diabetes (T2D) has focused on the pancreatic islet ß-cell. [1] Cross-sectional studies in adult and adolescent populations have shown inferior ß-cell function in individuals with impaired glucose tolerance (IGT) and even poorer function in those with T2D. [2–5] Longitudinal assessments to-date demonstrate progressive worsening of β-cell function among individuals with genetic and metabolic risk factors for T2D, and in patients with increasing hyperglycemia. [6–8] Methodologies for measuring β-cell function range in complexity from fasting measures, to protocols involving intravenous infusion of multiple stimulators of insulin release [9–16]. Each method brings strengths and weaknesses, and selection of the optimal approach must not only reflect pertinent physiology, but also factors such as cost and participant burden.
The Restoring Insulin Secretion (RISE) study was designed to test interventions to slow or reverse the progression of β-cell failure in individuals at high risk of T2D, or with recent onset T2D. [17] Here we present a review of available techniques for measurement of β-cell function, focusing on the methodologic and feasibility considerations that informed the selection of approaches utilized in RISE.
Physiologic Considerations for the Measurement of β-cell Function
β-cell function can be defined as the ability of pancreatic β-cells to produce, store and release insulin in concentrations sufficient to maintain euglycemia. Under normal physiologic conditions, circulating insulin concentrations are reciprocally related to insulin sensitivity, expressed as the body’s capacity for glucose disposal and ability to suppress hepatic glucose production in response to insulin. [10] When insulin sensitivity declines, the appropriate physiologic response is for insulin secretion to increase in a compensatory manner. The calculated line linking these factors, which exhibit a square hyperbolic relationship, is commonly expressed as the ‘disposition index’ (DI, insulin sensitivity * first-phase insulin secretion; Figure 1). [10, 14] The need to incorporate a measurement of insulin sensitivity into assessments of β-cell responses is widely accepted. With this in mind we will briefly review alternatives for measuring insulin sensitivity before turning to methods for assessing β-cell responses.
Figure 1.
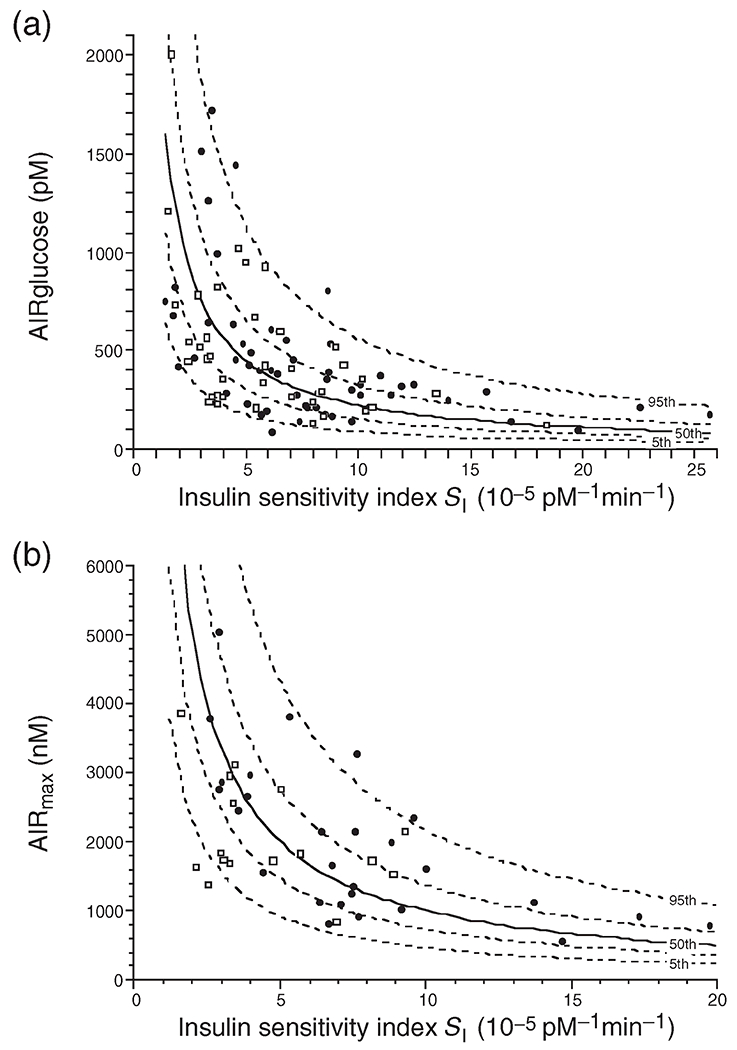
The hyperbolic relationship between insulin sensitivity (SI) and the first phase (acute) insulin response to glucose (AIRglucose) (a) and the maximal acute insulin response to arginine (AIRmax) (b) in a cohort of healthy individuals. The solid line depicts the best-fit relationship (50th percentile), while the broken lines represent the 5th, 25th, 75th, and 95th percentiles. A reduction in insulin sensitivity, as measured by a decrease in Si, results in a compensatory reciprocal and proportionate increase in glucose-stimulated insulin secretion and an increase in maximal acute insulin response to arginine, the latter a measure of β-cell secretory capacity. (Copyright 1993 American Diabetes Association. From Reference [10] Reprinted with permission from The American Diabetes Association)
Measuring Insulin Sensitivity
In order to appropriately adjust the β-cell reponse for the prevailing insulin sensitivity, a concurrent measure of insulin sensitivity is required. Robust discussions of different approaches to measuring insulin sensitivity have been previously published. [18–20] Here we present a brief exposition of available methods with a focus on the technical limitations and participant burden.
Hyperinsulinemic-Euglycemic Clamp –
This technique allows measurement of whole-body and tissue sensitivity to a steady-state concentration of insulin while the plasma glucose concentration is held constant (clamped), generally at physiologically normal (euglycemic) levels. [21] Performing the procedure in the euglycemic state obviates the need to correct for the impact of hyperglycemia on glucose disposal. This method produces measures of insulin-stimulated glucose disposal (M), and insulin sensitivity (M /I, where I denotes steady state plasma insulin concentration). Because this technique imposes plasma insulin and plasma glucose concentrations at defined experimental levels, independent of insulin production or release, it provides reliable measurements of insulin sensitivity in subjects across the full range of β-cell function.
The limitations of this technique include the need for two intravenous lines (one for infusion of insulin and glucose, and the other for blood sampling), high-precision glucose measures every 5 minutes, and personnel with expertise to make adjustments in the glucose infusion rate in order to maintain the target level of glycemia. The participant considerations include the need for two intravenous lines, and the duration of the procedure. Overall, this method is relatively resource-intensive (requiring nursing and investigator time and expertise), and it provides a measurement of insulin sensitivity without a simultaneous measure of β-cell function (as is provided in some methods discussed below).
Hyperglycemic Clamp-Derived Insulin Sensitivity –
The hyperglycemic clamp technique is discussed in detail below as a method for measurement of β-cell function. It also provides an indirect measure of insulin sensitivity, using the rate of glucose disposal under imposed steady-state hyperglycemic conditions, adjusted for the achieved endogenous insulin (or C-peptide) concentrations. [12, 22] Adjustments are needed for variations in achieved steady state glucose concentrations, and for urinary glucose losses. Typically, insulin sensitivity is calculated by dividing the glucose disposal rate by the plasma insulin (or C-peptide) concentration at steady state during the last 30 or 60 minutes of a 2-hr hyperglycemic clamp.
The principal limitation of measuring insulin sensitivity with this method is the dependence of the insulin sensitivity measure on the endogenous late-phase β-cell response. This is primarily an issue where poor late-phase insulin release provides an insufficient stimulus to drive glucose disposal in the face of poor insulin sensitivity, limiting accuracy of measurement of insulin sensitivity.
IVGTT - Minimal Model-Derived Insulin Sensitivity-
The minimal model of glucose kinetics developed by Bergman and colleagues allows insulin-mediated glucose disposal to be calculated from intravenous glucose tolerance test (IVGTT) data, with derivations of a model-derived measure of insulin sensitivity (SI). [23, 24] The minimal model has been extensively evaluated and widely adopted. In a modification of the original methodology, exogenous tolbutamide or (more commonly) insulin is administered after assessing the first-phase insulin response, to better characterize insulin dependent glucose disappearance where endogenous production is insufficient. [25, 26] However, in more severe insulin resistance, the standardized exogenous insulin bolus may be insufficient to produce data adequate for modeling.
Surrogate Measures of Insulin Sensitivity –
Indices of insulin sensitivity have been developed using fasting blood samples (e.g. inverse fasting insulin, homeostasis model assessment (HOMA) [27], quantitative insulin sensitivity check index (QUICKI)) [28], or the combined glucose and insulin excursions of the OGTT (e.g. Matsuda index) [19]). In cross-sectional evaluations, these fasting and OGTT-derived measures correlate reasonably well with hyperinsulinemic-euglycemic clamp or minimal model-derived measures (r=0.6-0.7) [29]. The utility of surrogate indices for longitudinal use has not been extensively evaluated. Some reports have described concurrent changes in multiple indices over time [30, 31], but to date correlations between longitudinal changes in surrogate indices of insulin resistance and more direct measures have only been formally evaluated in one publication. [20] This paper evaluated a cohort of Mexican-American women followed after gestational diabetes, and found changes in the surrogate indices to be less strongly correlated to changes in IVGTT-derived SI than is observed in cross-sectional settings.
Measuring β-cell Function
Glucose is the principal regulator of insulin secretion, via a well-described pathway linking β-cell glucose uptake to changes in ADP/ATP ratios and ultimately to changes in membrane potassium conductance and movement of insulin granules, producing a pulsatile and oscillatory pattern of insulin secretion in health. [32, 33]. Non-glucose β-cell stimuli include incretin hormones, acting through a cAMP system to potentiate the response to glucose [34, 35], and monobasic amino acids, fatty acids, and β-adrenergic agonists, which also act independent of the glucose sensing systems but converge on the same insulin secretion pathways. [36] These features are exploited in the many methods that have been developed for the measurement of β-cell function.
Hyperglycemic clamp -
Under this method, an exogenous glucose infusion is applied to raise blood glucose to a specified target concentration, or to achieve an increment above the individual’s fasting glucose. Both the magnitude and timing of the hyperglycemic stimulus are controlled, allowing for a precise and repeatable stimulus to insulin/C-peptide secretion and for clear separation of first- and second-phase responses to intravenous glucose (Figure 2). [21, 22]
Figure 2.
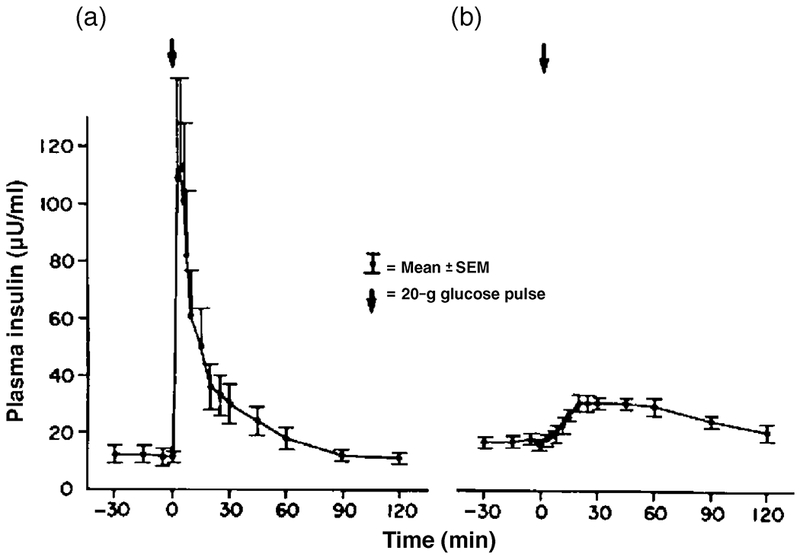
Plasma insulin response to a 20g intravenous glucose bolus in NGT (a) and T2D (b). The first-phase insulin response is absent in the subjects with diabetes while the second-phase response is relatively preserved, with a delayed maximal insulin response. (Reprinted from Reference [98], with permission from Excerpta Medica Inc.)
The first-phase insulin/C-peptide response primarily consists of release of stored insulin and occurs in the first few minutes after circulating glucose concentrations increase, subsiding within 10 minutes. [37] The first-phase response is measurably diminished in individuals with only modest elevations in fasting glucose and/or IGT, more severely diminished in individuals with fasting glucose concentrations >115 mg/dL (6.4 mmol/L), and absent in T2D. [38–40]
The second-phase insulin/C-peptide response begins concurrent with the first-phase response, and consists of a slow and sustained increase in insulin/C-peptide concentrations, reflecting pools of insulin granules with varying kinetic properties. [37, 41, 42] The second-phase response is not lost early in T2D, but declines over time with progressive reduction in β-cell function. [43, 44]
A DI can be calculated from data derived from the hyperglycemic clamp alone as long as a hyperbolic relationship exists between the measures of insulin/C-peptide secretion and insulin sensitivity. The DI is calculated using the measured insulin/C-peptide response and the indirect measure of insulin sensitivity (M/I) as explained above. [11]
Examples of application of the hyperglycemic clamp include understanding the progressive pathophysiology of β-cell dysfunction [12, 13, 30], and assessing the effects of pharmacologic interventions, weight loss and bariatric surgery on β-cell function. [44–46]
The ability to measure insulin/C-peptide secretion and insulin sensitivity in a single day is an advantage of the hyperglycemic clamp. Another advantage is that measures of β-cell function are accurate along the entire spectrum of NGT to prediabetes to diabetes, with reliable measures even in the low-response range seen in individuals with prediabetes and T2D. Despite impaired β-cell function, such individuals generally mount a sufficient second-phase response to provide a reliable measure of insulin-mediated glucose disposal. The principal technical limitations of the hyperglycemic clamp include the need for two intravenous lines (one for infusion of glucose and the other for blood sampling), rapid early sampling after the initial glucose bolus, high-precision glucose measures every 5-10 minutes, and personnel with expertise to make adjustments in the glucose infusion rate in order to maintain the target level of glycemia. The participant considerations include the need for two intravenous lines, and the 3-4 hour duration of the procedure (Table 1).
Table 1.
Methods of Assessing Human β-Cell Function In Vivo
| Method | Time* (hr) | Staff & Participant Burden | Directly Measures First- and Second-phase Insulin | Concurrent Measure of Insulin Sensitivity | Features of Note |
|---|---|---|---|---|---|
| Hyperglycemic Clamp | 2-3 | +++ | Yes | Yes | Direct measurement of traditional beta-cell function plus a measure of insulin sensitivity |
| IVGTT | 3-4 | +++ | Yes | Yes | Simpler procedure for combined measurement of beta-cell and insulin sensitvity |
| Graded Glucose Infusion | 2-4 | ++ | No | No | Direct measurement of beta-cell glucose sensitivity |
| Glucose-Potentiated Arginine Stimulation | 1-2 | ++ | No | No | Complementary, glucose-independent measurement of beta-cell function; may reflect beta-cell mass |
| OGTT or MMTT | 2-4 | + | No | Yes | Measuring physiologic response including incretin axis; OGTT can provide clinically diagnostic results |
| Fasting Measures | <1 | +/− | No | Yes | Simplest and least expensive, useful for epidemiologic studies |
Times do not include time necessary for catheter insertion and study preparation. The time for glucose-potentiated arginine secretion is in addition to the time needed for any preparatory procedures.
Intravenous glucose tolerance test (IVGTT) -
During the IVGTT, an intravenous bolus of dextrose is given and rapid sampling for measurement of glucose and insulin concentrations is performed during the first 10 minutes of the test to measure the acute (first-phase) insulin and C-peptide responses. Subsequent measurements across the remainder of the test are used to derive the late- (second) phase responses. As noted above, under current usage a bolus of exogenous insulin is generally applied in order to successfully model insulin sensitivity (SI).
Investigators have utilized the IVGTT minimal model to describe progressive loss of β-cell function in the development of diabetes [47–49], to describe the physiology of individuals at risk for diabetes [12, 47, 50–52], and to follow response to treatment. [53–55]
As with the hyperglycemic clamp, the IVGTT allows for derivation of measures of β-cell function and insulin sensitivity from a single testing day. This method works well when the endogenous β-cell response is sufficient to provide timely and effective control of the glucose excursion. However, important degrees of β-cell dysfunction are present earlier in the pathogenesis of diabetes than might be expected: In screen-detected T2D and in individuals with fasting glucose levels ≥115 mg/dL (6.4 mmol/L), the first-phase insulin response is characteristically low or unmeasureable. [38, 44] The insulin-modified protocol is intended to overcome this limitation, as discussed above, with its own pros and cons. This added insulin prevents assessment of the late-phase insulin secretion responses, as the exogenous insulin is measured together with the endogenous insulin. Because the test does not control for achieved levels of glycemia, the magnitude of the stimulus to β-cell secretion can differ between or within individuals. Overall, in the settings of low insulin release and/or low insulin sensitivity, the IVGTT methodology is less reliably able to provide accurate measures of β-cell function and insulin sensitivity than the hyperglycemic clamp.
The principal technical limitations of the IVGTT are the need for two intravenous lines, the need for rapid early sampling following the intravenous glucose bolus, the need for software and expertise to undertake the modeling analyses for each individual IVGTT to derive the SI . The participant considerations include the need for two intravenous lines, the 3-4 hour duration of the procedure, and the risks associated with insulin infusion (Table 1).
Graded glucose infusion –
With this method, the insulin/C-peptide response to a prolonged intravenous infusion of glucose is measured. Rather than targeting a particular level of glycemia, the graded glucose infusion imposes a series of pre-set glucose infusion rates. This produces acute, stepwise increments in blood glucose, engendering stepped insulin secretory responses. [56–58] The initial bolus generally differs from that used in the hyperglycemic clamp, and therefore first-phase insulin/C-peptide response measurements are not directly comparable between hyperglycemic clamps and graded glucose infusion tests. [59]
The graded glucose infusion has been used across the spectrum of glucose tolerance, and has the advantage of allowing derivation of a slope reflecting the β-cell sensitivity to glucose. [58] An indirect measure of insulin sensitivity can be obtained by extending the methodology, using an up and down graded glucose procedure together with minimal modeling. [58] Unless this approach is used, a separate measure of insulin sensitivity is required to calculate a DI. Due to differences in the achieved glucose concentrations, this measurement incorporates degrees of glucose mass action (glucose-mediated glucose disposal) that are different from the other methods. Thus, the results are parallel but not strictly comparable to other approaches to measure insulin sensitivity. [60]
The graded glucose infusion has been used principally in exploring the pathophysiologic progression of β-cell dysfunction [57], and in assessing the effects of treatment interventions on β-cell function [61].
The principal technical limitations of the graded glucose infusion are the need for two intravenous lines, and expertise with the mathematical approaches needed for data extraction. The personnel burden is comparable in terms of time but this method requires less methodologic expertise than the hyperglycemic clamp, and the graded glucose infusion requires less frequent blood sampling overall. Participant considerations include the need for two intravenous lines, and a time commitment of 3-4 hours (Table 1).
Glucose-potentiated arginine stimulation test -
L-arginine infused as a bolus while the participant is hyperglycemic at a level of 450 mg/dL (25 mmol/L) or greater produces a maximal insulin response considered to reflect the functional secretory capacity of β-cells. [36, 62–64] Individuals with blunted or absent first-phase insulin/C-peptide response to intravenous glucose maintain a brisk, although reduced response to arginine (Figure 3). [11]
Figure 3.
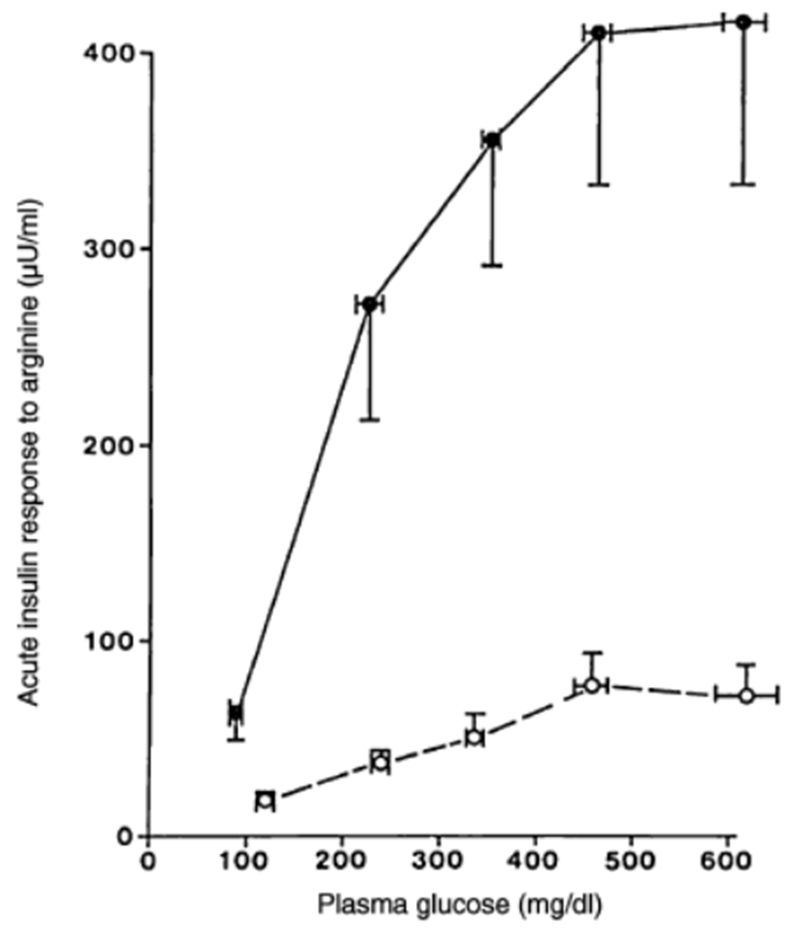
Comparison of the acute insulin response to a 5g intravenous L-arginine injection at different glucose levels in NGT participants compared with participants with T2D, with simiar age and body weight. (Reprinted with permission from Reference [39]).
Historically, stimulation with isoproterenol or glucagon was used to measure augmented insulin release, but resulted in unacceptable side effects. [63] Lower variability is observed in the insulin/C-peptide response with arginine versus glucagon; moreover, arginine stimulates glucagon release, allowing for a concurrent measure of α-cell function. [65] Recently, combinations of glucose with glucagon-like peptide 1 (GLP-1) or GLP-1 mimetics have also been used. [11, 34, 35] It is not clear whether these combination approaches offer an advantage in cost, safety, or measurement variability that may overcome the established experience with arginine.
A variation of the glucose-potentiated arginine stimulation test involves repeated applications of arginine under two or more achieved glucose concentrations. [66, 67] This approach measures stimulated responses at multiple levels of glycemia, allowing for derivation of slopes of glucose and arginine responsiveness. This provides complementary measures of β-cell function with more physiologic glucose exposures, and provides the statistical advantage of repeated within-subject measurements. It is feasible to undertake a glucose-potentiated arginine response immediately after completing a hyperglycemic clamp procedure, functionally performing one procedure but measuring multiple aspects of β-cell function.
The glucose-potentiated arginine response has been applied in assessing the function of a pancreas or islet cell transplant [67], and in assessing pharmacologic effects on β-cell function in T2D [68, 69]
The principal technical limitations of the glucose-potentiated arginine response are the same as for the hyperglycemic clamp, with the addition of the clinical supplies needed for the L-arginine infusion. Participant considerations include lengthening the hyperglycemic clamp procedure, and approximately 40% of participants experience mild side-effects (brief flushing or metallic taste) when L-arginine is administered. [65]
Oral Glucose Tolerance Test (OGTT) -
The OGTT can be employed to assess β-cell function. The relatively delayed appearance of glucose in the circulation prevents strict separation of first- and second-phase insulin responses; these components are therefore traditionally described as early and late insulin responses. The early response can be evaluated simply as the rise in insulin/C-peptide above basal at any time interval up to 30 minutes after commencing glucose ingestion, or as the “insulinogenic index” (the increment above basal insulin/C-peptide divided by the increment in glucose in the same time interval). [70] The early insulin response and the insulinogenic index are reduced in IGT and T2D. [71, 72] The late insulin or C-peptide response is generally evaluated as the integrated response over the entire sampled duration; this measure has been less widely used. [44, 71] The use of OGTT parameters to derive a DI is increasingly applied [72, 73], supported by mathematical evidence for an underlying hyperbolic relationship between specific measures of insulin secretion and insulin sensitivity from the OGTT. [74, 75]
The OGTT has been widely applied in the evaluation of the pathophysiologic progression of β-cell dysfunction [75, 76], and in assessing the effects of treatment interventions on β-cell function. [77]
A core advantage of the OGTT is that it incorporates the physiologic contributions of the gut-pancreas axis in the measure of ß-cell responses. When an OGTT is combined with an intravenous test (IVGTT or hyperglycemic clamp), this allows for comparisons of responses to parenteral versus enteral stimulation, enabling assessment of the contributions of the incretin effect to the overall response to ingested glucose. [78] OGTT-derived measures of insulin response can be adjusted for insulin sensitivity using a surrogate measure such as fasting insulin concentration or HOMA%S, or using a separate direct measurement. Minimal model methodologies have been developed that allow insulin response and insulin sensitivity to be simultaneously assessed with a multiple-sample, extended OGTT. [79–81] These models have been utilized to assess β-cell function during physiologic testing in subjects with NGT, prediabetes, and T2D.
A disadvantage of OGTT methodology is that differences in the rate of glucose absorption can modify the observed response. Due to the involvement of more biological systems and less direct control of the glycemic stimulus to insulin secretion, the variability in measures of β-cell function is high compared to that seen with intravenous testing. [13, 82–84]
Mixed meal tolerance test (MMTT) -
Analogous to the OGTT, a liquid or solid enteral stimulus consisting of a mixture of carbohydrate with other macronutrients can be delivered orally with subsequent sampling of blood glucose and insulin. As with OGTT, this method can be applied to assess contributions of the incretin effect to the overall mixed meal response. [85] The mixed nutrient load provides a more physiologically relevant comparison to human meal consumption than an isolated glucose load. [86] The same directly calculated and model-derived measures of β-cell responses can be derived from the MMTT dataset, with parallel advantages and disadvantages. [79, 87, 88] Despite the improved physiologic relevance of this method, the delivery of multiple nutrients involved in stimulation of gut hormones and in β-cell stimulation contributes to relatively high variability for this method as with the OGTT [9, 13]. Differences in size and composition of the enteral load lead to differences in insulin and incretin responses [86, 89], although there are recent efforts to standardize the test meal. [9]
The MMTT is widely utilized at present to assess β-cell function in therapeutic trials in type 1 diabetes. In contrast to high variability observed in other populations, results in this population have been highly reproducible.[90]
Simple indices and model-based estimates of β-cell function have been reported to be quantitatively higher when measured via MMTT as compared with OGTT with equal carbohydrate quantity among dysglycemic subjects. [13, 87, 88] (Figure 4) To date, no published data have formally demonstrated a hyperbolic relationship between MMTT derived β-cell function and insulin sensitivity to fully support their combination into a DI. Importantly, the MMTT glucose data cannot be interpreted using OGTT-based diagnostic criteria that define IFG, IGT and DM.
Figure 4:
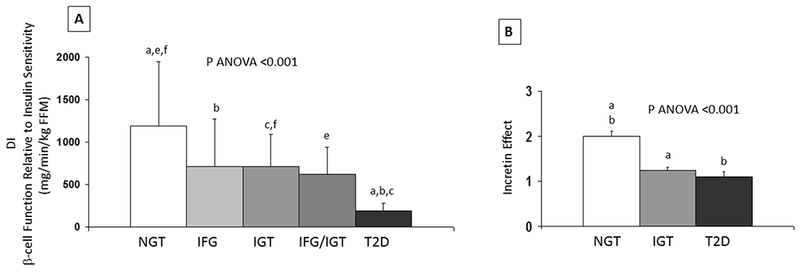
(A) DI in obese adolescents across the spectrum of glycemia. Letters indicate significant post hoc analyses (a: T2D vs. NGT; b: T2D vs. IFG; c: T2D vs. IGT; e: NGT vs. IFG/IGT; f: NGT vs. IGT). Adapted with permission from reference [99]. (B) Incretin effect in obese youth. Letters indicate significant post hoc analyses (a: NGT vs. IGT; b: NGT vs. T2D). Adapted with permission from reference [78].
Both the OGTT and MMTT require the placement of a single peripheral intravenous line for repeated blood sampling. Blood sampling frequency and timing is parallel to that for OGTT, reduced compared to glucose infusion based protocols, and there is no requirement for specific expertise in making adjustments to glucose infusions. Modeling methods require software and expertise. Participant considerations include the need for one intravenous line, and a time commitment of 3-4 hours.
Fasting proinsulin to insulin (or C-peptide) ratio
In subjects without diabetes, the molar proportion of circulating proinsulin to insulin is approximately 15% in the fasting state. [91, 92] As β-cell failure ensues, processing of proinsulin to insulin and C-peptide is impaired, and the fasting proinsulin to insulin ratio increases two- to three-fold in T2D. [91, 92] Interestingly, the ratio is not significantly increased in all individulas with IGT [93], possibly suggesting that an elevated ratio is an indicator of more established β-cell dysfunction or of increased β-cell demand.
Homeostatic model assessment (HOMA) –
The HOMA provides estimates of basal β-cell function and insulin sensitivity. [27] Updates to the model take into account variations in hepatic and peripheral glucose resistance, and other whole-body determinants of metabolic physiology.[94] The original linear equations are simplified approximations of the original nonlinear solution. [27] With modern computing the direct calculation is widely accessible, particularly with the availability of an online calculator (https://www.dtu.ox.ac.uk/homacalculator/). The use of the online methodology is preferred. [27, 95]
Because the model requires only basal glucose and insulin/C-peptide concentrations, it has obvious advantages in terms of cost and ease of application, and has been widely utilized in large epidemiologic studies, longitudinal cohort studies, and clinical trials. The HOMA%B is correlated (r=0.6-0.9) with direct measures of β-cell function in cross-sectional studies of healthy populations with NGT, but may be less reliably related in progressive dysglycemia and diabetes. [27, 83, 96]. Correlations with direct measures are weaker in longitudinal studies, even after accounting for increased variability of the measurements. [20]
These surrogate measures have been used primarily in epidemiologic studies, although in some instances they have been used to assess treatment effects on β-cell function in pharmacologic or surgical studies. [46, 77, 97]
These measures have advantages for cost and personnel burden, and require much less of individual participants. However, the compromises entailed make it an imperfect choice for studies primarily assessing β-cell function and response to interventions that may also improve insulin sensitivity.
A Case Study in Method Selection - The RISE Approach to Measuring ß-Cell Function
The Restoring Insulin Secretion (RISE) Consortium includes three studies assessing the hypothesis that glucose lowering will lead to sustained improvement in β-cell function in prediabetes and early T2D [17] :
RISE Adult Medication Study: Adult participants (ages 20-65) are randomized to one of the following treatment arms: (1) metformin alone, (2) early treatment with insulin glargine followed by metformin, (3) liraglutide plus metformin, or (4) placebo.
RISE Pediatric Medication Study: Pediatric participants (ages 10-19) are randomized to: 1) metformin alone, or 2) early treatment with insulin glargine followed by metformin.
RISE Adult Surgical Study (BetaFat Study): Adult participants (ages 20-65) are randomized to gastric banding or metformin.
The RISE Medication Studies will assess whether improvements in ß-cell function following 12 months of active treatment are maintained for 3 months following the withdrawal of therapy. The BetaFat trial will assess the same outcome variables after 12- and 24-months of active treatment with metformin or following gastic banding surgery.
The RISE studies use a shared set of measurements. The selection of methods to be applied in RISE incorporated the need to carefully assess β-cell function at repeated intervals, while balancing considerations for participant burden and resource constraints. Also of importance was the capacity to demonstrate change on repeated testing performed longitudinally. Incorporating the considerations and comparisons of methodologies outlined above, the RISE Consortium elected to undertake, as the primary method for measurement of ß-cell function, a two-stage hyperglycemic clamp including arginine stimulation. The first stage uses an initial weight-based glucose bolus followed by a 2-hour continuous glucose infusion targeting a sustained plasma glucose concentration of 200 mg/dL (11.1 mmol/L), to allow derivation of first- and second-phase insulin/C-peptide responses to intravenous glucose and the measurement of insulin sensitivity at the end of this 2-hour clamp. The second stage incorporates a 30-45 minute increase in plasma glucose concentration to at least 450 mg/dL (25 mmol/L) followed by a bolus of 5g of L-arginine, to allow measurement of maximal β-cell secretory capacity.
RISE also chose to perform, a separate 3-hour OGTT with rapid early sampling (10/20/30 minutes following ingestion) to evaluate glucose tolerance and ß-cell responses in the context of an enterally delivered stimulus. This increases the subject burden by adding an additional testing day, but provides information on glycemic control and responses that incorporate the incretin contributions to ß-cell function. The incretins were of interest as both the gastric-banding surgery and GLP-1 receptor agonist therapies could have treatment-specific effects to modify responses to enterally delivered nutrients that may not be adequately assessed using the hyperglycemic clamp. Comparisons of clamp versus OGTT responses will be used to evaluate whether changes in incretin response contribute to any observed effects of the RISE interventions. The OGTT was chosen over the MMTT to maximize standardization, and to allow a measure of glucose tolerance that can be evaluated against established clinical criteria.
The hyperglycemic clamp was chosen over the other methods described for the following reasons: 1) it allows for a controlled and repeatable hyperglycemic stimulus to the β-cell (minimizing variability), and allows the addition of arginine stimulation to measure β-cell secretory capacity; 2) the methodology is reproducible and amenable to standardization across study sites [21]; and 3) it simultaneously provides an indirect measure of insulin sensitivity. Including a hyperinsulinemic-euglycemic clamp would add considerable participant and staff burden over the course of a longitudinal study. We instead chose to evaluate insulin sensitivity using the insulin sensitivity index derived during the 200 mg/dL (11.1 mmol/L) steady-state period of the hyperglycemic clamp. Doing so allowed the inclusion of a second, less-intensive day of testing with an OGTT. Arginine-stimulated insulin secretion was included as a measure of the maximal ß-cell response, which could potentially show a different response to the various treatment approaches. Arginine was chosen as opposed to other available stimuli because it is an established method for this purpose, and it has superior technical performance.[65] Further, use of arginine would allow the evaluation of the glucagon response as a measure of α-cell function.
The IVGTT was ultimately not chosen because we anticipated very poor or absent first-phase insulin responses in the population to be evaluated, with attendant difficulties in modeling first-phase responses and missing data. We also considered graded-glucose infusion tests, given the unique advantage of directly quantifying β-cell glucose sensitivity, and the advantage of precise regulation of the glucose stimulus. However this method does not produce traditional measures of first- and second- phase insulin responses. In order to have a measure of insulin sensitivity, we also would have needed to use the model-derived measure from the up-down graded procedure, or perform a hyperinsulinemic clamp on a separate day. [58] Here again, there was a concern that modeling for individuals with poor β-cell function and poor insulin sensitivity would prove difficult, with loss of data and incomplete datasets even with this detailed method of measuring ß-cell glucose responses.
Conclusion
Many different methods have been developed for in vivo measurement of human β-cell function, each with strengths and weaknesses. The optimal selection of methods will be determined by the particular focus of study. Table 1 provides an overview of the main strengths and weaknesses of the methods discussed.
The RISE study is evaluating the effects of interventions including pharmacotherapeutics and metabolic surgery on β-cell function, in populations spanning from pediatrics to adults. We elected to measure our β-cell outcomes using a 2-day measurement procedure, namely a 3-hour 75g OGTT and a 2-stage hyperglycemic clamp with arginine. This combination of methods provides an assessment of: 1) first- and second-phase insulin/C-peptide responses; 2) insulin sensitivity; 3) maximal β-cell secretory capacity; 4) early and late insulin response to an enteral glucose stimulus. This protocol has been successfully implemented in a multi-center consortium, highlighting the feasibility of using these methods in treatment studies with multiple participating study sites.
Acknowledgements
RISE is supported by grants from the NIDDK (Chicago DK-094431, Denver DK-094467, Indiana DK-094438, Los Angeles DK-094430, and Seattle DK-094406) and the Department of Veterans Affairs. Additional financial and material support from the American Diabetes Association, Allergan Corporation, Abbott Laboratories, and Novo Nordisk A/S is gratefully acknowledged. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. RISE is registered in clinicaltrials.gov: NCT01779362, NCT01779375, and NCT01763346.
Footnotes
This is the peer reviewed version of the following article: Hannon TS, Kahn SE, Utzschneider KM, et al.; The RISE Consortium. Review of methods for measuringb-cell function: Design considerations from the Restoring Insulin Secretion (RISE) Consortium. Diabetes Obes Metab 2018;20:14–24, which has been published in final form at https://doi.org/10.1111/dom.13005. This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Use of Self-Archived Versions.
References
- [1].Kahn SE. The importance of the beta-cell in the pathogenesis of type 2 diabetes mellitus. The American journal of medicine. 2000; 108 Suppl 6a: 2S–8S [DOI] [PubMed] [Google Scholar]
- [2].Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006; 55: 1430–1435 [DOI] [PubMed] [Google Scholar]
- [3].Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes care. 2009; 32: 100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R. Beta-cell function across the spectrum of glucose tolerance in obese youth. Diabetes. 2005; 54: 1735–1743 [DOI] [PubMed] [Google Scholar]
- [5].Elder DA, Prigeon RL, Wadwa RP, Dolan LM, D’Alessio DA. Beta-cell function, insulin sensitivity, and glucose tolerance in obese diabetic and nondiabetic adolescents and young adults. The Journal of clinical endocrinology and metabolism. 2006; 91: 185–191 [DOI] [PubMed] [Google Scholar]
- [6].Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. The New England journal of medicine. 2006; 355: 2427–2443 [DOI] [PubMed] [Google Scholar]
- [7].Bacha F, Gungor N, Lee S, Arslanian SA. Progressive deterioration of beta-cell function in obese youth with type 2 diabetes. Pediatric diabetes. 2013; 14: 106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cali A, Dalla Man C, Cobelli C, et al. Primary Defects in b-Cell Function Further Exacerbated by Worsening of Insulin Resistance Mark the Development of Impaired Glucose Tolerance in Obese Adolescents diabetes care. 2009; 32: 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shankar SS, Vella A, Raymond RH, et al. Standardized Mixed-Meal Tolerance and Arginine Stimulation Tests Provide Reproducible and Complementary Measures of beta-cell Function: Results From the Foundation for the National Institutes of Health Biomarkers Consortium Investigative Series. Diabetes care. 2016; 39: 1602–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993; 42: 1663–1672 [DOI] [PubMed] [Google Scholar]
- [11].Kahn SE, Carr DB, Faulenbach MV, Utzschneider KM. An examination of beta-cell function measures and their potential use for estimating beta-cell mass. Diabetes, obesity & metabolism. 2008; 10 Suppl 4: 63–76 [DOI] [PubMed] [Google Scholar]
- [12].Sjaarda L, Lee S, Tfayli H, Bacha F, Bertolet M, Arslanian S. Measuring beta-cell function relative to insulin sensitivity in youth: does the hyperglycemic clamp suffice? Diabetes care. 2013; 36: 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bacha F, Gungor N, Arslanian SA. Measures of beta-cell function during the oral glucose tolerance test, liquid mixed-meal test, and hyperglycemic clamp test. The Journal of pediatrics. 2008; 152: 618–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bergman RN, Ader M, Heucking K, Van Citters G. Accurate Assessment of beta-cell function. The hyperbolic correction. Diabetes. 2002; 51 S212–S220 [DOI] [PubMed] [Google Scholar]
- [15].Wallace TM, Matthews DR. Coefficient of failure: a methodology for examining longitudinal beta-cell function in Type 2 diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2002; 19: 465–469 [DOI] [PubMed] [Google Scholar]
- [16].Davis SN, Piatti PM, Monti L, et al. A comparison of four methods for assessing in vivo beta-cell function in normal, obese and non-insulin-dependent diabetic man. Diabetes Res 1992; 19: 107–117 [PubMed] [Google Scholar]
- [17].Restoring Insulin Secretion (RISE): design of studies of beta-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes care. 2014; 37: 780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ferrannini E, Mari A. How to measure insulin sensitivity. Journal of hypertension. 1998; 16: 895–906 [DOI] [PubMed] [Google Scholar]
- [19].Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes care. 1999; 22: 1462–1470 [DOI] [PubMed] [Google Scholar]
- [20].Xiang AH, Watanabe RM, Buchanan TA. HOMA and Matsuda indices of insulin sensitivity: poor correlation with minimal model-based estimates of insulin sensitivity in longitudinal settings. Diabetologia. 2014; 57: 334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. The American journal of physiology. 1979; 237: E214–223 [DOI] [PubMed] [Google Scholar]
- [22].Elahi D In praise of the hyperglycemic clamp. A method for assessment of beta-cell sensitivity and insulin resistance. Diabetes care. 1996; 19: 278–286 [DOI] [PubMed] [Google Scholar]
- [23].Toffolo G, Bergman RN, Finegood DT, Bowden CR, Cobelli C. Quantitative estimation of beta cell sensitivity to glucose in the intact organism: a minimal model of insulin kinetics in the dog. Diabetes. 1980; 29: 979–990 [DOI] [PubMed] [Google Scholar]
- [24].Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. The Journal of clinical investigation. 1981; 68: 1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pfeifer MA, Halter JB, Beard JC, Porte D Jr. Differential effects of tolbutamide on first and second phase insulin secretion in noninsulin-dependent diabetes mellitus. The Journal of clinical endocrinology and metabolism. 1981; 53: 1256–1262 [DOI] [PubMed] [Google Scholar]
- [26].Prigeon RL, Roder ME, Porte D Jr., Kahn SE. The effect of insulin dose on the measurement of insulin sensitivity by the minimal model technique. Evidence for saturable insulin transport in humans. The Journal of clinical investigation. 1996; 97: 501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28: 412–419 [DOI] [PubMed] [Google Scholar]
- [28].Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. The Journal of clinical endocrinology and metabolism. 2000; 85: 2402–2410 [DOI] [PubMed] [Google Scholar]
- [29].Mather KJ, Hunt AE, Steinberg HO, et al. Repeatability characteristics of simple indices of insulin resistance: implications for research applications. The Journal of clinical endocrinology and metabolism. 2001; 86: 5457–5464 [DOI] [PubMed] [Google Scholar]
- [30].Giannini C, Weiss R, Cali A, et al. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes. 2012; 61: 606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tura A, Grassi A, Winhofer Y, et al. Progression to type 2 diabetes in women with former gestational diabetes: time trajectories of metabolic parameters. PLoS One. 2012; 7: e50419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].O’Meara NM, Sturis J, Van Cauter E, Polonsky KS. Lack of control by glucose of ultradian insulin secretory oscillations in impaired glucose tolerance and in non-insulin-dependent diabetes mellitus. The Journal of clinical investigation. 1993; 92: 262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. The Journal of clinical investigation. 1988; 81: 442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ahren B, Holst JJ, Mari A. Characterization of GLP-1 effects on beta-cell function after meal ingestion in humans. Diabetes care. 2003; 26: 2860–2864 [DOI] [PubMed] [Google Scholar]
- [35].Dalla Man C, Micheletto F, Sathananthan M, Vella A, Cobelli C. Model-Based Quantification of Glucagon-Like Peptide-1-Induced Potentiation of Insulin Secretion in Response to a Mixed Meal Challenge. Diabetes technology & therapeutics. 2016; 18: 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. The Journal of clinical investigation. 1984; 74: 1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ferrannini E, Pilo A. Pattern of insulin delivery after intravenous glucose injection in man and its relation to plasma glucose disappearance. The Journal of clinical investigation. 1979; 64: 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brunzell JD, Robertson RP, Lerner RL, et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. The Journal of clinical endocrinology and metabolism. 1976; 42: 222–229 [DOI] [PubMed] [Google Scholar]
- [39].Ward WK, Beard JC, Halter JB, Porte D Jr Pathophysiology of insulin secretion in diabetes mellitus. Advances in experimental medicine and biology. 1985; 189: 137–158 [DOI] [PubMed] [Google Scholar]
- [40].Kanat M, Norton L, Winnier D, Jenkinson C, DeFronzo RA, Abdul-Ghani MA. Impaired early- but not late-phase insulin secretion in subjects with impaired fasting glucose. Acta diabetologica. 2011; 48: 209–217 [DOI] [PubMed] [Google Scholar]
- [41].O’Connor MD, Landahl H, Grodsky GM. Comparison of storage- and signal-limited models of pancreatic insulin secretion. The American journal of physiology. 1980; 238: R378–389 [DOI] [PubMed] [Google Scholar]
- [42].Nesher R, Cerasi E. Modeling phasic insulin release: immediate and time-dependent effects of glucose. Diabetes. 2002; 51 Suppl 1: S53–59 [DOI] [PubMed] [Google Scholar]
- [43].Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003; 52: 102–110 [DOI] [PubMed] [Google Scholar]
- [44].Hannon TS, Kirkman MS, Patel YR, Considine RV, Mather KJ. Profound defects in beta-cell function in screen-detected type 2 diabetes are not improved with glucose-lowering treatment in the early diabetes prevention program (EDIP). Diabetes/metabolism research and reviews. 2014: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pennartz C, Schenker N, Menge BA, Schmidt WE, Nauck MA, Meier JJ. Chronic reduction of fasting glycemia with insulin glargine improves first- and second-phase insulin secretion in patients with type 2 diabetes. Diabetes care. 2011; 34: 2048–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Martinussen C, Bojsen-Moller KN, Dirksen C, et al. Immediate enhancement of first-phase insulin secretion and unchanged glucose effectiveness in patients with type 2 diabetes after Roux-en-Y gastric bypass. American journal of physiology Endocrinology and metabolism. 2015; 308: E535–544 [DOI] [PubMed] [Google Scholar]
- [47].Nijpels G, Boorsma W, Dekker JM, et al. Absence of an acute insulin response predicts onset of type 2 diabetes in a Caucasian population with impaired glucose tolerance. The Journal of clinical endocrinology and metabolism. 2008; 93: 2633–2638 [DOI] [PubMed] [Google Scholar]
- [48].Xiang AH, Kawakubo M, Trigo E, Kjos SL, Buchanan TA. Declining beta-cell compensation for insulin resistance in Hispanic women with recent gestational diabetes mellitus: association with changes in weight, adiponectin, and C-reactive protein. Diabetes Care. 2010; 33: 396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xiang AH, Kjos SL, Takayanagi M, Trigo E, Buchanan TA. Detailed physiological characterization of the development of type 2 diabetes in Hispanic women with prior gestational diabetes mellitus. Diabetes. 2010; 59: 2625–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bonadonna RC, Stumvoll M, Fritsche A, et al. Altered homeostatic adaptation of first- and second-phase beta-cell secretion in the offspring of patients with type 2 diabetes: studies with a minimal model to assess beta-cell function. Diabetes. 2003; 52: 470–480 [DOI] [PubMed] [Google Scholar]
- [51].Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. The Journal of clinical investigation. 1995; 96: 520–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Buchanan TA, Xiang A, Kjos SL, et al. Gestational diabetes: antepartum characteristics that predict postpartum glucose intolerance and type 2 diabetes in Latino women. Diabetes. 1998; 47: 1302–1310 [DOI] [PubMed] [Google Scholar]
- [53].Junqueira Vasques AC, Pareja JC, de Oliveira Mda S, et al. beta-Cell function improvements in grade I/II obese subjects with type 2 diabetes 1 month after biliopancreatic diversion: results from modeling analyses of oral glucose tolerance tests and hyperglycemic clamp studies. Diabetes care. 2013; 36: 4117–4124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006; 55: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002; 51: 2796–2803 [DOI] [PubMed] [Google Scholar]
- [56].Byrne MM, Sturis J, Polonsky KS. Insulin secretion and clearance during low-dose graded glucose infusion. The American journal of physiology. 1995; 268: E21–27 [DOI] [PubMed] [Google Scholar]
- [57].Ehrmann DA, Breda E, Cavaghan MK, et al. Insulin secretory responses to rising and falling glucose concentrations are delayed in subjects with impaired glucose tolerance. Diabetologia. 2002; 45: 509–517 [DOI] [PubMed] [Google Scholar]
- [58].Toffolo G, Breda E, Cavaghan MK, Ehrmann DA, Polonsky KS, Cobelli C. Quantitative indexes of beta-cell function during graded up&down glucose infusion from C-peptide minimal models. American journal of physiology Endocrinology and metabolism. 2001; 280: E2–10 [DOI] [PubMed] [Google Scholar]
- [59].Chen M, Porte D Jr The effect of rate and dose of glucose infusion on the acute insulin response in man. The Journal of clinical endocrinology and metabolism. 1976; 42: 1168–1175 [DOI] [PubMed] [Google Scholar]
- [60].Shankar SS, Shankar RR, Mixson LA, et al. Linearity of beta-cell response across the metabolic spectrum and to pharmacology: insights from a graded glucose infusion-based investigation series. American journal of physiology Endocrinology and metabolism. 2016; 310: E865–873 [DOI] [PubMed] [Google Scholar]
- [61].Cavaghan MK, Ehrmann DA, Byrne MM, Polonsky KS. Treatment with the oral antidiabetic agent troglitazone improves beta cell responses to glucose in subjects with impaired glucose tolerance. The Journal of clinical investigation. 1997; 100: 530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hollander PM, Asplin CM, Palmer JP. Glucose modulation of insulin and glucagon secretion in nondiabetic and diabetic man. Diabetes. 1982; 31: 489–495 [DOI] [PubMed] [Google Scholar]
- [63].Ward WK, Beard JC, Halter JB, Pfeifer MA, Porte D Jr. Pathophysiology of insulin secretion in non-insulin-dependent diabetes mellitus. Diabetes care. 1984; 7: 491–502 [DOI] [PubMed] [Google Scholar]
- [64].van Haeften TW, Voetberg GA, Gerich JE, van der Veen EA. Dose-response characteristics for arginine-stimulated insulin secretion in man and influence of hyperglycemia. The Journal of clinical endocrinology and metabolism. 1989; 69: 1059–1064 [DOI] [PubMed] [Google Scholar]
- [65].Robertson RP, Raymond RH, Lee DS, et al. Arginine is preferred to glucagon for stimulation testing of beta-cell function. American journal of physiology Endocrinology and metabolism. 2014; 307: E720–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rickels MR, Mueller R, Teff KL, Naji A. {beta}-Cell secretory capacity and demand in recipients of islet, pancreas, and kidney transplants. The Journal of clinical endocrinology and metabolism. 2010; 95: 1238–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes. 2015; 64: 565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vilsboll T, Brock B, Perrild H, et al. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with Type 2 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association. 2008; 25: 152–156 [DOI] [PubMed] [Google Scholar]
- [69].Diamant M, Van Gaal L, Guerci B, et al. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol 2014; 2: 464–473 [DOI] [PubMed] [Google Scholar]
- [70].Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabetic medicine : a journal of the British Diabetic Association. 1994; 11: 286–292 [DOI] [PubMed] [Google Scholar]
- [71].Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002; 51: 2170–2178 [DOI] [PubMed] [Google Scholar]
- [72].Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. The Journal of pediatrics. 2012; 161: 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cali AM, Man CD, Cobelli C, et al. Primary defects in beta-cell function further exacerbated by worsening of insulin resistance mark the development of impaired glucose tolerance in obese adolescents. Diabetes care. 2009; 32: 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring). 2008; 16: 1901–1907 [DOI] [PubMed] [Google Scholar]
- [75].Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes care. 2009; 32: 335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. Declining beta-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes care. 2011; 34: 2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kahn SE, Lachin JM, Zinman B, et al. Effects of rosiglitazone, glyburide, and metformin on beta-cell function and insulin sensitivity in ADOPT. Diabetes. 2011; 60: 1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Michaliszyn SF, Mari A, Lee S, et al. beta-cell function, incretin effect, and incretin hormones in obese youth along the span of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes. 2014; 63: 3846–3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes. 2014; 63: 1203–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of beta -cell function: modeling analysis in normal subjects. American journal of physiology Endocrinology and metabolism. 2002; 283: E1159–1166 [DOI] [PubMed] [Google Scholar]
- [81].Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001; 50: 150–158 [DOI] [PubMed] [Google Scholar]
- [82].Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. The Journal of clinical endocrinology and metabolism. 2008; 93: 4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hermans MP, Levy JC, Morris RJ, Turner RC. Comparison of tests of beta-cell function across a range of glucose tolerance from normal to diabetes. Diabetes. 1999; 48: 1779–1786 [DOI] [PubMed] [Google Scholar]
- [84].Utzschneider KM, Prigeon RL, Tong J, et al. Within-subject variability of measures of beta cell function derived from a 2 h OGTT: implications for research studies. Diabetologia. 2007; 50: 2516–2525 [DOI] [PubMed] [Google Scholar]
- [85].Jimenez A, Mari A, Casamitjana R, Lacy A, Ferrannini E, Vidal J. GLP-1 and glucose tolerance after sleeve gastrectomy in morbidly obese subjects with type 2 diabetes. Diabetes. 2014; 63: 3372–3377 [DOI] [PubMed] [Google Scholar]
- [86].Rijkelijkhuizen JM, McQuarrie K, Girman CJ, et al. Effects of meal size and composition on incretin, alpha-cell, and beta-cell responses. Metabolism: clinical and experimental. 2010; 59: 502–511 [DOI] [PubMed] [Google Scholar]
- [87].Rijkelijkhuizen JM, Girman CJ, Mari A, et al. Classical and model-based estimates of beta-cell function during a mixed meal vs. an OGTT in a population-based cohort. Diabetes research and clinical practice. 2009; 83: 280–288 [DOI] [PubMed] [Google Scholar]
- [88].Bacha F, Gungor N, Lee S, de Las Heras J, Arslanian S. Indices of Insulin Secretion during a Liquid Mixed-Meal Test in Obese Youth with Diabetes. The Journal of pediatrics. 2013: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bock G, Dalla Man C, Campioni M, et al. Effects of nonglucose nutrients on insulin secretion and action in people with pre-diabetes. Diabetes. 2007; 56: 1113–1119 [DOI] [PubMed] [Google Scholar]
- [90].Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes care. 2008; 31: 1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kahn SE, Leonetti DL, Prigeon RL, Boyko EJ, Bergstrom RW, Fujimoto WY. Proinsulin as a marker for the development of NIDDM in Japanese-American men. Diabetes. 1995; 44: 173–179 [DOI] [PubMed] [Google Scholar]
- [92].Ward WK, LaCava EC, Paquette TL, Beard JC, Wallum BJ, Porte D Jr Disproportionate elevation of immunoreactive proinsulin in type 2 (non-insulin-dependent) diabetes mellitus and in experimental insulin resistance. Diabetologia. 1987; 30: 698–702 [DOI] [PubMed] [Google Scholar]
- [93].Saad MF, Kahn SE, Nelson RG, et al. Disproportionately elevated proinsulin in Pima Indians with noninsulin-dependent diabetes mellitus. The Journal of clinical endocrinology and metabolism. 1990; 70: 1247–1253 [DOI] [PubMed] [Google Scholar]
- [94].Hill NR, Levy JC, Matthews DR. Expansion of the homeostasis model assessment of beta-cell function and insulin resistance to enable clinical trial outcome modeling through the interactive adjustment of physiology and treatment effects: iHOMA2. Diabetes care. 2013; 36: 2324–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes care. 1998; 21: 2191–2192 [DOI] [PubMed] [Google Scholar]
- [96].Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. The Journal of pediatrics. 2004; 144: 47–55 [DOI] [PubMed] [Google Scholar]
- [97].Derosa G, Carbone A, Franzetti I, et al. Effects of a combination of sitagliptin plus metformin vs metformin monotherapy on glycemic control, beta-cell function and insulin resistance in type 2 diabetic patients. Diabetes research and clinical practice. 2012; 98: 51–60 [DOI] [PubMed] [Google Scholar]
- [98].Pfeifer MA, Halter JB, Porte D Jr. Insulin secretion in diabetes. The American journal of medicine. 1981; 70: 579–588 [DOI] [PubMed] [Google Scholar]
- [99].Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes care. 2010; 33: 2225–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]


