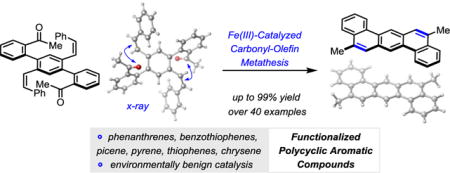
Abstract
Polycyclic aromatic hydrocarbons are important structural motifs in organic chemistry, pharmaceutical chemistry and materials science. The development of a new synthetic strategy toward these compounds is described based on the design principle of iron(III)-catalyzed carbonyl-olefin metathesis reactions. This approach is characterized by its operational simplicity, high functional group compatibility, and regioselectivity while relying on FeCl3 as an environmentally benign, earth-abundant metal catalyst. Experimental evidence for oxetanes as reactive intermediates in the catalytic carbonyl-olefin ring-closing metathesis has been obtained.
Graphical abstract
Authors are required to submit a graphic entry for the Table of Contents (TOC) that, in conjunction with the manuscript title, should give the reader a representative idea of one of the following: A key structure, reaction, equation, concept, or theorem, etc., that is discussed in the manuscript. Consult the journal’s Instructions for Authors for TOC graphic specifications.
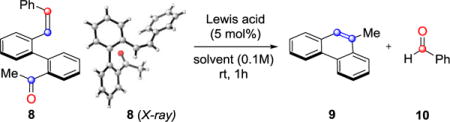
Polycyclic aromatic compounds (PACs),1 including phenanthrenes, pyrenes and chrysenes, are important structural motifs that exhibit desirable optical,2 electronic,3 and chelating4 properties. Consequently, diverse fields of research such as materials science,4 natural product synthesis,6 asymmetric catalysis,7 and molecular recognition8 rely on efficient strategies to access condensed polyaromatic compounds. Established procedures toward these motifs include McMurry coupling reactions9, 10 that are mediated by low-valent titanium reagents (Fig. 1A II) or oxidative photocyclization strategies11 of stilbene derivatives. These classical approaches12 have been hampered by the need for stoichiometric reagents, harsh reaction conditions, or competing substrate dimerization. Complementary approaches have been developed to overcome these challenges that are based on Diels-Alder cycloaddition reactions,13 radical cyclizations,14 and metal-mediated cycloisomerizations.15 Additionally, rhodium-and ruthenium-catalyzed procedures have been reported that rely on bis(N-tosylhydrazone)16 2 as substrate (Fig. 1A I) and olefin-metathesis reactions of bis(alkenes)17 4 (Fig. 1A III). We have recently reported the development of an efficient iron(III)-catalyzed carbonyl-olefin metathesis reaction18 that proceeds under mild reaction conditions and ambient temperature. Our synthetic strategy for ring-closing metathesis enables the direct coupling of carbonyl and olefin functional groups upon activation by a Lewis acid catalyst to forge the desired alkene bonds. Based on this design principle, we report the development of a new strategy for the synthesis of electronically and sterically diverse PACs. This strategy is compatible with both ketones and aldehydes, proceeding via intermediate oxetanes 6 to provide the corresponding metathesis products in good to excellent yields (Fig. 1B). While several Lewis acids were previously found capable of promoting carbonyl-olefin metathesis reactions,18 a fine-tuned combination of Lewis acidity19 and oxophilicity20 proved essential to give high yields of product. Indeed, when biaryl ketone 8 was reacted with numerous Lewis acids (e.g. TiCl4, SnCl4, FeCl2, Cu(OTf)2) no formation or only trace amounts of the metathesis product 9 was observed (entries 1–4, Table 1). Stronger Lewis acids, GaCl3 and AlCl3,18 were able to promote the desired transformation in 88% and 93% yield, respectively with complete conversion of starting material 8 (entries 7 and 8, Table 1). Notably, substoichiometric BF3·Et2O led to the formation of 9 in only modest yield and conversion (entry 6, Table 1).21 Ultimately, 5 mol% FeCl3 in either dichloroethane or toluene was identified as an optimal set of reaction conditions, resulting in quantitative formation of the product 9 in 97% and 99% yield, respectively (entries 9 and 11, Table 1). More dilute reaction conditions led to slightly lower yields of 9 (entry 10, Table 1). When the reaction was conducted in ethereal solvents (1,4-dioxane), or polar aprotic solvents (DMF), no formation of phenanthrene 9 was observed–presumably due to competing Lewis basicity of these solvents (entries 12 and 13, Table 1). Moreover, the Brønsted acids, anhydrous HCl22 and pTsOH in dichloroethane, did not form phenanthrene 9 and resulted in quantitative reisolation of starting material (entries 16 and 17, Table 1).
Figure 1.
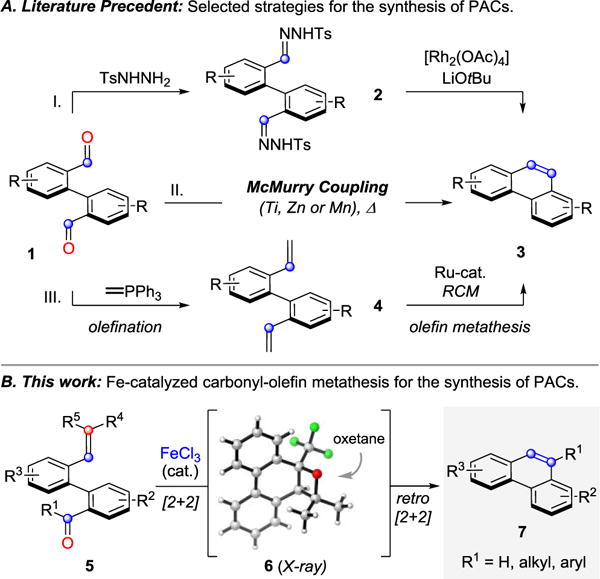
A. Select strategies to access PACs. B. Carbonyl-olefin metathesis approach reported.
Table 1.
Reaction optimization for synthesis of 9.
| ||||
|---|---|---|---|---|
|
| ||||
| entry | Lewis acid | solvent | yield 9 (%) | conversion (%) |
| 1 | TiCl4 | DCE | 3 | 7 |
| 2 | SnCl4 | DCE | 0 | 6 |
| 3 | FeCl2 | DCE | 0 | 2 |
| 4 | Cu(OTf)2 | DCE | 0 | 0 |
| 5 | ZnCl2 | DCE | 22 | 26 |
| 6 | BF3·Et2O | DCE | 31 | 35 |
| 7 | AlCl3 | DCE | 93 | 100 |
| 8 | GaCl3 | DCE | 88 | 100 |
| 9 | FeCl3 | DCE | 97 | 100 |
| 10 | FeCl3 | DCE (0.01M) | 95 | 100 |
| 11 | FeCl3 | toluene | 99 | 100 |
| 12 | FeCl3 | DMF | 0 | 0 |
| 13 | FeCl3 | 1,4-dioxane(0.1M) | 0 | 6 |
| 14 | HCl | DCE | 0 | 0 |
| 15 | pTsOH | DCE | 0 | 0 |
Conditions: biaryl 8 (0.13 mmol), Lewis or Brønsted acid (5 mol%) in solvent listed (0.1–0.01M), rt, 1h; yield determined by 1H NMR analysis with 1,3,5-trimethoxy-benzene as internal standard.
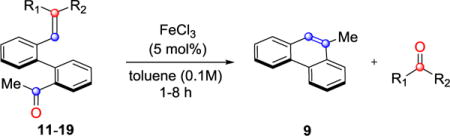
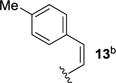
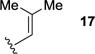
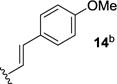
We next sought to investigate the ability of biaryl substrates with various olefin subunits (11–19) to undergo the metathesis reaction (Table 2). While both electron-rich and electron-poor styrenes (entries 1–6, Table 2) proved to be efficient substrates resulting in high yields of 9, all but styrene 11 and prenylated 17 required elevated temperatures of 50 ˚C to proceed to full conversion. Notably, no difference in reactivity between E- and Z-isomers was observed; both para-methyl styrenes 12 and 13 formed metathesis product 9 in yields up to 89% which indicates an indiscriminate reaction pathway of the carbonyl-olefin metathesis reaction. Although the formation of the respective benzaldehydes was observed as the corresponding metathesis byproducts in the course of the reaction, they did not impede reaction progress. Moreover, substrates 11–16 bearing styrenyl moieties proved superior to their prenylated analog 17, which resulted in the formation of 9 in only 79% yield (entries 1–7, Table 2). In comparison, no reaction was observed when terminal alkene 19 was subjected to the optimized reaction conditions (entry 9, Table 2). Conversion of biaryl 18 bearing a crotyl moiety under the reaction conditions resulted in low yields (18%) of the desired product. The hampered yields of the non-styrenyl substrates 17 and 18 were found to be caused by a competing carbonyl-ene reaction pathway which led to the formation of 20 and 21 in 21% and 47% yield, respectively, when subjected to the optimized reaction conditions (Fig. 2). These findings contrast distinctly with previous results obtained in our lab18 in the iron(III)-catalyzed carbonyl-olefin metathesis reaction of aliphatic aryl ketones, in which prenylated substrates proved superior to the analogous styrenes.
Table 2.
Alkene evaluation for formation of 9.
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | alkene | yield (%) | entry | alkene | yield (%) |
| 1 |
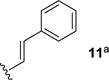
|
90 | 5 |
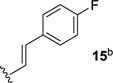
|
80 |
| 2 |
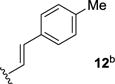
|
82 | 6 |
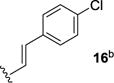
|
84 |
| 3 |
|
89 | 7 |
|
79 |
| 4 |
|
86 | 8 9 |

|
11 n.r. |
Conditions: biaryl (0.13 mmol), FeCI3 (5 mol%) in toluene (0.1 M);
mixture of E/Z (2:1) isomers;
reaction heated to 50°C.
Figure 2.
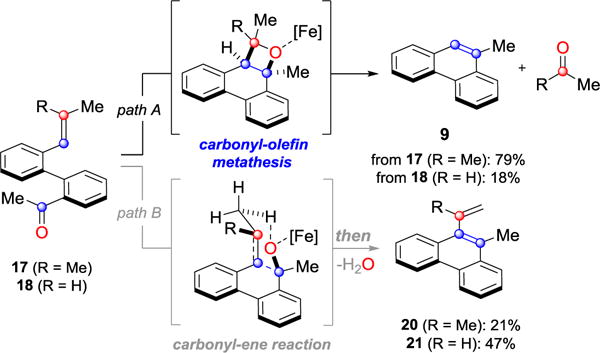
Competing metathesis and carbonyl-ene reactions.
Condition: biaryl (0.13 mmol), FeCl3 (5 mol%) in dichloroethane (0.1M), rt, 1h;
a)yield determined by 1H NMR analysis with 1,3,5-trimethoxybenzene as internal standard.
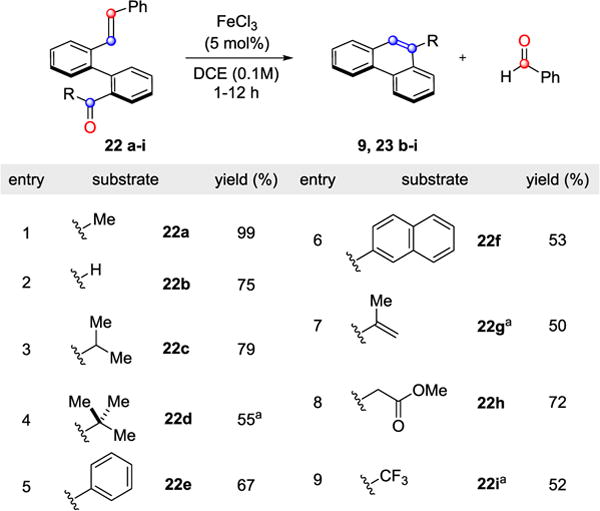
The conditions developed for the iron(III)-catalyzed carbonyl-olefin metathesis reaction proved efficient for a range of sterically and electronically differentiated ketones and aldehydes (entries 1–9, Table 3). Although aldehydes have previously been found unreactive in catalytic carbonyl-olefin ring-closing metathesis reactions,18 22b was found to yield the desired metathesis product 23b in 75% under the optimized conditions.
Table 3.
Evaluation of carbonyl substituents.
|
Conditions: biaryl (0.13 mmol), FeCl3 (5 mol%), in dichloroethane (0.1M), rt, 1–12 h;
reaction heated to 50°C;
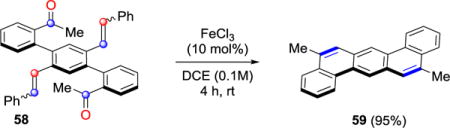
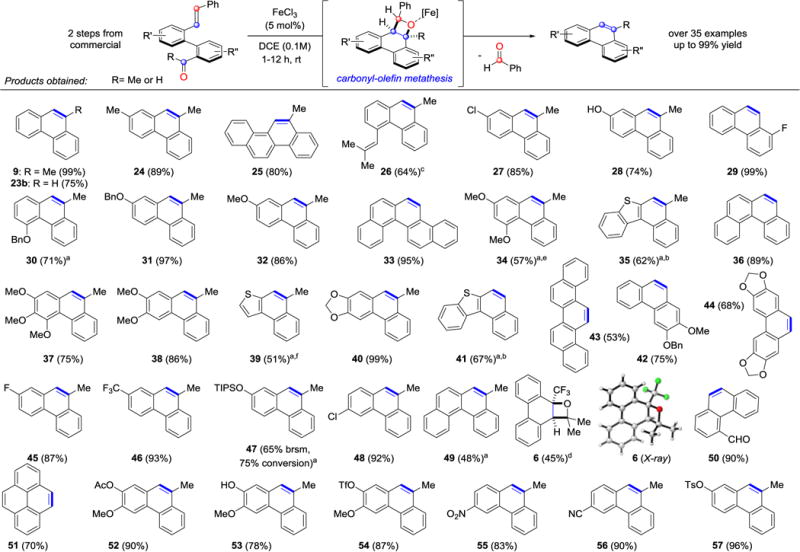
In addition to methyl ketone 22a and aldehyde 22b, substrates bearing sterically demanding isopropyl (22c) and tert-butyl (22d) moieties formed the alkylated phenanthrenes in 79% and 55%, respectively, although the latter required elevated temperatures for efficient conversion (entries 3 and 4, Table 3). Phenyl and naphthyl substituted carbonyl substrates (22e and 22f) were able to undergo metathesis in efficient yields (entries 5 and 6, Table 3). Importantly, biaryl enone 22g led to the corresponding polycycle 23g incorporating an exocyclic alkene as a functional handle in 50% yield, albeit at elevated temperatures (entry 7, Table 3). Additionally, β-ketoester 22h resulted in the formation of metathesis product 23h in satisfactory yield (72%), while electron-deficient trifluoromethyl ketone 22i also proved viable as a substrate converting to 9-trifluoromethyl phenanthrene 23i in 52% (entries 8 and 9, Table 3). Various PAC frameworks were accessible utilizing the optimal reaction conditions (Table 4). Upon subjection to metathesis conditions, the desired PACs were obtained with benzaldehyde as the corresponding byproduct. Electron-deficient phenanthrenes bearing halogen, trifluoromethyl, nitro, or nitrile substitution were formed in yields greater than 85% (27, 29, 45, 46, 55 and 56, Table 4). Similarly, electron-rich substrates incorporating methoxy or benzyl ether functionalities underwent the desired transformation in excellent yields (30, 31, 32, 38, 42, Table 4). However, diminished yields of 75% and 57% were observed for substrates bearing ortho-methoxy substitution (34 and 37, Table 4). Dioxoles 40 and 44 were formed in 99% and 68% yield, respectively, under the optimized reaction conditions. Moreover, sulfur-containing heterocycles proved viable substrates for metathesis and resulted in the formation of thiophene 39 and benzothiophenes 35 and 41 in good yields. Alternative strategies to these structural motifs are currently hampered by harsh reaction conditions and competing reaction pathways resulting in low overall yields.23 Unprotected phenols as well as aldehydes readily underwent metathesis resulting in the formation of phenanthrene 28 or aldehyde 50 in 74% and 90% yield, respectively. Furthermore, extended PACs are accessible employing this metathesis strategy. Specifically, methylchrysene 25 is generated in 80% yield, while benzo(c)phenanthrene 36 is accessible in 89% yield from the respective biaryl aldehyde (Table 4). Notably, dibenz[a,h]anthracene 59 is afforded in excellent yield via biscarbonyl-olefin metathesis (eq 1).
 |
(1) |
Table 4.
Scope of the iron(III)-catalyzed carbonyl-olefin metathesis reaction for the synthesis of PACs.
|
Conditions: biaryl (0.13 mmol), FeCl3 (5 mol%), in DCE (0.1M), rt, 1–12 h;
reaction heated to 50°C;
reaction was run with 20 mol% catalyst loading;
starting material is bis-prenylated biaryl ketone (see Supporting Information For details);
substrate is the prenylated analog of 22i; reaction was run in toluene as solvent;
starting material is reisolated;
substrate decomposition was observed at the elevated reaction temperatures;
low solubility in organic solvents.
Interestingly, when the prenylated analog of 22i was converted under the optimized reaction conditions, no formation of the desired carbonyl-olefin metathesis product 23i was observed. Oxetane 6 was identified as the major product (45% yield, Table 4). This result supports our hypothesis that iron(III)-catalyzed carbonyl-olefin metathesis reactions do proceed via oxetanes as reactive intermediates.18
The development of a new approach toward the synthesis of polyaromatic hydrocarbons is reported relying on the design principle of an iron(III)-catalyzed carbonyl-olefin metathesis reaction. This strategy is characterized by its operational simplicity, mild reaction conditions, as well as chemo- and regioselectivity. Analysis of the two reaction partners (olefin and carbonyl) revealed that the respective olefin moieties can readily couple to a variety of differentiated aryl-ketones or aryl aldehydes to garner the corresponding functionalized PACs as metathesis products. Isolation of aryl oxetane 6 supports the notion that this new strategy for the synthesis of polyaromatic hydrocarbons does indeed proceed via oxetanes as reactive intermediates.18
Supplementary Material
Acknowledgments
We thank the Petroleum Research Fund (PRF#54688-DNI1), the University of Michigan Office of Research and the NIH/National Institute of General Medical Sciences (GM118644) for financial support. P.S.R. thanks Eli Lilly for a summer predoctoral fellowship. We thank Dr. Jeff W. Kampf and Ren Wiscons for X-ray crystallographic studies.
Footnotes
Supporting Information
Experimental data as well as 1H and 13C NMR spectra for all new compounds prepared in the course of these studies are provided in the Supporting Information to this manuscript. The material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a) Harvey RG. Polycyclic Aromatic Hydrocarbons. Wiley-VCH; New York: 1997. [Google Scholar]; b) Gingras M. Chem Soc Rev. 2013;42:968. doi: 10.1039/c2cs35154d. [DOI] [PubMed] [Google Scholar]; c) Gingras M, Felix G, Peresutti R. Chem Soc Rev. 2013;42:1007. doi: 10.1039/c2cs35111k. [DOI] [PubMed] [Google Scholar]; d) Gingras M. Chem Soc Rev. 2013;42:1051. doi: 10.1039/c2cs35134j. [DOI] [PubMed] [Google Scholar]; e) Floyd AJ, Dyke SF, Ward SE. Chem Rev. 1976;76:509. [Google Scholar]; For additional examples, see:; e) Uchida K, Ito S, Nakano M, Abe M, Kubo T. J Am Chem Soc. 2016;138:2399. doi: 10.1021/jacs.5b13033. [DOI] [PubMed] [Google Scholar]; f) Ji F, Li X, Wu W, Jiang H. J Org Chem. 2014;79:11246. doi: 10.1021/jo502013s. [DOI] [PubMed] [Google Scholar]
- 2.Wigglesworth TJ, Sud D, Norsten TB, Lekhi VS, Branda NR. J Am Chem Soc. 2005;127:7272. doi: 10.1021/ja050190j. [DOI] [PubMed] [Google Scholar]
- 3.Furche F, Ahlrichs R, Wachsmann C, Weber E, Sobanski A, Vögtle F, Grimme S. J Am Chem Soc. 2000;122:1717. [Google Scholar]
- 4.Xu Y, Zhang YX, Sugiyama H, Umano T, Osuga H, Tanaka K. J Am Chem Soc. 2004;126:6566. doi: 10.1021/ja0499748. [DOI] [PubMed] [Google Scholar]
- 5.a) Lovinger AJ, Nuckolls C, Katz TJ. J Am Chem Soc. 1998;120:264. [Google Scholar]; b) Zöphel L, Enkelmann V, Müllen K. Org Lett. 2013;15:804. doi: 10.1021/ol303476g. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs A, Vasas A, Hohmann J. Phytochemistry. 2008;69:1084. doi: 10.1016/j.phytochem.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 7.For representative examples, see:; a) Knowles RR, Lin S, Jacobsen EN. J Am Chem Soc. 2010;132:5030. doi: 10.1021/ja101256v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Narcis MJ, Takenaka N. Eur J Org Chem. 2014;1:21. [Google Scholar]
- 8.Dreher SD, Katz TJ, Lam KC, Rheingold AL. J Org Chem. 2000;65:815. doi: 10.1021/jo001055m. [DOI] [PubMed] [Google Scholar]
- 9.McMurry JE, Lectka T, Rico JG. J Org Chem. 1989;54:3748. [Google Scholar]
- 10.Dubois F, Gingras M. Tetrahedron Lett. 1998;39:5039. [Google Scholar]
- 11.a) Flammang-Barbieux M, Nasielski J, Martin RH. Tetrahedron Lett. 1967;8:743. [Google Scholar]; b) Liu L, Yang B, Katz TJ, Poindexter MK. J Org Chem. 1991;56:3769. [Google Scholar]; c) Martin RH. Angew Chem Int Ed. 1974;13:649. [Google Scholar]
- 12.For homolytic aromatic substitution strategy, see; Harrowven DC, Guy IL, Nanson L. Angew Chem Int Ed. 2006;45:2242. doi: 10.1002/anie.200504287. [DOI] [PubMed] [Google Scholar]
- 13.a) Carreño MC, González-López M, Urbano A. Chem Comm. 2005;5:611. doi: 10.1039/b413879a. [DOI] [PubMed] [Google Scholar]; b) Liu L, Katz TJ. Tetrahedron Lett. 1990;31:3983. [Google Scholar]; c) Katz TJ, Liu L, Willmore ND, Fox JM, Rheingold AL, Shi S, Nuckolls C, Rickman BH. J Am Chem Soc. 1997;119:10054. [Google Scholar]; d) Fox JM, Goldberg NR, Katz TJ. J Org Chem. 1998;63:7456. doi: 10.1021/jo981380y. [DOI] [PubMed] [Google Scholar]; e) Dreher SD, Weix DJ, Katz TJ. J Org Chem. 1999;64:3671. doi: 10.1021/jo990065o. [DOI] [PubMed] [Google Scholar]
- 14.a) Harrowven DC, Guy IL, Nanson L. Angew Chem Int Ed. 2006;45:2242. doi: 10.1002/anie.200504287. [DOI] [PubMed] [Google Scholar]; b) Harrowven DC, Nunn MIT, Fenwick DR. Tetrahedron Lett. 2002;43:7345. [Google Scholar]
- 15.For examples, see:; a) Fürstner A, Mamane V. J Org Chem. 2002;67:6264. doi: 10.1021/jo025962y. [DOI] [PubMed] [Google Scholar]; b) Mamane V, Hannen P, Fürstner A. Chem Eur J. 2004;10:4556. doi: 10.1002/chem.200400220. [DOI] [PubMed] [Google Scholar]; c) Komeyama K, Igawa R, Takaki K. Chem Comm. 2010;46:1748. doi: 10.1039/b920695g. [DOI] [PubMed] [Google Scholar]; d) Chernyak N, Gevorgyan V. J Am Chem Soc. 2008;130:5636. doi: 10.1021/ja8006534. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Chernyak N, Gevorgyan V. Adv Synth Catal. 2009;351:1101. doi: 10.1002/adsc.200800765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia Y, Liu Z, Xiao Q, Qu P, Ge R, Zhang Y, Wang J. Angew Chem Int Ed. 2012;51:5714. doi: 10.1002/anie.201201374. [DOI] [PubMed] [Google Scholar]
- 17.a) Iuliano A, Piccioli P, Fabbri D. Org Lett. 2004;6:3711. doi: 10.1021/ol048668w. [DOI] [PubMed] [Google Scholar]; b) Donohoe TJ, Orr AJ, Bingham M. Angew Chem Int Ed. 2006;45:2664. doi: 10.1002/anie.200503512. [DOI] [PubMed] [Google Scholar]
- 18.This work was first reported as; a) Ludwig JR, Gianino JB, Schindler C. Metal-catalyzed carbonyl-olefin metathesis. Abstracts of Papers, 250th ACS National Meeting & Exposition; Boston, MA, United States. August 16th–20th, 2015; Proceedings of the American Chemical Society; Boston, MA. August 16th–20th; Washington, DC: American Chemical Society; 2015. p. 1328933. ORGN-447. [Google Scholar]; b) Ludwig JR, Zimmerman PM, Gianino JB, Schindler CS. Nature. 2016;533:374. doi: 10.1038/nature17432. [DOI] [PubMed] [Google Scholar]
- 19.For metal-mediated carbonyl-olefin metathesis reactions, see:; a) Schopov I, Jossifov C. Makromol Chem Rapid Commun. 1983;4:659. [Google Scholar]; b) Fu GC, Grubbs RH. J Am Chem Soc. 1993;115:3800. [Google Scholar]; For carbonyl-olefin metathesis reactions proceeding via oxetan photoadducts, see:; c) Jones G, II, Schwartz SB, Marton MT. J Chem Soc Chem Comm. 1973;11:374. [Google Scholar]; d) Jones G, II, Acquadro MA, Carmody MA. J Chem Soc Chem Comm. 1975;6:206. [Google Scholar]; e) Carless HAJ, Trivedi HS. J Chem Soc Chem Commun. 1979;8:382. [Google Scholar]; f) D’Auria M, Racioppi R, Viggiani L. Photochem Photobiol Sci. 2010;9:1134. doi: 10.1039/c0pp00076k. [DOI] [PubMed] [Google Scholar]; g) Pérez-Ruiz R, Gil S, Miranda MA. J Org Chem. 2005;70:1376. doi: 10.1021/jo048708+. [DOI] [PubMed] [Google Scholar]; h) Pérez-Ruiz R, Miranda MA, Alle R, Meerholz K, Griesbeck AG. Photochem Photobiol Sci. 2006;5:51. doi: 10.1039/b513875b. [DOI] [PubMed] [Google Scholar]; i) Valiulin RA, Arisco TM, Kutateladze AG. J Org Chem. 2011;76:1319. doi: 10.1021/jo102221q. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Valiulin RA, Arisco TM, Kutateladze AG. J Org Chem. 2013;78:2012. doi: 10.1021/jo301909j. [DOI] [PMC free article] [PubMed] [Google Scholar]; For Brønsted and Lewis acid mediated carbonyl-olefin metathesis reactions, see:; k) Soicke A, Slavov N, Neudörfl J-M, Schmalz H-G. Synlett. 2011;17:2487. [Google Scholar]; l) van Schaik HP, Vijn RJ, Bickelhaupt F. Angew Chem Int Ed. 1994;33:1611. [Google Scholar]; m) Bah J, Franzén J, Naidu VR. Eur J Org Chem. 2015;8:1834. [Google Scholar]; n) Jossifov C, Kalinova R, Demonceau A. Chim Oggi. 2008;26:85. [Google Scholar]; for catalytic carbonyl-olefin metathesis reactions proceeding via (3+2)/retro-(3+2)-cycloaddition, see:; o) Griffith AK, Vanos CM, Lambert TH. J Am Chem Soc. 2012;134:18581. doi: 10.1021/ja309650u. [DOI] [PubMed] [Google Scholar]; p) Hong X, Liang Y, Griffith AK, Lambert TH, Houk KN. Chem Sci. 2014;5:471. [Google Scholar]
- 20.a) Satchell DPN, Satchell RS. Chem Rev. 1969;69:251. [Google Scholar]; b) Jensen WB. Chem Rev. 1978;78:1. [Google Scholar]
- 21.Kepp KP. Inorg Chem. 2016;55:9461. doi: 10.1021/acs.inorgchem.6b01702. [DOI] [PubMed] [Google Scholar]
- 22.In the carbonyl-olefin metathesis reaction leading to cyclopentenes and cyclohexenes, catalytic amounts of BF3·Et2O formed the metathesis products in 71% (86% conversion).
- 23.Arnáiz FJ. J Chem Educ. 1995;72:1139. [Google Scholar]
- 24.a) Sanz R, Fernández Y, Castroviejo MP, Pérez A, Fañanás FJ. J Org Chem. 2006;71:6291. doi: 10.1021/jo060911c. [DOI] [PubMed] [Google Scholar]; b) Che R, Wu Z, Li Z, Xiang H, Zhou X. Chem Eur J. 2014;20:7258. doi: 10.1002/chem.201402265. [DOI] [PubMed] [Google Scholar]; c) Qiao Z, Ge N, Jiang X. Chem Comm. 2015;51:10295. doi: 10.1039/c5cc03038b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


