Abstract
Transposable elements, known colloquially as “jumping genes,” constitute approximately 45% of the human genome. Cells utilize epigenetic defenses to limit transposable element jumping, including formation of silencing heterochromatin and generation of piwi-interacting RNAs (piRNAs), small RNAs that facilitate clearance of transposable element transcripts. Here we identify transposable element dysregulation as a key mediator of neuronal death in tauopathies, a group of neurodegenerative disorders that are pathologically characterized by deposits of tau protein in the brain. Mechanistically, we find that heterochromatin decondensation and reduction of piwi/piRNAs drive transposable element dysregulation in tauopathy. We further report a significant increase in transcripts of the endogenous retrovirus class of transposable elements in human Alzheimer’s disease and progressive supranuclear palsy, suggesting that transposable element dysregulation is conserved in human tauopathy. Taken together, our data identify heterochromatin decondensation, piwi/piRNA depletion and consequent transposable element dysregulation as a novel, pharmacologically targetable, mechanistic driver of neurodegeneration in tauopathy.
Transposable elements are categorized as Class I, the retrotransposons, or Class II, the DNA transposons. Retrotransposons are structurally akin to retroviruses in that they require an RNA intermediate to mobilize. Unlike retroviruses, however, retrotransposons lack the ability to move between individuals. DNA transposons, which mobilize via a “cut and paste” mechanism, are thought to have lost the ability to mobilize in the human genome due to imprecise excision and insertion1. Organisms ranging from yeast to humans have developed cellular control mechanisms to limit potentially deleterious transposable element activation. Many transposable elements are embedded within highly condensed constitutive heterochromatin, and are thus epigenetically silenced2. In addition, transposable element transcripts are the targets of a well-conserved pathway involving piRNAs, small regulatory RNAs that bind to transposable element transcripts and mediate their degradation3.
The “transposon theory of aging” posits that transposable elements become deleteriously activated as cellular defense and surveillance mechanisms break down with age4,5. While transposable element activation has also been implicated in cancer6, and TDP-43 mediated neurodegeneration7–9, the extent to which transposable elements are involved in human disorders and drive disease pathogenesis is currently unknown. We have previously identified tau-induced decondensation of constitutive heterochromatin as a key event that mediates neuronal death in tauopathy10. We hypothesized that tau-mediated decondensation of constitutive heterochromatin causes epigenetic de-silencing of transposable elements in the context of Alzheimer’s disease and associated tauopathies.
Beginning with a simple model of tauopathy in Drosophila melanogaster11, we report significantly altered levels of transposable element transcripts as a consequence of human tau expression in the adult brain. We identify heterochromatin decondensation and depletion of piwi/piRNAs as major mechanistic links between pathogenic tau and loss of transposable element control, and demonstrate that pathogenic tau causes active transposable element mobilization in neurons. Dietary restriction and 3TC (lamivudine), a nucleoside analog inhibitor of reverse transcriptase that is FDA-approved for the treatment of HIV and Hepatitis B, suppress tau-induced transposable element dysregulation and tau-induced neurotoxicity. Using a systematic, unbiased approach, we identify transposable elements that are differentially expressed in postmortem human brain tissue from patients with Alzheimer’s disease and progressive supranuclear palsy, a “primary” tauopathy, and find that the endogenous retrovirus class of transposable elements is increased in the context of human tauopathy. Taken together, our studies identify heterochromatin decondensation and depletion of piwi and piRNAs as key mechanisms driving transposable element dysregulation and subsequent neuronal death in tau-mediated neurodegeneration. In addition, we show that that suppression of transposable element mobilization and resulting neurodegeneration can be achieved by environmental and pharmacological intervention.
RESULTS
Drosophila models of human tauopathy have altered levels of transposable element transcripts
Drosophila melanogaster provides a genetically tractable platform that can be used to identify cellular mechanisms implicated in disease states and to determine if they are causal for the disease process. To investigate a potential role for transposable element dysregulation as a consequence of pathogenic tau, we began with a Drosophila model of tauopathy11 involving neuron-specific expression of tauR406W, a mutant form of human tau that is associated with autosomal dominant tauopathy12. Drosophila models of human tauopathy have progressive, age-associated neuronal death, a shortened lifespan, and decreased locomotor activity10,11. In addition, neuronal phenotypes of tau transgenic Drosophila mimic features of human Alzheimer’s disease and associated tauopathies, including but not limited to aberrant tau phosphorylation13, oxidative stress14, DNA damage15,16, decondensation of constitutive heterochromatin10, synaptic dysfunction17 and activation of the cell cycle in post-mitotic neurons18.
We performed 100 bp, paired-end sequencing of RNA isolated from control and tauR406W transgenic Drosophila heads at day 10 of adulthood, an age at which neuronal death and locomotor deficits are detectable in tauR406W transgenic flies, but prior to the age at which survival is at exponential decline16. We identified 50 transposable elements that are significantly increased at the transcript level in tau transgenic Drosophila compared to controls, and 60 transposable elements that are significantly decreased (Fig. 1a, unscaled heatmaps are provided in Supplementary Fig. 1, full genotypes are provided in Supplementary Table 1, raw counts are provided in Supplementary Table 2). For several subgroups of transposable elements, we found that multiple members of the same subgroup, such as copia, HeT-A, and Quasimodo, are increased in tauR406W transgenic Drosophila, while members of other subgroups, such as Burdock and Blood, are decreased in tauR406W transgenic Drosophila. These data suggest that aberrant expression of transposable elements in tauopathy is a regulated, rather than stochastic, process. The most abundant class of differentially expressed elements in tauopathy are Class I long terminal repeat (LTR) retrotransposons, despite the fact that the majority of transposable elements in Drosophila are classified as Class II DNA transposons (Fig. 1b).
Figure 1 |. Transposable element transcription in tauR406W transgenic Drosophila.
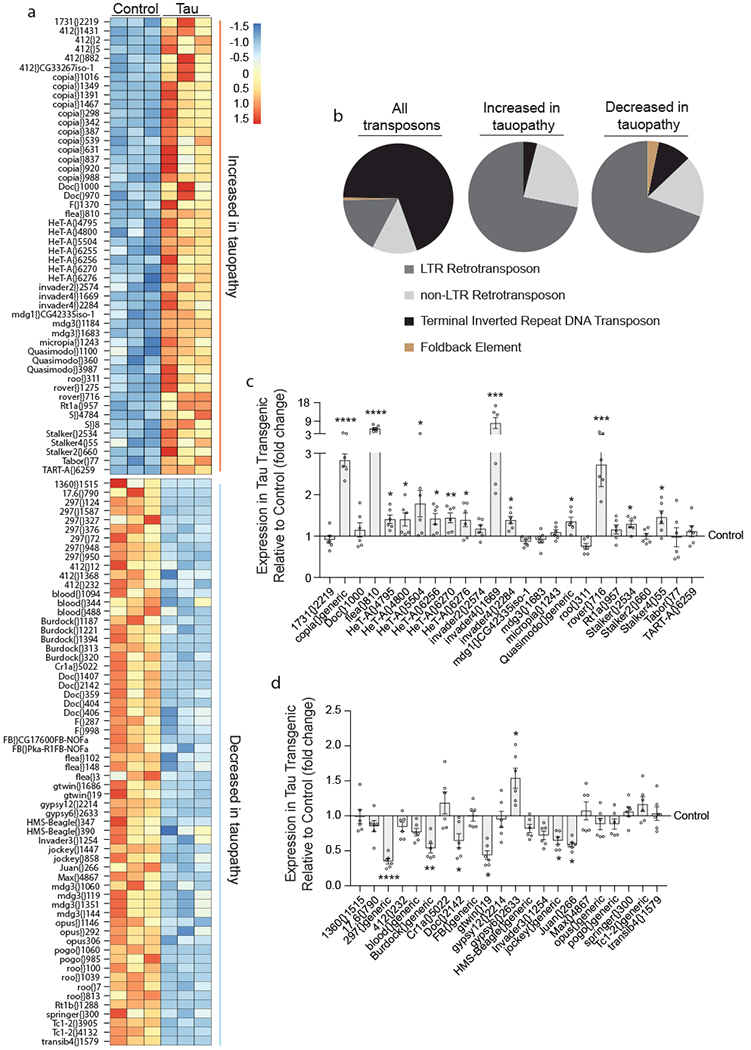
a, Transposable element transcripts that are differentially expressed in tauR406W transgenic Drosophila heads versus control based on RNA-seq (two-sided Wald test, FDR, P<0.01, n=3 biologically independent replicates, each consisting of RNA pooled from six heads). b, Pie charts depicting all classes of transposable elements in Drosophila, and classes of transposable elements that are increased or decreased in tauR406W transgenic Drosophila. NanoString-based validation of transposable element transcripts that are increased in tauopathy based on RNA-seq (c), and transposable elements transcripts that are decreased in tauR406W transgenic Drosophila based on RNA-seq (d), n=6 biologically independent replicates each consisting of RNA pooled from 6 heads, values are relative to control, which was set to 1. Unpaired, two-tailed Student’s t-test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Values are mean ± s.e.m. All flies are 10 days old. Full genotypes are listed in Supplementary Table 1. Transposable elements recognized by “generic” probes are listed in Supplementary Table 4.
The complexity and repetitive nature of transposable elements presents challenges to RNA-seq analysis, which is associated with a greater frequency of false positives and negatives compared to analysis of canonical messenger RNAs. As secondary validation of our RNA-seq analyses, we prepared a custom NanoString codeset consisting of a panel of probes recognizing transposable elements that were identified as differentially expressed in tau transgenic Drosophila based on RNA-seq (Supplementary Table 3). NanoString technology combines transcript-specific color-coded barcodes with fluorescent imaging to sensitively quantify transcript levels19. When possible, we created “generic” NanoString probes to recognize the differentially expressed transposable elements within a transposable element subgroup (Supplementary Table 4). While a calculation of the fold-change estimate for each element generated by RNA-seq versus the fold-change for NanoString (Supplementary Fig. 1b) suggests a moderate to strong relationship between RNA-seq and NanoString, not all transposable elements that were called as differentially expressed in tau transgenic Drosophila based on RNA-seq reach statistical significance based on NanoString analysis. 14 of 25 probes were confirmed by NanoString as significantly increased (Fig. 1c), while 6 of 22 probes were confirmed as significantly decreased in heads of tauR406W transgenic Drosophila (Fig. 1d). These analyses also revealed that the transposable elements transcripts that are increased in response to pathogenic tau generally have a greater magnitude of change than transposable element transcripts that are decreased in response to pathogenic tau.
We hypothesized that aberrant transposable element expression is relevant to the larger group of tauopathies, including Alzheimer’s disease, that are pathologically defined by deposition of wild-type tau in the brain. To test this hypothesis, we assayed transposable element transcript levels by NanoString in Drosophila expressing human wild-type tau (Supplementary Fig. 2a), which induces neuronal death in Drosophila11, albeit to a lesser extent than tauR406W. Multiple previous studies report that expression of human wild-type or R406W mutant tau involve the same major mechanisms of tau-induced neurotoxicity in Drosophila models10,16,20. Pan-neuronal expression of tauWT significantly increases 10 of 25, and decreases 8 of 22 probes recognizing transposable elements that were identified as increased or decreased, respectively, in tauR406W Drosophila based on RNA-seq (Supplementary Fig. 2b, c), suggesting that aberrant transposable element expression is relevant to the greater family of sporadic tauopathies that involve only wild-type tau.
Loss of transposable element silencing mediates tau-induced neurotoxicity in Drosophila.
RNA-seq and NanoString analyses clearly demonstrate that pathogenic tau disrupts baseline levels of transposable element transcripts in the brain. Transposable element activation is classically considered a deleterious event, as mobilization can cause genomic instability21. It is now understood, however, that transposable element RNAs have regulatory roles within the cell1. In addition, active transposable element mobilization during neurogenesis is thought to positively contribute to somatic diversification22. To establish if dysregulation of transposable element expression in the adult brain is beneficial, detrimental, or neutral in the context of tauopathy we determined if genetic manipulation of flamenco, a locus in Drosophila that is known to restrict transposable element mobilization, mediates tauR406W-induced neurotoxicity. Homozygous “permissive” loss of function alleles of flamenco allow transposable element mobilization and increase transposable element copy number within the Drosophila genome23,24. Two different heterozygous loss of function alleles of flamenco23,24 do not induce neuronal death or locomotor deficits in controls, but significantly enhance neuronal death in tauR406W transgenic Drosophila (Fig. 2a) and exacerbate tau-induced locomotor deficits (Fig. 2b). Importantly, flamenco mutations do not affect total protein levels of transgenic tau (Supplementary Fig. 3a).
Figure 2 |. Loss of function mutations in the flamenco locus enhance tauR406W-induced neurotoxicity.
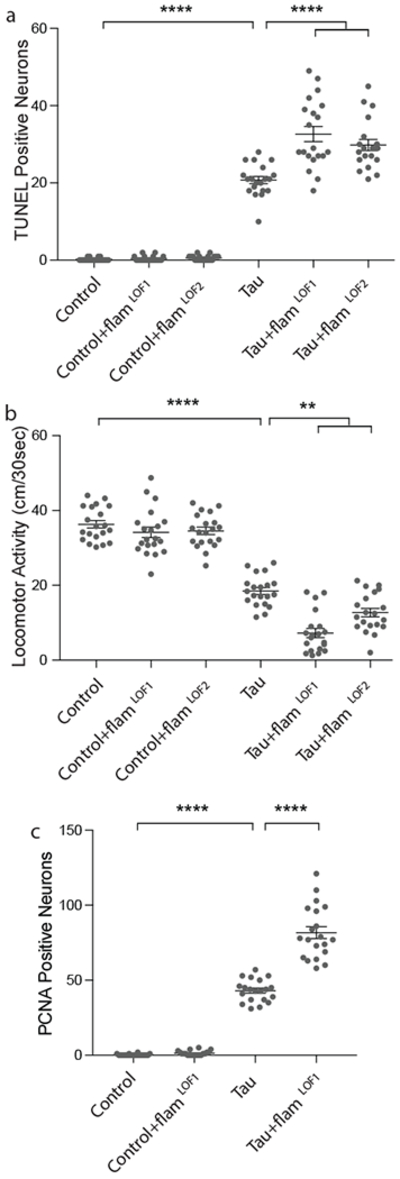
Compared to tauR406W expressed alone, tauR406W transgenic Drosophila harboring loss of function mutations in the flamenco locus have a, increased neuronal death, based on TUNEL (one-way ANOVA with Tukey’s multiple comparison test) and b, reduced locomotor activity (one-way ANOVA with Tukey’s multiple comparison test), and c, increased activation of the cell cycle based on PCNA staining (one-way ANOVA with Tukey’s multiple comparison test). n=20 animals per genotype, per assay. All flies are 10 days old. Values are mean ± s.e.m. n=20 animals per genotype, per assay, **P=0.005, ***P<0.001, ****P<0.0001. Full genotypes are listed in Supplementary Table 1.
Ectopic expression of proteins associated with aberrant activation of the cell cycle in post-mitotic neurons is a well-described feature of human tauopathy25. Studies in Drosophila indicate that cell cycle activation is causal for neuronal death in tauopathy, and that activation of the cell cycle in neurons is sufficient to induce neuronal death18. We find that heterozygous loss of flamenco function exacerbates tau-induced activation of the cell cycle in neurons based on staining with an antibody recognizing proliferating cell nuclear antigen (PCNA) (Fig. 2c). Taken together, these data suggest that loss of transposable element silencing in tau transgenic Drosophila is causally linked to neuronal death and promotes neuronal death through aberrant activation of the cell cycle in post-mitotic neurons.
The flamenco locus harbors piRNAs that specifically degrade gypsy, Idefix, and ZAM transposable element transcripts24, among others. To determine if flamenco mutation affects the specific panel of transposable elements that are aberrantly expressed in tauR406W transgenic Drosophila, we performed NanoString analyses on flamenco loss of function mutants (Supplementary Fig. 3). Rather than a general effect on the panel of transposable elements that are dysregulated by pathogenic tau, these analyses suggest that enhancement of tau-induced neurotoxicity by flamenco loss of function is due to activation of specific elements affected by pathogenic tau, and/or additional elements outside of our NanoString codeset.
Piwi/piRNA depletion is a mechanistic driver of transposable element transcription in tauopathy
Since flamenco is known to encode a major piRNA cluster3,26, and piRNAs have recently been reported to be differentially expressed in the human Alzheimer’s disease brain27,28, we next determined if piRNAs are involved in dysfunctional transposable element control in the tauopathy brain. Small RNA sequencing revealed a significant decrease in 46 of 50 differentially expressed piRNAs in heads of tau transgenic Drosophila at day 10 of adulthood (Fig. 3a, Supplementary Table 5, 6). In addition, protein levels of piwi, a central regulator of piRNA biogenesis, are depleted in brains of tau transgenic Drosophila based on both immunofluorescence (Fig. 3b) and western blotting (Fig. 3c).
Figure 3 |. Decreased expression of piwi and piRNAs mediate pathogenic tauR406W-induced increase in transposable element transcripts and drive neuronal death.
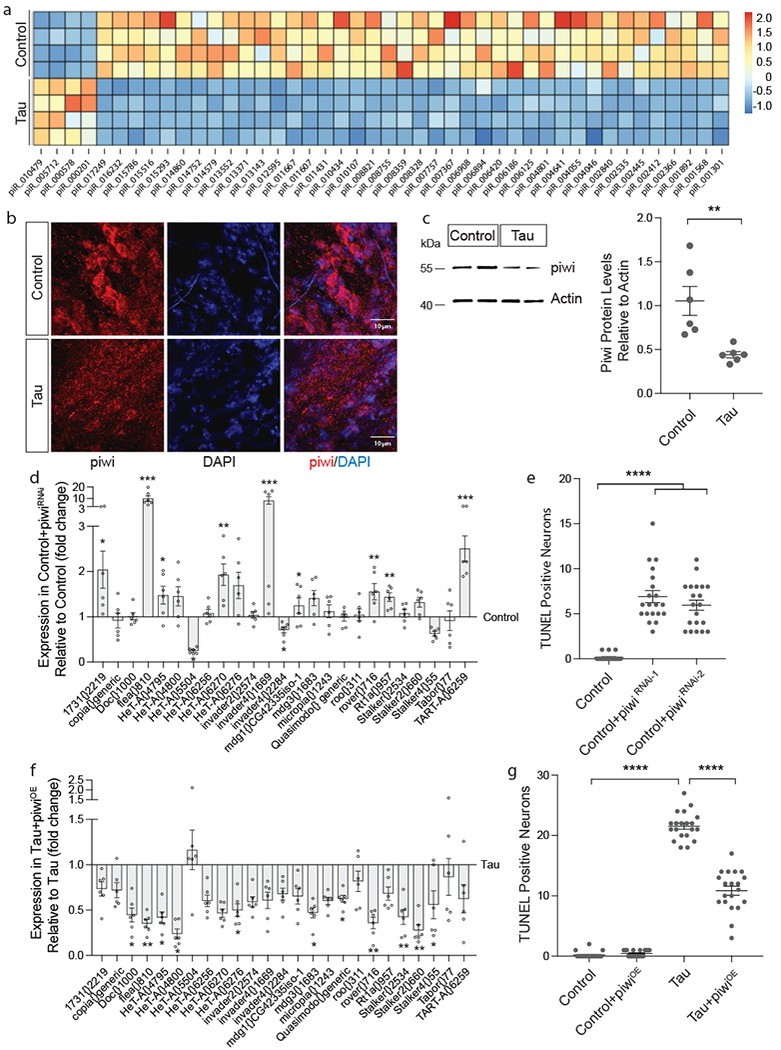
a, Heatmap reflecting fold change of piRNAs that are differentially expressed in tauR406W transgenic Drosophila heads versus controls based on small RNA-seq (two-sided Wald test, FDR, p<0.01, n=4 biologically independent replicates, each consisting of RNA pooled from 6 heads). b, Decreased levels of piwi protein (red) in cortex of the tauR406W transgenic Drosophila brain based on immunostaining and c, western blotting (unpaired, two-tailed Student’s t-test, **P=0.005, n=6 animals per genotype). In b, piwi immunostaining was repeated in 6 animals of each genotype with similar results. Western blot is cropped in c, full blot presented in Supplementary Figure 10. d, NanoString analysis of transposable element expression in response to RNAi-mediated knockdown of piwi versus control (unpaired, two-tailed Student’s t-test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, n=6 biologically independent replicates each consisting of RNA pooled from 6 heads, values are relative to control, which was set to 1). e, Neuronal death assayed by TUNEL in Drosophila caused by pan-neuronal RNAi-mediated knockdown of piwi (one-way ANOVA with Tukey’s multiple comparison test, ****P<0.0001, n=20 animals per genotype). f, NanoString analysis of transposable element expression in response to pan-neuronal piwi overexpression in tauR406W transgenic Drosophila versus tau expressed alone (unpaired, two-tailed Student’s t-test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, n=6 biologically independent replicates each consisting of RNA pooled from 6 heads, values are relative to tau expressed alone, which was set to 1). g, Neuronal death assayed by TUNEL in tauR406W transgenic Drosophila with pan-neuronal piwi overexpression (one-way ANOVA with Tukey’s multiple comparison test, ****P<0.0001, n=20 animals per genotype). All flies are 10 days old. Values are mean ± s.e.m. Full genotypes are listed in Supplementary Table 1. Transposable elements recognized by “generic” probes are listed in Supplementary Table 4.
While the function of piwi in regard to piRNA production and transposable element silencing is well established in the germline29, and piRNAs are known to exist in the Drosophila and mammalian brain30,31, it is currently unknown if piwi reduction affects transposable element transcripts in the brain. To directly quantify the effects of piwi reduction on the panel of transposable elements that are differentially expressed in tauR406W transgenic Drosophila, we utilized our custom NanoString codeset to assay transposable element transcript levels in response to pan-neuronal RNAi-mediated piwi knockdown in the absence of transgenic tau. These data indicate that pan-neuronal piwi knockdown (Supplementary Fig. 4a) is sufficient to elevate transcript levels of most of the transposable elements that are increased in tauR406W transgenic Drosophila (Fig. 3d), suggesting that tau-induced reduction of piwi and piRNAs is a major contributor to the transposable element expression profile in brains of tau transgenic Drosophila.
We next determined if piwi depletion is a causal mediator of neuronal death in tauopathy. RNAi-mediated piwi knockdown using two different non-overlapping RNAi lines is semi-lethal in the context of transgenic tau expression. In the absence of tau, piwi knockdown is sufficient to induce neuronal death by day 10 of adulthood (Fig. 3e). Based on PCNA staining, pan-neuronal piwi reduction is also sufficient to activate the cell cycle in neurons (Supplementary Fig. 4b), suggesting that pathogenic tau acts through piwi reduction to mediate aberrant cell cycle activation and consequent neuronal death.
We next determined if pan-neuronal overexpression of piwi32 reduces aberrant transposable element expression and neuronal death in brains of tau transgenic Drosophila. Compared to tau expressed alone, piwi overexpression significantly reduces transposable element transcripts that are elevated in tau transgenic Drosophila (Fig. 3f). In further support of piwi reduction as a causal contributor to tau-induced neurotoxicity, pan-neuronal piwi overexpression significantly reduces neuronal death in tau transgenic Drosophila (Fig. 3g) without affecting total levels of transgenic tau protein (Supplementary Fig. 4c). These data suggest that tau-induced piwi reduction depletes piRNAs, which significantly increases transposable element transcripts and causally contributes to tau-induced neurotoxicity.
Decondensation of heterochromatin contributes to aberrant transposable element transcription in the adult Drosophila brain
We have previously reported widespread relaxation of constitutive heterochromatin, a form of hyper-condensed DNA, in postmortem brains from patients with Alzheimer’s disease, which is the most common tauopathy, and in Drosophila and mouse models of tauopathy10. Tau-induced heterochromatin relaxation plays a causal role in mediating neurodegeneration, as loss of function mutations in Su(var)20533 and Su(var)3-934, genes encoding heterochromatin protein 1 (HP1) and a histone 3, lysine 9 methyltransferase, respectively, further deplete constitutive heterochromatin in tau transgenic Drosophila and enhance tau-induced neuronal death10.
Based on previous studies that identified constitutive heterochromatin as a silencing mechanism for transposable elements35, we next determined if genetically promoting heterochromatin relaxation affects the transposable elements that are differentially expressed in tau transgenic Drosophila. NanoString analyses reveal that genetically promoting heterochromatin relaxation by loss of function mutations in Su(var)205 or Su(var)3-9 causes an increase in most of the transposable element transcripts that were identified as significantly increased in tau transgenic Drosophila based on RNA-seq (Supplementary Fig. 5a, b). In the context of our previous report identifying tau-induced heterochromatin decondensation as a central mediator of neurotoxicity in tauopathy, these data suggest that tau-induced heterochromatin decondensation contributes to transposable element dysregulation in brains of tau transgenic Drosophila.
Active transposable element mobilization in neurons of tau transgenic Drosophila
56% of the transposable element transcripts that are significantly increased in tau transgenic Drosophila are full-length Class I retrotransposons that are fully capable of mobilization. As transcription of DNA to RNA is the first step in retrotransposon mobilization, we next determined if transposable elements actively mobilize in the context of tauopathy. Specifically, we utilized the “gypsy-TRAP” reporter, a GFP-based reporter of copia and/or gypsy insertion into the ovo locus that was developed to detect transposable element mobilization4. Because the gypsy-TRAP reporter utilizes the GAL4-UAS system that we normally use to express transgenic tau pan-neuronally, we instead utilized an existing tauopathy model that relies on direct fusion of the human tau transgene to GMR, a retinal neuron driver. These flies harbor the V337M-disease-associated12 form of human mutant tau.
Neuronal death in Drosophila models of tauopathy is age-dependent. Accordingly, we do not detect transposable element mobilization in tauV337M transgenic Drosophila based on either gypsy-TRAP GFP reporter fluorescence or immunoblotting at day one of adulthood, but detect a significant increase of transposable element mobilization in tau transgenic Drosophila based on both GFP fluorescence and immunoblotting at day ten of adulthood. The significant increase in transposable element mobilization in tauopathy compared to controls is sustained in 40-day-old adults (Fig. 4a, b).
Figure 4 |. Active mobilization of transposable elements in neurons of tau transgenic Drosophila.
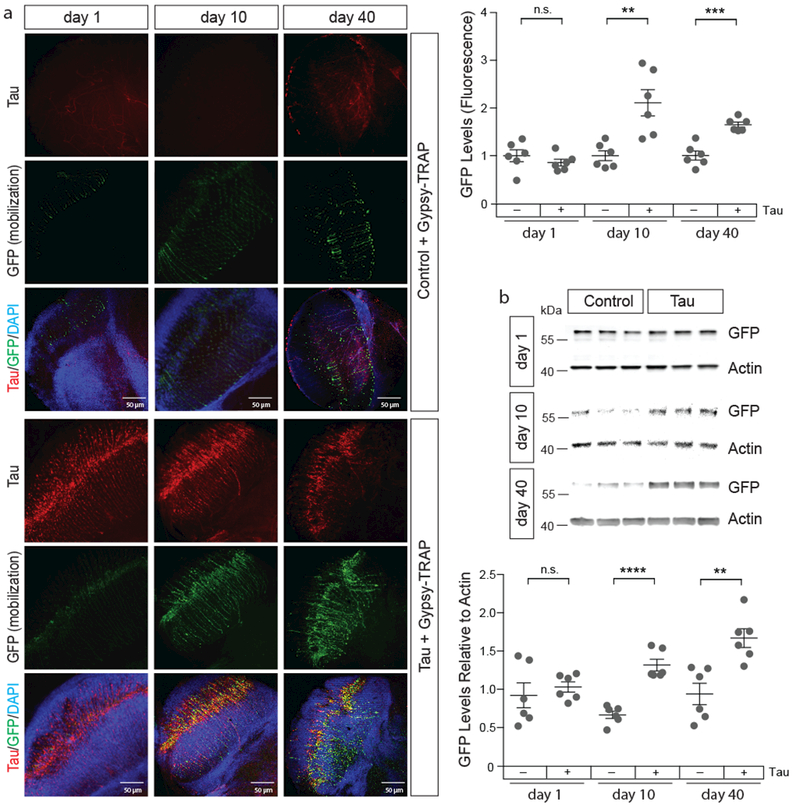
a, Gypsy-TRAP (GFP, green), a reporter of transposable element mobilization, is activated in retinal neurons of 10- and 40-day old tauV337M transgenic Drosophila compared to control. Brains were stained with the cTau antibody (red) to recognize transgenic tau (unpaired, two-tailed Student’s t-test, n=6 animals per genotype, per age, n.s.=not significant, **P=0.004, ***P=0.0001). b, Quantification of gypsy-TRAP activation based on western blotting with an antibody recognizing GFP (unpaired, two-tailed Student’s t-test, n.s.=not significant, **P=0.003, ****P<0.0001). Western blot is cropped, full blot presented in Supplementary Figure 10. n=6 animals per genotype, per age. Values are mean ± s.e.m. Full genotypes are listed in Supplementary Table 1.
Dietary restriction and inhibition of reverse transcriptase protect against tau-induced transposable element dysregulation and suppress neurotoxicity in tau transgenic Drosophila
Dietary restriction extends lifespan in invertebrate and vertebrate systems36, and reduces age-related transposable element mobilization events in the Drosophila fat body5. Using the gypsy-TRAP GFP-based reporter of transposable element mobilization4, we find that 66% dietary restriction reduces tau-induced transposable element mobilization in adult Drosophila neurons (Fig. 5a, b). Based on NanoString, dietary restriction significantly reduces the copia family at the transcript level, as well as several other transposable elements that are significantly increased in tau transgenic Drosophila (Supplementary Fig. 6). TUNEL reveals that dietary restriction suppresses tau-induced neuronal death (Fig. 5c).
Figure 5 |. Dietary restriction significantly suppresses tau-induced transposable element mobilization and tau-induced neurotoxicity in Drosophila.
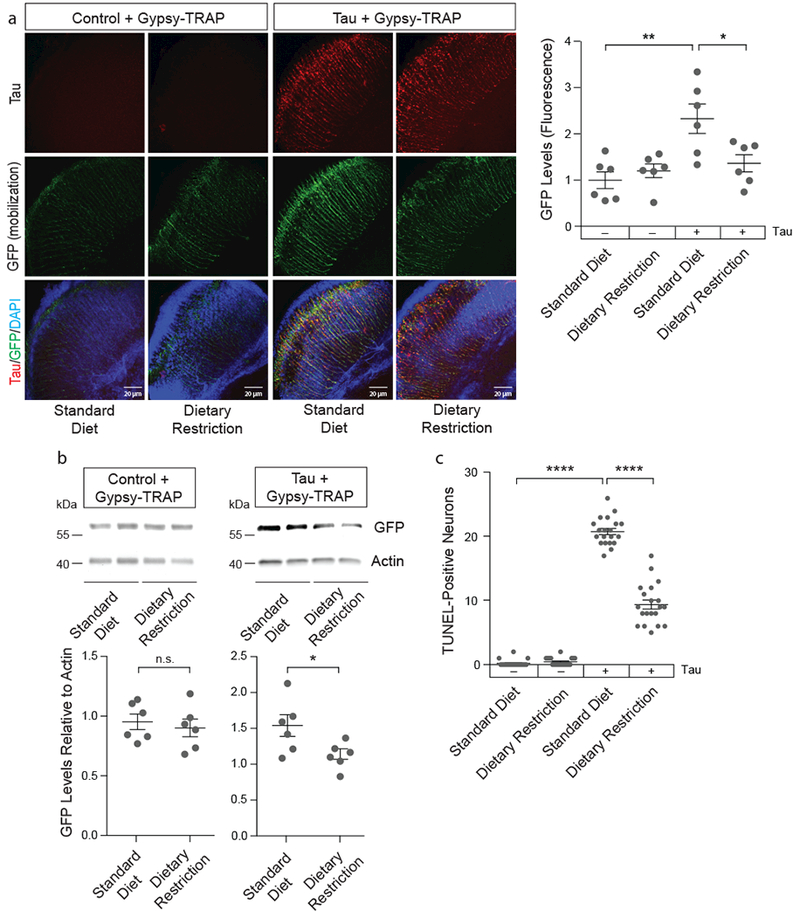
a, 66% dietary restriction reduces gypsy-TRAP reporter activation in retinal neurons of tauV337M transgenic Drosophila based on GFP fluorescence (one-way ANOVA with Tukey’s multiple comparison test, *P=0.03, **P=0.002, n=6 animals per genotype, per treatment) and b, western blotting (unpaired, two-tailed Student’s t-test, n.s.=not significant, *P=0.04, n=6 animals per genotype, per treatment). Western blot is cropped in b, full blot presented in Supplementary Figure 10. c, 66% dietary restriction significantly reduces tauR406W-induced neuronal death based on TUNEL (one-way ANOVA with Tukey’s multiple comparison test, ****P<0.0001, n=20 animals per genotype, per treatment). All flies are 10 days old. Values are mean ± s.e.m. Full genotypes are listed in Supplementary Table 1.
Having established that tau-induced transposable element dysregulation is amenable to suppression, we took a pharmacological approach to reduce transposable element mobilization in tauopathy. Like retroviruses, retrotransposons encode machinery, including a capsid protein, polymerase, integrase, and reverse transcriptase, needed to copy themselves and insert the new copy into the genome37. 3TC (Lamivudine) is a water-soluble nucleoside analog inhibitor of reverse transcriptase38 that is FDA-approved for treatment of HIV/AIDS and Hepatitis B, both of which are caused by retroviruses. Like dietary restriction, 3TC is reported to reduce age-associated transposable element mobilization in the Drosophila fat body5. Tau transgenic flies treated with 10 mM 3TC have significantly reduced gypsy-TRAP reporter activation in the brain (Fig. 6a, b). In addition, 3TC treatment significantly reduces tau-induced neuronal death (Fig. 6c), and significantly alleviates tau-induced locomotor deficits (Fig. 6d) in a dose-dependent manner (Supplementary Fig. 7a, b). Taken together, these data provide additional evidence that transposable element dysregulation is causal for the disease process in tauopathy and is responsive to environmental and pharmacological inhibition.
Figure 6 |. 3TC (Lamivudine), an FDA-approved nucleoside analog reverse transcriptase inhibitor, suppresses tau-induced transposable element mobilization and tau-induced neurotoxicity in Drosophila.
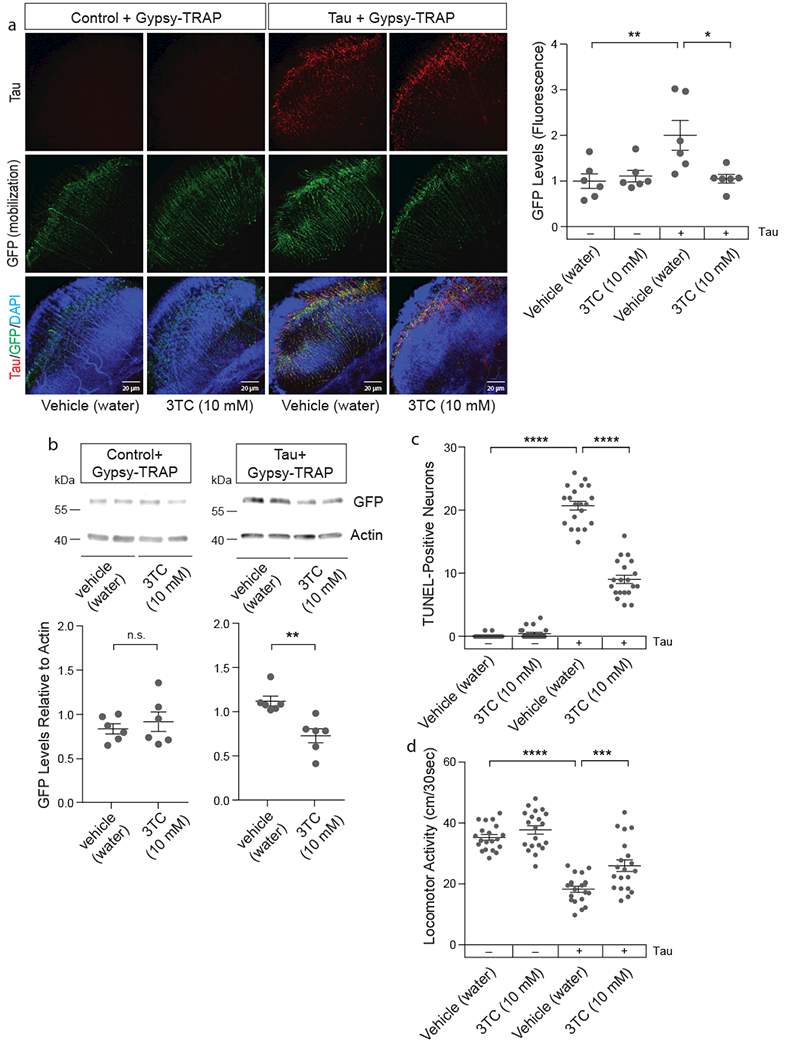
a, 10 mM 3TC reduces gypsy-TRAP reporter activation in retinal neurons of tauV337M transgenic Drosophila based on GFP fluorescence (one-way ANOVA with Tukey’s multiple comparison test, *P=0.01, **P<0.01, n=6 animals per genotype, per treatment) and b, western blotting (unpaired, two-tailed Student’s t-test, n.s.=not significant, **P=0.003, n=6 animals per genotype, per drug treatment). Western blot is cropped in b, full blot presented in Supplementary Figure 10. c, 10 mM 3TC significantly reduces tauR406W-induced neuronal death based on TUNEL (one-way ANOVA with Tukey’s multiple comparison test, ****P<0.0001, n=20 animals per genotype, per treatment). d, 10 mM 3TC significantly alleviates tauR406W-induced deficits in locomotor activity (one-way ANOVA with Tukey’s multiple comparison test, ***P=0.0008, ****P<0.0001, n=20 animals per genotype, per treatment). All flies are 10 days old. Values are mean ± s.e.m. Full genotypes are listed in Supplementary Table 1.
Aberrant transposable element transcription in human tauopathy
We next analyzed transposable element expression in human tauopathy using RNA-seq data from cortex (Fig. 7a, b) and cerebellum (Supplementary Fig. 8a, b) of postmortem human control, Alzheimer’s disease, and progressive supranuclear palsy patients (Supplementary Table 7). Similar to tau transgenic Drosophila, we found that specific subgroups of transposable elements are upregulated in human tauopathy, while other subgroups are downregulated. Among transposable elements that are upregulated in cortex and cerebellum of both tauopathies, we found a significant over-representation of endogenous retroviruses, while non-LTR retrotransposons are significantly over-represented among transposable elements that are downregulated in cortex and cerebellum of both tauopathies (Fig. 7c, Supplementary Fig. 8c).
Figure 7 |. Transposable element expression in cortex of human tauopathy.
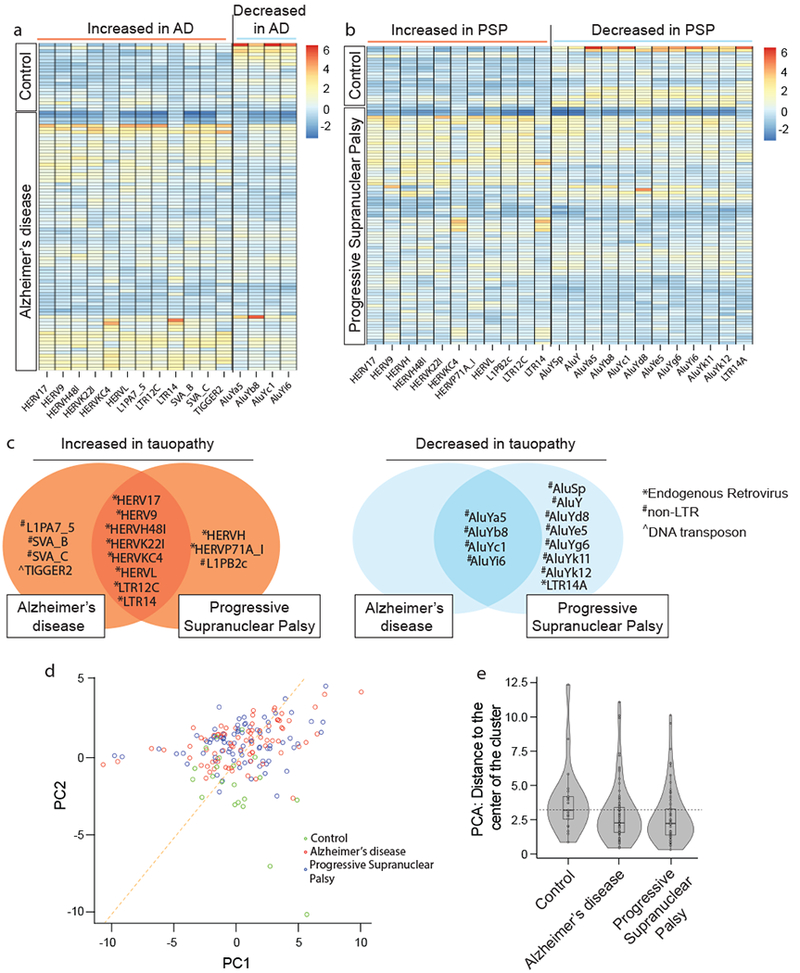
Heatmaps reflecting fold change of differentially expressed transposable elements in human cortex in a, control versus Alzheimer’s disease (AD), and b, control versus progressive supranuclear palsy (PSP), based on RNA-seq. (two-sided Wald test, FDR, P<0.01). c, Differentially expressed transposable elements in postmortem Alzheimer’s disease and progressive supranuclear palsy cortex compared to controls. HERVs are significantly over-represented among transposable element transcripts that are increased in tauopathy (hypergeometric test, adjusted P=0.004). Non-LTR are significantly over-represented among transposable elements that are decreased in tauopathy (hypergeometric test, adjusted P=4*10−8). d, Principal component analyses of differentially expressed transposable elements in control, Alzheimer’s disease, and progressive supranuclear palsy cortex (two-sided Kolmogorov-Smirnov test, P<10−13). e, Based on principal component analysis of transposable element expression in control versus tauopathy, violin plots show that control samples are relatively farther from the center of the cluster, as defined by the median of Alzheimer’s disease and progressive supranuclear palsy samples. Euclidian distance was computed using the first principal components of those transposable elements that are differentially expressed among the three conditions. For control, AD and PSP respectively, minima=0.8, 0.4, 0.3, maxima=12.3, 11.1, 10.1, median=3.2, 2.2, 2.2, mean=3.8, 2.8, 2.6, 1st quantile=2.5, 1.5, 1.3, 3rd quantile=4.2, 3.4, 3.2. Human control n=21, Alzheimer’s disease n=80, progressive supranuclear palsy n=82 biologically independent replicates in a-e.
We hypothesized that transposable element expression profiles of Alzheimer’s disease and progressive supranuclear palsy more closely resemble each other than controls. Principal component analysis supports this hypothesis; in particular, control samples have significantly lower values in the second principal component compared to Alzheimer’s disease and progressive supranuclear palsy in both cortex (Fig. 7d) and cerebellum (Supplementary Fig. 8d). We computed the median of the first two principal components over all tauopathy samples and found that the distance of control samples to the median center is significantly further compared to tauopathy samples in both cortex (Fig. 7e) and cerebellum (Supplementary Fig. 8e). Taken together, analyses of human transposable elements clearly reveal a significant transcriptional increase in endogenous retroviruses, and a significant decrease in non-LTR retroelements in postmortem human brain of both Alzheimer’s disease and progressive supranuclear palsy patients. In addition, these data suggest that transposable element dysregulation in human tauopathy is a regulated rather than stochastic process, consistent with our studies in tau transgenic Drosophila.
DISCUSSION
We have uncovered a novel, therapeutically targetable mechanism whereby pathogenic tau drives neuronal death (Supplementary Fig. 9, graphical summary). Specifically, our studies identify dysregulation of transposable elements as a consequence of pathogenic tau, and a driver of aberrant cell cycle activation in neurons and subsequent neuronal death. We identify genetic, dietary and pharmacological approaches to reduce transposable element dysregulation and suppress tau-induced neurotoxicity in Drosophila. We apply an unbiased transcriptomic approach to extend our findings to postmortem human brains and identify differentially expressed transposable elements in Alzheimer’s disease and progressive supranuclear palsy.
Because the complexity and repetitive nature of transposable elements presents challenges to RNA-seq analysis and is associated with a greater frequency of false positives and negatives compared to analysis of canonical messenger RNAs, we performed secondary validation of differentially expressed transposable element transcripts in tau transgenic Drosophila by NanoString. While NanoString data show a similar expression trend of most of the transposable elements that are identified as differentially expressed in tau transgenic Drosophila by RNA-seq, some of the elements are not confirmed as differentially expressed by NanoString. These data reveal the limitations of each assay when analyzing transposable element transcripts and stress the importance of rigorous secondary validation. Since many members of the copia family are increased at the transcript level based on both RNA-seq and NanoString analyses, we speculate that gypsy-TRAP reporter activation is a result of copia insertion into the ovo locus, rather than gypsy. Our attempts to sequence de novo copia insertions within the ovo locus in homogenates prepared from tau transgenic Drosophila heads resulted in a high frequency of mismatches (data not shown), which is likely a result of the stochastic nature of transposable element insertion.
Based on current understanding, cells have two layers of defense against potentially deleterious transposable element activation: 1) Transposable element transcription is limited by heterochromatin-mediated silencing, and 2) Transposable element transcripts are cleared from the cell by piRNA-mediated degradation. We find that both mechanisms of transposable element suppression are compromised in tauopathy. We speculate that tau-induced heterochromatin decondensation facilitates active transcription of transposable elements, and that tau-induced piwi/piRNA reduction allows those transcripts to persist. While our results are consistent with the effects of heterochromatin decondensation and piwi reduction on transposable element expression that have been reported in the Drosophila germline3,39, our studies reveal a previously undocumented role for heterochromatin- and piRNA-mediated transposable element silencing in the brain. Based on studies in the germline reporting a direct interaction between piwi and HP140, and a requirement for Rhino, a member of the HP1 subfamily, for piRNA production41, it is possible that a direct interaction between piwi and HP1 is also required to silence transposable elements in the brain.
Among upregulated transposable elements in human tauopathy, the human endogenous retrovirus (HERV) family, including HERV-K, is significantly over-represented. Elevated HERV-K transcripts are associated with amyotrophic lateral sclerosis (ALS)8 and many human cancers, including melanoma, breast cancer, germ cell tumors, renal cancer, and ovarian cancer42. A causal association between HERV-K and neuronal dysfunction has previously been established, as ectopic expression of HERV-K or the retroviral envelope protein that it encodes decreases synaptic activity and induces progressive motor dysfunction in mice8. Anti-retroviral reverse transcriptase inhibitors inhibit HERV-K activation in cultured cells43, and are currently in clinical trials for the treatment of ALS. Based on the data presented in the current study, reverse transcriptase inhibitors have significant potential as a novel therapeutic strategy for the treatment of neurodegenerative tauopathies, including Alzheimer’s disease.
The ability of flamenco loss of function mutations to enhance tau-induced neurotoxicity, and the ability of piwi overexpression, dietary restriction and inhibition of reverse transcriptase to reduce transposable element dysregulation and suppress tau-induced neurotoxicity suggest that tau-induced transposable element dysregulation is deleterious to neuronal survival. In addition to the detrimental effects of transposable element jumping, double stranded RNAs produced by transposable element transcripts, including HERVs, can trigger a type I interferon response through the innate immune system44. In light of the HERV increase in human tauopathy and the involvement of the innate immune response as a disease-promoting mechanism in Alzheimer’s disease45, it is tempting to speculate that expression of endogenous retroviruses in human tauopathy contributes to neuroinflammation in addition to promoting genomic instability. In future studies, it will be important to investigate a potential effect of transposable element activation on the innate immune response in the context of tauopathy.
ONLINE METHODS
Drosophila genetics.
Drosophila crosses and aging were performed at 25°C with a 12 h light/dark cycle at 60% relative humidity on a standard diet (Bloomington formulation). Full genotypes are listed in Supplementary Table 1. Pan-neuronal expression of transgenes, including RNAi-mediated knockdown, in Drosophila was achieved using the GAL4/UAS system46 with the elav promoter driving GAL4 expression. Retinal expression of transgenes in gypsy-TRAP studies was achieved using the retinal glass multiple reporter (GMR) promotor. elav-GAL4/+, GMR-TauV337M, UAS-GFP, flamKG00476, flamOR, Su(var)2055, and Su(var)3-92, were obtained from the Bloomington Stock Center. piwiv101658 and piwiv22235 were obtained from the Vienna Drosophila Resource Center47 (VDRC, www.vdrc.at). UAS-tauR406W and UAS-tauWT were a gift from Dr. Mel Feany. Gypsy-TRAP was a gift from Dr. Josh Dubnau. UAS-HA-piwi was a gift from Dr. Ruth Lehmann. An equal number of males and females were used in all Drosophila assays with the exception of gypsy-TRAP and flamenco genetic manipulations. Experiments utilizing the gypsy-TRAP reporter or flamenco require two genetic elements on the X chromosome, thus all data points are from female flies.
RNA sequencing and data analyses.
Library preparation and sequencing were performed by the Genome Sequencing Facility at Greehey Children’s Cancer Research Institute at the University of Texas Health San Antonio. For standard RNA-seq, three independent biologically independent replicates were sequenced, each consisting of 500 ng of total RNA from six pooled Drosophila heads (18 heads total). Extracted RNA was used for library preparation according to the KAPA Stranded RNA-seq Kit with RiboErase (HMR) sample preparation guide. After quantification by Qubit and Bioanalysis, libraries were pooled for cBot amplification and sequenced on the Illumina HiSeq 3000 platform with 100 base pair paired-end sequencing.
For small RNA-seq, four independent biological replicates were sequenced. 500 ng of total RNA from pooled Drosophila heads was used for library preparation according to the NEBNext small RNA sample preparation guide. Due to the abundance of the 2S rRNA in Drosophila, we included an additional 2S block step using the oligo (5’-TAC AAC CCT CAA CCA TAT GTA GTC CAA GCA/3SpC3/-3’) as described48. 2S rRNA blocking was performed directly after 3’ SR adapter ligation. 1 μM of the 2S rRNA block oligo was added directly to each ligation reaction on ice, and reactions were incubated at 90°C for 30 sec, then 65°C for 5 min. After 5 min, 1 μl of SR RT primer was added and we proceeded as described in the NEBNext protocol. After small RNA-seq libraries were subjected to quantification by Qubit and Bioanalysis, samples were pooled for cBot amplification and sequenced on the Illumina HiSeq 3000 platform with 50 base pair single-read sequencing. After standard and small-RNA sequencing, CASAVA was used for demultiplexing and fastq files were generated for each sample.
For data cleaning, SortMeRNA49 v2.1 was used to identify and exclude ribosomal RNA reads, and Trimmomatic50 v0.36 was used to remove Illumina adaptors. FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) v0.11.5 was used for a quality check before and after the above cleaning steps. The quality of bases in the cleaned reads was above 28 (Sanger/Illumina 1.9 encoding). Reads were aligned to the transposon reference FASTA files (FlyBase51 Release 6.12), and the quantity of each transposable element was calculated using Salmon52 v0.7.2. On average, 9 million reads were mapped per sample. DESeq253 v1.14.1 was used to identify sequences that were differentially expressed in tau transgenic Drosophila compared to controls. Ensembl BioMarts54 was used to assign genomic locations of differentially expressed transcripts (Supplementary Table 2). Pheatmap (https://CRAN.R-project.org/package=pheatmap) v1.0.8 and Pigengene55 v1.3.4 R56 packages were used to generate heatmaps. Values in transposable element heatmaps are standardized Transcripts Per Million57 (TPM). For presentation clarity in Fig. 1, we subtracted the TPM value of each transposable element from its average across all samples, and then divided the difference by the standard deviation of the TPM value of that particular transposable element. Unscaled heatmaps (Supplementary Fig. 1) represent raw TPM.
piRNA small RNA-seq analyses were similar to the above, with some exceptions. As some tRNAs are mis-annotated as piRNAs, piRNAs that were a subsequence of a tRNA (FlyBase51 Release 6.16) were removed from analysis. Reads were mapped to the remaining piRNA sequences (piRNABank58) as the reference. On average, 16 million reads were mapped per sample. piRNAs with low coverage were excluded as follows: If the sum of reads that mapped to a piRNA in all four tau transgenic Drosophila samples was less than three, we considered its expression “undetectable” in tau transgenic samples. Similarly, if the sum of reads that mapped to a piRNA in all four control normal samples was less than three, we considered its expression undetectable in controls. If a piRNA had undetectable expression values both tau transgenic and control samples, it was excluded from our analysis. Raw data for small RNA-seq are included in Supplementary Table 5, and genomic locations of differentially expressed piRNAs are included in Supplementary Table 6.
NanoString.
We worked with bioinformaticians at NanoString Technologies, Inc. to create a custom codeset consisting of 50 probes: 47 transposable elements identified as differentially expressed by RNA-seq, plus three internal control genes (RpL32, CG15117, cyp33). Codeset sequences are included in Supplementary Table 3. 100 ng of total RNA from pooled Drosophila heads was used for NanoString nCounter XT Codeset Gene Expression Assays according to the manufacturer’s protocol. Samples were analyzed by the nCounter™ Prep Station, and results were analyzed using the nCounter™ Digital Analyzer.
Immunofluorescence and histology.
For tau, piwi, and GFP immunofluorescence, Drosophila brains were dissected in PBS and fixed in methanol for 20 min. After blocking with 2% milk in 0.3% triton in PBS for 30 min, brains were incubated with primary antibody diluted in blocking solution overnight at 4°C. After washing with 0.3% triton in PBS, brains were incubated with Alexa488- or Alexa555-conjugated secondary antibodies for 2 h at room temperature. Slides were washed again and incubated with DAPI for 2 min to stain nuclei. Brains were visualized by confocal microscopy (Zeiss LSM 780 NLO with Examiner), and ImageJ was used for analysis. All images shown are a single slice. TUNEL staining was performed in 4 µm sections from formalin-fixed, paraffin embedded Drosophila heads. Secondary detection was performed with DAB. TUNEL-positive neurons were counted throughout the entire brain by bright field microscopy. Antibody concentrations and sources are listed in Supplementary Table 8.
Western blotting.
Frozen Drosophila heads were homogenized in 20 µl Laemmli sample buffer, boiled for 10 min, and analyzed by 4-20% SDS-PAGE. After transferring to nitrocellulose membranes, antigen retrieval was performed by microwaving membranes in one liter of PBS for 15 min. Equal loading was assessed by Ponceau S staining. After blocking membranes in PBS plus 0.05% Tween and 2% milk, membranes were incubated with primary antibodies overnight at 4°C. After washing, membranes were incubated with HRP- conjugated secondary antibodies for 2 h at room temperature. Blots were developed with an enhanced chemiluminescent substrate. Antibody concentrations and sources are listed in Supplementary Table 8. Full scans of western blots are provided in Supplementary Figure 10.
Locomotor activity.
Walking activity was performed as described previously10.
Dietary restriction and 3TC treatment.
“Standard” diet consists of 1.5% yeast, 6.6% light corn syrup, 0.9% soy flour, 6.7% yellow cornmeal, 0.5% agar; “Dietary restriction” diet consists of 0.5% yeast, 2.2% light corn syrup, 0.9% soy flour, 6.7% yellow cornmeal, 0.5% agar, (all wt/vol). Flies were collected at day one of adulthood and placed on a standard or restricted diet. Flies were transferred to fresh food every other day until they were 10 days old, at which point they were fixed, frozen, or assessed for locomotor activity.
For drug treatment, flies were collected at two days old and transferred to standard food or food containing 3TC (Fisher, #50731692, 10 mM except in Supplementary Fig. 3), which was dissolved in water. Flies were transferred to fresh food every other day until they were 10 days old, at which point they were fixed, frozen, or assessed for locomotor activity.
Analysis of human RNA-seq data.
The Mayo RNA-seq Study on Neuropathological Diseases generated whole transcriptome data for cerebellum and temporal cortex samples from 312 North American Caucasian subjects. These subjects were diagnosed with Alzheimer’s disease, progressive supranuclear palsy, pathologic aging or were elderly controls without neurodegenerative disorders59. We downloaded the corresponding clinical data (covariates) from the Accelerating Medicines Partnership – Alzheimer’s Disease (AMP-AD) Knowledge Portal60. Specifically, we used the synapseClient R package v1.15-0 (http://www.sagebase.org) to download temporal cortex (syn5223705) and cerebellum (syn3817650) clinical data. 12 cortex (syn6126114) and 10 cerebellum (syn6126119) samples were excluded due to low quality61,62. In this study, we also excluded 9 cortex and 15 cerebellum samples that had an RNA Integrity Number (RIN)63 less than 7. After this filtering, 80 Alzheimer’s disease, 82 progressive supranuclear palsy, and 21 control cortex samples were available, as well as 76 Alzheimer’s disease, 78 progressive supranuclear palsy, and 25 control cerebellum samples. Dr. Nilufer Taner at the Mayo Clinic provided us with unprocessed RNA-seq data. We converted raw bam files to fastq files using the Picard SamToFastq v2.10.10 tool (http://broadinstitute.github.io/picard). We downloaded human transposable elements from the Genetic Information Research Institute (GIRI) RepBase64 database in fasta format (http://www.girinst.org/repbase/update/browse.php?type=All&format=FASTA&autonomous=on&nonautonomous=on&simple=on&division=Homo+sapiens&letter=A). The file contains 1073 unique sequences including the 549 ancestral repeats that are shared among all mammals. Using the same pipeline that we described for the analysis of Drosophila RNA-seq data, we cleaned the fastq files, aligned them to the human transposable element sequences, and performed differential expression analysis.
Principal component analyses (PCA) of human RNA-seq data.
We used DESeq2 package and a likelihood ratio test to identify 19 transposable elements that have variable expression in any of the three Alzheimer’s disease, progressive supranuclear palsy, or control conditions in cortex (Supplementary Table 7). We then oversampled the control samples to balance the number of samples in each condition by repeating each control sample four times. We performed PCA using the 19 differentially expressed transposable elements, and the 80 Alzheimer’s disease, 82 progressive supranuclear palsy, and 84 control samples. A scatterplot of the first two principal components showed that Alzheimer’s disease and progressive supranuclear palsy samples cluster together (Fig. 7d). We considered the median of this cluster over all Alzheimer’s disease and progressive supranuclear palsy samples as the center of the cluster and computed the distance of each sample to the cluster center. A Kolmogorov–Smirnov Test showed that the control samples do not generally belong to this cluster (Fig. 7e, P<3*10−7). We used the ggplot265 R package v 2.2.1 to generate violin plots. We performed a similar analysis on cerebellum, which showed that 20 transposable elements have variable expression in Alzheimer’s disease, progressive supranuclear palsy, or control (Supplementary Fig. 4a, b, c, Supplementary Table 7). We repeated each control cerebellum sample three times, which provided 75 control samples for PCA. Similar to cortex, the 76 Alzheimer’s disease and 78 progressive supranuclear palsy samples cluster together in the scatter plot, and control samples do not generally belong to this cluster (Supplementary Fig. 4d, P<2*10−6). In particular, the majority of control samples are below the orange diagonal line.
Over-representation of specific transposable element classes in differential expression analyses.
All of the four transposable elements that are downregulated in Alzheimer’s disease cortex are non-LTR retrotransposons. This is significantly more than expected, since only 239 (22%) of the total 1073 human transposable elements are non-LTR (hypergeometric test, P=0.002). Similarly, the non-LTR retrotransposon are overrepresented among downregulated transposable elements in progressive supranuclear palsy cortex (P=5×10−7). We used the sumlog function from the metap package v0.8 to combine these two P-values by Fisher’s method (P=2×10−8). We multiplied the resulting P-value by two to adjust for the two tests conducted in cortex and cerebellum. Similarly, we confirmed that the non-LTR retrotransposon are also overrepresented in the decreased transposable elements in cerebellum (adjusted P=0.01). We used similar tests to show that endogenous retroviruses are overrepresented in the upregulated transposable elements in cortex (adjusted P=0.004) and cerebellum (adjusted P=0.0003).
Statistical analyses.
Every reported n is the number of biologically independent replicates. Except when noted otherwise, statistical analyses were performed using a one-way ANOVA with Tukey test when comparing among multiple genotypes, and a two tailed, unpaired Student’s t-test when comparing two genotypes. Data distribution was assumed to be normal but this was not formally tested. For RNA-seq analysis, a two-sided Wald test66 was used to calculate false discovery rates (FDR-adjusted P-value). For NanoString analyses, we used nSolver Analysis Software v3.0. The central tendency presented is the mean in all cases except NanoString data (median), and error bars represent standard error of the mean. A P-value less than 0.05 was considered significant unless otherwise specified. Sample sizes are similar to or greater than those reported in previous publications4,5,10. Samples were randomized in all Drosophila studies. Investigators were blinded to genotype in all immunohistochemistry, immunofluorescence, and locomotor activity assays. Full statistical analyses are provided in Supplementary Table 9. To improve transparency and increase reproducibility, detailed information on experimental design and reagents can be accessed in the Life Sciences Reporting Summary.
Supplementary Material
Acknowledgements.
We thank J. Dubnau (Stony Brook University) for gypsy-TRAP Drosophila stocks and R. Lehmann (New York University School of Medicine) for the piwiOE stock. We acknowledge the Texas Advanced Computing Center (TACC) at the University of Texas at Austin for providing high-performance computing resources: http://www.tacc.utexas.edu. This study was supported by the National Institute for Neurological Disorders and Stroke (BF) and the Owens Foundation (BF). The Mayo human RNAseq study data was led by N. Ertekin-Taner (Mayo Clinic) as part of the multi-PI U01 AG046139 (MPIs Golde, Ertekin-Taner, Younkin, Price) using samples from the following source:
The Mayo Clinic Brain Bank. Data collection was supported through funding by NIA grants P50 AG016574, R01 AG032990, U01 AG046139, R01 AG018023, U01 AG006576, U01 AG006786, R01 AG025711, R01 AG017216, R01 AG003949, NINDS grant R01 NS080820, CurePSP Foundation, and support from Mayo Foundation.
Footnotes
Accession codes. Full access to fastq files that include Drosophila transposable element and piRNA is provided through the Gene Expression Omnibus (GEO) database (GSE115606).
Competing Financial Interests Statement. The authors declare no competing financial interests.
Code availability. Custom codes that were created for cleaning and analyzing sequencing data are available in Supplementary Software and can also be accessed at https://bitbucket.org/habilzare/alzheimer.
Data availability. Raw counts from RNA sequencing are provided as Supplementary Tables. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Chuong EB, Elde NC & Feschotte C Regulatory activities of transposable elements: from conflicts to benefits. Nature reviews. Genetics 18, 71–86, doi: 10.1038/nrg.2016.139 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slotkin RK & Martienssen R Transposable elements and the epigenetic regulation of the genome. Nature reviews. Genetics 8, 272–285, doi: 10.1038/nrg2072 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Brennecke J et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128, 1089–1103, doi: 10.1016/j.cell.2007.01.043 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Li W et al. Activation of transposable elements during aging and neuronal decline in Drosophila. Nature neuroscience 16, 529–531, doi: 10.1038/nn.3368 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood JG et al. Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 113, 11277–11282, doi: 10.1073/pnas.1604621113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns KH Transposable elements in cancer. Nature reviews. Cancer 17, 415–424, doi: 10.1038/nrc.2017.35 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Li W, Jin Y, Prazak L, Hammell M & Dubnau J Transposable elements in TDP-43-mediated neurodegenerative disorders. PloS one 7, e44099, doi: 10.1371/journal.pone.0044099 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W et al. Human endogenous retrovirus-K contributes to motor neuron disease. Science translational medicine 7, 307ra153, doi: 10.1126/scitranslmed.aac8201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krug L et al. Retrotransposon activation contributes to neurodegeneration in a Drosophila TDP-43 model of ALS. PLoS genetics 13, doi: 10.1371/journal.pgen.1006635 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frost B, Hemberg M, Lewis J & Feany MB Tau promotes neurodegeneration through global chromatin relaxation. Nature neuroscience 17, 357–366, doi: 10.1038/nn.3639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittmann CW et al. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293, 711–714, doi: 10.1126/science.1062382 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Hutton M et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705, doi: 10.1038/31508 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Steinhilb ML, Dias-Santagata D, Fulga TA, Felch DL & Feany MB Tau phosphorylation sites work in concert to promote neurotoxicity in vivo. Molecular biology of the cell 18, 5060–5068, doi: 10.1091/mbc.E07-04-0327 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dias-Santagata D, Fulga TA, Duttaroy A & Feany MB Oxidative stress mediates tau-induced neurodegeneration in Drosophila. The Journal of clinical investigation 117, 236–245, doi: 10.1172/JCI28769 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khurana V et al. A neuroprotective role for the DNA damage checkpoint in tauopathy. Aging cell 11, 360–362, doi: 10.1111/j.1474-9726.2011.00778.x (2012). [DOI] [PubMed] [Google Scholar]
- 16.Frost B, Bardai FH & Feany MB Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Current biology : CB 26, 129–136, doi: 10.1016/j.cub.2015.11.039 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlo P et al. p53 prevents neurodegeneration by regulating synaptic genes. Proceedings of the National Academy of Sciences of the United States of America 111, 18055–18060, doi: 10.1073/pnas.1419083111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khurana V et al. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Current biology : CB 16, 230–241, doi: 10.1016/j.cub.2005.12.042 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Geiss GK et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nature biotechnology 26, 317–325, doi: 10.1038/nbt1385 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Bardai FH et al. A Conserved Cytoskeletal Signaling Cascade Mediates Neurotoxicity of FTDP-17 Tau Mutations In Vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience 38, 108–119, doi: 10.1523/jneurosci.1550-17.2017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly MT, Faulkner GJ, Dubnau J, Ponomarev I & Gage FH The role of transposable elements in health and diseases of the central nervous system. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 17577–17586, doi: 10.1523/jneurosci.3369-13.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muotri AR et al. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature 435, 903–910, doi: 10.1038/nature03663 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Pelisson A et al. About the origin of retroviruses and the co-evolution of the gypsy retrovirus with the Drosophila flamenco host gene. Genetica 100, 29–37 (1997). [PubMed] [Google Scholar]
- 24.Mevel-Ninio M, Pelisson A, Kinder J, Campos AR & Bucheton A The flamenco locus controls the gypsy and ZAM retroviruses and is required for Drosophila oogenesis. Genetics 175, 1615–1624, doi: 10.1534/genetics.106.068106 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost B, Gotz J & Feany MB Connecting the dots between tau dysfunction and neurodegeneration. Trends in cell biology 25, 46–53, doi: 10.1016/j.tcb.2014.07.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarot E, Payen-Groschene G, Bucheton A & Pelisson A Evidence for a piwi-dependent RNA silencing of the gypsy endogenous retrovirus by the Drosophila melanogaster flamenco gene. Genetics 166, 1313–1321 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy J, Sarkar A, Parida S, Ghosh Z & Mallick B Small RNA sequencing revealed dysregulated piRNAs in Alzheimer’s disease and their probable role in pathogenesis. Molecular BioSystems 13, 565–576, doi: 10.1039/C6MB00699J (2017). [DOI] [PubMed] [Google Scholar]
- 28.Qiu W et al. Transcriptome-wide piRNA profiling in human brains of Alzheimer’s disease. Neurobiology of aging 57, 170–177, doi: 10.1016/j.neurobiolaging.2017.05.020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siomi MC, Sato K, Pezic D & Aravin AA PIWI-interacting small RNAs: the vanguard of genome defence. Nature reviews. Molecular cell biology 12, 246–258, doi: 10.1038/nrm3089 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Ghildiyal M et al. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science 320, 1077–1081, doi: 10.1126/science.1157396 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee EJ et al. Identification of piRNAs in the central nervous system. RNA (New York, N.Y.) 17, 1090–1099, doi: 10.1261/rna.2565011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamparini AL et al. Vreteno, a gonad-specific protein, is essential for germline development and primary piRNA biogenesis in Drosophila. Development (Cambridge, England) 138, 4039–4050, doi: 10.1242/dev.069187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eissenberg JC, Morris GD, Reuter G & Hartnett T The heterochromatin-associated protein HP-1 is an essential protein in Drosophila with dosage-dependent effects on position-effect variegation. Genetics 131, 345–352 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuter G, Dorn R, Wustmann G, Friede B & Rauh G Third chromosome suppressor of position-effect variegation loci in Drosophila melanogaster. Molecular and General Genetics 202, 481–487 (1986). [Google Scholar]
- 35.Fedoroff NV Presidential address. Transposable elements, epigenetics, and genome evolution. Science 338, 758–767 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Longo VD et al. Interventions to Slow Aging in Humans: Are We Ready? Aging cell 14, 497–510, doi: 10.1111/acel.12338 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sultana T, Zamborlini A, Cristofari G & Lesage P Integration site selection by retroviruses and transposable elements in eukaryotes. Nature reviews. Genetics 18, 292–308, doi: 10.1038/nrg.2017.7 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Coates JA et al. (−)-2’-deoxy-3’-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrobial agents and chemotherapy 36, 733–739 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen PR, Tirian L, Vunjak M & Brennecke J A heterochromatin-dependent transcription machinery drives piRNA expression. Nature 549, 54–59, doi: 10.1038/nature23482 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brower-Toland B et al. Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes & development 21, 2300–2311, doi: 10.1101/gad.1564307 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klattenhoff C et al. The Drosophila HP1 homologue Rhino is required for transposon silencing and piRNA production by dual strand clusters. Cell 138, 1137–1149, doi: 10.1016/j.cell.2009.07.014 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez-Cao M et al. Human endogenous retroviruses and cancer. Cancer Biology & Medicine 13, 483–488, doi: 10.20892/j.issn.2095-3941.2016.0080 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyagi R, Li W, Parades D, Bianchet MA & Nath A Inhibition of human endogenous retrovirus-K by antiretroviral drugs. Retrovirology 14, doi: 10.1186/s12977-017-0347-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chuong EB, Elde NC & Feschotte C Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science 351, 1083–1087, doi: 10.1126/science.aad5497 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heneka MT, Golenbock DT & Latz E Innate immunity in Alzheimer’s disease. Nature immunology 16, 229–236, doi: 10.1038/ni.3102 (2015). [DOI] [PubMed] [Google Scholar]
METHODS-ONLY REFERENCES
- 46.Fischer JA, Giniger E, Maniatis T & Ptashne M GAL4 activates transcription in Drosophila. Nature 332, 853–856, doi: 10.1038/332853a0 (1988). [DOI] [PubMed] [Google Scholar]
- 47.Dietzl G et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156, doi: 10.1038/nature05954 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Wickersheim ML & Blumenstiel JP Terminator oligo blocking efficiently eliminates rRNA from Drosophila small RNA sequencing libraries. BioTechniques 55, 269–272, doi: 10.2144/000114102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopylova E, Noe L & Touzet H SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics (Oxford, England) 28, 3211–3217, doi: 10.1093/bioinformatics/bts611 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Bolger AM, Lohse M & Usadel B Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics (Oxford, England) 30, 2114–2120, doi: 10.1093/bioinformatics/btu170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gramates LS et al. FlyBase at 25: looking to the future. Nucleic acids research 45, D663–D671, doi: 10.1093/nar/gkw1016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patro R, Duggal G, Love MI, Irizarry RA & Kingsford C Salmon provides fast and bias-aware quantification of transcript expression. Nature methods 14, 417, doi:10.1038/nmeth.4197 10.1038/nmeth.4197https://www.nature.com/articles/nmeth.4197#supplementary-informationhttps://www.nature.com/articles/nmeth.4197#supplementary-information (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Love MI, Huber W & Anders S Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology 15, 550, doi: 10.1186/s13059-014-0550-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinsella RJ et al. Ensembl BioMarts: a hub for data retrieval across taxonomic space. Database : the journal of biological databases and curation 2011, bar030, doi: 10.1093/database/bar030 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foroushani A et al. Large-scale gene network analysis reveals the significance of extracellular matrix pathway and homeobox genes in acute myeloid leukemia: an introduction to the Pigengene package and its applications. BMC medical genomics 10, 16, doi: 10.1186/s12920-017-0253-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.R Development Core Team. R: A language and environment for statistical computing. (2010).
- 57.Wagner GP, Kin K & Lynch VJ Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory in Biosciences 131, 281–285, doi: 10.1007/s12064-012-0162-3 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Sai Lakshmi S & Agrawal S piRNABank: a web resource on classified and clustered Piwi-interacting RNAs. Nucleic acids research 36, D173–177, doi: 10.1093/nar/gkm696 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen M et al. Human whole genome genotype and transcriptome data for Alzheimer’s and other neurodegenerative diseases. Scientific data 3, 160089, doi: 10.1038/sdata.2016.89 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodes RJ & Buckholtz N Accelerating Medicines Partnership: Alzheimer’s Disease (AMP-AD) Knowledge Portal Aids Alzheimer’s Drug Discovery through Open Data Sharing. Expert opinion on therapeutic targets 20, 389–391, doi: 10.1517/14728222.2016.1135132 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Purcell S et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics 81, 559–575, doi: 10.1086/519795 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price AL et al. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics 38, 904–909, doi: 10.1038/ng1847 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Schroeder A et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC molecular biology 7, 3, doi: 10.1186/1471-2199-7-3 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bao W, Kojima KK & Kohany O Repbase Update, a database of repetitive elements in eukaryotic genomes. Mobile DNA 6, 11, doi: 10.1186/s13100-015-0041-9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wickham H ggplot2: Elegant Graphics for Data Analysis. (Springer International Publishing, 2016). [Google Scholar]
- 66.Wald A Tests of Statistical Hypotheses Concerning Several Parameters When the Number of Observations is Large. Transactions of the American Mathematical Society 54, 426–482, doi: 10.2307/1990256 (1943). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


