Abstract
Heat stress is a cause of major economic losses to cattle producers, especially in tropical and subtropical environments. The objectives of this study were to assess the phenotypic variability in core body temperature and sweating rate and to evaluate the effect of coat type, temperament, and BW on core body temperature and sweating rate in Brangus heifers. During August and September of 2016, 725 Brangus heifers were evaluated on pasture in four separate groups (n = 200, 189, 197, and 139). Environmental measurements of dry bulb temperature (Tdb) and relative humidity (RH) were measured every 15 min during the entire time of data collection and the temperature–humidity index (THI) was used to quantify heat-stress potential. Coat score, sweating rate, chute score, exit score, and live weight were recorded as the animals passed through the chute. Vaginal temperature was recorded every 5 min for five consecutive days. There was significant variation in vaginal temperature between heifers in the same environmental conditions (σ2u = 0.049), suggesting opportunities for selective improvements. A repeatability of 0.47 and 0.44 was estimated for sweating rate and vaginal temperature, respectively, suggesting that one measurement would be able to adequately describe the sweating capacity or ability to control the body temperature of an individual. Vaginal temperature increased as THI increased, with approximately 1 h lag time in the animal’s response. Vaginal temperature (−0.047 °C, P = 0.015) and sweating rate were lower (−5.49 ± 2.12 g/(m2·h), P < 0.01) for heifers that demonstrated a calmer behavior in the chute. Animals with shorter, smoother hair coats had significantly lower vaginal temperatures when compared to animals with longer hair coats (P < 0.01). Also, heavier heifers in this study maintained lower (P < 0.0001) vaginal temperature than the lighter heifers. Our results showed that hair coat, temperament, and weight influenced vaginal temperature regulation.
Keywords: Brangus, hair coat, heat stress, sweating rate, thermoregulation
INTRODUCTION
In 2003, it was estimated that the U.S. livestock industry suffers an annual economic loss of 1.69 to 2.36 billion dollars due to heat stress (St-Pierre et al., 2003). Most animal-producing areas in the United States are predicted to experience extreme summer conditions (Luber and McGeehin, 2008) and detrimental effects on livestock productivity associated with heat stress are expected to intensify upon the realization of predicted climate change (IPCC, 2007). Approximately 50% of the total world meat and 60% of milk originates from tropical and subtropical environments (FAO, 2010). Bos indicus genetics are commonly introduced into beef cattle herds in these areas, as they are better adapted to hot and humid conditions (Hammond et al., 1998; Gaughan et al., 1999; Hansen, 2004). This practice has improved the heat adaptability in the crossbred herds (Hammond et al., 1998; Olson et al., 2003); however, it also introduced other challenges primarily related to meat quality and reproduction (Freetly and Cundiff, 1997; Elzo et al., 2012; Elzo et al., 2014). Identification of animals with the ability to regulate body temperature in hot and humid environments, while still maintaining high performance for important production traits will hold the key to future success for cattle production in harsh environments. Hair type, sweating rate, and temperament are among the factors affecting the ability of animals to maintain their body temperature within normal physiological limits in environmental conditions with high heat stress potential. However, the magnitude of the impact these factors have on body core temperature of animals maintained on pastures has not been previously investigated. The first objective of this study was to assess the phenotypic variability of sweating rate and vaginal temperature of Brangus heifers grazing on pasture in South Florida. The second objective was to evaluate the effect of coat type, temperament behavior as described by chute and exit scores, and weight on sweating rate and vaginal temperature.
MATERIALS AND METHODS
Animals and Sample Collection
The University of Florida Institutional Animal Care and Use Committee approved the research protocol used in this study (number 201503578).
Two-yr-old Brangus heifers (n = 725) with an average weight of 382.69 ± 35.91 kg from the Seminole Tribe of Florida, Inc. were evaluated under hot and humid conditions during August and September 2016 at the Seminole Ranch, west of Lake Okeechobee (Brighton Reservation, FL, 27-04′46″ N; 081-04′11″ W). The heifers were randomly assigned to one of four groups and were maintained on pasture for the duration of the study. The first group (n = 200) was monitored from August 15 to August 19, the second group (n = 189) from August 22 to August 26, the third group (n = 197) from August 29 to September 2, and the fourth group (n = 139) from September 9 to September 12, 2016.
Environmental Measurements
Dry bulb temperature (Tdb) and relative humidity (RH) were measured every 15 min during the entire time of data collection from August 15 to September 12, 2016, using a HOBO U12 data logger (Onset Company, Bourne, MA).
The temperature–humidity index (THI) was used to quantify heat-stress potential and it was calculated as follows (NRC, 1971):
Physiological Measurements
Heifers within a group were gathered early in the morning and were individually restrained in a squeeze-chute for insertion of temperature-recording devices and measurement of coat color, coat score, sweating rate, chute score, and exit score. Additionally, live weight was recorded as they passed over the scale, immediately before entering the chute. Within each group, data were collected over a 4-h period.
Vaginal temperature, a direct measurement of an animal’s ability to prevent hyperthermia during heat stress, was recorded for five consecutive days using iButton data loggers, type DS1922L, temperature range −40 °C to 85 °C, accuracy ±0.5 °C, 11-bit for 0.0625 °C resolution (Maxim Integrated, San Jose, CA). The iButtons were attached to a blank controlled internal drug-releasing device (CIDR) and were inserted into the vagina. Each iButton was calibrated before the start of the study and pre-programmed to record body temperature at 5-min intervals on a 24-h cycle. At the end of the 5-d trial, the data were downloaded and iButtons and CIDR were sanitized for the next group.
Sweating rate was measured on the rump, 4 inches from spine and halfway along horizontal axis with a calibrated, digital moisture sensor (Vapometer; Delphin Tech. Ltd., Kuopio, Finland) that determines trans-epidermal water loss. The Vapometer uses a closed system approach, free of ambient airflow, to measure ambient relative humidity and temperature. The evaporation rate is displayed in g/(m2·h) with an accuracy of ±10%. The sweating rate was measured for all heifers in groups 2, 3, and 4.
Coat color and coat score were measured for each heifer in groups 1, 2, 3, and 4 while in the chute. The coat (COAT) was scored as 1 = very smooth, 2 = smooth, 3 = long, and 4 = woolly. A picture of the coat was taken and was used for final confirmation of the coat score.
The chute behavior (CHUTE) was scored as 1 = calm, no movement; 2 = slightly restless; 3 = squirming, occasionally shaking the squeeze chute; 4 = continuous, very vigorous movement and shaking of the squeeze chute; or 5 = rearing, twisting of the body and struggling violently (Grandin, 1993). Exit behavior (EXIT) was scored as 1 = slow exit, calm or 2 = jump, trot, or run.
Statistical Analysis
For each cow, the average vaginal temperature for 15-min windows of environmental data were calculated and matched with the measurements of environmental Tdb and THI recorded at the same time. Hourly averages were calculated for environmental measurements and vaginal temperature. Only data recorded during three 24-h periods starting at 2400 h the day of iButton insertion were used in subsequent analyses to reflect vaginal temperature of cows maintained on pastures without any human interaction.
Data were analyzed with the MIXED procedure in SAS 9.4 (SAS Inst. Inc., Cary, NC) and REML estimation. The linear mixed model (LMM) was:
where the design matrices X- and Z-related phenotypic observations in the vector Y to fixed (β) and random (u) effects, respectively. The vector e contained random residual effects specific to each animal. The vectors u and e were assumed to be normally distributed with 0 means and variances σ2u and σ2e, respectively.
The sweating rate was measured when heifers were in the chute and a time variable was created to represent the hour when a heifer was measured relative to the starting time of the protocol (HOUR = 1, 2, 3, or 4). The model included group and hour nested within group as random effects. The fixed effects included in LMM were coat score (COAT = 1, 2, 3, or 4), chute score (CHUTE = 1, ≥2), and exit score (EXIT = 1 or 2). Weight of the heifer was included in the model as a covariate. The sweating rate was also adjusted for linear and quadratic effect of time within each HOUR.
The hourly vaginal temperatures were analyzed using repeated measures in a LMM with a first-order autoregressive error structure. Each group of heifers was exposed to different environmental conditions during the 3 d of data recording used in the analysis. The model included group, day, and heifer nested within group and day as random effects. The RANDOM statement was used to model the crossover part of the data and REPEATED statement with a first-order autoregressive model AR(1) was used to model the covariance structure of repeated measures on the same heifer during a day. The fixed effects included in LMM were coat score (COAT = 1, 2, 3, or 4), chute score (CHUTE = 1, ≥2), and exit score (EXIT = 1 or 2). Weight of the heifer was included in the model as a covariate.
To evaluate the heat-stress potential, we calculated the hourly average THI during two 10-h periods (0200 to 1200 h, 1200 to 2200 h) for each day of the experiment, and calculated the difference in the hourly average THI between these two periods each day. The livestock weather hazard guide (LWSI; LCI, 1970) uses the following thresholds to define the thermal environment: minimal heat-stress potential when THI ≤75, moderate heat-stress potential for THI between 75 and 78, major heat-stress potential for THI between 79 and 83, and critical heat-stress potential THI ≥84. Days with moderate thermal stress potential during both periods and a difference hourly THI between the two periods less than 1.5 THI units were classified as “low heat stress”. Days with major heat-stress potential and a difference >5.0 THI units were classified as “high heat stress”. The response to a low and a high heat-stress potential day was evaluated for groups 2 and 3. Groups 1 and 4 were excluded from this analysis because they did not have 2-d meeting the low and high heat-stress potential criteria outlined. The random variable included in LMM was day within group and first-order autoregressive model AR(1) was used to model the covariance structure of repeated measures on the same heifer within each day.
RESULTS AND DISCUSSION
Sweating Rate
Summary statistics for sweating rate by group and hour are presented in Table 1. The heifer weight and the linear and quadratic effect of time on sweating rate for animals within each hour within a group were significant (P < 0.05) and kept in the model. Similar time effect was observed in recorded rectal temperature of beef cattle, which was dependent on the order animals were processed through the chute (Hammond and Olson, 1994; Magona et al., 2009; McManus et al., 2009). The estimated variance (σ2u) of groups and hours within group was 338.94 ± 155.58 and the estimated residual variance (σ2e) between heifers was 385.89 ± 26.07. The intraclass correlation, calculated as (σ2u)/(σ2u + σ2e), was 0.47. The intraclass correlation in these data represent the repeatability of the trait, or the proportion of variance that is due to permanent environmental and genetic differences among individuals and indicates that one measurement of sweating rate would adequately describe the sweating capacity of an individual.
Table 1.
Number of Brangus heifers, mean, and SE for sweating rate (g/[m2·h]) of animals in three different groups measured within the first, second, third, or fourth hour of the protocol
| Group/day | Hour | N | Mean | SE |
|---|---|---|---|---|
| Group 2 August 22, 2016 |
1 | 45 | 53.86 | 2.53 |
| 2 | 57 | 63.63 | 2.9 | |
| 3 | 48 | 83.41 | 4.03 | |
| 4 | 23 | 80.42 | 4.82 | |
| Group 3 August 29, 2016 |
1 | 33 | 69.49 | 3.25 |
| 2 | 56 | 68.25 | 2.42 | |
| 3 | 43 | 82.52 | 3.2 | |
| 4 | 44 | 118.55 | 3.94 | |
| Group 4 September 9, 2016 |
1 | 36 | 76.63 | 3.45 |
| 2 | 39 | 91.48 | 3.16 | |
| 3 | 51 | 104.76 | 2.3 |
Vaginal Temperature
The estimates of variance parameters for vaginal temperature are presented in Table 2. The between heifer’s variance was 0.049 and the within heifer’s variance was 0.06. The correlation between adjacent hourly measures of vaginal temperature on the same heifer was 0.79 and the correlation between any two observations on the same heifer in different days, or the repeatability of vaginal temperature was 0.44.
Table 2.
Covariance parameters estimates describing the variability of hourly vaginal temperature of Brangus heifers on pasture during summer in a subtropical environment
| Parameter | Estimate | SE | Z value | Pr Z |
|---|---|---|---|---|
| σ 2 u | 0.049 | 0.003 | 16.18 | <0.0001 |
| ρ | 0.791 | 0.003 | 275.63 | <0.0001 |
| σ 2 e | 0.062 | 0.001 | 73.44 | <0.0001 |
Estimate, SE and statistical significance for between heifer variance (σ2u), correlation between two adjacent vaginal temperature measures (ρ), and within heifer variance (σ2e).
There was a large variation in the THI over the time period evaluated, ranging from a minimum of 73 to a maximum of 89. Days within all heat-stress potential categories (minimal, moderate, major, and critical) were encountered in the present study. There was a high level of variation in the vaginal temperature, which ranged overall from 36.6 °C to 42.3 °C. Most importantly, there was a high level of variation in the maximum vaginal temperature for the individual animals ranging between 38.8 °C and 42.3 °C, allowing for investigation of factors responsible for this variation. Table 3 shows the mean vaginal temperatures, environmental temperature, and THI for each day and each group of cattle.
Table 3.
Vaginal temperature and meteorological conditions during the experiment
| Group | Day | Vaginal temperature | Dry bulb temperature | Relative humidity | THI |
|---|---|---|---|---|---|
| 1 | 1 | 38.88 (0.45) | 28.68 (0.37) | 81.35 (1.62) | 80.53 (0.35) |
| 2 | 39.01 (0.41) | 28.28 (0.33) | 82.01 (1.58) | 80.05 (0.30) | |
| 3 | 39.04 (0.41) | 28.24 (0.31) | 82.02 (1.39) | 80.08 (0.31) | |
| 2 | 1 | 39.01 (0.53) | 28.71 (0.4) | 79.47 (1.79) | 80.21 (0.37) |
| 2 | 38.90 (0.37) | 26.92 (0.23) | 90.55 (0.96) | 79.13 (0.25) | |
| 3 | 38.98 (0.43) | 26.53 (0.16) | 92.66 (0.55) | 78.83 (0.22) | |
| 3 | 1 | 38.73 (0.39) | 25.94 (0.23) | 94.80 (0.97) | 78.00 (0.33) |
| 2 | 38.81 (0.35) | 26.10 (0.30) | 94.84 (0.97) | 78.14 (0.39) | |
| 3 | 38.96 (3.95) | 27.93 (0.41) | 87.14 (1.42) | 80.12 (0.51) | |
| 4 | 1 | 38.95 (0.49) | 28.37 (0.49) | 81.70 (1.81) | 79.79 (0.54) |
| 2 | 39.03 (0.47) | 29.04 (0.46) | 80.41 (1.96) | 80.68 (0.46) | |
| 3 | 39.01 (0.44) | 28.98 (0.46) | 80.33 (1.90) | 80.61 (0.45) |
Means (SD) for three 24-h periods over all four groups of animals for vaginal temperature (°C), dry bulb temperature (°C), relative humidity (%), and temperature–humidity index (THI).
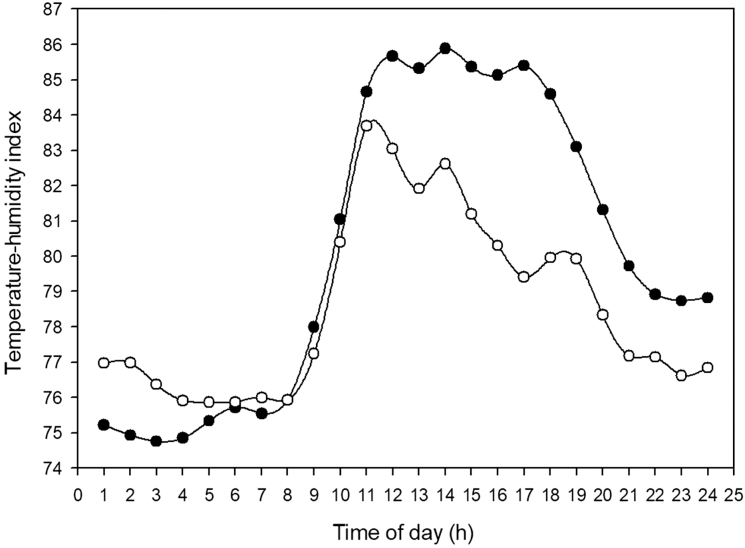
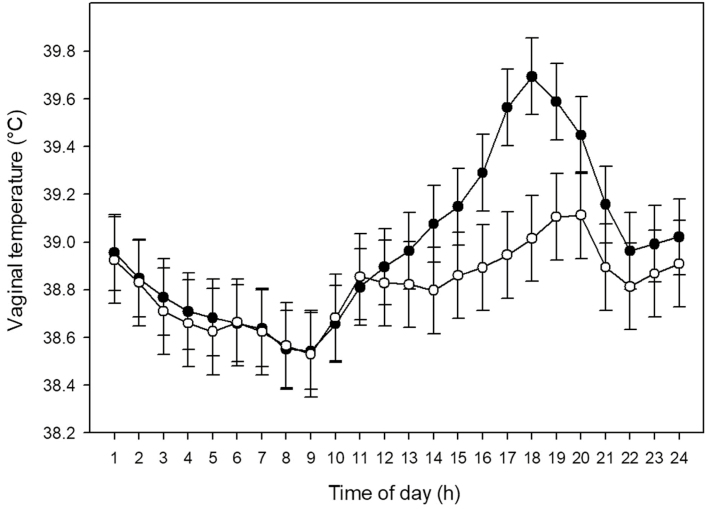
The THI was used to determine the heat-stress potential for each day. The intensity and duration of a thermal challenge determines an animal’s response to the environmental conditions. Two extreme days in their heat-stress potential were identified in groups 2 and 3 (Figure 1). The THI during the high heat-stress potential day was greater than 80 (major heat stress) for 11 h and greater than 85 (critical heat stress) for 6 h. The other extreme day in both groups had a low heat-stress potential with only 7 h of THI greater than 80 and no THI over 85. Because the same heifers within groups 2 and 3 were exposed to days with different heat-stress potential, the estimation of the response in vaginal temperature for an increase in THI was calculated. The maximum difference (5.99) in THI between these 2 d was recorded at hour 1700. The maximum difference in vaginal temperature (0.68 °C) between these 2 d (difference calculated within each cow) was recorded at hour 1800 (Figure 2) indicating that there is an approximately 1 h lag in the animal’s response to the increase in THI. This is in agreement with previous reports that changes in body temperature follow changes in the environmental temperature with some delay (Hahn, 1999; Brown-Brandl et al., 2005).
Figure 1.
Thermal environment during two extreme days in groups 2 and 3 of heifers. Data represent least squares means of temperature–humidity index during low heat-stress potential days (open circles) and high heat-stress potential days (closed circles).
Figure 2.
Least squares means ± SEM for vaginal temperature of the same group of heifers during a low heat-stress potential day (open circles) and a high heat-stress potential day (closed circles).
Coat Score, Chute Score, and BW
Among the fixed effects analyzed for sweating rate, only chute score and exit score were statistically significant (Table 4). The sweating rate for heifers with a calmer temperament in the chute was significantly lower (−5.49 ± 2.12 g/(m2·h), P < 0.01) relative to heifers that were more restless in the chute. The sweating rate was also significantly lower for heifers with a calm and slow exit from the chute (−4.13 ± 2.15 g/(m2·h), P < 0.05) when compared to heifers with a swifter exit. Little research has been previously conducted to analyze the relationship between temperament and sweating rate in cattle. Brown-Brandl et al. (2006) found that excitable heifers in a feedlot environment had a 3.2% higher heat-stress level than calm animals. This supports our findings and would suggest that excitable cattle with a higher chute score would be more sensitive to stress levels.
Table 4.
Effect of coat, chute, and exit score on vaginal temperature (°C) and sweating rate (g/[m2·h]) of Brangus heifers
| Trait/Effect | Estimate | SE | t value | Pr > |t| |
|---|---|---|---|---|
| Vaginal temperature | ||||
| Coat 1 vs. 2 | −0.097 | 0.021 | −4.64 | <0.0001 |
| Chute 1 vs. 2 | −0.047 | 0.019 | −2.42 | 0.015 |
| Exit 1 vs. 2 | 0.011 | 0.02 | 0.57 | 0.567 |
| Sweating rate | ||||
| Coat 1 vs. 2 | 0.32 | 2.12 | 0.15 | 0.879 |
| Chute 1 vs. 2 | −5.49 | 2.12 | −2.59 | 0.009 |
| Exit 1 vs. 2 | −4.13 | 2.15 | −1.92 | 0.055 |
Data represents differences of least squares means and their SE.
Coat score had a significant effect on vaginal temperature (P < 0.0001), where cows with a very smooth coat had significantly lower vaginal temperatures throughout the 3 d of continuous measurements relative to heifers with a less smooth coat type (Table 4). These findings are consistent with results from previous studies which observed a correlation between hair coat characteristics and body temperature. Turner and Schleger (1960) found that hair coat score significantly impacted body temperature of cattle, with animals possessing shorter hair coats maintaining a lower body temperature, suggesting that coat type plays an important role in the regulation of core body temperature. Similarly, the more extreme slick-hair phenotype observed in dairy cattle (Olson et al., 2003; Dikmen et al., 2008) was shown to confer superior thermoregulation. Riley et al. (2012) found a low positive genetic correlation between rectal temperature and coat score in Brahman cattle. A smooth coat can minimize heat gained from the sun by providing greater resistance to heat transfer to the skin (Finch, 1986). Additionally, a long, wooly coat minimizes conductive heat exchange and traps sweat interfering with an efficient evaporation process. Reducing thickness of the hair coat by clipping the hair increases sweating rate (Dikmen et al., 2008, 2014).
Chute behavior is an indicator of temperament for cattle (Grignard et al., 2001; Turner et al., 2011). Heifers with a calm temperament (CHUTE = 1) were able to maintain a significantly lower (P = 0.015) vaginal temperature during the entire duration of the experiment (Table 4). These results suggest that the temperament observed while heifers are handled is a good indicator of their temperament in the field and a restless animal would tend to have a higher internal body temperature.
The estimate of the regression coefficient for weight (−0.0011 ± 0.0003) was significantly different from zero (P < 0.0001), which indicates that heavier heifers are able to maintain a lower vaginal temperature relative to lighter weight heifers. The small regression coefficient is somewhat misleading, as weight has a meaningful impact on the vaginal temperature. For example, based on the estimated regression coefficient, a difference of 50 kg in live weight has a similar effect on vaginal temperature as one class difference in the chute score, and 100 kg difference in live BW has a similar effect as one class difference in the coat score. Numerous studies have found that cattle under heat stress decrease their DM intake and have a reduction in ADG (Hahn, 1999; Brown-Brandl et al., 2006). Heifers that are better adapted to hot and humid environments tend to have higher ADG and will be heavier than other cattle of similar age which are not adapted to these hot conditions. This could also explain the results observed in this study where heavier heifers were the ones with a lower core body temperature during the study.
CONCLUSION
This study found that several different characteristics of the animal including coat score and temperament influence vaginal temperature responses to heat stress in Brangus heifers. As expected, vaginal temperature increases as THI increases, with a 1-h lag time in the animal’s response. Additionally, there was significant variation in vaginal temperature among heifers in the same environmental conditions. Temperament played an important role in both sweating rate and vaginal temperatures, with calm cattle having lower sweating rates and maintaining lower body temperatures, suggesting heifers with a calmer demeanor respond better in hot conditions. Body weight was significantly associated with vaginal temperature, where heavier heifers maintained a lower core body temperature, probably because more thermotolerant heifers have a higher daily gain than those less adapted to the environment. These findings could be useful for producers trying to improve the thermotolerance in their herds by implying that selecting cattle with a calmer temperament and shorter hair coats could help increase thermotolerance. High level of variation in vaginal temperature in a population of same age Brangus heifers, managed uniformly and measured under similar environmental conditions is indicative of selective improvement opportunities.
Research was supported by Grant no. 2017-67007-26143 from the Agriculture and Food Research Initiative of USDA-NIFA, UF Agricultural Experimental Station, UF ANS Hatch Project; Seminole Tribe of Florida; International Brangus Breeders Association; Florida Beef Council, and Florida Cattlemen’s Association.
LITERATURE CITED
- Brown-Brandl T. M., Eigenberg R. A., and Nienaber J. A.. 2006. Heat stress risk factors of feedlot heifers. Livest. Sci. 105:57–68. doi: 10.1016/j.livsci.2006.04.025 [DOI] [Google Scholar]
- Brown-Brandl T. M., Eigenberg R. A., Nienaber J. A., and Hahn G. L.. 2005. Dynamic response indicators of heat stress in shaded and non-shaded feedlot cattle, part 1: analyses of indicators. Biosyst. Eng. 90:451–462. doi: 10.1016/j.biosystemseng.2004.12.006 http://linkinghub.elsevier.com/retrieve/pii/S1537511004002247. [DOI] [Google Scholar]
- Dikmen S., Alava E., Pontes E., Fear J. M., Dikmen B. Y., Olson T. A., and Hansen P. J.. 2008. Differences in thermoregulatory ability between slick-haired and wild-type lactating Holstein cows in response to acute heat stress. J. Dairy Sci. 91:3395–3402. doi: 10.3168/jds.2008-1072 http://www.sciencedirect.com/science/article/pii/S0022030208710543. [DOI] [PubMed] [Google Scholar]
- Dikmen S., Khan F. A., Huson H. J., Sonstegard T. S., Moss J. I., Dahl G. E., & Hansen P. J (2014). The SLICK hair locus derived from Senepol cattle confers thermotolerance to intensively managed lactating Holstein cows. J. Dairy Sci. 97:5508–5520. 10.3168/jds.2014-8087. [DOI] [PubMed] [Google Scholar]
- Elzo M. A., Lamb G. C., Johnson D. D., Thomas M. G., Misztal I., Rae D. O., Martinez C. A., Wasdin J. G., and Driver J. D.. 2012. Genomic-polygenic evaluation of Angus-Brahman multibreed cattle for feed efficiency and postweaning growth using the Illumina 3K chip. J. Anim. Sci. 90:2488–2497. doi: 10.2527/jas.2011-4730 [DOI] [PubMed] [Google Scholar]
- Elzo M. A., Thomas M. G., Martinez C. A., Lamb G. C., Johnson D. D., Rae D. O., Wasdin J. G., and Driver J. D.. 2014. Genomic-polygenic evaluation of multibreed angus-Brahman Cattle for postweaning feed efficiency and growth using actual and imputed Illumina50k SNP genotypes. Livest. Sci. 159:1–10. doi: 10.1016/j.livsci.2013.11.005 [DOI] [Google Scholar]
- FAO statistics 2010. [Accessed February 2018]. http://faostat.fao.org/.
- Finch V. A. 1986. Body temperature in beef cattle : its control and relevance to production in the tropics. J. Anim. Sci. 62:531–542. doi: 10.2134/jas1986.622531x [DOI] [Google Scholar]
- Freetly H. C., and Cundiff L. V.. 1997. Postweaning growth and reproduction characteristics of heifers sired by bulls of seven breeds and raised on different levels of nutrition. J. Anim. Sci. 75:2841–51. http://www.ncbi.nlm.nih.gov/pubmed/9374295. [DOI] [PubMed] [Google Scholar]
- Gaughan J. B., Mader, T. L., Holt, S. M., Josey, M. J., & Rowan, K. J (1999). Heat tolerance of Boran and Tuli crossbred steers. J. Anim. Sci. Technol. 77:2398–2405. 10.2527/1999.7792398x [DOI] [PubMed] [Google Scholar]
- Grandin T. 1993. Behavioral agitation during handling of cattle is persistent over time. Appl. Anim. Behav. Sci. 36:1–9. doi: 10.1016/0168-1591(93)90094-6 https://www.sciencedirect.com/science/article/pii/0168159193900946. [DOI] [Google Scholar]
- Grignard L., Boivin X., Boissy A., and Le Neindre P.. 2001. Do beef cattle react consistently to different handling situations?Appl. Anim. Behav. Sci. 71:263–276. doi: 10.1016/S0168-1591(00)00187-8 https://www.sciencedirect.com/science/article/pii/S0168159100001878. [DOI] [PubMed] [Google Scholar]
- Hahn G. L. 1999. Dynamic responses of cattle to thermal heat loads. J. Anim. Sci. 77 (Suppl 2):10–20. doi: 10.2527/1997.77suppl_210x [DOI] [PubMed] [Google Scholar]
- Hammond A. C., Chase C. C., Bowers E. J., Olson T. A., and Randel R. D.. 1998. Heat tolerance in Tuli-, Senepol-, and Brahman-sired F1 Angus heifers in Florida. J. Anim. Sci. 76:1568–77. http://www.ncbi.nlm.nih.gov/pubmed/9655576. [DOI] [PubMed] [Google Scholar]
- Hammond A. C., and Olson T. A.. 1994. Rectal temperature and grazing time in selected beef cattle breeds under tropical summer conditions in subtropical Florida. Trop. Agric. 71:128–134. https://geoscience.net/research/002/683/002683552.php. [Google Scholar]
- Hansen P. J. 2004. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci. 82-83:349–360. doi: 10.1016/j.anireprosci.2004.04.011 [DOI] [PubMed] [Google Scholar]
- IPCC 2007. Climate change 2007: the physical science basis. Cambridge University Press, Cambridge. [Google Scholar]
- LCI 1970. Patterns of transit losses. Livestock Conservation Inc, Omaha, NE. [Google Scholar]
- Luber G. and McGeehin M.. 2008. Climate change and extreme heat events. Am. J. Prev. Med. 35:429–435. doi: 10.1016/j.amepre.2008.08.021 [DOI] [PubMed] [Google Scholar]
- Magona J. W., Walubengo J., Olaho-Mukani W., Jonsson N. N., and Eisler M. C.. 2009. Diagnostic value of rectal temperature of African cattle of variable coat colour infected with trypanosomes and tick-borne infections. Vet. Parasitol. 160:301–305. doi: 10.1016/j.vetpar.2008.11.020 [DOI] [PubMed] [Google Scholar]
- McManus C., Prescott E., Paludo G. R., Bianchini E., Louvandini H., and Mariante A. S.. 2009. Heat tolerance in naturalized Brazilian cattle breeds. Livest. Sci. 120:256–264. doi: 10.1016/j.livsci.2008.07.014 [DOI] [Google Scholar]
- NRC 1971. A guide to environmental research on animals - National Research Council (U.S.). Committee on physiological effects of environmental factors on animals - Google books. Natl. Acad. Sci, Washington, DC: https://books.google.com/books/about/A_Guide_to_Environmental_Research_on_Ani.html?id=gzsrAAAAYAAJ. [Google Scholar]
- Olson T. A., Lucena C., Chase C. C. Jr, and Hammond A. C.. 2003. Evidence of a major gene influencing hair length and heat tolerance in Bos taurus cattle. J. Anim. Sci. 81:80–90. doi:/2003.81180x [DOI] [PubMed] [Google Scholar]
- Riley D. G., Chase J. C., Coleman S. W., and Olson T. A.. 2012. Genetic assessment of rectal temperature and coat score in Brahman, Angus, and Romosinuano crossbred and straightbred cows and calves under subtropical summer conditions. Livest. Sci. 148:109–118. doi: 10.1016/j.livsci.2012.05.017 http://www.livestockscience.com/article/S1871141312001825/fulltext. [DOI] [Google Scholar]
- St-Pierre N. R., Cobanov B., and Schnitkey G.. 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86:E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5 http://linkinghub.elsevier.com/retrieve/pii/S0022030203740405. [DOI] [Google Scholar]
- Turner S. P., Navajas E. A., Hyslop J. J., Ross D. W., Richardson R. I., Prieto N., Bell M., Jack M. C., and Roehe R.. 2011. Associations between response to handling and growth and meat quality in frequently handled Bos taurus beef cattle. J. Anim. Sci. 89:4239–4248. doi: 10.2527/jas.2010-3790 http://academic.oup.com/jas/article/89/12/4239/4772091. [DOI] [PubMed] [Google Scholar]
- Turner H. G., and Schleger A. V.. 1960. The significance of coat type in cattle. Aust. J. Agric. Econ. 11:645–663. doi: 10.1071/AR9600645 [DOI] [Google Scholar]