Abstract
Background
There are many epidemiological pieces of evidence that show IPF patients have the highest risk of lung cancer. We conducted a systematic review of all published data to define the characteristics of lung cancer that develops in IPF by performing a meta-analysis.
Method
This study was performed based on the PRISMA guideline. Documents gathered by searching through the Web of Sciences, Scopus, PubMed/Medline, OVID, and COCHRANE databases which published before 03/25/2018 that related to lung cancer in IPFs’ patients. Articles were searched using standard keywords as well as Mesh and Mesh Entry and all probabilistic combinations of words using Boolean operators. Data searching, extracting and quality appraising were done by two researchers, independently. At last, Random-effects size based on Cochrane test and I2 were used. The review protocol has been registered in PROSPERO with ID: CRD42018094037.
Results
Based on the meta-analysis conducted in 35 (0.18%) included studies, the total sample size of patients with IPF was estimated 131947 among whom 6384 had LC. The total rate of LC prevalence in IPF patients was estimated to be 13.54% (95% CI: 10.43–17.4) that was significantly 9 times higher in men vs. Women and smoker vs. non-smoker. Highest to lowest prevalence of cellular (histological) subtypes of lung cancer in IPF were SQCC (37.82%), ADC (30.79%), SmCC (20.48%), LCC (5.21%), and ADQC (4.81%), respectively. The highest and lowest stage of lung cancer in IPF patients was estimated at III and II, respectively. The highest involvement location of lung cancer in IPF patients was in the Peripheral. Also, the prevalence of the tumor region involved from the highest to the lowest was estimated to be in the RLL, LLL, RUL and LUL regions.
Conclusions
Lung cancer in IPF, most commonly SQCC, presents in elderly heavy smokers with a male, locating in peripheral regions and the lower part of lung predominance.
1. Introduction
Idiopathic Pulmonary Fibrosis (IPF), known as Cryptogenic Fibrosing Alveolitis (CFA), diffuse Fibrosing Alveolitis (diff FA), and Usual Interstitial Pneumonitis (UIP) is one of the most common forms of Interstitial Lung Disease (ILD) over the years [1–6]. IPF is an unknown chronic pulmonary disease with unknown origin. It is a chronic lung disease characterized by a progressive and irreversible decline in lung function [1, 7]. Due to the increasing prevalence of this disease in the United States of America, 48,000 new cases are diagnosed each year, with an annual mortality rate of 40,000 individuals (84%), which is as many as the mortality rate of breast cancer [8]. The incidence of IPF rises exponentially in the 50–85-year-old age groups (only 2–15% prevalence in patients under 50 [2, 9, 10]) [11–15].
Family history of disease (contrary to the common form, the prevalence in younger ages is higher[16–18])[16, 17, 19, 20], male gender, smoking, various types of environmental effects such as organic and inorganic dust, medical treatments, other medical disorders and microbial agents like Epstein-Barr viruses are risk factors that increase the risk of IPF [21]. Age over 50 years, dry and non-productive cough on exertion, progressive exertional dyspnea, dry inspiratory bibasilar crackles “Velcro-like” on auscultation, clubbing of the digits, hypoxemia, and abnormal results of lung function test and, constraint evidence and disturbance in gas exchange are symptoms, clinical and diagnostic features of IPF [11, 14, 15, 22].
Among radiographic features used in screening IPF patients, chest x-ray (CXR) can be mentioned, which the diagnosis and clinical manifestations may show bilateral interstitial opacities with the possibility of occurrence in peripheral and lower lung zones. This demonstration may not be visible in 2 to 10% of patients [1, 11, 22]. Also, HRCT is very important and crucial due to the high sensitivity and specificity which can differentiate IPF from other ILDs [1, 23, 24]. According to the ATS / ERS / JRS / ALAT 2011 protocol, the HRCT is the essential test for diagnosing IPF. Reticular opacities (mostly associated with bronchiectasis), honeycombing manifested (typically with 3–10 mm, occasionally large, and usually sub-pleural with well-defined walls), ground-glass opacities (more common, but less than reticulation) and distribution, particularly is in the basal and peripheral and often scattered areas that can be the diagnosis and clinical manifestations in the HRCT test of patients with IPF[1]. Also, forced vital capacity (FVC) ≥50%, diffusing capacity for carbon monoxide (DLCO) ≥ 30% and 6-minute walk test (6MWT) distance≥ 150 meters in the spirometry test represent the mild-to-moderate level of IPF disease [25–28]. Likewise, in laboratory testing issued for screening patients with ILDs, including IPF, the Krebs von den Lungen 6 (KL-6) biomarker is referred, which is not widely used[29, 30]. Since the diagnosis of IPF involves clinical, radiological and histopathological findings, the multi-diagnostic test increases the accuracy[31].
Among, lung cancer(LC)[32], pulmonary hypertension (PH)[33], obstructive sleep apnea syndrome[34], gastroesophageal reflux[35], coronary heart disease[1], pulmonic component of the second heart sound, right ventricular lift and tricuspid regurgitation[36, 37] can be mentioned as the comorbidities and common complications in patients with IPF. And, despite numerous conducted studies their correlation and cause have not been explicitly and directly specified[38].
There are many epidemiological pieces of evidence that show IPF patients have the highest risk of lung cancer[39, 40], that has been reported more often in older men smokers[8, 41–44]. Lung cancer detection in IPF patients due to fibrotic changes in the lung is difficult, which appears more often as nodular lesions with irregular or spiculated margins in peripheral lung zones[45, 46] that its diagnosis can be evaluated before, during and after treatment[8, 41–44]. The risk of IPF patients with LC during surgical treatment is much higher than those without IPF[47] that in severe cases causes acute respiratory distress syndrome[48, 49].
Despite previously published studies about the prevalence of LC in IPF patients in different countries (primary study) [3–6, 41, 44, 50–79], no comprehensive study and systematic review and meta-analysis have been conducted globally. One of the most important goals of systematic review and meta-analysis studies is combining the existing studies to increase sample size due to the increased number of related studies and to reduce the differences between the existing parameters and confidence interval, which ultimately leads to solving the review problems in the previous method. Certainly, such studies are a vital link between research studies and clinical decision making at the patient's bedside[80–86]. Considering the above mentioned cases and the prevalence, severity and extent of LC in IPF patients, as well as the presentation of the final conclusions for policy-making and correct management planning at the macro level, a systematic review of all documentation and their combination, is conducted via meta-analysis method to estimate the overall rate of LC in IPF patients and other risk factors.
2. Materials and methods
2.1. Study protocol
The present study is based on the Meta-analysis of Observational Studies in Epidemiology guideline [87] and it has been conducted in 5 steps according to the PRISMA statement[88] (S1 File) including design and search strategy, a collection of articles and their systematic review, evaluation of inclusion and exclusion criteria, qualitative evaluation and statistical analysis of data. All steps were carried out by two researchers independently and, any encounters were assessed by a specialist. The review protocol has been registered in PROSPERO: International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/PROSPERO/) Identifier: CRD42018094037 [89, 90](S2 File).
2.2. Search strategy
An advanced relevant search was conducted in international databases, such as Web of Sciences, Scopus, PubMed/Medline, OVID, and COCHRANE, to collect all of the studies which were related to LC in IPF patients. Articles were searched using standard keywords as well as Mesh and Mesh Entry and all probabilistic combinations of words using Boolean operators combined in accordance with the search syntax (S1 Table) on each database without time limit until 03/25/2018. And, a manual search was also done as reviewing the reference list of related articles. The important point in searching the databases was the high-sensitivity searching, and also the search was conducted by the researchers and a specialist which is expert in searching databases (A.R).
2.3. Inclusion and exclusion criteria
2.3.1. Inclusion criteria based on PICO (related to Evidence-Based Medicine) [91]
(1) Population: Cohort and retrospective studies that investigated LC in IPF patients; (2) Intervention: Surgical resection or radiological and pathologically confirmed cancer; (3) Comparison: That can show the rate of LC incidence in relation to non-LC in patients with IPF, which is called prevalence rate; (4) Outcome: Estimate the overall rate of LC in IPF patients and other risk factors.
2.3.2. Exclusion criteria
(1) Review articles, Letters, Comments, Case reports (only for estimating overall prevalence), or Conference proceedings; (2) Studies that did not focus on the prevalence of LC in IPF patients; (3) Duplicated papers; (4) Non-English full text; (5) Non-accessible full text.
2.4. IPF and LC detection criteria
2.4.1. IPF detection criteria
All patients after initial diagnosis of IPF based on CT-Scan and pathological criteria by examining the dossier or attending the clinic, according to the American Thoracic Society (ATS) and the European Respiratory Society (ERS)[1, 92, 93] and the official ATS / ERS / Japanese Respiratory Society / Latin American Thoracic Society statement on IPF[13, 94, 95] were also tested for their IPF disease (and other parameters of Pulmonary function tests such as: forced vital capacity (FVC) of ≥50% and DLCO of ≥30% and 6-minute walk test (6MWT) distance ≥150 meters[25–28]).
2.4.2. LC detection criteria
To diagnose the LC in patients, after presuming IPF, the pathology and cytology reports were evaluated and categorized into two groups of patients with and without LC. Cellular (histological) subtypes and clinical staging and region and location of LC were also evaluated in patients[3, 6, 55, 96].
2.5. Selection of studies
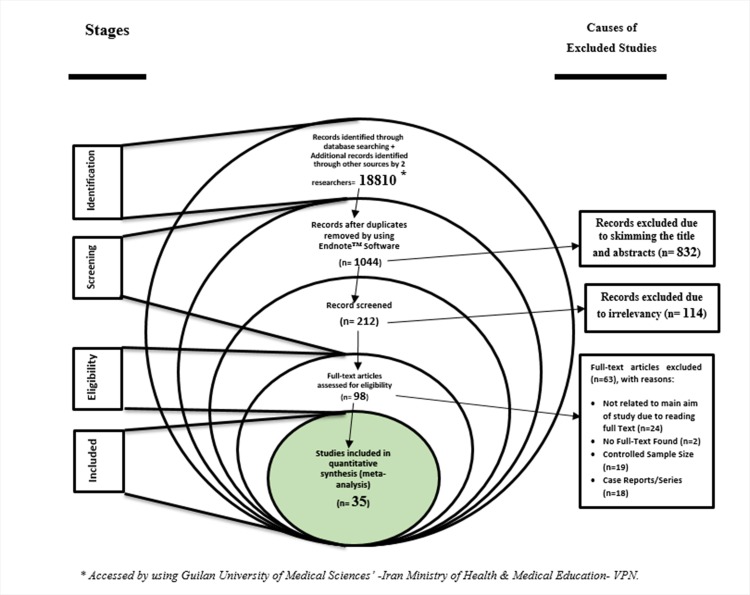
After the end of the search, the papers were entered into the EndNote, reference management software and, after “Find References Updates”, duplicates were removed. After blinding studies (hiding authors', the name of the journal and published year), each study was evaluated by two researchers independently in the screening stage, with skimming and scanning the study titles, to evaluate the inclusion and exclusion criteria and IPF and LC detection criteria (the eligibility stage). In the event of disagreement between the two researchers, the specialist researcher made the final decision (Fig 1).
Fig 1. A flow diagram (Stacked Venn) following the “PRISMA flow chart”.
2.6. Quality appraisal
After excluding irrelevant studies in the screening and eligibility stages, the quality of the final studies was examined. At this stage, the Newcastle-Ottawa Scale (NOS)[97] checklist (S3 File) was used which consists of 8 sections, and divides the studies with a scale score of 0 to 8 from poor to high quality, respectively. According to this scoring, the studies are divided into three levels of scoring: 1- Studies with a score of 5: poor quality; 2- studies with a score of 5–6: medium quality; 3- studies with a score of 7 to 8: high quality. Finally, (at the included stage), the articles that had medium to high quality were entered into the next stage (Fig 1).
2.7. Data extraction
At first, a checklist was designed according to the aims of the study and studying other available resources. Designed checklist includes items: Author name, Year, Place, Sample Size(SS), Periods of time, SS of IPF, SS of LC, Prevalence (LC in IPF), Cellular (Histological) Subtypes (ADC(Adenocarcinoma (%)), SQCC(Squamous-cell carcinoma (%)), SmCC(Small-cell carcinoma (%)), LCC(large-cell carcinoma (%)), ADSQC(Adeno-Squamous carcinoma (%)), Others), Clinical Staging (I, II, II, IV), Prevalence separated by Sex, Prevalence separated by Smoking status, FEV1(Forced Expiratory Volume in the first second) (Mean±SD), FVC1(Forced Vital Capacity in the first second) (Mean±SD), FEV1/FVC1 (Mean±SD), Age (Mean±SD), DLco(Diffusing capacity for carbon monoxide) (Mean±SD), Region (RUL(Right Upper Lobe), LUL(Left Upper Lobe), RLL(Right Lower Lobe), LLL(Left Lower Lobe), Upper, Center, Lower) and Location (Peripheral and Central), which were extracted by two independent researchers and blind for the name of the author, institute and journal. In necessary cases, further information and raw data were requested by contacting the author (the first author or responsible or the authors' department).
2.8. Statistical analysis
In each study, after considering the prevalence rate of LC in IPF patients as a binomial distribution probability, its variance was calculated by binomial distribution and for evaluating the heterogeneity of the studies Cochran test (Q) and I2 index were used. The I2 index less than 25% is low heterogeneity, between 75% -25% is the average heterogeneity and more than 75% are considered as heterogeneous[85, 98]. According to the heterogeneity of studies (high), the random effects model has been used to combine the results of studies. Sensitivity analysis (“One Study Removed” test) was conducted to investigate the impact of each study on total results for the overall prevalence and each of the risk factors. In order to evaluate the cause of heterogeneity, the subgroup analysis was performed based on the country, and gender. The Meta-Regression model was used to determine the prevalence rate based on the year of publication. The Egger and Begg's test were evaluated to examine the publication bias (by using the Funnel Plot). Data analysis was performed using the Comprehensive Meta-Analysis Ver.2, and the significance level of the test was considered less than 0.05.
3. Results
3.1. Search results and characteristics
In this systematic study, based on performed searches, 667 articles were identified and after conclusive investigation and evaluation according to the checklist, 35 (0.18%) articles[3–6, 44, 50–79](S2 Table)(Fig 1) were entered into the final list. The total sample size was estimated to be 38184 patients, with 131947 IPF patients, of which 6384 had LC. The mean age of patients, DLco, FEV, FVC and FEV1/ FVC1 was estimated to be 69.06±2.57, 59.61±12.52, 83.82±6.36, 85.44±6.44 and 76.9±6.47, respectively.
3.2. Overall prevalence of LC in IPF
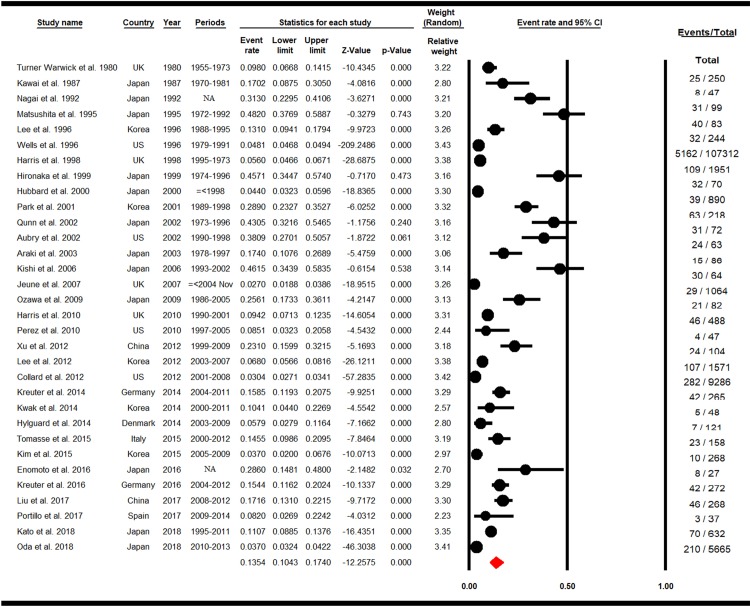
The overall prevalence rate of LC in patients with IPF was calculated at 13.54% (95% Confidence Interval (CI): 10.43–17.4, P = 0.000). The highest and lowest prevalence rate was observed in Matsushita et al. (1995) from Japan with a prevalence rate of 48.2% (95% CI: 37.69–58.87) and Jeune et al. (2007) from the UK with a prevalence rate of 2.7% (95% CI: 1.88–3.86), respectively (Fig 2).
Fig 2. Forest plot for overall prevalence of LC in IPF.
(Random effects model).
3.3. Prevalence of LC in IPF based on country
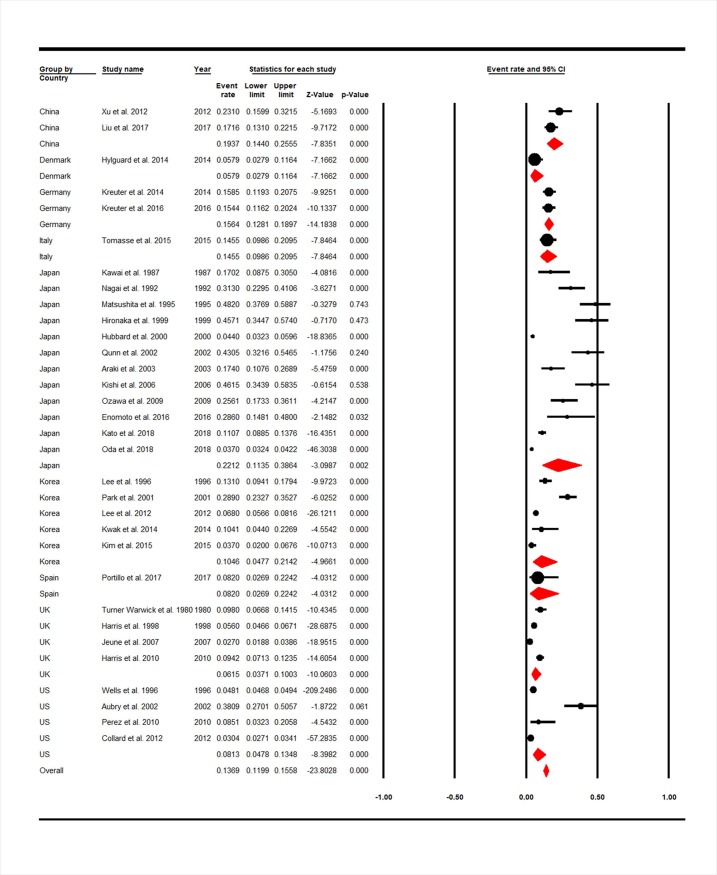
Among the studies in 8 countries, the highest and lowest prevalence of LC in IPF patients was in Japan with 12 studies and Denmark with 1 study that were estimated 22.12% (95% CI: 11.35–38.64) and 5.79% (95% CI: 2.79–11.64) which were statistically significant (P = 0.000) (Fig 3).
Fig 3. Forest plot for overall prevalence of LC in IPF sub-grouped based on country.
3.4. Cumulative meta-analysis and sensitivity analysis of studies
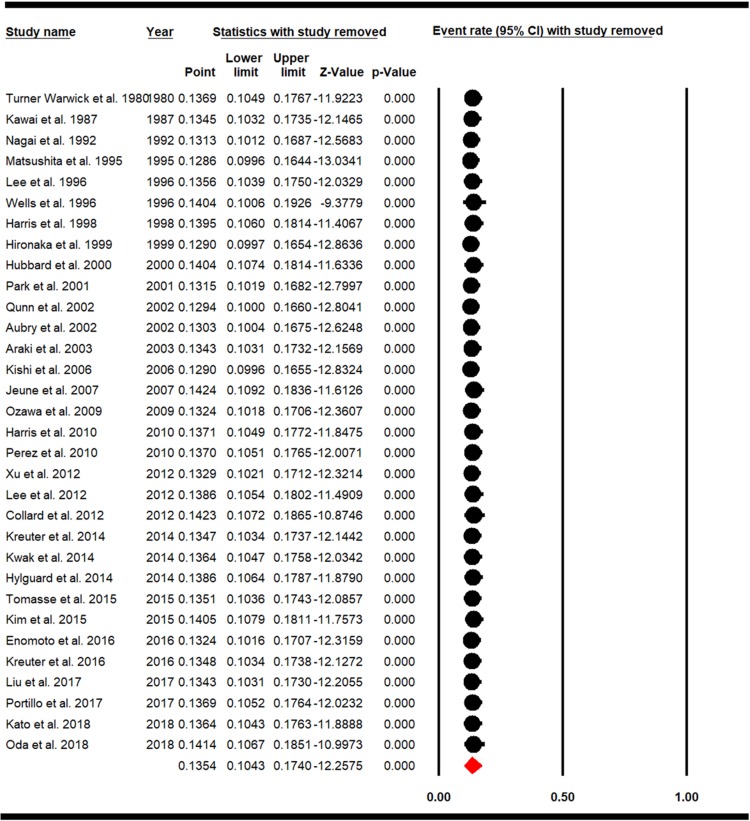
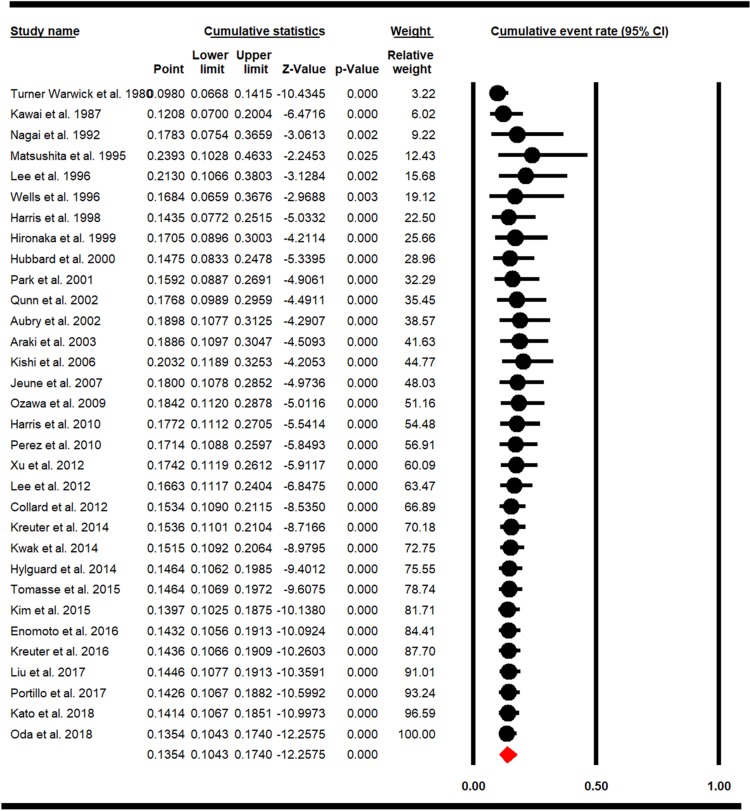
Sensitivity analysis of LC prevalence in IPF patients and the confidence interval of each study was calculated with a 95% confidence interval, and results showed that before and after exclusion of each study there had been no significant effect on the overall prevalence rate of LC in IPF patients (Fig 4). Cumulative meta-analysis is also estimated for the overall prevalence of LC in IPF patients based on the publication year and is represented in (Fig 5).
Fig 4. Sensitivity analysis for overall prevalence of LC in IPF.
Fig 5. Cumulative meta-analysis for overall prevalence of LC in IPF.
3.5. Meta-regression
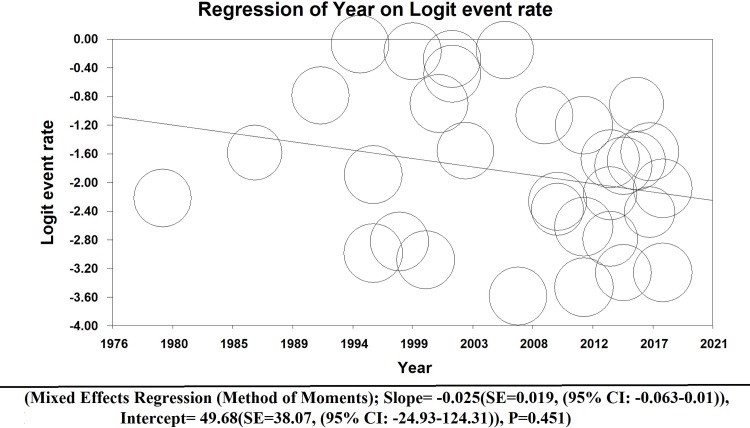
The Meta-Regression test shows that the prevalence of LC in IPF patients has been decreased by the year of publication, but this relationship is not statistically significant (Mixed effects regression (Method of moments); Slope = -0.025(SE = 0.019, (95% CI: -0.063–0.01)), Intercept = 49.68(SE = 38.07, (95% CI: -24.93–124.31)), P = 0.451)(Fig 6).
Fig 6. Meta-regression model of overall prevalence of LC in IPF (Method of moments).
3.6. Prevalence of LC in IPF based on gender
The prevalence rate of LC in IPF patients with female gender was estimated to be 9.48% (77 out of 890) (95% CI: 7.49–11.94) and in the male gender 91.22% (813 out of 890) (95% CI: 88.72–93.2) that this difference (1: 9; female: male) was statistically significant (P = 0.000) (S1 Fig).
3.7. Prevalence of smoking statue in LC-IPFs’ patients
The prevalence of smoking in LC-IPF patients were estimated to be 90.74% (95% CI: 87.07–93.44) (S2 Fig).
3.8. Cellular (histological) subtypes of LC in IPF
The prevalence of SQCC in IPF patients with LC was estimated 37.82% (95% CI: 32.65–43.29) (S3 Fig), which the highest prevalence was in France and the USA with 66.66% and the lowest was in China (27.17%). (S4 Fig). The prevalence of ADC in IPF patients with LC was estimated 30.79% (95% CI: 26.49–35.46) (S5 Fig), with the highest prevalence in China (41.58%), and the lowest in France (20%) (S6 Fig). The prevalence of SmCC in IPF patients with LC was estimated 20.48% (95% CI: 14.94–27.43) (S7 Fig), with the highest prevalence in Japan (24.93%) and the lowest prevalence in France (3.33%) (S8 Fig). The prevalence of LCC in IPF patients with LC was estimated 5.21% (95% CI: 2.91–9.17) (S9 Fig) that prevalence in Japan (6.21%) was estimated more than Korea (3.74%) (S10 Fig). The prevalence of ADQC in IPF patients with LC was estimated 4.81% (95% CI: 2.42–9.34) (S11 Fig.) that the highest prevalence was in China (8.3%) and the lowest was in Japan (3.15%) (S12 Fig).
3.9. Prevalence of LC in IPF according to clinical staging
The highest and lowest Stage of LC in IPF patients were estimated in III and II with a prevalence of 30.72% (95% CI: 22.68–40.14) and 13.33% (95% CI: 8.74–19.81), respectively (P = 0.000). The highest density was observed in Stage III and IV (S13 Fig).
3.10. Region and location of LC in IPF
The involvement location of LC in IPF patients was more pronounced in the peripheral area with a prevalence of 84.03% (95% CI: 75.47–89.99), which is statistically significant (P = 0.004) (S14 Fig). The tumor region involved in LC in IPF patients, from the highest to the lowest was estimated in the lower, upper and middle regions with a prevalence of 52.37% (95% CI: 47.54–57.15), 38.98% (95% CI: 33.2–45.05) and 5.37% (95% CI: 3.92–7.34), respectively, which is statistically significant (P = 0.000) (S15 Fig). The prevalence of the tumor region involved with the most detail from the highest to the lowest was estimated to be in the RLL, LLL, RUL and LUL regions with 32.74% (95% CI: 28.76–36.98), 23.46% (95% CI: 18.62–29.12), 19.99% (95% CI: 14.72–26.54) and 17.39% (95% CI: 14.26–21.03), respectively, which is statistically significant (P = 0.000) (S16 Fig).
3.11. Publication bias
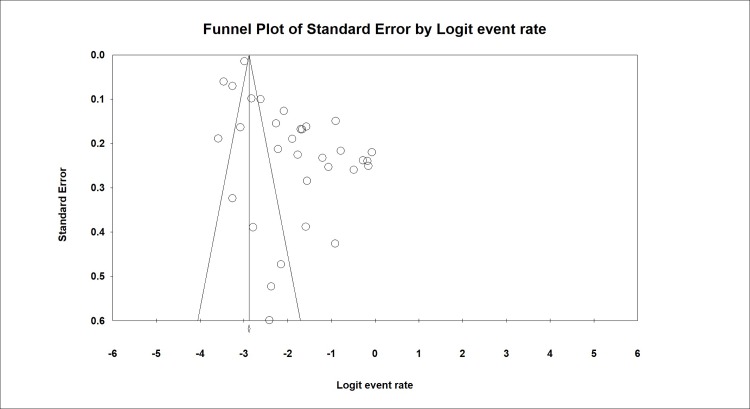
The publication bias was also evaluated by Begg and Egger’s tests and was estimated at P = 0.516 and P = 0.0521, respectively. In this test, the probability of publication bias was not statistically significant (Fig 7).
Fig 7. Publication bias of studies included due to the aim of prevalence of LC in IPF.
4. Discussion
The total sample size of patients with IPF was estimated 131947 among whom 6384 had LC. The total rate of LC prevalence in IPF patients based on the meta-analysis review was estimated to be 13.54% (95% CI: 10.43–17.4) that was significantly 9 times higher in men than women. Also, the prevalence of smoking in LC-IPF patients is estimated to be 9 times higher. Highest to lowest prevalence of cellular (histological) subtypes of lung cancer in IPF were SQCC (37.82%), ADC (30.79%), SmCC (20.48%), LCC (5.21%), and ADQC (4.81%), respectively. The highest and lowest stage of lung cancer in IPF patients was estimated at III and II, respectively. The highest involvement location of lung cancer in IPF patients was in the Peripheral region with highest to the lowest RLL, LLL, RUL and LUL regions respectively, that totally the highest to lowest was estimated to be in the lower, upper and middle regions.
Age and smoking status are also known to be the effective factors in the development of lung cancer in IPF patients [50, 70]. Nearly every patient with prostate cancer and lung cancer (95%) has finger clubbing, while the percentage of IPF patients are approximately only 60%, which is often known as the clinical evidence of lung cancer[40, 62].
Recent studies found that the progression of lung cancer in lower lobes is higher in IPF patients with[99, 100]. As also found in the findings of the present study, there is a significant relationship between the involved lobes, which can be a phenomenon called “scar–cinoma” between fibrotic areas and cancer progression[101]. Although further studies are required to prove this[101–105].
Evidence represent that IPF and LC have common pathological features[106]. Anticancer agents, like Nintedanib, show pleiotropic anti-fibrotic properties[28, 107, 108]. Among the common pathological characteristics of IPF and LC, the following can be mentioned: increased proliferation, proteus loss, immune dysregulation, senescence features and resistance against apoptosis, telomeric attrition, and disorder in bioenergetics of the cell[109–111]. Also, the key features of epithelial fibrosis are epithelial to mesenchymal transition (EMT), which plays an important role in lung cancer[101–105].
In another study, epigenetic and genetic changes, abnormal expression of microRNAs (miRNAs, cellular and molecular aberrances), like different responses to regulatory signals, apoptosis, delay or decrease in cell-to-cell correlation along with activation of specific signal transmission pathways lead to pathogenic features of both LC-IPF. Likewise, genetic analysis has shown that harmful and deleterious mutations in A1 or A2-surfactant proteins cause familial idiopathic interstitial pneumonia and lung cancer[112–114].
The heterogeneity rate (I2) in the present study was calculated at 90.71%, which is in the line of studies with high heterogeneity. It is assumed that the observed differences are due to various samplings and also the difference in the measured parameter in different societies[87, 88, 115].
According to the meta-regression, the prevalence of the LC-IPF was not statistically significant (p = 0.451) by the publication year. Even though the studies are in different countries and in years, but these findings cannot represent the reality in all countries, so further studies are needed to be conducted in this regard.
5. Limitations
One of the main limitations of this meta-analysis to be mentioned is the inclusion of studies with different inclusion and exclusion criteria, and there is no consensus definition of IPF expressed. Selection Bias is more discussed which can limit the generalization of these findings because the type of lung cancer in a country can be different with the other countries and could be related to descent diversities. Due to lack of resources and very few studies have investigated the survival rate and causes of mortality and the precise methods of treatment, according to the aim of the present study, we did not focus on these factors. Also, data were accessed by using Guilan University of Medical Sciences’ -Iran Ministry of Health & Medical Education- VPN which some databases are not fully accessible.
6. Conclusion
In conclusion, the high prevalence of the LC-IPF with 13.5% is more observed in older men who smoke, and is more evident in the progression of cancer, SQCC, and SmCC, and mostly affects the peripheral regions and the lower part of the lung. Studies have been conducted in limited countries, such as Japan, Korea, and UK and USA, which the weakness of a unit study of LC-IPF in different countries investigating the factors and important risk factors and reaching to a consensus and preparing a comprehensive global database for clinical decision-making is felt and is an essential need.
Supporting information
(DOC)
(PDF)
(PDF)
(DOCX)
(XLSX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
We would like to express sincere gratitude to thank Dr. Aboozar Ramezani (as an expert in Medical Library and Information) for his selfless and worthy collaboration.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. Epub 2011/04/08. 10.1164/rccm.2009-040GL ; PubMed Central PMCID: PMCPMC5450933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–6. Epub 2006/07/01. 10.1164/rccm.200602-163OC . [DOI] [PubMed] [Google Scholar]
- 3.Kato E, Takayanagi N, Takaku Y, Kagiyama N, Kanauchi T, Ishiguro T, et al. Incidence and predictive factors of lung cancer in patients with idiopathic pulmonary fibrosis. ERJ open research. 2018;4(1). Epub 2018/02/08. ; PubMed Central PMCID: PMCPMC5795191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreuter M, Ehlers-Tenenbaum S, Palmowski K, Bruhwyler J, Oltmanns U, Muley T, et al. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PloS one. 2016;11(3):e0151425 Epub 2016/03/31. 10.1371/journal.pone.0151425 ; PubMed Central PMCID: PMCPMC4811578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Jeune I, Gribbin J, West J, Smith C, Cullinan P, Hubbard R. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respiratory medicine. 2007;101(12):2534–40. Epub 2007/09/18. 10.1016/j.rmed.2007.07.012 . [DOI] [PubMed] [Google Scholar]
- 6.Park J, Kim DS, Shim TS, Lim CM, Koh Y, Lee SD, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. The European respiratory journal. 2001;17(6):1216–9. Epub 2001/08/09. . [DOI] [PubMed] [Google Scholar]
- 7.Ferri FF. Ferri's Clinical Advisor 2010 E-Book: 5 Books in 1: Elsevier Health Sciences; 2009.
- 8.Le Jeune I, Gribbin J, West J, Smith C, Cullinan P, Hubbard R. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respiratory medicine. 2007;101(12):2534–40. 10.1016/j.rmed.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 9.Pérez ERF, Daniels CE, Sauver JS, Hartman TE, Bartholmai BJ, Eunhee SY, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137(1):129–37. 10.1378/chest.09-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadrous HF, Myers JL, Decker PA, Ryu JH, editors. Idiopathic pulmonary fibrosis in patients younger than 50 years Mayo Clinic Proceedings; 2005: Elsevier. [DOI] [PubMed] [Google Scholar]
- 11.Ryu JH, Colby TV, Hartman TE, editors. Idiopathic pulmonary fibrosis: current concepts Mayo Clinic Proceedings; 1998: Elsevier. [DOI] [PubMed] [Google Scholar]
- 12.Society AT. Idiopathic pulmonary fibrosis: diagnosis and treatment: international consensus statement. Am J Respir Crit Care Med. 2000;161:646–64. 10.1164/ajrccm.161.2.ats3-00 [DOI] [PubMed] [Google Scholar]
- 13.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine. 2011;183(6):788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas WW, Ryu JH, Schroeder DR. Idiopathic pulmonary fibrosis: impact of oxygen and colchicine, prednisone, or no therapy on survival. American journal of respiratory and critical care medicine. 2000;161(4):1172–8. [DOI] [PubMed] [Google Scholar]
- 15.King TE Jr, Tooze JA, Schwarz MI, BROWN KR, CHERNIACK RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. American journal of respiratory and critical care medicine. 2001;164(7):1171–81. 10.1164/ajrccm.164.7.2003140 [DOI] [PubMed] [Google Scholar]
- 16.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172(9):1146–52. Epub 2005/08/20. 10.1164/rccm.200408-1104OC ; PubMed Central PMCID: PMCPMC2718398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HL, Ryu JH, Wittmer MH, Hartman TE, Lymp JF, Tazelaar HD, et al. Familial idiopathic pulmonary fibrosis: clinical features and outcome. Chest. 2005;127(6):2034–41. Epub 2005/06/11. 10.1378/chest.127.6.2034 . [DOI] [PubMed] [Google Scholar]
- 18.Borie R, Kannengiesser C, Crestani B. Familial forms of nonspecific interstitial pneumonia/idiopathic pulmonary fibrosis: clinical course and genetic background. Curr Opin Pulm Med. 2012;18(5):455–61. Epub 2012/07/12. 10.1097/MCP.0b013e328356b15c . [DOI] [PubMed] [Google Scholar]
- 19.Allam JS, Limper AH. Idiopathic pulmonary fibrosis: is it a familial disease? Current opinion in pulmonary medicine. 2006;12(5):312–7. 10.1097/01.mcp.0000239546.24831.61 [DOI] [PubMed] [Google Scholar]
- 20.Wahidi MM, Speer MC, Steele MP, Brown KK, Schwarz MI, Schwartz DA. Familial pulmonary fibrosis in the United States. Chest. 2002;121(3 Suppl):30S Epub 2002/03/15. . [DOI] [PubMed] [Google Scholar]
- 21.García-Sancho Figueroa MC, Carrillo G, Pérez-Padilla R, Fernández-Plata MR, Buendía-Roldán I, Vargas MH, et al. Risk factors for idiopathic pulmonary fibrosis in a Mexican population. A case-control study. Respiratory medicine. 2010;104(2):305–9. 10.1016/j.rmed.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–64. Epub 2000/02/15. 10.1164/ajrccm.161.2.ats3-00 . [DOI] [PubMed] [Google Scholar]
- 23.Lynch DA, Godwin JD, Safrin S, Starko KM, Hormel P, Brown KK, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172(4):488–93. Epub 2005/05/17. 10.1164/rccm.200412-1756OC . [DOI] [PubMed] [Google Scholar]
- 24.Silva CI, Muller NL. Idiopathic interstitial pneumonias. J Thorac Imaging. 2009;24(4):260–73. Epub 2009/11/26. 10.1097/RTI.0b013e3181c1a9eb . [DOI] [PubMed] [Google Scholar]
- 25.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–9. Epub 2011/05/17. 10.1016/S0140-6736(11)60405-4 . [DOI] [PubMed] [Google Scholar]
- 26.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE Jr., et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142(12 Pt 1):963–7. Epub 2005/06/22. . [DOI] [PubMed] [Google Scholar]
- 27.King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. New England Journal of Medicine. 2014;370(22):2083–92. 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 28.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. Epub 2014/05/20. 10.1056/NEJMoa1402584 . [DOI] [PubMed] [Google Scholar]
- 29.Tzouvelekis A, Kouliatsis G, Anevlavis S, Bouros D. Serum biomarkers in interstitial lung diseases. Respir Res. 2005;6(1):78 Epub 2005/07/27. 10.1186/1465-9921-6-78 ; PubMed Central PMCID: PMCPMC1215520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasse A, Muller-Quernheim J. Non-invasive biomarkers in pulmonary fibrosis. Respirology. 2009;14(6):788–95. Epub 2009/08/26. 10.1111/j.1440-1843.2009.01600.x . [DOI] [PubMed] [Google Scholar]
- 31.Flaherty KR, King TE Jr, Raghu G, Lynch III JP, Colby TV, Travis WD, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? American journal of respiratory and critical care medicine. 2004;170(8):904–10. 10.1164/rccm.200402-147OC [DOI] [PubMed] [Google Scholar]
- 32.Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. The European respiratory journal. 2015;46(4):1113–30. Epub 2015/10/02. 10.1183/13993003.02316-2014 . [DOI] [PubMed] [Google Scholar]
- 33.Seeger W, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D109–16. Epub 2013/12/21. 10.1016/j.jacc.2013.10.036 . [DOI] [PubMed] [Google Scholar]
- 34.Lancaster LH, Mason WR, Parnell JA, Rice TW, Loyd JE, Milstone AP, et al. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136(3):772–8. 10.1378/chest.08-2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savarino E, Carbone R, Marabotto E, Furnari M, Sconfienza L, Ghio M, et al. Gastro-oesophageal reflux and gastric aspiration in idiopathic pulmonary fibrosis patients. The European respiratory journal. 2013;42(5):1322–31. Epub 2013/03/09. 10.1183/09031936.00101212 . [DOI] [PubMed] [Google Scholar]
- 36.Behr J, Ryu JH. Pulmonary hypertension in interstitial lung disease. The European respiratory journal. 2008;31(6):1357–67. Epub 2008/06/03. 10.1183/09031936.00171307 . [DOI] [PubMed] [Google Scholar]
- 37.Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med. 2007;175(9):875–80. Epub 2007/01/27. 10.1164/rccm.200608-1153CC . [DOI] [PubMed] [Google Scholar]
- 38.Fulton BG, Ryerson CJ. Managing comorbidities in idiopathic pulmonary fibrosis. Int J Gen Med. 2015;8:309–18. Epub 2015/10/10. 10.2147/IJGM.S74880 ; PubMed Central PMCID: PMCPMC4590408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Archontogeorgis K, Steiropoulos P, Tzouvelekis A, Nena E, Bouros D. Lung cancer and interstitial lung diseases: a systematic review. Pulm Med. 2012;2012:315918 Epub 2012/08/18. 10.1155/2012/315918 ; PubMed Central PMCID: PMCPMC3414065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouros D, Hatzakis K, Labrakis H, Zeibecoglou K. Association of malignancy with diseases causing interstitial pulmonary changes. Chest. 2002;121(4):1278–89. Epub 2002/04/12. . [DOI] [PubMed] [Google Scholar]
- 41.Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med. 2000;161(1):5–8. Epub 2000/01/05. 10.1164/ajrccm.161.1.9906062 . [DOI] [PubMed] [Google Scholar]
- 42.Aubry M-C, Myers JL, Douglas WW, Tazelaar HD, Stephens TLW, Hartman TE, et al. , editors. Primary pulmonary carcinoma in patients with idiopathic pulmonary fibrosis Mayo Clinic Proceedings; 2002: Elsevier. [DOI] [PubMed] [Google Scholar]
- 43.Daniels CE, Jett JR. Does interstitial lung disease predispose to lung cancer? Current opinion in pulmonary medicine. 2005;11(5):431–7. [DOI] [PubMed] [Google Scholar]
- 44.Harris JM, Johnston ID, Rudd R, Taylor AJ, Cullinan P. Cryptogenic fibrosing alveolitis and lung cancer: the BTS study. Thorax. 2010;65(1):70–6. Epub 2009/12/10. 10.1136/thx.2009.121962 . [DOI] [PubMed] [Google Scholar]
- 45.Kishi K, Homma S, Kurosaki A, Motoi N, Yoshimura K. High-resolution computed tomography findings of lung cancer associated with idiopathic pulmonary fibrosis. Journal of computer assisted tomography. 2006;30(1):95–9. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida R, Arakawa H, Kaji Y. Lung cancer in chronic interstitial pneumonia: early manifestation from serial CT observations. AJR Am J Roentgenol. 2012;199(1):85–90. Epub 2012/06/27. 10.2214/AJR.11.7516 . [DOI] [PubMed] [Google Scholar]
- 47.Kushibe K, Kawaguchi T, Takahama M, Kimura M, Tojo T, Taniguchi S. Operative indications for lung cancer with idiopathic pulmonary fibrosis. The Thoracic and cardiovascular surgeon. 2007;55(8):505–8. Epub 2007/11/21. 10.1055/s-2007-965645 . [DOI] [PubMed] [Google Scholar]
- 48.Kushibe K, Kawaguchi T, Takahama M, Kimura M, Tojo T, Taniguchi S. Operative indications for lung cancer with idiopathic pulmonary fibrosis. The Thoracic and cardiovascular surgeon. 2007;55(08):505–8. [DOI] [PubMed] [Google Scholar]
- 49.Park JS, Kim HK, Kim K, Kim J, Shim YM, Choi YS. Prediction of acute pulmonary complications after resection of lung cancer in patients with preexisting interstitial lung disease. The Thoracic and cardiovascular surgeon. 2011;59(3):148–52. Epub 2011/04/12. 10.1055/s-0030-1250644 . [DOI] [PubMed] [Google Scholar]
- 50.Nagai A, Chiyotani A, Nakadate T, Konno K. Lung cancer in patients with idiopathic pulmonary fibrosis. The Tohoku journal of experimental medicine. 1992;167(3):231–7. Epub 1992/07/01. . [DOI] [PubMed] [Google Scholar]
- 51.Hironaka M, Fukayama M. Pulmonary fibrosis and lung carcinoma: A comparative study of metaplastic epithelia in honeycombed areas of usual interstitial pneumonia with or without lung carcinoma. Pathol Int. 1999;49(12):1060–6. 10.1046/j.1440-1827.1999.00989.x [DOI] [PubMed] [Google Scholar]
- 52.Wells C, Mannino DM. Pulmonary fibrosis and lung cancer in the United States: analysis of the multiple cause of death mortality data, 1979 through 1991. Southern medical journal. 1996;89(5):505–10. [DOI] [PubMed] [Google Scholar]
- 53.Ozawa Y, Suda T, Naito T, Enomoto N, Hashimoto D, Fujisawa T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology. 2009;14(5):723–8. Epub 2009/08/08. 10.1111/j.1440-1843.2009.01547.x . [DOI] [PubMed] [Google Scholar]
- 54.Qunn L, Takemura T, Ikushima S, Ando T, Yanagawa T, Akiyama O, et al. Hyperplastic epithelial foci in honeycomb lesions in idiopathic pulmonary fibrosis. Virchows Archiv: an international journal of pathology. 2002;441(3):271–8. Epub 2002/09/21. 10.1007/s00428-002-0618-9 . [DOI] [PubMed] [Google Scholar]
- 55.Goto T, Maeshima A, Oyamada Y, Kato R. Idiopathic pulmonary fibrosis as a prognostic factor in non-small cell lung cancer. International journal of clinical oncology. 2014;19(2):266–73. Epub 2013/05/11. 10.1007/s10147-013-0566-1 . [DOI] [PubMed] [Google Scholar]
- 56.Kishi K, Homma S, Kurosaki A, Motoi N, Yoshimura K. High-resolution computed tomography findings of lung cancer associated with idiopathic pulmonary fibrosis. Journal of computer assisted tomography. 2006;30(1):95–9. Epub 2005/12/21. . [DOI] [PubMed] [Google Scholar]
- 57.Portillo K, Perez-Rodas N, Garcia-Olive I, Guasch-Arriaga I, Centeno C, Serra P, et al. Lung Cancer in Patients With Combined Pulmonary Fibrosis and Emphysema and Idiopathic Pulmonary Fibrosis. A Descriptive Study in a Spanish Series. Archivos de bronconeumologia. 2017;53(6):304–10. Epub 2016/12/18. 10.1016/j.arbres.2016.10.004 . [DOI] [PubMed] [Google Scholar]
- 58.Harris JM, Cullinan P, McDonald J. Does cryptogenic fibrosing alveolitis carry an increased risk of death from lung cancer? J Epidemiol Community Health. 1998;52(9):602–3. 10.1136/jech.52.9.602 PubMed PMID: WOS:000075566400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collard HR, Ward AJ, Lanes S, Cortney Hayflinger D, Rosenberg DM, Hunsche E. Burden of illness in idiopathic pulmonary fibrosis. J Med Econ. 2012;15(5):829–35. Epub 2012/03/30. 10.3111/13696998.2012.680553 . [DOI] [PubMed] [Google Scholar]
- 60.Oda K, Yatera K, Fujino Y, Kido T, Hanaka T, Sennari K, et al. Respiratory comorbidities and risk of mortality in hospitalized patients with idiopathic pulmonary fibrosis. Respiratory investigation. 2018;56(1):64–71. Epub 2018/01/13. 10.1016/j.resinv.2017.09.006 . [DOI] [PubMed] [Google Scholar]
- 61.Kim ES, Choi SM, Lee J, Park YS, Lee CH, Yim JJ, et al. Validation of the GAP score in Korean patients with idiopathic pulmonary fibrosis. Chest. 2015;147(2):430–7. Epub 2014/09/12. 10.1378/chest.14-0453 . [DOI] [PubMed] [Google Scholar]
- 62.Turner-Warwick M, Lebowitz M, Burrows B, Johnson A. Cryptogenic fibrosing alveolitis and lung cancer. Thorax. 1980;35(7):496–9. Epub 1980/07/01. ; PubMed Central PMCID: PMCPMC471320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Zhu M, Geng J, Ban C, Zhang S, Chen W, et al. Incidence and radiologic-pathological features of lung cancer in idiopathic pulmonary fibrosis. The clinical respiratory journal. 2018;12(4):1700–5. Epub 2017/11/03. 10.1111/crj.12732 . [DOI] [PubMed] [Google Scholar]
- 64.Tomassetti S, Gurioli C, Ryu JH, Decker PA, Ravaglia C, Tantalocco P, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest. 2015;147(1):157–64. Epub 2014/08/29. 10.1378/chest.14-0359 . [DOI] [PubMed] [Google Scholar]
- 65.Enomoto Y, Inui N, Yoshimura K, Nishimoto K, Mori K, Kono M, et al. Lung cancer development in patients with connective tissue disease-related interstitial lung disease: A retrospective observational study. Medicine. 2016;95(50):e5716 Epub 2016/12/16. 10.1097/MD.0000000000005716 ; PubMed Central PMCID: PMCPMC5268067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Araki T, Katsura H, Sawabe M, Kida K. A clinical study of idiopathic pulmonary fibrosis based on autopsy studies in elderly patients. Internal medicine (Tokyo, Japan). 2003;42(6):483–9. Epub 2003/07/15. . [DOI] [PubMed] [Google Scholar]
- 67.Kwak N, Park CM, Lee J, Park YS, Lee SM, Yim JJ, et al. Lung cancer risk among patients with combined pulmonary fibrosis and emphysema. Respiratory medicine. 2014;108(3):524–30. Epub 2014/01/28. 10.1016/j.rmed.2013.11.013 . [DOI] [PubMed] [Google Scholar]
- 68.Lee KJ, Chung MP, Kim YW, Lee JH, Kim KS, Ryu JS, et al. Prevalence, risk factors and survival of lung cancer in the idiopathic pulmonary fibrosis. Thoracic cancer. 2012;3(2):150–5. Epub 2012/05/01. 10.1111/j.1759-7714.2011.00107.x . [DOI] [PubMed] [Google Scholar]
- 69.Kawai T, Yakumaru K, Suzuki M, Kageyama K. Diffuse interstitial pulmonary fibrosis and lung cancer. Acta Pathol Jpn. 1987;37(1):11–9. Epub 1987/01/01. . [DOI] [PubMed] [Google Scholar]
- 70.Aubry MC, Myers JL, Douglas WW, Tazelaar HD, Washington Stephens TL, Hartman TE, et al. Primary pulmonary carcinoma in patients with idiopathic pulmonary fibrosis. Mayo Clin Proc. 2002;77(8):763–70. Epub 2002/08/14. 10.4065/77.8.763 . [DOI] [PubMed] [Google Scholar]
- 71.Guyard A, Danel C, Theou-Anton N, Debray MP, Gibault L, Mordant P, et al. Morphologic and molecular study of lung cancers associated with idiopathic pulmonary fibrosis and other pulmonary fibroses. Respir Res. 2017;18(1):120 Epub 2017/06/18. 10.1186/s12931-017-0605-y ; PubMed Central PMCID: PMCPMC5472872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee T, Park JY, Lee HY, Cho YJ, Yoon HI, Lee JH, et al. Lung cancer in patients with idiopathic pulmonary fibrosis: clinical characteristics and impact on survival. Respiratory medicine. 2014;108(10):1549–55. Epub 2014/09/02. 10.1016/j.rmed.2014.07.020 . [DOI] [PubMed] [Google Scholar]
- 73.Lee HJ, Im JG, Ahn JM, Yeon KM. Lung cancer in patients with idiopathic pulmonary fibrosis: CT findings. J Comput Assist Tomogr. 1996;20(6):979–82. Epub 1996/11/01. . [DOI] [PubMed] [Google Scholar]
- 74.Tzouvelekis A, Bonella F, Spagnolo P. Update on therapeutic management of idiopathic pulmonary fibrosis. Ther Clin Risk Manag. 2015;11:359–70. Epub 2015/03/15. 10.2147/TCRM.S69716 ; PubMed Central PMCID: PMCPMC4354471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Y, Zhong W, Zhang L, Zhao J, Li L, Wang M. Clinical characteristics of patients with lung cancer and idiopathic pulmonary fibrosis in China. Thoracic cancer. 2012;3(2):156–61. Epub 2012/05/01. 10.1111/j.1759-7714.2012.00108.x . [DOI] [PubMed] [Google Scholar]
- 76.Fernandez Perez ER, Daniels CE, Schroeder DR, St Sauver J, Hartman TE, Bartholmai BJ, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137(1):129–37. Epub 2009/09/15. 10.1378/chest.09-1002 ; PubMed Central PMCID: PMCPMC2803118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kreuter M, Ehlers-Tenenbaum S, Schaaf M, Oltmanns U, Palmowski K, Hoffmann H, et al. Treatment and outcome of lung cancer in idiopathic interstitial pneumonias. Sarcoidosis, vasculitis, and diffuse lung diseases: official journal of WASOG. 2015;31(4):266–74. Epub 2015/01/16. . [PubMed] [Google Scholar]
- 78.Hyldgaard C, Hilberg O, Bendstrup E. How does comorbidity influence survival in idiopathic pulmonary fibrosis? Respiratory medicine. 2014;108(4):647–53. Epub 2014/02/18. 10.1016/j.rmed.2014.01.008 . [DOI] [PubMed] [Google Scholar]
- 79.Matsushita H, Tanaka S, Saiki Y, Hara M, Nakata K, Tanimura S, et al. Iung cancer associated with usual interstitial pneumonia. Pathol Int. 1995;45(12):925–32. 10.1111/j.1440-1827.1995.tb03417.x [DOI] [PubMed] [Google Scholar]
- 80.Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126(5):376–80. Epub 1997/03/01. . [DOI] [PubMed] [Google Scholar]
- 81.Mulrow CD, Cook D. Systematic reviews: synthesis of best evidence for health care decisions: ACP Press; 1998. [DOI] [PubMed] [Google Scholar]
- 82.Liberati A, Taricco M. How to do and report systematic reviews and meta-analysis Research in Physical & Rehabilitation Medicine Pavia: Maugeri Foundation Books; 2010:137–64. [Google Scholar]
- 83.YektaKooshali MH, Esmaeilpour-Bandboni M, Sharemi S, Alipour Z. Survival Rate and Average Age of the Patients with Breast Cancer in Iran: Systematic Review and Meta-Analysis. Journal of Babol University Of Medical Sciences. 2016;18(8):29–40. 10.22088/jbums.18.8.29 [DOI] [Google Scholar]
- 84.Azami M, Gh B, Mansouri A, Yekta Kooshali M, Kooti W, Tardeh Z, et al. Prevalence of chlamydia trachomatis in preg-nant iranian women: a systematic review and meta-analysis. Int J Fertil Steril. 2018;12(3):191–9. 10.22074/ijfs.2018.5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mansouri A, Adhami Mojarad MR, Badfar G, Abasian L, Rahmati S, Kooti W, et al. Epidemiology of Toxoplasma gondii among blood donors in Iran: A systematic review and meta-analysis. Transfus Apher Sci. 2017;56(3):404–9. Epub 2017/04/24. 10.1016/j.transci.2017.03.011 . [DOI] [PubMed] [Google Scholar]
- 86.Azami M, Shamloo MBB, Nasirkandy MP, Veisani Y, Rahmati S, Yektakooshali MH, et al. Prevalence of vitamin d deficiency among pregnant women in iran: A systematic review and meta-analysis. Koomesh. 2017;19(3):505–14. [Google Scholar]
- 87.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–12. Epub 2000/05/02. . [DOI] [PubMed] [Google Scholar]
- 88.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Editors PM. Best practice in systematic reviews: the importance of protocols and registration. PLoS medicine. 2011;8(2):e1001009 10.1371/journal.pmed.1001009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohammad Hossein YektaKooshali, AliReza JafariNezhad. Lung cancer in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. PROSPERO 2018 CRD42018094037 Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018094037. [DOI] [PMC free article] [PubMed]
- 91.Santos CMdC, Pimenta CAdM, Nobre MRC. The PICO strategy for the research question construction and evidence search. Revista latino-americana de enfermagem. 2007;15(3):508–11. [DOI] [PubMed] [Google Scholar]
- 92.Travis WD, Costabel U, Hansell DM, King TE Jr., Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48. Epub 2013/09/17. 10.1164/rccm.201308-1483ST ; PubMed Central PMCID: PMCPMC5803655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.European RS, Society AT. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. American Journal of Respiratory and Critical Care Medicine. 2002;165(2):277 10.1164/ajrccm.165.2.ats01 [DOI] [PubMed] [Google Scholar]
- 94.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192(2):e3–19. Epub 2015/07/16. 10.1164/rccm.201506-1063ST . [DOI] [PubMed] [Google Scholar]
- 95.Wells AU. The revised ATS/ERS/JRS/ALAT diagnostic criteria for idiopathic pulmonary fibrosis (IPF)—practical implications. Respir Res. 2013;14 Suppl 1(1):S2 Epub 2013/06/14. 10.1186/1465-9921-14-S1-S2 ; PubMed Central PMCID: PMCPMC3643186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, et al. Selection criteria for lung-cancer screening. New England Journal of Medicine. 2013;368(8):728–36. 10.1056/NEJMoa1211776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. Epub 2010/07/24. 10.1007/s10654-010-9491-z . [DOI] [PubMed] [Google Scholar]
- 98.ZakerJafari HR, YektaKooshali MH. Work-Related Musculoskeletal Disorders in Iranian Dentists: A Systematic Review and Meta-analysis. Safety and Health at Work. 2018;9(1):1–9. 10.1016/j.shaw.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kawasaki H, Nagai K, Yokose T, Yoshida J, Nishimura M, Takahashi K, et al. Clinicopathological characteristics of surgically resected lung cancer associated with idiopathic pulmonary fibrosis. Journal of surgical oncology. 2001;76(1):53–7. Epub 2001/02/27. . [DOI] [PubMed] [Google Scholar]
- 100.Masai K, Tsuta K, Motoi N, Shiraishi K, Furuta K, Suzuki S, et al. Clinicopathological, Immunohistochemical, and Genetic Features of Primary Lung Adenocarcinoma Occurring in the Setting of Usual Interstitial Pneumonia Pattern. J Thorac Oncol. 2016;11(12):2141–9. Epub 2016/08/31. 10.1016/j.jtho.2016.07.034 . [DOI] [PubMed] [Google Scholar]
- 101.Horowitz JC, Osterholzer JJ, Marazioti A, Stathopoulos GT. "Scar-cinoma": viewing the fibrotic lung mesenchymal cell in the context of cancer biology. The European respiratory journal. 2016;47(6):1842–54. Epub 2016/04/01. 10.1183/13993003.01201-2015 ; PubMed Central PMCID: PMCPMC5663641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vancheri C. Idiopathic pulmonary fibrosis and cancer: do they really look similar? BMC Med. 2015;13(1):220 Epub 2015/09/25. 10.1186/s12916-015-0478-1 ; PubMed Central PMCID: PMCPMC4581087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mittal V. Epithelial mesenchymal transition in aggressive lung cancers Lung Cancer and Personalized Medicine: Novel Therapies and Clinical Management: Springer; 2016. p. 37–56. [DOI] [PubMed] [Google Scholar]
- 104.Yu YY, Pinsky PF, Caporaso NE, Chatterjee N, Baumgarten M, Langenberg P, et al. Lung cancer risk following detection of pulmonary scarring by chest radiography in the prostate, lung, colorectal, and ovarian cancer screening trial. Arch Intern Med. 2008;168(21):2326–32; discussion 32. Epub 2008/11/26. 10.1001/archinte.168.21.2326 ; PubMed Central PMCID: PMCPMC2866505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proceedings of the National Academy of Sciences. 2006;103(35):13180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Antoniou KM, Tomassetti S, Tsitoura E, Vancheri C. Idiopathic pulmonary fibrosis and lung cancer: a clinical and pathogenesis update. Curr Opin Pulm Med. 2015;21(6):626–33. Epub 2015/09/22. 10.1097/MCP.0000000000000217 . [DOI] [PubMed] [Google Scholar]
- 107.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–87. Epub 2011/10/14. 10.1056/NEJMoa1103690 . [DOI] [PubMed] [Google Scholar]
- 108.Richeldi L, Cottin V, du Bois RM, Selman M, Kimura T, Bailes Z, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: combined evidence from the TOMORROW and INPULSIS® trials. Respiratory medicine. 2016;113:74–9. 10.1016/j.rmed.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 109.Drakopanagiotakis F, Xifteri A, Polychronopoulos V, Bouros D. Apoptosis in lung injury and fibrosis. The European respiratory journal. 2008;32(6):1631–8. Epub 2008/12/02. 10.1183/09031936.00176807 . [DOI] [PubMed] [Google Scholar]
- 110.Willis BC, Borok Z. TGF—induced EMT: mechanisms and implications for fibrotic lung disease. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2007;293(3):L525–L34. 10.1152/ajplung.00163.2007 [DOI] [PubMed] [Google Scholar]
- 111.Karampitsakos T, Tzilas V, Tringidou R, Steiropoulos P, Aidinis V, Papiris SA, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2017;45:1–10. Epub 2017/04/06. 10.1016/j.pupt.2017.03.016 . [DOI] [PubMed] [Google Scholar]
- 112.Kato E, Takayanagi N, Takaku Y, Kagiyama N, Kanauchi T, Ishiguro T, et al. Incidence and predictive factors of lung cancer in patients with idiopathic pulmonary fibrosis. ERJ open research. 2018;4(1):00111–2016. Epub 2018/02/08. ; PubMed Central PMCID: PMCPMC5795191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. American journal of human genetics. 2009;84(1):52–9. Epub 2008/12/23. 10.1016/j.ajhg.2008.11.010 ; PubMed Central PMCID: PMCPMC2668050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Moorsel CH, Ten Klooster L, van Oosterhout MF, de Jong PA, Adams H, Wouter van Es H, et al. SFTPA2 Mutations in Familial and Sporadic Idiopathic Interstitial Pneumonia. Am J Respir Crit Care Med. 2015;192(10):1249–52. Epub 2015/11/17. 10.1164/rccm.201504-0675LE . [DOI] [PubMed] [Google Scholar]
- 115.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–708. Epub 1999/10/16. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(PDF)
(DOCX)
(XLSX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.