Abstract
To examine the available evidence on the association between exposure to tobacco use in the womb and in infancy and the presence of caries in primary and permanent dentition in children and adolescents.
A systematic review was conducted through searches in 4 data bases (Pubmed, Scopus, Embase and Web of Science), complemented by hand-searching. Of the 559 articles identified, 400 were duplicates. Finally, 28 articles were included in the qualitative review and 21 in the meta-analysis. Their quality was assessed using the Newcastle-Ottawa scale. The quality was medium in 44% of the articles included and high in 56%.
The overall meta-analysis gave a significant odds ratio (OR = 1.53, 95% confidence interval 1.39–1.68, Z test p-value = 0.000) and high heterogeneity (Q = 200.3, p = 0.000; I2 = 86.52%). Separate meta-analyses were also performed for three subgroups: exposure in the womb (prenatal) and caries in primary dentition, which resulted in a significant OR = 1.46 with a 95% CI of 1.41–1.52 (Z test p = 0.000), without heterogeneity (Q = 0.91, p = 0.824; I2 = 0%); exposure in infancy (postnatal) and caries in primary dentition, with OR = 1.72 (95% CI 1.45–2.05) and high heterogeneity (Q = 76.59, p = 0.00; I2 = 83.01%); and postnatal exposure and caries in permanent dentition, with OR = 1.30 (95% CI 1.25–1.34) and no heterogeneity (Q = 4.48, p = 0.880; I2 = 0%). In children and adolescents, a significant though moderate association was found between passive tobacco exposure and caries.
Introduction
Dental caries is still one of the main diseases of the oral cavity, affecting a third of the population in the world[1]. Caries prevalence has fallen in industrialised countries in recent years, although it has increased in developing countries. The current epidemiological pattern of dental caries in the child population of developed countries shows lower caries levels but an unbalanced distribution, concentrated in the most socially-disadvantaged sector of the population[2]. The economic and social cost of caries is very high. Dental caries is considered a pandemic due to its global distribution and severe consequences[3].
Dental caries is a biofilm-mediated, sugar-driven, multifactorial, dynamic disease that results in the phasic demineralization and remineralization of dental hard tissues. The balance between pathological and protective factors influences the initiation and progression of caries[4]. The pathological factors include acidogenic bacteria, inhibition of salivary function, and frequency of ingestion of fermentable carbohydrates and the protective factors include salivary flow, numerous salivary components, antibacterials (both natural and applied), fluoride from extrinsic sources, and selected dietary components[5].
Over 1 billion people in the world smoke tobacco, and an enormous increase has been predicted, to 1.9 billion in 2025, with no great differences between genders. According to the WHO, smoking causes approximately 71% of lung cancer cases, 42% of chronic respiratory diseases and around 10% of cardiovascular diseases. Oral, throat and oesophagal cancers appear up to ten times more frequently in smokers than in non-smokers[3].
There is strong evidence that tobacco use has numerous negative effects on oral health, such as staining of teeth and tooth restorations by nicotine and tar, reduced senses of smell and taste, oral diseases such as smoker’s palate and smoker’s melanosis, precancerous lesions such as leukoplakias, oral cancer, oral candidiasis due to reduced saliva flow, changes in the bacterial flora and periodontal disease[6]. The WHO estimates that nearly 700 million children are exposed to tobacco smoke, mostly from their parents in their own homes[7]. Tobacco smoke has not passed through a filter, so it contains far higher concentrations of harmful substances than smoke which is inhaled through a cigarette, and is therefore potentially more dangerous for non-smokers[6,8]
Exposure to tobacco smoke is a major cause of paediatric morbidity and mortality around the world[9]. Children exposed to large quantities of environmental smoke are more likely to suffer respiratory infections, ear blockages, asthma attacks, hospitalisation and irritation of the eyes, throat and respiratory tract. Tobacco smoke has also been related to dental caries in children and adolescents[7].
Some controversy surrounds the possible association between tobacco and caries in children. Hanioka et al.[10] stated that the overall evidence in epidemiological studies is that a causal association in early childhood caries is possible, but the evidence for permanent teeth and the effect of maternal smoking during pregnancy are insufficient. A systematic review by Slayton[11] concluded that there is limited evidence concerning the possible aetiology.
Consequently, the main objective was to examine the available scientific evidence concerning on the association between exposure to tobacco use in the womb and in infancy and the presence of caries in primary and permanent dentition in children and adolescents.
A systematic review and meta-analysis has been designed to answer the following PECO question: “Is Passive smoking (Exposure) associated with the development of cavitated lesions of caries in teeth (Outcome) of children and adolescents (Participants) compared with those not exposed (Comparison/control)?”.
Materials and methods
Study registration
The present study was registered in the International prospective register of systematic reviews (PROSPERO) with the registration id. CRD42028090177.
Study selection criteria
The selection criteria for inclusion were: studies in humans in which the sample ages were children and adolescents (from 0 to 19 years-old), published since the year 2000, concerning passive tobacco exposure and dental caries. Unpublished resources were not considered. Longitudinal studies, case-control studies and cross-sectional studies were included provided they also had a control group of subjects not exposed to tobacco use for comparison and that the primary outcome of caries was expressed as odds ratios (or data provided on the number of events and totals for each group enabled the odds ratio to be calculated), or as hazard ratios or indices (DMFT, DMFS, dft or dfs).
Search strategies and article selection
To identify the most relevant studies, irrespective of language, a thorough electronic search from January 2000 to 2nd March 2018 was made in four databases: Pubmed, Scopus, Embase and Web of Science.
The search equation for Pubmed and Web of Science was: ((dental caries OR caries) AND (children OR adolescents) AND (Tobacco OR passive smoke OR passive smoking OR secondhand smoke OR secondhand smoking OR household smoke OR household smoking OR involuntary smoke OR involuntary smoking OR parental smoke OR parental smoking OR maternal smoke OR maternal smoking)). For Scopus, the equation was (TITLE-ABS-KEY (dental AND caries OR caries) AND TITLE-ABS-KEY (child OR adolescents) AND TITLE-ABS-KEY (passive AND smoking OR tobacco OR household AND smoking OR maternal AND smoking)) AND PUBYEAR > 1999 and finally, for Embase the search equation was 'dental caries' AND child AND smoking AND [2000–2018]/py.
Two calibrated reviewers (LGV and JMM-C) independently selected the articles. In the event of disagreement, they had to reach a consensus on whether or not to include the study in the review. Cohen’s kappa was used to determine the inter-reviewer reliability. The initial screening was performed by reading the titles and abstracts. If the information was insufficient, the decision was based on reading the full text of the article.
Data mining
For each of the articles selected, the following information was recorded: authors, year of publication, type of study, sample size, sample age, prenatal tobacco exposure (tobacco smoking during the pregnancy) or postnatal tobacco exposure (unwanted breathing in of parents and relatives’ cigarette smoke by children who do not smoke at home), primary or permanent dentition, dental caries (defined as a tooth with a definite cavity and undermined enamel) as the primary outcome of interest expressed as odds ratios, hazard ratios or DMFT (number of decayed, missing or filled teeth in permanent dentition), DMFS (number of decayed, missing or filled surfaces in permanent dentition), dft (number of decayed or filled teeth in primary dentition) or dfs (number of decayed or filled surfaces in primary dentition) caries indices, and the existence or otherwise of a significant association.
Study quality
The quality of the studies was assessed on the Newcastle-Ottawa Scale. This consists of three sections, each with a number of stars that can be awarded to the study. The three sections are selection, comparability and outcome or, for case-control studies, exposure. In the selection section, a cross-sectional study can obtain up to 5 stars but the maximum for cohort and case-control studies is 4 stars. All three types of study can achieve a maximum of 2 stars in the second section (comparability) and a maximum of 3 stars in the third section (outcome/exposure). The quality score is the sum of all the stars obtained by that study [12]. Articles with 8 or 9 stars were considered of high quality and those with 6 or 7 of medium quality; below 7 was considered poor quality.
Quantitative analysis of the studies (meta-analysis)
The studies included in each meta-analysis were combined using the random effects model, assuming the inter-studies heterogeneity. The significance of the meta-analysis was assessed through the Z test. The heterogeneity was measured by the I2 heterogeneity score, the Q statistic and the p-value. A Q statistic with p<0.1 was considered to indicate heterogeneity. In addition, I2 classifies the heterogeneity as mild (I2 25%-49%), moderate (50%-74%) or high (>75%).
For the presence of caries, the measure of effect size was the odds ratio, with 95% CI. When the study did not give the odds ratio, this was calculated from the events and totals. Whenever possible, the adjusted odds ratio was chosen. The estimate was considered significant when the Z test p-value was <0.05.
For the control of the possible heterogeneity sources, 3 meta-analysis divided into subgroups will be done, taking into account the dentition (primary or permanent) and the exposure (pre or postnatal).
The sensitivity of the meta-analysis was assessed by the one-study removed method, that assess the stability of the effect size obtained every time that a study is removed.
The publication bias in the meta-analysis was assessed through the classic fail-safe number, which estimates the number of statistically significant studies that would be required for a meta-analysis with a significant result (p<0.05) to cease to be significant, and through Egger’s regression intercept, when p<0.1. Additionally, symmetrical distribution of these studies was assessed through funnel plots with Duval and Tweedie‘s Trim and Fill that, after adjusting for missing studies, estimates a new overall effect size, that can be compared with the results obtained in the meta-analysis[13].
The meta-analyses were performed with Comprehensive Meta-analysis v3.0 software (Biostat).
Results
Flow chart
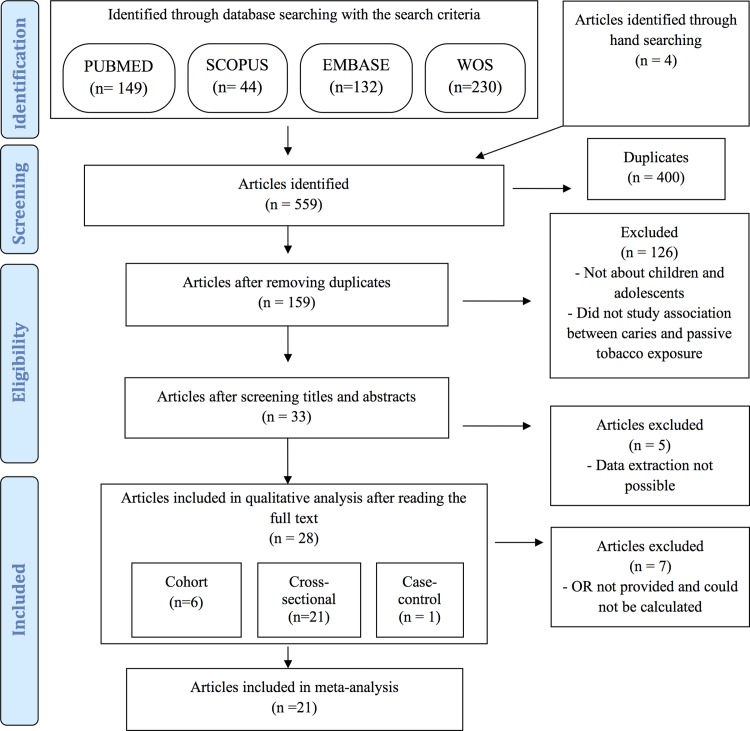
On applying the search criteria, 149 articles were identified in PubMed, 44 in Scopus, 132 in Embase and 230 in Web of Science, totalling 555 articles; this was complemented by hand-searching.
Following removal of 400 duplicate articles, 159 remained. Finally, 28 articles were included in the systematic review and 21 in the meta-analysis (Fig 1).
Fig 1. Flow diagram.
Cohen’s kappa value for the inter-reviewer reliability was 0.87
Qualitative analysis
Of the articles selected for systematic review (Table 1), 21 were cross-sectional studies[14–34], one was a case-control study[35] and six were longitudinal studies[36–41].
Table 1. Characteristics of studies included in the review, measuring the association between passive exposure to tobacco use and the presence of caries in children and adolescents.
| Study | Type | n | Age(yr) | Exposure | Dentition | Outcome | Association |
|---|---|---|---|---|---|---|---|
| Aida et al., 2008[14] | CS | 3086 | 3 | Postnatal | Primary | OR = 2.14 (1.59–2.87) | Yes |
| Aligne et al., 2003[15] | CS | 3531 | 4 to 11 | Postnatal | Primary | a: OR = 1.8 (1.2–2.7); ds | Yes |
| Permanent | b: OR = 1.2 (0.8–1.85); DS | No | |||||
| Almerich et al., 2013[16] | CS | 889 | 12 | Postnatal | Permanent | a: OR = 1.2 (0.82–1.75) | No |
| 15 | Permanent | b: OR = 1.24 (0.84–1.83) | |||||
| Avçar et al., 2008[35] | CC | 90 | 5 | Postnatal | Primary | Cases: dft = 10.58±2.12 | Yes |
| 90 | Controls: dft = 4.64±2.91 | ||||||
| Ayo-Yusuf et al., 2007[17] | CS | 1873 | 13 to 15 | Postnatal | Permanent | Caries in 2nd molars | Yes |
| OR = 2.02 (1.22–3.33) | |||||||
| Bakhurji et al., 2017 [33] | CS | 294 | 12–15 | Postnatal | Permanent | OR = 1.37 (0.46–4.08) | No |
| Bernabé et al., 2017[36] | L | 1102 | 1–4 | Postnatal | Primary | Data 4 years; Exposed dmfs = 5.18±9 | Yes |
| Non-exposed dmfs = 2.78 ±6.51 | |||||||
| Carbajosa et al., 2011[18] | CS | 380 | 10 to 15 | Postnatal | Permanent | Exposed DMFT = 1.62±2.2; df = 0.27±0.78 | Yes |
| Non-exposed DMFT = 0.92±1.40; df = 0.10±0.47 | |||||||
| Claudia et al., 2016 [41] | L | 1687 | 0.5–6 | Prenatal | Primary | RR = 1.42 (1.20–1.68) | Yes |
| Ditmyer et al. 2010[19] | CS | 4169 | 12 to 19 | Postnatal | Permanent | Highest DMFT group | Yes |
| OR = 1.42 (1.03–1.53) | |||||||
| Exposed DMFT = 7.23±0.06 | |||||||
| Non-exposed DMFT = 6.74±0.10 | |||||||
| Hanioka et al., 2008[20] | CS | 711 | 3 | Postnatal | Primary | OR = 2.25 (1.51–3.37) | Yes |
| Exposed dt = 2.1 (1.7–2.5) | |||||||
| Non-exposed dt = 1.2 (0.8–1.6) | |||||||
| Iida et al., 2007[21] | CS | 1576 | 2 to 5 | Prenatal | Primary | OR: 1.68 (1.01–2.79) | Yes |
| Julihn et al., 2009[37] | L | 15538 | 13 to 19 | Postnatal | Permanent | Interproximal caries | Yes |
| OR = 1.33 (1.22–1.44) | |||||||
| Leroy et al., 2008[22] | CS | 2533 | 3 | Postnatal | Primary | a: OR = 1.98 (0.68–5.76) | Yes |
| 5 | Postnatal | Primary | b: OR = 3.36 (1.49–7.58) | ||||
| Majorana et al., 2014[23] | CS | 2395 | 2–3 | Postnatal | Primary | Severe caries | Yes |
| OR = 1.62 (1.34–1.96) | |||||||
| Nakayama et al., 2015[24] | CS | 1801 | 3 | Postnatal | Primary | OR = 1.91 (1.43–2.54) | Yes |
| Exposed dfs = 1.27±2.98 | |||||||
| Non-exposed dfs = 0.53±1.69 | |||||||
| Nayani et al., 2018 [34] | CS | 500 | 5–14 | Postnatal | Permanent | Prevalence ratio <30min = 1.25 (1.07–1.45) | Yes |
| Prevalence ratio >30min = 1.34 (1.05–1.35) | |||||||
| Pita-Fernández et al., 2011[25] | CS | 281 | 6 to 10 | Postnatal | Primary | a: OR = 1.12 (0.55–2.28) | No |
| Postnatal | Permanent | b: OR = 1.47 (0.62–3.47) | |||||
| Plonka et al., 2012[38] | L | 1017 | 2–3 | Postnatal | Primary | Predictive variable in a logistic model | Yes |
| Shenkin et al., 2004[39] | L | 637 | 4 to 7 | Postnatal | Primary | OR = 3.38 (1.68–6.79) | Yes |
| Shulman et al. 2005[26] | CS | 7779 | 2 to 6 | Postnatal | Primary | Exposed dft = 3.19 (1.03) | No |
| Non-exposed dft = 2.10 (0.17) | |||||||
| Tanaka et al., 2006[27] | CS | 925 | 1 to 14 | Postnatal | Primary | OR = 1.26 (0.93–1.69) | No |
| Tanaka et al., 2009[28] | CS | 2015 | 3 | Prenatal | Primary | a: OR = 1.43 (1.07–1.91) | Yes |
| Postnatal | b: OR = 1.25 (1.04–1.50) | ||||||
| Tanaka et al., 2010[29] | CS | 20703 | 6 to 15 | Postnatal | Permanent | OR = 1.29 (1.24–1.34) | Yes |
| Tanaka K et al., 2015[30] | CS | 6412 | 3 | Prenatal | Primary | a: OR = 1.70 (1.15–2.48) | Yes |
| Postnatal | Primary | b: OR = 1.23 (1.05–1.45) | |||||
| Tanaka S et al., 2015[40] | RC | 76920 | 3 | Prenatal | Primary | a: HR = 1.46 (1.40–1.52) | Yes |
| Postnatal | Primary | b: HR = 2.14 (1.99–2.29) | |||||
| Wiener et al., 2013[31] | CS | 91642 | 1–15 | Postnatal | Permanent | Special health care needs children | Yes |
| OR = 1.23 (1.02–1.50) | |||||||
| Williams et al., 2000[32] | CS | 749 | 3 to 15 | Postnatal | Primary | OR: 1.54 (1.07–2.21) | Yes |
RC: retrospective cohorts; CS: cross-sectional; CC: case controls; L: longitudinal; OR: odds ratio; HR: hazard ratio; DMFT: permanent teeth decayed, missing and filled; DS: permanent tooth surfaces decayed; ds: primary tooth surfaces decayed; dft: primary teeth decayed and filled; dfs: primary tooth surfaces decayed and filled.
Most of their sample sizes were large, ranging from 180 in the study by Avçar et al.[35] to 76920 in Tanaka S et al.[40] and 91642 in Wiener et al.[31].
Only 5 articles investigated prenatal exposure (in the womb) [21,28,30,40,41] in the remaining 23 the exposure was postnatal (in infancy). Most of the studies examined caries in primary dentition[14,15,20–28,30,32,35,36,38–41], while the rest examined the permanent dentition. Different indices were used, such as DMFT or DS for permanent dentition and dft, ds or dt for primary dentition. Some odds ratios were calculated for the group with caries in second molars (Ayu-Yusuf et al.[17]), for those with the highest DMFT (Ditmyer et al.[19]) or for special health care needs children (Wiener et al.[31]); the rest of the studies did not effect any special selection.
On analysing the conclusions of each of the studies, it was observed that 80% of the articles included (in other words, all except five) concluded that an association does exist between passive smoking and a greater presence of caries in children, both in primary and in permanent dentition. Of the six studies that did not reach this conclusion, Aligne et al.[15] found an association with postnatal exposure for primary dentition but not for permanent dentition; Almerich-Torres et al.[16] and Bakhurji et al. [33] found no association between postnatal exposure and caries in permanent dentition in children 12–15 years old; Pita-Fernández et al.[25] found no association in either primary or permanent dentition, and Shulman et al.[26] and Tanaka et al.[27] did not find an association between postnatal exposure to environmental tobacco use and caries in primary dentition.
The quality assessment of the studies included in the systematic review is shown in Table 2. The quality of 43% of the articles included was medium and that of 57% was high. None of the studies presented low quality.
Table 2. Quality of the studies on the Newcastle-Ottawa scale.
| Study |
Selection *****/**** |
Comparability ** |
Exposure/Outcome *** |
Total Stars |
|---|---|---|---|---|
| Aida et al., 2008[14] | **** | ** | ** | 8 |
| Aligne et al., 2003[15] | ***** | ** | ** | 8 |
| Almerich et al., 2013[16] | ***** | * | ** | 8 |
| Avçar et al., 2008[35] | **** | ** | *** | 9 |
| Ayo-Yusuf et al., 2007[17] | **** | ** | ** | 8 |
| Bakhurji et al., 2017 [33] | *** | ** | ** | 7 |
| Bernabé et al., 2017[36] | ***** | * | *** | 8 |
| Carbajosa et al., 2011[18] | **** | * | ** | 7 |
| Claudia et al., 2016 [41] | *** | ** | *** | 8 |
| Ditmyer et al. 2010[19] | ***** | * | *** | 8 |
| Hanioka et al., 2008[20] | *** | * | *** | 7 |
| Iida et al., 2007[21] | **** | * | ** | 7 |
| Julihn et al., 2009[37] | **** | ** | *** | 9 |
| Leroy et al., 2008[22] | ***** | ** | ** | 9 |
| Majorana et al., 2014[23] | **** | * | ** | 7 |
| Nakayama et al., 2015[24] | ***** | * | *** | 9 |
| Nayani et al., 2018 [34] | **** | ** | ** | 8 |
| Pita-Fernández et al., 2011[25] | *** | * | *** | 7 |
| Plonka et al., 2012[38] | ***** | * | *** | 9 |
| Shenkin et al., 2004[39] | *** | ** | ** | 7 |
| Shulman et al. 2005[26] | **** | * | ** | 7 |
| Tanaka et al., 2006[27] | **** | * | * | 7 |
| Tanaka et al., 2009[28] | **** | ** | ** | 8 |
| Tanaka et al., 2010[29] | **** | ** | *** | 9 |
| Tanaka K et al., 2015[30] | **** | * | ** | 7 |
| Tanaka S et al., 2015[40] | **** | * | ** | 7 |
| Wiener et al., 2013[31] | **** | * | ** | 7 |
| Williams et al., 2000[32] | **** | ** | ** | 9 |
Quantitative analysis
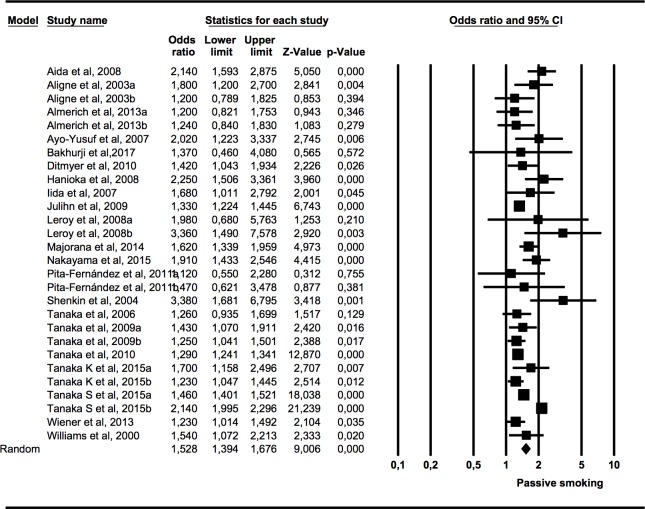
An overall meta-analysis was performed with data (odds ratios) from 21 studies (7 studies presented data from cohorts of different ages). Another three meta-analyses were performed with odds ratios data for the following subgroups: presence of caries in primary dentition in children exposed to tobacco use in the womb or prenatally (4 studies), presence of caries in primary dentition in children exposed to tobacco use postnatally (13 studies), and presence of caries in permanent dentition in children and adolescents exposed to tobacco use postnatally (1 studies, of which one presented data from two cohorts of different ages).
Association (OR) between the presence of caries in children and adolescents and exposure to tobacco use
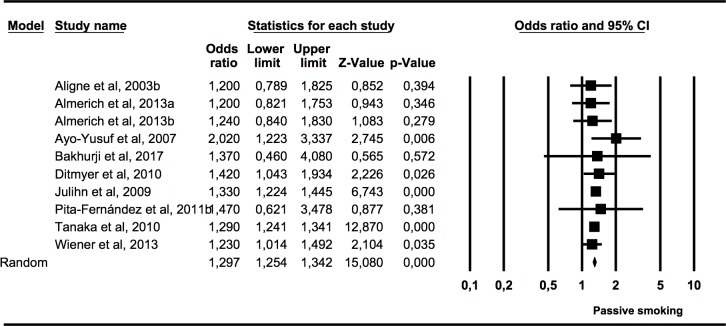
Data were included from 21 studies using the OR to measure the association between caries in the primary and permanent dentition of children and adolescents and their pre-or postnatal exposure to tobacco use (Fig 2). The meta-analysis presented Q = 200.3 (p = 0.000) and I2 = 86.52%, indicating high heterogeneity. Using the random effects model, an OR of 1.53 with a 95% of CI 1.39–1.68 was calculated, which was statistically significant (Z test p-value = 0.000) and signifies that the presence of caries in children and adolescents exposed to tobacco use was 1.5 times higher than in the non-exposed children.
Fig 2. Forest plot of OR of caries presence in primary or permanent dentition of children and adolescents exposed to tobacco use pre- or postnatally.
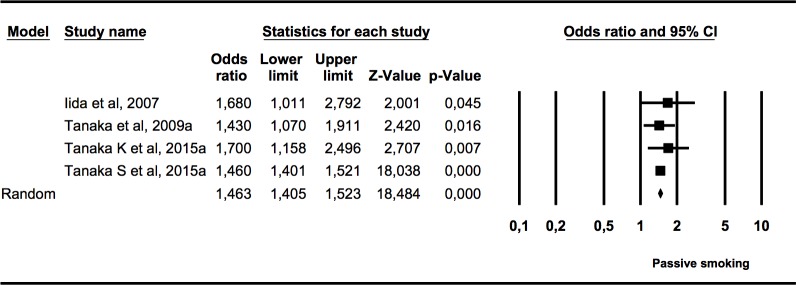
Association (OR) between caries presence in primary dentition and prenatal exposure to tobacco use in children
The first subgroup meta-analysis (Fig 3) included four studies that measured the presence of caries in primary dentition in children who had been exposed to tobacco use in the womb. Heterogeneity was not found (Q = 0.91, p = 0.824; I2 = 0%). The OR of 1.46 (95% CI 1.41–1.52) was statistically significant (Z test p = 0.000) and it was estimated that children exposed to tobacco use in the womb had 1.46 times more caries in primary teeth than the non-exposed group.
Fig 3. Forest plot of OR of caries presence in primary dentition of children exposed to tobacco use prenatally.
Association (OR) between caries presence in primary dentition and postnatal exposure to tobacco use in children
The second subgroup meta-analysis (Fig 4) included 13 studies that measured the presence of caries in primary dentition in children who had been exposed to tobacco use in infancy. The heterogeneity was high (Q = 76.59, p = 0.00; I2 = 83.01%). The estimate obtained through the combined random effects model was statistically significant (Z test p value = 0.000), with an OR of 1.72 (95% CI 1.45–2.05), meaning that the presence of caries in primary dentition in children exposed to tobacco use in infancy was 1.7 times higher than in the non-exposed children.
Fig 4. Forest plot of OR of caries presence in primary dentition of children exposed to tobacco use postnatally.
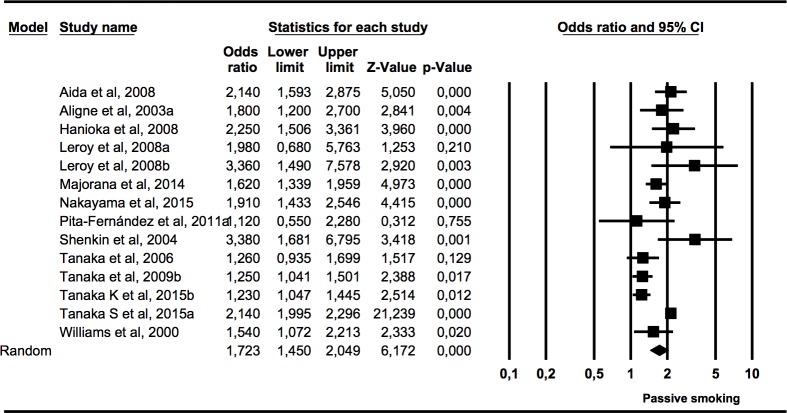
Association (OR) between caries presence in permanent dentition and postnatal exposure to tobacco use in children and adolescents
The third subgroup meta-analysis (Fig 5) included data from nine studies measuring the presence of caries in the permanent dentition of children and adolescents who had been exposed to tobacco use in infancy. Heterogeneity was not found (Q = 4.48; p = 0.88; I2 = 0%).
Fig 5. Forest plot of OR of caries presence in permanent dentition of children and adolescents exposed to tobacco use postnatally.
The calculation was statistically significant (p = 0.000), with an OR of 1.30 (95% CI 1.25–1.34), meaning that the presence of caries in children and adolescents exposed to tobacco use in infancy was 1.3 times higher than in the non-exposed children.
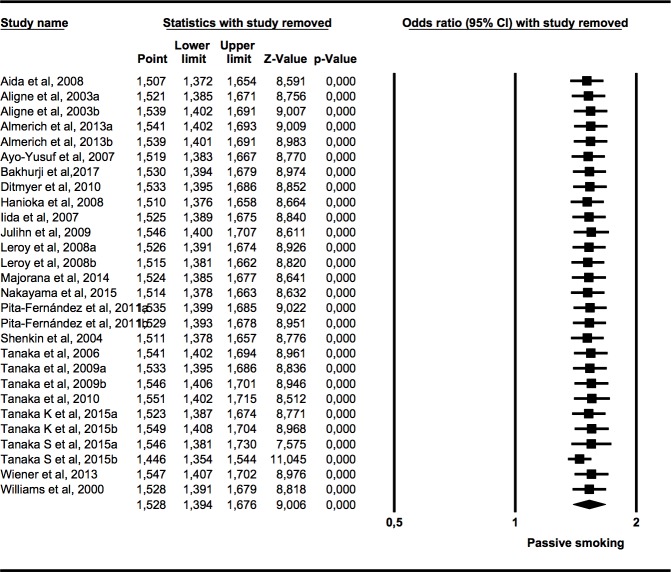
Sensitivity analysis
The one-study removed method did not detect any study that significantly affects the overall effect size estimated (Fig 6).
Fig 6. Forest plot “one-study removed” of OR of caries presence in primary or permanent dentition of children and adolescents exposed to tobacco use pre- or postnatally.
Publication bias
The overall meta-analysis gave a classic fail-safe number of 3434, so a very high number of studies would be needed to invalidate the statistical significance of the present results. Egger’s regression intercept was 0.670 (p = 0.323). For the first subgroup meta-analysis, the classic fail-safe number was 161 and Egger’s regression intercept was 0.453 (p = 0.278).
The classic fail-safe numbers for the second and third subgroup meta-analyses were 897 and 241, respectively. Their Egger’s regression intercept values were -0,893 (p = 0.394) and 0,233 (p = 0.442), respectively.
If we adjust the estimation of the meta-analysis using the Duval and Tweedie’s trim and fill, the estimated odds ratio for the overall meta-analysis is 1.50 (1.36–1.64). For the first subgroup, the estimated odds ratio is 1.46 (1.40–1.52), for the second subgroup is 1.72 (1.45–2.05) and 1.30 (1.25–1.33) for the third subgroup. No significant difference is observed between the adjusted odds ratio and the original estimations.
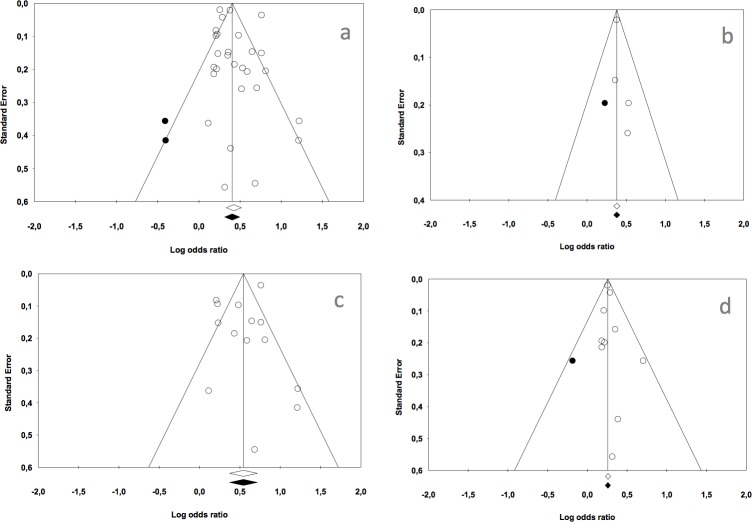
The funnel plots for the four meta-analyses are shown in Fig 7.
Fig 7. Funnel plots.
a: Caries presence in primary or permanent dentition of children and adolescents exposed to tobacco use prenatally or postnatally. b: Caries presence in primary dentition of children exposed to tobacco use prenatally. c: Caries presence in primary dentition of children exposed to tobacco use postnatally. d: Caries presence in permanent dentition of children and adolescents exposed to tobacco use postnatal.
Discussion
The present results confirm the existence of an association between passive tobacco exposure and dental caries in children and adolescents, for both prenatal and postnatal exposure and both primary and permanent dentition.
Most of the studies included in the meta-analysis were cross-sectional, making it impossible to study causal relation, although the four longitudinal studies[36–39] and the retrospective cohort study[40] concluded that this association did exist.
Several hypotheses support the biological plausibility of the association and could explain the causal mechanism of caries due to passive tobacco exposure. Chowdhury and Bromage[42] and Heikkinen et al.[43] state that exposure to tobacco use directly affects both the mineralisation of the developing tooth and the microorganisms, while Arora et al.[44] consider that exposure to environmental cadmium through cigarettes could play an important role. Impairment of the immune system could facilitate colonisation by Streptococcus mutans[45] and the lowering of vitamin C levels detected in the exposed children[46]. Avçar et al.[35] observed that children exposed to tobacco use had lower salivary pH, buffer capacity and saliva flow than non-exposed children, leading to a reduction in the capacity of saliva to protect against caries; this, together with a rise in S mutans and Lactobacilli levels, could explain the cause and effect relationship between tobacco and caries. In addition, according to Lindemeyer et al.[45], mothers who are smokers and have high levels of cariogenic bacteria transmit them more easily to their children in the first few years of life.
Despite all these plausible aetiological mechanisms, the tendency is to consider that the association between passive exposure to tobacco use and caries in children is mainly due to shared socio-economic, educational or behavioural factors. It is clear that children from a low socio-economic level show significantly higher caries rates[2].
Jakhete and Gitterman[47] found that exposure to tobacco use and poor nutrition were associated with higher caries prevalence in children from a low socio-economic level. Delpisheh[48] confirmed that passive exposure to tobacco use in children is significantly associated with low socio-economic level. Hanioka et al.[20] and Nakayama et al. [24] observed that the children of parents who smoked had poorer hygiene habits, brushed their teeth less frequently and consumed more sugar, favouring the appearance of caries. Leroy et al.[22] detected that children growing up with smoker parents brushed their teeth less and ate more between meals, so had poorer oral hygiene. Majorama et al.[23] found that children who were bottle-fed, lived in families from a low socio-economic level and were exposed to tobacco use had a greater likelihood of suffering severe caries.
The strong relationship between caries and socio-economic and behavioural factors does not help to clarify the relationship between passive exposure to tobacco use and caries in children. However, the estimate of this association in the present results is mostly derived from studies with OR values adjusted through logistic regression using variables such as toothbrushing frequency, age, gender, educational level, application of fluoride, visits to the dentist, socio-economic level, place of residence, etc. [14,15,17,19–23,25,26,29,39]. Few studies that presented only the raw OR were included in the meta-analysis [16,35]. Consequently, this confirms not only the strength of the association but also its independence from the other factors that have been related to the aetiology of dental caries.
The dose-response relationship between levels of exposure to tobacco use and dental caries has been studied by several authors [14,15,20,28,29,34], all of whom have confirmed this relationship. The studies by Tanaka et al.[28,29] examined exposure in terms of years of smoking in the household, number of cigarettes smoked and whether smoking had taken place only during the child’s infancy or also during the pregnancy. With the exception of Tanaka et al.[29], who focused on permanent dentition, all the studies examined the presence of caries in primary dentition. Nayani et al. [34]observed a higher prevalence ratio in children exposed more than 30 minutes to tobacco smoke than in children exposed less time. Aligne et al.[15] established a significant positive relationship between blood cotinine levels and the odds ratio for caries presence.
An important point to note is how exposure is measured. Most of the studies were based on questionnaires addressed to the parents, which could affect the results by introducing memory or response biases. Cotinine, on the other hand, is a primary metabolite of nicotine with a longer half-life and is considered a reliable biomarker when screening for passive exposure to tobacco use. It can be measured in body fluids such as plasma, saliva and urine[49]. For this reason, measuring the cotinine level is recommended in addition to collecting information from the parents’ answers to the questionnaires. Despite this, only two of the studies included (Aligne et al.[15] and Avçar et al.[35]) used cotinine levels to quantify passive exposure to tobacco use.
When analysing the data in the present study, three subgroup meta-analyses were performed to check for heterogeneity, distinguishing between exposure in the womb (prenatal) or in infancy (postnatal) and between caries in primary teeth and in permanent teeth. No studies that examined prenatal exposure and caries in permanent teeth were identified. The only meta-analysis that showed heterogeneity was that for association between postnatal exposure and caries in primary teeth, even though the large sample size of most of the studies led to narrow OR confidence intervals, which facilitate heterogeneity between the studies. The classic fail-safe numbers and Egger regression values indicated little risk of publication bias in the present results.
The cross-sectional design of most of the studies did not allow confirmation of a possible aetiology of passive exposure to tobacco use, despite the association found with caries, the positive dose-response relationship, the possible hypotheses of biological plausibility, and even that the few longitudinal studies included also showed this association. Prospective studies are needed to identify passive tobacco exposure in the home, measured through the joint use of questionnaires and cotinine level testing, as a direct risk factor for caries in children.
Conclusions
In view of the foregoing, after examining the available evidence and bearing in mind the above-mentioned methodological limitations, it may be asserted that a moderate association (estimated OR: 1.5) between the presence of caries in primary and permanent dentition and passive exposure (prenatal or postnatal) to tobacco smoke in children and adolescents does exist.
Supporting information
(DOC)
Acknowledgments
The authors wish to thank Mary Georgina Hardinge for translating the manuscript into English.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94: 650–658. 10.1177/0022034515573272 [DOI] [PubMed] [Google Scholar]
- 2.Marthaler TM. Changes in dental caries 1953–2003. Caries Res. 2004;38: 173–181. 10.1159/000077752 [DOI] [PubMed] [Google Scholar]
- 3.Edelstein BL. The dental caries pandemic and disparities problem. BMC Oral Health. 2006;6 Suppl 1: S2 1472-6831-6-S1-S2 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Primers. 2017;3: 17030 10.1038/nrdp.2017.30 [DOI] [PubMed] [Google Scholar]
- 5.Featherstone JD. The continuum of dental caries—evidence for a dynamic disease process. J Dent Res. 2004;83 Spec No C: 39. doi: 83/suppl_1/C39 [pii]. [DOI] [PubMed] [Google Scholar]
- 6.Zhou S, Rosenthal DG, Sherman S, Zelikoff J, Gordon T, Weitzman M. Physical, behavioral, and cognitive effects of prenatal tobacco and postnatal secondhand smoke exposure. Curr Probl Pediatr Adolesc Health Care. 2014;44: 219–241. 10.1016/j.cppeds.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Tobacco free initiative Consultative report International consultation on environmental tobacco smoke and child health. 1st ed Geneve: WHO; 1999. [Google Scholar]
- 8.Vellappally S, Fiala Z, Smejkalova J, Jacob V, Shriharsha P. Influence of tobacco use in dental caries development. Cent Eur J Public Health. 2007;15: 116–121. [DOI] [PubMed] [Google Scholar]
- 9.Wilson Karen M, Weis Emily. The Epidemiology and Health Effects of Tobacco Smoke Exposure. Current Pediatric Reviews. 2011;7: 76–80. 10.2174/157339611795735594 [DOI] [Google Scholar]
- 10.Hanioka T, Ojima M, Tanaka K, Yamamoto M. Does secondhand smoke affect the development of dental caries in children? A systematic review. Int J Environ Res Public Health. 2011;8: 1503–1519. 10.3390/ijerph8051503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slayton RL. Exposure to secondhand smoke may cause dental caries in children. J Evid Based Dent Pract. 2012;12: 8–9. 10.1016/j.jebdp.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 12.GA Wells, B Shea, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 13.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56: 455–463. [DOI] [PubMed] [Google Scholar]
- 14.Aida J, Ando Y, Oosaka M, Niimi K, Morita M. Contributions of social context to inequality in dental caries: a multilevel analysis of Japanese 3-year-old children. Community Dent Oral Epidemiol. 2008;36: 149–156. 10.1111/j.1600-0528.2007.00380.x [DOI] [PubMed] [Google Scholar]
- 15.Aligne CA, Moss ME, Auinger P, Weitzman M. Association of pediatric dental caries with passive smoking. JAMA. 2003;289: 1258–1264. doi: joc21865 [pii]. [DOI] [PubMed] [Google Scholar]
- 16.Almerich-Torres T, Montiel-Company JM, Almerich-Silla JM. Relación entre caries y tabaquismo pasivo en los escolares de 12 y 15 años de la Comunidad Valenciana: Primer Accésit en los XII Premios Fin de Carrera de Odontología GACETA DENTAL. Gaceta dental: Industria y profesiones. 2013: 116–124. [Google Scholar]
- 17.Ayo-Yusuf OA, Reddy PS, van Wyk PJ, van den Borne B W. Household smoking as a risk indicator for caries in adolescents' permanent teeth. J Adolesc Health. 2007;41: 309–311. doi: S1054-139X(07)00184-X [pii]. 10.1016/j.jadohealth.2007.04.012 [DOI] [PubMed] [Google Scholar]
- 18.Carbajosa Garcia S, Llena Puy C. Relationship between tobacco smoke and dental caries in school children at the Valencian Country. Rev Esp Salud Publica. 2011;85: 217–225. 10.1590/S1135-57272011000200009 [DOI] [PubMed] [Google Scholar]
- 19.Ditmyer M, Dounis G, Mobley C, Schwarz E. A case-control study of determinants for high and low dental caries prevalence in Nevada youth. BMC Oral Health. 2010;10: 24 10.1186/1472-6831-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanioka T, Nakamura E, Ojima M, Tanaka K, Aoyama H. Dental caries in 3-year-old children and smoking status of parents. Paediatr Perinat Epidemiol. 2008;22: 546–550. 10.1111/j.1365-3016.2008.00950.x [DOI] [PubMed] [Google Scholar]
- 21.Iida H, Auinger P, Billings RJ, Weitzman M. Association between infant breastfeeding and early childhood caries in the United States. Pediatrics. 2007;120: 944. doi: 120/4/e944 [pii]. [DOI] [PubMed] [Google Scholar]
- 22.Leroy R, Hoppenbrouwers K, Jara A, Declerck D. Parental smoking behavior and caries experience in preschool children. Community Dent Oral Epidemiol. 2008;36: 249–257. 10.1111/j.1600-0528.2007.00393.x [DOI] [PubMed] [Google Scholar]
- 23.Majorana A, Cagetti MG, Bardellini E, Amadori F, Conti G, Strohmenger L, et al. Feeding and smoking habits as cumulative risk factors for early childhood caries in toddlers, after adjustment for several behavioral determinants: a retrospective study. BMC Pediatr. 2014;14: 45 10.1186/1471-2431-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakayama Y, Mori M. Association of environmental tobacco smoke and snacking habits with the risk of early childhood caries among 3-year-old Japanese children. J Public Health Dent. 2015;75: 157–162. 10.1111/jphd.12085 [DOI] [PubMed] [Google Scholar]
- 25.Pita-Fernández S, Pombo-Sánchez A, Pértega-Díaz S. Exposición pasiva al tabaco y caries dental de los niños. Archivos de Bronconeumologia. 2011;47: 419–420. 10.1016/j.arbres.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 26.Shulman JD. Is there an association between low birth weight and caries in the primary dentition? Caries Res. 2005;39: 161–167. doi: 84792 [pii]. 10.1159/000084792 [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K, Hanioka T, Miyake Y, Ojima M, Aoyama H. Association of smoking in household and dental caries in Japan. J Public Health Dent. 2006;66: 279–281. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka K, Miyake Y, Sasaki S. The effect of maternal smoking during pregnancy and postnatal household smoking on dental caries in young children. J Pediatr. 2009;155: 410–415. 10.1016/j.jpeds.2009.03.032 [DOI] [PubMed] [Google Scholar]
- 29.Tanaka K, Miyake Y, Arakawa M, Sasaki S, Ohya Y. Household smoking and dental caries in schoolchildren: the Ryukyus Child Health Study. BMC Public Health. 2010;10: 335 10.1186/1471-2458-10-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka K, Miyake Y, Nagata C, Furukawa S, Arakawa M. Association of prenatal exposure to maternal smoking and postnatal exposure to household smoking with dental caries in 3-year-old Japanese children. Environ Res. 2015;143: 148–153. 10.1016/j.envres.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 31.Wiener RC. Children with Special Health Care Need's Association of Passive Tobacco Smoke Exposure and Dental Caries: 2007 National Survey of Children's Health. J Psychol Abnorm Child. 2013;1: 1000104. doi: 1000104 [pii]. 10.4172/2329-9525.1000104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams SA, Kwan SY, Parsons S. Parental smoking practices and caries experience in pre-school children. Caries Res. 2000;34: 117–122. doi: 16578 [pii]. 10.1159/000016578 [DOI] [PubMed] [Google Scholar]
- 33.Bakhurji Eman A., El Tantawi Maha M., Gaffar Balgis O., Al-Khalifa Khalifa S., Al-Ansari Asim A. Carious lesions of permanent molars and oral health practices of parents and peers in Saudi male adolescents. Saudi Medical Journal. 2017;38: 748–754. 10.15537/smj.2017.7.17601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nayani AA, Iqbal R, Azam SI, Khan FR, Khan AH, Janjua N, et al. Association between environmental tobacco smoke and dental caries amongst 5–14 years old children in Karachi, Pakistan. J Pak Med Assoc. 2018;68: 203–209. doi: 8555 [pii]. [PubMed] [Google Scholar]
- 35.Avçar A, Darka O, Topaloglu B, Bek Y. Association of passive smoking with caries and related salivary biomarkers in young children. Arch Oral Biol. 2008;53: 969–974. 10.1016/j.archoralbio.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 36.Bernabe E, MacRitchie H, Longbottom C, Pitts NB, Sabbah W. Birth Weight, Breastfeeding, Maternal Smoking and Caries Trajectories. J Dent Res. 2017;96: 171–178. 10.1177/0022034516678181 [DOI] [PubMed] [Google Scholar]
- 37.Julihn A, Ekbom A, Modeer T. Maternal overweight and smoking: prenatal risk factors for caries development in offspring during the teenage period. Eur J Epidemiol. 2009;24: 753–762. 10.1007/s10654-009-9399-7 [DOI] [PubMed] [Google Scholar]
- 38.Plonka KA, Pukallus ML, Barnett AG, Holcombe TF, Walsh LJ, Seow WK. A longitudinal case-control study of caries development from birth to 36 months. Caries Res. 2013;47: 117–127. 10.1159/000345073 [DOI] [PubMed] [Google Scholar]
- 39.Shenkin JD, Broffitt B, Levy SM, Warren JJ. The association between environmental tobacco smoke and primary tooth caries. J Public Health Dent. 2004;64: 184–186. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka S, Shinzawa M, Tokumasu H, Seto K, Tanaka S, Kawakami K. Secondhand smoke and incidence of dental caries in deciduous teeth among children in Japan: population based retrospective cohort study. BMJ. 2015;351: h5397 10.1136/bmj.h5397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Claudia C, Ju X, Mejia G, Jamieson L. The relationship between maternal smoking during pregnancy and parental-reported experience of dental caries in Indigenous Australian children. Community Dent Health. 2016;33: 297–302. 10.1922/CDH_3937Claudia06 [DOI] [PubMed] [Google Scholar]
- 42.Chowdhury IG, Bromage TG. Effects of fetal exposure to nicotine on dental development of the laboratory rat. Anat Rec. 2000;258: 397–405. 10.1002/(SICI)1097-0185(20000401)258:43.0.CO;2-I [pii]. [DOI] [PubMed] [Google Scholar]
- 43.Heikkinen T, Alvesalo L, Osborne RH, Tienari J. Maternal smoking and tooth formation in the foetus. III. Thin mandibular incisors and delayed motor development at 1 year of age. Early Hum Dev. 1997;47: 327–340. doi: S0378-3782(96)01792-6 [pii]. [DOI] [PubMed] [Google Scholar]
- 44.Arora M, Weuve J, Schwartz J, Wright RO. Association of environmental cadmium exposure with pediatric dental caries. Environ Health Perspect. 2008;116: 821–825. 10.1289/ehp.10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindemeyer RG, Baum RH, Hsu SC, Going RE. In vitro effect of tobacco on the growth of oral cariogenic streptococci. J Am Dent Assoc. 1981;103: 719–722. doi: S0002-8177(81)35020-X [pii]. [DOI] [PubMed] [Google Scholar]
- 46.Strauss RS. Environmental tobacco smoke and serum vitamin C levels in children. Pediatrics. 2001;107: 540–542. [DOI] [PubMed] [Google Scholar]
- 47.Jakhete N, Gitterman BA. Environmental smoke exposure associated with increased prevalence of dental caries in low-income children. International Journal on Disability and Human Development. 2012;11 10.1515/ijdhd-2012-0049 [DOI] [Google Scholar]
- 48.Delpisheh A, Kelly Y, Brabin BJ. Passive cigarette smoke exposure in primary school children in Liverpool. Public Health. 2006;120: 65–69. doi: S0033-3506(05)00151-4 [pii]. 10.1016/j.puhe.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 49.Binnie V, McHugh S, Macpherson L, Borland B, Moir K, Malik K. The validation of self-reported smoking status by analysing cotinine levels in stimulated and unstimulated saliva, serum and urine. Oral Diseases. 2004;10: 287–293. 10.1111/j.1601-0825.2004.01018.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.