Abstract
Wnt signaling plays critical roles in dorsoventral fate specification and anteroposterior patterning, as well as in morphogenetic cell movements. Dishevelled proteins, or Dvls, mediate the activation of Wnt/ß-catenin and Wnt/planar cell polarity pathways. There are at least three highly conserved Dvl proteins in vertebrates, but the implication of each Dvl in key early developmental processes remains poorly understood. In this study, we use genome-editing approach to generate different combinations of maternal and zygotic dvl mutants in zebrafish, and examine their functions during early development. Maternal transcripts for dvl2 and dvl3a are most abundantly expressed, whereas the transcript levels of other dvl genes are negligible. Phenotypic and molecular analyses show that early dorsal fate specification is not affected in maternal and zygotic dvl2 and dvl3a double mutants, suggesting that the two proteins may be dispensable for the activation of maternal Wnt/ß-catenin signaling. Interestingly, convergence and extension movements and anteroposterior patterning require both maternal and the zygotic functions of Dvl2 and Dvl3a, but these processes are more sensitive to Dvl2 dosage. Zygotic dvl2 and dvl3a double mutants display mild axis extension defect with correct anteroposterior patterning. However, maternal and zygotic double mutants exhibit most strongly impaired convergence and extension movements, severe trunk and posterior deficiencies, and frequent occurrence of cyclopia and craniofacial defects. Our results suggest that Dvl2 and Dvl3a products are required for the activation of zygotic Wnt/ß-catenin signaling and Wnt/planar cell polarity pathway, and regulate zygotic developmental processes in a dosage-dependent manner. This work provides insight into the mechanisms of Dvl-mediated Wnt signaling pathways during early vertebrate development.
Author summary
The embryogenesis of most animals is first supported by maternal gene products accumulated in the oocyte, and then by the expression of genes from the zygote. In all vertebrates, there are at least three Dishevelled (Dvl) proteins, which play critical roles in normal development and human diseases. They are both maternally and zygotically expressed, and can activate the ß-catenin-dependent Wnt pathway that regulates gene expression and cell fate, and the ß-catenin-independent Wnt pathway that orchestrates cell movements. In zebrafish embryo, Dvl2 and Dvl3a are most abundant, but their functions are not fully understood. We find that maternally and zygotically expressed Dvl2 plays a predominant role in the elongation of the anteroposterior axis, and the expression of genes involved in the development of the posterior region. Dvl3a cooperates with Dvl2 in these processes. Analyses after loss-of-function of these genes indicate that deficiency of maternal and zygotic Dvl2 and Dvl3a results in embryos with cyclopia, craniofacial defects, and severe abnormality in the trunk and posterior regions. Many human birth defects and other diseases, like cancer, are attributed to the dysfunction of the Wnt pathways. Our results help to understand the mechanisms of Dvl-mediated Wnt pathway activation, and the causes of developmental disorders.
Introduction
The specification of the dorsoventral (DV) axis is tightly linked to, and ultimately determines, the processes that establish the anteroposterior (AP) pattern in vertebrate embryos. A large body of work has elucidated the induction and patterning processes underlying DV and AP polarities [1, 2]. It is well established that, in Xenopus and zebrafish, maternal canonical Wnt/ß-catenin signaling is activated on the future dorsal side following fertilization, and ß-catenin is absolutely required for the establishment of the primary DV asymmetry through cooperation with other factors, such as members of the Nodal family [1–5]. During gastrulation, dorsolateral cells converge toward the dorsal midline, while dorsal midline cells undergo extension along the AP axis. These movements, called convergence and extension (CE), not only provide the driving force for gastrulation, but also make an important contribution to the elongation of AP axis. The conserved non-canonical Wnt/PCP (planar cell polarity) signaling plays a key role in CE movements in all vertebrates [6–13]. Thus, disruption of Wnt/ß-catenin and Wnt/PCP signaling pathways can result in severe defects in the formation of embryonic axes.
Dishevelled (Dvl) is a key intracellular signaling molecule that mediates the activation of both Wnt/ß-catenin and Wnt/PCP pathways during early development [6, 8, 14–18]. Functional analyses in Xenopus suggest that Dvl2 (also called Xdsh) exhibits dorsalizing and neuralizing activity [14], and controls polarized cell behaviors that are required for CE movements during gastrulation [15]. However, its requirement for the activation of maternal Wnt/ß-catenin signaling in dorsal axis specification has not been clearly established, and remains largely enigmatic [17]. Simultaneous depletion of maternally expressed dvl2 and dvl3 from Xenopus oocytes using morpholino antisense oligonucleotides has no obvious effect on the expression of maternal Wnt/ß-catenin target genes and the specification of dorsal axis [19]. This negative result may be due to the presence of stored punctae of Dvl proteins in the oocyte cortex, which translocate to the dorsal region soon after fertilization [20], or due to the insufficiency of maternal dvl mRNA depletion. Therefore, appropriate genetic approaches will be necessary to determine whether Dvls function upstream of ß-catenin in early dorsal fate specification.
There are three dvl genes (dvl1, dvl2 and dvl3) in human, mouse, and Xenopus, and at least five in zebrafish [21, 22]. They are highly conserved and broadly expressed throughout early development [21, 23]. Extensive analyses of mutant phenotypes in mice have uncovered both unique and redundant functions among the three Dvl genes [24–27]. Although single mutants for these mouse Dvl genes generally survived to adulthood, abnormal neural tube closure and defective organogenesis were observed in different combinations of double mutants, which also cause embryonic lethality [25–27]. This, combined with the development in utero, make it less convenient to assay Dvl implication in early axis patterning and morphogenetic processes. In Xenopus, triple knockdown of dvl1, dvl2 and dvl3 led to CE defects [21], which were similar as those resulted from overexpression of Xdd1, a Dvl2 (Xdsh) mutant lacking the PDZ domain [28]. Interestingly, Dvl1 and Dvl2 were found to be involved in neural crest specification and somite segmentation, while Dvl3 was required to maintain muscle gene expression [21]. These observations reveal a distinct requirement of Dvl proteins for Wnt signaling in regulating the expression of developmental genes. However, at present, the relative contribution of individual Dvl protein in CE movements that are dependent on the Wnt/PCP pathway has not been clearly determined, making this important question quite open for further investigation.
One of the critical functions of Wnt signaling during early development is the requirement of zygotic Wnt/ß-catenin pathway for ventroposterior development in Xenopus and zebrafish embryos. In contrast to maternal Wnt/ß-catenin signaling that specifies dorsal fate, zygotic Wnt/ß-catenin signaling inhibits anterior development by activating the expression of different target genes that specify ventral and posterior tissues [1–5]. How different dvl genes are implicated in this process also remains poorly understood. Another difficulty in studying Dvl function is the presence of abundant maternal dvl transcripts, as shown both in Xenopus [14, 19], and in zebrafish [29, 30]. These maternal products play an important role to support early developmental processes, and when translated into proteins and stored during oogenesis, could not be targeted by knockdown approaches. Also, zygotic homozygous mutants often do not survive to fertile adulthood to produce maternal and zygotic (MZ) mutant embryos for the analysis of maternal gene function. Taken together, it is clear that the maternal and zygotic contributions of dvl genes in different patterning and morphogenetic processes remain elusive, and merits further investigation.
In the present study, we take advantage of the genome-editing approach to generate different combinations of maternal and zygotic dvl mutants in zebrafish. Transcriptomic analysis revealed that, among the five dvl genes, only dvl2 and dvl3a are maternally and abundantly expressed, whereas the transcript levels of the other three dvl genes (dvl1a, dvl1b, dvl3b) are negligible [29]. By creating targeted mutations, we find that MZdvl2 mutants display most severe CE and craniofacial defects, but with correct AP patterning. In dvl2 and dvl3a double mutants, dvl3a dosage exerts a permissive effect on the loss of dvl2 in CE movements and posterior development. By further targeting the wild-type (WT) allele in triallelic mutant embryos to generate mosaic germline transmissible double homozygous adults, we obtained MZdvl2;MZdvl3a offspring. These mutants show correct dorsal fate specification, but display severe CE defects and develop trunk and posterior deficiencies, as well as cyclopia. These observations indicate that Dvl2 and Dvl3a may be not required for maternal Wnt/ß-catenin pathway activation. Instead, Dvl2 plays a major role in Wnt/PCP and zygotic Wnt/ß-catenin signaling. Our findings thus help to better understand the function of Dvl proteins in different patterning and morphogenetic processes.
Results
MZdvl2 mutants display axis extension and craniofacial defects
We used transcription activator-like effector nucleases (TALENs) genome-editing approach to generate mutant lines for the five zebrafish dvl genes (dvl1a, dvl1b, dvl2, dvl3a, dvl3b). All indel mutations led to premature stop codons in the transcripts, and resulted in proteins truncated either at the DIX or the PDZ domain (S1–S5 Figs). Analysis of the phenotypes at different stages indicated that, except for dvl2, maternal and zygotic mutants for the other dvl genes developed normally, and could survive to adulthood and were fertile (Fig 1 and S6 Fig). Because dvl2 and dvl3a represent the most abundantly expressed maternal transcripts (S7 Fig), we focused our analyses on these two mutant lines. Compared with WT embryos at different stages (Fig 1A–1C), Zdvl2 mutants showed weakly reduced AP axis extension at 11.5 hpf (hours post-fertilization), as judged by the degree of the angle between the anterior end and posterior end, with vertex at the geometric center of the embryo (Fig 1M). This defect was largely recovered at 30 hpf (Fig 1E). At 5 dpf (days post-fertilization), Zdvl2 mutants displayed essentially a normal AP axis, except for the presence of a smaller gas-filled swim bladder (Fig 1F). However, only about half of these Zdvl2 mutants could survive to adulthood, and only about two-third of the survived adult female fish could spawn, whereas all male Zdvl2 mutants were not fertile due to the absence of courtship behavior.
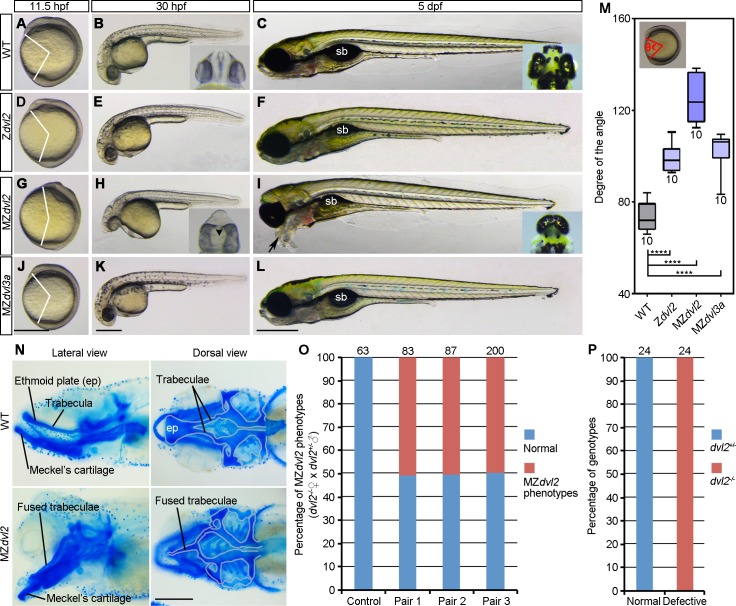
Fig 1. Analysis of dvl2 and dvl3a mutant phenotypes.
(A-C) WT embryos, the insets show the eyes in ventral view at 30 hpf and in dorsal view at 5 dpf. (D-F) Zdvl2 mutants, obtained by crosses between heterozygous dvl2+/- carriers, display weak axis extension defect at 11.5 hpf. They are normal at 30 hpf, and show reduced swim bladder (sb) at 5 hpf. (G-I) MZdvl2 mutants, obtained from crosses between female dvl2-/- fish and male dvl2+/- fish, display a reduced AP axis at 11.5 hpf and 30 hpf. They present fused eyes at 30 hpf (inset, ventral view; arrowhead indicates fused lenses), and develop craniofacial defects and cyclopia (inset, dorsal view), with pharyngeal cartilages protruding outward (arrow) at 5 dpf. (J-L) MZdvl3a mutants from crosses between dvl3a-/- carriers display weak axis extension defect, but are indistinguishable from WT embryos at 30 hpf and 5 dpf. (M) Statistical analysis of the extent of axis extension delay. The embryos were imaged at 11.5 hpf followed by genotyping. Those embryos with expected genotypes were used to measure the angle between the anterior end and posterior end, with vertex at the geometric center of the embryo (inset). Bars represent the mean ± s.d. from indicated numbers of embryos (****, P<0.0001). (N) Alcian blue staining of head cartilages at 5 dpf. Cartilage structures of the basicranium are outlined in grey, showing the fusion of trabeculae and the absence of ethmoid plate (ep) in MZdvl2 mutants. (O) Quantitative analysis of MZdvl2 mutant phenotypes at 5 dpf in offspring from three independent female dvl2-/- and male dvl2+/- fish pairs. Control embryos were obtained from crosses between female WT fish and male dvl2+/- fish. Numbers on the top of each column indicate total embryos analyzed. (P) Genotyping of dvl2 mutants with normal and defective phenotypes. All embryos with a normal phenotype are dvl2+/- mutants, whereas all defective embryos are MZdvl2 mutants. Numbers on the top of each column indicate total embryos genotyped from three independent fish pairs. Scale bars: (A, D, G, J) 400 μm; (B, E, H, K) 400 μm; (C, F, I, L) 400 μm; (N) 100 μm.
To obtain MZdvl2 embryos, we crossed female dvl2-/- fish with male dvl2+/- fish. At 11.5 hpf, about half of the resulting embryos showed more severe axis extension defect (Fig 1G and 1M). At 30 hpf, they exhibited a shortened AP axis, associated with a reduced yolk extension, which are characteristics of defective CE movements (Fig 1H). At this stage, MZdvl2 mutants also displayed an obvious cyclopic phenotype (compare insets in Fig 1B and 1H). At 5 dpf, although MZdvl2 mutants had a similar length of AP axis as WT embryos, abnormalities in the head region were clearly apparent. These include severe craniofacial defects, and cyclopia or fused eyes (compare insets in Fig 1C and 1I). In particular, pharyngeal arches were not correctly positioned, and eventually protruded outward (Fig 1I). Alcian blue staining of larval head cartilages indicated that, among other abnormalities, the pair of trabeculae was fused and the ethmoid plate was absent (Fig 1N). All these phenotypes are reminiscent of impaired Wnt/PCP signaling and defective extension of axial tissues, which are frequently observed in other Wnt/PCP-specific mutants, such as trilobite/vangl2, and slb/wnt11 [31–33]. Most strikingly, the protrusion outward of pharyngeal cartilages is much similar as the “bulldog” facial phenotype described in slb/wnt11 mutant [32–34]. A more detailed analysis of these late phenotypes is beyond the scope of this study, but it will be interesting for future work. Due to these defects, MZdvl2 mutant embryos could not survive beyond 5 dpf. Genotyping by allele-specific PCR (S1 Table and S8 Fig) of large numbers of severely affected embryos derived from three independent fish pairs confirmed that they were indeed MZdvl2 mutants (Fig 1O and 1P). In contrast to MZdvl2 mutants, the late phenotype of MZdvl3a mutants was indistinguishable from that of WT embryos (Fig 1J–1L), although statistical analysis revealed a weakly reduced AP axis extension at 11.5 hpf (Fig 1M). These results show that both maternal and zygotic Dvl2 make an important contribution to CE movements. They also suggest that deficiency of maternal and zygotic Dvl2 or Dvl3a is not sufficient to affect DV and AP patterning.
By RT-PCR analysis, we found that maternal dvl2 mutant transcripts were subjected to nonsense-mediated mRNA decay (NMD) at cleavage stages, which was further confirmed by in situ hybridization. Maternal dvl3a mutant transcripts also underwent NMD, but to a lesser extent. We then checked whether there was a mutual compensation between dvl2 and dvl3a. No significant change in the level of maternal dvl2 transcripts was found in MZdvl3a mutants, whereas the level of maternal dvl3a transcripts showed a weak decrease in Mdvl2 mutants (S9 Fig). We also attempted to verify if Dvl2 or Dvl3a protein was absent in the mutants by western blotting using available antibodies, but failed to detect any specific signal either in WT or in mutant embryos. Alternatively, to examine whether dvl2 and dvl3a mutant transcripts may be translated by using an alternative ATG or by a stop codon bypass mechanism, we cloned WT and mutant dvl2 and dvl3a coding sequences upstream of myc sequences, and injected the corresponding mRNA (100 pg) into zebrafish embryos. Analysis by western blotting did not detect any signal in embryos injected with these mutant mRNAs (S10A and S10B Fig). This suggests that dvl2 and dvl3a mutant transcripts should not be translated into proteins.
Zygotic Dvl2 and Dvl3a cooperate to regulate axis extension
To analyze the function of Dvl2 and Dvl3a dosages in AP axis extension and patterning, we first generated double heterozygous dvl2+/-;dvl3a+/- mutants by crosses between female Zdvl2 and male Zdvl3a. Compared to WT embryos (Fig 2A–2C), dvl2+/-;dvl3a+/- mutants showed weak, but significant delay in axis extension at 11.5 hpf (Fig 2D and 2P). They were completely normal at 30 hpf and 5 dpf (Fig 2E and 2F), and developed to fertile adults, indicating that Wnt/ß-catenin signaling was not affected, and Wnt/PCP signaling was weakly affected during gastrulation. We then intercrossed dvl2+/-;dvl3a+/- fish to analyze the phenotypes of triallelic mutants. Similar as dvl2+/-;dvl3a+/- mutants, dvl2+/-;Zdvl3a mutants displayed weak axis extension defect at 11.5 hpf (Fig 2G and 2P), and slightly shortened AP axis at 30 hpf (Fig 2H). These mutants also recovered to a nearly normal phenotype at 5 dpf (Fig 2I). However, the extent of axis extension delay was more pronounced in Zdvl2;dvl3a+/- mutant embryos at 11.5 hpf (Fig 2J and 2P), which further developed a shortened AP axis at 30 hpf (Fig 2K), and a compressed head at 5 dpf (Fig 2L). These embryos, which were grouped as type I, presented a more severely affected phenotype than Zdvl2 mutants and did not survive to adulthood. This suggests that removal of one dvl3a allele could enhance CE defects in Zdvl2 mutants.
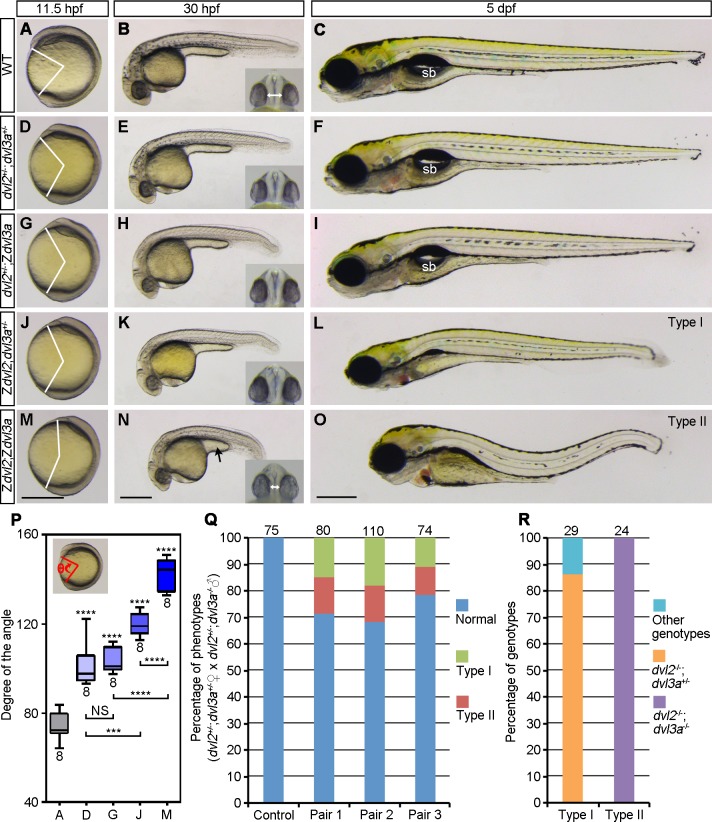
Fig 2. Zygotic Dvl2 and Dvl3a in axis extension.
Mutant phenotypes were compared with WT embryos at indicated stages. Insets show eye phenotypes in ventral view, with bidirectional arrows indicating the distance of the two eyes. (A-C) WT embryos. (D-F) Double heterozygous dvl2+/-;dvl3a+/- mutants, from crosses between female dvl2-/- fish and male dvl3a-/- fish, show weakly delayed axis extension at 11.5 hpf, but are phenotypically normal at later stages. (G-I) Triallelic dvl2+/-;Zdvl3a mutants,from crosses between dvl2+/-;dvl3a+/- carriers, display weak axis extension defect at 11.5 hpf and 30 hpf, and recover at 5 dpf. (J-L) Triallelic Zdvl2;dvl3a+/- mutants, from crosses between dvl2+/-;dvl3a+/- carriers, exhibit more obvious axis extension defect at 11.5 hpf and 30 hpf, and develop shortened AP axis, compressed head, and reduced swim bladder at 5 dpf, which are referred as type I phenotype. (M-O) Zdvl2;Zdvl3a mutants, from crosses between female dvl2+/-;dvl3a+/- fish and male dvl2+/-;Zdvl3a fish, show more strong axis extension defect at 11.5 hpf and 30 hpf, and present a severe type II phenotype, with shortened and wavy axis, craniofacial defects, and complete disappearance of swim bladder (sb) at 5 dpf. (P) Statistical analysis of the extent of axis extension delay, after genotyping of imaged embryos at 11.5 hpf. Capital letters of the abscissa correspond to the images at 11.5 hpf. Bars represent the mean ± s.d. from indicated numbers of embryos, and asterisks above the bars refer to comparison with WT embryos (***, P<0.001; ****, P<0.0001; NS, not significant). (Q) Quantitative analysis of type I and type II phenotypes at 5 dpf from three independent female dvl2+/-;dvl3a+/- and male dvl2+/-;dvl3a-/- fish pairs. Control embryos were from crosses between female WT fish and male dvl2+/-;dvl3a-/- fish. (R) Genotypes of type I and type II embryos. Numbers on the top of each column indicate total embryos analyzed. Scale bars: (A, D, G, J, M) 400 μm; (B, E, H, K, N) 400 μm; (C, F, I, L, O) 400 μm.
Since dvl2+/-;Zdvl3a mutants could survive to adulthood and were fertile, the male fish could be used for generating Zdvl2;Zdvl3a double mutants, after crosses with female dvl2+/-;dvl3a+/- fish. We found that double Zdvl2;Zdvl3a mutants displayed more strongly affected axis extension at 11.5 hpf (Fig 2M and 2P), and developed a shortened AP axis, with a reduced yolk extension at 30 hpf (Fig 2N). The distance between the two eyes was also reduced (compare insets in Fig 2B and 2N). All these phenotypes are suggestive of impaired CE movements. These embryos, grouped as type II, further developed bent axis and craniofacial defects at 5 dpf (Fig 2O). From three independent fish pairs, we found that the occurrence of the defective axis extension phenotype in the resulting offspring was quite reproducible because similar proportions of type I (13%) and type II (15%) mutant embryos have been obtained (Fig 2Q and 2R), which could be expected from the crosses between dvl2+/-;dvl3a+/- and dvl2+/-;Zdvl3a fish. Since both head and tail were present in zygotic double homozygous mutants, this indicates that half of the maternal Dvl2 and Dvl3a products should be largely sufficient to support AP patterning.
Maternal contribution of Dvl2 and Dvl3a in axis extension and AP patterning
Since AP patterning does not seem to be affected in Zdvl2;Zdvl3a mutants, we examined the maternal contribution of Dvl2 and Dvl3a in this process. Intercrosses between dvl2+/-;Zdvl3a triallelic fish could generate three types of mutant offspring, including MZdvl3a, dvl2+/-;MZdvl3a, and Zdvl2;MZdvl3a. Analysis of the phenotypes followed by genotyping indicated that, compared to WT embryos (Fig 3A–3C), dvl2+/-;MZdvl3a mutants displayed mild axis extension defect at 11.5 hpf (Fig 3D). At 30 hpf, these embryos were short in length, but AP patterning was not affected because head and tail regions were correctly formed (Fig 3E). At 5 dpf, they completely recovered to a normal phenotype (Fig 3F), and eventually developed to fertile adult. This indicates that, in the absence of Dvl3a, maternal and zygotic Dvl2 product derived from one allele is sufficient for AP patterning, although Wnt/PCP pathway activation is reduced at early stages.
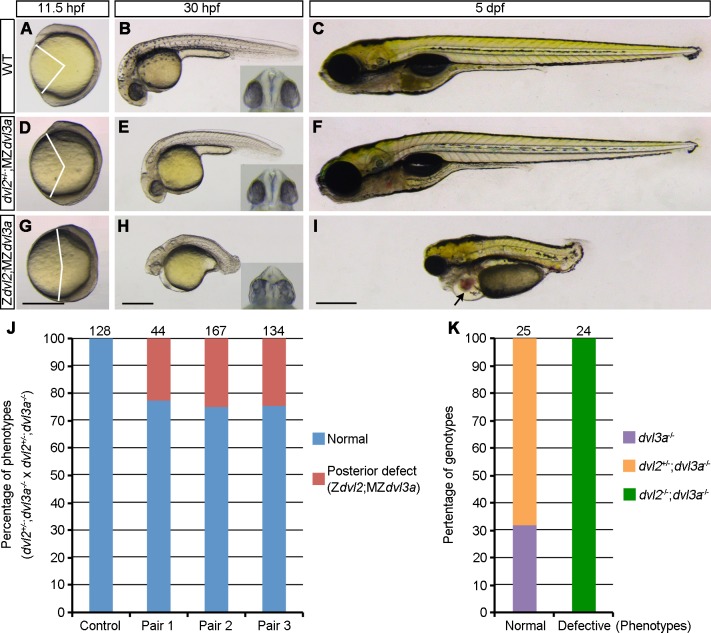
Fig 3. Dvl2 and Dvl3a dosages in axis extension and AP patterning.
Both dvl2+/-;MZdvl3a and Zdvl2;MZdvl3a mutants were from crosses between dvl2+/-;dvl3a-/- carriers. Representative mutant embryos were imaged at indicated stages. Eye phenotypes are shown in the insets as ventral view. (A-C) WT embryos. (D-F) dvl2+/-;MZdvl3a mutants display moderate axis extension defect at 11.5 hpf and 30 hpf, and recover to a normal phenotype at 5 dpf. (G-I) Zdvl2;MZdvl3a mutants show strong axis extension defect at different stages, and display caudal truncation, craniofacial defects, cardiac edema (arrow), and fused eyes or cyclopia (inset) at 30 hpf and at 5 dpf. (J) Quantitative analysis of the posterior truncation phenotype at 5 dpf in offspring derived from three independent dvl2+/-;dvl3a-/- carriers. Control embryos were from crosses between female WT fish and male dvl2+/-;dvl3a-/- fish. Posterior deficiency is present in the offspring from all three fish pairs with a proportion that follows the Mandel inheritance (about 25%). Numbers on the top of each column indicate total embryos analyzed. (K) Genotyping of normal and posteriorly truncated embryos. All defective embryos are dvl2-/-;dvl3a-/- mutants. Scale bars: (A, D, G) 400 μm; (B, E, H) 400 μm; (C, F, I) 400 μm.
Strikingly, Zdvl2;MZdvl3a mutants displayed strong axis extension defect at 11.5 hpf (Fig 3G), indicating severely impaired Wnt/PCP signaling. At 30 hpf and 5 dpf, axis extension and AP patterning defects became particularly prominent. These mutants displayed a reduced head size with cyclopia or fused eyes (Fig 3H and 3I). This phenotype is likely caused by perturbed midline development, and is a fish-specific consequence independent of the affected genetic pathway [35]. Cardiac edema was also evident at 5 dpf (Fig 3I). Importantly, Zdvl2;MZdvl3a mutants exhibited a severely reduced body length, with posterior truncation (Fig 3H and 3I). We then intercrossed three independent dvl2+/-;Zdvl3a fish pairs, and found that the appearance of severe axis extension defect associated with posterior deficiency was quite reproducible in the resulting offspring, with an average of 24.6% that corresponds to the Mendel inheritance (Fig 3J). Genotyping of these severely affected embryos confirmed that they were indeed Zdvl2;MZdvl3a mutants (Fig 3K). This analysis indicates that, in the MZdvl3a background, the reduction of maternal Dvl2 dosage along with the absence of zygotic Dvl2 products strongly impairs Wnt/PCP-dependent axis extension, and leads to defective posterior patterning.
Generation of MZdvl2;MZdvl3a double mutants
Zdvl2;Zdvl3a double mutants could not survive to adulthood, this prevented us from obtaining maternal and zygotic double mutants to fully address the maternal contribution of Dvl2 and Dvl3a in DV and AP patterning. To circumvent this obstacle, we used a strategy to generate mosaic dvl2-/-;dvl3a-/- fertile adult fish, by disrupting the remaining dvl2 WT allele in dvl2+/-;dvl3a-/- embryos derived from intercrosses between triallelic dvl2+/-;dvl3a-/- fish (Fig 4A). To obtain viable mosaic adult fish, low amounts of the original dvl2 TALENs mRNAs (100 pg each) were injected in these triallelic mutant embryos at 1-cell stage. The resulting adult female fish were crossed with male dvl2+/-;dvl3a-/- fish, and genotyping of the offspring by allelic-specific PCR followed by sequencing was performed to screen female fish carrying a new germline transmissible dvl2 mutant allele (Fig 4B). Those positive female fish were designated as mdvl2+(-)/-;dvl3a-/-, for mosaic dvl2 homozygous genotype. By this approach, if mutations of the remaining dvl2 WT allele occur in some germ cells, MZdvl2;MZdvl3a offspring could be obtained through crosses between female mdvl2+(-)/-;dvl3a-/- fish and male dvl2+/-;dvl3a-/- fish (S11 Fig). Indeed, this strategy has allowed us to generate rather efficiently mdvl2+(-)/-;dvl3a-/- fish. Among a total of 45 female adults tested, 7 female fish produced offspring with a varied proportion of extremely severe phenotype. At 11.5 hpf, these embryos displayed most strongly reduced axis extension associated with sharply widened paraxial mesoderm (Fig 4C–4F). At 30 hpf, they exhibited severe trunk and posterior deficiencies, but the head region was still present (Fig 4G and 4H). At 5 dpf, all these embryos developed cyclopia or fused eyes (S12 Fig). Other siblings either presented posterior truncation, which were genotyped as Zdvl2;MZdvl3a mutants (Fig 4I), or developed relatively normally, which were genotyped as dvl2+/-;MZdvl3a triallelic mutants or MZdvl3a mutants. These embryos could be expected from the crosses, since a high proportion of germ cells with one dvl2 WT allele should be still present in mdvl2+(-)/-;dvl3a-/- fish.
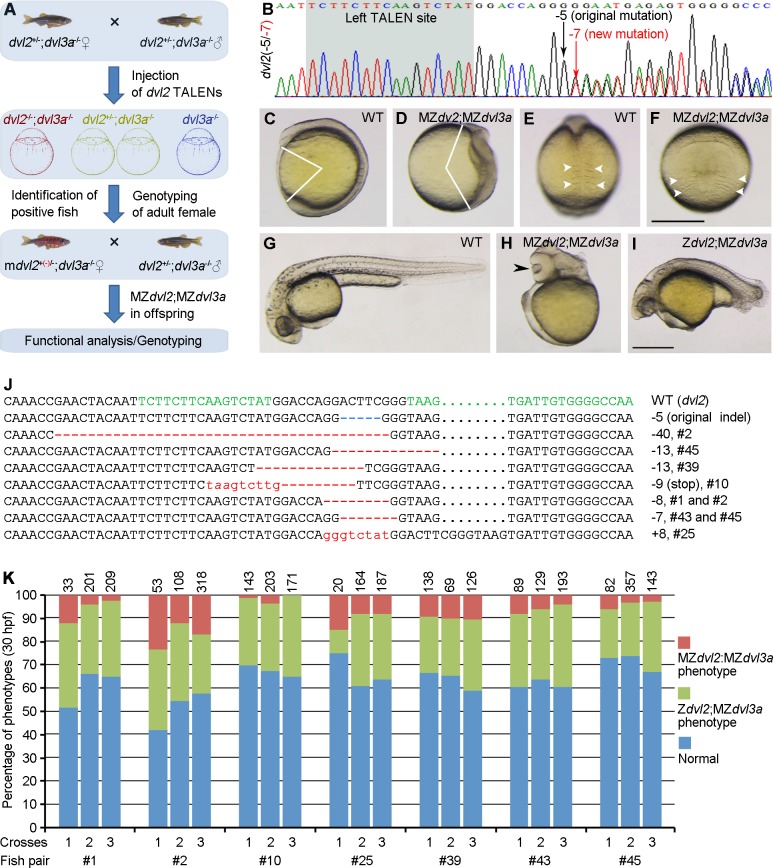
Fig 4. Analysis of MZdvl2;MZdvl3a mutants.
(A) Schema illustrating the strategy to generate female mosaic mdvl2+(-)/-;dvl3a-/- adult fish with a new mutant allele (red) in dvl2. MZdvl2;MZdvl3a mutant embryos are present at varied proportions in the offspring from crosses between female mdvl2+(-)/-;dvl3a-/- and male dvl2+/-;dvl3a-/- fish, depending on the efficiency of germline mutations in the remaining dvl2 WT allele. (B) An example of the sequencing chromatogram with both the original mutant allele and a new indel in dvl2 locus. (C-F) Lateral (C, D) and dorsal (E, F) views of a representative WT embryo (C, E), and an MZdvl2;MZdvl3a mutant (D, F) at 11.5 hpf. Notice that the mutant embryo displays most severely impaired AP axis and convergence of paraxial mesoderm, with strongly widened somites (arrowheads). (G) A WT embryo at 30 hpf. (H) Lateral view of a representative MZdvl2;MZdvl3a mutant at 30 hpf shows deficiency of trunk and posterior regions, and cyclopia (arrowhead; see also S12 Fig). (I) The phenotype of a Zdvl2;MZdvl3a mutant with characteristic caudal truncation at 30 hpf. (J) Genotyping of dvl2 alleles in MZdvl2;MZdvl3a mutant embryos from 7 independent fish pairs. A novel indel (red) along with the original mutation (blue) are present in the mutants. The left and right TALEN targeting sites are indicated in green. Dots are introduced to optimize sequence alignment. (K) Quantitative analyses of the occurrence of MZdvl2;MZdvl3a and Zdvl2;MZdvl3a mutants among offspring from 7 independent fish pairs. Each fish pair was crossed three times, and numbers on the top of each column indicate total embryos scored. Scale bar: (C-F) 400 μm; (G-I) 400 μm.
We then genotyped all the most severely affected embryos to confirm the presence of separate mutations in the dvl2 alleles. As expected, analysis of the sequencing chromatograms revealed that, in addition to the original mutated allele that has a deletion of 5 nucleotides, different new indels were also detected in these embryos (Fig 4J). It is unlikely that the re-injected dvl2 TALENs could target the original mutant allele in dvl2+/-;dvl3a-/- embryos, since we verified that they had no effect in dvl2-/- embryos, and it has been shown the TALEN pair had no gene modification activity when separated by 11 nucleotides or less [36], which is the case for the original dvl2 mutant allele. Thus, we can conclude that the extremely severe defects were specific to MZdvl2;MZdvl3a mutants, and were caused by the deficiency of maternal and zygotic Dvl2 and Dvl3a products. When the phenotypes of offspring from three independent crosses between a fixed pair of female mdvl2+(-)/-;dvl3a-/- and male dvl2+/-;dvl3a-/- fish were analyzed, we reproducibly obtained most severely affected embryos, although the proportion varied among fish pairs, or between crosses from a fixed fish pair (Fig 4K). Some female mdvl2+(-)/-;dvl3a-/- fish (#2, #25, #39) produced a relatively high proportion of MZdvl2;MZdvl3a mutant embryos, ranging from 10% to 24%, depending on the crosses. Thus, by generating mosaic double mutants, we revealed a maternal requirement for Dvl2 and Dvl3a in AP patterning, and in axis extension.
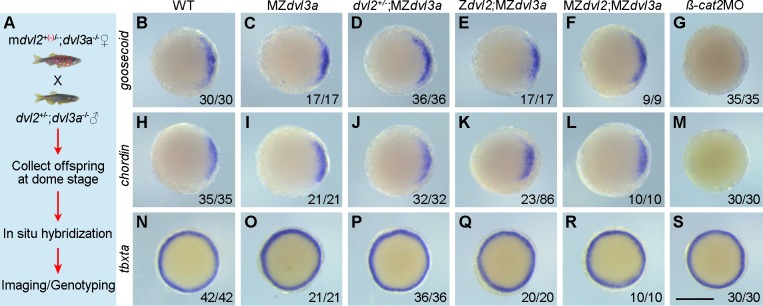
Dorsal fate specification is not affected in MZdvl2;MZdvl3a mutants
An implication of Dvl in activating maternal Wnt/ß-catenin signaling for dorsal fate specification has been unclear in Xenopus [17, 19, 20, 28], and this issue has not been addressed in zebrafish. Unlike the maternal effect mutant, ichabod, that reduces ß-catenin2 transcripts and results in severe dorsal and anterior deficiencies during early development [37, 38], anterior structures such as eyes were present in MZdvl2;MZdvl3a mutants, although they were fused or cyclopic. This implies that dorsal and anterior fate specification should not be disrupted in the absence of maternal and zygotic Dvl2 and Dvl3a products. To further test this possibility, we examined the expression of dorsal mesoderm genes, goosecoid and chordin, and the pan-mesoderm gene, tbxta (ntla), at dome stage by in situ hybridization. Since only a small proportion of MZdvl2;MZdvl3a embryos was present in the offspring, and no phenotype difference could be observed among siblings at early stages, all the offspring from the crosses between female mdvl2+(-)/-;dvl3a-/- and male dvl2+/-;dvl3a-/- fish were collected, and divided into three parts for hybridization with each probe. Following in situ hybridization, the embryos were individually imaged and genotyped by sequencing (Fig 5A). The experiment was performed using three independent fish pairs and the results did not reveal any obvious difference in the expression patterns of goosecoid, chordin, and tbxta between WT embryos and MZdvl2;MZdvl3a mutants (Fig 5B–5F, 5H–5L and 5N–5R). By contrast, in the embryos injected with 2 ng ß-catenin2 morpholino (ß-cat2MO), goosecoid expression was strongly reduced, chordin expression was absent, but tbxta expression was not affected (Fig 5G, 5M and 5S), confirming that maternal ß-catenin2 is required for dorsal fate specification. Together with phenotype analysis, this result suggests that maternal Dvl2 and Dvl3a may be not required for dorsal axis specification in zebrafish.
Fig 5. The expression of organizer genes is not affected in MZdvl2;MZdvl3a mutants.
In situ hybridization analysis of the expression patterns of goosecoid, chordin, and tbxta at dome stage. Animal pole viewed embryos with dorsal region on the right. (A) Schematic representation for the analysis of MZdvl2;MZdvl3a embryos. (B-F) Representative images show similar expression patterns of goosecoid in WT embryos and in different mutants. (G) Knockdown of ß-catenin2 strongly inhibits goosecoid expression. (H-L) Similar expression patterns of chordin in WT embryos and all indicated mutants. (M) Knockdown of ß-catenin2 blocks chordin expression. (N-R) The expression patterns of tbxta are similar between WT embryos and different mutants. (S) Knockdown of ß-catenin2 has no effect on tbxta expression. Scale bar: (B-J) 400 μm.
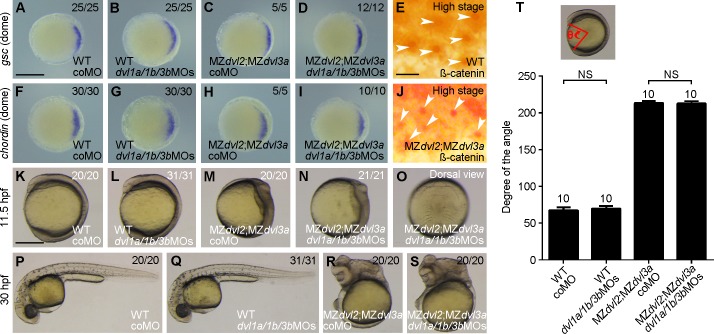
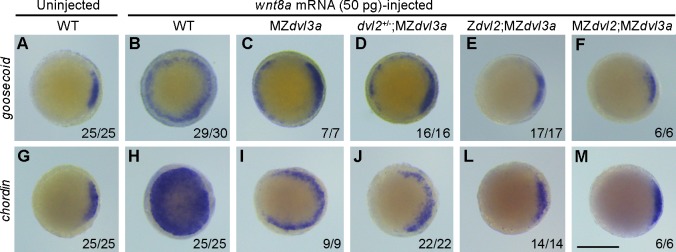
The negative result could be interpreted by the presence of residual amounts of other Dvl proteins. To test this possibility, we injected 2 ng of each dvl1a, dvl1b, and dvl3b morpholino oligonucleotides (referred to as dvl1a/1b/3bMOs) in 1-cell stage offspring derived from the crosses between female mdvl2+(-)/-;dvl3a-/- fish and male dvl2+/-;dvl3a-/- fish. In situ hybridization analysis of the expression of dorsal organizer genes goosecoid and chordin was performed at dome stage. The results showed that simultaneous knockdown of dvl1a, dvl1b, and dvl3 in WT or MZdvl2;MZdvl3a embryos did not change the expression of goosecoid and chordin, compared to the embryos injected with 2.5 ng control morpholino (Fig 6A–6D and 6F–6I). Consistent with this observation, analysis by immunocytochemistry indicated that the nuclear accumulation of endogenous ß-catenin in dorsal marginal cells was comparable between WT and MZdvl2;MZdvl3a embryos at high stage (compare Fig 6E and 6J). Similarly, injection of dvl1a/1b/3bMOs had no effect on axis extension in WT embryos (Fig 6K, 6L, 6P, 6Q and 6T), and did not aggravate the defective phenotype of MZdvl2;MZdvl3a mutants (Fig 6M–6O and 6R–6T). Moreover, quantitative RT-PCR analysis at different stages, and analysis by RNA sequencing at 12 hpf all showed that there was no upregulation of dvl1a, dvl1b and dvl3b transcripts in MZdvl2;MZdvl3a mutants (S13 Fig). These results suggest that Dvl1a, Dvl1b, and Dvl3b could not compensate for the loss of Dvl2 and Dvl3a in early dorsal fate specification and in axis extension. To further confirm the absence of Dvl activity in MZdvl2;MZdvl3a early embryos, we injected synthetic wnt8a mRNA (50 pg) in 1-cell stage offspring obtained as above. In situ hybridization analysis of goosecoid and chordin ectopic expression clearly showed that overexpression of Wnt8a was able to strongly induce ectopic expression of these genes in WT embryos (Figs 7A, 7B, 7G and 6H). However, reducing Dvl dosage progressively decreased the activity of Wnt8a to activate ectopic organizer gene expression (Fig 7C, 7D, 7I and 7J). In particular, Wnt8a had no effect in Zdvl2;MZdvl3a or MZdvl2;MZdvl3a embryos, which showed similar goosecoid and chordin expression patterns as in WT embryos (Fig 7E, 7F, 7L and 7M). The fact that Wnt8a failed to activate maternal Wnt/ß-catenin signaling leading to ectopic organizer gene expression suggests that Dvl activity should be absent in these mutants.
Fig 6. Knockdown of other dvl genes has no effect in MZdvl2;MZdvl3a mutants.
The embryos were injected with coMO (2.5 ng) or a mixture of equal amount of dvl1a/1b/3bMOs (6 ng in total) at 1-cell stage, except for immunostaining. All mutant embryos were imaged after different analyses, followed by genotyping. (A-D) In situ hybridization analysis of goosecoid (gsc) expression in indicated embryos at dome stage. Animal pole view with dorsal region on the right. (E) Endogenous ß-catenin nuclear accumulation (arrows) in dorsal marginal cells of a WT embryo at high stage. (F-I) In situ hybridization analysis of chordin expression in indicated embryos at dome stage. Animal pole view with dorsal region on the right. (J) Endogenous ß-catenin nuclear accumulation (arrows) in dorsal marginal cells of an MZdvl2;MZdvl3a mutant at high stage. (K-O) Phenotypes of indicated embryos at 11.5 hpf. Lateral view, with a dvl1a/1b/3bMOs-injected MZdvl2;MZdvl3a embryo also shown in dorsal view (O). (P-S) Phenotypes of indicated embryos at 30 hpf. Lateral view, note that injection of dvl1a/1b/3bMOs does not change the phenotype of WT and MZdvl2;MZdvl3a embryos. (T) Statistical analysis of the extent of axis extension delay in indicated embryos at 11.5 hpf. Bars represent the mean ± s.d. from indicated numbers of embryos (NS, not significant). Scale bars: (A-D, F-I) 400 μm; (E, J) 25 μm; (K-S) 400 μm.
Fig 7. The inducing-activity of Wnt8a is blocked in MZdvl2;MZdvl3a mutants.
In situ hybridization analysis of ectopic organizer gene expression at dome stage following Wnt8a overexpression in indicated embryos. Animal pole viewed embryos with dorsal region on the right. (A-F) goosecoid expression pattern in indicated embryos. (G-M) chordin expression pattern in indicated embryos. Notice that reduction of Dvl dosage progressively inhibits the inducing activity of Wnt8a, and MZdvl2;MZdvl3a embryos display a complete absence of ectopic goosecoid and chordin expression. All mutant embryos were derived from crosses between a female mdvl2+(-)/-;dvl3a-/- and a male dvl2+/-;dvl3a-/- fish, and were genotyped after in situ hybridization. Scale bar: 400 μm.
Maternal Dvl2 and Dvl3a regulate AP patterning
Inhibition of zygotic Wnt/ß-catenin signaling, in particular Wnt8, is known to cause dorsalization and anteriorization [1, 3, 5]. However, this does not seem to occur in MZdvl2;MZdvl3a embryos, since the expression domains of goosecoid and chordin at 60% epiboly did not show obvious lateral expansion, although lateral and ventral expression of axin2 was inhibited (S14 Fig). This suggests that downregulation of Wnt8 and Dvl activity exerts distinct effects to restrict the organizer domain.
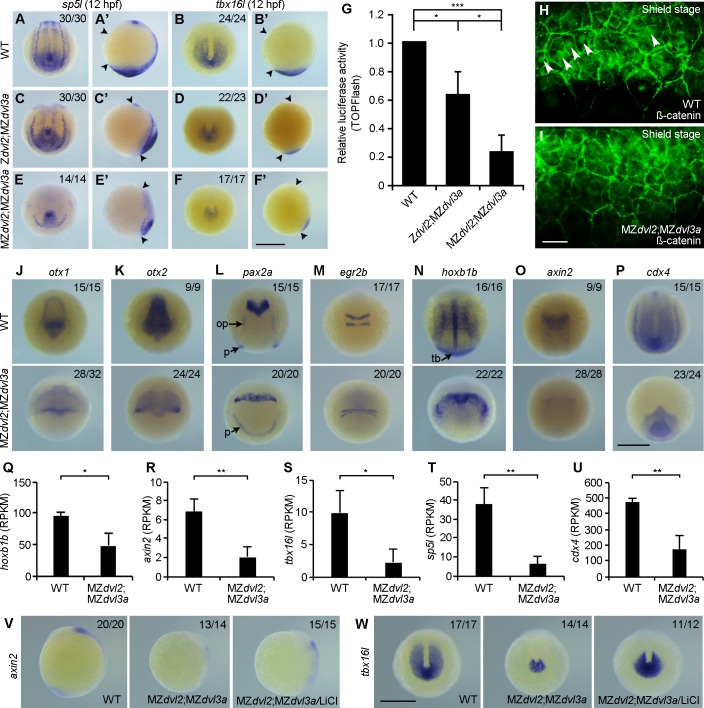
To analyze how AP patterning is affected in MZdvl2;MZdvl3a mutants, we first performed in situ hybridization using two well characterized posterior markers, sp5l (spr2) and tbx16l (tbx6l), which mediate zygotic Wnt/ß-catenin signaling in posterior patterning [39–41]. At 12 hpf, the phenotypes specific to Zdvl2;MZdvl3a or MZdvl2;MZdvl3a mutants were easily discernible, which allowed us to select sufficient mutant embryos from different crosses. Confirmed by genotyping after in situ hybridization, Zdvl2;MZdvl3a mutants showed no obvious or only weak alternation in the expression of sp5l (Fig 8A, 8A’, 8C and 8C’), but they displayed a markedly reduced expression of tbx16l (Fig 8B, 8B’, 8D and 8D’). In MZdvl2;MZdvl3a mutants, however, the expression of sp5l was strongly decreased (Fig 8E and 8E’), and the expression of tbx16l was reduced to residual level (Fig 8F and 8F’). Consistently, TOPFlash luciferase assay revealed that there was approximately a 30% decrease of Wnt/ß-catenin transcriptional activity in Zdvl2;MZdvl3a mutants at 12 hpf, and about a 75% decrease in MZdvl2;MZdvl3a mutants (Fig 8G). This decrease of reporter activity correlated well with a reduction of endogenous ß-catenin nuclear accumulation in ventral marginal cells at shield stage, when zygotic transcription has already started, as assayed by immunofluorescence staining (Fig 8H and 8I). These observations strongly suggest that maternal Dvl2 and Dv3a play an important role in the activation of zygotic Wnt/ß-catenin signaling, and that Dvl2 may exert a predominant role.
Fig 8. Cooperation of maternal and zygotic Dvl2 and Dvl3a in AP patterning.
Mutant embryos were selected by the extent of CE defects at 12 hpf, and genotyped after in situ hybridization. Immunostaining was performed at shield stage, followed by genotyping. (A-B’) Expression patterns of sp5l and tbx16l in WT embryos. Arrowheads in lateral viewed embryos indicate the anterior end and posterior end. Other embryos are dorsal-posterior view. (C, C’) The expression of sp5l is not affected in Zdvl2;MZdvl3a mutants. (D, D’) Strongly reduced tbx16l expression in Zdvl2;MZdvl3a mutants. (E, E’) Strongly reduced sp5l expression in MZdvl2;MZdvl3a mutants. (F, F’) Residual expression of tbx6l in MZdvl2;MZdvl3a mutants. (G) TOPFlash luciferase reporter activity in Zdvl2;MZdvl3a and MZdvl2;MZdvl3a mutants at 12 hpf. Bars represent the mean ± s.d. from three independent experiments (*, P<0.05; ***, P<0.001). (H) Endogenous ß-catenin nuclear accumulation (arrows) in ventral marginal cells of a WT embryo at shield stage. (I) Absence of ß-catenin nuclear accumulation in ventral marginal cells of an MZdvl2;MZdvl3a mutant at shield stage. (J-P) In situ hybridization analysis of indicated genes in WT and MZdvl2;MZdvl3a embryos at 12 hpf. Dorsal view with anterior to the top (J, K), dorsal view (L-O), and dorsal-posterior view (P). (R-U) Analysis of hoxb1b and zygotic Wnt/ß-catenin target genes by RNA-sequencing at 12 hpf. Note the significant decrease in RPKM (reads per kilobase million) for these genes in MZdvl2;MZdvl3a mutants. Bars represent the mean ± s.d. from three independent samples (*, P<0.05; **, P<0.01). (V, W) Lateral (V) and dorsal-posterior (W) views show rescue of axin2 and tbx16l expression in MZdvl2;MZdvl3a mutants at 12 hpf, following LiCl (0.3 M) treatment for 8 minutes at 5 hpf. op, otic placode; p, pronephric mesoderm; tb, tailbud. Scale bar: (A-F’) 400 μm; (H, I) 20 μm; (J-P) 400 μm; (V, W) 400 μm.
We further examined the expression of a panel of AP genes in MZdvl2;MZdvl3a mutants at 12 hpf by in situ hybridization. This indicated that the expression of otx1 (forebrain and midbrain), otx2 (forebrain, midbrain and midbrain-hindbrain boundary), and pax2a (midbrain-hindbrain boundary, otic placode and pronepheric mesoderm) was reduced, and widened mediolaterally due to impaired axis extension (Fig 8J–8L). The expression of egr2b (krox20) in rhombomeres 3 and 5, and hoxb1b in the notochord and paraxial mesoderm was compressed and widened (Fig 8M and 8N). Notably, the expression of hoxb1b in the tailbud was absent (Fig 8N). The expression of axin2 in the neural plate, and of cdx4 in the posterior paraxial mesoderm was strongly inhibited (Fig 8O and 8P). Analysis by RNA sequencing confirmed that axin2, tbx16l, sp5l and cdx4, which are zygotic Wnt/ß-catenin target genes in AP patterning [3, 39–41], as well as hoxb1b, were all downregulated (Fig 8Q–8U). These indicate that AP patterning, and in particular posterior development, are strongly affected in MZdvl2;MZdvl3a mutants.
To determine whether AP patterning defect in MZdvl2;MZdvl3a mutants was due to a decreased zygotic Wnt/ß-catenin signaling, offspring at 5 hpf derived from the crosses between female mdvl2+(-)/-;dvl3a-/- fish and male dvl2+/-;dvl3a-/- fish were treated with LiCl, which activates Wnt/ß-catenin signaling downstream of Dvl. In situ hybridization was performed to examine the expression of axin2 and tbx16l at 12 hpf. Following genotyping, we found that MZdvl2;MZdvl3a mutants treated with LiCl displayed increased expression of axin2 and tbx16l, compared to untreated mutants (Fig 8V and 8W). Injection of a low dose of synthetic mRNA (50 pg) encoding the constitutively active ß-catenin into 1-cell stage embryos also rescued the expression of axin2 and tbx16l, but to a lesser extent (S15A–S15F Fig), likely due to the mosaic distribution of injected mRNA. However, phenotypic examination indicated that this injection could effectively rescue tail development in Zdvl2;MZdvl3a mutants (S15G–S15I Fig). These observations further demonstrate that Dvl2 and Dvl3a deficiency causes defective AP patterning by preventing zygotic Wnt/ß-catenin signaling.
Maternal Dvl2 and Dvl3a contribute to CE movements
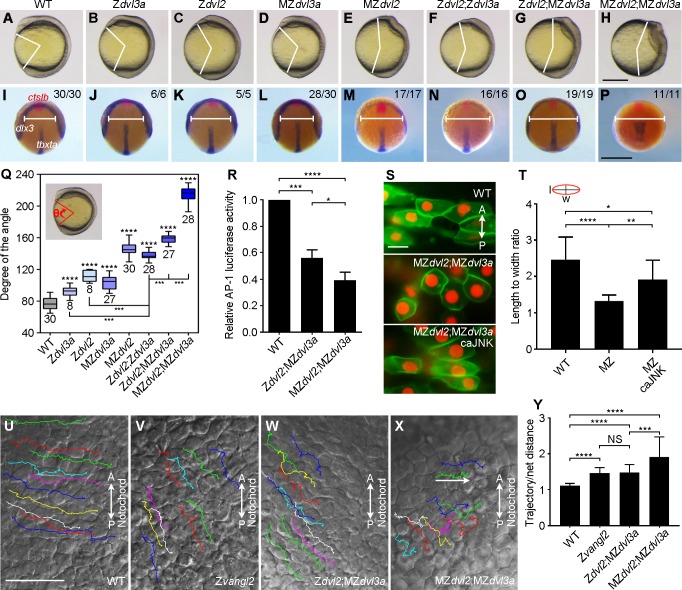
The most severely impaired axis elongation in MZdvl2;MZdvl3a mutants clearly suggests an important maternal contribution of Dvl2 and Dvl3a for CE movements. To further clarify this aspect and to determine Dvl dosages in Wnt/PCP signaling, we first compared the extent of axis extension defect between different mutants by phenotype analysis, and by simultaneous in situ hybridization using tbxta as a marker of the notochord, dlx3 as a marker of the neural plate borders, and ctslb (hgg1) as a marker of the prechordal plate mesoderm. At 11.5 hpf, Zdvl3a, Zdvl2 or MZdvl3a mutants displayed weak, but obvious delay in neural plate convergence and notochord elongation (Fig 9A–9D, 9I–9L and 9Q). However, MZdvl2 mutants showed more severe defect (Fig 9E, 9M and 9Q). Thus, it is clear that either MZdvl2 or MZdvl3a mutants present more severely affected CE phenotypes than the respective zygotic mutants. The same situation was also observed in double mutants. MZdvl2;MZdvl3a mutants displayed most severely impaired neural plate convergence and axis extension, compared with Zdvl2;Zdvl3a and Zdvl2;MZdvl3a mutants (Fig 9F–9H and 9N–9Q), indicating that maternal Dvl dosage is important in Wnt/PCP signaling.
Fig 9. Dvl2 and Dvl3a dosages in CE movements and Wnt/PCP signaling activity.
(A-H) Representative live images of WT and mutant embryos at 11.5 hpf. Lateral view, with anterior region on the top. (I-P) Dorsal view of indicated embryos simultaneously hybridized with ctslb, dlx3, and tbxta probes to reflect the position of prechordal plate mesoderm, neural plate borders, and notochord, respectively. MZdvl3a mutants were from intercrosses between dvl3a-/- carriers; MZdvl2 mutants were from crosses between female dvl2-/- fish and male dvl2+/- fish; Zdvl2;Zdvl3a mutants were from crosses between female dvl2+/-;dvl3a-/- fish and male dvl2+/-;dvl3a+/- fish; Zdvl2;MZdvl3a mutants were from intercrosses between dvl2+/-;dvl3a-/- carriers; MZdvl2;MZdvl3a mutants were from crosses between female mdvl2+(-)/-;dvl3a-/- fish and male dvl2+/-;dvl3a-/- fish. (Q) Statistical analysis shows that progressive reduction of Dvl2 and Dvl3a dosages increasingly aggravates axis extension defect. The embryos were imaged at 11.5 hpf, and genotyped before measuring the angle (inset). Bars represent the mean ± s.d. from indicated numbers of embryos collected from three independent experiments, and asterisks above the bars indicate significance with respect to WT embryos (***, P<0.001; ****, P<0.0001). (R) Reduced AP1 reporter activity in Zdvl2;MZdvl3a and MZdvl2;MZdvl3a mutants at 12 hpf. Bars represent the mean ± s.d. from three independent experiments (*, P<0.05, ***, P<0.001, ****, P<0.0001). (S) Rescue of cell polarity of MZdvl2;MZdvl3a mutant cells by caJNK. Vertical bidirectional arrows indicate AP orientation. (T) Statistical analysis of the length (l) to width (w) ratio in indicated cells. Bars represent the mean ± s.d. from at least 10 cells in two representative images (*, P<0.05, **, P<0.01, ****, P<0.0001). (U-X) Still frames from live time-lapse images show the dorsal convergence and movement behaviors of lateral cells in indicated embryos (see also S1–S4 Movies). The trajectories of 10 randomly selected cells are traced. (Y) Statistical analysis of the ratio between the trajectory and the net mediolateral distance (as indicated by a horizontal arrow). Bars represent the mean ± s.d. from at least 15 cells in two or three representative images (***, P<0.001; ****, P<0.0001). Scale bars: (A-H) 400 μm; (I-P) 400 μm; (S) 20 μm; (U-X) 50 μm.
Dvl was shown to regulate cytoskeletal architecture and cell polarity upstream of Rac and Jun N-terminal kinase (JNK) both in Xenopus and zebrafish [42, 43]. When the AP1 luciferase reporter was used to monitor JNK activation [44], we found a 40% decrease of AP1 reporter activity in Zdvl2;MZdvl3a mutants, and a 60% decrease in MZdvl2;MZdvl3a mutants (Fig 9R). Consistent with this result, analysis of cell polarity indicated that WT midline cells at 12 hpf were elongated mediolaterally, while MZdvl2;MZdvl3a mutant cells were rounded with a strongly reduced length to width ratio (Fig 9S and 9T), which was much similar as dvl2 and dvl3a knockdown cells [43]. However, injection of synthetic mRNA (200 pg) encoding a constitutively active JNK, along with synthetic mRNAs encoding Histone2B and membrane GFP in one blastomere at 64-cell stage, significantly rescued mediolateral elongation of fluorescently-labeled descendent MZdvl2;MZdvl3a mutant cells (Fig 9S and 9T). This suggests that deficiency of Dvl2 and Dvl3a affects cell polarity by preventing at least partially JNK activation. Furthermore, we performed live time-lapse analysis of cell movement behaviors in Zdvl2;MZdvl3a and MZdvl2;MZdvl3a mutants, in comparison with the PCP mutant trilobite/vangl2 [45]. Since the AP axis was strongly shortened in these mutants, and the notochord became severely irregular, we examined the dorsal convergence behaviors of lateral cells. In WT embryos, these cells moved toward the notochord with regular trajectories (Fig 9U and S1 Movie). In zygotic trilobite/vang2 mutants, lateral cells displaced along irregular trajectories and moved in more posterior direction (Fig 9V and S2 Movie). Lateral cells in Zdvl2;MZdvl3a mutants moved similarly as in zygotic trilobite/vang2 mutants (Fig 9W and S3 Movie). Strikingly, the directional movement of lateral cells in MZdvl2;MZdvl3a mutants was most severely affected. These cells moved along zigzagging trajectories, and displayed forward and back movements (Fig 9X and S4 Movie), with a more strong increase in the ratio of trajectory distance relative to net distance toward the notochord (Fig 9Y). These analyses strongly indicate an important maternal contribution of Dvl2 and Dv3a in Wnt/PCP signaling and CE movements. The fact that lateral cells in these mutants tend to move in more vegetal direction is likely due to an impaired AP patterning. Thus, it could not be excluded that the severely affected CE movements may result from the combined effects of defective Wnt/PCP and zygotic Wnt/ß-catenin signaling.
We further demonstrated the maternal contribution of Dvl2 and Dvl3a in axis extension using maternal double mutants. By crossing female mdvl2+(-)/-;dvl3a-/- fish with male WT fish, the resulting double heterozygous embryos that carry a new mutation in one dvl2 allele are maternal double mutants because of the absence of maternal Dvl2 and Dvl3a products (see S11 Fig). These Mdvl2;Mdvl3a mutants displayed strongly impaired axis extension at 11.5 hpf. However, they only presented a slightly shortened AP axis at 30 hpf (S16A–S16D and S16I Fig), suggesting that zygotic Dvl2 and Dvl3a can rescue axis extension at late stages. By contrast, Mdvl3a mutants or dvl2+/-;Mdvl3a mutants (lacking half of the maternal Dvl2 products), obtained from the crosses between female dvl2+/-;dvl3a-/- fish and male WT fish, were either normal or displayed weak axis extension defect at 11.5 hpf, and were completely normal at 30 hpf (S16E–S16I Fig). These observations indicate a predominant contribution of maternal Dvl2 to embryonic axis elongation, and Dvl3a exerts a permissive effect. Altogether, our results show that both maternal and zygotic Dvl2 and Dvl3a cooperate to orchestrate CE movements and AP patterning.
Discussion
Dvl proteins play key roles in both Wnt/ß-catenin and Wnt/PCP signaling pathways, and they are highly conserved and broadly expressed during early development in all vertebrates. However, many aspects of their involvement in early developmental processes remain elusive and enigmatic. In this study, we resolved some of the unanswered issues through comprehensive mutational analyses. Our results demonstrate that the two most abundantly expressed Dvl proteins, Dvl2 and Dv3a, are not required for early dorsal fate specification, which is dependent on the activation of maternal Wnt/ß-catenin signaling. Instead, maternal and zygotic Dvl2 and Dvl3a, in particular Dvl2, are important in CE movements, which are regulated by Wnt/PCP signaling, and in AP patterning. These findings help to clarify the implication of Dvl proteins in Wnt-regulated developmental events, and provide insight into the mechanisms underlying embryonic axis formation.
The early development in many vertebrates and invertebrates is supported by maternal products accumulated during oogenesis, zygotic transcription does not occur until the start of mid-blastula transition [1–5]. Inactivation of key genes implicated in early developmental processes frequently leads to embryonic lethality, or unproductive adults, preventing the analysis of maternal gene function. The situation becomes more complex when multiple gene paralogs are expressed. This is particularly true for functional analyses of Dvl paralogs during early development. Before this work, no dvl mutant has been reported in zebrafish, and the maternal and zygotic functions of Dvl proteins are not clear. We have used TALENs genome-editing technology to generate single mutants for all five zebrafish dvl paralogs, as well as a panel of dvl2 and dvl3a triallelic and double homozygous mutants, and examined the maternal and zygotic contributions of Dvl2 and Dvl3a in embryonic patterning and morphogenetic movements. These mutants represent a valuable resource for the study of important developmental processes, which are dependent on the activation of the Wnt pathways.
A significant finding is the absence of implication for maternal Dvl2 and Dvl3a in early dorsal fate specification. This suggests that they are not required for the activation of maternal Wnt/ß-catenin signaling. Indeed, the expression of the dorsal organizer genes, goosecoid and chordin, is not affected in these mutants, whereas it is strongly decreased in ß-catenin2 morphants. Moreover, the phenotypes of MZdvl2;MZdvl3a or Mdvl2;Mdvl3a mutants completely differ from those of zebrafish ichabod mutants, which display an absence of anterior structures caused by a reduced ß-catenin2 activity [37, 38]. This further argues against a requirement of Dvl2 and Dvl3a in dorsal axis formation. Our results from mutational analyses are consistent with the observations showing that simultaneous knockdown of dvl2 and dvl3a in zebrafish does not apparently affect head formation [43, 46, 47], but mostly affects AP axis elongation and tail development [43]. They are supported by functional studies in Xenopus, which show that depletion of maternally expressed Dvl2 and Dvl3 from oocytes also has no effect on dorsal fate specification [19]. There is a possibility that these negative outcomes could be due to the inefficiency of the approaches to inhibit endogenous Dvl function [20]. However, our genetic evidence now suggests that the activation of maternal Wnt/ß-catenin signaling is independent of Dvl2 and Dvl3a. Although it was extremely difficult to assay maternal Wnt/ß-catenin activity in MZdvl2;MZdvl3a mutants at blastula stages, the correct expression of dorsal organizer genes is suggestive of an unaffected maternal Wnt/ß-catenin signaling.
Zebrafish genome contains at least five dvl genes, but transcriptomic analysis has revealed that dvl2 and dvl3a represent approximately 98% of total dvl transcripts from fertilization until before the end of gastrulation [29], indicating that they are the major dvl genes expressed in the early embryo. It is unlikely that the loss-of-function of Dvl2 and Dvl3a could be compensated by other Dvl proteins, since they are expressed at an extremely low level, and their maternal and zygotic mutants do not result in any discernable phenotype at all stages examined. Moreover, our quantitative analyses indicate that the maternal and zygotic expression of dvl1a, dvl1b and dvl3b is not upregulated in MZdvl2;MZdvl3a mutants, and that simultaneous knockdown of these genes has no effect. A recent study indicates that Wnt/ß-catenin signaling is not affected in mouse ependymal cells lacking 5 of the 6 Dvl alleles [48], there is thus a possibility that trace amounts of Dvl protein could be sufficient for dorsal fate specification. However, several studies in zebrafish suggest a dose-dependent function of maternal Wnt/ß-catenin activity in organizer formation. In ichabod embryos with reduced ß-catenin2 level, dorsal and anterior deficiencies occur with variable expressivity [37], and knockdown of ß-catenin2 increases the severity of ichabod phenotypes [38]. By contrast, MZdvl2;MZdvl3a embryos displayed correct organizer gene expression at the onset of zygotic transcription, suggesting that maternal Wnt/ß-catenin signaling should not be affected by the deficiency of Dvl activity. Thus, our present results support the model that early dorsal axis formation is a consequence of dorsal accumulation of ß-catenin caused by asymmetrical translocation of vegetally localized dorsal determinants [1–5]. They suggest that Dvls may be dispensable for the activation of maternal Wnt/ß-catenin signaling in dorsal fate specification. Nevertheless, there may be a possibility that maternal Wnt/ß-catenin signaling is activated by other mechanisms that are independent of maternal Wnt ligand/receptor signaling.
In Xenopus, Wnt11 was shown to be required for the activation of maternal Wnt/ß-catenin signaling in dorsal axis formation [49]. In zebrafish, vegetally localized maternal wnt8a is transported to the dorsal region and has been thought to play a role in specifying dorsal fate [50]. In this regard, it would be interesting to analyze the maternal effect following removal of the bicistronic wnt8 locus [51]. While this work was in progress, it was reported that maternal mutants for the two wnt8a open reading frames did not show axis formation defect [52]. Thus, our present results are consistent with this observation, and in particular, the trunk and posterior deficiencies in MZwnt8a mutants are much similar as those observed in our MZdvl2;MZdvl3a mutants. However, it is worth to mention that MZwnt8a mutants show dorsalized phenotype [52], whereas MZdvl2;MZdvl3a mutants exhibit CE defects with strongly reduced ventroposterior gene expression, without obvious dorsalizing effect. There are at least two credible explanations that may account for these differences. First, zygotic Wnt8a only activates Wnt/ß-catenin signaling and participates in ventral and posterior tissue formation, but not in CE movements. Second, extracellular Wnt8a ligand should also function to antagonize organizer-secreted Wnt inhibitors during gastrulation, and its absence leads to an expansion of organizer activity [5]. However, the absence of maternal and zygotic Dvl2 and Dvl3a should not disturb this functional antagonism, thus keeping early dorsal fate unaffected. Also as a consequence, MZdvl2;MZdvl3a mutants do not show anteriorization at late stages.
It is well established that Dvl plays a critical role in mediating the activation of Wnt/PCP signaling in CE movements during gastrulation [17]. Several studies in mice suggest that functional redundancy exists between Dvl proteins, and that the Wnt/PCP pathway during neurulation is more readily affected following reduction of Dvl dosage [25–27]. However, it is still unclear how Dvl dosage influences CE movements during gastrulation, and how Dvl proteins functionally interact in these processes in zebrafish. Our analyses by using single and double mutants clearly indicate that Dvl2 plays a predominant role in embryonic axis extension. There is only a partial functional redundancy between Dvl2 and Dvl3a, because MZdvl2 mutants display obvious CE defects, whereas MZdvl3a mutants are phenotypically normal. Nevertheless, reducing Dvl3a dosage in zygotic dvl2 mutants sensibly aggravates the defective CE phenotypes, indicating that Dvl3a exerts a permissive effect on Dvl2 in Wnt/PCP signaling. In mice, Dvl2-/-;Dvl3+/- triallelic mutants exhibit more severely shortened AP axis than Dvl3-/- or Dvl2+/-;Dvl3-/- mutants [26]. Taken together, our present results suggest that Dvl2 plays an important role in Wnt/PCP signaling during CE movements, which may be conserved in vertebrates.
Interestingly, mutational analyses indicate that the absence of zygotic Dvl2 and Dvl3a function only results in moderate CE defects. However, removal of both maternal and zygotic Dvl2 and Dvl3a produces the most severely affected CE phenotypes, indicating clearly an important maternal contribution of these proteins in Wnt/PCP-mediated CE movements. This is further confirmed by analyzing the phenotypes of maternal dvl2 and dvl3a double mutants, which show severe axis extension defect during gastrulation. Thus, by comparison of the extent of axis extension defect between Zdvl2;Zdvl3a, Mdvl2;Mdvl3a, and MZdvl2;MZdvl3a at different stages, it can be concluded that, to a large extent, maternal Dvl2 and Dvl3a may be sufficient to activate Wnt/PCP signaling in CE movements during gastrulation, whereas zygotic Dvl2 and Dvl3a are implicated to a lesser extent. Consistently, MZdvl2;MZdvl3a mutants display strongly reduced ability to activate the AP1 reporter, which monitors JNK activation [44], and the disrupted cell polarity can be rescued by a constitutively active JNK. However, the activity of the AP1 reporter was not completely blocked in MZdvl2;MZdvl3a mutants at late gastrula stages. This may be due to an independent activation of JNK signaling by other proteins such as the paraxial protocadherin that regulates morphogenesis and signals through the small GTPases RhoA and Rac1 to JNK [53, 54]. Altogether, our analyses indicate that Wnt/PCP-mediated CE movements are particularly sensitive to both maternal and zygotic Dvl dosages.
Another striking observation is the requirement for maternal Dvl function in AP patterning that is dependent on zygotic Wnt/ß-catenin signaling to activate region-specific gene expression. It is well established that an endogenous Wnt/ß-catenin signaling gradient, with highest activity in the posterior region, is important for AP patterning [3, 5]. At present, there is only limited evidence implicating Dvl in AP axis specification. In Xenopus, overexpression study shows that graded amounts of Dvl elicit AP fates in the prospective ectoderm [55], suggesting that Dvl dosage may be important to differentially activate zygotic gene expression along the AP axis. This is supported by the present observation showing an implication of Dvl2 and Dvl3a in this process in a dosage-dependent manner. We find that progressive reduction of Dvl dosage gradually elicits AP patterning defect, ranging from posterior deficiency to a complete lack of trunk and tail. The maternal contribution of Dvl2 and Dvl3a is clearly evident. While Zdvl2;Zdvl3a mutants display a relatively normal AP axis, Zdvl2;MZdvl3a mutants begin to show caudal truncation. The most severe defect of AP patterning in MZdvl2;MZdvl3a mutants is clearly caused by a strongly impaired Wnt/ß-catenin signaling, which results in a severely decreased expression of target genes.
Maternal and zygotic Wnt/ß-catenin signaling has opposite functions in the specification of embryonic axes. While maternal Wnt/ß-catenin signaling specifies dorsal fate, zygotic Wnt/ß-catenin signaling induces ventroposterior mesoderm and inhibits anterior development [5]. This apparently contradicts with the requirement of maternal Dvl in AP axis specification, but it could be explained by the region-specific expression and regulation of other components of the Wnt/ß-catenin pathway. Following zygotic gene activation, the ventral region expresses several Wnt ligands. Thus, maternal Dvl proteins may serve to relay extracellular Wnt signals for region-specific activation of the pathway. Another possibility is that maternal Dvl may play a role in the specification of AP cell fates in the prospective ectoderm, as observed in Xenopus embryo [19, 55]. In overexpression experiments, high levels of Xdsh (Dvl2) activate posterior neural markers, whereas low levels induce the expression of anterior neural genes. Accordingly, in our zebrafish dvl mutants, deficiency of posterior and trunk tissues could be obtained by substantially reducing maternal Dvl dosage.
In the present study, we have revealed a predominant role for Dvl2 dosage both in CE movements and in AP patterning, however, it is clear that Dvl3a also cooperates with Dvl2 in these processes. Our results are consistent with previous studies indicating that Dvl proteins differentially activate the Wnt pathways and regulate distinct developmental processes. Indeed, the three mammalian DVL proteins differentially mediate the activation of Wnt/ß-catenin signaling in cultured cells [56]. In Xenopus, knockdown of dvl1 and dvl2 causes severe neural crest defects, while knockdown of dvl3 affects muscle gene expression and sclerotome development [21]. In this regard, it is of interest to note that MZdvl2 mutants develop craniofacial defects that at least partially result from fusion or absence of neural crest-derived cartilages. In addition, the heart abnormality in dvl2 and dvl3a double mutants is consistent with previous observations showing that Dvl2 mutant mice display defects in cardiac neural crest development [25]. At present, it is still intriguing that why Dvl2 plays a major role both in Wnt/ß-catenin and Wnt/PCP signaling, and how it distinguishes these two pathways? Our previous structural and functional analyses have provided some clues as to how Dvl2 activity in Wnt/PCP signaling is regulated by its C-terminus [57, 58]. The present observations further indicate that the activaty of Wnt/ß-catenin or Wnt/PCP signaling during development may be regulated by Dvl dosages.
In summary, our findings have uncovered, to a significant extent, the manner in which these Dvl proteins are implicated in regulating the activation of different Wnt signaling pathways. In particular, we clarified that they are not required for dorsal fate specification, and demonstrated that maternal and zygotic Dvl2 dosages, in cooperation with Dvl3a, play a predominant role in regulating important zygotic events, such as AP patterning and morphogenetic movements.
Materials and methods
Ethical statement
All experiments using zebrafish adults and embryos were performed according to the ARRIVE guidelines and approved by the Ethics Committee for Animal Research of Life Science of Shandong University (permit number SYDWLL-2018-05).
Zebrafish and microinjections
Zebrafish adult of the AB strain were maintained at 28.5°C. The embryos were staged as described [59], and for most experiments, were injected at 1-cell stage in the yolk using a PLI-100A Picoliter microinjector (Harvard Apparatus).
Expression constructs and morpholino oligonucleotides
TALENs were assembled through Golden Gate Assembly [60], using the Golden Gate TALEN and TAL Effector Kit (cat#1000000016) from Addgene. TALEN repeat variable di-residues targeting sequences were cloned into modified pCS2+KKR and pCS2+ELD vectors [61]. Zebrafish wnt8a coding sequence was PCR-amplified and cloned in pCS2 vector. WT and mutant dvl2 and dvl3a coding sequences were cloned in pCS2MT vector such that the proteins are C-terminally myc-tagged. Constructs for Histone2B-RFP, mGFP, JNKK2-JNK1 (encoding a constitutively active Jun kinase) and ΔN-ß-catenin (encoding a constitutively active ß-catenin) have been previously described [43, 62–64]. Capped mRNAs were synthesized from linearized plasmids by in vitro transcription using appropriate RNA polymerases.
Translation-blocking morpholino antisense oligonucleotides against ß-catenin2 [38], dvl1a (5′-AATCATTGACAGAAGAAGGAGCAAG-3′), dvl1b (5′-GGTATATGATTTTAGTCTCCGCCAT-3′), dvl3b (5′-TCTCCCTTCAGACAGCGACAATAAC-3′), and standard control morpholino (5′-CCTCTTACCTCAGTTACAATTTATA-3′) were synthesized by Gene Tools, and suspended in sterile water.
Targeted gene mutations and generation of MZdvl2;MZdvl3a mutants
The two TALEN mRNAs were mixed at equal amounts (200 pg each) and injected into 1-cell stage embryos. The targeting efficiency was determined by Sanger sequencing of PCR products amplified from genomic DNA extracted from 15 randomly selected F0 embryos at 24 hpf. When the result indicates a positive targeting effect, other embryos were reared to adulthood for outcross to screen F1 heterozygotes using genomic DNA extracted from the tail fin.
To generate MZdvl2;MZdvl3a mutant lines, we first used germline replacement approach by transplanting blastoderm cells from Zdvl2 donors at dome stage into dvl2+/-;dvl3a-/- hosts. Since this only generated 21 male chimera fish, we next used a strategy to target the remaining dvl2 WT allele in dvl2+/-;dvl3a-/- embryos. The offspring obtained from crosses between dvl2+/-;dvl3a-/- carriers were injected at 1-cell stage with dvl2 TALEN mRNAs (100 pg each) and raised to adulthood. Due to the mosaic distribution of TALEN mRNAs and incomplete targeting efficiency, mosaic fish with mixed dvl2+/-;dvl3a-/- and dvl2-/-;dvl3a-/- genotypes could be obtained, and denoted as mdvl2+(-)/-;dvl3a-/-, for mosaic homozygous dvl2 mutations. Female mdvl2+(-)/-;dvl3a-/- fish were then crossed with male dvl2+/-;dvl3a-/-, and the resulting offspring were screened by PCR using allele-specific primers (S1 Table), followed by sequencing to detect de novo mutations in the dvl2 allele. The offspring that contained a new indel along with the original indel were MZdvl2;MZdvl3a mutants, and the parental fish were selected for further experiments. To obtain Mdvl2;Mdvl3a mutants, female mdvl2+(-)/-;dvl3a-/- fish were crossed with male WT fish. The resulting dvl2 and dvl3a heterozygous offspring that carry either the original indel or a new indel in one dvl2 allele were devoid of maternal Dvl2 and Dvl3a gene products.
Whole-mount in situ hybridization and immunostaining
Whole-mount in situ hybridization was performed as previously described [65]. The constructs for goosecoid, chordin, tbxta, dlx3, ctslb, otx2, pax2a and egr2b were reported previously [65, 66], and otx1, axin2, hoxb1b, cdx4, sp5l, tbx16l and dvl2 constructs were generated by cloning PCR framents in pZeroBack/Blunt Vector (Tiangen). They were labeled using digoxigenin-11-UTP (Roche Diagnostics). Staining of embryos simultaneously hybridized with tbxta, dlx3, and ctslb probes was performed using NBT/BCIP and Fast Red as substrates (Roche Diagnostics), respectively.
For immunostaining, the embryos were fixed in 4% paraformaldehyde at 4°C overnight, and washed with PBST (PBS, 0.1% Triton X-10), they were then incubated in mouse monoclonal anti-ß-catenin antibody (1/250, Sigma-Aldrich, C7207) at 4°C overnight. After several washes in PBST, embryos at high stage were incubated with horseradish peroxidase conjugated secondary antibody (1/500, INTERCHIM), followed by incubation in diaminobenzidine substrate, and embryos at shield stage were incubated with Alexa-488 conjugated secondary antibody (1/1000, INTERCHIM), followed by confocal microscopic imaging (Zeiss, LSM700).
Cartilage staining
Larvae at 5 dpf were fixed in 4% paraformaldehyde at 4°C overnight, then washed twice in PBS for 10 minutes. The larvae were incubated in alcian blue solution (0.37% HCl, 70% ethanol, 0.1% alcian blue) for 6 hours, and washed in destaining solution (1% HCl, 70% ethanol). Following dehydration in ethanol, the larvae were cleared in benzyl benzoate, and imaged using an upright microscope (Leica DM2500).
Live time-lapse imaging
Embryos at 90% epiboly stage were mounted in a cavity microscope slide in 1% low-melting agarose as described [43]. Cell movements were recorded using an upright light microscope (Leica, LM2500) equipped with a CCD digital camera (Leica, IC180), under differential interference contrast. The embryos were imaged every 30 seconds for a period of 60 minutes, and mutant embryos were then subjected to genotyping by Sanger sequencing. Time-lapse movies were generated using ImageJ software (NIH Image).
Analysis of cell polarity
At 64-cell stage, a single marginal cell was injected with a mixture of Histone2B-RFP (50 pg) and mGFP (100 pg) mRNAs, with or without caJNK mRNA (200 pg). At 12 hpf, mosaically labeled embryos were dechorionated and placed on a microscope slide in a drop of Ringer’s solution. The yolk was removed, and the embryos were flat mounted with neuroectoderm facing upward. Following image acquisition using an upright fluorescence microscope (Leica LM2500), the embryos were subjected to genotyping.
Semi-quantitative and quantitative RT-PCR, and RNA sequencing
Total RNA was reverse transcribed using M-MLV reverse transcriptase (Invitrogen). Semi-quantitative PCR was performed using gene-specific primers, with ß-actin as a loading control (S1 Table). The intensity of PCR products was analyzed using the Lane 1D software (Sagecreation). Quantitative PCR was performed using Quant qRT-PCR Kit (Tiangen) with gene-specific primers (S1 Table). RNA sequencing was performed on Illumina HiSeq 2000, using 12 hpf mRNA libraries constructed by TruSeq RNA Library Preparation Kit. The data were aligned and analyzed as described [65].
Western blotting
Zebrafish embryos were lysed in ice-cold lysis buffer (100 mM NaCl, 10 mM Tris-HCl, pH 7.5, 5 mM EDTA, 1% Triton X-100) containing 1 x protease inhibitor cocktail (Sigma-Aldrich). The samples were separated by polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, probed with anti-myc (1/1000, Santa Cruz Biotechnology) and anti-α-tubulin (1/1000, GeneTex, GTX124303) antibodies, and detected using the Western-Lightning Plus-ECL substrate (PerkinElmer).
PCR-based genotyping
Single embryo was placed in an Ependorf tube containing 40 μl of lysis buffer (10 mM Tris-HCl, pH 8.0, 2 mM EDTA, 0.2% Triton X-100, 100 μg/ml proteinase K), and homogenized by pipetting. The tube was heated at 50°C for 2 hours, then at 94°C for 10 minutes. After a brief centrifugation, 1 μl of the solution was used for PCR reaction.
Luciferase assays
WT and mutant embryos at 1-cell stage were injected with 50 pg TOPFlash or AP1 reporter DNA, along with 5 pg pRL-TK DNA as an internal control. Fifteen to twenty embryos at 12 hpf were manually dechorionated and lysed in 60 μl lysis buffer (Promega). The lysate was clarified by centrifugation and luciferase activities were measured using the Dual-Luciferase® Reporter Assay System (Promega), according to the manufacturer’s instruction. The values were normalized with respect to Renilla luciferase activities, and the value in control condition was set as 1, and expressed as relative luciferase activity.
Statistical analysis
All data were obtained from at least three independent experiments, and analyzed using paired Student’s t test.
Supporting information
(A) TALENs-targeted sites (red) in the seventh exon and adjacent intron. Nucleotides in italic indicate intron sequence. (B) A deletion of 201 nucleotides in the seventh exon and the adjacent intron. Dots are introduced in WT sequence to optimize alignment, and dashes represent deleted nucleotides. (C) Schematic of Dvl domains shows truncated Dvl1a protein.
(JPG)
(A) TALENs-targeted sites (red) in the first exon. (B) A deletion of 8 nucleotides (dashes) within the exon. (C) Schematic of Dvl domains shows truncated Dvl1b protein.
(JPG)
(A) TALENs-targeted sites (red) in the first exon and adjacent intron. Nucleotides in italic indicate intron sequence. (B) A deletion of 5 nucleotides (dashes) in the first exon. (C) Schematic of Dvl domains shows truncation of Dvl2 protein.
(JPG)
(A) TALENs-targeted sites (red) in the first exon. (B) A deletion of 5 nucleotides (dashes) within the exon. (C) Schematic of Dvl domains shows truncation of Dvl3a protein.
(JPG)
(A) TALENs-targeted sites (red) in the first exon. (B) An insertion of 13 nucleotides (lowercases) within the exon. Dots are introduced in WT sequence to optimize alignment. (C) Schematic of Dvl domains shows truncated Dvl3b protein.
(JPG)
(A-C) WT embryos. (D-F) MZdvl1a mutants. (G-I) MZdvl1b mutants. (J-L) MZdvl3b mutants. All embryos are lateral view. The anterior region of 11.5 hpf embryos is positioned on the top. Scale bars: (A, D, G, J) 400 μm; (B, E, H, K) 400 μm; (C, F, I, L) 400 μm.
(JPG)
The graph was obtained by analyzing published RNA-seq data (Harvey et al., 2013. See reference 29 in the main text). FPKM, fragments per kilobase million.
(JPG)
Shown are the PCR products that should be amplified from genomic DNA in WT, heterozygous, and homozygous adult fish, by using allele-specific primers (see S1 Table for primer sequences).
(JPG)
(A) Semi-quantitative RT-PCR analysis to detect mutant dvl2 and dvl3a transcripts at 1-cell stage. ß-actin served as a loading control. NMD can be observed for dvl2 and dvl3a transcripts, respectively. (B, C) Quantification of mutant dvl2 and dvl3a mRNA levels in Mdvl2 and MZdvl3a mutants. The expression level in WT embryo is set as 1 after normalization with ß-actin. Bars represent the mean ± s.d. from three experiments (*, P<0.05; ****, P<0.0001). (D, E) In situ hybridization analysis of dvl2 transcripts in WT and Mdvl2 embryos. RT, reverse transcriptase.
(JPG)
Synthetic mRNAs encoding C-terminally myc-tagged WT and mutant Dvl2 and Dvl3a were expressed in zebrafish embryos. (A, B) Western blotting shows that mutant dvl2 and dvl3a transcripts are not translated.
(JPG)
(A) In the crosses between a mosaic female mdvl2+(-)/-;dvl3a-/- fish and a male dvl2+/-;dvl3a-/- fish, when the germline of the female fish contains a novel mutation for dvl2, the gametes that it produces will give rise to an MZdvl2;MZdvl3a offspring when fertilized by a male gamete with the original mutation, and an Mdvl2;MZdvl3a offspring when fertilized by a male gamete with WT dvl2 allele. The same female gametes will give rise to an Mdvl2;Mdvl3a offspring when fertilized by a male gamete from WT fish. (B) When a female mdvl2+(-)/-;dvl3a-/- fish is crossed with a male dvl2+/-;dvl3a-/- fish, if the germline of the female fish only contains the original mutation, the genotypes of offspring should include dvl2+/-;MZdvl3a-/-, MZdvl3a, or Zdvl2;Mdvl3a zygotes, depending on the genotype of the male gamete. These female gametes will give rise to Mdvl3a offspring when fertilized by a male gamete from WT fish. In all these cases, the early embryos should lack half of the dvl2 gene product. Only the dvl2 alleles are indicated in the schema.
(JPG)
(A-E) Ventral view of representative images of normal and different degrees of eye phenotypes at 3 dpf. (F) Quantitative analysis of different degrees of eye phenotypes in indicated mutants. Except for WT embryos, all mutants were analyzed from three independent crosses using the same fish pair (indicated below the horizontal line). Numbers on the top of each column indicate total embryos carrying the indicated genotypes (above the horizontal line). Scale bar: (A-E) 400 μm.
(JPG)
(A-I) Quantitative RT-PCR analysis of dvl1a (A, D, G), dvl1b (B, E, H), and dvl3b (C, F, I) at 1-cell stage (A-C), 50% epiboly (D-F) and 12 hpf (G-I). The expression level in WT embryo is set as 1 after normalization with ß-actin, and bars represent the mean ± s.d. from three independent experiments (NS, not significant). (J-L) Analysis of dvl1a (J), dvl1b (K), and dvl3b (L) expression levels by RNA sequencing at 12 hpf. Bars represent the mean ± s.d. from three independent samples (NS, not significant). RPKM, reads per kilobase million
(JPG)
In situ hybridization analysis of dorsoventral markers. (A, B) The expression of axin2 at 50% epiboly is inhibited in MZdvl2;MZdvl3a mutants. (C-F’) The expression domains of goosecoid (gsc) and chordin in MZdvl2;MZdvl3a mutants at 60% epiboly do not show ventral expansion, but reflect impaired AP extension. Scale bar: 400 μm.
(JPG)
Embryos at 1-cell stage derived from crosses between female mdvl2+(-)/-;dvl3a-/- fish and male dvl2+/-;dvl3a-/- fish were injected with ΔN-ß-catenin mRNA (50 pg). Following in situ hybridization or phenotype analysis, the embryos were subjected to genotyping. (A-F) ΔN-ß-catenin partially rescues axin2 and tbx16l expression in MZdvl2;MZdvl3a mutants. Arrowheads indicate the axin2 anterior expression domain. (G-I) ΔN-ß-catenin partially rescues tail development in Zdvl2;MZdvl3a mutants (arrow). Scale bar: (A-F) 400 μm; (G-I) 400 μm.
(JPG)
The embryos were imaged at 11.5 hpf and 30 hpf, followed by genotyping, and the extent of axis extension defect was reflected by the angle between the anterior end and the posterior end at 11.5 hpf. (A, B) WT embryos. (C, D) Mdvl2;Mdvl3a mutants from crosses between female mdvl2+(-)/-;dvl3a-/- fish and male WT fish were genotyped for the presence of a novel indel (red) along with a WT allele in the dvl2 locus. They have dvl2 and dvl3a heterozygous mutations. (E, H) The offspring with the two possible genotypes (dvl2+/+;dvl3a+/- and dvl2+/-;dvl3a+/-; the effects of these mutations are indicated in parenthesis), derived from a cross between female dvl2+/-;dvl3a-/- fish and male WT fish, are maternal mutants for dvl3a, with a reduced dosage of maternal dvl2 in both cases, despite of the genotype. (I) Statistical analysis of the extent of axis extension delay in three types of maternal mutants. Bars represent the mean ± s.d. from indicated numbers of embryos, and asterisks above the bars show significance with respect to WT embryos (***, P<0.001; ****, P<0.0001). (J) Quantitative analysis of defective axis extension at 11.5 hpf. Each type of cross was done using three independent fish pairs, and total numbers of embryos analyzed are indicated on the top of each column. Subjective measures of axis extension defect are shown on the embryos at 11.5 hpf. Scale bar: (A, C, E, G) 400 μm; (B, D, F, H) 400 μm.
(JPG)
This file contains statistical data corresponding to all graphs presented in the manuscript.
(ZIP)
(DOCX)
Time-lapse recording of cell movement toward the notochord (right side) was performed at 90% epiboly.
(AVI)
Time-lapse recording of cell movement toward the notochord (right side) was performed at 90% epiboly.
(AVI)
Time-lapse recording of cell movement toward the notochord (right side) was performed at 90% epiboly.
(AVI)
Time-lapse recording of cell movement toward the notochord (right side) was performed at 90% epiboly.
(AVI)
Acknowledgments
We thank members of the laboratories for technical assistance and zebrafish care, C.H.K Cheng, Q. Wang for providing the plasmid constructs used in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by grants from the National Natural Science Foundation of China (http://www.nsfc.gov.cn; 31471360 and 31671509; DLS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Langdon YG, Mullins MC. Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu. Rev. Genet. 2011; 45: 357–377. 10.1146/annurev-genet-110410-132517 [DOI] [PubMed] [Google Scholar]
- 2.Slack J. Establishment of spatial pattern. Wiley Interdiscip. Rev. Dev. Biol. 2014; 3: 379–388. 10.1002/wdev.144 [DOI] [PubMed] [Google Scholar]
- 3.Hikasa H, Sokol SY. Wnt signaling in vertebrate axis specification. Cold Spring Harb. Perspect. Biol. 2013; 5: a007955 10.1101/cshperspect.a007955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuazon FB, Mullins MC. Temporally coordinated signals progressively pattern the anteroposterior and dorsoventral body axes. Semin. Cell Dev. Biol. 2015; 42: 118–133. 10.1016/j.semcdb.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carron C, Shi DL. Specification of anteroposterior axis by combinatorial signaling during Xenopus development. Wiley Interdiscip. Rev. Dev. Biol. 2016; 5: 150–168. 10.1002/wdev.217 [DOI] [PubMed] [Google Scholar]
- 6.Heisenberg CP, Tada M, Rauch GJ, Saúde L, Concha ML, Geisler R, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000; 405: 76–81. 10.1038/35011068 [DOI] [PubMed] [Google Scholar]
- 7.Djiane A, Riou J, Umbhauer M, Boucaut J, Shi DL. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2000; 127: 3091–3100. [DOI] [PubMed] [Google Scholar]
- 8.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000; 127: 2227–2238. [DOI] [PubMed] [Google Scholar]
- 9.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, et al. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev. Cell. 2001; 1: 251–264. [DOI] [PubMed] [Google Scholar]
- 10.Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin. Cell Dev. Biol. 2009; 20: 986–997. 10.1016/j.semcdb.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev. Cell. 2011; 21: 120–133. 10.1016/j.devcel.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 2012; 28: 627–653. 10.1146/annurev-cellbio-092910-154208 [DOI] [PubMed] [Google Scholar]
- 13.Solnica-Krezel L, Sepich DS. Gastrulation: making and shaping germ layers. Annu. Rev. Cell Dev. Biol. 2012; 28: 687–717. 10.1146/annurev-cellbio-092910-154043 [DOI] [PubMed] [Google Scholar]
- 14.Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995; 121: 1637–1647. [DOI] [PubMed] [Google Scholar]
- 15.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000; 405: 81–85. 10.1038/35011077 [DOI] [PubMed] [Google Scholar]
- 16.Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech. Dev. 1999; 83: 27–37. [DOI] [PubMed] [Google Scholar]
- 17.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005; 132: 4421–4436. 10.1242/dev.02068 [DOI] [PubMed] [Google Scholar]
- 18.Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell. Signal. 2009; 22: 717–727. 10.1016/j.cellsig.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 19.Tadjuidje E, Cha SW, Louza M, Wylie C, Heasman J. The functions of maternal Dishevelled 2 and 3 in the early Xenopus embryo. Dev. Dyn. 2011; 240: 1727–1736. 10.1002/dvdy.22671 [DOI] [PubMed] [Google Scholar]
- 20.Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J. Cell Biol. 1999; 146: 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray RS, Bayly RD, Green SA, Agarwala S, Lowe CJ, Wallingford JB. Diversification of the expression patterns and developmental functions of the dishevelled gene family during chordate evolution. Dev. Dyn. 2009; 238: 2044–2057. 10.1002/dvdy.22028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mlodzik M. The Dishevelled protein family: Still rather a mystery after over 20 years of molecular studies. Curr. Top. Dev. Biol. 2016; 117: 75–91. 10.1016/bs.ctdb.2015.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wynshaw-Boris A. Dishevelled: in vivo roles of a multifunctional gene family during development. Curr. Top. Dev. Biol. 2012; 101: 213–235. 10.1016/B978-0-12-394592-1.00007-7 [DOI] [PubMed] [Google Scholar]
- 24.Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997; 90: 895–905. [DOI] [PubMed] [Google Scholar]
- 25.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002; 129: 5827–5838. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006; 133: 1767–1778. 10.1242/dev.02347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008; 4: e1000259 10.1371/journal.pgen.1000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol. 1996; 6: 1456–1467. [DOI] [PubMed] [Google Scholar]
- 29.Harvey SA, Sealy I, Kettleborough R, Fenyes F, White R, Stemple D, et al. Identification of the zebrafish maternal and paternal transcriptomes. Development. 2013; 140: 2703–2710. 10.1242/dev.095091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White RJ, Collins JE, Sealy IM, Wali N, Dooley CM, Digby Z, et al. A high-resolution mRNA expression time course of embryonic development in zebrafish. Elife. 2017; 6: e30860 10.7554/eLife.30860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marlow F, Zwartkruis F, Malicki J, Neuhauss SC, Abbas L, Weaver M, et al. Functional interactions of genes mediating convergent extension, knypek and trilobite, during the partitioning of the eye primordium in zebrafish. Dev. Biol. 1998; 203: 382–399. 10.1006/dbio.1998.9032 [DOI] [PubMed] [Google Scholar]
- 32.Heisenberg CP, Brand M, Jiang YJ, Warga RM, Beuchle D, van Eeden FJ, et al. Genes involved in forebrain development in the zebrafish, Danio rerio. Development. 1996; 123: 191–203. [DOI] [PubMed] [Google Scholar]
- 33.Piotrowski T, Schilling TF, Brand M, Jiang YJ, Heisenberg CP, Beuchle D, et al. Jaw and branchial arch mutants in zebrafish II: anterior arches and cartilage differentiation. Development. 1996; 123: 345–356. [DOI] [PubMed] [Google Scholar]
- 34.Kimmel CB, Miller CT, Moens CB. Specification and morphogenesis of the zebrafish larval head skeleton. Dev. Biol. 2001; 233: 239–257. 10.1006/dbio.2001.0201 [DOI] [PubMed] [Google Scholar]
- 35.Brand M, Heisenberg CP, Warga RM, Pelegri F, Karlstrom RO, Beuchle D, et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development. 1996; 123: 129–142. [DOI] [PubMed] [Google Scholar]
- 36.Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, et al. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 2011; 29: 143–148. 10.1038/nbt.1755 [DOI] [PubMed] [Google Scholar]
- 37.Kelly C, Chin AJ, Leatherman JL, Kozlowski DJ, Weinberg ES. Maternally controlled (beta)-catenin-mediated signaling is required for organizer formation in the zebrafish. Development. 2000; 127: 3899–3911. [DOI] [PubMed] [Google Scholar]
- 38.Bellipanni G, Varga M, Maegawa S, Imai Y, Kelly C, Myers AP, et al. Essential and opposing roles of zebrafish beta-catenins in the formation of dorsal axial structures and neurectoderm. Development. 2006; 133: 1299–1309. 10.1242/dev.02295 [DOI] [PubMed] [Google Scholar]
- 39.Szeto DP, Kimelman D. Combinatorial gene regulation by Bmp and Wnt in zebrafish posterior mesoderm formation. Development. 2004; 131: 3751–3760. 10.1242/dev.01236 [DOI] [PubMed] [Google Scholar]
- 40.Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr. Biol. 2005; 15: 489–500. 10.1016/j.cub.2005.01.041 [DOI] [PubMed] [Google Scholar]
- 41.Li HY, Bourdelas A, Carron C, Gomez C, Boucaut JC, Shi DL. FGF8, Wnt8 and Myf5 are target genes of Tbx6 during anteroposterior specification in Xenopus embryo. Dev. Biol. 2006; 290: 470–481. 10.1016/j.ydbio.2005.11.020 [DOI] [PubMed] [Google Scholar]
- 42.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003; 17: 295–309. 10.1101/gad.1022203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng XN, Shao M, Li JT, Wang YF, Qi J, Xu ZG, et al. Leucine repeat adaptor protein 1 interacts with Dishevelled to regulate gastrulation cell movements in zebrafish. Nat. Commun. 2017; 8: 1353 10.1038/s41467-017-01552-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, et al. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996; 85: 403–414. [DOI] [PubMed] [Google Scholar]
- 45.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol. 2002; 4: 610–615. 10.1038/ncb828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ, et al. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 2006; 8: 348–357. 10.1038/ncb1381 [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Thorpe C. BMP and non-canonical Wnt signaling are required for inhibition of secondary tail formation in zebrafish. Development. 2011; 138: 2601–2611. 10.1242/dev.058404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohata S, Nakatani J, Herranz-Pérez V, Cheng J, Belinson H, Inubushi T, et al. Loss of Dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron. 2014; 83: 558–571. 10.1016/j.neuron.2014.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, et al. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005; 120: 857–871. 10.1016/j.cell.2005.01.013 [DOI] [PubMed] [Google Scholar]
- 50.Lu FI, Thisse C, Thisse B. Identification and mechanism of regulation of the zebrafish dorsal determinant. Proc. Natl. Acad. Sci. USA. 2011; 108: 15876–15880. 10.1073/pnas.1106801108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell. 2001; 1: 103–114. [DOI] [PubMed] [Google Scholar]
- 52.Hino H, Nakanishi A, Seki R, Aoki T, Yamaha E, Kawahara A, et al. Roles of maternal wnt8a transcripts in axis formation in zebrafish. Dev. Biol. 2018; 434: 96–107. 10.1016/j.ydbio.2017.11.016 [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto A, Amacher SL, Kim SH, Geissert D, Kimmel CB, De Robertis EM. Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm. Development. 1998; 125: 3389–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Unterseher F, Hefele JA, Giehl K, De Robertis EM, Wedlich D, Schambony A. Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK. EMBO J. 2004; 23: 3259–3269. 10.1038/sj.emboj.7600332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Itoh K, Sokol SY. Graded amounts of Xenopus dishevelled specify discrete anteroposterior cell fates in prospective ectoderm. Mech. Dev. 1997; 61: 113–125. [DOI] [PubMed] [Google Scholar]
- 56.Lee YN, Gao Y, Wang HY. Differential mediation of the Wnt canonical pathway by mammalian Dishevelleds-1, -2, and -3. Cell. Signal. 2008; 20: 443–452. 10.1016/j.cellsig.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee HJ, Shi DL, Zheng JJ. Conformational change of Dishevelled plays a key regulatory role in the Wnt signaling pathways. Elife. 2015; 4: e08142 10.7554/eLife.08142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qi J, Lee HJ, Saquet A, Cheng XN, Shao M, Zheng JJ, et al. Autoinhibition of Dishevelled protein regulated by its extreme C terminus plays a distinct role in Wnt/β-catenin and Wnt/planar cell polarity (PCP) signaling pathways. J. Biol. Chem. 2017; 292: 5898–5908. 10.1074/jbc.M116.772509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995; 203: 253–310. 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- 60.Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011; 39: e82 10.1093/nar/gkr218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lei Y, Guo X, Liu Y, Cao Y, Deng Y, Chen X, et al. Efficient targeted gene disruption in Xenopus embryos using engineered transcription activator-like effector nucleases (TALENs). Proc. Natl. Acad. Sci. USA. 2012; 109: 17484–17489. 10.1073/pnas.1215421109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng C, Xiang J, Hunter T, Lin A. The JNKK2-JNK1 fusion protein acts as a constitutively active c-Jun kinase that stimulates c-Jun transcription activity. J. Biol. Chem. 1999; 274: 28966–28971. [DOI] [PubMed] [Google Scholar]
- 63.Carron C, Bourdelas A, Li HY, Boucaut JC, Shi DL. Antagonistic interaction between IGF and Wnt/JNK signaling in convergent extension in Xenopus embryo. Mech. Dev. 2005; 122: 1234–1247. 10.1016/j.mod.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 64.Wei S, Dai M, Liu Z, Ma Y, Shang H, Cao Y, et al. The guanine nucleotide exchange factor Net1 facilitates the specification of dorsal cell fates in zebrafish embryos by promoting maternal β-catenin activation. Cell Res. 2017; 27: 202–225. 10.1038/cr.2016.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shao M, Wang M, Liu YY, Ge YW, Zhang YJ, Shi DL. Vegetally localised Vrtn functions as a novel repressor to modulate bmp2b transcription during dorsoventral patterning in zebrafish. Development. 2017; 144: 3361–3374. 10.1242/dev.152553 [DOI] [PubMed] [Google Scholar]
- 66.Cao JM, Li SQ, Zhang HW, Shi DL. High mobility group B proteins regulate mesoderm formation and dorsoventral patterning during zebrafish and Xenopus early development. Mech. Dev. 2012; 129: 263–274. 10.1016/j.mod.2012.07.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) TALENs-targeted sites (red) in the seventh exon and adjacent intron. Nucleotides in italic indicate intron sequence. (B) A deletion of 201 nucleotides in the seventh exon and the adjacent intron. Dots are introduced in WT sequence to optimize alignment, and dashes represent deleted nucleotides. (C) Schematic of Dvl domains shows truncated Dvl1a protein.
(JPG)
(A) TALENs-targeted sites (red) in the first exon. (B) A deletion of 8 nucleotides (dashes) within the exon. (C) Schematic of Dvl domains shows truncated Dvl1b protein.
(JPG)
(A) TALENs-targeted sites (red) in the first exon and adjacent intron. Nucleotides in italic indicate intron sequence. (B) A deletion of 5 nucleotides (dashes) in the first exon. (C) Schematic of Dvl domains shows truncation of Dvl2 protein.
(JPG)
(A) TALENs-targeted sites (red) in the first exon. (B) A deletion of 5 nucleotides (dashes) within the exon. (C) Schematic of Dvl domains shows truncation of Dvl3a protein.
(JPG)
(A) TALENs-targeted sites (red) in the first exon. (B) An insertion of 13 nucleotides (lowercases) within the exon. Dots are introduced in WT sequence to optimize alignment. (C) Schematic of Dvl domains shows truncated Dvl3b protein.
(JPG)
(A-C) WT embryos. (D-F) MZdvl1a mutants. (G-I) MZdvl1b mutants. (J-L) MZdvl3b mutants. All embryos are lateral view. The anterior region of 11.5 hpf embryos is positioned on the top. Scale bars: (A, D, G, J) 400 μm; (B, E, H, K) 400 μm; (C, F, I, L) 400 μm.
(JPG)
The graph was obtained by analyzing published RNA-seq data (Harvey et al., 2013. See reference 29 in the main text). FPKM, fragments per kilobase million.
(JPG)
Shown are the PCR products that should be amplified from genomic DNA in WT, heterozygous, and homozygous adult fish, by using allele-specific primers (see S1 Table for primer sequences).
(JPG)
(A) Semi-quantitative RT-PCR analysis to detect mutant dvl2 and dvl3a transcripts at 1-cell stage. ß-actin served as a loading control. NMD can be observed for dvl2 and dvl3a transcripts, respectively. (B, C) Quantification of mutant dvl2 and dvl3a mRNA levels in Mdvl2 and MZdvl3a mutants. The expression level in WT embryo is set as 1 after normalization with ß-actin. Bars represent the mean ± s.d. from three experiments (*, P<0.05; ****, P<0.0001). (D, E) In situ hybridization analysis of dvl2 transcripts in WT and Mdvl2 embryos. RT, reverse transcriptase.
(JPG)
Synthetic mRNAs encoding C-terminally myc-tagged WT and mutant Dvl2 and Dvl3a were expressed in zebrafish embryos. (A, B) Western blotting shows that mutant dvl2 and dvl3a transcripts are not translated.
(JPG)
(A) In the crosses between a mosaic female mdvl2+(-)/-;dvl3a-/- fish and a male dvl2+/-;dvl3a-/- fish, when the germline of the female fish contains a novel mutation for dvl2, the gametes that it produces will give rise to an MZdvl2;MZdvl3a offspring when fertilized by a male gamete with the original mutation, and an Mdvl2;MZdvl3a offspring when fertilized by a male gamete with WT dvl2 allele. The same female gametes will give rise to an Mdvl2;Mdvl3a offspring when fertilized by a male gamete from WT fish. (B) When a female mdvl2+(-)/-;dvl3a-/- fish is crossed with a male dvl2+/-;dvl3a-/- fish, if the germline of the female fish only contains the original mutation, the genotypes of offspring should include dvl2+/-;MZdvl3a-/-, MZdvl3a, or Zdvl2;Mdvl3a zygotes, depending on the genotype of the male gamete. These female gametes will give rise to Mdvl3a offspring when fertilized by a male gamete from WT fish. In all these cases, the early embryos should lack half of the dvl2 gene product. Only the dvl2 alleles are indicated in the schema.
(JPG)
(A-E) Ventral view of representative images of normal and different degrees of eye phenotypes at 3 dpf. (F) Quantitative analysis of different degrees of eye phenotypes in indicated mutants. Except for WT embryos, all mutants were analyzed from three independent crosses using the same fish pair (indicated below the horizontal line). Numbers on the top of each column indicate total embryos carrying the indicated genotypes (above the horizontal line). Scale bar: (A-E) 400 μm.
(JPG)
(A-I) Quantitative RT-PCR analysis of dvl1a (A, D, G), dvl1b (B, E, H), and dvl3b (C, F, I) at 1-cell stage (A-C), 50% epiboly (D-F) and 12 hpf (G-I). The expression level in WT embryo is set as 1 after normalization with ß-actin, and bars represent the mean ± s.d. from three independent experiments (NS, not significant). (J-L) Analysis of dvl1a (J), dvl1b (K), and dvl3b (L) expression levels by RNA sequencing at 12 hpf. Bars represent the mean ± s.d. from three independent samples (NS, not significant). RPKM, reads per kilobase million
(JPG)
In situ hybridization analysis of dorsoventral markers. (A, B) The expression of axin2 at 50% epiboly is inhibited in MZdvl2;MZdvl3a mutants. (C-F’) The expression domains of goosecoid (gsc) and chordin in MZdvl2;MZdvl3a mutants at 60% epiboly do not show ventral expansion, but reflect impaired AP extension. Scale bar: 400 μm.
(JPG)
Embryos at 1-cell stage derived from crosses between female mdvl2+(-)/-;dvl3a-/- fish and male dvl2+/-;dvl3a-/- fish were injected with ΔN-ß-catenin mRNA (50 pg). Following in situ hybridization or phenotype analysis, the embryos were subjected to genotyping. (A-F) ΔN-ß-catenin partially rescues axin2 and tbx16l expression in MZdvl2;MZdvl3a mutants. Arrowheads indicate the axin2 anterior expression domain. (G-I) ΔN-ß-catenin partially rescues tail development in Zdvl2;MZdvl3a mutants (arrow). Scale bar: (A-F) 400 μm; (G-I) 400 μm.
(JPG)
The embryos were imaged at 11.5 hpf and 30 hpf, followed by genotyping, and the extent of axis extension defect was reflected by the angle between the anterior end and the posterior end at 11.5 hpf. (A, B) WT embryos. (C, D) Mdvl2;Mdvl3a mutants from crosses between female mdvl2+(-)/-;dvl3a-/- fish and male WT fish were genotyped for the presence of a novel indel (red) along with a WT allele in the dvl2 locus. They have dvl2 and dvl3a heterozygous mutations. (E, H) The offspring with the two possible genotypes (dvl2+/+;dvl3a+/- and dvl2+/-;dvl3a+/-; the effects of these mutations are indicated in parenthesis), derived from a cross between female dvl2+/-;dvl3a-/- fish and male WT fish, are maternal mutants for dvl3a, with a reduced dosage of maternal dvl2 in both cases, despite of the genotype. (I) Statistical analysis of the extent of axis extension delay in three types of maternal mutants. Bars represent the mean ± s.d. from indicated numbers of embryos, and asterisks above the bars show significance with respect to WT embryos (***, P<0.001; ****, P<0.0001). (J) Quantitative analysis of defective axis extension at 11.5 hpf. Each type of cross was done using three independent fish pairs, and total numbers of embryos analyzed are indicated on the top of each column. Subjective measures of axis extension defect are shown on the embryos at 11.5 hpf. Scale bar: (A, C, E, G) 400 μm; (B, D, F, H) 400 μm.
(JPG)
This file contains statistical data corresponding to all graphs presented in the manuscript.
(ZIP)
(DOCX)
Time-lapse recording of cell movement toward the notochord (right side) was performed at 90% epiboly.
(AVI)
Time-lapse recording of cell movement toward the notochord (right side) was performed at 90% epiboly.
(AVI)
Time-lapse recording of cell movement toward the notochord (right side) was performed at 90% epiboly.
(AVI)
Time-lapse recording of cell movement toward the notochord (right side) was performed at 90% epiboly.
(AVI)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.