Abstract
Background
Malaria burden remains high in the sub-Saharan region where helminths are prevalent and where children are often infected with both types of parasites. Although the effect of helminths on malaria infection is evident, the impact of these co-infections is not clearly elucidated yet and the scarce findings are conflicting. In this study, we investigated the effect of schistosomiasis, considering soil-transmitted helminths (STH), on prevalence and incidence of Plasmodium falciparum infection.
Methodology
This longitudinal survey was conducted in school-age children living in two rural communities in the vicinity of Lambaréné, Gabon. Thick blood smear light microscopy, urine filtration and the Kato-Katz technique were performed to detect malaria parasites, S. haematobium eggs and, STH eggs, respectively. P. falciparum carriage was assessed at inclusion, and incidence of malaria and time to the first malaria event were recorded in correlation with Schistosoma carriage status. Stratified multivariate analysis using generalized linear model was used to assess the risk of plasmodium infection considering interaction with STH, and survival analysis to assess time to malaria.
Main findings
The overall prevalence on subject enrolment was 30%, 23% and 9% for S. haematobium, P. falciparum infections and co-infection with both parasites, respectively. Our results showed that schistosomiasis in children tends to increase the risk of plasmodium infection but a combined effect with Trichuris trichiura or hookworm infection clearly increase the risk (aOR = 3.9 [95%CI: 1.7–9.2]). The incidence of malaria over time was 0.51[95%CI: 0.45–0.57] per person-year and was higher in the Schistosoma-infected group compared to the non-infected group (0.61 vs 0.43, p = 0.02), with a significant delay of time-to first-malaria event only in children aged from 6 to 10-years-old infected with Schistosoma haematobium.
Conclusions
Our results suggest that STH enhance the risk for P. falciparum infection in schistosomiasis-positive children, and when infected, that schistosomiasis enhances susceptibility to developing malaria in young children but not in older children.
Author summary
Despite the progress made in the last decade, malaria remains a serious public health issue in sub-Saharan region, where it overlaps with helminths infections. The interactions between both are manifold and complex, requiring further investigation. We report here that Trichuris trichiura or hookworm associated with schistosomiasis increase the risk of Plasmodium falciparum infection; whilst schistosomiasis is independently associated with a malaria increase in young children, but not in the older children. Our finding is an additional evidence that optimizing helminth control mainly in children contributes to overcoming malaria in areas endemic for both parasitic infections where children are those who bear the highest burden of these infections.
Introduction
Over the past fifteen years, morbidity and mortality due to malaria have globally decreased. However, sub-Saharan Africa, where 90% cases of malaria and 92% of deaths related to malaria have occurred in 2015, still bears the highest burden of the disease [1]. Most of these cases remain confined to rural and semi-urban areas [2–4] where helminths are co-prevalent [5–7]. In these areas where malaria significantly overlaps with helminth infections, several studies have reported interactions between the two parasitic infections at both immunological [8–12] and epidemiological [13–16] levels. Studies have reported an effect of helminths on the cellular and humoral immune responses to the malaria parasites mainly in children [8–12,17]. Some authors have reported that this effect leads to the aggravation of clinical manifestations of malaria. Indeed, it has been shown that Trichuris trichiura infection was associated with increased malaria prevalence, while an increased helminth burden was associated with increased Plasmodium falciparum or Plasmodium vivax parasitemia [18], or enhanced anemia in co-infected children [19]. Another author has reported a positive effect of helminths on malaria outcomes. Indeed, Nacher et al found that helminths, particularly Ascaris, may have a role in the establishment of malaria tolerance in Thai patients [20]. However HIV co-infection complicated the picture further. Indeed, high prevalences of helminth infections and malaria have been reported in HIV positive people, particularly in pregnant women under anti-retroviral therapy (ART) in Rwanda [21–23] with 10% co-infections with both [23]. These high infection prevalences are found to be associated with a low CD4 counts and moreover, each of these infections is a risk factor for the other [22].
The situation is similarly unclear when it comes to Schistosoma spp. infections. It has been reported that the effect of schistosomiasis on malaria may depend on the Schistosoma species [24], or may be conflicting even for the same species [15,25–27]. Indeed, some reports have indicated that infection with S. haematobium can confer protection against severe malaria in children [25], reducing the risk of progression to symptomatic disease in long-term asymptomatic carriers of P. falciparum [15], or can delay the occurrence of a malaria episode in children [26]; whereas others found that S. haematobium may increase the prevalence of P. falciparum parasites in co-infected children [27]. In contrast, Schistosoma mansoni was reported to significantly increase the malaria incidence rate in children [28]. These finding provide evidence of the effect of schistosomiasis on Plasmodium infection. Current results on schistosomiasis and plasmodial co-infection are conflicting as reviewed recently by Adegnika et al. [24]. Most studies conducted to address these co-infections are cross-sectional in nature and could be limited in their capacity to precisely examine interactions between schistosomiasis and malaria. In this study, we conducted a longitudinal survey in order to address this issue in an area where S. haematobium and P. falciparum are the main prevalent species of schistosomiasis and malaria [13,14]. We thus assessed the effect of S. haematobium on clinical and parasitological aspects of P. falciparum infection in school aged children living in this co-endemic area, including the effect of soil-transmitted helminths (STH) in this association.
Methods
Ethics statement
The study was approved by the institutional ethics committee of CERMEL, reference number: CEI-MRU 002/2012. Parents or legal representatives of the participant gave a written informed consent. The study was conducted in line with the Good Clinical Practice (GCP) principles of the International Conference on Harmonisation (ICH) [29] and the Declaration of Helsinki [30].
Study site
The study took place at CERMEL (Centre de Recherches Médicales de Lambaréné). Data and samples were collected from May 2012 to December 2014 in Bindo-Makouké villages (BM) and Zilé-PK villages, two settlements in the vicinity of Lambaréné [14] situated approximately at 60 km and 120 km, respectively, to the South of the Equator. The rainfall is perennial except for the long dry season (from June to September) with a mean of 1,216 mm per year [31]. The region is irrigated by the Ogooué River and its tributaries, with many ponds, lakes and streams constituting favourable conditions for fresh-water snail habitation. Recent published data demonstrate that the prevalence in the area for S. haematobium range from 15% to 75% [9,13,14]. Water supply, fishing, household work, fetching water and playing are some activities which expose the local population to schistosomiasis. Malaria transmission is perennial and the dominant malaria parasite species is P. falciparum [32,33].
Study design
The study was designed as a prospective longitudinal study.
Study population and inclusion criteria
School-age children living in two vicinities of Lambaréné (Nzilé-PK villages and Bindo-Makouké villages) were invited to participate in the study. Volunteers without any known chronic diseases other than possible helminth co-infections, and living in the study area for at least one year before inclusion were eligible to take part to the study. During the survey, participants found with a recurrent or severe disease other than malaria or helminth infection were excluded from the study.
Sample size determination
A previous study conducted in the vicinities of Lambaréné reported a 42% prevalence for plasmodium infection among school age children [9]. To be able to detect a minimum of 12.5% prevalence difference of plasmodium infection between children with schistosomiasis and those without, with a minimum of 80% power, we needed to include in the study at baseline at least 249 children for each study group, giving a total of 498 volunteers school age children.
Study procedure
Field-workers went to each house and school of both villages to invite through their parents or legal representatives school-age children to participate in the study. Eligible and consenting volunteers were included. At baseline, demographics (age, sex and location) and anthropological (weight, height) data were collected. Axillary temperature was recorded. S. haematobium infection, P. falciparum infection and soil-transmitted helminths (STH) status were assessed. Participants were treated if they were found to harbour either of those parasitic infections. The follow-up consisted of two kinds of visit: active visits consisted of monthly home visits for any malaria-like symptoms assessment and recording of any medication intake; and passive visits were ad-hoc presentations of participating children at the research centre for any health issues, including flu-like symptoms.
Malaria status was defined as positive thick blood smear (TBS) associated with fever, or history of fever in the past 48h from the time of visit. Fever was defined as an axillary temperature of 37.5°C or higher. In case malaria was diagnosed, urine filtration was performed to assess evidence of co-infection with urogenital schistosomiasis. Urine filtration was also performed during the follow-up every time the children had visible haematuria.
Study groups were determined based on the schistosomiasis status. This was done differently for baseline analysis and for longitudinal analysis. At baseline and for baseline analysis, participants found infected with S. haematobium were assigned to the ‘Schistosoma-positive’ (S+) group and the others to the ‘Schistosoma-negative’ (S-) group. For longitudinal analysis, study groups were formed at the end of the follow-up period, and we assigned any participants found with infection at baseline and at any time point of the study course to the ‘Schistosoma-infected’ (SI) group. Those found negative at baseline and who did not experienced schistosomiasis during the study course were assigned to the ‘Schistosoma-uninfected’ (SU) group. Time of exposure to malaria infection for incidence calculation did not include the first 28 days after each malaria treatment.
In accordance with the national guidelines, treatment of schistosomiasis consisted of the administration of 40 mg of praziquantel per kilogram body weight once; asymptomatic P. falciparum parasitemia and malaria episodes were treated with tablets of 20/120mg of artemether-lumefantrine combination therapy given according to the body weight twice a day, in three consecutive days. Treatment of STH was a once-daily dose of 400 mg of albendazole for three consecutive days [34]. For any other cause of a disease episode, the participant was referred to the appropriate health centre.
Samples collection and laboratory assays
Detection of malaria parasites was done microscopically by TBS using the Lambaréné method as described elsewhere [35–37]. Detection of S. haematobium eggs was done by filtration of 10ml of fresh urine using a 12μm pore-size filter as previously described [38–40]. For the diagnosis of urogenital schistosomiasis, urine samples were collected over three consecutive days, unless the participant was found positive with at least one parasite egg in any sample before the second or the third day. The Kato-Katz technique was performed to assess the presence of A. lumbricoides, T. trichiura and hookworm in fresh stool samples [41]. For each time point of STH assessment, one stool sample was collected. For each stool sample, two slides were performed and each slide was independently read by two readers.
Statistical analysis
Data were captured on the patient report form (PRF), entered in Access 2013 software and transferred to R software version 3.2.4 for analysis. Univariate and multivariate analysis were performed applying the Generalized Linear Model (GLM). For multivariate analysis, first we considered the interaction between asymptomatic P. falciparum infection as the main variable and each explanatory variable. In case of effect measure modification, the analysis was stratified on the variable, and the Breslow test was done to assess the homogeneity of the strata. Otherwise, the variable was evaluated as confounding factor to be include in the final model. Ten per cent (10%) or more difference of estimated measure of association before and after adjustment was used to define confounding factors. The effect of STH infection was assessed separately with respect to the species. Incidence of malaria was estimated in person-year according to each variable. A Kaplan Meier curve was drawn to assess time-to-malaria occurrence. The Log-rank test was used to compare the curves and the Cox model was used for adjusted analysis.
Results
Study population at baseline
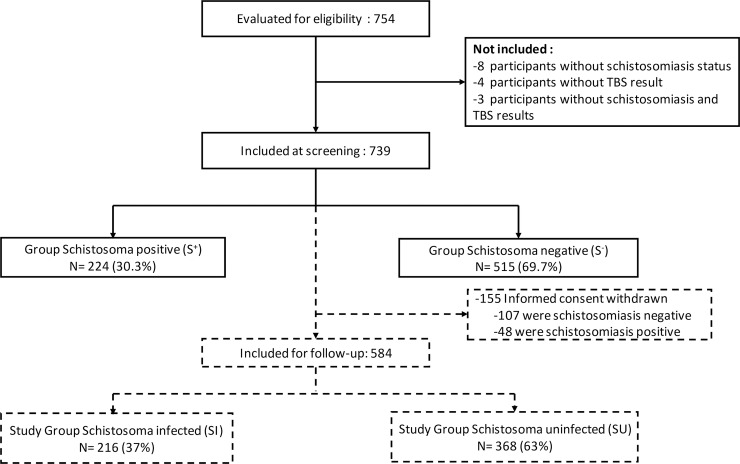
Among the participants who were invited to participate in the study, informed consent was granted for 754 children by their parents or their legal representatives. Of those, a total of 739 children with schistosomiasis and P. falciparum status available were included at baseline (Fig 1). Among participants enrolled, 68 (9%) children were not able to provide sample stool at baseline and from the others, 31% [95%CI: 27%-35%] were infected with STH. The most prevalent infection was trichuris with 21% [95%CI: 18%-24%] followed by ascaris and hookworm with 19% [95%CI: 16%-22%] and 6% [95%CI: 5%-8%], respectively. Mean age of these study population was 10.4 (SD = 3.1) years, the boy-to-girl sex ratio was 1.1:1 (Table 1). Of participants included, 586 (79%) agreed to be followed-up for malaria incidence.
Fig 1. Flow chart of the participants during the study course.
The inclusion phase is shown as a solid line and the follow-up phase is shown as a broken line.
Table 1. Characteristic of 739 participants seen at inclusion.
| Study population | ||||
|---|---|---|---|---|
| n | (%) | 95%CI(%) | ||
| Age | ||||
| [6–10] | 382 | (51.7) | [48.0–55.3] | |
| [10–16] | 357 | (48.3) | [44.6–52.0] | |
| Age (mean, sd) | (10.4, 3.1) | / | / | |
| Sex | ||||
| Female | 351 | (47.5) | [43.8–51.2] | |
| Male | 388 | (52.5) | [48.8–56.1] | |
| Sex ratio (M/F) | (1.1) | |||
| Location | ||||
| Bindo-Makouké villages | 420 | (56.8) | [53.2–60.4] | |
| Zilé-PK villages | 319 | (43.2) | [39.6–46.8] | |
| S. haematobium infection | ||||
| Negative | 515 | (69.7) | [66.2–73.0] | |
| Positive | 224 | (30.3) | [27.0–33.8] | |
| P. falciparum infection | ||||
| Negative | 572 | (77.4) | [74.2–80.4] | |
| Positive | 167 | (22.6) | [19.6–25.8] | |
| Soil-transmitted helminths* | ||||
| A. lumbricoides | 127 | (18.9) | [16.0–22.1] | |
| T. trichiura | 139 | (20.7) | [17.7–24.0] | |
| Hookworm | 43 | (06.4) | [04.7–08.5] | |
| Any STH | 208 | (31.0) | [27.5–34.6] | |
*68 missing data
Distribution of P. falciparum and S. haematobium infections
The prevalence of P. falciparum and S. haematobium at baseline was 23% [95%CI: 20%-26%] and 30% [95%CI: 27%-34%], respectively; with 67 (9%, [95%CI: 07%-11%]) participants co-infected with both parasites. Eight per cent of participants infected with P. falciparum had fever. As shown in Table 2, both infections were more prevalent in Zilé-PK villages compared to Bindo and Makouké villages with 29% [95%CI: 24%-34%] vs 18% [95%CI: 14%-22%], respectively, for P. falciparum and 45% [95%CI: 40%-51%] vs 19% [95%CI: 15%-23%], respectively, for S. haematobium. The prevalence of both infections was similar for age and sex groups.
Table 2. Distribution of P. falciparum and S. haematobium infections among the 739 participants seen at baseline.
| P. falciparum infection | S. haematobium infection | ||||||
|---|---|---|---|---|---|---|---|
| n | % [95%CI(%)] | p | n | % [95%CI(%)] | p | ||
| Total | 167 | 22.6 [19.6–25.6] | / | 224 | 30.3 [27.1–33.7] | / | |
| Sex | 0.16 | 0.74 | |||||
| Female | 71 | 20.2 [16.3–24.8] | 104 | 29.6 [25.1–34.6] | |||
| Male | 96 | 24.7 [20.7–29.3] | 120 | 30.9 [26.5–35.7] | |||
| Age group | 0.93 | 0.17 | |||||
| [6–10] | 87 | 22.8 [18.8–27.0] | 107 | 28.0 [23.7–32.7] | |||
| [10–16] | 80 | 22.7 [18.4–27.0] | 117 | 32.8 [28.1–37.8] | |||
| (Mean,sd) | (10.1, 3.1) | (10.7, 3.0) | |||||
| Location | <0.001 | <0.001 | |||||
| Bindo-Makouké villages | 75 | 17.9 [14.5–21.8] | 80 | 19.0 [15.6–23.1] | |||
| Zilé-PK villages | 92 | 28.8 [24.1–34.0] | 144 | 45.1 [39.8–50.6] | |||
| STH* | |||||||
| Ascaris | 30 | 23.6 [16.5–32.0] | 0.88 | 43 | 33.9 [25.7–42.8] | 0.52 | |
| Trichuris | 41 | 29.5 [22.1–37.8] | 0.05 | 49 | 35.2 [27.3–43.8] | 0.28 | |
| Hookworm | 11 | 25.6 [13.5–41.2] | 0.70 | 21 | 48.8 [33.3–64.5] | 0.13 | |
| Any STH | 52 | 25.0 [19.3–31.5] | 0.43 | 72 | 34.6 [28.2–41.5] | 0.24 | |
*68 missing data
Study group characteristics
Among the participants followed-up for malaria incidence assessment, 216 (37%) were found positive for Schistosoma infection during the study period, including 176 cases on inclusion and additionally 40 new cases during follow-up and assigned to SI group. The 368 (63%) others participants who remained negative during the whole study course were thus assigned to the SU group (Fig 1). As shown in Table 3, the two study groups were comparable for all parameters except for location and P. falciparum parasite carrier status. Indeed, 168 (78%) of SI children came from Zilé-PK villages while 253(69%) of SU children came from Bindo and Makouké villages (p<0.001). Additionally, prevalence of P. falciparum parasite carriage was significantly higher in the SI group compared to the SU group (31% vs 20%, p = 0.004).
Table 3. Characteristics of study groups considered for longitudinal analysis regarding Schistosoma status (N = 586).
| Schistosoma Infected (n = 216) | Schistosoma Uninfected (n = 368) | P | ||||
|---|---|---|---|---|---|---|
| n | % [95%CI (%)] | n | % [95%CI (%)] | |||
| Sex | 0.49 | |||||
| Female | 111 | 51.4 [44.5–58.2] | 177 | 48.1 [42.9–53.3] | ||
| Male | 105 | 48.6 [41.8–55.5] | 191 | 51.9 [46.7–57.1] | ||
| Age | 0.14 | |||||
| [6–10] | 110 | 50.9 [44.0–57.8] | 211 | 57.3 [52.1–62.4] | ||
| [10–16] | 106 | 49.1 [42.2–55.9] | 157 | 42.7 [37.5–47.9] | ||
| n, (mean, sd) | 216 | (10.4, 3.1) | 368 | (10.1, 3.0) | 0.19 | |
| Location | < 0.001 | |||||
| Bindo-Makouké villages | 48 | 22.2 [16.9–28.4] | 253 | 68.7 [63.7–73.4] | ||
| Zilé-PK villages | 168 | 77.8 [71.6–83.1] | 115 | 31.3 [26.5–36.3] | ||
| P. falciparum parasite carriage | 0.004 | |||||
| Positive | 66 | 30.6 [24.5–37.2] | 74 | 20.1 [16.1–24.6] | ||
| STH species | ||||||
| A. lumbricoïdes | 30 | 13.9 [09.6–19.2] | 60 | 16.3 [12.7–20.5] | 0.47 | |
| T. trichiura | 38 | 17.6 [12.8–23.3] | 58 | 15.8 [12.2–19.9] | 0.56 | |
| Hookworm | 17 | 07.9 [04.6–12.3] | 14 | 03.8 [02.1–06.3] | 0.05 | |
| Any species | 58 | 26.9 [21.1–33.3] | 97 | 26.4 [21.9–31.2] | 0.92 | |
Association between S. haematobium and P. falciparum parasitic infections
At crude analysis as given in Table 4, Schistosoma infection (p = 0.002) and location (p<0.001) were associated with P. falciparum infection. Children infected with S. haematobium had a 1.8 [95%CI: 1.2–2.5] times odds of being co-infected with P. falciparum parasites compared to their non-infected counterpart. After adjustment (Table 4), P. falciparum infection remains associated only with location (p = 0.02) while a trend of association with schistosomiasis infection was found (p = 0.06).
Table 4. Potential risk factors including S. haematobium infection associated with P. falciparum infection among the 739 participants seen at baseline.
| Crude analysis | Adjusted analysis | ||||
|---|---|---|---|---|---|
| OR [95%CI(OR)] | p | aOR [95%CI(aOR)] | p | ||
| S. haematobium status | 0.002 | 0.06 | |||
| Negative | 1 | 1 | |||
| Positive | 1.8 [1.23–2.53] | 1.5 [0.98–2.18] | |||
| Location | <0.001 | 0.02 | |||
| Bindo-Makouké villages | 1 | 1 | |||
| Zilé-PK villages | 1.9 [1.32–2.64] | 1.6 [1.09–2.37] | |||
| Sex | 0.14 | 0.38 | |||
| Female | 1 | 1 | |||
| Male | 1.3 [0.92–1.84] | 1.2 [0.82–1.72] | |||
| Age (years) | 1.1 [0.99–1.11] | 0.09 | 0.9 [0.89–1.01] | 0.08 | |
| T. trichiura | 0.06 | 0.09 | |||
| Negative | 1 | 1 | |||
| Positive | 1.5 [0.98–2.28] | 1.5 [0.94–2.38] | |||
| A. lumbricoïdes | 0.93 | 0.72 | |||
| Negative | 1 | 1 | |||
| Positive | 1.0 [0.64–1.59] | 0.9 [0.55–1.49] | |||
| Hookworm | 0.72 | 0.76 | |||
| Negative | 1 | 1 | |||
| Positive | 1.1 [0.54–2.25] | 0.9 [0.39–1.88] | |||
In the following analysis, we found effect modification of Trichuris or hookworm infections on P. falciparum and S. haematobium infections association. As presented in Table 5, analysis stratified on those two infections showed that among study participants without T. trichiura and hookworm infections, there is no effect of S. haematobium on P. falciparum parasite carriage; while among those infected with either hookworm or T. trichiura or a combination, children co-infected with S. haematobium had a 3.1 ([95%CI: 1.5–6.4], p = 0.002) time odds of being infected with P. falciparum. This finding remained statistical significant when adjusted for age, sex, ascariasis and location (aOR = 3.9 [95%CI: 1.75–9.19], p < 0.001). Age, sex and Ascaris infection were forced in the final model of the GLM analysis.
Table 5. Association between asymptomatic Plasmodium falciparum infection and Schistosoma haematobium infection stratified on Trichuris trichiura and hookworm infections among the 671 participants with known STH infection status and seen at baseline.
| N | Crude analysis+ | Adjusted analysis* | |||||
|---|---|---|---|---|---|---|---|
| OR [95%CI(OR)] | p | aOR [95%CI(aOR)] | p | ||||
| T. trichiura and hookworm negative (N = 516) | |||||||
| S. haematobium status | 0.27 | 0.84 | |||||
| Negative | 360 | 1 | 1 | ||||
| Positive | 156 | 1.3 [0.83–2.01] | 1.1 [0.65–1.67] | ||||
| T. trichiura or hookworm positive (N = 155) | |||||||
| S. haematobium status | 0.002 | <0.001 | |||||
| Negative | 100 | 1 | 1 | ||||
| Positive | 55 | 3.1. [1.48–6.44] | 3.9 [1.75–9.19] | ||||
+Breslow-test, p = 0.046
*Adjusted for location, sex, age and A. lumbricoïdes infection
Effect of S. haematobium Infection on P. falciparum malaria incidence
During the 19 months follow-up phase for P. falciparum malaria incidence assessment, 210 (36%) participants had developed a total of 318 new cases of malaria (Table 6). The overall incidence was 0.51 [95%CI: 0.47–0.55] per person-year. Taking into account the study groups, participants in the SI group had 1.4 [95%CI: 1.1–1.8] times the risk of developing malaria compared to their counterparts in the SU group.
Table 6. Malaria risk and malaria incidence among the 584 participants according to Schistosoma status and other risk factors.
| Study group | Number of participants exposed | Participants who developed malaria attack | Malaria attack cases | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | RR [95%CI(RR)] | Number of cases | Exposure Time + | Incidence [95%CI(I)] | RR [95%CI(RR)] | P-value | |||
| Total | 584 | 210 (36.0) | / | 318 | 627 | 0.51 [0.47–0.55] | / | ||
| Schistosoma status | 0.002 | ||||||||
| Uninfected | 365 | 109 (29.9) | 1 | 162 | 373 | 0.43 [0.38–0.48] | 1 | ||
| Infected | 219 | 101 (46.1) | 1.54 [1.17–2.02] | 156 | 254 | 0.61 [0.55–0.67] | 1.42 [1.14–1.77] | ||
| Location | 0.76 | ||||||||
| Bindo-Makouké villages | 301 | 107 (35.5) | 1 | 163 | 316 | 0.51 [0,45–0,56] | 1 | ||
| Zilé-PK villages | 283 | 103 (36.4) | 1.02 [0.78–1.34] | 155 | 311 | 0.50 [0,44–0,55] | 0.98 [0.79–1.22] | ||
| Age | 0.73 | ||||||||
| [6–10] | 321 | 123 (38.3) | 1 | 178 | 345 | 0.52 [0.47–0.57] | 1 | ||
| [10–16] | 263 | 87 (33.1) | 0.86 [0.65–1.13] | 140 | 282 | 0.50 [0.44–0.56] | 0.96 [0.77–1.20] | ||
| Sex | 0.21 | ||||||||
| Female | 288 | 97 (33.7) | 1 | 143 | 304 | 0.47 [0.41–0.53] | 1 | ||
| Male | 296 | 113 (38.2) | 1.13 [0.86–1.48] | 175 | 323 | 0.54 [0.48–0.60] | 1.15 [0.92–1.43] | ||
+In person-year
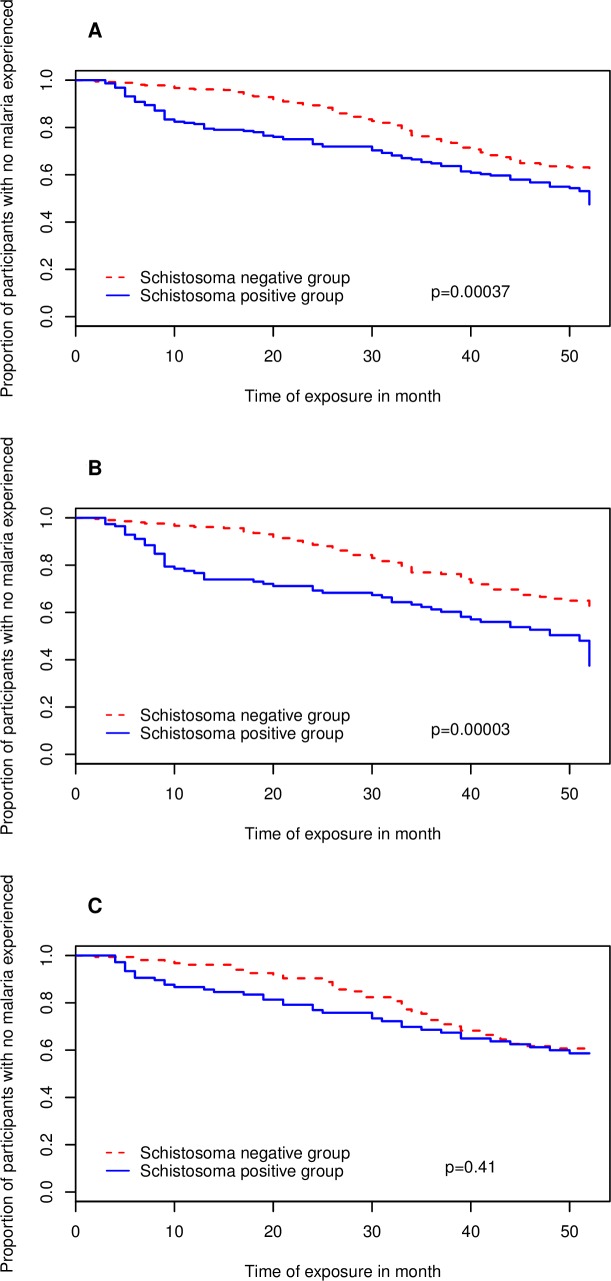
The time-to-first malaria episode was assessed for the first twelve months of follow-up of each participant and those who did not develop malaria before the end of that time were censored. Our results show that in the SI group, among the 101 (46%) participants developed malaria, the median time to first malaria episode was 52 weeks. For their counterparts of SU group where 109 (30%) participants developed malaria, the median time to first malaria episode was not reached at the end of 52 weeks of follow-up. As presented in Fig 2, we found a significant delay to malaria in the SU group compared to SI group (Log-Rank test: p = 0.00037). Assessing the delay until development of malaria according to schistosomiasis status, we found that SI group participants had a 1.6 (Cox model: p = 0.0004) times increased risk of early development of malaria as compared to their counterparts of the SU group. This association remains significant when adjusted for location, age and sex (aRR = 1.9, p = 0.000034). Stratifying for age yielded a significant delay in time-to-first-malaria episode in the SU group (median time not reach) compared to the SI group, where the median time was 51 weeks (p = 0.00003) for 6–10 year old children. Children with schistosomiasis had a 2.1 (Mantel-Cox test: p = 0.00004) times increased risk of developing malaria compared to children without schistosomiasis. On the contrary, there was no difference in terms of delay in time-to-first malaria episode between the two study groups (p = 0.41) in children aged 11–16 years.
Fig 2. Depicts estimates of time to malaria after 52 weeks of follow-up.
Depicted in the vertical axis the proportion of children who did not experience malaria and in the horizontal axis, the follow-up time in months. In red, children in S. haematobium non-infected group and in blue children in S. haematobium infected group. 2A) Kaplan Meier curve for time-to-first malaria case for overall study population. 2B) Kaplan Meier curve for time-to-first malaria case for children aged from 6 to 10 years old. 2C) Kaplan Meier curve for time-to-first malaria case for children aged from 11 to 16 years old.
Discussion
In area where schistosomiasis is endemic, the question of its effect on malaria outcome is a growing concern. Our study area is endemic for both infections [14], and our study reveals that up to 11% of school-age children could be co-infected with P. falciparum and S. haematobium parasites, comparing to up to 15% of pregnant women co-infected in Cameroon [42] or 23% of children co-infected with S. mansoni in a co-endemic area of North-Western Tanzania [43]. In our study area, poly-parasitism is evident [8,9,13,14,24]. The prevalence of STH species ranged from 32% to 48% among children infected with S. haematobium. This finding is not surprising since STH is commonly reported to be prevalent in rural areas [43]. Therefore, the risk to be infected by multiple parasites including S. haematobium is high [44]. Since these intestinal parasites are known to be able to modulate the immune system of the host, it would be necessary to assess the effect of these infections on Schistosoma-P. falciparum association.
Everything else equal, we found a trend of association between risk of P. falciparum carriage and Schistosoma infection. In univariate analysis, Adedoja et al. found that children infected with S. heamatobium have equal chances of being infected with P. falciparum as children with no worm infection [45], while Ateba and collaborators found a significant increase of plasmodium asexual parasite prevalence among Schistosoma infected children in comparison to the uninfected [9]. Conflicting results found in the literature on schistosomiasis-malaria co-infection issue suggest that there are potential confounding factors not yet established which need to be taken into account. In this study, we found an effect-measure modification of Trichuris and Hookworm infections on the association between S. haematobium and P. falciparum. As well, location was identified as confounding factor. Some authors have previously shown that T. trichiura [18] and hookworm [45,46] individually can affect the association between schistosomiasis and malaria co-infection by increasing the risk of being infected with P. falciparum parasites. Our analysis was stratified for those two STH infections and adjusted for age and sex which could affect malaria infection [47], and for location found in our analysis as confounding factor. The result shows that when considered only schistosomiasis infection, there is a trend on the risk of being infected with P. falciparum. But, in combination with trichuriasis or hookworm infection, schistosomiasis clearly increases the risk of being infected with P. falciparum. This result shows that S. haematobium alone does not predispose to P. falciparum infection in children instead of combined effect. We hypothesize that the cumulative effects of Schistosoma, Trichuris and Hookworm infections on P. falciparum parasite carriage acts at the immunological level. A potential immuno-modulation effect of a poly-parasitism not measured in our study could explain the combined effect of helminths we have observed. Indeed, there is evidence that Schistosoma infection can modulate the immune system in response to P. falciparum [9,11]. There is also evidence that T. trichiura can exert an influence on the immune response, and for instance negatively affect the antibody response to malaria vaccine candidate in children [17]. However, these potential cumulative effects of helminth infections on plasmodium infection need to be properly investigated at immunological level.
The overall incidence of malaria was 0.51 per person-year. This incidence was higher among people infected with S. haematobium compared to the uninfected, suggesting that schistosomiasis infection increases the risk of developing malaria. Children infected with S. haematobium infection had 1.4 times the risk of developing malaria than uninfected.
Regarding time-to-first malaria infection, we found that malaria occurred earlier in participants infected with Schistosoma than those uninfected even after adjustment for age, sex and location. The main symptom we have considered to define malaria was fever, which is one of the results of some endogenous pyrogen molecules activities, notably pro-inflammatory cytokine TNF-α during the infection. Some authors reported that during malaria infection, the production of pro-inflammatory cytokines as well as of anti-inflammatory cytokines can be affected by co-infection with schistosomiasis infection in an age-dependent manner [11,48]. In our study population, the assessment of the delay-to-malaria in relation to age group shows that there is no difference in terms of delay in time-to-first malaria in children aged from 11 to 16 years, while the difference was significant in children from 6 to 10 years. Children aged from 6 to 10 years infected with S. haematobium developed malaria earlier than those without S. haematobium infection. This finding could support the possible effect of age on the immune responses of malaria in co-infected subjects. However, since the finding is based on statistical significance, biological assessment is suitable for confirmation.
We can retain that exposure to schistosomiasis enhances incidence of, and susceptibility to develop malaria in our study population. This finding corroborates with previous reports like the one by Sokhna and collaborators who reported an increased in susceptibility to developing malaria in co-infected children, even though it was only in children excreting high S. mansoni eggs loads [28]; supporting therefore the hypothesis that schistosomiasis negatively affects the outcome of malaria. This stands in opposition to the idea that schistosomiasis possibly improves the outcome of malaria. Indeed, it was reported, for instance, that protection from malaria is conferred by asymptomatic P. falciparum infection or co-infection with S. haematobium in a Malian study cohort [15]. In the study presented here, we assessed the effect of having been S. haematobium-infected on malaria instead of becoming infected at time of malaria, which could affect our conclusion compared to the studies mentioned above. On the other hand, it has been shown that STH can affect susceptibility to malaria infection by acting at the immunological level [49,50]. Not having considered the STH status of participants in our analysis could have affected our results; however, since all participants were assessed and infected ones were treated for STH at inclusion, we assume that the effect of STH was minimized. The prevalence of STH was similar between the both study groups at baseline, and the STH treatment effect was considered as equally distributed between groups.
We have assessed the effect of schistosomiasis on clinical and parasitological aspects of P. falciparum infection based on prevalence of P. falciparum parasite carriage and malaria incidence. We therefore grouped our study population in relation to the schistosomiasis status. If it was easy at baseline to discriminate children infected or non-infected with S. haematobium, the problem we faced during the follow-up phase was to appropriately group our population in accordance with schistosomiasis status. Subjects infected at baseline or during the follow-up phase were treated systematically. However, they were considered as Schistosoma-infected for the whole follow-up phase and those who were not found positive throughout the survey were considered as non-infected. This approach was sustained by the fact that schistosomiasis is known as a chronic infection and, in areas where schistosomiasis is prevalent, the risk factors as playing habits, swimming, taking baths, washing clothes, distance from river are usually constant [51,52] and therefore the probability to be re-infected after treatment is high [53]. Thus, we have assessed the effect of schistosomiasis infection on P. falciparum parasite carriage at baseline and on malaria infection during the follow-up study phase.
An earlier conducted study in the same population showed that PCR has a better sensitivity than microscopy for the detection of P. falciparum parasites [9]. We have used the light microscopy Lambaréné method for the detection of P. falciparum parasites as it is the clinical gold standard. However, this may lead to potential misclassification of the participants regarding P. falciparum status at baseline. We think that if prevalence of P. falciparum carriage could be underestimated, this potential misclassification of participants would have been equally distributed in both groups and would not therefore affect the trend of our results.
This study confirms that the transmission of schistosomiasis is not evenly distributed in the vicinity of Lambaréné. Schistosomiasis infection is present in many villages but the prevalence varies significantly from one point to another. For example, we found a moderate prevalence for Bindo and Makouké villages where 19% of our study participants were found to be positive when compared to Zilé-PK villages, where 45% of our study participants were found to be positive. This corroborates with a previous pilot study conducted in the same population in 2012. The earlier-indicated prevalences of 15% and 43% for Bindo and Zilé-PK villages, respectively [14], suggest that prevalence of schistosomiasis infection is stable on each location. It was suggested that the difference observed could be explained by the fact that in Zilé-PK villages, streams represent the first source of water compared to Bindo village. The same observation could be applied to Makouké village where piped water is available for the majority of the population. Indeed, the lack of pipe water supply observed in the PK area promotes daily open freshwater contact by the population for household activities, bathing and playing, using the streams well known as schistosomiasis foci. In addition to humans, other ecological factors influence Schistosoma host snail density [54], which affect schistosomiasis prevalence. Therefore, we can assume that such factors may also sustain the difference of prevalence for schistosomiasis observed between the both locations, which requires further research. On the other hand, we have observed that areas where S. haematobium prevalence is high, a high prevalence of P. falciparum carriage was also found. Indeed, the difference in prevalence observed in favour of the PK area for S. haematobium infection was also observed for P. falciparum. This observation suggest a correlation of factors affecting both infections as either a consequence of the presence of same environmental risk factors. Another explanation could be indeed the effect of S. haematobium infection on P. falciparum infection, as demonstrated above. However, this need to be more investigated.
In summary, this study demonstrates that S. haematobium infection alone does not increase the risk of being infected with P. falciparum parasite but when associated with STH particularly with T. trichiura and hookworm, the risk does increase. On the other hand, in people exposed to schistosomiasis infection, risk and susceptibility of developing a malaria event increase in an age-dependent manner. Our results suggest that Schistosoma and probably STH co-infections in general cumulatively impact on malaria outcome in school-age children and therefore need to be accounted for when designing malaria control programs. Thus, in areas of co-endemicity and in support of higher efficiency, STH and schistosomiasis control should be considered as an additional tool of malaria control.
Supporting information
(PDF)
(XLSX)
Acknowledgments
We would like to thank the children and their parents or legal representatives from Bindo, Makouké and Zilé-PK villages for their participation in this study. We also want to thank the field workers, lab technicians and data managers of CERMEL for their commitment to this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the funding granted to AAA by EDCTP through a Senior Fellowship training award TA_11_40200 (www.edctp.org) and Deutsche Forschungsgemeinschaft, GZ:MO 1071/12-1 AOBJ: 620617. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Organization WH. World Malaria Report 2015 World Health Organization; 2016. 283 p. [Google Scholar]
- 2.De Beaudrap P, Nabasumba C, Grandesso F, Turyakira E, Schramm B, Boum Y, et al. Heterogeneous decrease in malaria prevalence in children over a six-year period in south-western Uganda. Malar J. 2011. May 18;10:132 10.1186/1475-2875-10-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govoetchan R, Gnanguenon V, Azondékon R, Agossa RF, Sovi A, Oké-Agbo F, et al. Evidence for perennial malaria in rural and urban areas under the Sudanian climate of Kandi, Northeastern Benin. Parasit Vectors. 2014. February 24;7:79 10.1186/1756-3305-7-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maghendji-Nzondo S, Kouna L-C, Mourembou G, Boundenga L, Imboumy-Limoukou R-K, Matsiegui P-B, et al. Malaria in urban, semi-urban and rural areas of southern of Gabon: comparison of the Pfmdr 1 and Pfcrt genotypes from symptomatic children. Malar J. 2016. August 18;15(1):420 10.1186/s12936-016-1469-1 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Barra M, Bustos L, Ossa X. [Inequality in the prevalence of intestinal parasitic infections among schoolchildren from urban and rural schools]. Rev Med Chil. 2016. July;144(7):886–93. 10.4067/S0034-98872016000700009 [DOI] [PubMed] [Google Scholar]
- 6.Cooper PJ, Amorim LD, Figueiredo CA, Esquivel R, Tupiza F, Erazo S, et al. Effects of environment on human cytokine responses during childhood in the tropics: role of urban versus rural residence. World Allergy Organ J. 2015;8(1):22 10.1186/s40413-015-0071-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO | Schistosomiasis [Internet]. WHO. [cited 2017 Jul 6]. Available from: http://www.who.int/mediacentre/factsheets/fs115/en/
- 8.Ateba-Ngoa U, Adegnika AA, Zinsou JF, Kassa Kassa RF, Smits H, Massinga-Loembe M, et al. Cytokine and chemokine profile of the innate and adaptive immune response of Schistosoma haematobium and Plasmodium falciparum single and co-infected school-aged children from an endemic area of Lambaréné, Gabon. Malar J. 2015. February 25;14:94 10.1186/s12936-015-0608-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ateba-Ngoa U, Jones S, Zinsou JF, Honkpehedji J, Adegnika AA, Agobe J-CD, et al. Associations Between Helminth Infections, Plasmodium falciparum Parasite Carriage and Antibody Responses to Sexual and Asexual Stage Malarial Antigens. Am J Trop Med Hyg. 2016. August 3;95(2):394–400. 10.4269/ajtmh.15-0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diallo TO, Remoue F, Gaayeb L, Schacht A-M, Charrier N, De Clerck D, et al. Schistosomiasis coinfection in children influences acquired immune response against Plasmodium falciparum malaria antigens. PloS One. 2010. September 15;5(9):e12764 10.1371/journal.pone.0012764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diallo TO, Remoue F, Schacht AM, Charrier N, Dompnier J-P, Pillet S, et al. Schistosomiasis co-infection in humans influences inflammatory markers in uncomplicated Plasmodium falciparum malaria. Parasite Immunol. 2004. September;26(8–9):365–9. 10.1111/j.0141-9838.2004.00719.x [DOI] [PubMed] [Google Scholar]
- 12.Imai N, Rujeni N, Nausch N, Bourke CD, Appleby LJ, Cowan G, et al. Exposure, infection, systemic cytokine levels and antibody responses in young children concurrently exposed to schistosomiasis and malaria. Parasitology. 2011. October;138(12):1519–33. 10.1017/S0031182011001181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adegnika AA, Ramharter M, Agnandji ST, Ateba Ngoa U, Issifou S, Yazdanbahksh M, et al. Epidemiology of parasitic co-infections during pregnancy in Lambaréné, Gabon. Trop Med Int Health TM IH. 2010. October;15(10):1204–9. 10.1111/j.1365-3156.2010.02598.x [DOI] [PubMed] [Google Scholar]
- 14.Ateba Ngoa U, Zinsou JF, Kassa RFK, Ngoune Feugap E, Honkpehedji YJ, Massinga-Loembe M, et al. Assessment of the effect of Schistosoma haematobium co infection on malaria parasites and immune responses in rural populations in Gabon: study protocol SpringerPlus; 2014;3:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doumbo S, Tran TM, Sangala J, Li S, Doumtabe D, Kone Y, et al. Co-infection of long-term carriers of Plasmodium falciparum with Schistosoma haematobium enhances protection from febrile malaria: a prospective cohort study in Mali. PLoS Negl Trop Dis. 2014. September;8(9):e3154 10.1371/journal.pntd.0003154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Getie S, Wondimeneh Y, Getnet G, Workineh M, Worku L, Kassu A, et al. Prevalence and clinical correlates of Schistosoma mansoni co-infection among malaria infected patients, Northwest Ethiopia. BMC Res Notes. 2015. September 28;8:480 10.1186/s13104-015-1468-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esen M, Mordmüller B, de Salazar PM, Adegnika AA, Agnandji ST, Schaumburg F, et al. Reduced antibody responses against Plasmodium falciparum vaccine candidate antigens in the presence of Trichuris trichiura. Vaccine. 2012. December 14;30(52):7621–4. 10.1016/j.vaccine.2012.10.026 [DOI] [PubMed] [Google Scholar]
- 18.Mulu A, Legesse M, Erko B, Belyhun Y, Nugussie D, Shimelis T, et al. Epidemiological and clinical correlates of malaria-helminth co-infections in Southern Ethiopia. Malar J. 2013. July 3;12:227 10.1186/1475-2875-12-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deribew K, Tekeste Z, Petros B, Huat LB. Urinary schistosomiasis and malaria associated anemia in Ethiopia. Asian Pac J Trop Biomed. 2013. April;3(4):307–10. 10.1016/S2221-1691(13)60068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nacher M, Singhasivanon P, Traore B, Vannaphan S, Gay F, Chindanond D, et al. Helminth infections are associated with protection from cerebral malaria and increased nitrogen derivatives concentrations in Thailand. Am J Trop Med Hyg. 2002. March;66(3):304–9. [DOI] [PubMed] [Google Scholar]
- 21.Ivan E, Crowther NJ, Mutimura E, Rucogoza A, Janssen S, Njunwa KK, et al. Effect of deworming on disease progression markers in HIV-1-infected pregnant women on antiretroviral therapy: a longitudinal observational study from Rwanda. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015. January 1;60(1):135–42. [DOI] [PubMed] [Google Scholar]
- 22.Ivan E, Crowther NJ, Mutimura E, Osuwat LO, Janssen S, Grobusch MP. Helminthic infections rates and malaria in HIV-infected pregnant women on anti-retroviral therapy in Rwanda. PLoS Negl Trop Dis. 2013;7(8):e2380 10.1371/journal.pntd.0002380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivan E, Crowther NJ, Rucogoza AT, Osuwat LO, Munyazesa E, Mutimura E, et al. Malaria and helminthic co-infection among HIV-positive pregnant women: prevalence and effects of antiretroviral therapy. Acta Trop. 2012. December;124(3):179–84. 10.1016/j.actatropica.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 24.Adegnika AA, Kremsner PG. Epidemiology of malaria and helminth interaction: a review from 2001 to 2011. Curr Opin HIV AIDS. 2012. May;7(3):221–4. 10.1097/COH.0b013e3283524d90 [DOI] [PubMed] [Google Scholar]
- 25.Lemaitre M, Watier L, Briand V, Garcia A, Le Hesran JY, Cot M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: Additional Evidence of the Protective Effect of Schistosomiasis on Malaria in Senegalese Children. Am J Trop Med Hyg. 2014. February 5;90(2):329–34. 10.4269/ajtmh.12-0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyke KE, Dicko A, Dabo A, Sangare L, Kone A, Coulibaly D, et al. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am J Trop Med Hyg. 2005. December;73(6):1124–30. [PMC free article] [PubMed] [Google Scholar]
- 27.Sangweme DT, Midzi N, Zinyowera-Mutapuri S, Mduluza T, Diener-West M, Kumar N. Impact of schistosome infection on Plasmodium falciparum Malariometric indices and immune correlates in school age children in Burma Valley, Zimbabwe. PLoS Negl Trop Dis. 2010. November 9;4(11):e882 10.1371/journal.pntd.0000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokhna C, Le Hesran J-Y, Mbaye PA, Akiana J, Camara P, Diop M, et al. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar J. 2004. November 15;3:43 10.1186/1475-2875-3-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ICH Official web site: ICH [Internet]. [cited 2017 Oct 6]. Available from: http://www.ich.org/home.html
- 30.WMA—The World Medical Association-Déclaration d’Helsinki de L’AMM–Principes éthiques applicables à la recherche médicale impliquant des êtres humains [Internet]. [cited 2018 Apr 23]. Available from: https://www.wma.net/fr/policies-post/declaration-dhelsinki-de-lamm-principes-ethiques-applicables-a-la-recherche-medicale-impliquant-des-etres-humains/
- 31.Climate & Weather Averages in Lambaréné, Gabon [Internet]. [cited 2017 Jun 18]. Available from: https://www.timeanddate.com/weather/gabon/lambarene/climate
- 32.Dal-Bianco MP, Köster KB, Kombila UD, Kun JFJ, Grobusch MP, Ngoma GM, et al. High prevalence of asymptomatic Plasmodium falciparum infection in Gabonese adults. Am J Trop Med Hyg. 2007. November;77(5):939–42. [PubMed] [Google Scholar]
- 33.Wildling E, Winkler S, Kremsner PG, Brandts C, Jenne L, Wernsdorfer WH. Malaria epidemiology in the province of Moyen Ogoov, Gabon. Trop Med Parasitol Off Organ Dtsch Tropenmedizinische Ges Dtsch Ges Tech Zusammenarbeit GTZ. 1995. June;46(2):77–82. [PubMed] [Google Scholar]
- 34.Adegnika AA, Zinsou JF, Issifou S, Ateba-Ngoa U, Kassa RF, Feugap EN, et al. Randomized, controlled, assessor-blind clinical trial to assess the efficacy of single- versus repeated-dose albendazole to treat ascaris lumbricoides, trichuris trichiura, and hookworm infection. Antimicrob Agents Chemother. 2014. May;58(5):2535–40. 10.1128/AAC.01317-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kremsner PG, Zotter GM, Feldmeier H, Graninger W, Rocha RM, Wiedermann G. A comparative trial of three regimens for treating uncomplicated falciparum malaria in Acre, Brazil. J Infect Dis. 1988. December;158(6):1368–71. [DOI] [PubMed] [Google Scholar]
- 36.Planche T, Krishna S, Kombila M, Engel K, Faucher JF, Ngou-Milama E, et al. Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hyg. 2001. November;65(5):599–602. [DOI] [PubMed] [Google Scholar]
- 37.Joanny F, Löhr SJZ, Engleitner T, Lell B, Mordmüller B. Limit of blank and limit of detection of Plasmodium falciparum thick blood smear microscopy in a routine setting in Central Africa. Malar J. 2014. June 14;13:234 10.1186/1475-2875-13-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Riet E, Adegnika AA, Retra K, Vieira R, Tielens AGM, Lell B, et al. Cellular and humoral responses to influenza in gabonese children living in rural and semi-urban areas. J Infect Dis. 2007. December 1;196(11):1671–8. 10.1086/522010 [DOI] [PubMed] [Google Scholar]
- 39.Peters PA, Warren KS, Mahmoud AA. Rapid, accurate quantification of schistosome eggs via nuclepore filters. J Parasitol. 1976. February;62(1):154–5. [PubMed] [Google Scholar]
- 40.Peters PA, Mahmoud AA, Warren KS, Ouma JH, Siongok TK. Field studies of a rapid, accurate means of quantifying Schistosoma haematobium eggs in urine samples. Bull World Health Organ. 1976;54(2):159–62. [PMC free article] [PubMed] [Google Scholar]
- 41.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972. December;14(6):397–400. [PubMed] [Google Scholar]
- 42.Anchang-Kimbi JK, Elad DM, Sotoing GT, Achidi EA. Coinfection with Schistosoma haematobium and Plasmodium falciparum and Anaemia Severity among Pregnant Women in Munyenge, Mount Cameroon Area: A Cross-Sectional Study. J Parasitol Res. 2017;2017:6173465 10.1155/2017/6173465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazigo HD, Waihenya R, Lwambo NJ, Mnyone LL, Mahande AM, Seni J, et al. Co-infections with Plasmodium falciparum, Schistosoma mansoni and intestinal helminths among schoolchildren in endemic areas of northwestern Tanzania. Parasit Vectors. 2010. May 19;3:44 10.1186/1756-3305-3-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babamale OA, Ugbomoiko US, Heukelbach J. High prevalence of Plasmodium falciparum and soil-transmitted helminth co-infections in a periurban community in Kwara State, Nigeria. J Infect Public Health. 2017. April 22; [DOI] [PubMed] [Google Scholar]
- 45.Adedoja A, Tijani BD, Akanbi AA, Ojurongbe TA, Adeyeba OA, Ojurongbe O. Co-endemicity of Plasmodium falciparum and Intestinal Helminths Infection in School Age Children in Rural Communities of Kwara State Nigeria. PLoS Negl Trop Dis. 2015;9(7):e0003940 10.1371/journal.pntd.0003940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinung’hi SM, Magnussen P, Kaatano GM, Kishamawe C, Vennervald BJ. Malaria and helminth co-infections in school and preschool children: a cross-sectional study in Magu district, north-western Tanzania. PloS One. 2014;9(1):e86510 10.1371/journal.pone.0086510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briand V, Watier L, LE Hesran J-Y, Garcia A, Cot M. Coinfection with Plasmodium falciparum and schistosoma haematobium: protective effect of schistosomiasis on malaria in senegalese children? Am J Trop Med Hyg. 2005. June;72(6):702–7. [PubMed] [Google Scholar]
- 48.Lyke KE, Dabo A, Sangare L, Arama C, Daou M, Diarra I, et al. Effects of concomitant Schistosoma haematobium infection on the serum cytokine levels elicited by acute Plasmodium falciparum malaria infection in Malian children. Infect Immun. 2006. October;74(10):5718–24. 10.1128/IAI.01822-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faye B, Ndiaye JL, Tine RC, Lô AC, Gaye O. [Interaction between malaria and intestinal helminthiasis in Senegal: influence of the carriage of intestinal parasites on the intensity of the malaria infection]. Bull Soc Pathol Exot 1990. 2008. December;101(5):391–4. [DOI] [PubMed] [Google Scholar]
- 50.Spiegel A, Tall A, Raphenon G, Trape JF, Druilhe P. Increased frequency of malaria attacks in subjects co-infected by intestinal worms and Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2003. April;97(2):198–9. [DOI] [PubMed] [Google Scholar]
- 51.Ismail HAHA, Hong S-T, Babiker ATEB, Hassan RMAE, Sulaiman MAZ, Jeong H-G, et al. Prevalence, risk factors, and clinical manifestations of schistosomiasis among school children in the White Nile River basin, Sudan. Parasit Vectors. 2014. October 15;7:478 10.1186/s13071-014-0478-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kapito-Tembo AP, Mwapasa V, Meshnick SR, Samanyika Y, Banda D, Bowie C, et al. Prevalence distribution and risk factors for Schistosoma hematobium infection among school children in Blantyre, Malawi. PLoS Negl Trop Dis. 2009;3(1):e361 10.1371/journal.pntd.0000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya: survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg. 2006. July;75(1):83–92. [PMC free article] [PubMed] [Google Scholar]
- 54.Monde C, Syampungani S, van den Brink PJ. Natural and human induced factors influencing the abundance of Schistosoma host snails in Zambia. Environ Monit Assess [Internet]. 2016;188 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4882361/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.