Abstract
Background
Buruli ulcer (BU) is a chronic necrotizing infectious skin disease caused by Mycobacterium ulcerans. The treatment with BU-specific antibiotics is initiated after clinical suspicion based on the WHO clinical and epidemiological criteria. This study aimed to estimate the predictive values of these criteria and how they could be improved.
Methodology/Principal findings
A total of 224 consecutive patients presenting with skin and soft tissue lesions that could be compatible with BU, including those recognized as unlikely BU by experienced clinicians, were recruited in two BU treatment centers in southern Benin between March 2012 and March 2015. For each participant, the WHO and four additional epidemiological and clinical diagnostic criteria were recorded. For microbiological confirmation, direct smear examination and IS2404 PCR were performed. We fitted a logistic regression model with PCR positivity for BU confirmation as outcome variable. On univariate analysis, most of the clinical and epidemiological WHO criteria were associated with a positive PCR result. However, lesions on the lower limbs and WHO category 3 lesions were rather associated with a negative PCR result (respectively OR: 0.4, 95%CI: 0.3–0.8; OR: 0.5, 95%IC: 0.3–0.9). Among the additional characteristics studied, the characteristic smell of BU was strongest associated with a positive PCR result (OR = 16.4; 95%CI = 7.5–35.6).
Conclusion/Significance
The WHO diagnostic criteria could be improved upon by differentiating between lesions on the upper and lower limbs and by including lesion size and the characteristic smell recognized by experienced clinicians.
Author summary
Buruli ulcer (BU) is a neglected necrotizing skin disease caused by Mycobacterium ulcerans. The treatment with BU-specific antibiotics is initiated after clinical suspicion based on WHO diagnostic criteria. In this study we evaluated the WHO diagnostic guidelines for BU and how these criteria could be improved. A total of 224 patients presenting with skin lesions were recruited in two BU treatment centers in southern Benin between March 2012 and March 2015. Most of the clinical and epidemiological WHO criteria were associated with a confirmed BU diagnosis although lesions on the lower limbs were rather associated with a negative PCR result. Among the additional characteristics studied, the characteristic smell of BU was most strongly associated with a positive PCR result. The WHO diagnostic criteria could therefore be improved upon by discriminating between lesions on the upper and lower limbs and by including lesion size and the characteristic smell recognized by experienced clinicians. The volatiles responsible for this smell could serve as a Point-of-Care diagnostic test, useful for non-invasive confirmation during active case-finding activities, and for training of clinicians.
Introduction
Buruli ulcer (BU) is a chronic necrotizing infectious disease of the skin caused by Mycobacterium ulcerans. After tuberculosis and leprosy, BU is the third most common mycobacterial disease worldwide [1,2]. BU has been reported in over 30 countries, typically in warm and humid intertropical regions where it predominantly affects children among poor and rural populations with difficult access to health care [3–5]. The diagnosis of BU is based on epidemiological and clinical criteria defined by the World Health Organization (WHO) [2,4]. The epidemiological WHO criteria are (i) residence or stay in a known BU endemic area and (ii) age between 0 and 15 years old since over 50% of all BU patients in Africa are children [6–8]. The clinical WHO criteria are (i) lesions on the upper or lower limbs since these represent about 85% of BU cases [4,8–11]; (ii) painless nodules, plaques or edema of the skin that, without treatment, evolve to a necrotic ulceration with [4,8] (iii) undermined and often hyperpigmented edges; (iv) lesions that are generally not accompanied by adenopathy, nor fever, and (v) that may become painful in case of superinfection [2,4].
Among the four tests recommended to confirm a BU diagnosis, two are most often used: direct smear examination (DSE) to detect acid-fast bacilli (AFB) and IS2404 PCR, which is the most sensitive test to date yet with associated delays in the availability of results of more than 10 days [12–14]. Despite having the highest sensitivity of all laboratory tests, PCR does not detect all BU cases. Patients have been described that fulfill the epidemiological and clinical WHO criteria yet repeatedly test negative by PCR [7,15–17]. Given the limited sensitivity of PCR to confirm BU, and to avoid under-treatment of BU if relying on laboratory confirmation, the WHO recommends national BU programs to initiate treatment with BU-specific antibiotics even in the absence of confirmation guided by the WHO clinical and epidemiological criteria described above [4]. However, WHO does recommend to enforce the laboratory confirmation of BU, aiming for at least 70% of notified BU cases to be confirmed by a positive PCR result [12]. In the current context of a declining BU incidence observed in several endemic countries with good surveillance in place, and consequently a proportional increase of non-BU lesions being treated in BU facilities [18,19], we expect waning clinical expertise in the recognition of BU. This can result in diagnostic and therapeutic errors as has been observed for leprosy [20].
Smelling may be among the oldest diagnostic methods, as different pathologies, such as infectious and endogenous metabolic disorders, can affect human body odors [21]. A study in Cameroon found the characteristic smell of BU to be strongly associated with a confirmed BU diagnosis and described it as the smell of rotten fish, cassava or cheese, mixed with that of pyocyanic bacteria [22]. In our clinical experience, this characteristic smell draws our attention to a BU diagnostic, even in atypical presentations.
We recently reported on the accuracy of the clinical and microbiological diagnosis of BU and found that clinicians recognized BU with a sensitivity of 92% (95%CI 85%-96%) which was higher than the sensitivity of any of the laboratory tests. However, 14% (95%CI 7%-24%) of patients not suspected to have BU at diagnosis were classified as BU by a clinical expert panel [17]. We have therefore further investigated the WHO clinical and epidemiological criteria of the cohort of patients with lesions compatible with BU from our previous study and explored how these could be improved.
Methods
Ethical statements
The study was approved by the Provisional National Committee for Ethics in Health Research of Benin (registration n°: IRB 00006860), the Institutional Review Board of the ITM (code: 11 25 4 778) and the Committee for Medical Ethics of the Antwerp University Hospital (registration n°: B300201213080). The study also received an administrative authorization of the Benin Ministry of Health Ethics Board (N°IORG 0005695). All patients included in the study provided informed written consent. Parents or guardians provided consent on behalf of their children if participants were under the age of 18.
Study type, population, location, and sampling
This is an analytical prospective study of 224 consecutive patients presenting with skin and soft tissue lesions that could be compatible with BU (nodules, plaques, edemas, ulcers or osteomyelitis, including those recognized as unlikely BU by experienced clinicians), living in a BU endemic region, who were recruited in the “Centre de Dépistage et de Traitement de l’Ulcère de Buruli” (CDTUB) of Allada and Lalo in southern Benin between March 2012 and March 2015. Traumatic lesions of less than two weeks duration and relapses of BU were excluded from the study. Depending on whether the clinical and epidemiological characteristics of BU were met, the lesions were diagnosed clinically as BU or non-BU by experienced clinicians who had been trained on the WHO BU diagnostic criteria. Two swabs were taken from ulcers, or two fine-needle aspirates from closed lesions for the microbiological confirmation by IS2404 PCR and DSE after auramine staining in the Mycobacteriology Reference Laboratory in Cotonou (Benin), with quality control for molecular analyses performed by the Institute of Tropical Medicine in Antwerp (Belgium).
Variables, data collection and statistical methods
The epidemiological, clinical and microbiological results of the patients were collected using standard WHO forms and entered in a Microsoft Access database by dedicated staff. We collected data related to the WHO diagnostic criteria (age, type and location of the lesion, pain, fever, adenopathy) and additional clinical and epidemiological information (size of lesion, WHO category, gender, and functional limitation). These results were documented for each patient by a team of two clinicians and three nurses, experienced in the diagnosis, treatment and management of BU. The presence of a characteristic smell was discussed systematically between clinicians at the time of sampling, before treatment initiation. The clinical team was not aware of laboratory information (not available yet at the time of clinical examination) but was unblinded to clinical and epidemiological information.
The statistical analysis was done using Epi Info 7.2.2.6 (Database and statistics software for public health professionals, Centers for Disease Control and Prevention (CDC), Atlanta, USA) and STATA/SE 11.0. As a reference standard for BU confirmation we used IS2404 PCR. We performed univariate, bivariate and multivariate analysis using logistic regression with PCR result as dependent variable in order to establish a predictive model for BU. We also tested for interaction and confounding among variables associated with the PCR result using stratified analysis in bivariate analysis. All variables associated with a positive PCR result in the univariate analysis with a p-value <0.10 were considered in the multivariate model. We used a backward elimination procedure, probability for removal was set at <0.05. Odds ratios (OR) and their 95% confidence intervals (95%CI) were used as a measure of strength of the associations with a positive PCR result. We studied two predictive models of a positive PCR result and estimated the discriminative ability of both predictive models using the ROC analysis.
Results
Among the 224 participants included in this study, 120 (53.6%) were male and 108 (48.2%) were ≤15 years old. Median age was 18 years (IQR: 9–42 years). A total of 201 (89.7%) patients had ulcerated lesions and 201 (89.7%) had lesions on their limbs. A clinical BU diagnosis was made in 134 (59.8%) patients. PCR was positive for 98 (43.7%) participants among whom 9 participants had been clinically diagnosed as non-BU (10.0% of patients diagnosed as clinically non-BU). DSE was positive for AFB for 37 participants (16.6%) (Table 1).
Table 1. Clinical characteristics and their association with positive PCR results.
| Variables | Total (%) n = 224 | PCR+ (%) n = 98 | PCR- (%) n = 126 | OR (95%CI) | p-value |
|---|---|---|---|---|---|
| WHO clinical and epidemiological characteristics | |||||
| Age ≤ 15 years | 108 (48.2) | 70 (71.4) | 38 (30.2) | 5.8 (3.2–10.3) | < 0.001 |
| Located on limbs | 201 (89.7) | 87 (88.8) | 114 (90.5) | 0.8 (0.3–2.0) | 0.678 |
| Located on lower limbs | 146 (65.2) | 54 (55.1) | 92 (73.0) | 0.4 (0.3–0.8) | 0.006 |
| Located on upper limbs | 55 (24.5) | 33 (33.7) | 22 (17.5) | 2.4 (1.3–4.5) | 0.006 |
| Absence of pain | 134 (59.8) | 84 (85.7) | 50 (39.7) | 9.1 (4.7–17.8) | < 0.001 |
| Absence of fever | 212 (94.6) | 96 (98.0) | 116 (92.1) | 4.1 (0.9–19.3) | 0.071 |
| Absence of satellite adenopathy | 223 (99.5) | 98 (100.0) | 125 (99.2) | NA | NA |
| Ulcerated lesion | 201 (89.7) | 87 (88.8) | 114 (90.5) | 0.8 (0.3–2.0) | 0.678 |
| Necrotic base (n = 199)a | 165 (82.9) | 82 (95.3) | 83 (73.4) | 7.4 (2.5–22.0) | < 0.001 |
| Undermined edge (n = 194)a | 143 (73.7) | 75 (89.3) | 68 (61.8) | 5.1 (2.3–11.3) | < 0.001 |
| Additional clinical and epidemiological characteristics | |||||
| Characteristic smell (n = 190)a | 107 (56.3) | 74 (88.1) | 33 (31.1) | 16.4 (7.5–35.6) | < 0.001 |
| WHO category of lesion (n = 223)a | |||||
| WHO Category 3 (diameter > 15cm) | 81 (36.3) | 27 (27.5) | 54 (43.2) | 1 | - |
| WHO Category 2 (5 < diameter ≤ 15cm) | 111 (49.8) | 60 (61.2) | 51 (40.8) | 2.35 (1.30–4.26) | 0.005 |
| WHO Category 1 (diameter ≤ 5cm) | 31 (13.9) | 11 (11.2) | 20 (16.0) | 1.1 (0.46–2.62) | 0.830 |
| Male sex | 120 (53.6) | 51 (52.0) | 69 (54.7) | 0.9 (0.5–1.5) | 0.685 |
| Functional limitations | 96 (42.9) | 41 (41.8) | 55 (43.6) | 0.9 (0.5–1.6) | 0.786 |
| Diagnostic test | |||||
| Initial clinical BU diagnosis | 134 (59.8) | 89 (90.8) | 45 (35.7) | 17.8 (8.2–38.7) | < 0.001 |
| Positive direct smear examination (n = 223)a | 37 (16.6) | 37 (38.1) | 0 (0.0) | NA | NA |
aThe number of patients with available data varies because of missing data
On bivariate analysis we confirmed that most of the WHO clinical and epidemiological criteria of BU were indeed associated with a positive PCR result (Table 1). Age ≤ 15 years was significantly associated with a positive PCR result with an OR of 5.8 (95%CI: 3.2–10.3). Painless lesions were also significantly associated with a positive PCR result with an OR of 9.1 (95%CI: 4.7–17.8). For factors related to ulcerated lesions such as “necrotic base” and “undermined edge”, the associations were also strong and statistically significant with an OR of respectively 7.4 (95%CI: 2.5–22.0) and 5.1 (95%CI: 2.3–11.3). There was no statistically significant association with the localization of the lesions on the upper or lower limbs (OR: 0.8, 95%CI: 0.3–2.0) while a localization on the lower limbs was negatively associated with a PCR confirmed BU (OR: 0.4, 95%CI: 0.3–0.8) and a localization on the upper limbs was positively associated with a PCR confirmed BU (OR: 2.4, 95%IC: 1.3–4.5). There was no association with “absence of satellite adenopathy” and “absence of fever”.
Among the additional characteristics studied, the characteristic smell of ulcerated BU lesions was strongly associated with a positive PCR result with an OR of 16.4 (95%CI: 7.5–35.6), as was the initial clinical BU diagnosis made by the clinicians (OR: 17.8, 95%CI: 8.2–38.7), essentially a synthesis of the WHO criteria and clinical experience. A lesion size between 5–15 cm (WHO category 2) had a significant positive association with a positive PCR result with an OR of 2.35 (95%IC: 1.30–4.26). Gender and functional limitation were not associated with a positive PCR result (Table 1). There was no effect modification among the biologically plausible interactions we tested for (characteristic smell/necrotic base and characteristic smell/WHO category 3).
In a multivariate predictive logistic regression model exploring the associations between the WHO criteria and a positive PCR result, only “age ≤ 15years”, “absence of pain” and “necrotic base of ulcerative lesion” were retained with ORs of respectively 3.3 (95%IC: 1.6–6.7), 5.9 (95%IC: 2.7–12.8) and 3.7 (95%IC: 1.1–12.2). In a second multivariate predictive model, exploring all variables associated with a positive PCR result at a p <0.10 on univariate analysis, only the clinical criteria “characteristic smell”, “necrotic base” and “WHO category 2” were retained (Table 2).
Table 2. Predictive model of positive PCR result based on WHO criteria (model A) and predictive model of positive PCR result based on WHO criteria and additional characteristic (model B).
| Variables | OR | 95% C.I. | p-value |
|---|---|---|---|
| Model A (included variables: age, absence of pain, absence of fever, localization, necrotic base, undermined edge) | |||
| Absence of pain | 6.3 | 2.8–14.2 | < 0.001 |
| Necrotic base | 3.9 | 1.2–12.8 | 0.026 |
| Age ≤ 15 years | 2.7 | 1.3–5.7 | 0.007 |
| Model B (included variables: age, absence of pain, absence of fever, localization, necrotic base, undermined edge, characteristic smell, WHO category). | |||
| Characteristic smell | 15.7 | 6.6–37.4 | < 0.001 |
| Necrotic base | 3.8 | 1.0–14.2 | 0.046 |
| WHO Category | |||
| WHO Category 3 | 1 | - | - |
| WHO Category 2 | 2.5 | 1.1–5.7 | 0.032 |
| WHO Category 1 | 0.4 | 0.1–1.4 | 0.153 |
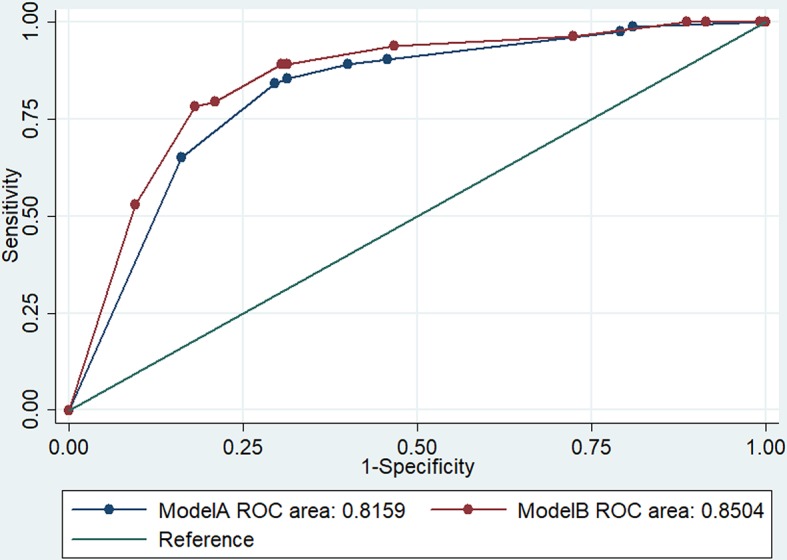
To estimate the discriminative ability of both predictive models A and B, we estimated the areas under their ROC curves and found that model A (AUC: 0.82, 95%CI: 0.76–0.88) discriminated equally well as model B (AUC: 0.85, 95%CI: 0.79–0.91) between BU and non-BU patients (p = 0.1940) (Fig 1).
Fig 1. ROC analysis for both model A and model B.
Discussion
Our study results confirm the validity of the WHO epidemiological and clinical criteria to guide the diagnostic process for BU. However, we found that the localization of lesions on the lower limbs and WHO category 3 lesions are inversely associated with a positive PCR result. In addition, the characteristic smell of ulcerated lesions recognized by experienced clinicians was an even stronger predictor. We also showed that even experienced clinicians can miss the diagnosis of BU in PCR confirmed BU patients.
Most of the epidemiological and clinical criteria for a BU diagnosis defined by WHO [4] were associated with a positive PCR result with varying OR’s aligning with the clinical and epidemiological description of BU patients in literature [2,3,12,22,23]. The localization of lesions on the limbs was not associated with a positive PCR result and was therefore not predictive of BU. As also mentioned by the WHO [4], lesions on the lower limbs are more prone to being PCR negative than those on the upper limbs or other parts of the body and we indeed found them to be negatively associated with a PCR confirmation. Kibadi et al. [15] made a similar observation in a study including clinically suspected BU patients with large ulcers. Among their 25 PCR negative patients, 23 had lesions on the lower limbs. It is thus likely that these PCR negative patients did not have BU. Lesions on the lower limbs have a broader range of etiologies with proportionally less BU [4,24]. The clinical suspicion for BU can moreover be broadened since 10.0% of patients clinically diagnosed as non-BU in our study turned out to have BU by PCR, as we reported before [17].
We found the characteristic smell of ulcerated BU lesions to be strongly associated with a PCR confirmation, suggesting that it is specific for BU. This was also reported by Mueller et al. in a BU treatment centre in Cameroon [22]. In our clinical experience, this characteristic smell, distinct from the smell of “putrid” wounds, draws our attention to a BU diagnosis even in atypical presentations. Moreover, this characteristic odor can be sensed during surgical excisions of non-ulcerated BU lesions, particularly on edematous lesions. According to Mueller et al. the characteristic BU smell was described by clinicians as strong, like the smell of rotten fish, cassava or cheese, mixed with that of pyocyanic bacteria [22]. Ribera et al. described this smell as “unpleasant” and stated that it was recognized also by the BU patient's family and is a stigmatizing factor [25]. In a short survey we held among 15 health care workers from the CDTUBs of Allada and Lalo, they all recognized that ulcerative lesions of BU have a characteristic smell and that this smell differs from the smell of other chronic wounds. However, the description varied between disagreeable, nauseating, strongly penetrating and rotten. Specific smells have been described in other pathologies [26–28] and, if reproducible and validated by experienced clinicians, the BU specific volatiles could be an important tool to train health care workers, especially in the current context of a decreasing BU incidence [29]. Capturing such specific volatiles could allow the development of a Point-of-Care diagnostic test and thus improve BU diagnosis in the field [30,31].
In a preliminary study, we analyzed the organic volatiles in used gauzes of bandages of 13 presumptive BU and 17 non-BU patients using a hand-held chemical vapor sensing instrument which predicted the clinical BU diagnosis in 66.7% of samples [32]. For tuberculosis, the recognition of specific volatiles from sputa by trained Gambian pouched rats has been shown to be a helpful diagnostic test [26], although we are not aware of publications on clinicians distinguishing tuberculosis patients from those with other pulmonary diseases based on smell. In order to test whether smell could be used as a diagnostic tool, M. ulcerans volatiles should be characterized in vitro, in parallel with characterization of air sampled in operation theaters, with appropriate controls. This would allow the development of an «electronic-nose» that could be useful as a non-invasive diagnostic tool that can be easily used during active case-finding activities, as for other diseases [33–35]. In carcinoma diagnosis for example, an electronic-nose was used to differentiate head and neck carcinoma from lung carcinoma [36] and also to discriminate head and neck squamous carcinoma from colon and bladder carcinoma [37]. An electronic-nose allowed the detection of cannabis use on the human skin surface [38]. A non-invasive test based on smell will only be applicable to ulcerated lesions, although for the differential diagnosis of ulcerated forms which make up the majority of BU compatible lesions, a smell-based Point-of-Care test could contribute to the discrimination between BU and non-BU lesions. Early non-ulcerated lesions are expected to become more common with increasing BU control efforts and will not be testable by an electronic nose unless after validation on fine-needle aspirations.
Apart from the characteristic smell, lesions with diameter between 5–15 cm (WHO category 2) were more likely PCR positive while lesions with diameter > 15 cm were inversely associated with a positive PCR. This might result from the impact of BU control strategies focusing on early detection of BU lesions [39] by involving community volunteers in active case-finding [40,41], as BU lesions can be quite extensive when detected late. Another explanation could be that in large ulcers the bacterial load reduces due to the natural course of spontaneous healing of BU resulting in lower PCR confirmation rates.
Limitations of this study include the fact that our reference standard of BU diagnosis, PCR, is not perfect although it is currently the best available test [12,17,42,43]. The proportion of confirmed BU in our population of suspected BU patients may have been higher than what would be seen among patients suspected of having BU in routine clinical practice in an endemic area since the prevalence of BU is probably higher in the referral centers of our study. Moreover, the clinicians of this study are probably more experienced in recognizing BU clinically. This could lead to overestimating the discriminative value of the predictive model. Strictly speaking, our model is thus predictive of PCR positive BU rather than of a BU diagnosis. With regards to specificity, quality controls on the molecular laboratory performing the IS2404 PCR consistently yielded excellent results. Our team of clinicians and nurses evaluating the patients was not blinded to clinical and epidemiological information when identifying the characteristic smell, possibly introducing a bias in their appreciation of the BU specific volatiles. However, blinding the clinicians to features of the lesion that in clinical practice are observed anyway, would underestimate the true accuracy of smell, resulting in test review bias [44]. The estimates of smell accuracy in such an experimental setting would not mimic its performance in a clinical setting. Also, the inter-observer variability in identifying the characteristic BU smell cannot be analyzed in this dataset.
Conclusion
The current BU diagnostic criteria can benefit from revision by differentiating between lesions on the upper and lower limbs and by including lesion size and the characteristic smell recognized by experienced clinicians. Although the characteristic BU smell, strongly associated with a positive PCR result, will be difficult to describe in guidelines that need to be understandable to non-experienced clinicians. Further studies need to clarify if M. ulcerans indeed releases specific volatiles that can serve for the development of Point-of-Care diagnostic tests useful for non-invasive confirmation during active case-finding activities. Reproduced BU volatiles, if safe, may moreover serve for training purposes. Both could be important tools for health care workers, especially in the present context of decreasing BU incidence.
Supporting information
(XLSX)
(DOC)
Acknowledgments
We are grateful to the participants of this study, to the staff of CDTUB’s of Allada and Lalo, and of the Institute of Tropical Medicine in Antwerp. The authors acknowledge M. Alphonse Biaou Chabi for his contribution to the data analysis.
Data Availability
All necessary data are uploaded with this manuscript as supplementary files.
Funding Statement
This work was supported by the Medicor Foundation (www.medicor.li) and UBS Optimus Foundation (www.ubs.com/microsites/optimus-foundation) through the BU differential diagnosis project in Benin. Fondation Follereau Luxembourg (www.ffl.lu/) gives core support to the CDTUB in Allada. M. E was supported by the Department of Economy, Science and Innovation (www.ewi-vlaanderen.be/) of the Flemish government (Belgium). B. d. J. was supported by the European Research Council-INTERRUPTB (starting grant number 311725). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Buruli ulcer. In: Investing to overcome the global impact of neglected tropical diseases: Third WHO report on neglected tropical diseases. Geneva, Switzerland; 2015. p. 70–4. Available from: WHO/HTM/NTD/2015.1
- 2.Portaels F, Silva MT, Meyers WM. Buruli ulcer. Clin Dermatol. 2009;27(3):291–305. 10.1016/j.clindermatol.2008.09.021 [DOI] [PubMed] [Google Scholar]
- 3.van der Werf TS, Stienstra Y, Johnson RC, Phillips R, Adjei O, Fleischer B, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83(10):785–91. Ref. No. 04–020099 [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Treatment of Mycobacterium Ulcerans Disease (Buruli Ulcer). Geneva World Heal Organ. 2012;33. WHO/HTM/NTD/IDM/2012.1 [Google Scholar]
- 5.World Health Organization. Distribution of Buruli ulcer, worldwide 2016 [Internet]. 2017 [cited 2018 Mar 13]. Available from: http://gamapserver.who.int/mapLibrary/Files/Maps/Buruli_2016.png
- 6.Debacker M, Portaels F, Aguiar J, Steunou C, Zinsou C, Meyers W, et al. Risk factors for Buruli ulcer, Benin. Emerg Infect Dis. 2006;12(9):1325–31. 10.3201/eid1209.050598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent QB, Ardant MF, Adeye A, Goundote A, Saint-André JP, Cottin J, et al. Clinical epidemiology of laboratory-confirmed Buruli ulcer in Benin: a cohort study. Lancet Glob Heal. 2014;2:e422–30. 10.1016/S2214-109X(14)70223-2 [DOI] [PubMed] [Google Scholar]
- 8.Capela C, Sopoh GE, Houezo JG, Fiodessihoué R, Dossou AD, Costa P, et al. Clinical epidemiology of Buruli ulcer from Benin (2005–2013): Effect of time-delay to diagnosis on clinical forms and severe phenotypes. PLoS Negl Trop Dis. 2015;9(9):e4005 10.1371/journal.pntd.0004005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sica A, Kaba L, Toho E, Kobenan A. Ulcère de Buruli: résultats du traitement chirurgical à l’Institut Raoul Follereau D’Adzopé en Côte - d’Ivoire. Res fr. 2015;2:1299 10.13070/rs.fr.2.1299 [DOI] [Google Scholar]
- 10.Bratschi MW, Bolz M, Minyem JC, Grize L, Wantong FG, Kerber S, et al. Geographic distribution, age pattern and Sites of Lesions in a cohort of Buruli ulcer patients from the Mapé Basin of Cameroon. PLoS Negl Trop Dis. 2013;7(6):e2252 10.1371/journal.pntd.0002252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bretzel G, Huber KL, Kobara B, Beissner M, Piten E, Herbinger K-H, et al. Laboratory confirmation of Buruli ulcer disease in Togo, 2007–2010. PLoS Negl Trop Dis. 2011;5(7):e1228 10.1371/journal.pntd.0001228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Laboratory diagnosis of Buruli ulcer disease: A manual for health care providers [Internet]. Portaels F, editor. WHO Library; Geneva, Switzerland; 2014. 105p. WHO/HTM/NTD/IDM/2014.1 [Google Scholar]
- 13.Herbinger K, Adjei O, Nienhuis WA, Kunaa L, Siegmund V, Thompson W, et al. Comparative study of the sensitivity of different diagnostic methods for the laboratory diagnosis of Buruli ulcer disease. Clin Infect Dis. 2009;48:1055–64. 10.1086/597398 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Buruli ulcer: progress report, 2004–2008. Wkly Epidemiol Rec. 2008;83(17):145–54 [PubMed] [Google Scholar]
- 15.Kibadi K, Boelaert M, Fraga AG, Kayinua M, Longatto-Filho A, Minuku J-B, et al. Response to treatment in a prospective cohort of patients with large ulcerated lesions suspected to be Buruli Ulcer (Mycobacterium ulcerans disease). PLoS Negl Trop Dis. 2010. January;4(7):e736 10.1371/journal.pntd.0000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegmund V, Adjei O, Nitschke J, Thompson W, Klutse E, Herbinger KH, et al. Dry reagent-based polymerase chain reaction compared with other laboratory methods available for the diagnosis of Buruli ulcer disease. Clin Infect Dis. 2007;45:68–75. 10.1086/518604 [DOI] [PubMed] [Google Scholar]
- 17.Eddyani M, Sopoh GE, Ayelo G, Brun LVC, Roux J, Barogui Y, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;ciy197 10.1093/cid/ciy197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sopoh G, Barogui YT, Houezo JG, Wadagni A, Ayelo G, Dossou A, et al. Noncommunicable or Negleted tropical diseases with cutaneous manifestation: Epidemiology, diagnosis and integrated management of chronic ulcers often misdiagnosed as Buruli ulcer. In: WHO meeting on Buruli ulcer. 23–25 march 2015. WHO Headquarters, Geneva, Switzerland; 2015
- 19.World Health Organization. Summary report of the Buruli ulcer control group. In: WHO meeting on Buruli ulcer: lutte and research. 2015
- 20.Portaels F, de Jong B. Nouveaux défis dans la lutte contre la lèpre. Bull des séances Académie R d’Outre-Mer. 2014;60(3):sous presse [Google Scholar]
- 21.Shirasu M, Touhara K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem 2011;150:257–266. 10.1093/jb/mvr090 [DOI] [PubMed] [Google Scholar]
- 22.Mueller YK, Bastard M, Nkemenang P, Comte E, Ehounou G, Eyangoh S, et al. The “Buruli Score”: Development of a Multivariable Prediction Model for Diagnosis of Mycobacterium ulcerans Infection in Individuals with Ulcerative Skin Lesions, Akonolinga, Cameroon. PLoS Negl Trop Dis. 2016;10(4):e0004593 10.1371/journal.pntd.0004593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marion E, Carolan K, Adeye A, Kempf M, Chauty A, Marsollier L. Buruli Ulcer in South Western Nigeria: A Retrospective Cohort Study of Patients Treated in Benin. PLoS Negl Trop Dis. 2015;9(1):e3443 10.1371/journal.pntd.0003443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toutous Trellu L, Nkemenang P, Comte E, Ehounou G, Atangana P, Mboua DJ, et al. Differential Diagnosis of Skin Ulcers in a Mycobacterium ulcerans Endemic Area: Data from a Prospective Study in Cameroon. PLoS Negl Trop Dis. 2016;10(4):e0004385 10.1371/journal.pntd.0004385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribera JM, Peeters Grietens K, Toomer E, Hausmann-Muela S. A word of caution against the stigma trend in neglected tropical disease research and control. PLoS Negl Trop Dis. 2009;3(10):e445 10.1371/journal.pntd.0000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weetjens BJC, Mgode GF, Machang’u RS, Kazwala R, Mfinanga G, Lwilla F, et al. African pouched rats for the detection of pulmonary tuberculosis in sputum samples. Int J Tuberc Lung Dis. 2009;13(6):737–43 [PubMed] [Google Scholar]
- 27.Schroeder W. Volatile S-nitrosothiols and the typical smell of cancer. J Breath Res [Internet]. 2015;9(1):16010 10.1088/1752-7155/9/1/016010 [DOI] [PubMed] [Google Scholar]
- 28.Mcnerney R, Mallard K, Okolo PI, Turner C. Production of volatile organic compounds by mycobacteria. FEMS Microbiol Lett. 2012;328:150–6. 10.1111/j.1574-6968.2011.02493.x [DOI] [PubMed] [Google Scholar]
- 29.World Health Organisation. Number of new reported cases: Data by country [Internet]. WHO. World Health Organization; 2017. [cited 2018 Feb 8]. Available from: http://apps.who.int/gho/data/view.main.95000 [Google Scholar]
- 30.World Health Organization. Report of a WHO–FIND consultative meeting on diagnostics for Buruli ulcer. Geneva, Switzerland; 2013. WHO/HTM/NTD/IDM/2014.2
- 31.O’Brien DP, Comte E, Serafini M, Ehounou G, Antierens A, Vuagnat H, et al. The urgent need for clinical, diagnostic, and operational research for management of Buruli ulcer in Africa. Lancet Infect Dis. 2014;14:435–40. 10.1016/S1473-3099(13)70201-9 [DOI] [PubMed] [Google Scholar]
- 32.Chudy S, de Jong BC, Sopoh GE. Pilot Study on E-nose Detection of Buruli Ulcer. In: WHO Meeting on Buruli Ulcer control and Research, March 2017. WHO Headquarters. Geneva, Switzerland; 2017
- 33.Kolk AHJ, van Berkel JJBN, Claassens MM, Walters E, Kuijper S, Dallinga JW, et al. Electronic-nose technology using sputum samples in diagnosis of patients with tuberculosis. Int J Tuberc lung Dis. 2010;16(6):777–82. 10.5588/ijtld.11.0576 [DOI] [PubMed] [Google Scholar]
- 34.Fend R, Kolk AHJ, Bessant C, Buijtels P, Klatser PR, Woodman AC. Prospects for Clinical Application of Electronic-Nose Technology to Early Detection of Mycobacterium tuberculosis in Culture and Sputum. J Clin Microbiol. 2006;44(6):2039–45. 10.1128/JCM.01591-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dymerski T, Gębicki J, Wardencki W, Namieśnik J. Application of an Electronic Nose Instrument to Fast Classification of Polish Honey Types. Sensors. 2014;14(6):10709–24. 10.3390/s140610709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Hooren MRA, Leunis N, Brandsma DS, Dingemans AMC, Kremer B, Kross KW. Differentiating Head and Neck carcinoma from Lung Carcinoma with an Electronic Nose: A Proof of Cpncept Study. Eur Arch Otorhinolaryngol. 2016;273:3897–3903. 10.1007/s00405-016-4038-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Goor RMGE, Leunis N, van Hooren MRA, Francisca E, Masclee A, Kremer B and al. Feasibility of Electronic Nose Technology for Discriminating between Head and Neck, Bladder and Colon carcinomas. Eur Arch Otorhinolaryngol. 2017;274:1053–60. 10.1007/s00405-016-4320-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voss A, Witt K, Kaschowitz T, Poitz W, Ebert A, Patrik R and al. Detecting Cannabis Use on the Human Skin Surface via an Electronic Nose System. Sensors 2014;14:13256–72. 10.3390/s140713256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Programme National de Lutte contre la Lèpre et l’Ulcère de Buruli. Plan stratégique de lutte contre l’ulcère de buruli 2012–2016. Cotonou, Bénin; 2012
- 40.Barogui YT, Sopoh GE, Johnson RC, de Zeeuw J, Dossou AD, Houezo JG, et al. Contribution of the community health volunteers in the control of Buruli ulcer in Benin. PLoS Negl Trop Dis. 2014;8(10):e3200 10.1371/journal.pntd.0003200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vouking MZ, Takougang I, Mbam LM, Mbuagbaw L, Tadenfok CN, Tamo CV. The contribution of community health workers to the control of Buruli ulcer in the Ngoantet area, Cameroon. Pan Afr Med J. 2013;16:1–5. 10.11604/pamj.2013.16.1.3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips RO, Sarfo FS, Osei-Sarpong F, Boateng A, Tetteh I, Lartey A, et al. Sensitivity of PCR targeting Mycobacterium ulcerans by use of Fine-needle aspirates for diagnosis of Buruli ulcer. J Clin Microbiol. 2009;47(4):924–6. 10.1128/JCM.01842-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeboah-Manu D, Asante-Poku A, Asan-Ampah K, Ampadu EDE, Pluschke G. Combining PCR with microscopy to reduce costs of laboratory diagnosis of Buruli ulcer. Am J Trop Med Hyg. 2011;85(5):900–4. 10.4269/ajtmh.2011.11-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moons KGM, Grobbee DE. When should we remain blind and when should our eyes remain open in diagnostic studies? J Clin Epidemiol. 2002;55(7):633–6. 10.1016/S0895-4356(02)00408-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOC)
Data Availability Statement
All necessary data are uploaded with this manuscript as supplementary files.


