Abstract
Post-traumatic stress disorder (PTSD) is a devastating disorder with symptoms such as flashbacks, hyperarousal, and avoidance of reminders of the traumatic event. Exposure therapy, which attempts to extinguish fear responses, is a commonly used treatment for PTSD but relapse following successful exposure therapy is a frequent problem. In rodents, spontaneous recovery (SR), where extinguished fear responses resurface following extinction treatment, is used as a model of fear relapse. Previous studies from our lab showed that chronic nicotine impaired fear extinction and acute nicotine enhanced SR of contextual fear in adult male mice. In addition, we showed that acute nicotine’s effects were specific to SR as acute nicotine did not affect recall of contextual fear conditioning in the absence of extinction. However, effects of chronic nicotine administration on SR are not known. Therefore, in the present study, we investigated if chronic nicotine administration altered SR or recall of contextual fear in adult male and female C57BL/6J mice. Our results showed that chronic nicotine significantly enhanced SR in female mice and significantly decreased SR in males. Chronic nicotine had no effect on recall of contextual fear in males or females. Female sham mice also had significantly less baseline SR than male sham mice. Overall, these results demonstrate sex differences in SR of fear memories and that chronic nicotine modulates these effects on SR but nicotine does not alter recall of contextual fear.
Keywords: Nicotine, Fear learning, Sex Difference, Spontaneous Recovery, Recall, PTSD
1.1 Introduction
Post-traumatic stress disorder (PTSD) is precipitated by a traumatic event, after which the person is unable to properly extinguish negative emotional responses associated with cues and contexts related to that event. Exposure therapy is a common treatment for PTSD that attempts to extinguish maladaptive fear responses by repeatedly presenting the patient with trauma-associated cues and contexts in a safe environment. While exposure therapy is an effective form of treatment, relapse commonly occurs. Anxiety disorders have a 19–62% rate of relapse of fear symptoms following successful treatment with the type of disorder and the different metrics used to define “relapse” influencing the rate (Vervliet et al., 2013). Relapse seems to occur because of a failure of extinction memories to suppress the original fear memory. Certain populations may be more vulnerable to PTSD relapse. For example, individuals with PTSD are more likely to smoke than the healthy population; 45.3% of PTSD patients smoke compared to 22.5% of the healthy population (Ziedonis et al., 2008), and daily smoking rates increased with reported severity of symptoms (Thorndike et al., 2006). Individuals may initially use nicotine as a form of self-medication for its anxiolytic effects but with a transition to chronic use, adaptations may occur resulting in a loss of anxiolytic effects and a worsening of symptoms.
PTSD patients show several fear-related symptoms such as hypervigilance, re-experiencing trauma-related memories and avoidance of trauma-associated cues and contexts, which elicit behavioral symptoms such as exaggerated startle response (Forbes et al., 2010). Some aspects of these behavioral outcomes may be captured in rodent fear response (e.g., freezing) exhibited in the presence of a cue or context associated with fearful stimulus (e.g., a mild footshock). Utilizing fear conditioning models, previous studies investigated the effects of nicotine exposure on acquisition, extinction, and recovery of fear memories. These studies showed that acute nicotine enhanced contextual fear conditioning, indicating that nicotine may be a modulatory factor in the development of PTSD (Gould & Higgins, 2003). Extinction treatment is also affected by nicotine in rodents. Studies from our lab have shown that adult male mice given acute (0.18 mg/kg) and chronic (12.6 mg/kg) nicotine have deficits in contextual fear extinction (Kutlu & Gould, 2014; Kutlu et al., 2016a). We also ran a study looking at acute nicotine’s effect on contextual SR in male mice and found that nicotine significantly enhanced SR. Using c-fos immunohistochemistry, we showed that mice that had received nicotine and undergone contextual extinction had altered levels of recent neuronal activity in the infralimbic cortex, ventral hippocampus, and basolateral amygdala, regions of the brain implicated in extinction learning and memory retrieval, relative to saline and homecage controls while nicotine administration alone had no effect (Kutlu et al., 2016b). This indicates that acute and chronic nicotine use could lead to changes in extinction and spontaneous recovery, which could cause relapse.
However, the effects of chronic nicotine on SR and the effects of nicotine on SR in females are not known. While acute nicotine is a model of smoking initiation, chronic nicotine is more analogous to long-term nicotine use in humans. The current study investigated the effects of chronic nicotine on SR in male and female mice.
1.2 Materials and Methods
1.2.1 Subjects
Subjects were naïve adult (8–10 week) male and female C57BL/6J mice. Mice were group-housed with access to food and water ad-libitum. Procedures used in this study were approved by the Temple University Institutional Animal Care and Use Committee.
1.2.2 Apparatus
Behavioral experiments took place in four identical chambers (18.8 × 20 × 18.3 cm) located in sound attenuating boxes. Ventilation fans produced the background noise (65 dB) and the white noise (85 dB) conditioned stimulus (CS) was delivered by a speaker. The chambers were composed of Plexiglas and the chamber floors were metal grids (0.20 cm in diameter and 1.0 cm apart) connected to a shock generator, which delivered a 2-sec, 0.57-mA foot shock unconditioned stimulus (US).
1.2.3 Behavioral Procedures
Freezing, defined as lack of all movement except respiration, was the dependent variable. Mice were scored using a time-sampling method in which they were observed every 10 seconds for a duration of 1 second. Experimenters were blind to group assignments. Following our previous studies showing nicotine-induced impaired contextual fear extinction (Kutlu & Gould, 2014; Kutlu et al., 2016a), in both Experiment 1 and 2, mice were trained in background contextual fear conditioning, in which they were placed in the conditioning chambers and baseline freezing was measured for 120 seconds. Mice then received two white noise–foot shock pairings where the white noise co-terminated with the 2-sec foot shock with a 120-second interval between pairings. All mice remained in the chamber for another 30 seconds following the final CS-US pairing. The next day, all mice were placed back in the training conditioning chamber for 5 minutes to score freezing to the context in the absence of the foot shock. For the five following days, mice underwent contextual extinction trials within the training chambers in which their freezing was again scored in the absence of foot shocks. The day after extinction, the experimental group underwent osmotic mini-pump surgeries with a 12.6 mg/kg dose of nicotine while the control group received sham surgeries. On the eighth day following the surgeries, the mice were tested for SR by being placed back into the same chambers and scoring their freezing levels. In Experiment 2, separate groups of mice underwent the same procedure except they were not given the five extinction sessions.
1.2.4 Osmotic mini-pump surgeries
Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) dissolved in saline was administered subcutaneously through osmotic mini-pumps (Alzet, Model 1002, Durect, Cupertino, CA). Mini-pumps were surgically inserted through an incision on the lower back of the mouse while mice were under 3% isoflurane anesthetic. Surgeries were performed under sterile conditions. Mice were either implanted with subcutaneous mini-pumps delivering a nicotine solution (12.6 mg/kg/day) or received sham surgeries the day after the final extinction session, 7 days prior to SR testing. The nicotine doses are reported as freebase weights and were chosen based on the previous research form our laboratories showing deficits in contextual fear learning during extinction testing with this dose (Kutlu et al., 2016a).
1.2.5 Statistical Analysis
In Experiment 1 and 2, freezing behavior was converted to percent freezing and SR was expressed as percent rebound compared to freezing levels measured during testing (%Rebound; average freezing during retesting × 100/average freezing during initial testing; Santini, 2001). To examine main effect, one-way ANOVAs were employed and two-way ANOVAs were used to examine Drug × Sex interaction.
1.3 Results
1.3.1 Experiment 1
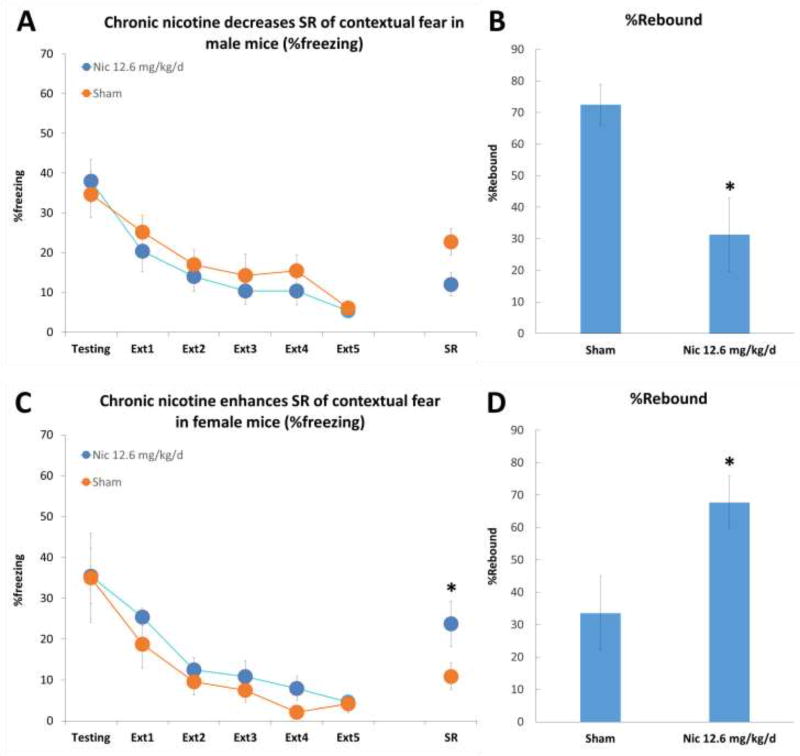
The results showed that chronic nicotine enhances SR of contextual fear in female mice, decreases SR in male mice, and that male mice who did not receive nicotine had significantly increased SR compared to females control subjects. A two-way ANOVA showed a Drug × Sex interaction (F(1, 33) = 7.734, P < .05). One-way ANOVAs showed a significant main effect of Drug on males’ freezing levels (F(1,18) = 10.481, P < .05) and females’ (F(1, 14) = 6.812, P < .05).
1.3.2 Experiment 2
In Experiment 2, mice were trained and tested in contextual fear in the same manner as Experiment 1 but they did not undergo extinction. Mice were then either implanted with osmotic nicotine pumps or given sham surgery. Seven days later, recall was tested. The results showed that chronic nicotine had no effect on recall of fear in either sex. A two-way ANOVA showed no Drug × Sex interaction (F(1,33) = .018, p>.05). One-way ANOVAs found that male nicotine subjects had no significant difference in recall from male sham subjects (F(1, 19) = 1.234, P>.05) and that female groups did not differ (F(1, 13) = 1.289, P>.05).
1.4 Discussion
Results from our study show that chronic nicotine (12.6 mg/kg) administration reduces SR of contextual fear in adult male mice and enhances SR in adult female mice but has no effect on recall for either sex. Furthermore, female mice in the control group showed significantly less SR than male mice. For recall subjects, these results are in line with previous work from our lab showing that acute nicotine has no effect on recall of contextual fear in males (Kutlu et al., 2016b). However, the same study showed that that acute nicotine (0.18 mg/kg) enhances SR in adult males, indicating that there may be different neurobiological mechanisms for SR following chronic nicotine administration compared to acute (Kutlu et al., 2016b). It is possible that upregulation of nicotinic acetylcholine receptors (nAChRs) underlies these effects. A previous study from our lab shows that 12.6 mg/kg chronic nicotine administration for 4 days upregulated nAChRs in the dorsal and ventral hippocampus (Kutlu et al., 2016a). This could also be a factor in the behavioral differences between males and females; another study found that male rats given chronic nicotine showed upregulation of nicotinic acetylcholine receptors while female rats did not (Koylu et al., 1997). However, the samples were from the whole brain, not specific to the hippocampus or other brain regions associated with fear extinction or SR, and the rats were given repeated injections of nicotine as opposed to continuous administration through osmotic mini-pumps. Additional experiments would need to investigate the possible role of nAChR upregulation in both the sex differences and the differences between the effects of acute and chronic nicotine on SR. It is also important to note that in this initial study, we used only a dose that is effective in impairing contextual fear extinction in male mice. Therefore, it is possible that lower or higher doses of chronic nicotine may have differential effects on spontaneous recovery of contextual fear in male and female mice. Future studies will clarify this potential effect and neurobiological mechanisms that underlie sex differences in the effects of chronic nicotine on spontaneous recovery.
A possible reason for the difference in SR between male and female control groups may be related to activation of the dorsal anterior cingulate cortex (dACC). An fMRI study conducted on men and women with PTSD found that males had deficits in extinction recall whereas females did not. The males also had significantly increased activation in the dACC, which could underlie the behavioral difference between sexes (Schvil et al., 2014). Furthermore, the estrous cycle could influence females’ SR due to its effects on consolidation of extinction memories. One study showed that extinction memories may be more fully consolidated during the proestrus phase (highest estrogen/progesterone) compared to the other phases of the cycle, as they had lower levels of freezing upon SR testing. Rats injected with estrogen and progesterone during the metestrus phase (lowest estrogen/progesterone) performed similarly to mice in the proestrus phase, further showing that hormones and the estrus cycle could influence extinction consolidation (Milad, et al., 2009). However, for this initial study, we chose not to track estrous cycle in our subjects because the estrous cycle tracking procedure could introduce behavioral changes that may not be mimicked in male mice. Therefore, future studies are required to establish links between nicotine’s effects on SR and specific estrous cycle phases.
There are several clinical implications from the results of this experiment. It is possible that women using nicotine products may be at a higher risk for the re-emergence of PTSD symptoms after treatment. In nonsmokers, men may be more likely to show the re-emergence of PTSD symptoms after treatment than women, even though women have higher rates of PTSD (Kessler et al., 1995). Further clinical studies will need to be conducted to confirm if the results of this experiment translate into human subjects.
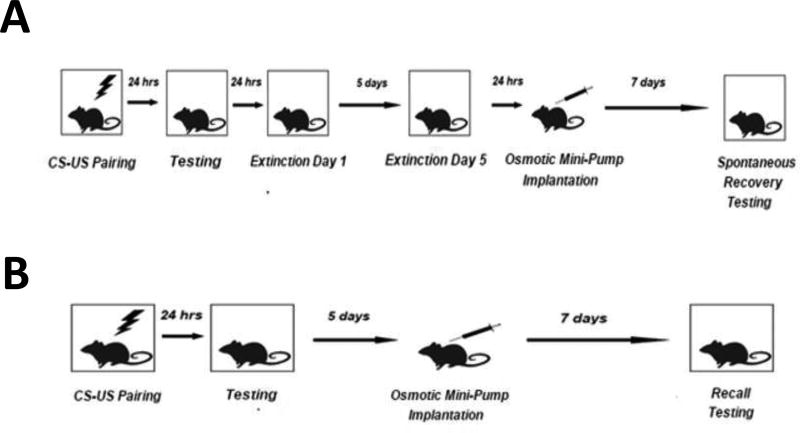
Figure 1. Schematic description of experimental design.
Panel A: SR subjects were trained and tested in contextual fear conditioning, underwent extinction training for five days, and the following day underwent either osmotic mini-pump implantation or sham surgery. After a delay of 7 days, subjects were tested for SR. Panel B: Recall subjects were trained and tested in contextual fear conditioning, had a delay of 5 days, and then underwent either osmotic mini-pump implantation or sham surgery. Seven days later, subjects were tested for recall.
Figure 2. Chronic nicotine differentially affects spontaneous recovery of contextual fear in male and female mice.
Panel A: Spontaneous recovery of contextual fear in males was significantly decreased following chronic nicotine administration (n = 10–11 per group), expressed %percent freezing. Panel B: Spontaneous recovery in males following chronic nicotine administration or sham surgery, expressed as %rebound. Panel C: Female mice had significantly increased spontaneous recovery after chronic nicotine administration (n = 8 per group), expressed as %freezing. Asterisks indicate a significant difference between groups at p < .05 and error bars represent standard error of the mean (SEM). Panel D: Female mice in the chronic nicotine group had significantly increased SR, expressed as %rebound. Asterisks indicate a significant difference between groups at p < .05 and error bars represent standard error of the mean (SEM).
Figure 3. Chronic nicotine does not affect retrieval of contextual fear memories in male and female mice.
Panel A: There was no significant difference of recall of contextual fear (F(1, 19) = 1.234, P>.05) between drug groups in male subjects (n = 10–12 per group). Panel B: Male nicotine and sham group recall as expressed by %rebound. Panel C: Female subjects showed no difference in recall results (F(1, 13) = 1.289, P>.05) following chronic nicotine administration (n = 7–8 per group). Panel D: Female subjects’ recall as expressed by %rebound. Error bars represent standard error of the mean (SEM).
Highlights.
Female mice given chronic nicotine had higher rates of spontaneous recovery of contextual fear compared to sham controls
Male mice given chronic nicotine had lower rates of spontaneous recovery compared to sham controls
Chronic nicotine had no effect on recall of contextual fear
Acknowledgments
This work was funded with grant support from the National Institute on Drug Abuse (T.J.G., DA017949; 1U01DA041632), Jean Phillips Shibley Endowment, and Penn State Biobehavioral Health Department.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We declare no potential conflict of interest.
References
- Forbes D, Parslow R, Creamer M, O'Donnell M, Bryant R, McFarlane A, Shalev A. A longitudinal analysis of posttraumatic stress disorder symptoms and their relationship with fear and anxious-misery disorders: implications for DSM-V. Journal of Affective Disorders. 2010;127(1):147–152. doi: 10.1016/j.jad.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiology of Learning and Memory. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Koylu E, Demirgoren S, London E, Pogun S. Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sciences. 1997;61(12):PL185–PL190. doi: 10.1016/s0024-3205(97)00665-6. [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ. Acute nicotine delays extinction of contextual fear in mice. Behavioral Brain Research. 2014;263:133–137. doi: 10.1016/j.bbr.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Oliver C, P H, Liu-Chen L, Gould TJ. Impairment of contextual fear extinction by chronic nicotine and withdrawal from chronic nicotine is associated with hippocampal nAChR upregulation. Neuropharmacology. 2016a;109:341–348. doi: 10.1016/j.neuropharm.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Tumolo JM, Holliday E, Garrett B, Gould TJ. Acute nicotine enhances spontaneous recovery of contextual fear and changes c-fos early gene expression in infralimbic cortex, hippocampus, and amygdala. Learning and Memory. 2016b;23:405–414. doi: 10.1101/lm.042655.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164(3):887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. Journal of Neuroscience. 2001;21(22):9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvil E, Sullivan GM, Schaffer S, Markowitz JC, Campeas M, Wager TD, Neria Y. Sex differences in extinction recall in posttraumatic stress disorder: A pilot fMRI study. Neurobiology of Learning and Memory. 2014;113:101–108. doi: 10.1016/j.nlm.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike FP, Wernicke R, Pearlman MY, Haaga DA. Nicotine dependence, PTSD symptoms, and depression proneness among male and female smokers. Addictive Behaviors. 2006;31:223–231. doi: 10.1016/j.addbeh.2005.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: state of the art. Annual Review of Clinical Psychology. 2013;9:215–248. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain- McGovern J, Calhoun PS. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine & Tobacco Research. 2008;10(12):1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]