Abstract
Background
Distinguishing desmoplastic melanomas (DMs) from neurofibromas (NFs) can be histologically challenging in some cases. To date, a reliable marker to differentiate the two entities has remained elusive. S100 subtyping and CD34 fingerprinting have been proposed, but controversy remains as to their reliability. Missense mutations in TP53 are often found in DMs, resulting in a dominant negative effect and paradoxical accumulation of the tumor suppressor protein p53.
Hypothesis
We hypothesized that p53 may be expressed differentially in DMs, making it a valuable tool in differentiating DMs from NFs. Using immunohistochemistry, we compared p53 protein expression in 20 DMs and 20 NFs retrieved from our tissue archives and stained with p53 antibody (Monoclonal, DO-7).
Results
Patients with DM included 18 men and 2 women (age 36–95 years (mean=70.5 years, median = 70 years). Fifteen (15/20) tumors occurred in head and neck area; 2 (2/20) on the trunk; and 3 (3/ 20) on the extremities. Patients with NF included 12 men and 8 women (age 47–85 years (mean=65.2 years, median=69.5 years). Eleven (11/20) tumors occurred on the trunk, 6 (6/20) on the extremities, and 3 (3/20) on the head and neck area. A total of 19/20 (95%) desmoplastic melanomas were positive for p53. Desmoplastic melanoma H-scores ranged from 0-300 (mean=203, median=260). Nuclear accumulation of p53 was seen in all 19 positive DMs. None of the 20 neurofibromas were positive for p53 (two-tailed t-test p-value<0.0001).
Conclusion
Detection of p53 by immunohistochemistry can help to distinguish desmoplastic melanomas from neurofibromas.
Keywords: p53, desmoplastic melanoma, neurofibroma
Introduction
First described in 1971 by Conway et al., desmoplastic melanoma (DM) is a rare variant of melanoma that is characterized by an infiltrative malignant spindle cell tumor with marked interstitial fibrosis and collagenization1,2. Clinically, DM can simulate amelanotic lesions resembling scars, making the diagnosis difficult. Histologically, DM also presents a diagnostic challenge as it is often confused with neurofibroma (NF)3-5. These two entities share similar immune-phenotyping profiles: S100 and SOX-10 positive but Melan-A and HMB-45 negative, making the differentiation between DM and NF difficult in some cases, even with immunohistochemistry6. Previous studies have suggested that various S100 family protein members may be differentially expressed in DMs compared to NFs, and that the subtype S100A1 is often found in DM and not NF4. However, the commonly used polyclonal S100 antibody does not differentiate the subtypes and also stains immature fibroblasts, epithelioid granulomas and histiocytic proliferations in scars and may be inferior to SOX-107. CD34 fingerprint immune-reactivity has been shown to be more prominent in NFs compared to DM5 but this has been controversial as a similar pattern was observed in an early desmoplastic melanoma by a different group3. Thus, a reliable marker to differentiate between DM and NF remains elusive.
From a genetic standpoint, DM is unique from conventional melanomas. It lacks classic mutations such as BRAF, NRAS and KIT, instead harboring a higher frequency of loss of function NF1 mutations 8-11. Exome sequencing showed that DM carries a significantly higher mutation burden compared to other melanomas with ultraviolet radiation as the dominant mutagen12. It was also shown that missense mutations in TP53 are often found in DMs, resulting in a dominant negative effect and paradoxical accumulation of the tumor suppressor protein p53. Given these findings, we hypothesized that p53 staining may be expressed differentially in DMs, making it a valuable tool in differentiating DMs from NFs. To test our hypothesis, we compared p53 protein expression in 20 DMs and 20 NFs using immunohistochemistry.
Materials and Methods
The study was approved by the University of California Irvine's Institutional Review Board (IRB). Twenty desmoplastic melanomas and 20 neurofibromas were analyzed. DMs were retrieved from the Dermatopathology and Pathology Databases at the University of California Irvine Medical Center and the Laguna Pathology Medical Group in Laguna Hills, California. The search term “Desmoplastic melanoma” was used, and years “2010-2017”. Cutaneous NFs were retrieved from the Dermatopathology and Pathology Database at the University of California Irvine Medical Center. The search term “Neurofibroma” was used, and years “2015-2017” were searched. Sections for all specimens were taken from formalin-fixed, paraffin-embedded tissue, and stained with p53 antibody (Monoclonal, DO-7) at the University of California Irvine Department of Pathology Laboratories. A number of specimens were also stained with CD34 (Monoclonal: My10) and Sox-10 (Monoclonal: N-20) antibody.
Appropriate positive and negative controls were included with study sections. p53 staining intensity was qualitatively graded by a dermatopathologist where: 0, no tumor cells staining; 1+, weak tumor cell staining; 2+, moderate tumor cells staining; 3+, strong tumor cells staining. The percentage of tumor cells staining positive in each staining intensity category (0, 1+, 2+, and 3+) was qualitatively determined by dermatopathologist review. Using the staining intensities observed for each DM, the Histo (H)-Score was then calculated using the following formula: [1 × (% cells staining 1+) + 2 × (% cells staining 2+) + 3 × (% cells staining 3+)], resulting in a final score ranging from 0-30013,14. A two-tailed t-test was performed to determined statistical significance in p53 staining between the two groups (DMs and NFs).
Results
Twenty DMs were analyzed. Patient age ranged from 36 – 95 years (mean = 70.5 years, median = 70 years). They included 18 men and 2 women. Fifteen (15/20) tumors occurred in head and neck area; 2 (2/20) on the trunk; and 3 (3/ 20) on the extremities (Table 1). Twenty NFs were analyzed. Patient age ranged from 47 – 85 years (mean = 65.2 years, median = 69.5 years). They included 12 men and 8 women. Eleven (11/20) tumors occurred on the trunk, 6 (6/20) on the extremities, and 3 (3/20) on the head and neck area (Table 2). A total of 19/20 (95%) desmoplastic melanomas were positive for p53. Desmoplastic melanoma H-scores ranged from 0-300 (mean=203, median=260). Nuclear accumulation of p53 was seen in all p53 positive DMs (19/19); one (1/19) of which showed both nuclear and cytoplasmic staining. A total of 0/20 neurofibromas were positive for p53 (two-tailed t-test p-value < 0.0001) (Figures 1,2). Clinical and immunohistochemical features are summarized in Table 3.
Table 1. Clinicopathologic characteristics associated with desmoplastic melanomas.
| Case | Age | Gender | Site | Morphology | Presence of MIS | Weak (1+) | Moderate (2+) | Strong (3+) | H-score | P53 staining positive |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 90 | Male | Arm | Spindle, pleomorphic | Yes | 0% | 0% | 100% | 300 | Yes |
| 2 | 95 | Male | Scalp | Spindle, pleomorphic | No | 10% | 0% | 1% | 13 | Yes |
| 3 | 70 | Male | Neck | Mixed epithelioid spindle pleomorphic | No | 0% | 10% | 0% | 20 | Yes |
| 4 | 89 | Male | Scalp | Mixed epithelioid spindle | No | 0% | 0% | 100% | 300 | Yes |
| 5 | 54 | Female | Chest | Spindle | No | 10% | 0% | 0% | 10 | Yes |
| 6 | 70 | Male | Scalp | Epithelioid/focal spindle | Yes | 69% | 30% | 1% | 132 | Yes |
| 7 | 69 | Male | Ear | Mixed epithelioid spindle pleomorphic | Yes | 0% | 0% | 100% | 300 | Yes |
| 8 | 60 | Male | Face | Mixed epithelioid spindle pleomorphic | No | 0% | 50% | 50% | 250 | Yes |
| 9 | 69 | Male | Face | Mixed epithelioid spindle pleomorphic | No | 0% | 0% | 100% | 300 | Yes |
| 10 | 66 | Male | Back | Spindle | No | 0% | 50% | 20% | 160 | Yes |
| 11 | 80 | Male | Scalp | Pleomorphic spindle | Yes | 0% | 0% | 100% | 300 | Yes |
| 12 | 36 | Male | Neck | Mixed epithelioid spindle | Yes | 0% | 10% | 90% | 290 | Yes |
| 13 | 58 | Male | Scalp | Mixed epithelioid spindle pleomorphic | No | 0% | 0% | 100% | 300 | Yes |
| 14 | 77 | Male | Scalp | Mixed epithelioid spindle | Yes | 0% | 0% | 25% | 75 | Yes |
| 15 | 78 | Male | Scalp | Mixed epithelioid spindle | No | 0% | 0% | 80% | 240 | Yes |
| 16 | 83 | Male | Scalp | Spindle, pleomorphic | No | 0% | 25% | 75% | 275 | Yes |
| 17 | 74 | Male | Scalp | Mixed epithelioid spindle pleomorphic | No | 0% | 0% | 100% | 300 | Yes |
| 18 | 81 | Male | Arm | Spindle, pleomorphic | No | 0% | 30% | 70% | 270 | Yes |
| 19 | 57 | Male | Arm | Spindle | No | 0% | 80% | 20% | 220 | Yes |
| 20 | 54 | Female | Face | Spindle | No | 0% | 0% | 0% | 0 | No |
Table 2. Clinicopathologic characteristics associated with neurofibromas.
| Case | Age | Gender | Site | Intensity of p53 staining | % of p53+ tumor cells | P53 staining positive |
|---|---|---|---|---|---|---|
| 1 | 61 | Female | Leg | 0 | 0% | No |
| 2 | 57 | Male | Back | 0 | 0% | No |
| 3 | 71 | Male | Chest | 0 | 0% | No |
| 4 | 72 | Male | Abdomen | 0 | 0% | No |
| 5 | 58 | Female | Face | 0 | 0% | No |
| 6 | 50 | Male | Neck | 0 | 0% | No |
| 7 | 71 | Female | Arm | 0 | 0% | No |
| 8 | 84 | Female | Arm | 0 | 0% | No |
| 9 | 85 | Male | Back | 0 | 0% | No |
| 10 | 73 | Male | Back | 0 | 0% | No |
| 11 | 28 | Male | Leg | 0 | 0% | No |
| 12 | 69 | Female | Arm | 0 | 0% | No |
| 13 | 81 | Male | Back | 0 | 0% | No |
| 14 | 47 | Female | Back | 0 | 0% | No |
| 15 | 61 | Female | Abdomen | 0 | 0% | No |
| 16 | 63 | Male | Arm | 0 | 0% | No |
| 17 | 70 | Male | Back | 0 | 0% | No |
| 18 | 70 | Male | Back | 0 | 0% | No |
| 19 | 73 | Male | Scalp | 0 | 0% | No |
| 20 | 60 | Female | Back | 0 | 0% | No |
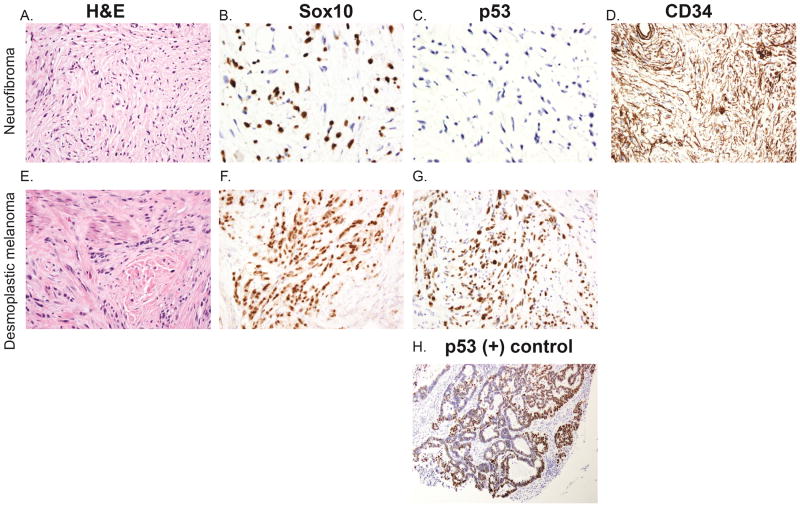
Figure 1.
H&E of desmoplastic melanoma (A) and neurofibroma (E) at 200x magnification. p53 and SOX-10 staining of desmoplastic melanomas x 200x magnification (F,G) and neurofibromas at 400x magnification (B,C). Desmoplastic melanomas demonstrate strong positive p53 staining (G) while neurofibromas remain negative (C). In contrast, SOX-10 staining is comparable in desmoplastic melanoma (F) and neurofibroma (B). Neurofibromas also demonstrate CD34 fingerprinting pattern, 200x magnification (D). Positive control of p53 staining (H).
Figure 2.
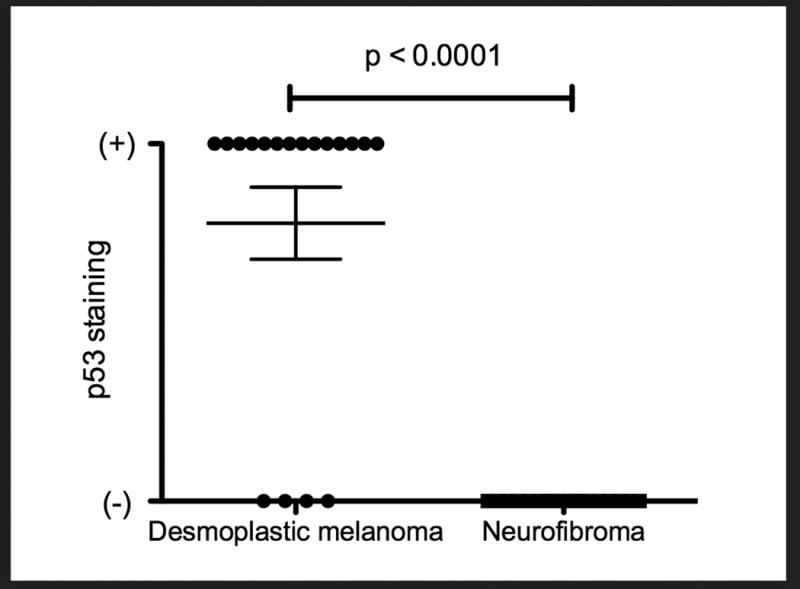
Two-tailed t-test of p53 positivity in desmoplastic melanomas versus neurofibromas, p-value <0.0001.
Table 3. Summary of clinicopathologic characteristics of desmoplastic melanoma and neurofibroma cases.
| Desmoplastic Melanoma | Neurofibroma | |
|---|---|---|
| Age (median) | 70 years | 69.5 years |
| Site | Head and neck - 15 | Head and neck - 3 |
| Trunk - 2 | Trunk - 11 | |
| Extremities - 3 | Extremities - 6 | |
| Gender | Male - 18 | Male - 12 |
| Female - 2 | Female - 8 | |
| + p53 | 19/20 (95%) | 0/20 (0%) |
Discussion
Distinguishing neurofibromas (NF) from desmoplastic melanomas (DM) can be challenging in some cases. Differentiating DMs from NFs proves particularly challenging in the following scenarios: 1.) An early DM that may not show significant cytological atypia to be readily differentiated from NF; 2.) When a superficial or limited biopsy of a DM is taken; 3.) When a NF-like proliferation arises within severely sun-damaged skin, a location where DMs typically develop, and 4.) When an intraepidermal group of melanocytes is located above a dermal population of spindled S100-positive cells. In these particular scenarios, a marker of differentiation would be desirable. To date, a reliable histologic marker to differentiate the two entities has remained elusive. S100 subtyping and CD34 fingerprinting have been proposed as potential avenues, but controversy remains about the practicality and reliability of these methods.
Based upon our immunohistochemical analysis— which showed p53 to be positive in 95% of DMs and negative in 100% of NFs— we conclude that p53 can help to distinguish desmoplastic melanomas from neurofibromas. In addition, we observed nuclear accumulation of p53 in all p53 positive DMs, except in one case that showed both nuclear and cytoplasmic accumulation. This finding differs from previous reports of melanoma showing predominately cytoplasmic overexpression of p5315. We hypothesize that this stress-induced nuclear accumulation of p53 may be due to mutations resulting in decreased nuclear export and or enhanced nuclear import of p53 in this melanoma subtype16.
Acknowledgments
Funding: None
Footnotes
Conflicts of Interest: None
References
- 1.Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of spindle cell melanoma) Cancer. 1971;28(4):914–936. doi: 10.1002/1097-0142(1971)28:4<914::aid-cncr2820280415>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Bastian BC, Lazar A. Melanoma. In: Calonje E, Brenn T, Lazar A, PH M, editors. McKee's Pathology of the Skin. Edinburgh: Elsevier Saunders; 2012. [Google Scholar]
- 3.Husain S, Silvers DN. Fingerprint CD34 immunopositivity to distinguish neurofibroma from an early/paucicellular desmoplastic melanoma can be misleading. J Cutan Pathol. 2013;40(11):985–987. doi: 10.1111/cup.12206. [DOI] [PubMed] [Google Scholar]
- 4.Nonaka D, Chiriboga L, Rubin BP. Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors. J Cutan Pathol. 2008;35(11):1014–1019. doi: 10.1111/j.1600-0560.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 5.Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38(8):625–630. doi: 10.1111/j.1600-0560.2011.01700.x. [DOI] [PubMed] [Google Scholar]
- 6.Busam KJ, Iversen K, Coplan KC, Jungbluth AA. Analysis of microphthalmia transcription factor expression in normal tissues and tumors, and comparison of its expression with S-100 protein, gp100, and tyrosinase in desmoplastic malignant melanoma. Am J Surg Pathol. 2001;25(2):197–204. doi: 10.1097/00000478-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Herberth FI, Karamchandani J, Kim J, Dadras SS. SOX10 immunostaining distinguishes desmoplastic melanoma from excision scar. J Cutan Pathol. 2010;37(9):944–952. doi: 10.1111/j.1600-0560.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- 8.Davison JM, Rosenbaum E, Barrett TL, et al. Absence of V599E BRAF mutations in desmoplastic melanomas. Cancer. 2005;103(4):788–792. doi: 10.1002/cncr.20861. [DOI] [PubMed] [Google Scholar]
- 9.Wiesner T, Kiuru M, Scott SN, et al. NF1 Mutations Are Common in Desmoplastic Melanoma. Am J Surg Pathol. 2015;39(10):1357–1362. doi: 10.1097/PAS.0000000000000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutzmer R, Herbst RA, Mommert S, et al. Allelic loss at the neurofibromatosis type 1 (NF1) gene locus is frequent in desmoplastic neurotropic melanoma. Hum Genet. 2000;107(4):357–361. doi: 10.1007/s004390000374. [DOI] [PubMed] [Google Scholar]
- 11.Kiuru M, McDermott G, Berger M, Halpern AC, Busam KJ. Desmoplastic melanoma with sarcomatoid dedifferentiation. Am J Surg Pathol. 2014;38(6):864–870. doi: 10.1097/PAS.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shain AH, Garrido M, Botton T, et al. Exome sequencing of desmoplastic melanoma identifies recurrent NFKBIE promoter mutations and diverse activating mutations in the MAPK pathway. Nat Genet. 2015;47(10):1194–1199. doi: 10.1038/ng.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch FR, Varella-Garcia M, Bunn PA, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: Correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 14.John T, Liu G, Tsao MS. Overview of molecular testing in non-small-cell lung cancer: Mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene. 2009;28:S14–S23. doi: 10.1038/onc.2009.197. [DOI] [PubMed] [Google Scholar]
- 15.Weiss J, Heine M, Körner B, Pilch H, Jung EG. Expression of p53 protein in malignant melanoma: clinicopathological and prognostic implications. Br J Dermatol. 1995;133(1):23–31. doi: 10.1111/j.1365-2133.1995.tb02487.x. [DOI] [PubMed] [Google Scholar]
- 16.Marchenko N, Hanel W, Li D, Becker K, Reich N, Moll U. Stress-mediated nuclear stabilization of p53 is regulated by ubiquitination and importin-α3 binding. Cell Death Differ. 2010;17(2):255–267. doi: 10.1038/cdd.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]