Abstract
Primary progressive apraxia of speech (PPAOS) is a neurodegenerative disorder in which AOS is the sole presenting complaint. We report clinical and neuroimaging data spanning 10-years from disease onset-to-death in a 49 year-old male PPAOS patient, DY, who died with corticobasal degeneration. He presented with AOS with normal neuroimaging. Abnormalities in the caudate nucleus, supplementary motor area, cingulate, insula and Broca’s area were observed after five years, with involvement of motor cortex and development of agrammatism, parkinsonism and dysarthria three years later. Cognitive impairment and temporoparietal atrophy were late features. This data provides important insight into disease progression of corticobasal degeneration when presenting as PPAOS.
Keywords: apraxia of speech, MRI, FDG PET, longitudinal, corticobasal degeneration
Introduction
Apraxia of speech (AOS) is a motor speech disorder that typically presents with distorted or segmented speech, articulatory difficulties and groping, and slow speech rate, reflecting difficulties in the motor planning and programming necessary for speech(Duffy, 2005). While AOS is commonly associated with vascular insults, it can be the predominant manifestation of neurodegenerative disease(Duffy, 2006). In such cases it often presents with agrammatic aphasia but can sometimes be the first, dominant, or sole manifestation of neurodegeneration, in which case it is denoted as primary progressive AOS (PPAOS)(Josephs et al., 2012). Group-level studies have shown that PPAOS is associated with cortical atrophy on magnetic resonance imaging (MRI) and hypometabolism on [18F]fluorodeoxyglucose PET localized in the superior premotor region, including supplementary motor area and lateral premotor cortex(Josephs et al., 2013; Josephs, et al., 2012). PPAOS is typically related to the underlying tau pathologies of progressive supranuclear palsy and corticobasal degeneration(Deramecourt et al., 2010; Josephs et al., 2005; Josephs et al., 2006).
Despite the recent characterization and recognition of PPAOS, little is known about disease progression in this syndrome. We have previously reported longitudinal data over a two year interval in patients with PPAOS in order to investigate patterns of disease progression(Whitwell et al., 2017; Zalewski et al., 2014). We showed that some patients maintained an isolated AOS over time, while some developed agrammatic aphasia, and others developed parkinsonian and other features akin to progressive supranuclear palsy and corticobasal syndrome. However, these studies only assessed a relatively short window of the disease’s duration and thus did not permit a complete description of disease progression from onset to death. Understanding the full evolution of the disease process is critically important to developing prognostic information for patients, and to help patients and families to better understand and plan for the challenges that they will face. In this case report, we present longitudinal findings from speech/language, neurological and neuropsychological assessments, as well as structural MRI and FDG-PET, spanning the entire 10-year disease course in a patient with PPAOS who ultimately died with corticobasal degeneration. This patient provides a unique opportunity to assess progressive patterns of degeneration and characterize the order in which the various signs and symptoms develop and how they relate to underlying changes in neuroanatomy and metabolism.
Case Report
DY was first evaluated neurologically in March 2006 at the age of 49. He was a right-handed male with ten years of formal education who began noticing problems with his speech while attending a party six months prior. The speech problem had been progressive and had begun causing him some embarrassment in his work as a salesman. He described his speech as slurred and said he had trouble “getting the words out right … as though I have gravel in my mouth.” He denied problems with writing or typing. He denied changes or problems with walking, swallowing, or movements of his limbs; he played racquetball frequently and noted no deterioration in his game. On examination his speech was characterized by vowel distortions and distorted sound substitutions and additions, particularly during the production of multisyllabic words. Voice quality, resonance and speaking rate were normal, as were his receptive and expressive language abilities. There were no other neurological abnormalities. He was diagnosed with mild PPAOS. Laboratory studies including CSF studies and a MRI head scan were normal.
A year later, at the age of 50, he returned for follow-up examination. He said his speech problem was relatively stable, judging it to be about 70% normal as compared to 75% the previous year. He noted occasions when his speaking problems would uncontrollably snowball if he had been anxious. Because of his speech difficulty he had left his sales job and was working in a prison. Speech-language examination revealed little change in the previously noted abnormal features, but speech rate was now mildly slow and syllables in multisyllabic words were sometimes segmented. There was no evidence of aphasia, dysarthria, or other neurologic abnormalities. The diagnosis remained mild PPAOS, with slight worsening compared to the previous year.
One year later, at the age of 51, he returned for re-evaluation. He thought his speech problem had worsened to about 50% normal. He admitted to considerable frustration and trying to hide his speech impairment because “people look at me like I have a psychological problem.” He was taking alprazolam for anxiety, which he felt was helpful because his speech problems were most severe when he was anxious. Speech-language examination revealed worsening of the previously noted abnormalities, with further slowing of speech rate and frequent attempts to correct articulatory errors. There was no evidence of dysarthria. He complained of occasional word-finding difficulty and his word fluency performance was below average, but there was no other suggestion of aphasia. He did not have any limb apraxia or Parkinsonism.
In 2009, at the age of 52, he was evaluated once again for his progressive speech problem. He was still working but stated, “It’s tough at work. It sets me up for defeat. I look stupid, sound stupid, and feel stupid, especially on the phone.” He was considering going on disability because of his speech and felt his employer would prefer that he not work. He denied problems with spoken or written language comprehension and noted no trouble with writing. Speech-language examination revealed unequivocal worsening of the previously noted speech characteristics, with further slowing of speech rate and increased efforts to correct errors. Language performance was stable. There was no evidence for agrammatism, and word fluency was slightly better than the previous year when it was in the borderline normal range. There was no unambiguous evidence of dysarthria. He had very mild hypomimia and slightly reduced rapid alternating movements of the left fingers only. His diagnosis remained PPAOS.
He returned in 2010, reporting that his speech problems had again progressed. He had now developed some depression in addition to his anxiety and was still considering work disability due to his near-inability to communicate effectively at work. There was no unequivocal evidence of aphasia on testing. There was no evidence for dysarthria. His AOS was rated as moderately severe and intelligibility was compromised. His neurologic examination was essentially identical to that of the year prior and, as such, he maintained a diagnosis of PPAOS.
His next evaluation was not until 2013, at the age of 56. At this time his speech was very hesitant. He was now having problems with grammar, spelling, constructing sentences, and finding words. He had also developed excessive daytime drooling. Neurological examination showed worsening of his AOS, in addition to the presence of agrammatic aphasia, which was less severe than his apraxia. He was again noted to have hypomimia, as well as mild slowing of finger and now hand alternating movements.
In 2014, at the age of 57, he returned for follow-up. At that time, his movements, including walking, had slowed, and he was having problems with coordination and motor function. For example, he had difficulty squeezing water from a rag or mowing his lawn. He was also described as being slightly more aggressive towards his wife. He started craving sweets and carbs and would often eat peanut butter and honey sandwiches. His gate and balance remained intact, and he reported walking 2 miles per day, 4 times a week. He would occasionally cough when drinking; pseudobular laughter was evident and his smile was sometimes exaggerated and prolonged. He had no urinary problems. He now had severe AOS, mild-moderate agrammatic aphasia, and mild-moderate spastic dysarthria. To communicate, he was primarily using an electronic device with a speaking application, as well as signs and gestures. In addition to this, he also had increased muscle tone of the cogwheel type that was mild on the left and slight on the right. He had mild-moderate axial rigidity and mild limb ideomotor apraxia. His extraocular eye movements and gait were normal. No myoclonus or dystonia were observed. Diagnosis was now dominant apraxia of speech with aphasia (DAOS), dysarthria, and Parkinsonism. His secondary diagnosis was corticobasal syndrome.
In 2015, at the age of 58, he was once again evaluated. He was now nearly mute, only able to make some distorted sounds but no comprehensible words. His spoken and written language comprehension was fairly good, although not normal. Expressive language assessment was very limited by his muteness and inability to write or type. He had developed problems with swallowing and was frequently choking. In fact, on one occasion, he actually turned blue and had to be taken to the emergency room. His wife also mentioned that he occasionally had a “goofy” look to his face. He was no longer walking 2 miles, 4 times a week, although it was unclear why he stopped exercising, as he had not had any falls and could still walk. He had now developed a grasp reflex, and sometimes would grasp things with his teeth, for example a spoon, and would not let go of it. His diet was fixed and consisted primarily of oatmeal, milk, orange juice, blueberries, wheat toast, and grapes. He would occasionally eat chicken, salmon, and hamburgers. On neurological examination he was essentially mute. He was somewhat unsteady while walking and required some assistance to stand up from a seated position. There was increased latency of initiation of vertical and horizontal eye movements and severe slowing of vertical eye movement velocity with mild slowing of velocity of horizontal saccades. He had developed gegenhalten rigidity of his limbs, and his alternating motor rates of his fingers and hands were now moderately slow. He had marked-severe limb ideomotor apraxia. He was diagnosed as having a Parkinson-plus condition which was most consistent with corticobasal syndrome(Josephs et al., 2016).
DY passed away in April 2016 at the age of 59 years. Autopsy examination revealed tau immunoreactive inclusions, including astrocytic plaques, consistent with corticobasal degeneration. Details of the pathological findings have been described(Josephs, et al., 2016).
Methods
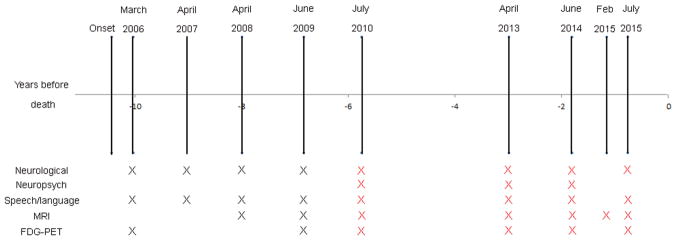
A timeline showing when all DY’s clinical and neuroimaging examinations were performed is shown in Figure 1. Beginning in 2010, DY entered a longitudinal NIH-funded research study at Mayo Clinic, Rochester, MN. Research visits included neurological, neuropsychological, speech/language, MRI and FDG-PET examinations. Some of the speech and language findings from the first two study evaluations have been previously reported (Duffy et al., 2015).
Figure 1.
Time-line showing all clinical and neuroimaging evaluations performed by DY. All evaluations from 2010 onwards (shown by red crosses) were research visits during which standardized clinical evaluations were performed and all MRI were performed at 3T.
Clinical assessments
The neurological evaluation was performed by a Behavioral Neurologist and Movement Disorder Specialist (KAJ) and included the Mini-Mental State Examination (MMSE)(Folstein, Folstein, & McHugh, 1975) and Montreal Cognitive Assessment battery (MoCA)(Nasreddine et al., 2005), which measure general cognition; the Clinical Dementia Rating (CDR)(Hughes, Berg, Danziger, Coben, & Martin, 1982) to assess functional abilities; the short version of the Neuropsychiatric Inventory Questionnaire (NPI-Q)(Kaufer et al., 2000) to assess psychiatric and behavioral traits; the Frontal Assessment Battery (FAB)(Dubois, Slachevsky, Litvan, & Pillon, 2000) to assess executive dysfunction, the Western Aphasia Battery (WAB)(Kertesz, 2007) apraxia rating scale to assess limb and orobuccal apraxia; and the Movement Disorder Society Sponsored revision of the Unified Parkinson’s disease Rating Scale III (MDS-UPDRS III)(Goetz et al., 2008) to assess Parkinsonism. The PSP saccadic impairment scale (PSIS)(Whitwell et al., 2011) was used to rate severity of eye movement abnormalities. The neuropsychological test battery included the Auditory Verbal Learning Test(Rey, 1964) to assess recognition memory; Trail Making Test (TMT) A and B(Spreen and Strauss, 1998) to assess processing speed and executive function and Visual Object and Space Perception Battery (VOSP)(Warrington and James, 1991) incomplete letters and cubes tests to assess visuospatial and visuoperceptual processing. The Wide Range Achievement Test (WRAT) – 3rd edition(Wilkinson, 1993) was used to estimate premorbid levels of cognitive function.
The speech-language battery was performed by one of two speech-language pathologists (JRD or EAS). The Western Aphasia Battery (WAB), revised(Kertesz, 2007), Part 1, served as the primary measure of global language ability. The Token Test, Part V(De Renzi and Vignolo, 1962), served as a measure of grammatic/syntactic comprehension, and the 15-item Boston Naming Test(Lansing, Ivnik, Cullum, & Randolph, 1999) as a measure of confrontation-naming ability. Action (verb)(Woods et al., 2005) and Letter (FAS) fluency(Loonstra, Tarlow, & Sellers, 2001) tasks served as indices of rapid-word retrieval ability. The severity of his motor speech disorder was quantified using the Motor Speech Disorder (MSD) scale(Yorkston, Strand, Miller, Hillel, & Smith, 1993) and the AOS rating scale (ASRS)(Duffy, Strand, & Josephs, 2014). Dysarthria severity was measured on a 0–4 scale based on all spoken language tests of the WAB plus additional speech tasks. Non-verbal oral apraxia (NVOA) was rated based on eight items, each on a 0–4 scale. In order to assess speech rate, we measured the average rate of syllable production from three consecutive repetitions of the multisyllabic word “catastrophe” using an acoustic protocol previously described(Duffy et al., 2017). The word catastrophe was selected as we have shown that it is a sensitive and specific stimulus to differentiate PPAOS from primary progressive aphasia(Duffy, et al., 2017).
At each of his speech-language evaluations from 2010 to 2015, as part of the WAB, DY was asked to describe in writing a pictured picnic scene using full sentences. Later in each session, he was asked to complete the same task with a spoken response, which was video recorded. DY’s written and spoken picture description language samples were transcribed and coded in CHAT transcription format for use in CLAN (Computerized Language ANalysis)(MacWhinney, 2000). The transcriptions included word-level codes for each grammatical category of nouns, verbs, and function or closed-class words (e.g. articles, pronouns, prepositions). Syntactic errors were also marked. Additionally, each utterance was coded as grammatical or ungrammatical. CLAN was then used to calculate the mean length of utterance (MLU), verb count, noun count, closed-class word count, the number of syntactic errors, and the number of grammatical utterances. The numbers from these language variable measurements were converted into ratios to control for the differences in number of words and utterances produced at different test dates. For the spoken language samples, DY’s language production was compared to that of normal controls (Thompson, Ballard, Tait, Weintraub, & Mesulam, 1997).
Neuroimaging assessments
Quantitative analysis was performed using all the volumetric MRI performed at 3T. The MRI protocol included a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence. All MPRAGE images underwent pre-processing correction for gradient non-linearity and intensity non-uniformity. All scans were co-registered using nine degrees of freedom rigid-body registration with differential bias correction(Gunter, Senjem, Vemuri, & Jack, 2012). An in-house developed version of tensor-based morphometry using symmetric normalization (TBM-SyN)(Cash et al., 2015) was used to compute, for each pair of scans, a nonlinear deformation required to warp the late image to the early image and to warp the early image to the late image. The warped early image and original late image were averaged together to obtain a synthetic late image, and similarly, the warped late image and original early image were averaged together to obtain a synthetic early image. The synthetic images were then segmented using SPM5 unified segmentation, and the resulting spatial normalization parameters were used to propagate a set of ROI labels from template space to subject space for each synthetic image. The gray matter segmentations of each synthetic image were then parcellated into ROIs and the volume of gray matter in each ROI was quantified for each synthetic image. All calculated volumes for each scan date were averaged to provide an estimated volume at each time-point. These volumes were divided by total intracranial volume, and corrected volumes were converted to Z scores compared to an age matched cohort of 10 male healthy controls that had a median age at MRI of 53 years (range 53–54 years).
DY underwent research FDG-PET scans using a PET/CT scanner (GE Healthcare, Milwaukee, Wisconsin) as previously described(Josephs, et al., 2012). Individual patterns of hypometabolism were assessed using the clinical tool of 3-dimensional stereotactic surface projections (SSP)(Minoshima, Frey, Koeppe, Foster, & Kuhl, 1995). PET uptake was normalized to the pons and compared with an age-segmented normative database, yielding a 3-dimensional SSP z-score image. The software package used to perform these analyses was CortexID (GE Healthcare, Waukesha, Wisconsin).
Results
Quantitative Neurological and Neuropsychological Data
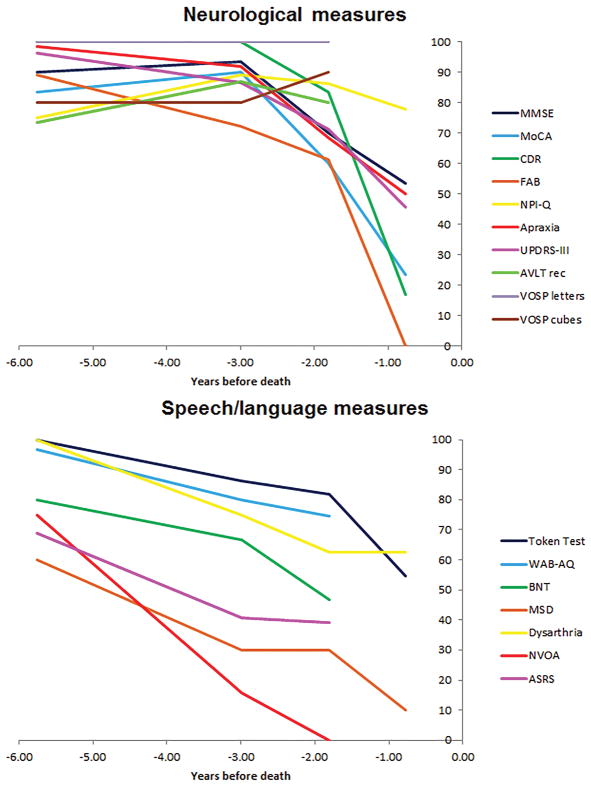
In 2010, five years after onset, DY performed relatively well on all neurological and neuropsychological measures (Figure 2A, Table 1). His performance remained normal at the following examination in 2013, three years before death, except with some decline on the FAB. Performance declined on every measure in 2014, nine years after onset, with the exception of the NPI-Q, AVLT recognition memory and VOSP tests, which remained stable. This trend continued in 2015, with performance on all neurological measures falling significantly between 2014 and 2015, except the NPI-Q. We were unable to obtain neuropsychological test scores in 2015. DY performed in the low average range on the WRAT reading (Standard Score = 83), a test of premorbid ability.
Figure 2.
Longitudinal decline on neurological, neuropsychological and speech and language variables in DY. Each clinical test score was converted to a percentage of the maximum possible score, with each scale transformed so a normal score would be 100%, to allow the trajectory of decline to be compared across tests.
Table 1.
Neurological and Speech and Language test scores for DY
| July 2010 | April 2013 | June 2014 | July 2015 | |
|---|---|---|---|---|
| Age, years | 53 | 56 | 57 | 58 |
| Disease duration, years | 5 | 8 | 9 | 10 |
| Years before death | 5.8 | 3.0 | 1.8 | 0.8 |
| Neurological/neuropsychological tests | ||||
| Mini Mental State Examination (/30) | 27 | 28 | 21 | 16 |
| Montreal Cognitive Assessment Battery (/30) | 25 | 27 | 18 | 7 |
| Clinical Dementia Rating Scale sum of boxes (/18) | 0 | 0 | 3.0 | 15 |
| Neuropsychiatric Inventory - Q (/36) | 9 | 4 | 5 | 8 |
| Frontal Assessment Battery (/18) | 16 | 13 | 11 | 0 |
| Western Aphasia Battery (WAB)-apraxia (/60) | 59 | 55 | 41 | 30 |
| Unified Parkinson’s Disease Rating Scale III (/132) | 5 | 18 | 38 | 72 |
| PSP Saccadic Impairment Scale (/5) | 0 | 1 | 1 | 3 |
| Auditory Verbal Learning Test - recognition (/15) | 11 | 13 | 12 | Untestable |
| Trail Making Test A | 19 | 29 | 51 | Untestable |
| Trail Making Test B | 60 | 118 | 244 | Untestable |
| Visual Object and Space Perception Battery -Incomplete letters (/20) | 20 | 20 | 20 | Untestable |
| Visual Object and Space Perception Battery - cubes (/10) | 8 | 8 | 9 | Untestable |
| Speech and language tests | ||||
| Token Test (/22) | 22 | 19 | 18 | 12 |
| WAB scores | ||||
| Aphasia quotient (/100) | 96.6 | 80 | 74.6 | Untestable |
| Fluency (/10) | 9 | 4 | 5.0 | Untestable |
| Spon. Speech (/20) | 19 | 12 | 13.0 | Untestable |
| AV comp (/10) | 10 | 10 | 10.0 | 9.8 |
| Repetition (/10) | 9.3 | 8.6 | 5.8 | Untestable |
| Naming (/10) | 10 | 9.4 | 8.5 | Untestable |
| Reading comp (/40) | 40 | 26 | 34.0 | 20.0 |
| Read commands (/20) | 20 | 14 | Untestable | Untestable |
| Write output (/10) | 33 | 32 | 13.5 | Untestable |
| Read Irregular (/10) | 9 | 10 | Untestable | Untestable |
| Read non-words (/10) | 8 | 9 | Untestable | Untestable |
| Write Irregular (/10) | 4 | 2 | 2.0 | Untestable |
| Write non-words (/10) | 7 | 8 | 0.0 | Untestable |
| Agrammatism in writing | No | Yes | Yes | Untestable |
| Agrammatism in speech | No | Yes | Yes | Untestable |
| Boston Naming Test (/15) | 12 | 10 | 7 | Untestable |
| Motor Speech Disorders Scale (/10) | 6 | 3 | 3 | 1 |
| Apraxia of Speech Rating Scale (/64) | 20 | 38 | 39 | Untestable |
| Action fluency | 16 | 12 | Untestable | Untestable |
| Letter fluency | 11 | 11 | Untestable | Untestable |
| Dysarthria (0–4) | 0 | 1 | 1.5 | Untestable |
| Non-verbal oral apraxia (/32) | 24 | 5 | 0 | 4 |
Quantitative Speech and Language Data
In 2010, five years after onset, DY performed normally on the WAB-AQ, Token Test and BNT and showed no evidence of dysarthria (Figure 2B, Table 1). However, he was moderately impaired on the MSD and ASRS rating scales, reflecting effects of his AOS. He also scored poorly on the test of NVOA. Between 2010 and 2014, he showed a steady decline in performance on all of the speech and language tests, with a particularly fast rate of decline in NVOA (Figure 2B, Table 1). He performed in the abnormal range for the WAB-AQ and Token Test, and had mild dysarthria by his 2013 visit. His speech rate was judged perceptually as slow in 2010 and reduced over time, with syllable rate per second for production of the word “catastrophe,” declining from 4.0 per second in 2010, to 1.9 in 2013, to 1.0 2014. At his final examination in 2015, he was untestable on many of the measures due to his muteness and significant language difficulties. However, he showed a steep decline in performance on the Token Test and the MSD rating scale.
Linguistic analysis
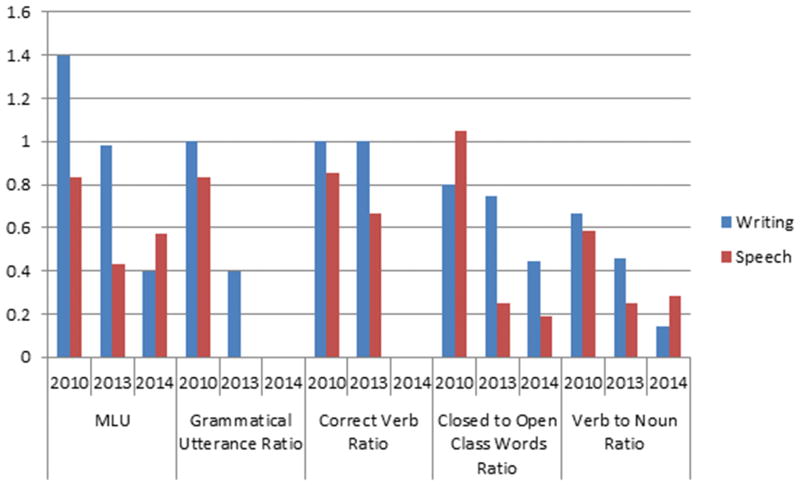
At his first language exam in 2010, his spoken language performance was in the normal range; DY was within one standard deviation of normal for every variable except verb to noun ratio, where he produced more nouns than the controls (Figure 3). Upon reviewing his speech sample, however, he tended to use many descriptive prepositional phrases, which included nouns but not verbs. His writing sample was also normal. By 2013, in his spoken language sample, he showed decreased MLU, fewer correct verbs, fewer closed-class words, fewer verbs compared to nouns, and none of his utterances were grammatical without errors. Errors were more pronounced in speech compared to writing. DY’s agrammatism further worsened by his follow-up in 2014, at which point he produced no correct verbs or grammatical utterances in his spoken or written language samples (Figure 3). His production of closed-class words also declined in both modalities. His ratio of verbs to nouns was relatively the same as the previous year in his speech sample but further worsened in his writing, which initially was more preserved. At his final examination in 2015, DY was unable to complete the picture description tasks.
Figure 3.
Longitudinal comparison of language variables in speech versus writing samples from DY
MRI
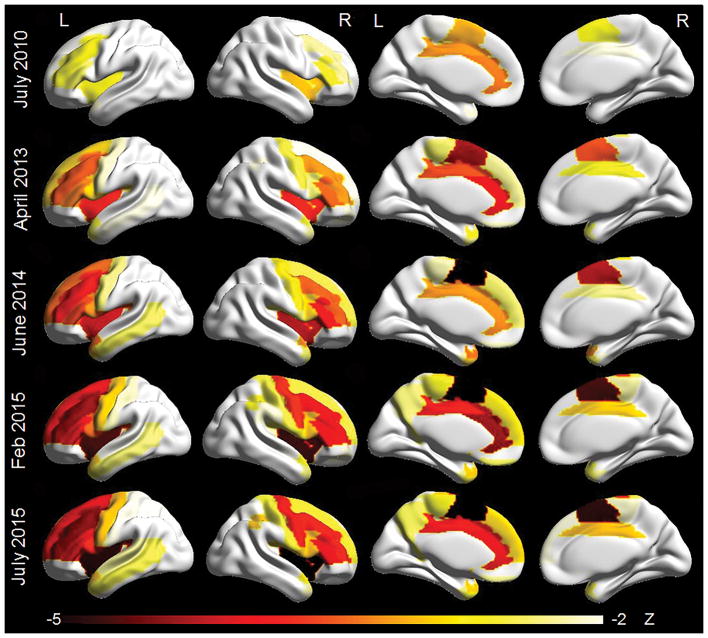
DY’s MRI scans in 2008 and 2009 were read as normal, with no clear regions of atrophy. However, in 2010, five years after onset, our quantitative analysis demonstrated cortical atrophy, particularly targeting the frontal lobes (Figure 4). Atrophy was most severe in left supplementary motor area, anterior cingulate, middle cingulate and right insula, but also involved middle frontal gyrus, frontal inferior triangularis and opercularis, left superior frontal gyrus, left paracentral lobule, and right caudate and putamen. A steady decline in grey matter volume over time was observed across all the regions that were abnormal in 2010, and these regions remained among the most affected regions in 2015.
Figure 4.
Three-dimensional renders showing regional patterns of grey matter atrophy on each MRI. Each region in the AAL atlas has been assigned a Z score representing atrophy compared to matched controls. Renders were created using BrainNet viewer.
In addition to these regions, in 2013, atrophy was observed in precentral cortex bilaterally and left superior medial frontal lobe, rolandic operculum and caudate. The right superior frontal gyrus and frontal inferior opercularis also became involved. At this time-point regions within the temporal lobe started to become involved. The bilateral postcentral cortex, right paracentral lobule and regions in the parietal lobe first showed atrophy in 2015. Orbitofrontal cortex and occipital lobe remained relatively unaffected throughout the disease course.
FDG-PET
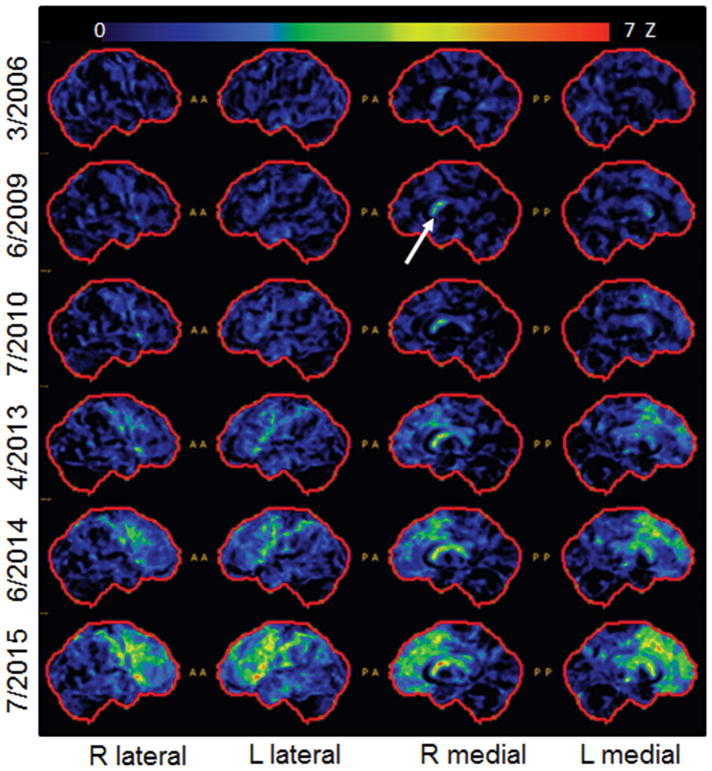
DY’s first FDG-PET was performed in 2006, six months after onset, and it was unremarkable, showing no evidence for hypometabolism (Figure 5). By 2009, mild hypometabolism was observed in caudate nucleus, particularly in the right hemisphere. The FDG-PET remained stable in 2010, however, by 2013 mild hypometabolism was also now observed in the supplementary motor area, lateral frontal lobes and lateral motor cortex. Hypometabolism in these regions progressed in severity and area of involvement in 2014. DY’s final FDG PET scan was performed in 2015, one year before his death. At this point, hypometabolism had progressed and now involved lateral and medial posterior frontal lobes in both hemispheres, which was most striking in Broca’s area, left premotor cortex and supplementary motor area. Hypometabolism also extended into prefrontal cortex and left lateral temporal lobe.
Figure 5.
Serial FDG-PET scans for DY. Cortex ID Z score maps are shown for each date. Arrow points to early involvement of right caudate.
General discussion and conclusion
This report has detailed the neurological, speech-language, neuropsychological, and neuroimaging findings that describe the disease evolution in a patient who originally presented with PPAOS and died with autopsy confirmed corticobasal degeneration. Observation of this patient taught us a number of things about the disease process: 1) AOS can remain the only neurologic symptom for several years, 2) subtle parkinsonism can be detected relatively early in the disease course, although frank symptoms of corticobasal syndrome may develop only a couple of years before death, 3) cognition can be relatively spared until the very end of the disease course, except for some executive dysfunction, 4) early in its course AOS can occur in the absence of any structural or metabolic abnormalities on neuroimaging, and 5) the disease predominantly targets frontal, premotor and motor cortices before eventually involving temporoparietal cortex.
The clinical and neuroimaging data collected on DY provided a unique opportunity to document and describe the entire ten-year disease course of a patient with PPAOS. His disease started with the isolated presentation of AOS, with no evidence for dysarthria, aphasia or any other neurological deficits on examination. The AOS remained the only prominent symptom for the first five years, with speech worsening during that time, leading to depression and anxiety, and ultimately leaving his job. His speech rate was below average(Duffy, et al., 2017) at his first visit and continued to slow over time, as has been previously observed in a patient with progressive AOS(Code, Ball, Tree, & Dawe, 2013). While there have been no direct comparisons, most of his AOS speech characteristics were not obviously different from those that have been described for stroke-induced or nondegenerative AOS(Duffy and Josephs, 2012; McNeil, Robin, & Schmidt, 2009). Given the absence of evidence for aphasia during this time, our patient would not meet clinical criteria for primary progressive aphasia (Gorno-Tempini et al., 2011; Mesulam, 2001). Although he only scored 12 on the BNT at baseline this is still considered a normal score and within 1 standard deviation of the mean for his age and years of education (M = 12.8; SD = 2.4)(Lansing, et al., 1999). NVOA also presented relatively early and subsequently declined rapidly. We have previously shown that NVOA is a common finding in PPAOS and that is has overlapping anatomical substrates with AOS(Botha et al., 2014).
The first time we found qualitative or quantitative evidence of aphasia was at his 2013 visit, eight years post-onset. Performance then declined over time, but aphasia remained less severe than the AOS throughout the disease course. Agrammatism was evident in his picture description samples, with the proportion of grammatical sentences being the first variable to decline. Beyond this, his production of correct verbs steadily declined, as did his production of closed-class words. Notably, although DY’s language declined in both spoken and written modalities, his writing was preserved for a longer time. This pattern has been previously reported in three cases with agrammatic aphasia(Hillis, Tuffiash, & Caramazza, 2002). For DY’s case, this difference is likely partly an artifact of his more severe AOS. His laborious and inconsistent articulations, his reduced intelligibility, and his acute awareness of those deficiencies may have altered his language as expressed through speech.
DY developed clinical features of corticobasal syndrome, and he eventually met diagnostic criteria for corticobasal syndrome, but notably this only occurred 1–2 years before death, after disease duration of almost ten years. Given the oculomotor slowing, he would have also met the Movement Disorder Society criteria for possible progressive supranuclear palsy with predominant speech/language dysfunction(Hoglinger et al., 2017). However, features of Parkinsonism were noted before this time with hypomimia, and reduced rapid alternation of his fingers was observed three years into his disease, perhaps representing early clinical signs of corticobasal syndrome. Early hypomimia has also been observed in another patient who started with speech problems and eventually developed corticobasal syndrome(Gorno-Tempini, Murray, Rankin, Weiner, & Miller, 2004). Around the time that DY’s Parkinsonism worsened considerably at nine years, he also developed some behavioral features. These features were, however, relatively mild compared to the severity of his Parkinsonism and AOS, and his scores on the NPI-Q were relatively stable over time. Behavioral and psychiatric features, therefore, can occur late in PPAOS but may not be prominent features of the disease. DY also showed relatively intact cognitive abilities throughout most of his disease course, with decline in general cognitive function only occurring in the last year or two before death.
DY underwent extensive neuroimaging examinations throughout his disease course. Notably, his very earliest FDG-PET and MRI scans showed no detectable abnormalities, despite the fact that he had a mild-moderate AOS at the time. Typically AOS is associated with atrophy and hypometabolism in the supplementary motor area and lateral premotor cortex(Josephs, et al., 2013; Josephs, et al., 2012). We did observe striking involvement of these regions later in the disease course but not for the first few years of the illness. It is possible that structural abnormalities were small enough to escape detection, or that abnormalities in other brain processes may proceed the development of atrophy, such as disruptions in structural or functional connectivity. Regardless, the clinical results show that a normal FDG-PET or MRI should not reduce confidence in the clinical diagnosis of PPAOS. An unexpected finding was that, at least on FDG-PET, abnormalities in the right caudate nucleus were observed before abnormalities in the premotor cortex. It is possible that involvement of this structure may be related to the eventual development of corticobasal syndrome in DY; atrophy and hypometabolism of the striatum is a common finding in corticobasal syndrome(Josephs et al., 2008; Zalewski, et al., 2014).
Our quantitative analysis of regional atrophy in DY demonstrates how the disease progressed through the brain from 2010 to 2015, less than one year before death. In concordance with the observed deficits in FDG-PET scans, we observed atrophy in 2010 predominantly in the striatum, frontal lobes, particularly supplementary motor area, inferior, middle and superior frontal gyri and anterior and middle cingulate, and insula. Findings in the frontal regions were predominantly left-sided. This demonstrates involvement of regions we have previously shown to be associated with AOS(Whitwell et al., 2013), but also shows involvement of Broca’s area, which has been associated with agrammatic aphasia(Amici et al., 2007; Whitwell, et al., 2013). The fact that Broca’s area showed abnormalities before the development of agrammatic aphasia in DY suggests that involvement of this region could be a useful predictor for the future development of aphasia in PPAOS. We have previously shown this to also be the case in a larger cohort of patients with PPAOS(Whitwell, et al., 2017). Over time we observed progression in these regions but also the gradual involvement of other regions, starting with the motor cortex, lateral temporal lobe and thalamus in 2013, and then with later involvement of sensory cortex and parietal lobes in 2015. The gradual spreading of atrophy to involve temporal and parietal lobes coincided with the development of more striking cognitive impairment and Parkinsonism in DY, suggesting a possible association between these features. It is also possible that the spread of atrophy into the motor cortex may have been related to the development of the mild dysarthria, which we have shown to be related to degeneration of motor cortex white matter tracts(Clark et al., 2014).
In summary, longitudinal assessment and observation of patient DY has provided invaluable information about the clinical evolution of PPAOS and how this devastating disease progresses within the brain. Although speech deficits can remain the predominant feature for many years; other cognitive, behavioral, motor and swallowing features will evolve quite rapidly in the final years of the disease, which will present many challenges to the patient and caregivers. Understanding this evolution will be critical for clinicians to help educate and assist future patients and their families.
Acknowledgments
We would like to thank Dr. Clifford Jack, Jr, for allowing the use of neuroimaging pipelines developed in his laboratory and Dr. Val Lowe for overseeing the PET imaging. We would also like to express our gratitude to patient DY and his family for their dedication to our research program. Without the generosity and support of patients like DY research into this devastating disease would not be possible.
Footnotes
Disclosure statement
The authors report no conflicts of interest. This work was supported by the National Institutes of Health under grants R01-DC12519, R01-DC010367 and R21-NS89757.
References
- Amici S, Brambati SM, Wilkins DP, Ogar J, Dronkers NL, Miller BL, Gorno-Tempini ML. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci. 2007;27(23):6282–6290. doi: 10.1523/JNEUROSCI.1331-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA. Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology. 2014;82(19):1729–1735. doi: 10.1212/WNL.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash DM, Frost C, Iheme LO, Unay D, Kandemir M, Fripp J, … Ourselin S. Assessing atrophy measurement techniques in dementia: Results from the MIRIAD atrophy challenge. Neuroimage. 2015;123:149–164. doi: 10.1016/j.neuroimage.2015.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HM, Duffy JR, Whitwell JL, Ahlskog JE, Sorenson EJ, Josephs KA. Clinical and imaging characterization of progressive spastic dysarthria. Eur J Neurol. 2014;21(3):368–376. doi: 10.1111/ene.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Code C, Ball M, Tree J, Dawe K. The effects of initiation, termination and inhibition impairments on speech rate in a case of progressive nonfluent aphasia with progressive apraxia of speech with frontotemporal degeneration. J Neurolinguistics. 2013;26:602–618. [Google Scholar]
- De Renzi E, Vignolo LA. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, … Pasquier F. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74(1):42–49. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55(11):1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders: substrates, differetial diagnois, and management. 2. St Louis, MI: Mosby; 2005. [Google Scholar]
- Duffy JR. Apraxia of Speech in degenerative neurologic disease. Aphasiology. 2006;20(6):511–527. [Google Scholar]
- Duffy JR, Hanley H, Utianski R, Clark H, Strand E, Josephs KA, Whitwell JL. Temporal acoustic measures distinguish primary progressive apraxia of speech from primary progressive aphasia. Brain Lang. 2017;168:84–94. doi: 10.1016/j.bandl.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, Josephs KA. The diagnosis and understanding of apraxia of speech: why including neurodegenerative etiologies may be important. J Speech Lang Hear Res. 2012;55(5):S1518–1522. doi: 10.1044/1092-4388(2012/11-0309). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, Strand EA, Clark H, Machulda M, Whitwell JL, Josephs KA. Primary progressive apraxia of speech: Clinical features and acoustic and neurologic correlates. American journal of speech-language pathology. 2015;24(2):88–100. doi: 10.1044/2015_AJSLP-14-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, Strand EA, Josephs KA. Motor Speech Disorders Associated with Primary Progressive Aphasia. Aphasiology. 2014;28(8–9):1004–1017. doi: 10.1080/02687038.2013.869307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, … LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders: official journal of the Movement Disorder Society. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, … Grossman M. Classification of primary progressive aphasia and its variants. [Consensus Development Conference Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Murray RC, Rankin KP, Weiner MW, Miller BL. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase. 2004;10(6):426–436. doi: 10.1080/13554790490894011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter JL, Senjem ML, Vemuri P, Jack CR., Jr Comparison of mask-based differences, boundary shift integral and symmetric normalization jacobian integration. MICCAI 2012 workshop on novel imaging biomarkers for Alzheimer’s disease and related disorders (NIBAD’12).2012. [Google Scholar]
- Hillis AE, Tuffiash E, Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. J Cogn Neurosci. 2002;14(7):1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE … Movement Disorder Society-endorsed PSPSG. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32(6):853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Boeve BF, Duffy JR, Smith GE, Knopman DS, Parisi JE, … Dickson DW. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11(4):283–296. doi: 10.1080/13554790590963004. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, … Whitwell JL. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81(4):337–345. doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, … Whitwell JL. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(Pt 5):1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, … Petersen RC. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, … Jack CR., Jr Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29(2):280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Tacik P, Duffy JR, Senjem ML, Tosakulwong N, … Murray ME. [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol. 2016;132(6):931–933. doi: 10.1007/s00401-016-1618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, … DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery (Revised) San Antonio, Tx: PsychCorp; 2007. [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 1999;14(6):481–487. [PubMed] [Google Scholar]
- Loonstra AS, Tarlow AR, Sellers AH. COWAT metanorms across age, education, and gender. [Review] Applied neuropsychology. 2001;8(3):161–166. doi: 10.1207/S15324826AN0803_5. [DOI] [PubMed] [Google Scholar]
- MacWhinney B. The CHILDES project: The database. Psychology Press; 2000. [Google Scholar]
- McNeil MR, Robin DA, Schmidt RA. Apraxia of speech: definition and differential diagnosis. In: McNeil MR, editor. Clinical management of sensorimotor speech disorders. New York: Thieme; 2009. [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Annals of neurology. 2001;49(4):425–432. [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36(7):1238–1248. [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, … Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- Spreen O, Strauss E. Compendium of Neuropsychological tests, second edition: administration, norms and commentary. New York: Oxford University Press; 1998. [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam MM. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11(4–5):297–321. [Google Scholar]
- Warrington EK, James M. The visual object and space perception battery. Bury St Edmonds, UK: Thames Valley Test Company; 1991. [Google Scholar]
- Whitwell JL, Duffy JR, Machulda MM, Clark HM, Strand EA, Senjem ML, … Josephs KA. Tracking the development of agrammatic aphasia: A tensor-based morphometry study. Cortex. 2017;90:138–148. doi: 10.1016/j.cortex.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, Machulda MM, … Josephs KA. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang. 2013;125(3):245–252. doi: 10.1016/j.bandl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Master AV, Avula R, Kantarci K, Eggers SD, Edmonson HA, … Josephs KA. Clinical correlates of white matter tract degeneration in PSP. Arch Neurol. 2011;68(6):753–760. doi: 10.1001/archneurol.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test 3. Wilmington, Delaware: Wide Range, Inc; 1993. [Google Scholar]
- Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Troster AI. Action (verb) fluency: test-retest reliability, normative standards, and construct validity. Journal of the International Neuropsychological Society: JINS. 2005;11(4):408–415. [PubMed] [Google Scholar]
- Yorkston KM, Strand EA, Miller R, Hillel A, Smith K. Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. Journal of Medical Speech-Language Pathology. 1993;1(1):35–46. [Google Scholar]
- Zalewski N, Botha H, Whitwell JL, Lowe V, Dickson DW, Josephs KA. FDG-PET in pathologically confirmed spontaneous 4R-tauopathy variants. J Neurol. 2014;261(4):710–716. doi: 10.1007/s00415-014-7256-4. [DOI] [PubMed] [Google Scholar]